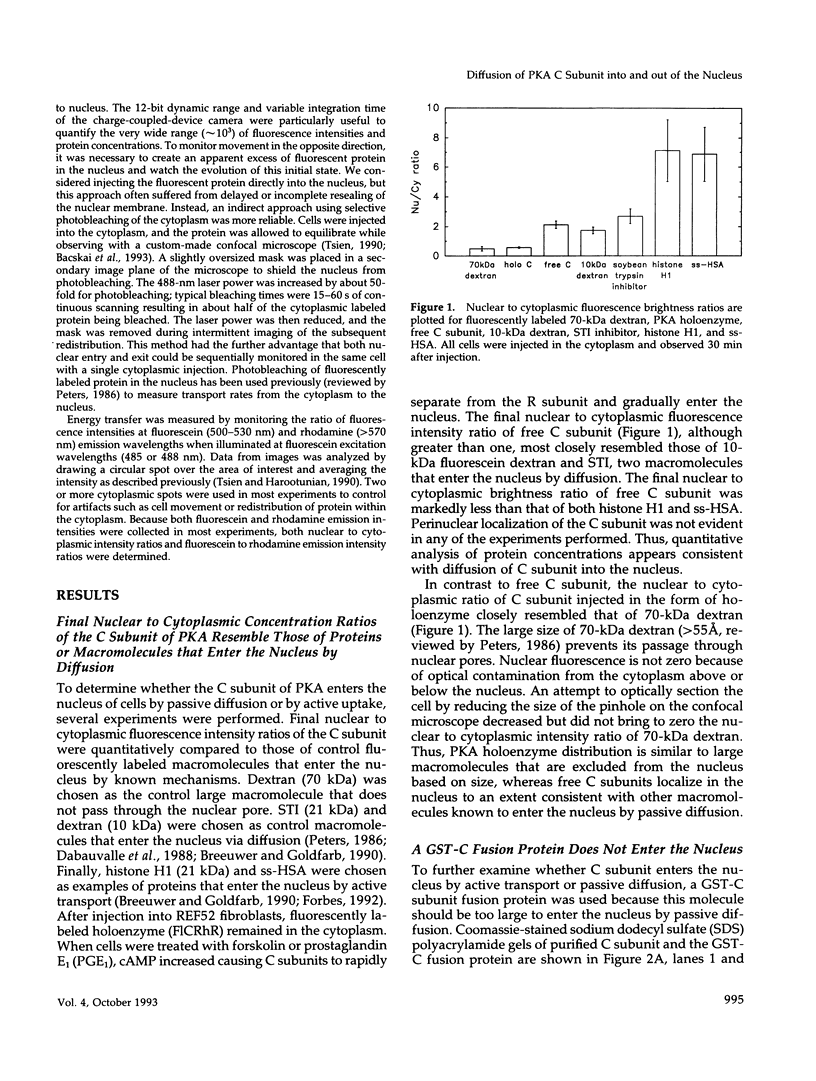
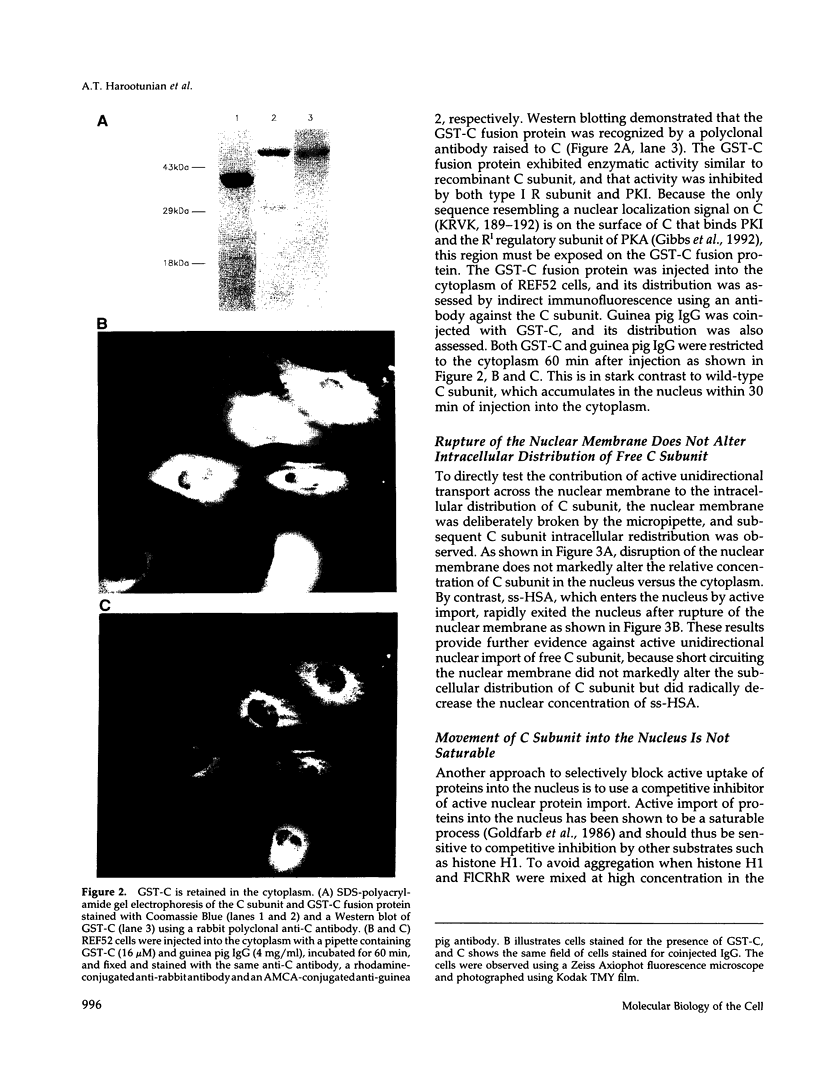
Abstract
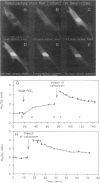
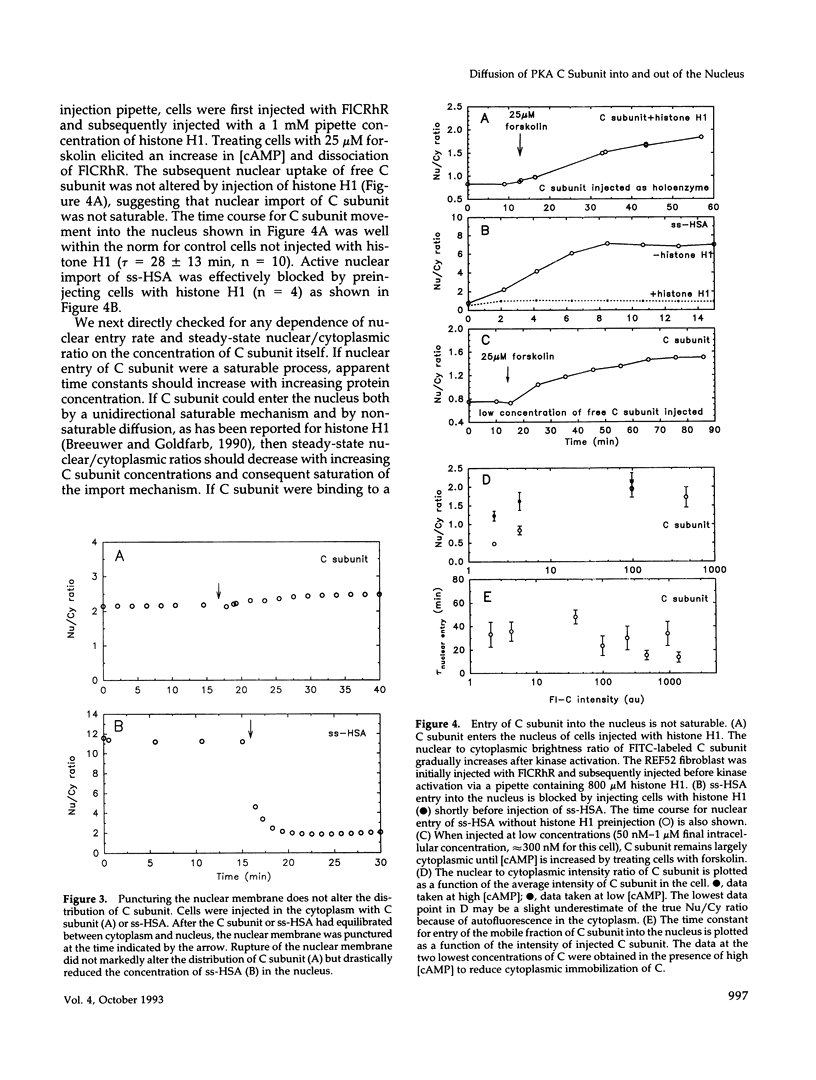
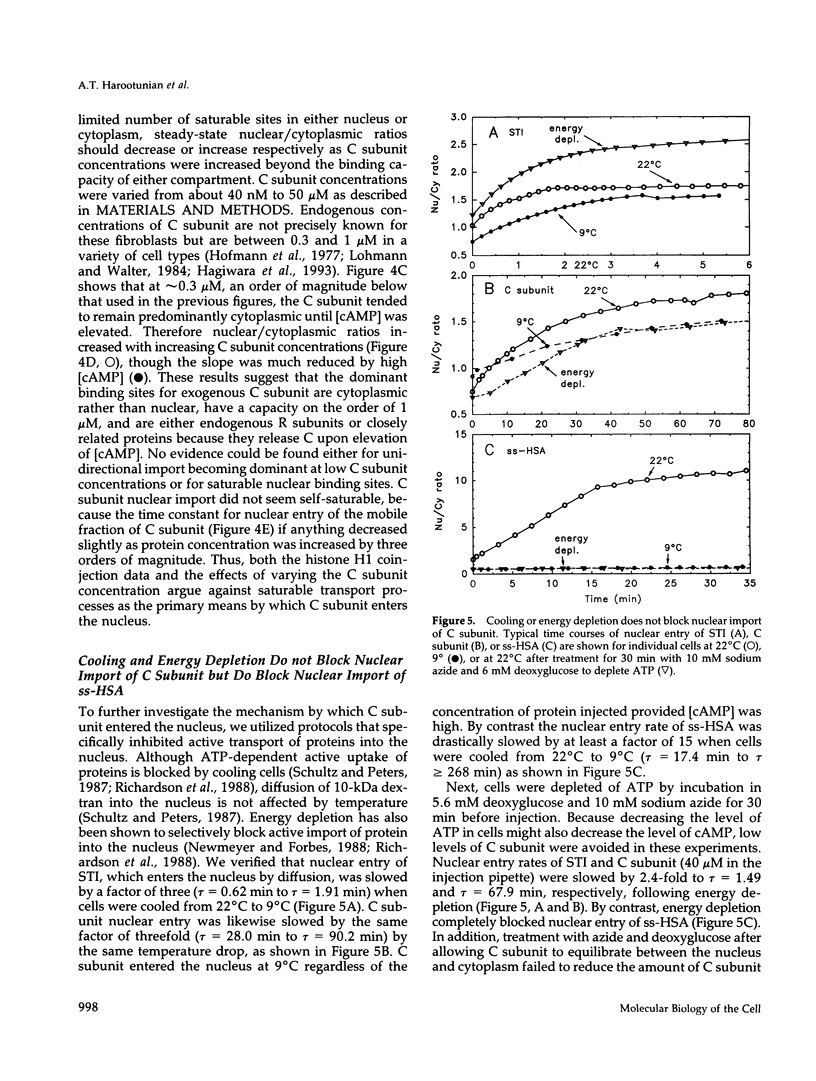
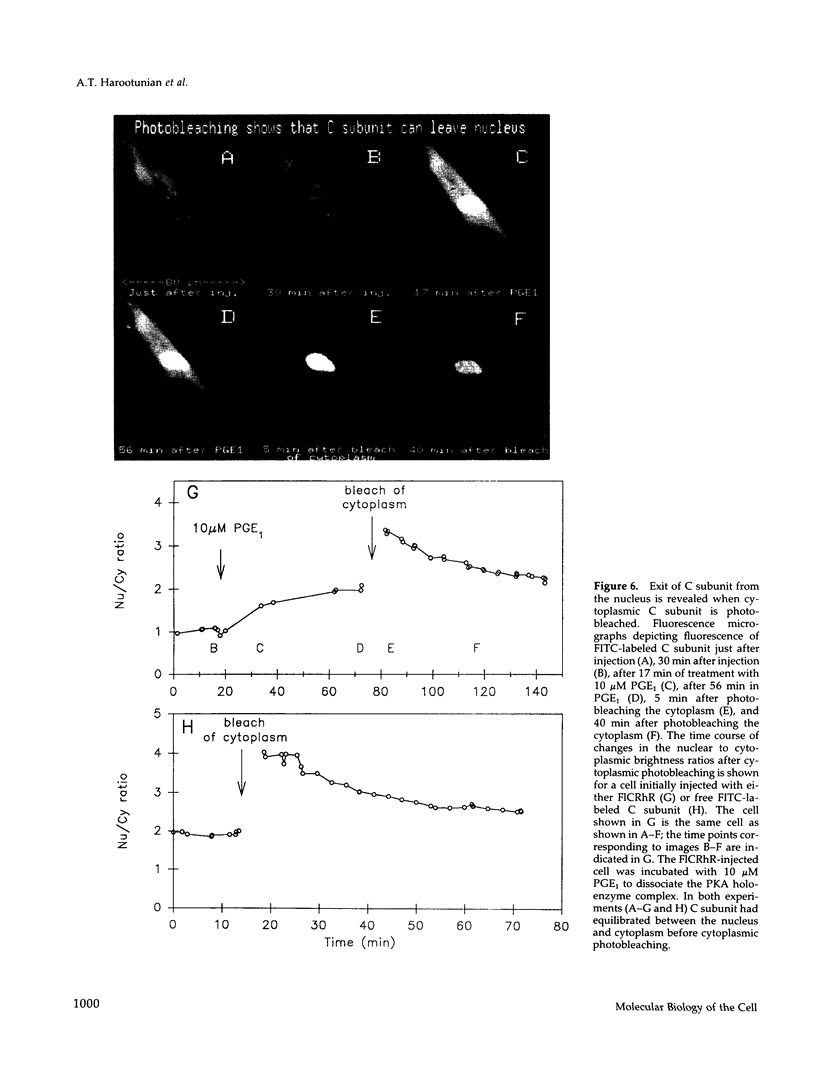
The catalytic (C) subunit of cyclic AMP (cAMP) dependent protein kinase (PKA) has previously been shown to enter and exit the nucleus of cells when intracellular cAMP is raised and lowered, respectively. To determine the mechanism of nuclear translocation, fluorescently labeled C subunit was injected into living REF52 fibroblasts either as free C subunit or in the form of holoenzyme (PKA) in which the catalytic and regulatory subunits were labeled with fluorescein and rhodamine, respectively. Quantification of nuclear and cytoplasmic fluorescence intensities revealed that free C subunit nuclear accumulation was most similar to that of macromolecules that diffuse into the nucleus. A glutathione S-transferase-C subunit fusion protein did not enter the nucleus following cytoplasmic microinjection. Puncturing the nuclear membrane did not decrease the nuclear concentration of C subunit, and C subunit entry into the nucleus did not appear to be saturable. Cooling or depleting cells of energy failed to block movement of C subunit into the nucleus. Photobleaching experiments showed that even after reaching equilibrium at high [cAMP], individual molecules of C subunit continued to leave the nucleus at approximately the same rate that they had originally entered. These results indicate that diffusion is sufficient to explain most aspects of C subunit subcellular localization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. R., Harootunian A. T., Buechler Y. J., Taylor S. S., Tsien R. Y. