Abstract
The deficits of Alzheimer’s disease (AD) are believed to result, at least in part, from neurotoxicity of β-amyloid (Aβ), a set of 38–43 amino acid fragments derived from the β-amyloid precursor protein (APP). In addition, APP generates the APP-C31 and Jcasp toxic fragments intracellularly by cleavage at Asp664. We reported that mutation of Asp664 to A in a FAD-human APP transgene prevented AD-like deficits but did not affect Aβ production or deposition in PDAPP mice, arguing that D664A plays a crucial role in the generation of AD-like deficits. Whether D664A simply delays or completely prevents AD-like deficits, however, remained undefined. To address this question, we performed behavioral studies longitudinally on a pretrained mouse cohort at 9 and 13 months of age. While behavioral deficits were present in PDAPP mice, performance of Tg PDAPP(D664A) mice was not significantly different from non-Tg littermates’ across all ages tested. Moreover, aberrant patterns in non-cognitive components of behavior in PDAPP mice were ameliorated in PDAPP(D664A) animals as well. A trend towards poorer retention at 9 mo and poorer learning at 13 mo that did not reach statistical significance was observed in PDAPP(D664A) mice. These results support and extend recent studies showing that cleavage of APP at Asp664 (or protein-protein interactions dependent on Asp664) is a crucial event in the generation of AD-like deficits in PDAPP mice. Our results thus further demonstrate that the D664A mutation either completely precludes, or markedly delays (beyond 13 mo) the appearance of AD-like deficits in this mouse model of AD.
Keywords: Memory, transgenic mice, amyloid, Morris water maze, caspase, behavior
Alzheimer’s disease (AD) is the most common form of dementia, and a serious healthcare issue. Deficits in AD are believed to result in large part from the toxic effects of amyloid-beta (Aβ), a peptide released after proteolysis of the amyloid precursor protein (APP). APP may also be cleaved intracytoplasmically at Asp664 by caspases [8,20], liberating a cytotoxic carboxy (C)-terminal peptide, APP-C31[6,20], which is thought to activate apoptosis through an N-terminal Smac/DIABLO-like motif[9]. The remaining membrane-associated fragment, Jcasp, was also shown to be toxic in vitro[23]. Various mechanisms mediating Aβ toxicity have been proposed on the basis of in vitro data, but genetic evidence for their relevance in vivo is still largely lacking. We recently showed[7,32] that a significant component of Aβ toxicity in vivo may be dependent on the cleavage of APP at Asp664, since transgenic mice otherwise identical to a well-characterized model of AD, PDAPP mice[13,27], but carrying a mutation that obliterates the Asp664 cleavage site in APP [PDAPP(D664A)mice] continue to produce and deposit Aβ, but do not develop AD-like deficits. One potential link between Aβ production and APP Asp664 cleavage has been described, with the demonstration that Aβ binds APP and induces APP multimerization[2,19,21,22,34,35], leading in turn to cleavage of the APP cytosolic tail at Asp664[21,22,34], followed by synaptic and neuronal damage[22,34].
We had previously shown[7,32] that AD-like deficits were abolished in two independent PDAPP(D664A) transgenic mouse lines, in the moderate-expressing PDAPP(D664A)B21 line up to 12 months of age (mo) and in the high-expressing PDAPP(D664A)B254 line until 7 mo. To determine whether mutation of Asp664 in high-expressing PDAPP(D664A)B254 mice delays or completely prevents AD-like pathology, we examined cognitive function in transgenic PDAPP and PDAPP(D664A)B254 animals at 9 and 13 mo and AD-like histopathology after completion of the study at 13 mo. Spatial learning and memory were assayed longitudinally on a mouse cohort that was trained and tested at 7[7], 9 and 13 mo with the Morris water maze (MWM) paradigm[26]. Our results indicate that obliteration of the Asp664 cleavage site ameliorates AD-like spatial learning and memory deficits in the high-expressor PDAPP(D664A)B254 transgenic line until late in life (13 mo), when Aβ-plaque deposition was increased by 50% and gliosis was similar to that in brains of the high-expressor PDAPP(J20) line. Moreover, aberrant patterns of behavior involving non-cognitive (emotional) processes in Tg PDAPP mice were also alleviated in Tg PDAPP(D664A) animals at all ages tested. These observations indicate that Asp664 cleavage site significantly contributes to the generation of cognitive and non-cognitive aspects of AD-like behavioural deficits in the PDAPP transgenic mouse model until late in life. It has been proposed that a significant component of Aβ toxicity is mediated by its interaction with APP and requires Asp664 in vitro[21,22,34] and in vivo[7,32]. The present study provides further support for the hypothesis that a major pathway mediating Aβ toxicity requires Asp664 and suggests that additional, non Asp664-dependent pathway(s) of Aβ toxicity may be operant that could underlie trends to poorer performance observed in aged Tg PDAPP(D664A) mice.
METHODS
Mice
A G-to-C mutation was introduced in the PDGF β-chain promoter-driven human APP minigene carrying the Swedish and Indiana mutations[13,27] that mutated Asp664 (APP695 numbering) to Ala (PDAPP(D664A)) to generate PDAPP(D664A) transgenic mice, as described[7]. Mice from the high-expressing PDAPP(D664A)B254 transgenic line were used in this study. Mice from the high-expressing PDAPP(J20) line were kindly provided by Dr. Lennart Mucke, Gladstone Institutes for Neurological disease, San Francisco, CA. Non-Tg littermates were culled in equal proportions from both transgenic lines and used as controls. All transgenic lines were maintained by heterozygous crosses with C57BL/6J breeders (Jackson Laboratories, Bar Harbor, ME). All transgenic animals were heterozygous with respect to the transgene. After removal of overt ‘floaters’ (see Methods, Behavioral Testing, below), experimental groups at 9 mo were: non-Tg, n=15 (11 males, 4 females); Tg PDAPP(D664A), n=13 (10 males, 3 females); Tg PDAPP, n=10 (6 males, 4 females). All animals had been trained in the MWM previously. Experimental groups at 13 months were: non-Tg, n=10; Tg PDAPP(D664A), n=10; Tg PDAPP, n=8. All animals were males. Two Tg PDAPP animals showed no motivation to swim (“floaters”) at 13 months and were removed from the group. Two additional Tg PDAPP animals that had been trained at 9 mo in the MWM previously were added to the 13 mo group to preserve sample size.
