Abstract
Background
Obstructive sleep apnea (OSA) is prevalent in patients with heart failure. Treatment with continuous positive airway pressure (CPAP) improves systolic function in patients with heart failure. Bilevel positive airway pressure (PAP) is another treatment modality for OSA. The intermediate-term effect of bilevel PAP on left ventricular ejection fraction (LVEF) in patients with stable heart failure and OSA has not been compared to the effect of CPAP.
Methods
In this pilot randomized controlled trial, patients with stable systolic dysfunction and newly diagnosed OSA (n = 24) were randomized to receive either CPAP or bilevel PAP. Titration was done in the sleep laboratory using a CPAP-based algorithm. Primary outcome was the improvement in LVEF after 3 months of treatment. Other measurements included 6-min walk test, Epworth sleepiness scale score, and the Minnesota Living With Heart Failure questionnaire.
Results
Bilevel PAP increased LVEF 7.9% (LVEF percentage scale) more than CPAP (95% confidence interval [CI], 2.3 to 13.4; p = 0.01). In the bilevel PAP group, LVEF increased 8.5% (95% CI, 3.7 to 13.4; p = 0.002). In the CPAP group, LVEF did not change significantly (0.5%; 95% CI, − 2.7 to 3.7; p = 0.7). The difference in LVEF improvement between the two groups was still significant after adjustment for adherence, level of treatment positive pressure, body mass index, and severity of OSA.
Conclusion
This pilot randomized controlled trial suggests that bilevel PAP is superior to CPAP in improving LVEF in patients with systolic dysfunction and OSA. Larger trials are required to evaluate the mechanism behind this effect.
Keywords: bilevel positive airway pressure, continuous positive airway pressure, left ventricular ejection fraction, obstructive sleep apnea
Obstructive sleep apnea (OSA) is present in 9 to 24% of middle-age adults.1 The severe cardiovascular impact of OSA is increasingly recognized.2–4 OSA has a strong etiologic relation with hypertension5,6 and worsens BP control.7,8 Patients with OSA are at increased risk for atrial fibrillation9,10 and recurrence of atrial fibrillation after cardioversion.10 OSA increases the risk for coronary artery disease11,12 and worsens coronary artery disease outcomes.13–15 OSA may be independently responsible for a significant increase in fatal and nonfatal cardiovascular events.4,16 The prevalence of OSA is even higher in patients with heart failure,17,18 and its impact on morbidity and mortality may be more severe.2–4,19
Treatment with continuous positive airway pressure (CPAP) reverses the negative cardiovascular consequences of OSA.2–4 CPAP improves control of hypertension and reduces the risk of fatal and nonfatal cardiovascular events.2–4 In patients with heart failure and OSA, CPAP improves sympathetic activity,20–22 cardiac sympathetic tone,20–22 cardiac afterload,23 and ventricular arrhythmia.24 Within several weeks of treatment with CPAP, patients with heart failure and OSA have an improvement in systolic function and autonomic control.25,26
Along with traditional fixed-pressure CPAP, other positive pressure delivery modalities are now available and commonly used for OSA in patients with and without heart failure. Newer CPAP devices now deliver a variable pressure pattern with minor reduction in the expiratory pressure.27 Bilevel positive airway pressure (PAP) is a positive pressure modality that delivers two levels of pressure: higher during inspiration and lower during expiration. Bilevel PAP has been compared to CPAP in the treatment of OSA in patients without heart failure.28 While CPAP improves systolic function in patients with heart failure and OSA,25,26 the cardiac effect of bilevel PAP has not been evaluated in the intermediateterm setting. Several studies evaluated the cardiac effects of bilevel PAP after very short-term application,29–31 or in the acute setting as a form of noninvasive ventilation for patients with acutely decompensated heart failure.32 It emerged from these studies that bilevel PAP was generally equivalent to CPAP in improving the cardiac and respiratory dysfunction in the acute setting.29,30 In one nonrandomized trial, Acosta et al33 demonstrated a significant and immediate effect of bilevel PAP on left ventricular ejection fraction (LVEF) in stable outpatients with systolic dysfunction and no OSA. They attributed the effect to reduction in afterload. In some studies34,35 comparing CPAP and bilevel PAP for the treatment of acute heart failure, bilevel PAP was more effective in reducing respiratory load than CPAP.