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991 Feb 21;349(6311):694–697. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- Bacskai B. J., Hochner B., Mahaut-Smith M., Adams S. R., Kaang B. K., Kandel E. R., Tsien R. Y. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993 Apr 9;260(5105):222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989 Feb 10;56(3):379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Breeuwer M., Goldfarb D. S. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990 Mar 23;60(6):999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- Brindle P. K., Montminy M. R. The CREB family of transcription activators. Curr Opin Genet Dev. 1992 Apr;2(2):199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- Cook P. F., Neville M. E., Jr, Vrana K. E., Hartl F. T., Roskoski R., Jr Adenosine cyclic 3',5'-monophosphate dependent protein kinase: kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochemistry. 1982 Nov 9;21(23):5794–5799. doi: 10.1021/bi00266a011. [DOI] [PubMed] [Google Scholar]
- Dabauvalle M. C., Schulz B., Scheer U., Peters R. Inhibition of nuclear accumulation of karyophilic proteins in living cells by microinjection of the lectin wheat germ agglutinin. Exp Cell Res. 1988 Jan;174(1):291–296. doi: 10.1016/0014-4827(88)90163-2. [DOI] [PubMed] [Google Scholar]
- Fantozzi D. A., Taylor S. S., Howard P. W., Maurer R. A., Feramisco J. R., Meinkoth J. L. Effect of the thermostable protein kinase inhibitor on intracellular localization of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1992 Aug 25;267(24):16824–16828. [PubMed] [Google Scholar]
