Abstract
The evolution of eusociality, especially how selection would favor sterility or subfertility of most individuals within a highly social colony, is an unresolved paradox. Eusociality evolved independently in diverse taxa, including insects (all ants and termites; some bees, wasps, thrips, and beetles), snapping shrimp, and naked mole rats. Termites have received comparatively less focus than the haplodiploid Hymenoptera (ants, bees, and wasps); however, they are the only diploid group with highly complex colonies and an extraordinary diversity of castes. In this study we staged encounters between unrelated colonies of primitive dampwood termites, Zootermopsis nevadensis, mimicking natural meetings that occur under bark. During encounters, kings and/or queens were killed and surviving members merged into one colony. After encounters, members of both unrelated colonies cooperated as a single social unit. We determined the colony of origin of replacement reproductives that emerged after death of kings and/or queens. Here, we document that replacement reproductives developed from workers in either or both original colonies, inherited the merged resources of the colony, and sometimes interbred. Because this species shares many characteristics with ancestral termites, these findings demonstrate how ecological factors could have promoted the evolution of eusociality by accelerating and enhancing direct fitness opportunities of helper offspring, rendering relatedness favoring kin selection less critical.
Keywords: ancestral termites, evolution of eusociality, replacement reproductives, selection, Zootermopsis
The evolution of eusociality, specifically how dramatic reproductive skew evolved such that most individuals within a colony never reproduce, is a key question in evolutionary biology (1). Hamilton (2) offered insight into the evolution of eusociality when he noted that individuals gain indirect benefits by helping relatives other than their offspring. He also hypothesized that eusociality may have been favored in the Hymenoptera due to its genetic system in which females are more closely related to their sisters than they are to their own offspring. However, asymmetrical relatedness among relatives does not fully explain the evolution of eusociality (3–6). Eusociality not only evolved within haplodiploid groups, but it also evolved, although rarely, in several independent diploid lineages (7, 8). Five phylogenetically diverse diploid animal taxa include eusocial species [termites, naked mole rats (9, 10), beetles (11), shrimp (12, 13), and aphids (14)]. The inability of the haplodiploid hypothesis to explain all eusocial evolution has returned some attention to the ecological benefits of eusocial behavior (3, 7). How relatedness, colony-level selection, and ecology interact to facilitate the evolution of eusociality is still the focus of active debate (3, 7, 15–20).
Termites present an especially interesting evolutionary problem because they are a diploid group without asymmetric degrees of relationship between siblings and offspring of either sex; thus, they do not fit Hamiltonian models (21–23). No comprehensive, broadly accepted theory has emerged to explain the convergent evolution of highly social colonies in this order of ≈3,000 species of insects (21, 23–26). No extant termite species are solitary or subsocial. Therefore, we do not have the option of studying “stepping stones” toward eusociality in this clade, but we can make inferences about eusocial evolution by studying primitive living groups as model systems (23, 25). One of the most primitive termite families is Termopsidae, which is similar to ancestral termites in features such as colony size, nesting biology, development, and caste polyphenism (21–23, 27–29). Colony members of the Termopsid Zootermopsis nevadensis (Hagen) nest and feed within a single log, never foraging elsewhere (30). Fertile winged alates are the only individuals to leave the natal colony when they fly to find mates and found new colonies as young kings and queens. Hundreds of king and queen pairs begin colonies simultaneously under the bark of the same decaying tree or log and never leave again. Remaining in a single piece of wood imposes major ecological constraints, because as colonies grow, they deplete an already decaying, limited resource. Neighboring Zootermopsis colonies encounter each other as they grow, leading to interactions between colonies that can result in deaths of the king and queen from either or both colonies. Surviving members form a larger merged colony (25, 31). This merging behavior is intriguing because neighboring colonies are typically unrelated (32).
The ability of a helper to become reproductive and inherit the resources of a colony is a fundamental element in hypotheses on the evolution of eusociality (12, 22–25, 33) and the evolution of cooperative breeding in many animals (34–37). Development is highly flexible in Termopsidae: All nonreproductive individuals, except soldiers, can differentiate into any other caste (23). Because of this developmental plasticity, Termopsidae does not have true workers in formal termite terminology (28), but we refer to them here as workers in the functional sense that they help and work within the colony. Workers can develop into normal soldiers that are unable to reproduce, or alates that can disperse and found new colonies. After death of the founding kings and queens, workers may also develop into replacement reproductives (neotenics), including reproductive soldiers, a caste unique to Termopsidae. In this manner, workers can replace kings and/or queens killed during interactions between colonies, giving helper offspring early opportunities to inherit the colony resources [“Accelerated Inheritance” (25), parallel results are described in the more derived kalotermitid Cryptotermes secundus Hill (38)]. Zootermopsis kings and queens in isolated colonies tend to live for many years (31). These intraspecific interactions that occur naturally within a limited resource and cause death of young parents are significant in creating opportunities for workers to differentiate into replacement reproductives and inherit colonies.