There were no intermediate- or long-term studies evaluating the effect of bilevel PAP on cardiac function in patients with stable heart failure and OSA. As these newer positive pressure modalities are increasingly used in patients with heart failure, it is important to determine, in a pilot study, whether they differ in effect on cardiac function. We hypothesized that bilevel pressure may be better tolerated and may reduce filling pressure less than CPAP, resulting in superior improvement in left ventricular systolic function over time as measured by echocardiography at 3 months. Therefore, we sought to compare the cardiac effects of a bilevel PAP device to the current standard pressure modality, fixed CPAP, in the treatment of OSA in patients with systolic dysfunction.
MATERIALS AND METHODS
Subjects
Patients with left ventricular systolic dysfunction, stable American Heart Association class II or III, and newly diagnosed OSA were targeted for recruitment. Inclusion criteria were baseline LVEF ≤ 45%; no hospitalizations or medication changes in the 3 months prior to enrollment; and newly diagnosed OSA (diagnostic polysomnography with apnea-hypopnea index [AHI] > 10/h; > 50% of total events obstructive). Additionally, adequate resolution of respiratory events with PAP titration was required. This was defined as reduction of AHI > 50% on the titration night.
Exclusion Criteria
Exclusion criteria were as follows: (1) medical instability, including recent ischemic episode or unstable angina, recent episode of acute heart failure (within past 6 months), concomitant large hemispheric or brainstem stroke, and renal failure requiring dialysis; (2) long-term CPAP or bilevel PAP therapy; (3) central sleep apnea or Cheyne-Stokes respiration; and (4) COPD or need for supplemental oxygen. Patients were recruited from the Ohio State University (OSU) Sleep Disorders Center.
The study took place from May 2005 to May 2007. The initial protocol planned on enrolling 30 patients. The sponsor withdrew funding after 24 patients were enrolled and already randomized. This was due to a change in management, and it occurred before any results were available. Randomization was by permuted blocks of 10, and stopping at 24 instead of 30 resulted in a slight difference in the number randomly assigned to bilevel PAP (n = 13) and to CPAP (n = 11). The protocol was approved by the OSU Institutional Review Board.
Protocol
Patients with systolic dysfunction and OSA were recruited after baseline polysomnography. Participants presented to the OSU Pulmonary Clinical Trials Office within 1 week of a positive pressure titration sleep study to provide baseline measurement and receive their randomization treatment device. Patients were provided with the same PAP device (BiPAP-Pro; Respironics; Murrysville, PA). Patients randomized to the bilevel PAP arm received bilevel PAP with the Bi-Flex (Respironics) feature activated on the machine. Patients randomized to CPAP received fixed CPAP. Pressure level determination is discussed below. Using the same device for both groups was intended to achieve double blinding of the patient and the investigators because only the research coordinator was aware of the device assignment. Data entry was performed by a coordinator who was blinded to patient randomization. The cardiologist interpreting the echocardiograms was also blinded to patient assignment. The use of the same machine also mitigated against any preconceived belief regarding superiority of either device that participants may have had. The research coordinators contacted participants at regular 2-week intervals and received their device card memory for regular download. Patients returned after 90 days of randomization for conclusion measurements.
Polysomnography
All patients underwent polysomnography twice. Baseline polysomnography was standard nocturnal polysomnography performed in the OSU Sleep Laboratory. The polysomnography montage (MedCare; Buffalo, NY) included airflow measured by nasal pressure and oronasal thermistor. Thoracoabdominal wall movements were measured by inductance plethysmography, arterial oxygen saturation was measured by pulse oximetry; ECG, bilateral electrooculography, four channels of EEG, chin and tibial electromyograms, and body position were all part of the montage. Scoring of respiratory events in the polysomnography was in accordance to the event definitions provided by the American Academy of Sleep Medicine (1999).36
Titration polysomnography was a clinical positive pressure titration study with a modified titration algorithm. Continuous positive pressure was used for all patients on the titration night and the titration-targeted elimination of apneas. Once apneas were eliminated, the pressure was then increased to eliminate hypopneas followed by snoring. The sleep specialist generated both CPAP and bilevel PAP prescriptions for every patient following the titration study. Pressure determination was as follows: the lowest pressure required to eliminate apneas was used for expiratory PAP (EPAP) level; the pressure required to eliminate hypopneas and snoring was used for either inspiratory PAP (IPAP) or CPAP levels. Patient who required bilevel PAP during the titration night, at the technician’s discretion, were automatically disqualified from enrollment. On the day of randomization, the research coordinator used either the CPAP or the bilevel PAP prescription for device setup depending on the randomization.
