Abstract
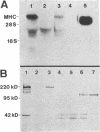
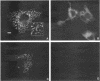
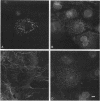
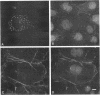
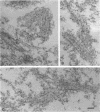
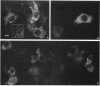
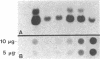
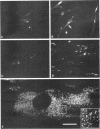
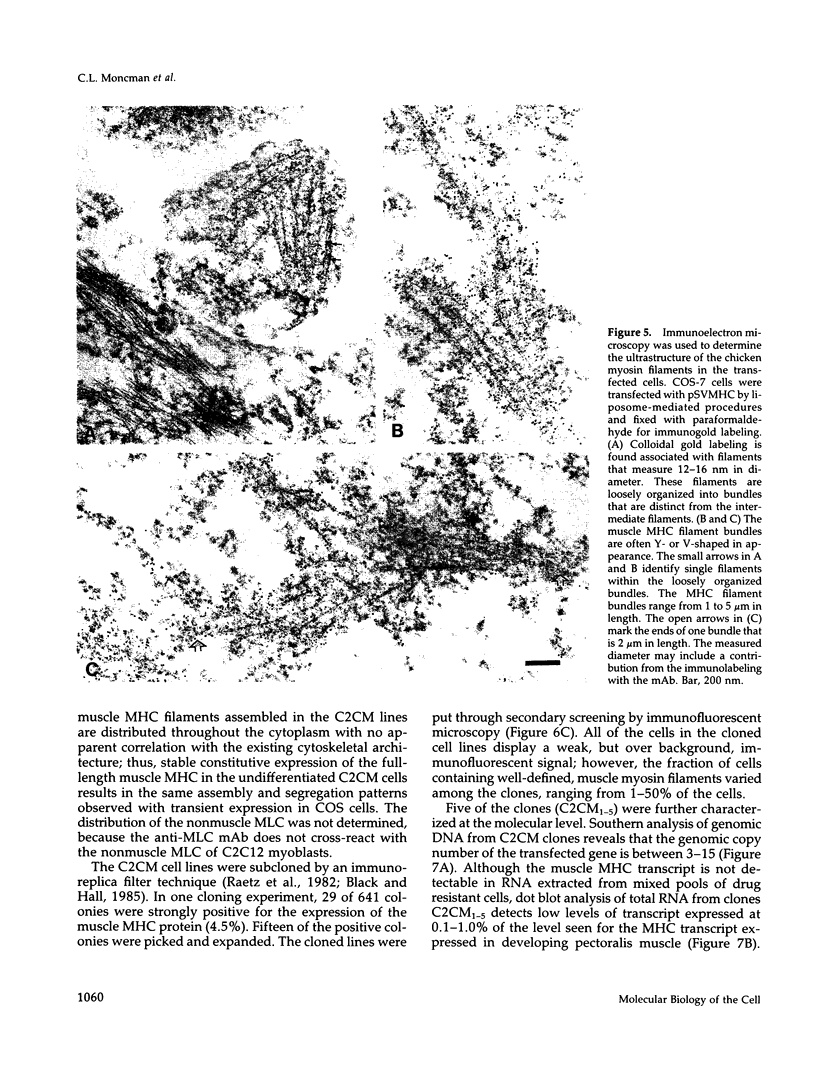
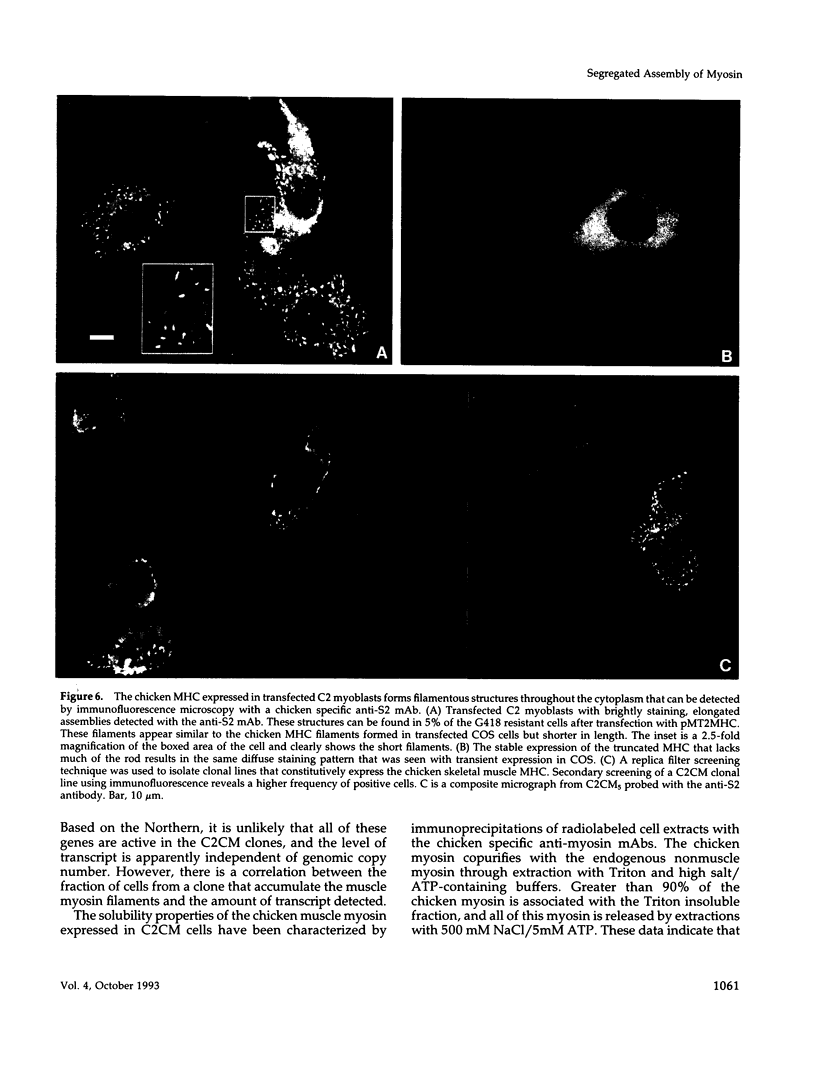
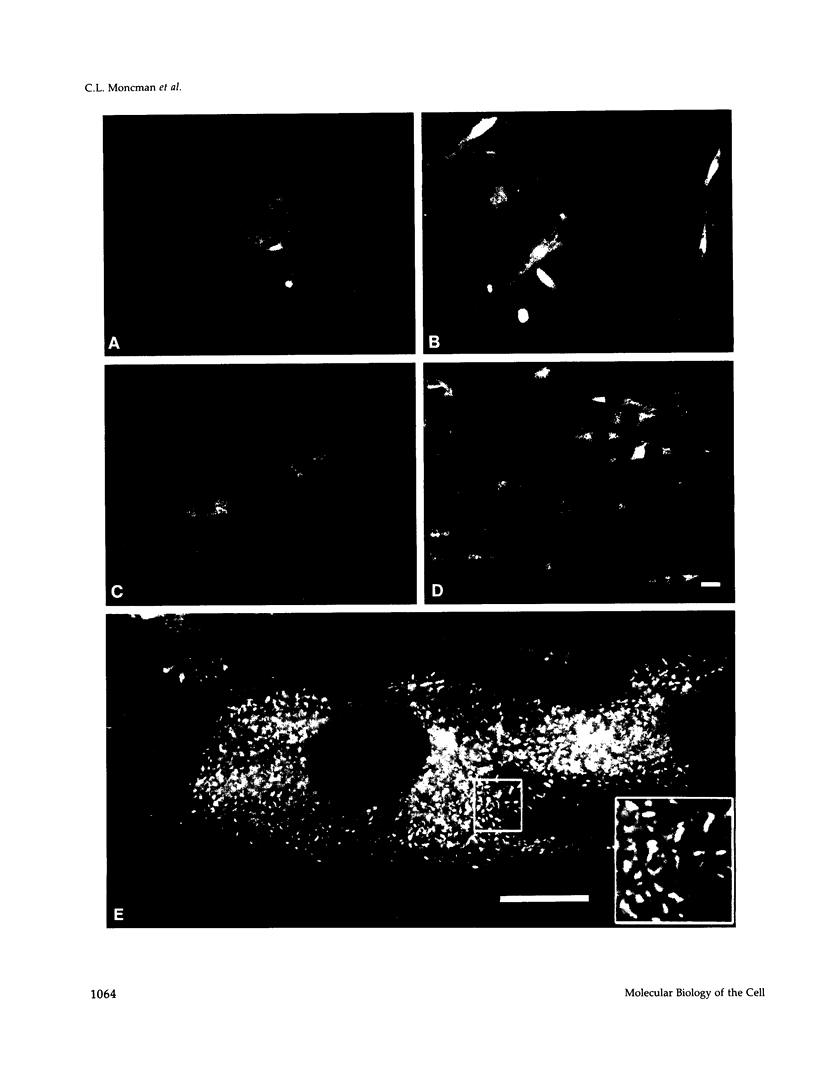
Skeletal muscle myosin cDNAs were expressed in a simian kidney cell line (COS) and a mouse myogenic cell line to investigate the mechanisms controlling early stages of myosin filament assembly. An embryonic chicken muscle myosin heavy chain (MHC) cDNA was linked to constitutive promoters from adenovirus or SV40 and transiently expressed in COS cells. These cells accumulate