- Forbes D. J. Structure and function of the nuclear pore complex. Annu Rev Cell Biol. 1992;8:495–527. doi: 10.1146/annurev.cb.08.110192.002431. [DOI] [PubMed] [Google Scholar]
- Gibbs C. S., Knighton D. R., Sowadski J. M., Taylor S. S., Zoller M. J. Systematic mutational analysis of cAMP-dependent protein kinase identifies unregulated catalytic subunits and defines regions important for the recognition of the regulatory subunit. J Biol Chem. 1992 Mar 5;267(7):4806–4814. [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Lescop P., Christin-Maitre S., Loosfelt H., Perrot-Applanat M., Milgrom E. Nucleocytoplasmic shuttling of the progesterone receptor. EMBO J. 1991 Dec;10(12):3851–3859. doi: 10.1002/j.1460-2075.1991.tb04954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
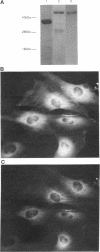
- Hagiwara M., Brindle P., Harootunian A., Armstrong R., Rivier J., Vale W., Tsien R., Montminy M. R. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993 Aug;13(8):4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Horowitz S. B., Paine P. L. Cytoplasmic exclusion as a basis for asymmetric nucleocytoplasmic solute distributions. Nature. 1976 Mar 11;260(5547):151–153. doi: 10.1038/260151a0. [DOI] [PubMed] [Google Scholar]
- Kuettel M. R., Squinto S. P., Kwast-Welfeld J., Schwoch G., Schweppe J. S., Jungmann R. A. Localization of nuclear subunits of cyclic AMP-dependent protein kinase by the immunocolloidal gold method. J Cell Biol. 1985 Sep;101(3):965–975. doi: 10.1083/jcb.101.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Feramisco J. R., Welch W. J. Modulation of vimentin containing intermediate filament distribution and phosphorylation in living fibroblasts by the cAMP-dependent protein kinase. J Cell Biol. 1989 Jun;108(6):2409–2422. doi: 10.1083/jcb.108.