Merging of colonies after colony interactions results in workers from different colonies cooperating although they are unrelated. Despite this observed cooperation, it is still not clear whether workers from both colonies develop into reproductives and gain fitness benefits as a result of the merger. Workers can gain direct fitness benefits if they develop into replacement reproductives or alates. Even the first brood of workers remaining to help in their parents' nest may have opportunities to reproduce if young colonies interact with other young colonies and kings and queens are killed in the process. Because these young colonies are relatively small, individuals have a nontrivial chance of becoming a reproductive. Workers may also gain indirect fitness benefits if close relatives become replacement reproductives.
Here, we determined whether workers from one or both original colonies have opportunities for direct and indirect fitness after encounters between pairs of unrelated young termite colonies in the primitive termite, Z. nevadensis. We grew colonies from unrelated pairs of alates, and staged interactions between pairs of similarly sized complete colonies, simulating natural meetings between neighboring families (25, 31). We recorded which kings and queens were killed when colonies met, and collected new replacement reproductives and alates as they differentiated in the merged colony. We used microsatellite markers to identify the colony of origin of all newly differentiated replacement reproductives and alates, determining whether they developed from one or both original colonies. We also sampled workers and soldiers in merged colonies to reveal potential interbreeding between the two original families. If unrelated reproductives inherit colony resources, and potentially even interbreed, then ecological circumstances can favor cooperative behavior and eusocial evolution in termites even when indirect fitness benefits are low or nonexistent.
Results
Every 2 months for 18 months after the initial staged encounters between pairs of colonies, we performed complete censuses on the merged colonies. In each replicate, at least one of the four original kings or queens had died by the time the first replacement reproductive differentiated (Table 1). An intact royal pair (king and queen from the same original colony) survived in 14 of 25 (56%) interactions, including three interactions where a second queen from the other colony survived. In 11 (44%) of the interactions, no intact royal pair survived, including three where only a single king survived, six where only a single queen survived, and two where only the king from one colony and queen from the other were alive when the first replacement reproductive differentiated. We designated the colony from which fewer kings or queens survived until the appearance of the first new reproductive as the “deposed” colony and the other colony as the “surviving” colony (Table 1).
Table 1.
Surviving primary reproductives after interactions between two colonies at the point when the first replacement reproductive(s) differentiated (up to 18 months)
| Outcome | n | Colony |
|||
|---|---|---|---|---|---|
| Surviving primary |
Deposed primary |
||||
| King | Queen | King | Queen | ||
| Intact royal pair | 11 | + | + | — | — |
| 3 | + | + | — | + | |
| No intact royal pair | 3 | + | — | — | — |
| 6 | — | + | — | — | |
| 2 | + | — | — | + | |
| Total | 25 | ||||
Interactions fell into two categories: a king and queen from one colony survived (intact royal pair); and only the king or the queen from either colony survived (no intact royal pair). We identify the colony with the most surviving primary reproductives as the ″surviving primary's″ colony, and the colony that lost at least one king or queen as the ″deposed″ colony. If only a king from one colony and a queen from the other survived (n = 2), we do not identify either colony as deposed. Survival is indicated by a +.
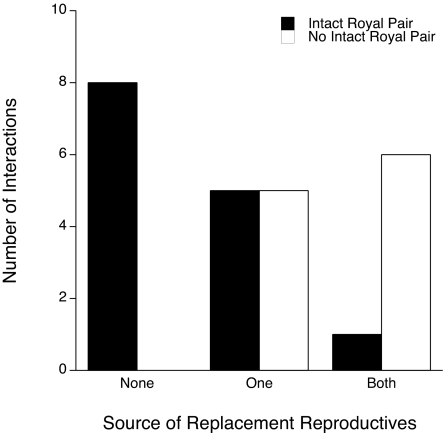
The first censuses in which new reproductives differentiated revealed 22 replacement reproductives that developed after 17 interactions; only two merged colonies produced replacements from both original colonies. When we included the next 4 months in our analysis, 10 of 25 (40%) merged colonies produced replacement reproductives from only one colony, whereas seven (28%) merged colonies produced replacement reproductives from both original colonies (Fig. 1). Twenty-five of all 48 (52%) replacement reproductives to differentiate over 4 months were reproductive soldiers, and all replacement reproductives (n = 9) that differentiated after interactions resulting in an intact royal pair were reproductive soldiers.
Fig. 1.