Measurements
Cardiovascular
LVEF was measured using echocardiography. Baseline echocardiography was accepted if it was obtained within 6 months of randomization; otherwise, it was obtained at baseline before randomization. A conclusion echocardiogram was obtained on all patients at 3 months after randomization. Additionally, a Minnesota Living With Heart Failure (MLWHF) questionnaire was administered the day of randomization (again prior to randomization) and 3 months after randomization. The technologist and echocardiographer were blinded to device randomization.
Functional
A 6-min walk test and Epworth sleepiness scale (ESS) score were obtained at baseline (prior to randomization) and at 3 months after randomization.
Adherence to Treatment
Adherence was determined by biweekly download of device memory. Adherence was mainly expressed as average hours of nightly use in the 90 nights of the study period.
Statistical Methods
To model the conclusion LVEF at 3 months, we used a linear mixed model (Proc Mixed; SAS Institute; Cary NC) to account for baseline LVEF, which is almost identical to an analysis of covariance approach.37 Our primary hypothesis test was based on a simple two-group model (bilevel PAP vs CPAP) with 3-month and baseline LVEF (two-sided α = 0.05). Since this was a randomized trial, no adjustment for other covariates was required. However, we planned on performing adjustments for selected covariates as part of the sensitivity analysis. In particular, we determined the impact of pressure and adherence differences on the bilevel PAP vs CPAP effect.
Missing Data Strategy
One patient in the CPAP group did not return for the conclusion visit, and subsequently no echocardiography was performed for this patient. We relied on the missing-at-random assumption of Proc Mixed for primary analyses but also used last observation carried forward in a sensitivity analysis.
RESULTS
Patient Characteristics
Participants were 24 patients with systolic dysfunction and newly diagnosed OSA. Table 1 details participant characteristics. Thirteen participants (6 women) were enrolled in the bilevel PAP group, and 11 patients were in the CPAP group (4 women). All patients had LVEF ≤ 45%. There were no apparent imbalances between the two groups in LVEF, weight, severity of OSA, or baseline score on ESS score. All patients were followed up by the OSU Heart Failure Program and were receiving optimal doses of angiotensin-converting enzyme inhibitors and β-blockers. Table 2 details sleep characteristics in both groups on the baseline polysomnogram.
Table 1.
Baseline Characteristics of Participants*
| Characteristics | Bilevel Group (n = 13) | CPAP Group (n = 11) | 95% CI for Difference |
|---|---|---|---|
| LVEF, % | 26.0 (2.7) | 29.0 (2.9) | − 5.3, 11.3 |
| BMI, kg/m2 | 35 (1.6) | 32 (1.8) | − 8.5, 1.6 |
| Baseline AHI, events/h | 33 (4.5) | 30 (4.9) | − 17.9, 9.6 |
| ESS score | 11.8 (1.8) | 13.5 (1.8) | − 3.5, 7.1 |
| Age, yr | 51.3 (2.7) | 54.8 (3.0) | − 4.9, 11.9 |
| Baseline systolic BP, mm Hg | 115 (4.8) | 110 (5.2) | − 19.7, 9.6 |
| Baseline diastolic BP, mm Hg | 68 (3.3) | 68 (3.6) | − 10.0, 10.0 |
| Baseline heart rate, beats/min | 74 (3.8) | 73 (3.4) | − 12.3, 9.3 |
| Type of cardiomyopathy: dilated/ischemic/other | 8/2/3 | 7/4/0 | |
| Patients receiving β-blockers: carvedilol/metoprolol | 9/4 | 9/2 | |
| Patients receiving angiotensin-converting enzyme or angiotensin inhibitors |
13 | 11 | |
| Patients receiving spironolactone | 6 | 5 |
Data are presented as mean (SE) or No. unless otherwise indicated.
Table 2.