Behavioral testing
The Morris water maze[26] (MWM) was used to test spatial memory. All animals had normal motor and visual skills as determined by sensorimotor tasks performed prior to testing. Swimming ability was assessed with a straight water alley (15 by 200 cm) containing a submerged (1 cm) 12 × 12 cm platform. No significant differences were observed in swimming abilities between groups. The procedure described by Morris et al. [26] was followed. All groups had been tested in the MWM at 7 mo previously[7]. Details are included as Supporting Text. Animals were monitored daily and weights recorded weekly. Mice showing no motivation to swim (“floaters”, spending more than 60% of trial time making no attempt to swim) were taken out of the study. All “floaters” were in the transgenic PDAPP(J20) group.
Quantitation of Aβ deposits and GFAP immunoreactivity
Standard immunohistochemical methods were used. Details are included as Supporting Text.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (GraphPad, San Diego, CA). In two-variable experiments, as when scoring performance of different genotypes across several days of training, repeated measures two-way ANOVA followed by Bonferroni’s post hoc tests were used to evaluate significance of differences between group means. When analyzing one-variable experiments with more than 2 groups, as when scoring retention across genotypes in probe trials, significance of differences between means was evaluated using one-way ANOVA followed by Tukey’s post-hoc test. Significance of differences in means between two different groups or between the same group at two testing times were done with Student’s t test. Values of P < 0.05 were considered significant. Slopes (m) of curves were calculated using the LINEST function in MS EXCEL. Pearson correlation coefficients between variables were calculated with regression analyses performed using GraphPad Prism (GraphPad, San Diego, CA).
RESULTS
Reference memory in 9 mo TgPDAPP and TgPDAPP(D664A) mice
We previously showed that PDAPP(D664A) transgenic (Tg) mice carrying the D664A mutation do not develop learning and spatial memory deficits in the Morris water maze test[6,32], a widely used tool in the assessment of hippocampal-dependent learning and memory in rodents[26]. Whereas AD-like anatomical, histopathological and cognitive parameters had been examined in aged (12 mo) mice from the moderate-expressing PDAPP(D664A)B21 Tg line[7,32], AD-like deficits had been assessed in the high-expressing PDAPP(D664A)B254 Tg line only at the earliest age at which Tg PDAPP mice begin to show detectable impairments, which for spatial learning and memory deficits is ~7 mo. At this age, soluble Aβ levels in PDAPP(D664A) mice from the high-expressor B254 line were increased by approximately 1.5-fold with respect to high-expressing PDAPP(J20) mice, in a manner proportional to their levels of expression of the FAD-hAPP transgene[7]. In this study, plaque deposition was not assayed in the 7 mo cohort examined in behavioural studies since at that age Tg PDAPP mice do not show overt plaque deposition. Saganich et al., however, described plaque deposits in Tg PDAPP(D664A)B254 mice as early as at 6 mo[32]. Whether plaque deposition, learning and memory would be affected by the D664A mutation in Tg animals from the high-expressing PDAPP(D664A)B254 line at later times in life remained to be determined. To answer this question, we used a longitudinal experimental design[3] to examine a mouse cohort that was trained and tested in the MWM at different times across their lifespan (7[7], 9 and 13 mo).
First, we examined groups of Tg PDAPP(J20), Tg PDAPP(D664A)B254 (PDAPP and PDAPP(D664A) mice henceforth) and non-Tg littermates at 9 mo. Nine mo Tg PDAPP, Tg PDAPP(D664A) and non-Tg littermates showed no deficiencies in swimming abilities, directional swimming or climbing onto a cued platform during pretraining and had no sensorimotor deficits as determined with a battery of neurobehavioral tasks. However, Tg PDAPP mice, but not the other experimental groups consistently showed a higher tendency to float (swimming at speeds lower than 0.025 m/s), which was exacerbated in some animals. Tg PDAPP mice showing no motivation to swim (“floaters”) were taken out of the study. Although we removed overt ‘floaters’ from the Tg PDAPP group, the tendency to spend a portion of trial time making no attempt to swim was still present in this group (Figure 5b). Tg PDAPP mice also showed an increased tendency to thigmotactic swim (with swim paths restricted to distances ~10 cm from the tank wall) (Figure 5a), which is frequent in animals that don’t learn well[1,3,40]. As shown in Figure 5, the percentage of time spent floating and engaged in thigmotactic swim was increased in the Tg PDAPP group at all ages tested. Floating artificially reduces distance travelled, resulting in short distances (hence better scores) even for animals that do not find the platform, and also decreases the measured average swim speeds in each trial. Thigmotactic swim artificially increases the time spent in the target quadrant in probe trials. Thus, to avoid potential confounds arising from these patterns of behavior in the Tg PDAPP group, we used latency to reach the escape platform as a measure of performance during both conventional and cued MWM training, and number of crosses over the former platform location as measure of performance during probe trials.
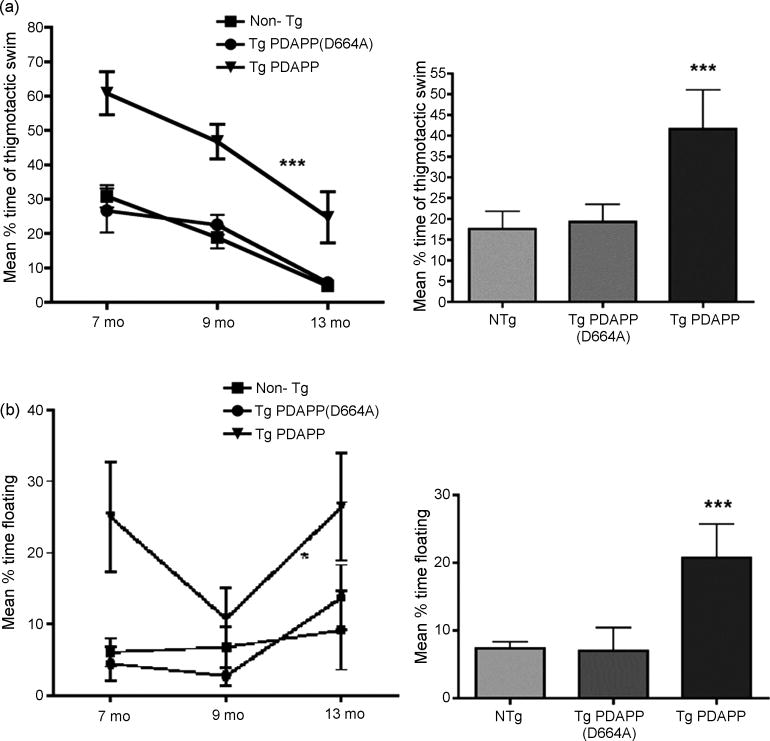
Figure 5. Non-cognitive components of behavior in Tg PDAPP and Tg PDAPP(D664A) mice.
a. Thigmotaxis Percent time spent engaged in thigmotactic swim decreased significantly as a function of age at testing [F(2,2)=25.64, P=0.0001, two-way ANOVA]. Tg PDAPP mice spent a significantly larger percent of time during trials swimming close to the tank wall than all other groups across the ages tested [P<0.001 with respect to both non-Tg and Tg PDAPP(D664A) groups respectively as a result of Bonferroni’s post hoc test applied to a significant effect of genotype (F(2,2)=31.82; ***P<0.0001, two-way ANOVA]. b, Floating. Percent time spent floating increased significantly as a function of age at testing [F(2,75)=3.3, P=0.04, two-way ANOVA]. Tg PDAPP mice spent a significantly larger percent of trial time floating than all other groups across the ages tested (P<0.05 with respect to both non-Tg and Tg PDAPP(D664A) mice respectively as a result of Bonferroni’s post hoc test applied to a significant effect of genotype (F(2,2)=8.49; P=0.0005; two-way ANOVA). “Floaters” were excluded from all analyses. Data are means ± SEM.