Colony of origin, as determined by genetic markers, of newly differentiated replacement reproductives after interactions (n = 25) between pairs of unrelated colonies. None, replacement reproductives did not differentiate after the colony interaction; One, replacement reproductives differentiated from termites belonging to only one of the two interacting colonies; and Both, replacement reproductives differentiated from both interacting colonies. Interactions that resulted in a surviving king and queen (“intact royal pair”) from one of the preinteraction colonies (n = 14) are shown in black; interactions resulting in only a single surviving king or queen from either colony (n = 11), including two interactions resulting in a surviving king from one colony and queen from the other, are shown in white.
Interactions between colonies accelerated the differentiation of replacement reproductives, particularly when kings and/or queens from both original colonies died. In cases where an intact royal pair survived and replacement reproductives differentiated within 4 months (n = 6), only 1.5 ± 0.34 SEM replacement reproductives differentiated; merged colonies with no surviving royal pair (n = 11) produced 3.6 ± 2.9 SEM. In the 11 merged colonies in which multiple replacement reproductives differentiated within a single census over the 18-month collection period, eight (73%) simultaneously produced replacement reproductives from both colonies. These results demonstrate that offspring from both colonies often take advantage of the early death of kings and queens and inherit the resources of the merged group.
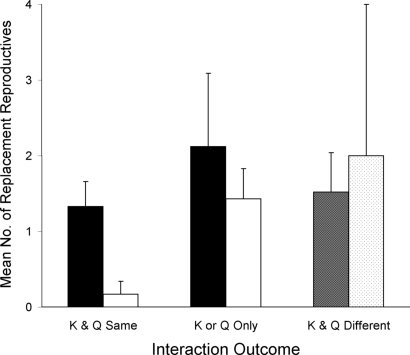
All interactions resulted in at least one surviving king or queen. If replacement reproductives differentiate from the deposed colony, then subsequent eggs can be the product of matings between individuals of each original colony. This interbreeding would also allow workers from both colonies to gain some fitness benefit from the early death of kings or queens. In two of the nine interactions where only a single king or queen survived (Table 1), all replacement reproductives differentiated from the deposed colony. However, of those encounters that result in replacement reproductives over a 4-month period, approximately twice as many differentiate from the colonies with the surviving kings or queens (1.8 ± 0.58 SEM) as from deposed colonies (0.93 ± 0.28 SEM; n = 15 excluding the two interactions resulting in a lone primary reproductive from each original colony), although this difference was not significant (paired t = 1.42, P = 0.18). If a king and queen from the same colony survived an interaction, and replacement reproductives differentiated over 4 months, then more differentiated from the surviving colony than the deposed (paired t = 2.9, P = 0.034). Only one of nine replacement reproductives to develop after such interactions developed from the deposed colony. Similar numbers of replacement reproductives differentiated from either colony when no pair of primary reproductives survived (P > 0.2 for each; Fig. 2).
Fig. 2.
Effect of colony of origin of kings and queens that survive interactions on mean number and colony of origin of replacement reproductives. Data are represented as mean ± SEM. Interactions resulted in a surviving king and queen from the same colony (K & Q Same), only one surviving king or one surviving queen (K or Q Only), or only one surviving king from one colony and one surviving queen from the other colony (K & Q Different). In the K & Q Same and K or Q Only, black bars indicate replacement reproductives differentiating from the surviving king and/or queen's colony (surviving primaries; Table 1); white bars indicate replacement reproductives differentiating from the colony whose king and queen were killed (deposed primaries; Table 1). In K & Q Different, crosshatched bar indicates replacement reproductives differentiating from the surviving king's colony, and stippled bar indicates replacement reproductives differentiating from the surviving queen's colony.
In addition to replacement reproductives, alates differentiated within 18 months of several colony encounters. An average of 6.0 ± 1.4 SEM alates developed after six out of 25 interactions (24%). In half of those interactions, alates developed from both original colonies. The development of alates from either colony demonstrates that nonreproductive castes from both interacting families have two opportunities for direct fitness: (i) develop into a replacement reproductive, or (ii) develop into alates that disperse to found new colonies. All individuals within a merged colony appeared to behave normally; we did not observe any agonism or decrease in food processing or nest maintenance. Nor did we observe workers unrelated to surviving kings and/or queens developing en masse into the wingbud instar preceding alates.
We also examined the colony of origin of 20 workers and all nonreproductive soldiers present in each of 25 merged colonies 18 months after interactions. On average, 78 ± 6% SEM of workers were produced by the colonies with a surviving king and/or queen, 6 ± 2% were produced by the deposed colony, and 16 ± 5% resulted from interbreeding between colonies. Fourteen merged colonies produced at least one worker with alleles consistent with interbreeding between the original colonies, and in three merged colonies, this kind of worker was the most common genotypic form. Interestingly, the skew in soldiers was less extreme than the workers: Only 53 ± 11% were produced by the surviving royals' original colonies, 36 ± 11% were produced by the deposed colony, and 11 ± 6% resulted from interbreeding between colonies (two-way ANOVA on arcsine root transformed data, caste x source colony interaction, F = 8.7, P = 0.0003). Soldiers may live longer than other nonreproductives, and their origins may reflect genotypic ratios before new workers are produced postinteraction. Alternatively, soldiers may be more likely to develop from the deposed colony, or workers from the deposed colony may be more likely to die or be killed.