Sleep Characteristics on Baseline Polysomnography*
| Characteristics | Bilevel PAP (n = 13) | CPAP (n = 11) | 95% CI for CPAP-Bilevel PAP |
|---|---|---|---|
| Total sleep time, min | 249 (32) | 296 (31) | − 47, 140 |
| Sleep efficiency, % | 80 (3.6) | 82 (3.3) | − 8, 12 |
| Sleep latency, min | 18 (4.4) | 17 (3.6) | − 13, 11 |
| Rapid eye movement latency, min | 95 (22) | 65 (10) | − 84, 24 |
| Rapid eye movement, % | 12.3 (3.7) | 15.4 (1.9) | − 6.0, 12.2 |
| Arousal index, % | 24.5 (5.4) | 24.8 (3.3) | − 13.4, 14.0 |
| AHI, events/h | 34 (4.8) | 30 (4.4) | − 18, 10 |
| Central apnea index, events/h | 1.3 (0.5) | 1.9 (0.6) | − 0.9, 2.2 |
| Obstructive apnea index, events/h | 12.4 (4.3) | 6.8 (2.7) | − 16.6, 5.4 |
| Hypopnea index, events/h | 19.0 (3.2) | 20.0 (2.1) | − 7.3, 9.2 |
| Desaturation index (> 4%) | 22.8 (5.8) | 19.1 (4.0) | − 18.8, 11.3 |
| Desaturation nadir, % | 78 (2.3) | 80 (2.2) | − 5, 8 |
Data are presented as mean (SE) or No. unless otherwise indicated.
Comparison of Effects of Bilevel PAP and CPAP on Left Ventricular Systolic Function
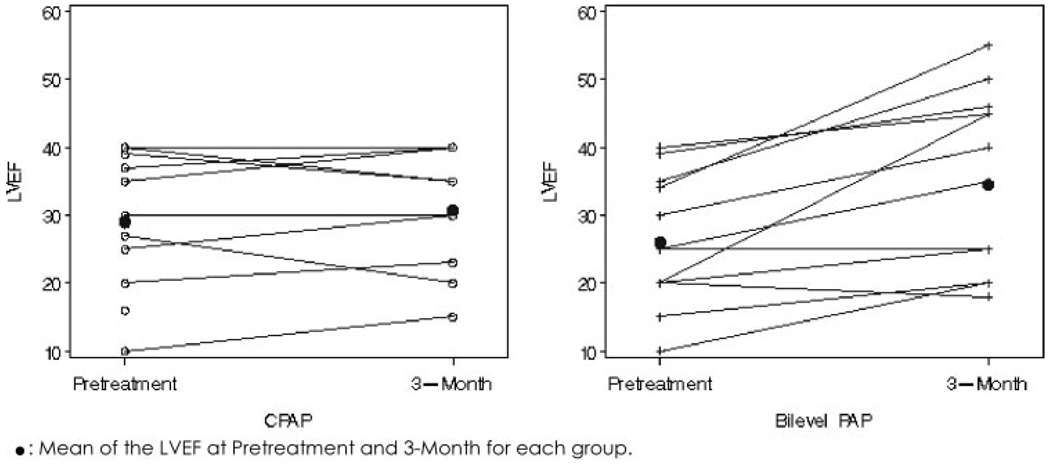
Bilevel PAP increased LVEF by 7.9% (on the LVEF percentage scale) more than CPAP (95% confidence interval [CI], 2.3 to 13.4; p = 0.01). In the bilevel PAP group, LVEF increased 8.5% (95% CI, 3.7 to 13.4; p = 0.002). In the CPAP group, the LVEF change was not significant (0.5%; 95% CI, − 2.7 to 3.7; p = 0.7). Note that the CI for the CPAP change includes values that are consistent with results of prior studies.25,26 Figure 1 provides the individual change in LVEF in both groups. Table 3 summarizes the sensitivity analysis of the difference in LVEF change between the two groups. After adjustment for baseline characteristics (severity of OSA or body mass index [BMI]), the improvement in LVEF was still significantly greater in the bilevel PAP group than in the CPAP group (6.3%; 95% CI, 0.9 to 11.8). The greater beneficial effect of bilevel PAP over CPAP on LVEF was still present after adjustment for potential mediating factors (level of positive pressure and adherence). Difference in LVEF improvement of 7.9 was only slightly reduced to 5.9 (95% CI, 0.2 to 11.6) after adjustment for adherence and pressure (Table 3). We expected that adjustment to these factors would explain some of the superiority of bilevel PAP. Adjustments for other potential mediating variables (not reported in Table 3), such as type of cardiomyopathy, change in ESS score, 6-min walk test distance, or residual AHI did not explain the superior effect of bilevel PAP. Since the one missing LVEF happened to be in the CPAP group, carrying forward the baseline value (zero change) in a sensitivity analysis increased the superiority of bilevel PAP to 9.8. There were no differences in the effect of both devices on BP and heart rate (Table 4).