Consistent with our previous observations[7], Tg PDAPP mice showed significant deficits in performance both during training (Figure 1a) and in the probe trial (Figure 1b) at 9 mo. Performance of Tg PDAPP mice was significantly impaired with respect non-Tg littermates’ (P<0.01) and to Tg PDAPP(D664A) animals [P<0.001, as a result of Bonferroni’s post-hoc test applied to a significant effect of genotype (F(2, 35) =10.71; P<0.0002), repeated measures two-way ANOVA]. Performance of 9 month-old Tg PDAPP(D664A) mice during training was indistinguishable from non-Tg littermates’. No significant interaction was observed between day number and genotype, thus genotype had roughly the same effect at all times during training. Overall, session number did not significantly affect performance at 9 months (F(3,105)=0.28, P=0.83; repeated measures two-way ANOVA), suggesting that all groups might have reached their optimum attainable performance, albeit at different levels, after training at 7 mo. Even though percent time spent floating was higher for Tg PDAPP mice during the first 2 days of training, it decreased thereafter to levels similar to those of non-Tg and Tg PDAPP mice. Thus, no significant effect of genotype on percent time spent floating was observed (P=0.27, F(3,105)=1.32, repeated measures two-way ANOVA). This biphasic pattern was present, albeit at lower levels, in the latter groups as well (significant effect of swim number on percent time spent floating, P<0.0001, F(3, 105)=10.21, repeated measures two-way ANOVA). Percent time spent in thigmotactic swim was significantly higher in Tg PDAPP mice than in all other groups (Figure 5a) and this effect was uniform across days of training (P<0.001 and P<0.01 with respect to non-Tg and Tg PDAPP(D664A) groups respectively, Bonferroni’s post test applied to a significant effect of genotype on percent time spent in thigmotactic swim, F(2,35)=13.34, P<0.0001, repeated measures two-way ANOVA].
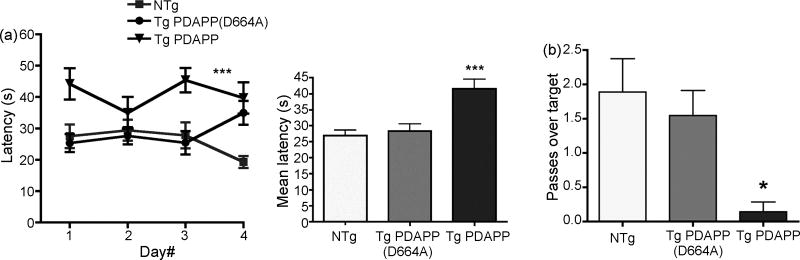
Figure 1. Performance of 9 mo Tg PDAPP, Tg PDAPP(D664A) and non-Tg littermates in the MWM.
a. Spatial training Left panel, mean latencies to reach a hidden platform were significantly different for the Tg PDAPP mouse group with respect to non-Tg littermates (P<0.01) and Tg PDAPP(D664A) animals [P<0.001, as a result of Bonferroni’s post-hoc test applied to a significant effect of genotype, F(2,35)=10.71; *** P<0.0002; repeated measures two-way ANOVA]. Right panel, mean latencies for all genotypes. b. Probe trial. Retention of the former platform site was impaired in Tg PDAPP mice with respect to the non-Tg group [P<0.05, Tukey’s multiple comparisons test applied to a significant effect of genotype (P<0.01) in one-way ANOVA] but was not significantly different for the non-Tg and Tg PDAPP(D664A) groups nor between the Tg PDAPP and Tg PDAPP(D664A) groups. Data are mean ± SEM.
Spatial memory for location of the escape platform was then evaluated by counting the number of crosses over the former platform location for each group in a probe trial administered after the last training trial. Retention of the former platform site was not significantly different for the non-Tg and Tg PDAPP(D664A) groups (Figure 1b). In contrast, Tg PDAPP mice crossed the former platform site significantly fewer times than Non-Tg mice (P<0.05, Tukey’s multiple comparisons test applied to a significant effect of genotype (P<0.01) in one-way ANOVA), suggesting impaired retention of the former platform site. Albeit retention in Tg PDAPP(D664A) mice was not significantly different from non-Tg littermates’, in contrast to our observations at 7 mo[7], at this age retention in Tg PDAPP(D664A) mice was not significantly different from that of the Tg PDAPP group either. These results suggest that mild spatial memory impairments may have developed in Tg PDAPP(D664A) mice at 9 mo.