Discussion
Our primary finding is that workers from both original unrelated colonies can take advantage of the early death of kings and queens after intercolony interactions in a primitive termite considered a model for ancestral species. These accelerated inheritance opportunities provide a selective incentive for hopeful reproductive helpers (39) to remain in their parents' colony rather than attempt the high risk of dispersal and low probability of reproductive success of founding their own colony (22, 23, 25, 38). Because dead trees and logs are simultaneously colonized by hundreds of founding pairs, interactions are likely to occur most frequently between small, young colonies, increasing the chance that any given worker in a young colony will differentiate into a replacement reproductive. Workers that do not differentiate into replacement reproductives still stand to gain indirect fitness benefits by being related to newly differentiated replacement reproductives, even if a worker's parents (the king and queen) were killed in an interaction (25).
The potential fitness benefits of remaining to cooperate with nonrelatives are magnified by the ecology of Zootermopsis. Merged colonies achieve a larger colony size than colonies that have not interacted with neighboring colonies. Within a single log, larger colonies have advantages both in survivorship and in future battles against neighbors (25). Young fire ant colonies achieve similar advantages by stealing pupae from smaller neighboring colonies to supplement their worker population, such that success of developing colonies in densely populated areas depends greatly on this brood-raiding behavior (40). However, victimized colonies of ants are unlikely to experience any future benefits as a result of being raided because stolen worker pupae have no further developmental plasticity and cannot reproduce.
Given that multiple dampwood termite families colonize a single tree, nearly all colonies will encounter intraspecific neighbors as they grow. The premium on colony size and early growth raises the question of why reproductives are killed during such interactions. It is well established that primary queens and kings inhibit the differentiation of replacement reproductives of their same sex within families (41). Eliminating some of the primary reproductives reduces the hormonal constraints and permits helpers to develop into replacement reproductives. If both the king and queen from the same colony survive an interaction, then very few replacement reproductives differentiate (25), and workers from the deposed colony have little chance of developing into replacement reproductives (Fig. 2). Although the motivation to attack reproductives could be dampened by potential reductions in egg-laying rates at a critical period of colony growth and risks of injury to the attackers, workers from either colony in an interaction have a better chance at inheriting the resources of the nest if at least one primary reproductive from the opposing colony dies. Also, surviving primaries in colonies that participated in an interaction do not live as long as founding reproductives in isolated control colonies (31). Thus, opportunities to inherit a colony will typically arise faster in merged colonies than in isolated families even if both primaries from one colony initially survive an interaction.
Genetic evidence presented here suggests that, in Zootermopsis, both original families can benefit from merging with neighbors because both may develop new reproductives and fertile alates within the larger colony. Kings and queens that survive intercolony interactions dominate the production of nymphs that can eventually develop into reproductives. Even so, the deposed colonies generally produce some reproductives, suggesting that these colonies benefit from merging. When a king or queen loses its original partner after an intercolony interaction, new mates differentiate, an important dynamic because female reproductive termites must be periodically reinseminated. There are several cases in our study where the new partners are replacement reproductives from the deposed colony. Therefore, it is more likely that both colonies will have non-zero fitness after mergers, which could favor remaining to help both before and after mergers if dispersal success is extremely low. These results demonstrate that helpers from both original families can benefit after colony encounters, and that accelerated inheritance opportunities could have driven proto-termite offspring to remain as workers in their natal colonies rather than attempt high risk dispersal and independent colony initiation.
Inheritance opportunities favoring worker philopatry have also been reported in the Kalotermitid termite C. secundus (38). Korb and Schneider (38) also conclude that the incentives of inheritance opportunities select for workers remaining in their natal nests, although it is not yet clear whether replacement reproductives come from one or both colonies in this species. Korb and Schneider (38) evaluated fitness trade-offs of individual C. secundus workers remaining as helpers in monogamous controlled (predicted average relatedness among offspring 0.5), experimentally fused (average relatedness expected as 0.25 after exchange of half of offspring from an unrelated colony), and inbred colonies (expected average relatedness among offspring 0.75). As expected by kinship theory, workers in inbred colonies were more likely to remain as helpers and less likely to develop into dispersing alates. However, workers also remained as helpers in high numbers (relative to the number of individuals molting into alates) in fused groups where the skew toward alate production was expected due to low relatedness within the colony. We interpret Korb and Schneider's (38) findings to support the theory of accelerated inheritance for termite eusocial evolution (25). The large number of C. secundus workers staying as helpers rather than dispersing as alates, even in fused colonies, suggests that the fitness prospects from inheriting a breeding position as a replacement reproductive outweigh the risks of successful dispersal as an alate.