FIGURE 1.
Change in LVEF with treatment in both groups; individual plot of LVEF change with each treatment modality. Left panel: CPAP group. Right panel: bilevel PAP group. One patient is missing a final LVEF measurement in the CPAP group. Means of LVEF at pretreatment and 3 months for each group are given.
Table 3.
Difference in LVEF Improvement Between Bilevel PAP and CPAP Groups
| Variables | Bilevel PAP-CPAP |
95% CI |
|---|---|---|
| Final LVEF minus baseline LVEF, % | 7.9 | 2.3, 13.4 |
| Basic model without adjustments for any covariables |
p = 0.01 | |
| Final LVEF minus baseline LVEF, % | 6.3 | 0.9, 11.8 |
| Adjustment for baseline AHI and BMI |
p = 0.03 | |
| Final LVEF minus baseline LVEF, % | 5.9 | 0.2, 11.6 |
| Adjustment for adherence and pressure |
p = 0.05 |
Table 4.
Effect of CPAP and Bilevel PAP on Sleep, Functional, and Cardiovascular Parameters
| Treatment Features | CPAP | Bilevel PAP | Bilevel PAP-CPAP | 95% CI | p Value |
|---|---|---|---|---|---|
| Residual AHI, events/h | 3.95 | 1.43 | − 2.5 | − 5.2, 0.2 | 0.06 |
| Average pressure (inspiratory pressure for bilevel group), cm H2O |
8.36 | 10.9 | 2.6 | 0.1, 5.0 | 0.04 |
| Adherence (average hourly use in 90 d) | 3.6 | 4.5 | 0.9 | − 1.6, 3.4 | 0.46 |
| Improvement in ESS score | − 4.7 | − 2.6 | 2.1 | − 2.7, 6.9 | 0.38 |
| Improvement in MLWHF questionnaire | − 2.9 | − 11.1 | − 8.2 | − 32.6, 16.3 | 0.49 |
| Change in 6-min walk distance, feet | 5.8 | 35.5 | 29.7 | − 59,118 | 0.49 |
| Change in systolic BP, mm Hg | − 1.4 | − 6.3 | 4.9 | − 10.9, 20.6 | 0.53 |
| Change in diastolic BP, mm Hg | − 2.2 | − 7.5 | 5.3 | − 5.4, 15.9 | 0.31 |
| Change in heart rate, beats/min | − 5.6 | − 1.3 | 4.3 | − 6.6, 6.9 | 0.42 |
Effect of CPAP and Bilevel PAP on Sleep Parameters
Both devices reduced AHI effectively. AHI reductions from baseline were 25.6/h in the CPAP group and 33.6/h in the bilevel PAP group. There was no significant difference in the AHI change between the two groups (p = 0.24). Adherence to the treatment device was slightly higher in the bilevel PAP group but did not reach significance (Table 4). In the CPAP group, the conclusion ESS score difference between the groups was 2.1 in favor of CPAP (p = 0.38).
Patients with CPAP required a fixed pressure of 8.4 ± 2 cm H2O. Patients randomized to bilevel PAP required an average IPAP of 11 ± 3 cm H2O (± SE). Average EPAP in the bilevel group was 8 ± 2.7 cm H2O, with an average pressure difference (IPAP – EPAP) of 3 ± 1 cm H2O. As noted above, adjustment for the difference in pressure (IPAP and CPAP) did not explain the superiority of bilevel PAP. Similarly, there was a slight difference in adherence in favor of bilevel PAP (p = 0.46), but this also did not explain the superiority of bilevel PAP as noted above.