Reference memory in 13 mo TgPDAPP and TgPDAPP(D664A) mice
We next determined spatial learning and memory on Tg PDAPP, Tg PDAPP(D664A) and non-Tg littermates at 13 mo. Thirteen month-old Tg PDAPP, Tg PDAPP(D664A) and non-Tg littermates showed no sensorimotor deficits as determined with a battery of neurobehavioral tasks. Similar to our observations at 7 (Galvan et al. 2006) and at 9 mo (Figure 1), performance of Tg PDAPP animals during conventional training was significantly impaired with respect to that of non-Tg littermates’ (P<0.05) as a result of Bonferroni’s post-hoc test applied to a significant effect of genotype (F(2,25) = 4.56; P<0.02; repeated measures two-way ANOVA), Figure 2a]. Consistent with our observations at 9 mo, performance of Tg PDAPP(D664A) was indistinguishable from non-Tg littermates’ (P>0.05). At 13 mo, however, performance of the PDAPP(D664A) group during training was not significantly different from that of Tg PDAPP mice either (P>0.05). Thus, and although performance of the Tg PDAPP(D664A) group was not significantly different from non-Tg littermates’, the observed trend suggests that, in spite of an overall significant improvement in performance with respect to Tg PDAPP mice, deficits in spatial learning may develop in Tg PDAPP(D664A) with increasing age. Performance improved through session 2 for all experimental groups during training at 13 mo and mean latencies remained constant for the PDAPP(D664A) group or actually increased for the Tg PDAPP and non-Tg groups hereafter (Figure 2a). In contrast to our observations during training at 9 mo, session number significantly affected performance (F(4,100)=3.77; P=0.007; repeated measures two-way ANOVA), with improvements observed only for the PDAPP(D664A) and non-Tg groups, arguing that these groups had not attained optimal performance during training at 7 mo and that cumulative carry-over (savings) effects of experience on performance were not negated by aging. The interaction between genotype and session number was not significant (F(8,100)=0.57; P=0.79, repeated measures two-way ANOVA), thus genotype had the same effect at all sessions. The lack of interaction between genotype and session number during training both at 9 and 13 mo may have resulted from a tendency of Tg PDAPP mice to display a biphasic pattern of performance during training, with decreased average latencies in the first 4-6 trials followed by increased latencies in subsequent trials (Figures 1 and 2). This behavioral pattern was absent in younger Tg PDAPP(D664A) mice, which showed monotonically decreasing latencies in successive trials at 7[7] and 13 mo. The same was true at 9 mo except for a trend to worse performance on the last session of training (Figure 1a) which did not reach statistical significance. These observations suggest that mild deficits in learning and a trend to a biphasic pattern of performance at 9 mo may develop with increasing age in Tg PDAPP(D664A) mice, but that these deficits in learning may be modified by savings (carry-over) effects of experience. On the other hand, while average latencies of the non-transgenic group monotonically decreased across sessions both at 7 and 9 mo, they also showed a trend to a biphasic pattern at 13 mo. In contrast to our observations at 7 [7] and 9 mo, percent time spent floating increased for all groups at 13 mo (Figure 5b) and for all experimental groups across sessions [significant effect of session number on percent time spent floating, P<0.0001, F(4,100)=6.35, repeated measures two-way ANOVA]. Interestingly, and similar to our observations at 9 mo, percent time spent floating was higher in the Tg PDAPP group only during the first two sessions of training. Thigmotactic swim was uniformly higher for the Tg PDAPP group throughout training at 13 mo as well (P<0.01 with respect to both non-Tg and Tg PDAPP(D664A) groups, Bonferroni’s post hoc test applied to a significant effect of genotype on percent time spent in thigmotactic swim (F(2,4)=8.04, P=0.002, repeated measures two-way ANOVA)]. At 13 mo, however, percent time engaged in thigmotactic swim monotonically decreased across sessions for all groups [F(2,100)=3.5, P=0.009, repeated measures two-way ANOVA].
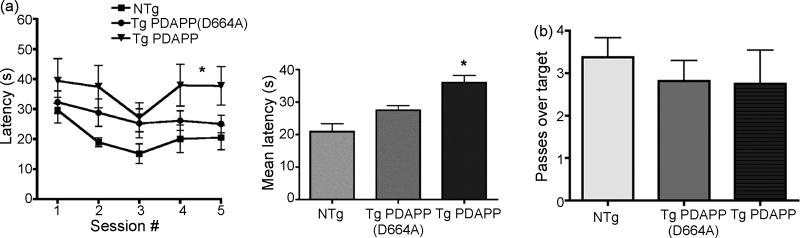
Figure 2. Performance of 13 mo Tg PDAPP, Tg PDAPP(D664A) and non-Tg littermates in the MWM.
a. Spatial trainingLeft panel, mean latencies to reach a hidden platform were significantly different in Tg PDAPP mice with respect to non-Tg littermates [(P<0.05 as a result of Bonferroni’s post-hoc test applied to a significant effect of genotype (F(2, 25)=4.56; *P<0.02, repeated measures two-way ANOVA)]. Performance of 13 mo Tg PDAPP(D664A) was indistinguishable from non-Tg littermates’ (P>0.05) and from Tg PDAPP mice (P>0.05). Right panel, mean latencies for all genotypes. Session number significantly affected performance of all groups (F(4,100)=3.77, P=0.007, repeated measures two-way ANOVA). b. Probe trial. No significant difference in number of crosses over the target were observed between groups. Data are means ± SEM.
Percent time engaged in thigmotactic swim decreased with increased experience (Figure 5a), and percent time floating was not strongly correlated with latency (R2=0.13). Thus, effects of anxiety (Figure 5a) or helplessness (Figure 5b) do not explain the observed pattern of performance of non-Tg mice at 13 mo. Therefore, our results suggest that aging per se may negatively affect learning in mice.
Spatial memory for location of the escape platform was then evaluated by counting the number of crosses over the former platform location for each group in a probe trial administered after the last session of spatial training at 13 mo. Surprisingly, retention of the former platform site was not significantly different for any experimental group at 13 mo (Figure 2b). In contrast to the memory impairments observed in the Tg PDAPP group at 7 and 9 mo, all experimental groups crossed the former platform site a similar number of times at 13 mo and this effect was due to improved retention in the Tg PDAPP group (Figures 1b and 2b), regardless of the observed deficits of this group during training (Figure 2a). These observations suggest that, in spite of showing significant deficits during acquisition, retention impairments in Tg PDAPP mice may be compensated or positively affected by carry-over (savings) effects of prior experience due to practice [24]. In cued conditions (Figure 3), all groups readily navigated to the visible platform and showed a significant difference in performance over trials (significant effect of trial number on performance, F(5,125) =5.72; P=0.0001; repeated measures two-way ANOVA). Both transgenic groups, however, showed a tendency to longer latencies in the first two sessions that were improved in later ones, although this differences reached significance only for the first swim when the non-Tg and the Tg PDAPP(D664A) groups were compared (P<0.05 as a result of Bonferroni’s post hoc test applied to a significant effect of genotype on performance, F(2,25)=6.34; P<0.0059, repeated measures two-way ANOVA). It is possible that the initial difference in performance observed for the transgenic groups, which has been documented for other hAPP transgenic models [16], could be caused by differences in their reactivity to novel testing conditions. Although latencies for the Tg PDAPP mice decreased monotonically across trials, this group performed significantly worse than non-Tg and Tg PDAPP(D664A) animals during the last trial of cued training (P<0.05 as a result of Bonferroni’s post hoc test applied to a significant effect of genotype on performance, F(2,25)=6.33; P<0.0059, repeated measures two-way ANOVA). This increase in average latency of Tg PDAPP mice in the last trial of cued training was not due to increased floating, since genotype did not have a significant effect on percent time spent floating during cued training, and percent time spent floating decreased significantly across trials for all groups [significant effect of trial number on percent time spent floating, F(5,125)=5.7, P=0.001, repeated measures two-way ANOVA]. The increase in average latency observed for the Tg PDAPP group in the last trial was not due to an increase in percent time engaged in thigmotactic swim at that trial either (P>0.05, Bonferroni’s post hoc test applied to a significant effect of genotype on percent time spent in thigmotactic swim, F(2,25)=5.91, P=0.008, repeated measures two-way ANOVA], although time spent in thigmotactic swim was significantly increased for the Tg PDAPP group during cued training with respect to non-Tg and Tg PDAPP(D664A) mice (P<0.001 and P<0.05 respectively). Thus, the increased latencies observed for the Tg PDAPP group during the last trial of cued training might be explained by learning deficits (Palop, Galvan, etc.) present in this group.