hybrid myosin molecules composed of muscle MHCs and endogenous, nonmuscle, myosin light chains. The muscle myosin is found associated with a Triton insoluble fraction from extracts of the COS cells by immunoprecipitation and is detected in 2.4 +/- 0.8-micron-long filamentous structures distributed throughout the cytoplasm by immunofluorescence microscopy. These structures are shown by immunoelectron microscopy to correspond to loosely organized bundles of 12-16-nm-diameter myosin filaments. The muscle and nonmuscle MHCs are segregated in the transfected cells; the endogenous nonmuscle myosin displays a normal distribution pattern along stress fibers and does not colocalize with the muscle myosin filament bundles. A similar assembly pattern and distribution are observed for expression of the muscle MHC in a myogenic cell line. The myosin assembles into filament bundles, 1.5 +/- 0.6 micron in length, that are distributed throughout the cytoplasm of the undifferentiated myoblasts and segregated from the endogenous nonmuscle myosin. In both cell lines, formation of the myosin filament bundles is dependent on the accumulation of the protein. In contrast to these results, the expression of a truncated MHC that lacks much of the rod domain produces an assembly deficient molecule. The truncated MHC is diffusely distributed throughout the cytoplasm and not associated with cellular stress fibers. These results establish that the information necessary for the segregation of myosin isotypes into distinct cellular structures is contained within the primary structure of the MHC and that other factors are not required to establish this distribution.
