Abstract
Circadian clock genes are regulated by glucocorticoids; however, whether this regulation is a direct or secondary effect and the physiological consequences of this regulation were unknown. Here, we identified glucocorticoid response elements (GREs) at multiple clock genes and showed that 3 were directly regulated by the glucocorticoid receptor. We determined that a GRE within the core clock gene Per2 was continuously occupied during rhythmic expression and essential for glucocorticoid regulation of that gene in vivo. We further demonstrated that mice with a genomic deletion spanning this GRE expressed elevated leptin levels and were protected from glucose intolerance and insulin resistance on glucocorticoid treatment but not from muscle wasting. We conclude that Per2 is an integral component of a particular glucocorticoid regulatory pathway and that glucocorticoid regulation of the peripheral clock is selectively required for some actions of glucocorticoids.
Keywords: circadian rhythm, diabetes, nuclear receptors, metabolic syndrome
Steroid hormones confer diverse physiological actions by binding to nuclear hormone receptors and interacting with response elements in the genome to regulate target gene expression [reviewed by Mangelsdorf et al. (1)]. Glucocorticoids are a class of steroid hormones that function in virtually all vertebrate cells and tissues, affecting physiological homeostasis in a highly cell- and gene-specific manner. Dysregulation of glucocorticoid signaling has been implicated in the pathogenesis of diseases, such as metabolic syndrome and diabetes (2, 3). Therefore, elucidating the molecular basis of glucocorticoid action holds promise for understanding how tissue-specific regulation is achieved and may identify precise pathways important in disease.
In mammals, glucocorticoids are secreted from the adrenal gland in circadian cycles, producing temporally regulated peaks and troughs in systemic hormone levels (4). Although the function of this oscillation in secretion is not well understood, it is thought to be important for energy balance, because diet and feeding time have an impact on secretion rhythm (5–7). Diet composition (8) and timing of feeds have also been shown to modulate the circadian pacemaker (9, 10), and a connection between the pacemaker and the oscillations in glucocorticoid secretion has been observed (8, 11, 12). In turn, glucocorticoids can themselves induce cycling of circadian clock components in peripheral tissues, suggesting that there are situations in which glucocorticoids may dictate cycling rhythmicity (13).
This intimate connection with and feedback to the circadian clock raised the possibility that regulation of the clock by glucocorticoids might be direct and that glucocorticoid control of clock genes may be embedded mechanistically within certain glucocorticoid-regulated gene circuits, such as those involved in energy balance and metabolism. We therefore set out to characterize GR regulation of peripheral circadian clock genes, to investigate the systemic relevance of this regulation in vivo, and to assess the connection of this pathway to certain metabolic effects of glucocorticoids.
Results
Glucocorticoids Stimulate Oscillation of Specific Clock Genes in Mesenchymal Stem Cells.
For these studies, we exploited the fact that primary mesenchymal stem cells [also called marrow stromal cells (MSCs)] are readily purified, proliferate in culture, and are responsive to glucocorticoids (14). To test if the circadian clock is responsive to glucocorticoids in this tissue, as shown in other peripheral cells, (13) and to define the components that are regulated by glucocorticoids in MSCs, we treated primary mouse cells with the synthetic glucocorticoid dexamethasone (dex) and monitored the transcript levels of circadian clock genes over a 48-h time course.
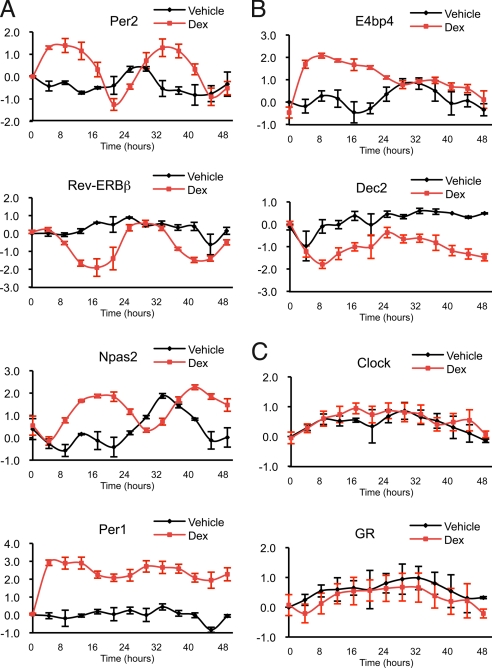
We found that dex stimulated transcriptional oscillation for 10 of the known core clock components (Per1, Per2, Per3, Cry1, Cry2, Rev-Erbα, Rev-Erbβ, Dbp, Npas2, and Bmal1) in MSCs (Fig. 1A and Fig. S1A]. The clock is an intricate network of positive and negative feedback loops [reviewed by Ko and Takahashi (15)]. As predicted by this network, dex stimulated distinct transcriptional oscillatory phases consistent with the network positions and functions of the different genes (Fig. S2). For example, the heterodimeric positive regulators (Npas2 and Bmal1) resided together in one phase, and the negative regulators (Cry1 and Cry2) were grouped in another.