Figure 3. Performance of Tg PDAPP and Tg PDAPP(D664A) mice in the cued version of the MWM.
Tg PDAPP, Tg PDAPP(D664A) and non-Tg animals performed similarly during training in cued conditions, showing significantly reduced latencies over sessions [significant effect of session number on performance, F(5,125)=5.72; P=0.0001, repeated measures two-way ANOVA]. Data are means ± SEM.
Effect of experience on performance of Tg PDAPP and Tg PDAPP(D664A) mice
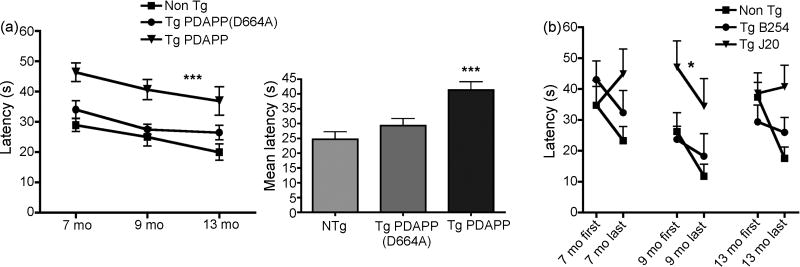
Although Tg PDAPP mice showed significantly impaired learning of the platform location during conventional training with respect to non-Tg littermates (P<0.001) and with respect to Tg PDAPP(D664A) mice (P<0.05, Bonferroni’s post hoc test applied to a significant effect of genotype, F(2,2)=23.76, P<0.0001, repeated measures two-way ANOVA) at all ages tested, all groups significantly reduced their average escape latencies across training sessions between 7 and 13 mo as evidenced by a significant effect of age at testing on performance [Figure 4a, F(2,79)=6.57, P=0.002, two-way ANOVA], suggesting that a positive effect of prior experience on learning can also be observed in Tg PDAPP mice across ages at testing. The interaction between genotype and session number was not significant (F(4,79)=0.13; P=0.96, two-way ANOVA), thus genotype had the same effect at all ages. Average latencies across training were not significantly different between non-Tg and Tg PDAPP(D664A) mice, indicating that impairments in spatial learning of Tg PDAPP mice are rescued in PDAPP(D664A) animals up to 13 mo.
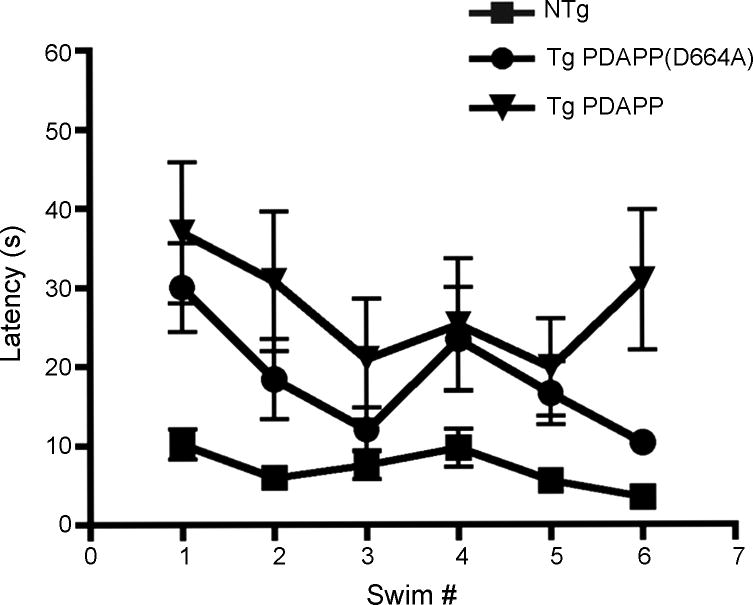
Figure 4. Effect of prior experience on performance of Tg PDAPP, Tg PDAPP(D664A) and non-Tg littermates in the MWM.
aLeft panel, performance of all experimental groups was significantly improved with increasing age and experience [significant effect of age at testing on performance, F(2,79)=6.57, P=0.002, repeated measures two-way ANOVA]. Tg PDAPP mice were significantly impaired with respect to non-Tg controls (P<0.001) and with respect to Tg PDAPP(D664A) mice [P<0.05, as a result of Bonferroni’s post test applied to a significant effect of genotype, F(2,2)=23.76; ***P<0.0001, repeated measures two-way ANOVA]. Right panel, mean latencies for all genotypes. b. Performance at first and last swim during training at 7, 9 and 13 mo. Latencies to find the escape platform were reduced at last swim with respect to the first swim of each training period for non-Tg and Tg PDAPP(D664A), but not for Tg PDAPP mice except at 9 mo, when performance of Tg PDAPP mice also improved as a result of training. *, significant effect of genotype on performance, F(2,5)=2.45, P=0.0003, two-way ANOVA. Retention was observed between 7 and 9 mo, but not between 9 and 13 mo for non-Tg and Tg PDAPP(D664A) groups. Data are means ± SEM
Long-term retention in Tg PDAPP and Tg PDAPP(D664A) mice
To determine long-term retention of the platform location between different experiments, we compared average latencies of all groups at first and last trials during training at 7 [7], 9 and 13 mo. Consistent with the deficits observed in the Tg PDAPP group in a longitudinal analysis of performance (Figure 4a) Tg PDAPP mice showed significant differences in long-term retention of the platform location between experiments with respect to non-Tg and Tg PDAPP(D664A) animals across all ages tested (P=0.0003 as a result of Bonferroni’s post hoc test applied to a significant effect of genotype [F(2,5)=2.45, two-way ANOVA, Figure 4b)]. Although learning was significantly impaired in Tg PDAPP mice at 7 mo, the performance of this group improved during the last 3 sessions of training[7]. In spite of this improvement, which was consistent with prior studies showing that spatial learning is impaired but not absent in Tg PDAPP mice[29,31] average latencies of Tg PDAPP mice at the end of training at 7 mo were still higher than those at the beginning of training ([7], Figure 4b). Non-Tg and Tg PDAPP(D664A) animals showed long-term retention of the platform location between 7 and 9 mo. Average latencies of the Tg PDAPP group in the first trial at 9 mo were also similar to those at the end of training at 7 mo[7], suggesting long-term retention of the platform location at 9 mo (Figure 4b), albeit at significantly decreased levels than transgenic PDAPP(D664A) mice (P<0.05) and non-Tg animals [P<0.05, Bonferroni’s post hoc test test applied to a significant effect of genotype, F(2,1)=5.85, two-way ANOVA, P<0.005)]. Interestingly, performance of transgenic PDAPP(D664A) and non-Tg mice at the beginning of training at 13 mo was worse than their performance at the end of training at 9 mo, being the difference significant for the non-Tg group (P=0.013) but not for the PDAPP(D664A) group (P=0.3, unpaired Student’s t test), suggesting an age-related effect on long-term retention of the platform location for non-Tg and possibly for Tg PDAPP(D664A) animals, which may be associated with normal aging. No further decline was observed in performance of the Tg PDAPP group between the last trial at 9 months and the first one at 13 mo (Figure 4b). A detectable effect of age in long-term retention was therefore not observed in Tg PDAPP mice. Since learning is impaired in Tg PDAPP mice at all ages analyzed, it is possible that the lack of effect of age on retention is due to limitations of the experimental design (e.g., scores for latency cannot be higher than 60 s).