Recent reviews have suggested that lifetime monogamy is essential to the evolution of eusociality (42, 43). Our data are difficult to reconcile with this hypothesis. We found genetic evidence that reproductives from one colony mated with reproductives from the other colony after 14 of 25 intercolony encounters. Most of the “hybrid” offspring were juveniles at 18 months, but may have developed into reproductives in the future. Contrary to some theoretical predictions (43), colony fusions have been reported in several termite families (25, 38, 44–49) that recent phylogenies place among basal termite lineages (50). This finding suggests that colony fusion is not a derived condition in Termopsidae, but reflects an ancestral ecological circumstance where multiple alate pairs initiate colonies within a limited resource. In Zootermopsis, one third of field colonies collected from isolated stumps derived from more than one founding pair (51), demonstrating that our results are not an artifact of laboratory conditions. In the primitive termite, C. secundus, Korb and Schneider (38) report evidence of fusion in 25% of 510 field colonies, although they did not identify the colony of origin of surviving secondary reproductives nor measure interbreeding between colonies. In more derived termites, fused colonies seem to be rare. DeHeer and Vargo (52) found that only eight of 354 natural Reticulitermes flavipes colonies showed evidence of fusing, and they found no evidence of interbreeding within those eight fused colonies. Colony fusing is largely a phenomenon of more primitive lineages.
Genetic relatedness is not necessary for the evolution of reciprocal altruism and mutualism (53). Our results support the hypothesis that ecological factors promoted termite eusocial evolution by favoring cooperation between colonies to monopolize resources after neighboring colony interactions. Relatedness is an inherent component of the selective topography because individuals are related to some of their nestmates in the merged colony, including some kings, queens, or replacement reproductives. However, cooperation in merged colonies after interactions in Z. nevadensis suggests that cooperation evolved and persisted due to selective incentives driven by ecological circumstances with potentially high direct as well as indirect fitness payoffs, and without high relatedness between all helpers.
Early in the evolution of eusociality in termites, multiple young families likely occupied the same limited resource the way young colonies do in the primitive termite Z. nevadensis. Mortality of parents resulting from interactions between neighbors and competition for food and nest resources provided opportunities for offspring, and/or their siblings, to inherit fused colonies and the resources they occupied. This process increased the potential for direct fitness benefits to helpers, making high relatedness favoring indirect benefits less critical. As termites evolved from nesting in one piece of wood to foraging among food sources, moving colonies to exploit new resources became easier, thus reducing incentives for battles between (and merging of) colonies. By that time, helpers had already evolved, and termites had passed the “point of no return” of eusocial evolution, meaning that reversion to a solitary or subsocial life history would be highly unlikely (7, 54).
Fusions between Zootermopsis colonies may represent one of the few situations in which it is possible to contrast the importance of relatedness and ecological pressures on eusocial evolution. As shown by our results, it is possible for cooperation to evolve and be maintained in termite societies despite low relatedness between cooperating individuals. In general, theories emphasizing genetic relatedness to explain eusocial evolution have not been well-supported by evidence from primitive termites. This lack of evidence does not mean that relatedness is generally unimportant, but rather that above-average relatedness is not a prerequisite. It is likely that ecological factors, including competition among families living within limited food and nesting resources, had a prominent role in the evolution of eusociality in termites.
Materials and Methods
Experimental Procedures: Dry Lab.
We staged encounters between colonies using methods similar to Thorne et al. (31) and Thorne et al. (25). Animals descended from laboratory families derived from samples of isolated field colonies of Z. nevadensis (Hagen) collected near Placerville, CA. Unrelated pairs of alates were placed in 55 × 15 mm plastic Petri dishes with field-collected decayed paper birch (Betula papyrifera Marsh.). These incipient colonies were allowed to grow for ≈12 months in progressively larger containers, feeding ad libitum on moist birch. Year old colonies were completely censused, ranging in size from 20 to 82 animals, and similar sized colonies (within three individuals) were paired for each interaction.
To simulate the natural encounters between young colonies, interaction chambers consisted of two 15-cm plastic Petri dishes connected by 8 cm of Tygon tubing (12-mm outside diameter, 10-mm inside diameter). Tubing interiors were abraded with acetone-washed sand to provide traction for the termites. Before interactions, five individuals from each colony were collected and preserved in 95% EtOH for later genotyping. All individuals in each colony larger than third instar were painted with a dot of Testor's model paint. A colony, including its food and fecal material, was transferred to one of the interaction chambers 24 h before interaction. The tubing between chambers was clamped for 24 h of equilibration, then unclamped to allow interaction.