Fig. 1.
Glucocorticoids stimulate transcriptional rhythmicity of specific circadian clock genes in primary mouse MSCs. The transcript levels at 4-h intervals were measured by quantitative PCR and normalized to time = 0 h. Graphs represent the relative transcriptional levels of genes averaged over at least 3 independent experiments with MSCs isolated from different mice (plotted in log2 scale, and error bars represent ± SEM). (A) Per2, Rev-ERBβ, Npas2, and Per1 expression levels oscillate in response to glucocorticoids. (B) E4bp4 and Dec2 respond to glucocorticoids without oscillation. (C) Clock and GR expression levels do not respond to glucocorticoids. See Table S1 for primer sequences.
Interestingly, the regulatory patterns conferred by dex were specific to the different clock components; some clock genes (E4bp4, Dec1, Dec2, and Timeless) were responsive to dex but displayed no oscillation, whereas others were unresponsive to dex (Cnsk1d, Cnsk1e, and Clock) (Fig. 1 B and C and Fig. S1 B and C). Other peripheral tissues appear to display different sets of oscillating and nonoscillating components [reviewed by Ko and Takahashi (15)], perhaps indicating that there are tissue-specific variations in the clock mechanism that may produce specialized functions. The particular oscillating and nonoscillating clock genes in our study may reflect, in part, the nature of the clock in MSCs. Alternatively, components that are nonresponsive at the level of transcript accumulation may achieve rhythmicity by other mechanisms, such as posttranslational modification [reviewed by Reppert and Weaver (16)].
Glucocorticoids Directly Regulate the Circadian Clock.
Primary glucocorticoid receptor (GR) target genes are those at which GR occupies a nearby genomic glucocorticoid response element (GRE) and regulates target gene transcription (17). Subsequently, other genes might be indirectly regulated by glucocorticoids, lacking GR-occupied GREs and, instead, responding to the actions of the primary target gene products.
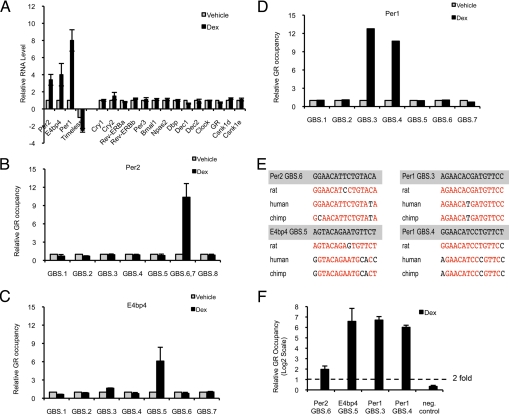
We found significant changes in transcript levels in Per1, Per2, E4bp4, and Timeless within 4 h of dex exposure, whereas expression from other clock genes was not altered detectably at this time point (Fig. 2A). We then tested whether the early responding genes that we identified are directly regulated by GR, using a recently developed and validated approach (18): we scanned in silico for glucocorticoid receptor binding sequences (GBSs), which collectively comprise a 15-bp sequence motif within 64-kb segments of genomic DNA centered on the transcription start site (TSS) of each candidate target gene. The mouse genomic segments bearing GBSs were then aligned with the corresponding segment of other mammalian genomes (rat, human, chimpanzee, and dog), and the highly conserved GBSs were tested for GR occupancy using ChIP (18).
Fig. 2.