Non-cognitive components of behavior in Tg PDAPP and Tg PDAPP(D664A) mice
Making no attempt to swim (floating) and swimming in close proximity to the pool wall (‘wall hugging’ or thigmotaxis) are frequent performance deficits to which certain strains of mice are prone that are frequently more pronounced in mice that have been genetically manipulated[38,41]. These patterned behaviours are believed to be manifestations of emotional responses, such as anxiety and helplessness in stressing situations. Thigmotaxis is a pattern of behavior frequently displayed by animals that do not learn well[3]. Immobility, defined as the absence of active, escape-oriented behaviors such as swimming in the water tank is considered indicative of depressive-like behavior in experimental paradigms such as the forced swim test[4,30]. Percent time spent engaged in thigmotactic swim decreased significantly for all groups as a function of age [Figure 5a, significant effect of age at testing on percent time spent engaged in thigmotactic swim (F(2,99)=25.64; P=<0.0001; two-way ANOVA], suggesting long-term effects of increased experience that may result in decreased anxiety. This effect was more pronounced for Tg PDAPP than for all other groups (slopes m=−6.9, m=−6.5, m=−15.5 for non-Tg, Tg PDAPP(D664A) and Tg PDAPP respectively). Tg PDAPP mice, however, spent a significantly larger percent of time engaged in thigmotactic swim than all other groups at all ages tested [P<0.001 with respect to both non-Tg controls and Tg PDAPP(D664A) mice as a result of Bonferroni’s post hoc test applied to a significant effect of genotype (F(2,2)=31.82; P<0.0001; two-way ANOVA], suggesting that, in contrast to non-Tg littermates and Tg PDAPP(D664A) mice, Tg PDAPP animals may experience increased anxiety when facing challenging or relatively stressful situations such as swimming in a brightly lit open pool. In contrast, percent time engaged in thigmotactic swim in Tg PDAPP(D664A) mice was indistinguishable from the non-Tg group at all ages tested, suggesting that obliteration of the Asp664 site at the C-terminus of FAD-hAPP prevents the emergence of this behavioral pattern. It is possible that compensatory mechanisms such as hyperactivity may be activated in Tg PDAPP(D664A) mice that ‘mask’ the anxiety response and produce a behavior that mimics that of non-Tg animals. This alternative hypothesis is, however, unlikely since increased spontaneous activity is present both in PDAPP and PDAPP(D664A) mice[7,31] and may represent a general effect of overexpression of the FAD-hAPP transgene.
Even though overt ‘floaters’ were taken out of the study, significantly increased floating was observed in the Tg PDAPP group across the ages tested [Figure 5b, P<0.05 with respect to both non-Tg controls and Tg PDAPP(D664A) mice, Bonferroni’s post hoc test applied to a significant effect of genotype (F(2,2)=8.49; P=0.0005; two-way ANOVA], suggesting that Tg PDAPP mice experience increased helplessness as compared to non-Tg littermates’ in mildly stressful conditions such as those present during water maze training. However, and in contrast to the effect of age at testing on thigmotaxis, floating significantly increased in all groups with increasing age (F(2,75)=3.3, P=0.04, two-way ANOVA). Percent time spent floating in Tg PDAPP(D664A) mice was not significantly different from that of the non-Tg group, suggesting that obliteration of the Asp664 site at the C-terminus of FAD-hAPP largely prevents the appearance of this pattern of behavior, possibly by decreasing reactivity to stress. Adequate learning may act to reduce reactivity to stress. Consistent with this notion and with their improved retention at 9 mo (Figure 1b), Tg PDAPP mice showed reduced floating at this age. This effect, however, was negated by increasing age (Figure 5b). A trend to increased floating, that nevertheless did not reach statistical significance in a pair-wise comparison within the group, was also observed in non-Tg animals, suggesting that aging per se may increase floating behavior. Albeit at lower levels than Tg PDAPP mice, Tg PDAPP(D664A) mice also showed an age-dependent increase in the proportion of time spent floating between 9 and 13 mo (P<0.04, paired Student’s t test, with a mean of differences of −10.9). Rates of increase in floating time were, however, significantly different between Tg PDAPP and Tg PDAPP(D664A) mice, with a 2X higher rate of increase in floating time for Tg PDAPP with respect to the Tg PDAPP(D664A) group (slopes m=21.6 for the rate increase in Tg PDAPP, m=11 for that in Tg PDAPP(D664A) mice). While the observed increase in floating time for all groups with increasing age suggests that augmented helplessness in the face of stress may be a feature of ‘normal’ aging processes in mice, our results indicate that Tg mice expressing FAD-hAPP may experience significantly higher levels of helplessness with respect to non-Tg littermates with increasing age. This behavioral pattern, however, was significantly attenuated in Tg PDAPP(D664A) animals.
Percent time spent engaged in thigmotactic swim and floating were not correlated across subjects at any age.
Aβ deposition in 13 month-old PDAPP and PDAPP(D664A) transgenic mice
Previous studies have shown that the D664A mutation does not affect overall Aβ40 and Aβ42 production in cultured cells[36,39], and similar results were obtained by us in vivo[7,32]. In those studies, plaque deposition was examined in older (12 mo) mice from the moderate-expressing PDAPP(D664A)B21 transgenic line and in younger (6 mo) mice from the high-expressing PDAPP(D664A)B254 transgenic line. While plaque deposition was documented in the high-expressing PDAPP(D664A)B254 transgenic line at ages that preceded overt plaque deposition in Tg PDAPP mice (6 mo), whether plaque deposition would be affected by the D664A mutation in transgenic animals from the PDAPP(D664A)B254 line at more advanced ages remained to be determined.