Interactions were observed and recorded for the first 2 h. After 24 h, the new, merged social units were censused. Complete censuses were conducted every 2 months during the first 18 months after the interaction. Any replacement reproductives or alates were removed and preserved in 95% EtOH.
The social and hormonal environment within the merged colony changed as kings and queens died, and as replacement reproductives differentiated and were removed, potentially altering patterns of differentiation. We controlled for these changes in three ways. First, we determined the colony of origin of only the first replacement reproductives to develop. Eight of 25 merged colonies produced no replacement reproductives. Only three merged colonies produced multiple “first” replacement reproductives in a given census (all from interactions where no intact royal pair survived), which limited our ability to estimate how often reproductives differentiated from both colonies. Therefore, our second measure was to extend our sample interval to include the 4 months after the differentiation of the first replacement after colony interaction. Last, we included any sampling interval in which more than one replacement reproductive differentiated in the same merged colony, reasoning that environmental conditions influencing which workers differentiate would be similar for reproductives that differentiate during the same census interval.
Experimental Procedures: DNA Extraction and Evaluation.
We extracted DNA from workers collected before interactions, and from primary and replacement reproductives, alates, workers, and soldiers after interactions, using Qiagen DNeasy Kits (Qiagen). We amplified microsatellite loci Zoot28, Zoot29, Zoot73, Zoot101, and Zoot117 (55), and compared the genotypes of workers collected before interactions and primary reproductives from each colony to replacement reproductives, alates, and workers collected after interactions, to determine which colony was the source of individuals collected after interactions. These loci were allele-rich, containing five to nine alleles each. Because we performed controlled crosses to generate our unrelated interacting colonies, we were able to use simple exclusion, based on alleles unique to one or the other colony in an interaction and the assumption of Mendelian inheritance, to determine colony sources of all animals of interest. To perform PCR, we used 10 μL volume reactions containing 1 μL template DNA, 0.125 U Taq polymerase (Invitrogen), 1× PCR buffer (Invitrogen), 0.2 mM of each dNTP, 2.5 mM MgCl2 and 0.5 μM of primers, one of which was labeled with a fluorescent dinucleotide. Amplification was initiated at 95 °C for 2 min, followed by 30 cycles of 95 °C for 30 s, the annealing temperature for 30 s, and 72 °C for 30 s. Labeled PCR products were separated on a 3730 Genetic Analyzer (Applied Biosystems) and evaluated using Genescan 3.1.2, Genotyper 2.5, and Genemapper software (Applied Biosystems). Amplification was robust, and there was little or no ambiguity in calling peaks.
Acknowledgments.
We thank R. Abdul-Haqq, J. Baxley, M. Jones, A. Kelman, N. Lemanski, K. MacBride-Gill, C. Malabanan, P. Miller, M. Muscedere, S. Pinsky, B. Puszkiewicz, L. Rodriguez, J. Sandt, M. TerAvest, and A. Tkaczuk for assistance in this research and A. Greene, R. Jeanne, T. Leon, S. Suryanarayanan, B. Taylor, and G. Wilkinson for constructive comments on previous versions of the manuscript. This work was supported by National Science Foundation Grant IBN-0414596 (to B.L.T.), which supported P.M.J. and K.J.H. as postdoctoral researchers, and the Howard Hughes Medical Institute through the University of Maryland's Undergraduate Biological Sciences Education Program.
Footnotes
The authors declare no conflict of interest.