GR directly regulates transcription of circadian clock components in mouse and human primary MSCs. (A) Per2, E4bp4, Per1, and Timeless rapidly respond to glucocorticoid stimulation. Relative transcript levels of mouse primary MSCs treated with vehicle (DMSO) or 1 μM dex for 4 h were measured by quantitative PCR. The transcript levels are normalized to the vehicle-treated samples, and the data represent the average of 3 independent experiments (mean + SEM). (B–D) GR occupies genomic sites at Per2, E4bp4, and Per1. ChIP was performed with primary mouse MSCs treated with vehicle (DMSO) (gray) or 1 μM dex (black) for 4 h. Quantitative PCR was used to quantify enrichment of computationally identified putative GBSs surrounding 64 kb of the corresponding gene TSSs (Fig. S3). The samples were normalized to amplification of a genomic region near the Hsp70 gene (mean + SEM). (E) GR-occupied GBSs at Per1, Per2, and E4bp4 are conserved in sequence across species. Aligned rat, human, and canine sequences of GBSs were obtained from the UCSC Genome Browser (20). Conserved bases are highlighted in red. (F) GR occupancy of GBSs at Per1, Per2, and E4bp4 is conserved in human primary MSCs. ChIP experiments with primary human MSCs [treated with 1 μM dex for 1.5 h] were performed and analyzed as indicated previously (mean + SEM).
We identified 8 GBS motifs in the mouse Per2 gene (Fig. S3), of which 1 was highly conserved (Per2 GBS6) and overlapping with another less conserved motif (Per2 GBS7) (Fig. 2E). As predicted by our prior studies of GBS motifs (18), we detected GR occupancy in the region containing the conserved GBS (Per2 GBS6,7; Fig. 2B). This region resides in an intron 22.8 kb downstream from the TSS. Several nonconserved sites were examined and were not occupied by GR (Fig. 2B). Similarly, we identified 7 GBS motifs in the E4bp4 gene (Fig. S3) and confirmed GR occupancy at a conserved GBS located ≈5 kb upstream from the TSS (Fig. 2 C and E).
At the Per1 locus, we identified 5 previously undescribed and 2 known (19) GBS motifs (Fig. S3) and observed GR binding at 2 conserved GBSs located +0.5 kb and −1.9 kb from the TSS found in RefSeq (Fig. 2 D and E); Per1 expression is also initiated at an alternative TSS (20), and both are responsive to dex in mouse MSCs. The 2 GR-occupied GBSs might direct TSS-specific expression of Per1 or collaborate to drive transcription at both TSSs.
In view of the strong sequence conservation of the GR-occupied GBSs at the mouse Per2, E4bp4, and Per1 genes (Fig. 2E), we tested the corresponding sites for GR occupancy in primary human MSCs and found them indeed to be occupied (Fig. 2F). We also confirmed that glucocorticoids stimulated synchronized cycling of clock genes in the human primary MSCs (Fig. S3). Collectively, our data suggest that GR directly activates Per2, E4bp4, and Per1 gene expression to stimulate circadian rhythmicity in mammalian MSCs. Our studies imply that these direct GR target genes serve as “entry points” to overall regulation of the clock by glucocorticoids, where synchronized oscillations may be initiated by hormonal regulation of specific core clock components.
Endogenous Per2 GBS Is Functional.
Primary response elements for GR and androgen receptor, inferred from sites of receptor occupancy within or close to target genes, typically appear to be rather remote from the regulated TSS, with ≈70% of the receptor binding sites positioned >10 kb upstream or downstream from the TSS (18, 21, 22); others have obtained similar results with estrogen receptors (23). Indeed, “assignments” of response elements to particular target genes have been made solely by this relatively loose geographical correlation.
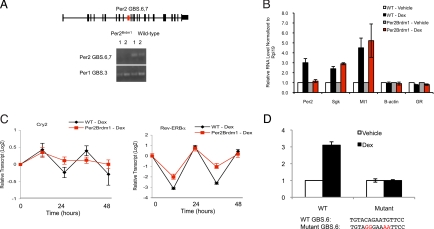
To assess whether the GR-occupied GBS located 22.8 kb downstream from the TSS of Per2 (Per2 GBS.6,7; Fig. 2B) indeed confers hormonal control over Per2 expression, we compared cells from Per2 WT and Per2Brdm1 mutant mice; the mutant mouse carries a 2.1-kb deletion that fortuitously includes GBS.6,7 (24) (Fig. 3A). Strikingly, although dex induced a robust circadian oscillation of Per2 transcription in WT cells, it failed to stimulate any response in the mutant cells over a 48-h time course (representative 4-h time point in Fig. 3B). Importantly, GR-mediated regulation of other primary target genes (18, 21) (Sgk and Mt1, Fig. 3B) was normal in the Per2 GBS.6,7-deleted cells. We conclude that the ≈2-kb genomic deletion in Per2Brdm1 mice that spans GBS.6,7 contains a Per2-specific GRE in vivo. To our knowledge, this is the only direct confirmation and gene-specific assignment of a mammalian chromosomal hormone response element reported to date.