There was a small to moderate (Pearson correlation = − 0.38) between the improvement in MLWHF and the improvement in LVEF (p = 0.09). Adjustment for the change in MLWHF did not explain the bilevel PAP effect.
DISCUSSION
This pilot randomized controlled trial compared the cardiac effect of bilevel PAP to CPAP in patients with systolic dysfunction and OSA. Patients were randomized to 3 months of either fixed CPAP or bilevel PAP. There were no apparent imbalances in baseline characteristics between the two groups. LVEF improved significantly more in the bilevel PAP group than the CPAP group. The change in LVEF was not significant in the CPAP group. This pilot study is the first intermediate-term evaluation of the cardiovascular effects of bilevel PAP in patients with heart failure and OSA.
This study does not provide a specific mechanism for the superior cardiac effect of bilevel PAP. AHI was reduced to similar levels in both groups, with no significant difference in the degree of AHI improvement. There was a non-significant increase in adherence in the bilevel PAP group, but this did not explain the superior effect of bilevel PAP on LVEF. We had expected that the difference in LVEF effect between bilevel PAP and CPAP may be explained by a combined effect of both adherence and the level of positive pressure used. Adjustment for both of these factors did not explain the bilevel PAP effect. These results suggest a unique effect of bilevel PAP that is not available with continuous pressure.
The immediate- and short-term cardiovascular effects of CPAP are better understood than those of bilevel PAP. Administration of CPAP in patients with severe decompensated heart failure reduced left ventricular filling pressure,34,38 and mitral regurgitation,32 and increased LVEF.34,38 Also, within 2 h of administration, CPAP improves systolic function, myocardial energy consumption,39 and respiratory muscles workload40 in stable patients with heart failure. After few weeks of administration, CPAP improves LVEF, left ventricular transmural pressure,23 and left ventricular hypertrophy and cardiac work index.41 An additional added benefit of eliminating underlying OSA would further enhance these effects of CPAP. Treatment with CPAP in patients with heart failure and OSA improves sympathetic activity,20–22 cardiac after-load,23 and reduces ventricular arrhythmia.24 Within several weeks of treatment with CPAP in patients with heart failure and OSA, there was a consistent improvement in ejection fraction.25,26
Several speculations can be made regarding the mechanism of this unexpected superior bilevel PAP effect. Studies42–44 of CPAP have suggested that a subgroup of patients with systolic dysfunction may not have the same improvement in systolic function. This lack of benefit was attributed to a negative effect of CPAP on stroke volume in patients with low filling pressures (pulmonary capillary wedge pressure < 12 mm Hg in one series43). While we did not have a consistent measurement of filling pressure in our patients, it remains plausible that this negative effect of CPAP was present in some of our patients randomized to CPAP. An alternative explanation is that the pressure-delivery modality of bilevel PAP mitigates against this negative effect and perhaps prevents the reduction in stroke volume in patients with lower filling pressures. Bilevel PAP provides lower intrathoracic pressure during expiration that may reduce the effect of CPAP on filling pressures. Whether this effect is beneficial in the acute or even the long-term setting is not yet clear. Another speculation regarding the superior effect of bilevel PAP may be related to its effect on respiratory muscles. It emerged from some studies34,35 that bilevel PAP may be superior to CPAP in unloading the respiratory muscles and improving the work of breathing. This unloading effect on the respiratory muscle was implicated at least partially in the beneficial effect of CPAP on systolic function.40 Whether this acute respiratory effect of bilevel PAP is relevant in the intermediate term in stable patients is not clear. Furthermore, there are no data on the relation between the long-term effect of CPAP on LVEF and the underlying cardiac filling pressure. Larger and longer-term trials comparing CPAP and bilevel PAP with measurements of other hemodynamic and respiratory parameters will be needed to evaluate this superior effect.