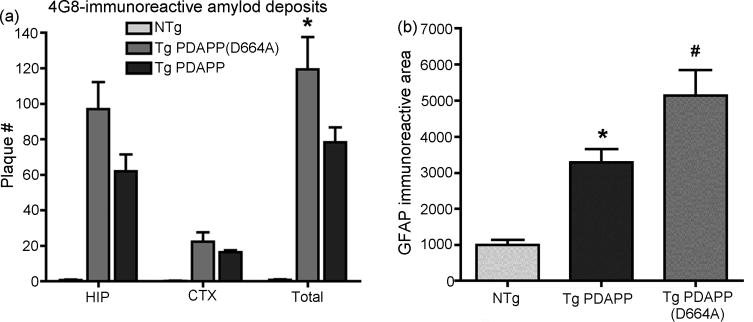
To ascertain whether cleavage of APP at Asp664 had an effect on plaque deposition in older animals, we examined brain sections from aged (13 mo) Tg PDAPP and PDAPP(D664A) animals after completion of behavioral studies by immunohistochemistry, using an antibody directed against the middle portion of Aβ (4G8). Quantitative determinations of 4G8 immunoreactivity in brain sections of 13 mo Tg PDAPP and PDAPP(D664A) mice showed multiple 4G8-immunoreactive Aβ deposits restricted to the hippocampus and cortex (PDAPP=78.3 ± 8.3, PDAPP(D664A)=119.3 ± 18.2 total; PDAPP=62 ± 9.5, PDAPP(D664A)=97 ± 15.3 hippocampal; PDAPP=16.3 ± 1.2, PDAPP(D664A)=22.3 ± 5.3 cortical plaques per section, Figure 6a). Plaque deposition in brains of Tg PDAPP(D664A) mice from the high-expressor PDAPP(D664A)B254 line was thus ~50% higher than in Tg PDAPP animals from the J20 line at 13 mo, consistent with the levels of expression of the hAPP transgene in each Tg line as well as with their Aβ production levels, which are ~20–25% higher and ~60% higher for Aβ42 in PDAPP(D664A)B254 mice with respect to PDAPP(J20) mice respectively[7,32]. Amyloid plaques in brain sections from both Tg PDAPP and Tg PDAPP(D664A) mice were fibrillar in nature, as evidence by thioflavin-S staining (data not shown). Taken together, these results confirm our previous observations in aged animals from the moderate-expressing PDAPP(D664A)B21 transgenic line [7,32], indicating that mutation of Asp664 in the intracytoplasmic domain of APP has neither an overt quantitative nor qualitative effect on the deposition of β-amyloid in vivo.
Figure 6. Plaque deposition in 13 mo Tg PDAPP and Tg PDAPP(D664A) mice.
a Total hippocampal (HIP) and cortical (CTX) Aβ plaques (4G8-immunoreactive objects) were quantitated in 13 mo Tg PDAPP, Tg PDAPP(D664A) and non-Tg littermate control brain sections using ImageJ by investigators blinded to genotype. *, P<0.05 as a result of Bonferroni’s post-hoc test applied to a significant effect of genotype on number of plaques [F(2,42)=74.26, P<0.0001, two-way ANOVA]. b. Astrogliosis in 13 mo Tg PDAPP and Tg PDAPP(D664) mice. Total GFAP-immunoreactive area was quantitated in 13 mo Tg PDAPP, Tg PDAPP(D664A) and non-Tg littermate control brain sections using Image J by investigators blinded to genotype. Total GFAP-immunoreactive area was higher both in Tg PDAPP and Tg PDAPP(D664A) animals as compared to non-Tg littermates (*, P<0.05; #, P<0.01 respectively as a result of Tukey’s post-hoc test applied to a significant difference between genotypes in one-way ANOVA (P<0.0001). GFAP-immunoreactive areas were not significantly different between Tg PDAPP(D664A) and Tg PDAPP groups. Data are means ± SEM.
Astrogliosis in hippocampi of 13 month-old PDAPP and PDAPP(D664A) transgenic mice
Astrogliosis occurs in many neurodegenerative diseases, including AD[37]. This phenomenon is recapitulated in transgenic PDAPP mice as described[7]. In those studies, increased astrogliosis was detected in 12 mo Tg PDAPP mice from the J20 line but not in Tg PDAPP(D664A) mice from the moderate-expressing PDAPP(D664A)B21 line at the same age[7]. To determine whether astrogliosis would be present in 13 mo animals from the high-expressing PDAPP(D664A)B254 transgenic line, we stained brain sections from Tg PDAPP(J20) and Tg PDAPP(D664A)B254 mice with GFAP antibodies after completion of behavioral studies at 13 mo. Quantitative determinations of GFAP immunoreactivity revealed that the area of GFAP immunoreactive signal in hippocampi of Tg PDAPP mice was significantly increased with respect to non-transgenic littermates’ (P<0.05, as a result of Tukey’s post hoc test applied to a significant effect of genotype (P<0.0001) in one-way ANOVA), as previously shown[7]. However, GFAP immunoreactivity in hipppocampi from 13 mo Tg PDAPP(D664A)B254 mice was also significantly increased with respect to non-transgenic littermates’ (P<0.001, Tukey’s post hoc test applied to a significant effect of genotype (P<0.0001) in one-way ANOVA), but was not significantly different from PDAPP Tg mice (Figure 6b). Thus, and in contrast to our observations in Tg PDAPP(D664A) mice from the moderate-expressing B21 line[7], astrogliosis was present in Tg PDAPP(D664A)B254 mice in a manner roughly proportional to the levels of expression of the hAPP transgene and consistent with the observed 50% increase in plaque deposition in these brains (Figure 6a). These results suggest that astrocytic proliferation may constitute a response to the levels of expression of the hAPP transgene and/or to the levels of Aβ production and deposition in this mouse model of AD. Therefore, we conclude that the D664A mutation did not affect astrogliosis in the PDAPP mouse model of AD. Since astrogliosis was present in brains from Tg PDAPP(D664A) mice in the absence of detectable behavioral abnormalities, our results suggest that astrogliosis does not have a pathogenic role in this mouse model of AD.