Fig. 3.
Endogenous GR-occupied GBS in Per2 is functional in vivo. (A) Diagram of Per2 is displayed (exons shown as solid black bars, and introns shown as lines) along with the GR-occupied GBS (Per2 GBS.6,7) located at the +22.8-kb position (red box). PCR genotyping using primers spanning Per2 GBS.6,7 demonstrates that the 2.1-kb region containing Per2 GBS.6,7 is deleted in mutant cells but not in WT cells. A GR-occupied site at Per1 (Per1 GBS.3) was used as a positive control for DNA amplification. (B) Deletion of the endogenous genomic region harboring the GR-occupied Per2 GBS.6,7 specifically abolishes the glucocorticoid transcriptional response of Per2 but not other targets occupied by GR (Sgk, Mt1). Cells were treated with vehicle (DMSO) (white and gray) or 1 μM dex (black and red) for 4 h. Transcript levels from 3 independent experiments were measured by quantitative PCR (mean ± SEM). (C) Deletion of Per2 (red) dampens oscillations of other clock components induced by glucocorticoids compared with the response in WT cells (black) (mean ± SEM.). (D) A 500-bp DNA fragment from the endogenous Per2 gene that spans Per2 GBS.6,7 is responsive to dex in a luciferase reporter assay. Site-directed mutagenesis of Per3 GBS6 results in ablation of the dex response (mean ± SD).
We then tested the effect of this deletion in Per2 on the glucocorticoid-induced cycling of other clock genes. We found that the Per2Brdm1 lesion significantly dampened the synchronized cycling of other clock genes (Fig. 3C). Thus, analogous to the blunted transcriptional cycling of clock genes in the central pacemaker of Per2 mutant mice (25, 26), Per2 activity appears to influence the general function of the peripheral clock. Collectively, our data demonstrate that GR directly induces Per2 circadian rhythm and that this is an entry point to glucocorticoid regulation of the peripheral clock. It seemed possible that pursuing the mechanism and downstream consequences of Per2 regulation by glucocorticoids further might reveal a physiological rationale for this mode of control over the circadian clock.
To validate that the conserved Per2 GBS6 is the functional GRE, we subcloned an endogenous 500-bp DNA fragment containing the response element fused to a luciferase reporter and assayed for dex responsiveness. Indeed, addition of dex to cells containing the reporter construct resulted in a 3-fold increase in luciferase activity (Fig. 3D). We then performed site-directed mutagenesis on the conserved GBS6 sequence. This mutation completely ablated the dex-induced activity in the reporter assay, indicating that this GBS sequence is essential for the dex response of the reporter (Fig. 3D).
GR Is Required to Maintain Synchronized Phase of Circadian Rhythm.
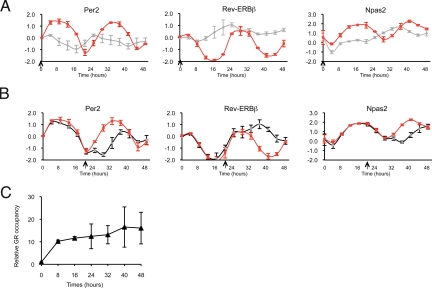
To probe mechanistic aspects of glucocorticoid regulation of rhythmicity, we tested whether GR activity was required to initiate and maintain transcriptional oscillations provoked by glucocorticoids. We found that coadministration of dex with a 10-fold molar excess of the competitive antagonist RU486 abrogated dex-stimulated circadian rhythmicity, consistent with GR's role in initiating this process (Fig. 4A). We then tested the role of GR in maintenance of the synchronized oscillations. We treated dex-stimulated cells with excess RU486 before (t = 22 h, Fig. 4B) or after (t = 24 h, Fig. S4) completion of the initial circadian cycle to examine whether GR activity was required to maintain oscillation after the rhythm was synchronized. At both times, addition of RU486 produced immediate amplitude dampening and cycle phase shifting, reminiscent of the results of zeitgeber removal, such as the shift from light entrainment to constant darkness (27). These results indicate that continuous GR activity is essential both to establish and to maintain the glucocorticoid-induced peripheral cell circadian oscillation and phase.
Fig. 4.