Full text
PDF




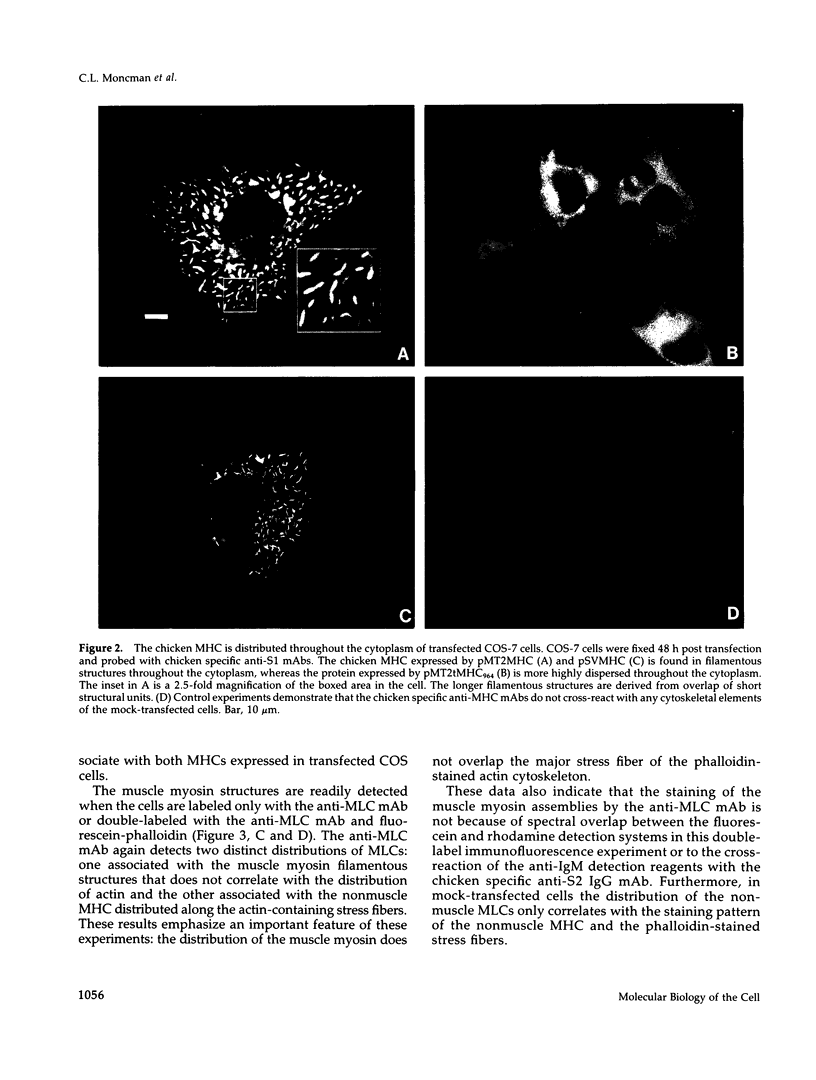
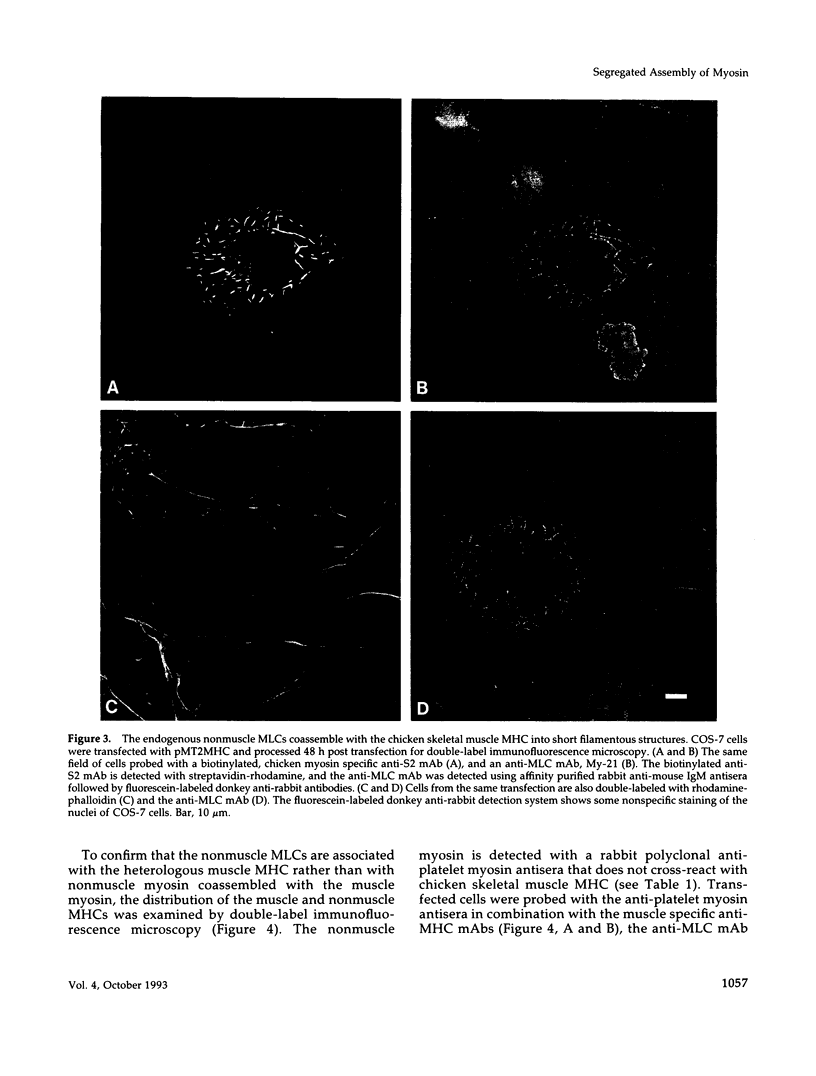
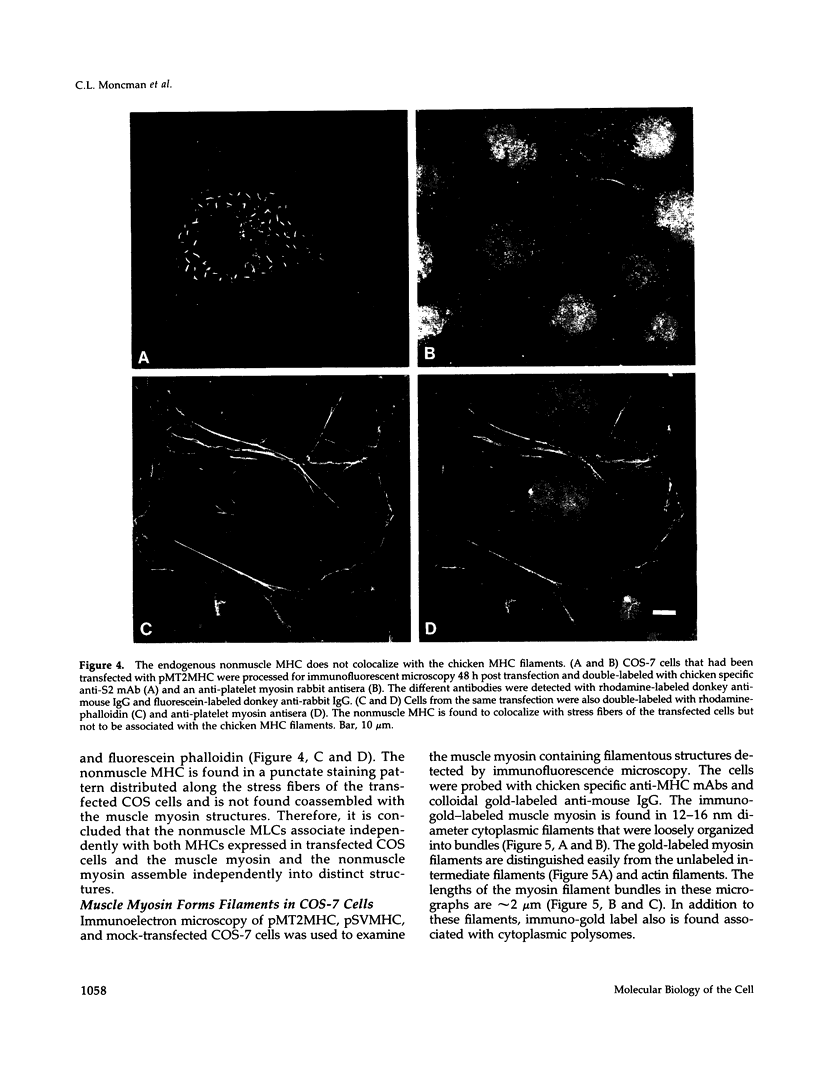
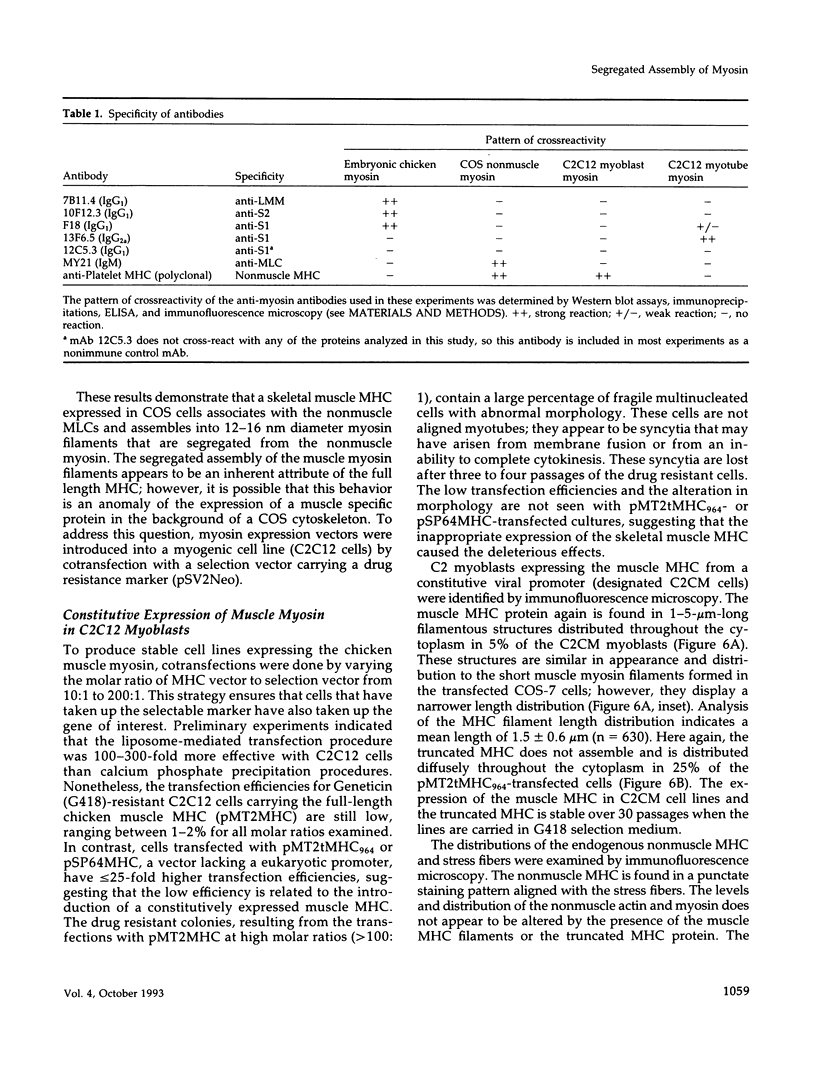





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amasino R. M. Acceleration of nucleic acid hybridization rate by polyethylene glycol. Anal Biochem. 1986 Feb 1;152(2):304–307. doi: 10.1016/0003-2697(86)90413-6. [DOI] [PubMed] [Google Scholar]
- Antin P. B., Tokunaka S., Nachmias V. T., Holtzer H. Role of stress fiber-like structures in assembling nascent myofibrils in myosheets recovering from exposure to ethyl methanesulfonate. J Cell Biol. 1986 Apr;102(4):1464–1479. doi: 10.1083/jcb.102.4.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson S. J., Stewart M. Expression in Escherichia coli of fragments of the coiled-coil rod domain of rabbit myosin: influence of different regions of the molecule on aggregation and paracrystal formation. J Cell Sci. 1991 Aug;99(Pt 4):823–836. doi: 10.1242/jcs.99.4.823. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Beall C. J., Sepanski M. A., Fyrberg E. A. Genetic dissection of Drosophila myofibril formation: effects of actin and myosin heavy chain null alleles. Genes Dev. 1989 Feb;3(2):131–140. doi: 10.1101/gad.3.2.131. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Fitch J. M., Babiarz J. P., Linsenmayer T. F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol. 1988 Mar;106(3):999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A., Hall Z. W. Use of a replica technique to isolate muscle cell lines defective in expressing the acetylcholine receptor. Proc Natl Acad Sci U S A. 1985 Jan;82(1):124–128. doi: 10.1073/pnas.82.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Citi S., Smith R. C., Kendrick-Jones J. Effects of light chain phosphorylation and skeletal myosin on the stability of non-muscle myosin filaments. J Mol Biol. 1987 Nov 20;198(2):253–262. doi: 10.1016/0022-2836(87)90311-1. [DOI] [PubMed] [Google Scholar]