References
- 1.Darwin C. The Origin of Species. 6th Ed. London: John Murray; 1872. [Google Scholar]
- 2.Hamilton WD. The genetical evolution of social behavior I, II. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 3.Wilson DS, Wilson EO. Rethinking the theoretical foundation of sociobiology. Quart Rev Biol. 2007;82:327–348. doi: 10.1086/522809. [DOI] [PubMed] [Google Scholar]
- 4.Queller DC, Strassmann JE. Kin selection and social insects. Bioscience. 1998;48:165–175. [Google Scholar]
- 5.Gadagkar R. On testing the role of genetic asymmetries created by haplodiploidy in the evolution of eusociality in the Hymenoptera. J Genet. 1991;70:1–31. [Google Scholar]
- 6.Andersson M. The evolution of eusociality. Ann Rev Ecol Syst. 1984;15:165–189. [Google Scholar]
- 7.Wilson EO, Hölldobler B. Eusociality: Origin and consequences. Proc Natl Acad Sci USA. 2005;102:13367–13371. doi: 10.1073/pnas.0505858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe JC, Crespi BJ, editors. The Evolution of Social Behavior in Insects and Arthropods. New York: Cambridge Univ Press; 1997. [Google Scholar]
- 9.Jarvis JUM. Eusociality in a mammal–cooperative breeding in naked mole- rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 10.Sherman PW, Jarvis JUM, Alexander RD. The Biology of the Naked Mole Rat. Princeton, NJ: Princeton Univ Press; 1991. [Google Scholar]
- 11.Kent DS, Simpson JA. Eusociality in the beetle Austroplatypus incompertus (Coleoptera: Platypodidae) Naturwissenschaften. 1992;79:86–87. [Google Scholar]
- 12.Duffy JE. In: Genes, Behavior, and Evolution in Social Insects. Kikuchi T, editor. Sapporo, Japan: Univ of Hokkaido Press; 2002. pp. 1–38. [Google Scholar]
- 13.Duffy JE. Eusociality in coral-reef shrimp. Nature. 1996;381:512–514. [Google Scholar]
- 14.Stern DL, Foster WA. In: The Evolution of Social Behavior in Insects and Arachnids. Choe JC, Crespi BJ, editors. Cambridge, MA: Cambridge Univ Press; 1997. pp. 150–165. [Google Scholar]
- 15.Wilson EO. One giant leap: How insects achieved altruism and colonial life. Bioscience. 2008;58:17–25. [Google Scholar]
- 16.Ratnieks FLW, Wenseleers T. Altruism in insect societies and beyond: Voluntary or enforced? Trends Ecol Evol. 2008;23:45–52. doi: 10.1016/j.tree.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher JA, Zwick M, Doebeli M, Wilson DS. What's wrong with inclusive fitness? Trends Ecol Evol. 2006;21:597–598. doi: 10.1016/j.tree.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Foster KR, Wenseleers TF, Ratnieks LR, Queller DC. There is nothing wrong with inclusive fitness. Trends Ecol Evol. 2006;21:599–600. [Google Scholar]
- 19.Foster KR, Wenseleers T, Ratnieks FLW. Kin selection is the key to altruism. Trends Ecol Evol. 2005;21:57–60. doi: 10.1016/j.tree.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Wilson EO. Kin selection as the key to altruism: Its rise and fall. Soc Res. 2005;72:159–168. [Google Scholar]
- 21.Thorne BL, Traniello JFA. Comparative social biology of basal taxa of ants and termites. Annu Rev Entomol. 2003;48:283–306. doi: 10.1146/annurev.ento.48.091801.112611. [DOI] [PubMed] [Google Scholar]
- 22.Shellman-Reeve JS. In: The Evolution of Social Behavior in Insects and Arachnids. Choe JC, Crespi BJ, editors. Cambridge, MA: Cambridge Univ Press; 1997. pp. 52–93. [Google Scholar]
- 23.Thorne BL. Evolution of eusociality in termites. Annu Rev Ecol Syst. 1997;28:27–54. [Google Scholar]
- 24.Korb J. In: Ecology of Social Evolution. Korb J, Heinze J, editors. Berlin: Springer; 2008. pp. 151–74. [Google Scholar]
- 25.Thorne BL, Breisch NL, Muscedere M. Evolution of eusociality and the soldier caste in termites: Influence of intraspecific competition and accelerated inheritance. Proc Natl Acad Sci USA. 2003;100:12808–12813. doi: 10.1073/pnas.2133530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashi M, Yamamura N, Abe T. In: Termites: Evolution, Sociality, Symbioses, Ecology. Abe T, Bignell D E, Higashi M, editors. The Netherlands: Kluwer, Dordrecht; 2000. pp. 169–187. [Google Scholar]
- 27.Noirot C. Social structure in termite societies. Ethol Ecol Evol. 1989;1:1–17. [Google Scholar]
- 28.Noirot C, Pasteels JM. Ontogenetic development and evolution of the worker caste in termites. Experientia. 1987;43:851–860. [Google Scholar]
- 29.Emerson AE, Krishna K. The termite family Serritermitidae (Isoptera) Am Mus Novit. 1975;2570:1–31. [Google Scholar]
- 30.Abe T. In: Termites: Evolution, Sociality, Symbioses, Ecology. Abe T, Bignell DE, Higashi M, editors. The Netherlands: Kluwer, Dordrecht; 1987. pp. 125–148. [Google Scholar]