Continuous GR activity is required to initiate circadian rhythm and maintain a synchronized phase. (A) Simultaneous treatment with RU486 antagonized dex-stimulated transcriptional oscillation. Cells were treated with 100 nM dex alone (red) or 100 nM dex and 1 μM RU486 simultaneously (gray) at time = 0 h (arrows). The samples were quantified as described previously (average and range of 2 independent experiments). (B) Addition of RU486 after dex treatment disrupts glucocorticoid-stimulated circadian rhythm. One micromolar RU486 (black) or vehicle (DMSO) (red) was added to the culture medium after 22 h of treatment with 100 nM dex (arrows). Transcript levels from 3 independent experiments were measured by quantitative PCR (mean ± SEM). (C) GR remains bound at GR-occupied Per2 GBS.6,7 during cycling of expression. ChIP experiments were performed in a time course after treatment with 1 μM dex. The data were processed as described previously and normalized to ChIP samples treated with vehicle (DMSO) for 8 h (mean ± SEM).
We next examined whether the rhythmic expression of Per2 reflects rhythmic GR occupancy of the Per2 GRE. Interestingly, ChIP followed by quantitative PCR of GR occupancy at 8-h intervals for 48 h revealed that GR maintained similar levels of binding at every time point (Fig. 4C). Thus, we conclude that continuous GR occupancy of the Per2 GRE stimulates circadian rhythm and that rhythmicity, per se, is imparted downstream of receptor binding.
Per2 in Glucocorticoid-Altered Glucose Homeostasis.
Pharmacological doses of glucocorticoids are highly efficacious in treating a multitude of inflammatory and autoimmune disorders (28, 29). Unfortunately, long-term treatment can provoke severe morbidity, including muscle wasting, metabolic syndrome, and diabetes (30). Understanding precisely how these complications arise could facilitate development of more specific therapies and provide insight into the pathogenesis of metabolic diseases in general (31, 32). Our identification of Per2 as a direct target for glucocorticoids created the potential to determine whether control of circadian rhythm is an essential component of any or all glucocorticoid effects and, in particular, to assess the role of this pathway in the development of disease. Therefore, we treated WT and Per2Brdm1 mice with glucocorticoids and monitored the animals for systemic effects. The dex injections were administered every other day for 2 months (see Materials and Methods for details), because dex has a biological half-life of 36–54 h and this regimen produces systemic effects in WT mice (33); we then monitored glucose tolerance, insulin sensitivity, and muscle wasting.
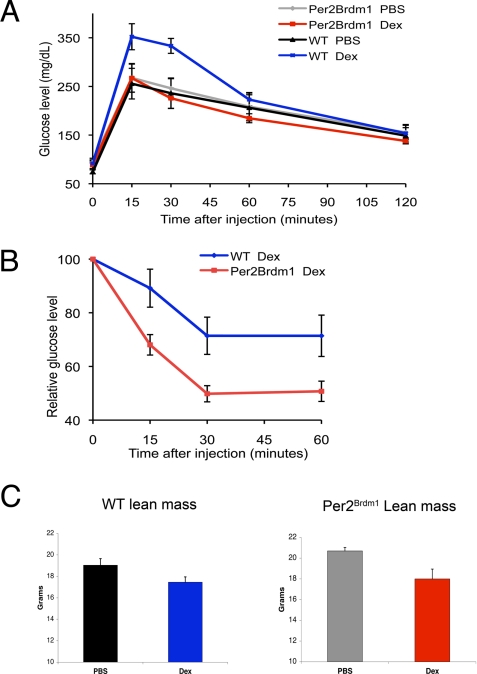
As expected, we found that the WT mice injected with dex developed glucose intolerance, a sign of diabetes. In striking contrast, Per2Brdm1mice treated with dex were protected from the glucose intolerance caused by dex because they maintained homeostasis in response to receiving a bolus of glucose (Fig. 5A). We also found that Per2Brdm1 mice treated with dex were more sensitive to insulin compared with dex-treated WT animals, as measured by blood glucose levels after an injection of insulin; Per2Brdm1 mice had a more substantial decrease in blood sugar in response to the insulin than did their WT counterparts (Fig. 5B).
Fig. 5.
Per2 is required and specific for alterations in glucose homeostasis induced by glucocorticoids in vivo. (A) Blood glucose levels were monitored in mice following an injection with 2 mg/kg glucose. Glucocorticoid-induced glucose intolerance depends on Per2. Per2Brdm1 mice (n = 10) are protected from glucocorticoid-induced glucose intolerance (red) compared with WT mice (n = 6) (blue) (mean ± SEM; P = 0.0574 at 15 min, P = 0.002 at 30 min). Per2Brdm1 (n = 7) and WT mice (n = 8) injected with PBS (gray and black) had similar glucose tolerance. (B) The decline in blood glucose from basal levels of mice was monitored following injections with 0.75 units/kg insulin. Per2Brdm1 mice (red) were more sensitive to insulin than WT mice (blue) (mean ± SEM; P = 0.0104 at 15 min, P = 0.0048 at 30 min, P = 0.021 at 60 min). (C) DEXA scans of mice injected with glucocorticoids were compared with those of PBS-injected animals. Per2Brdm1 mice treated with glucocorticoids had a similar decrease in lean mass (P = 0.0014) as WT animals. Probability values were calculated using unpaired Student's t tests.