- Colley N. J., Tokuyasu K. T., Singer S. J. The early expression of myofibrillar proteins in round postmitotic myoblasts of embryonic skeletal muscle. J Cell Sci. 1990 Jan;95(Pt 1):11–22. doi: 10.1242/jcs.95.1.11. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Dlugosz A. A., Antin P. B., Nachmias V. T., Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984 Dec;99(6):2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane K. J., Birk D. E. Fibroblasts retain their tissue phenotype when grown in three-dimensional collagen gels. Exp Cell Res. 1991 Aug;195(2):432–442. doi: 10.1016/0014-4827(91)90394-a. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J., Kurowski T. T., Zak R. Synthesis and assembly of native myosin on muscle polyribosomes. FEBS Lett. 1989 Jul 3;250(2):549–555. doi: 10.1016/0014-5793(89)80794-x. [DOI] [PubMed] [Google Scholar]
- Handel S. E., Wang S. M., Greaser M. L., Schultz E., Bulinski J. C., Lessard J. L. Skeletal muscle myofibrillogenesis as revealed with a monoclonal antibody to titin in combination with detection of the alpha- and gamma-isoforms of actin. Dev Biol. 1989 Mar;132(1):35–44. doi: 10.1016/0012-1606(89)90202-9. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Duran S., Lin Z. X., Weber K., Holtzer H. Titin and myosin, but not desmin, are linked during myofibrillogenesis in postmitotic mononucleated myoblasts. J Cell Biol. 1986 Dec;103(6 Pt 1):2185–2196. doi: 10.1083/jcb.103.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs W. B., Fulton A. B. Cotranslational assembly of myosin heavy chain in developing cultured skeletal muscle. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6174–6178. doi: 10.1073/pnas.84.17.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Manstein D. J., Ruppel K. M., Spudich J. A. Expression and characterization of a functional myosin head fragment in Dictyostelium discoideum. Science. 1989 Nov 3;246(4930):656–658. doi: 10.1126/science.2530629. [DOI] [PubMed] [Google Scholar]
- McNally E. M., Goodwin E. B., Spudich J. A., Leinwand L. A. Coexpression and assembly of myosin heavy chain and myosin light chain in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7270–7273. doi: 10.1073/pnas.85.19.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Teal S. B., Stockdale F. E. Evolutionarily conserved sequences of striated muscle myosin heavy chain isoforms. Epitope mapping by cDNA expression. J Biol Chem. 1989 Aug 5;264(22):13122–13130. [PubMed] [Google Scholar]
- Mittal B., Sanger J. M., Sanger J. W. Visualization of myosin in living cells. J Cell Biol. 1987 Oct;105(4):1753–1760. doi: 10.1083/jcb.105.4.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M. I., Kropp K. E., Gulick J., Robbins J. The sequence of an embryonic myosin heavy chain gene and isolation of its corresponding cDNA. J Biol Chem. 1987 May 15;262(14):6478–6488. [PubMed] [Google Scholar]
- Moss P., Micou-Eastwood J., Strohman R. Altered synthesis of myosin light chains is associated with contractility in cultures of differentiating chick embryo breast muscle. Dev Biol. 1986 Apr;114(2):311–314. doi: 10.1016/0012-1606(86)90195-8. [DOI] [PubMed] [Google Scholar]