- 31.Thorne BL, Breisch NL, Haverty MI. Longevity of kings and queens and first time of production of fertile progeny in dampwood termite (Isoptera; Termopsidae; Zootermopsis) colonies with different reproductive structures. J Anim Ecol. 2002;71:1030–1041. [Google Scholar]
- 32.Shellman-Reeve JS. Genetic relatedness and partner preference in a monogamous, wood-dwelling termite. Anim Behav. 2001;61:869–876. [Google Scholar]
- 33.Alexander RD, Noonan KM, Crespi BJ. In: The Biology of the Naked Mole Rat. Sherman PW, Jarvis JUM, Alexander RD, editors. Princeton, NJ: Princeton Univ Press; 1991. pp. 1–44. [Google Scholar]
- 34.Kokko H, Ekman J. Delayed dispersal as a route to breeding: Territorial inheritance, safe havens, and ecological constraints. Am Nat. 2002;160:468–484. doi: 10.1086/342074. [DOI] [PubMed] [Google Scholar]
- 35.Kokko H, Johnstone RA, Wright J. The evolution of parental and alloparental effort in cooperatively breeding groups: When should helpers pay to stay? Behav Ecol. 2002;13:291–300. [Google Scholar]
- 36.Queller DC, et al. Unrelated helpers in a social insect. Nature. 2000;405:784–787. doi: 10.1038/35015552. [DOI] [PubMed] [Google Scholar]
- 37.Kokko H, Johnstone RA. Social queuing in animal societies: A dynamic model of reproductive skew. Proc R Soc London B Biol Sci. 1999;266:571–578. [Google Scholar]
- 38.Korb J, Schneider K. Does kin structure explain the occurrence of workers in a lower termite? Evol Ecol. 2007;27:817–828. [Google Scholar]
- 39.West-Eberhard MJ. Temporary queens in Metapolybia wasps: Nonreproductive helpers without altruism? Science. 1978;200:441–443. doi: 10.1126/science.200.4340.441. [DOI] [PubMed] [Google Scholar]
- 40.Tschinkel WR. The Fire Ants. Cambridge, MA: Harvard Univ Press; 2006. [Google Scholar]
- 41.Light SF. The determination of the castes of social insects (Concluded) Quart Rev Biol. 1943;18:46–63. [Google Scholar]
- 42.Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science. 2008;320:1213–1216. doi: 10.1126/science.1156108. [DOI] [PubMed] [Google Scholar]
- 43.Boomsma JJ. Kin selection versus sexual selection: Why the ends do not meet. Curr Biol. 2007;17:673–683. doi: 10.1016/j.cub.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 44.Goodisman MAD, Crozier RH. Population and colony genetic structure of the primitive termite Mastotermes darwiniensis. Evolution. 2002;56:70–83. doi: 10.1111/j.0014-3820.2002.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 45.Thorne BL, Lenz M. Population and colony structure of Stolotermes inopinus and S. ruficeps (Isoptera: Stolotermitinae) in New Zealand. N Z Entomol. 2001;24:63–70. [Google Scholar]
- 46.Broughton RE. Mitochondrial DNA variation within and among species of termites in the genus Zootermopsis (Isoptera: Termopsidae) Ann Ent Soc Am. 1995;88:120–128. [Google Scholar]
- 47.Gay FJ, Calaby JH. In: The Biology of Termites. Krishna K, Weesner FM, editors. Vol 1. New York: Academic; 1970. pp. 393–448. [Google Scholar]
- 48.Morgan FD. The ecology and external morphology of Stolotermes ruficeps Brauer (Isoptera: Hodotermitidae) Trans R Soc N Z. 1959;86:155–195. [Google Scholar]
- 49.Imms AD. On the structure and biology of Archotermopsis, together with descriptions of new species of intestinal protozoa and general observations on the Isoptera. Phil Trans R Soc London. 1919;209:75–180. [Google Scholar]
- 50.Inward DJG, Vogler AP, Eggleton P. A comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol Phylogenet Evol. 2007;44:953–967. doi: 10.1016/j.ympev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Aldrich BT, Kambhampati S. Population structure and colony composition of two Zootermopsis nevadensis subspecies. Heredity. 2007;99:443–451. doi: 10.1038/sj.hdy.6801022. [DOI] [PubMed] [Google Scholar]
- 52.DeHeer CJ, Vargo EL. Strong mitochondrial DNA similarity but low relatedness at microsatellite loci among families within fused colonies of the termite Reticulitermes flavipes. Insect Soc. 2008;55:190–199. [Google Scholar]
- 53.Foster KR, Wenseleers TJ. A general model for the evolution of mutualisms. Evol Biol. 2006;19:1283–1293. doi: 10.1111/j.1420-9101.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 54.Wilson EO. Insect Societies. Cambridge, MA: Harvard Univ Press; 1971. [Google Scholar]
- 55.Aldrich BT, Kambhampati S. Microsatellite markers for two species of dampwood termites in the genus Zootermopsis (Isoptera: Termopsidae) Mol Ecol Notes. 2004;4:719–721. [Google Scholar]