Importantly, the Per2Brdm1 mice were not protected from all systemic effects of glucocorticoids. In particular, Per2Brdm1 mice injected with dex developed muscle wasting similar to the WT animals, as assessed by dual-energy x-ray absorptiometry (DEXA) scanning (Fig. 5C). Decreased muscle mass is often implicated in poor glucose tolerance (34). However, we found that Per2Brdm1 mice maintained glucose tolerance despite the muscle wasting, demonstrating that the 2 can be uncoupled by inhibition of circadian cycling. More specifically, we conclude that control of Per2 expression by glucocorticoids is an important component of the pathway by which glucocorticoids regulate glucose homeostasis but is not essential for glucocorticoid-mediated muscle wasting.
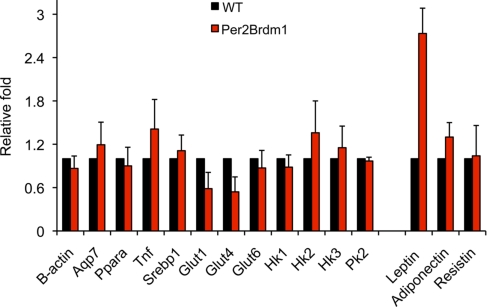
To begin to explore the mechanism by which dex-treated Per2Brdm1 mice achieve improved glucose homeostasis, we examined the expression of 14 genes that affect glucose utilization and insulin sensitivity (Fig. 6). Of this set, we found that only leptin expression was significantly altered in the Per2Brdm1 animals. Leptin was previously reported to be elevated in Per1;Per 2 double-mutant mice, but a connection to glucose homeostasis was not explored in that study (35). However, other studies have demonstrated that elevated leptin levels improve insulin sensitivity and glucose tolerance (36, 37) and that leptin resistance causes obesity and diabetes (38, 39). Intriguingly, leptin displays a circadian cycling pattern (35, 40, 41) that is antiphasic to the cycling of cortisol levels (the primary glucocorticoid in humans) (42). Taken together, our results imply that glucocorticoids regulate glucose homeostasis, in part, through direct control of the circadian clock, which, in turn, modulates leptin levels.
Fig. 6.
Per2Brdm1 mice have elevated leptin levels. A panel of candidate genes was screened for differential expression levels in adipose tissue with quantitative PCR. Adipose leptin expression was 2.7-fold higher in Per2Brdm1 mice compared with WT mice. Five mice from each genotype were used, and the experiment was repeated 3 times (mean ± SD). The same genes (except for the adipokines) were also screened in muscle, with similar results.
Discussion
Although it was previously established that glucocorticoids trigger or synchronize oscillations of circadian clock genes in peripheral tissues, neither the mechanism nor its broader physiological implications were defined. We found that several of the core circadian clock genes are primary GR target genes (i.e., regulated by glucocorticoids and linked to GR-occupied GREs) in primary mesenchymal stem cells. Indeed, using a deletion in the Per2 gene that covered its putative GRE, we were able to ascribe regulation of the core clock component Per2 to a particular genomic hormone response element. Because higher metazoan response elements typically appear to reside >10 kb from their target gene promoters (21) [and may even reside on different chromosomes (43)], it will be essential to develop robust methods to assign response elements to their in vivo targets.
The discovery that the peripheral circadian clock can be controlled directly by GR raised interesting questions about whether this regulatory circuit is isolated or, instead, might interact with, or perhaps even be embedded within, other physiological circuits that are regulated by glucocorticoids. Consistent with the latter view are classical studies showing that glucocorticoids play a central role in energy balance, coupled with more recent findings that appear to insinuate circadian rhythms in this same process (44–48). Our results revealed that glucocorticoid regulation of circadian rhythm is integral to glucocorticoid regulation of at least 1 component of energy balance, glucose homeostasis. The identification of this pathway also provides insight into the pathogenesis of certain adverse effects characteristic of glucocorticoid excess (Cushing's syndrome) and has implications for metabolic disease in general, including previously unrecognized potential therapeutical targets.