- Nyitray L., Mocz G., Szilagyi L., Balint M., Lu R. C., Wong A., Gergely J. The proteolytic substructure of light meromyosin. Localization of a region responsible for the low ionic strength insolubility of myosin. J Biol Chem. 1983 Nov 10;258(21):13213–13220. [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. Purification of nonmuscle myosins. Methods Enzymol. 1982;85(Pt B):331–356. doi: 10.1016/0076-6879(82)85033-7. [DOI] [PubMed] [Google Scholar]
- Pomeroy M. E., Lawrence J. B., Singer R. H., Billings-Gagliardi S. Distribution of myosin heavy chain mRNA in embryonic muscle tissue visualized by ultrastructural in situ hybridization. Dev Biol. 1991 Jan;143(1):58–67. doi: 10.1016/0012-1606(91)90054-7. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Nagai K., Kendrick-Jones J. Site-directed mutagenesis of the regulatory light-chain Ca2+/Mg2+ binding site and its role in hybrid myosins. Nature. 1986 Jul 3;322(6074):80–83. doi: 10.1038/322080a0. [DOI] [PubMed] [Google Scholar]
- Rindt H., Bauer B. J., Robbins J. In vitro production of enzymatically active myosin heavy chain. J Muscle Res Cell Motil. 1993 Feb;14(1):26–34. doi: 10.1007/BF00132177. [DOI] [PubMed] [Google Scholar]
- Samarel A. M., Ferguson A. G., Vander Heide R. S., Davison R., Ganote C. E. Release of unassembled rat cardiac myosin light chain 1 following the calcium paradox. Circ Res. 1986 Jan;58(1):166–171. doi: 10.1161/01.res.58.1.166. [DOI] [PubMed] [Google Scholar]
- Sanger J. W., Mittal B., Sanger J. M. Analysis of myofibrillar structure and assembly using fluorescently labeled contractile proteins. J Cell Biol. 1984 Mar;98(3):825–833. doi: 10.1083/jcb.98.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevzov G., Lloyd C., Gunning P. High level expression of transfected beta- and gamma-actin genes differentially impacts on myoblast cytoarchitecture. J Cell Biol. 1992 May;117(4):775–785. doi: 10.1083/jcb.117.