There was no significant improvement in LVEF in the CPAP group in this study. However, the design of this trial allows only for a conclusion regarding superiority of bilevel PAP. Two previous studies25,26 have shown a significant improvement of LVEF in patients with systolic dysfunction and OSA after several weeks of treatment with CPAP. The CI of LVEF change in this current study, while not significant, is wide and includes values in the range of improvement reported by these two previous studies. A more recent and larger trial45 comparing therapeutic CPAP to sham CPAP in the same population also reported CIs of change identical to our study, and identified a subgroup of patients with heart failure who have baseline LVEF < 30% to be less likely to have improvement in LVEF with CPAP.45
This pilot study has several limitations. Mainly, there were limited measurements of cardiovascular parameters that precluded a full assessment of the impact of bilevel PAP. Additional echocardiographic measurements including filling pressures and left ventricular remodeling parameters could have further characterized the effect of bilevel PAP. This is the first study to compare the intermediate cardiovascular impact of bilevel PAP and CPAP in the treatment of OSA in patients with systolic dysfunction, and so must be considered a first-stage (pilot) study. Larger studies with longer follow-up and more hemodynamic measurements are required to arrive at a strong conclusion about the superiority of bilevel PAP. Many factors may influence improvement levels in LVEF. Such factors may include the type of underlying cardiomyopathy, age, sex, or cardiac filling pressures. In this pilot study, we did not adjust for all of these, but focused on adjustment for BMI, pressure level, and apnea severity (AHI). Adjustment for these had very little impact on the estimate of the superiority of bilevel PAP.
Abbreviations
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- EPAP
expiratory positive airway pressure
- ESS
Epworth sleepiness scale
- IPAP
inspiratory positive airway pressure
- LVEF
left ventricular ejection fraction
- MLWHF
Minnesota Living With Heart Failure Questionnaire
- OSA
obstructive sleep apnea
- OSU
Ohio State University
- PAP
positive airway pressure
Footnotes
This project was supported by research grant from Respironics, Inc. Drs. Khayat and Abraham have received research grants from Respironics, Inc.
Reprints Information about ordering reprints can be found online: http://www.chestjournal.org/site/misc/reprints.xhtml
REFERENCES
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, et al. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest. 2005;128:624–633. doi: 10.1378/chest.128.2.624. [DOI] [PubMed] [Google Scholar]
- 3.Doherty LS, Kiely JL, Swan V, et al. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 7.Wilcox I, Grunstein RR, Hedner JA, et al. Effect of nasal continuous positive airway pressure during sleep on 24-hour blood pressure in obstructive sleep apnea. Sleep. 1993;16:539–544. doi: 10.1093/sleep/16.6.539. [DOI] [PubMed] [Google Scholar]
- 8.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 9.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 10.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 11.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 12.Peker Y, Kraiczi H, Hedner J, et al. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–184. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 13.Yumino D, Tsurumi Y, Takagi A, et al. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99:26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 14.Peker Y, Hedner J, Kraiczi H, et al. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–86. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 15.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg O, Lamp B, Faber L, et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128:2116–2122. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 19.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 20.Spaak J, Egri ZJ, Kubo T, et al. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension. 2005;46:1327–1332. doi: 10.1161/01.HYP.0000193497.45200.66. [DOI] [PubMed] [Google Scholar]
- 21.Usui K, Bradley TD, Spaak J, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45:2008–2011. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 22.Kaye DM, Mansfield D, Aggarwal A, et al. Acute effects of continuous positive airway pressure on cardiac sympathetic tone in congestive heart failure. Circulation. 2001;103:2336–2338. doi: 10.1161/01.cir.103.19.2336. [DOI] [PubMed] [Google Scholar]
- 23.Tkacova R, Rankin F, Fitzgerald FS, et al. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. 1998;98:2269–2275. doi: 10.1161/01.cir.98.21.2269. [DOI] [PubMed] [Google Scholar]