Interestingly, not all glucocorticoid-controlled circuits depend on glucocorticoid regulation of Per2 and its consequent effects on circadian rhythm. For example, the capacity for glucocorticoids to alter lean mass proceeded normally in Per2Brdm1 mice, whereas leptin expression is misregulated in the mutant animals, contributing to the maintenance of glucose tolerance and insulin sensitivity despite the glucocorticoid challenge. Although the uncoupling of glucose homeostasis from muscle wasting is itself informative, the more broadly significant point is that glucocorticoid-regulated genes appear to divide into 2 general classes: those that require hormonal control of circadian rhythm and those that do not. It will be interesting in future studies to populate the 2 classes of genes more fully in hopes of inferring the function and rationale for this cross-talk circuit.
In the meantime, it is tempting to speculate that embedding circadian clock control within the regulation of glucose homeostasis by glucocorticoids might confer selective advantage by ensuring a coupling of activity and feeding. Thus, because cycling of glucocorticoid secretion is modified by feeding times, glucocorticoid levels are highest during fasting when blood sugar is lowest (6). During these times, energy is diverted from the periphery to the brain, and glucocorticoids assist by antagonizing insulin action and stimulating gluconeogenesis. In turn, this is best accomplished if fasting is concurrent with decreased physical activity so that energy needs are minimized in the periphery. In this model, glucocorticoids act as a synchronization signal to coordinate the circadian rhythm with energy balance. In contrast, significant breakdown of muscle protein is invoked only after prolonged fasting and other major physiological stressors that substantially alter the relatively modest circadian fluctuations in energy needs and glucocorticoid levels (49, 50). Therefore, linkage of muscle wasting to circadian cycling might be disadvantageous.
Methods
Mice.
The Per2 mutant and WT control mice (both in C57BL/6 background) were purchased from Jackson Laboratories. For the in vivo studies, PBS or dex (Sigma) was injected into the peritoneal cavity of either WT or Per2Brdm1 mice every other day. Physiological experiments (i.e., glucose tolerance testing and insulin tolerance testing) were done on the day following an injection as described below. All experiments were approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
Cell Culture.
Mouse MSCs were harvested from the femurs and tibias of mice between 2 and 3 months of age. The ends of the bone were cut off, and the bone marrow was ejected by inserting a 23-gauge needle into the bone marrow cavity and flushing out the marrow with ≈2 mL of Iscove's medium with 2% vol/vol FBS per bone. Flushed bone marrow was placed in culture flasks with mesenchymal stem cell enrichment media (Stem Cell Technologies). The media were changed daily for 3 days to remove the hematopoietic/nonadherent cells and then every other day thereafter. When large circular colonies of MSCs grew in flasks, the cells were trypsinized and passaged to enrich for MSCs.
Circadian Rhythm Experiments.
MSCs were seeded in 12-well plates (Falcon). After cells grew to confluence, the media were replaced with mesenchymal stem cell enrichment media (Stem Cell Technologies) containing either vehicle (DMSO) or 1 μM dex dissolved in DMSO. Cells were harvested at time points, and total RNA was isolated using the RNeasy mini kit (Qiagen). An equal amount of RNA was reverse transcribed (≈500 ng of RNA for a 20-μL reverse transcription reaction) using random priming, and the relative transcript level was measured by quantitative PCR using a 7300 Real Time PCR system (Applied Biosystems) with SYBR green detection of amplicons. Fold change in transcript level was calculated by ΔΔCt normalized to vehicle-treated samples. Amplification of RPL19 was used as a loading control.
ChIP.
Cells were exposed to formaldehyde for 10 min to cross-link DNA and protein. The reaction was stopped with glycine. The cells were then collected by scraping into a conical tube, and chromatin was sheared by sonication. GBSs were immunoprecipitated with 8 μg of N499 anti-GR antibody with Protein A/G beads (Santa Cruz Biotechnology) per 15-cm plate. Samples were extracted with phenol-chloroform and purified with Qiaquick columns (Qiagen). GR occupancy was confirmed by quantitative PCR comparing dex-treated samples with vehicle-treated samples using ΔΔCt.
DEXA Scanning.
The lean masses of the mice were measured using DEXA (Lunar PIXImus2). The mice were fasted for 4 hours and anesthetized with isoflurane before scanning.
Glucose Tolerance Testing.