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver G., Etlinger J. D. Regulation of myofibrillar accumulation in chick muscle cultures: evidence for the involvement of calcium and lysosomes in non-uniform turnover of contractile proteins. J Cell Biol. 1985 Dec;101(6):2383–2391. doi: 10.1083/jcb.101.6.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati T., Perriard J. C. Intracompartmental sorting of essential myosin light chains: molecular dissection and in vivo monitoring by epitope tagging. Cell. 1991 Jul 26;66(2):277–289. doi: 10.1016/0092-8674(91)90618-9. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spudich J. A. In pursuit of myosin function. Cell Regul. 1989 Nov;1(1):1–11. doi: 10.1091/mbc.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Wachsberger P. R., Pepe F. A. Interaction between vertebrate skeletal and uterine muscle myosins and light meromyosins. J Cell Biol. 1980 Apr;85(1):33–41. doi: 10.1083/jcb.85.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. M., Greaser M. L., Schultz E., Bulinski J. C., Lin J. J., Lessard J. L. Studies on cardiac myofibrillogenesis with antibodies to titin, actin, tropomyosin, and myosin. J Cell Biol. 1988 Sep;107(3):1075–1083. doi: 10.1083/jcb.107.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Lowey S., Press J. L. Monoclonal antibodies localize changes on myosin heavy chain isozymes during avian myogenesis. Cell. 1983 Aug;34(1):295–306. doi: 10.1016/0092-8674(83)90160-5. [DOI] [PubMed] [Google Scholar]
- Winkelmann D. A., Lowey S. Probing myosin head structure with monoclonal antibodies. J Mol Biol. 1986 Apr 20;188(4):595–612. doi: 10.1016/s0022-2836(86)80009-2. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Blough R. Assessment of protein turnover by use of radioisotopic tracers. Physiol Rev. 1979 Apr;59(2):407–447. doi: 10.1152/physrev.1979.59.2.407. [DOI] [PubMed] [Google Scholar]