- 24.Ryan CM, Usui K, Floras JS, et al. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax. 2005;60:781–785. doi: 10.1136/thx.2005.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield DR, Gollogly NC, Kaye DM, et al. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. 2004;169:361–366. doi: 10.1164/rccm.200306-752OC. [DOI] [PubMed] [Google Scholar]
- 27.Aloia MS, Stanchina M, Arnedt JT, et al. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–2093. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gay PC, Herold DL, Olson EJ. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep. 2003;26:864–869. doi: 10.1093/sleep/26.7.864. [DOI] [PubMed] [Google Scholar]
- 29.Moritz F, Brousse B, Gellee B, et al. Continuous positive airway pressure versus bilevel noninvasive ventilation in acute cardiogenic pulmonary edema: a randomized multicenter trial. Ann Emerg Med. 2007;50:666–675. doi: 10.1016/j.annemergmed.2007.06.488. [DOI] [PubMed] [Google Scholar]
- 30.Park M, Sangean MC, Volpe Mde S, et al. Randomized, prospective trial of oxygen, continuous positive airway pressure, and bilevel positive airway pressure by face mask in acute cardiogenic pulmonary edema. Crit Care Med. 2004;32:2407–2415. doi: 10.1097/01.ccm.0000147770.20400.10. [DOI] [PubMed] [Google Scholar]
- 31.Becker H, Grote L, Ploch T, et al. Intrathoracic pressure changes and cardiovascular effects induced by nCPAP and nBiPAP in sleep apnoea patients. J Sleep Res. 1995;4:125–129. doi: 10.1111/j.1365-2869.1995.tb00201.x. [DOI] [PubMed] [Google Scholar]
- 32.Bellone A, Barbieri A, Ricci C, et al. Acute effects of non-invasive ventilatory support on functional mitral regurgitation in patients with exacerbation of congestive heart failure. Intensive Care Med. 2002;28:1348–1350. doi: 10.1007/s00134-002-1424-1. [DOI] [PubMed] [Google Scholar]
- 33.Acosta B, DiBenedetto R, Rahimi A, et al. Hemodynamic effects of noninvasive bilevel positive airway pressure on patients with chronic congestive heart failure with systolic dysfunction. Chest. 2000;118:1004–1009. doi: 10.1378/chest.118.4.1004. [DOI] [PubMed] [Google Scholar]
- 34.Chadda K, Annane D, Hart N, et al. Cardiac and respiratory effects of continuous positive airway pressure and noninvasive ventilation in acute cardiac pulmonary edema. Crit Care Med. 2002;30:2457–2461. doi: 10.1097/00003246-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Mehta S, Jay GD, Woolard RH, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25:620–628. doi: 10.1097/00003246-199704000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research; the Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 37.Crager MR. Analysis of covariance in parallel-group clinical trials with pretreatment baselines. Biometrics. 1987;43:895–901. [PubMed] [Google Scholar]
- 38.Bendjelid K, Schutz N, Suter PM, et al. Does continuous positive airway pressure by face mask improve patients with acute cardiogenic pulmonary edema due to left ventricular diastolic dysfunction? Chest. 2005;127:1053–1058. doi: 10.1378/chest.127.3.1053. [DOI] [PubMed] [Google Scholar]
- 39.Yoshinaga K, Burwash IG, Leech JA, et al. The effects of continuous positive airway pressure on myocardial energetics in patients with heart failure and obstructive sleep apnea. J Am Coll Cardiol. 2007;49:450–458. doi: 10.1016/j.jacc.2006.08.059. [DOI] [PubMed] [Google Scholar]
- 40.Naughton MT, Rahman MA, Hara K, et al. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91:1725–1731. doi: 10.1161/01.cir.91.6.1725. [DOI] [PubMed] [Google Scholar]
- 41.Dursunoglu N, Dursunoglu D, Ozkurt S, et al. Effects of CPAP on left ventricular structure and myocardial performance index in male patients with obstructive sleep apnoea. Sleep Med. 2007;8:51–59. doi: 10.1016/j.sleep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Baratz DM, Westbrook PR, Shah PK, et al. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest. 1992;102:1397–1401. doi: 10.1378/chest.102.5.1397. [DOI] [PubMed] [Google Scholar]
- 43.Bradley TD, Holloway RM, McLaughlin PR, et al. Cardiac output response to continuous positive airway pressure in congestive heart failure. Am Rev Respir Dis. 1992;145(2 pt 1):377–382. doi: 10.1164/ajrccm/145.2_Pt_1.377. [DOI] [PubMed] [Google Scholar]
- 44.Liston R, Deegan PC, McCreery C, et al. Haemodynamic effects of nasal continuous positive airway pressure in severe congestive heart failure. Eur Respir J. 1995;8:430–435. doi: 10.1183/09031936.95.08030430. [DOI] [PubMed] [Google Scholar]
- 45.Egea CJ, Aizpuru F, Pinto JA, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med. 2008;9:660–666. doi: 10.1016/j.sleep.2007.06.018. [DOI] [PubMed] [Google Scholar]