Per2Brdm1 and WT mice were fasted for 15 h (overnight) after a dex injection day. Fasting blood sugar was measured using a One Touch Ultra 2 glucometer (Johnson & Johnson) with blood from a nicked tail vain. Dextrose (2 mg/kg) was injected into the peritoneal cavity of each animal. Blood sugar was measured at the following times: 0, 15, 30, 60, and 120 min after dextrose injection.
Insulin Tolerance Testing.
Per2Brdm1 and WT mice were fasted for 6 h during the day (the day after an injection day). Fasting blood sugar was measured using a One Touch Ultra 2 glucometer (Johnson & Johnson) with blood from a nicked tail vein. Humalog (Lilly) insulin was injected into the peritoneal cavity of each animal at a rate of 0.75 units/kg. Blood sugar was measured at the following times: 0, 15, 30, and 60 min after insulin injection.
Supplementary Material
Acknowledgments.
We thank Marc Diamond, David Feldman, Stefan Taubert, and Lisa Watson for their comments on the manuscript. A.Y.-L.S., M.L.P., and K.R.Y. are members of the Chemistry and Chemical Biology Graduate Program at the University of California, San Francisco. M.L.P. was funded in part by National Institutes of Health training grant GM 56847. This work was funded by National Institutes of Health grants DK 73697 (to B.J.F.) and CA 20535 (to K.R.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909733106/DCSupplemental.
References
- 1.Mangelsdorf DJ, et al. The nuclear receptor superfamily: The second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotelevtsev Y, et al. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA. 1997;94:14924–14929. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masuzaki H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 4.Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab. 1971;32:266–284. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- 5.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallman MF, et al. Feast and famine: Critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 7.Leal AM, Moreira AC. Food and the circadian activity of the hypothalamic-pituitary-adrenal axis. Braz J Med Biol Res. 1997;30:1391–1405. doi: 10.1590/s0100-879x1997001200003. [DOI] [PubMed] [Google Scholar]
- 8.Kohsaka A, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza J. Circadian clocks: Setting time by food. J Neuroendocrinol. 2007;19:127–137. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- 11.Abe K, Kroning J, Greer MA, Critchlow V. Effects of destruction of the suprachiasmatic nuclei on the circadian rhythms in plasma corticosterone, body temperature, feeding and plasma thyrotropin. Neuroendocrinology. 1979;29:119–131. doi: 10.1159/000122913. [DOI] [PubMed] [Google Scholar]
- 12.Ishida A, et al. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 14.Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair—Current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 15.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. Spec No 2. [DOI] [PubMed] [Google Scholar]
- 16.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto KR. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- 18.So AY, Cooper SB, Feldman BJ, Manuchehri M, Yamamoto KR. Conservation analysis predicts in vivo occupancy of glucocorticoid receptor-binding sequences at glucocorticoid-induced genes. Proc Natl Acad Sci USA. 2008;105:5745–5749. doi: 10.1073/pnas.0801551105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto T, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 20.Karolchik D, et al. The UCSC Genome Browser Database: 2008 update. Nucleic Acids Res. 2008;36:D773–D779. doi: 10.1093/nar/gkm966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:e94. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolton EC, et al. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21:2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll JS, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 24.Zheng B, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 25.Bae K, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 26.Zheng B, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 28.Townsend HB, Saag KG. Glucocorticoid use in rheumatoid arthritis: Benefits, mechanisms, and risks. Clin Exp Rheumatol. 2004;22(Suppl):S77–S82. [PubMed] [Google Scholar]
- 29.Warrell RP, Jr, de The H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 30.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 31.Masuzaki H, Flier JS. Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)—A promising drug target for the treatment of metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:255–262. doi: 10.2174/1568008033340135. [DOI] [PubMed] [Google Scholar]
- 32.Masuzaki H, et al. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest. 2003;112:83–90. doi: 10.1172/JCI17845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gounarides JS, et al. Effect of dexamethasone on glucose tolerance and fat metabolism in a diet-induced obesity mouse model. Endocrinology. 2008;149:758–766. doi: 10.1210/en.2007-1214. [DOI] [PubMed] [Google Scholar]
- 34.Harrison BC, Leinwand LA. Fighting fat with muscle: Bulking up to slim down. Cell Metab. 2008;7:97–98. doi: 10.1016/j.cmet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Shimabukuro M, et al. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz MW, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 39.Lee GH, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 40.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 41.Sinha MK, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Licinio J, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 43.Lomvardas S, et al. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126:403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 44.Turek FW, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1[agr] integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 47.Yin L, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 48.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129(1S Suppl):227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- 50.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.