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U. Regulation of the cellular and subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- Mandell R. B., Feldherr C. M. Identification of two HSP70-related Xenopus oocyte proteins that are capable of recycling across the nuclear envelope. J Cell Biol. 1990 Nov;111(5 Pt 1):1775–1783. doi: 10.1083/jcb.111.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. T., Blobel G. A nuclear localization signal binding protein in the nucleolus. J Cell Biol. 1990 Dec;111(6 Pt 1):2235–2245. doi: 10.1083/jcb.111.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J. L., Ji Y., Taylor S. S., Feramisco J. R. Dynamics of the distribution of cyclic AMP-dependent protein kinase in living cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9595–9599. doi: 10.1073/pnas.87.24.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Hilz H., Eppenberger H. M., Dutly F. Rapid and reversible translocation of the catalytic subunit of cAMP-dependent protein kinase type II from the Golgi complex to the nucleus. EMBO J. 1985 Nov;4(11):2801–2806. doi: 10.1002/j.1460-2075.1985.tb04006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. Mechanisms of signal transduction to the cell nucleus. Adv Cancer Res. 1990;55:271–310. doi: 10.1016/s0065-230x(08)60471-6. [DOI] [PubMed] [Google Scholar]
- Peters R. Fluorescence microphotolysis to measure nucleocytoplasmic transport and intracellular mobility. Biochim Biophys Acta. 1986 Dec 22;864(3-4):305–359. doi: 10.1016/0304-4157(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T. Transcription factor activity during cellular aging of human diploid fibroblasts. Biochem Cell Biol. 1992 Oct-Nov;70(10-11):1064–1072. doi: 10.1139/o92-151. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Sammak P. J., Adams S. R., Harootunian A. T., Schliwa M., Tsien R. Y. Intracellular cyclic AMP not calcium, determines the direction of vesicle movement in melanophores: direct measurement by fluorescence ratio imaging. J Cell Biol. 1992 Apr;117(1):57–72. doi: 10.1083/jcb.117.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B., Peters R. Nucleocytoplasmic protein traffic in single mammalian cells studied by fluorescence microphotolysis. Biochim Biophys Acta. 1987 Oct 1;930(3):419–431. doi: 10.1016/0167-4889(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Stofko R. E., McDonald J. R., Comer J. D., Vitalis E. A., Mangili J. A. Type II regulatory subunit dimerization determines the subcellular localization of the cAMP-dependent protein kinase. J Biol Chem. 1990 Dec 15;265(35):21561–21566. [PubMed] [Google Scholar]
- Slice L. W., Taylor S. S. Expression of the catalytic subunit of cAMP-dependent protein kinase in Escherichia coli. J Biol Chem. 1989 Dec 15;264(35):20940–20946. [PubMed] [Google Scholar]
- Tsien R. Y., Harootunian A. T. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990 Feb-Mar;11(2-3):93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Waeber G., Habener J. F. Nuclear translocation and DNA recognition signals colocalized within the bZIP domain of cyclic adenosine 3',5'-monophosphate response element-binding protein CREB. Mol Endocrinol. 1991 Oct;5(10):1431–1438. doi: 10.1210/mend-5-10-1431. [DOI] [PubMed] [Google Scholar]