DISCUSSION
We showed previously[7,32] that mutation of Asp664 in the C-terminal domain of a FAD-hAPP transgene abolishes AD-like deficits in the PDAPP mouse model. AD-like deficits were absent in PDAPP(D664A) transgenic mice from the moderate-expressor B21 line up to 12 mo (mo)[32] and in the high-expressor PDAPP(D664A) transgenic line B254 until 7 mo [7]. Tg PDAPP(D664A)B254 mice express ~1.5X higher levels of the FAD-hAPP transgene and produce proportionally higher levels of Aβ than Tg PDAPP(J20) mice [7]. To determine whether mutation of Asp664 completely abolishes or only delays the appearance of AD-like deficits in high-expressing PDAPP(D664A)B254 mice, we examined cognitive function longitudinally in transgenic PDAPP and PDAPP(D664A) at 9 and 13 mo and features of AD-like histopathology at 13 mo after completion of behavioral studies. Consistent with previous reports[7,29,31,32], transgenic PDAPP mice exhibited spatial learning and memory deficits with respect to non-Tg animals at all ages studied (Figures 1, 2 and 4). Examination of these features of the AD-like phenotype in PDAPP(D664A)B254 mice revealed an overall normalization of learning and memory deficits in animals carrying a D664A mutation until 13 mo. These results are in agreement with our previous observations in younger Tg PDAPP(D664A)B254 mice (2.5 to 7 mo, [7,32]). Although overall performance of Tg PDAPP(D664A) mice across all ages examined in the present study was not significantly different from that of non-Tg littermates’ (Figure 4), a trend towards poorer performance during retention at 9 mo and during spatial learning at 13 mo was present in Tg PDAPP(D664A)B254 mice (Figure 2a). None of these changes, however, resulted in significant differences with respect to non-Tg littermates’ performance during training or at testing. Performance of Tg PDAPP(D664A) was therefore significantly improved with respect to Tg PDAPP animals at all ages examined. While retention in PDAPP(D664A) mice was not significantly different from the non-Tg group at 9 mo, it was not significantly different from the Tg PDAPP group either, suggesting that a trend to impairment in spatial memory may have developed in Tg PDAPP(D664A) mice at that age. This trend, however, was reversed by carry-over (savings) effects of experience (Figure 2b). Interestingly, experience had a positive impact on performance in all groups (Figure 4a), suggesting that long-term retention is operant in PDAPP mice even in the presence of significant learning and memory deficits. Retention of the platform location in Tg PDAPP mice was improved at 13 mo, in spite of the sustained deficits observed for this group during training (Figure 2). These observations are in agreement with the observed amelioration in cognitive deficits resulting from enriched experience in other Tg mouse models of AD[15], are consistent with the concept of “cognitive reserve” proposed for human subjects[33] and may provide support for cognitive training approaches to the prevention of AD.
PDAPP(D664A) mice from the high-expressing line used in these studies(B254), produce very high levels of soluble Aβ [7,32]. As would be expected from their higher levels of soluble Aβ production, we found that Aβ plaque deposition was increased by ~50% in 13 month-old Tg PDAPP(D664A) mice as compared to Tg PDAPP(J20) mice (Figure 6a). These observations are in agreement with those of Saganich et al. [32], who showed that Aβ deposition was present in Tg PDAPP(D664A)B524 mice at 6 mo, when it is still undetectable in Tg PDAPP(J20) mice. The distribution of Aβ deposits in hippocampus and in cortex was roughly equivalent in both transgenic lines (Figure 6a). Taken together, these results support and extend previous observations[7,32] and provide strong support for the hypothesis that Asp664 cleavage is required for the generation of AD-like deficits in the PDAPP mouse model of AD, possibly by mediating a significant component of Aβ toxicity. Considering the very high levels of Aβ production and accumulation in PDAPP(D664A), it is likely that Asp664-independent pathways of Aβ damage may be responsible for the emerging trends to poorer performance observed in aged Tg PDAPP(D664A) mice.
In addition to cognitive deficits, Tg PDAPP mice displayed aberrant patterns of behavior (increased floating and thigmotactic swim) during training (Figure 5). These may be associated with non-cognitive components of behavior such as anxiety and helplessness, which are thought to underlie thigmotactic swim and floating behaviors respectively. Supporting the notion that effective learning is associated with decreased anxiety, time spent engaged in thigmotactic swim decreased monotonically with increased experience in all groups (Figure 5a). Percent time spent swimming close to the tank wall was highest in the Tg PDAPP group at all ages, but their responsiveness to increased experience was also more pronounced than any other group, suggesting that, in spite of their deficits in learning and memory, long-term savings effects operant in Tg PDAPP mice may have an impact in their responses to stressful situations. Thus, our results suggest that effective learning may have an effect in decreasing anxiety that is not affected by aging processes in mice, and that this effect of learning is operant also in Tg PDAPP mice modelling AD. Moreover, a carry-over (savings) effect was present for this non-cognitive component of behavior across ages at training for all groups, with the lowest percent times spent engaged in thigmotactic swim at the end of the experiment in the last session at 13 mo.
Although increased floating and thigmotactic swim patterns were absent in non-Tg and PDAPP(D664A) mice at all ages tested, a trend to increased floating was observed both in non-Tg and Tg PDAPP(D664A) groups at 13 mo (Figure 5b). This trend may reflect processes associated with normal aging in mice.
We also observed a positive effect of prior experience on increased helplessness (floating) associated with testing conditions that was pronounced in Tg PDAPP mice between 7 and 9 mo; this decreasing effect on helplessness, however, and in contrast to our observations for anxiety, disappeared with increasing age (Figure 5b). A closer examination of percent spent floating in all groups at 9 and 13 mo, however, revealed that the overall increase in floating for the Tg PDAPP group arose from a stereotypical response during the first and second days of training that disappeared as training progressed at both ages. Thus, our results suggest that the observed increase in helplessness in mice modelling AD may be ascribed to a lack of familiarity with the conditions of training, arising from a deficit in learning [absent carry-over (savings) effect] between ages at training. Long-term effects (retention between days at training) are operant in Tg PDAPP mice, however, since helplessness subsided as training progressed both at 9 and 13 mo. Thus, Tg PDAPP mice, in contrast to non-Tg and Tg PDAPP(D664A) mice, did not show carry-over (savings) effect between 9 and 13 mo, arguing that this non-cognitive component of behavior is not significantly affected by prior experience in aged animals undergoing AD-like processes. Interestingly, and in contrast to our observations for anxiety (Figure 5a), floating also showed a trend to monotonic increase in both non-Tg and Tg PDAPP(D664A) mice during training at 13 mo, possibly as a consequence of normal aging processes in mice.
Astrogliosis is commonly present in neurodegenerative conditions. Both beneficial and detrimental effects have been attributed to increased numbers of reactive astrocytes[28]. Astrogliosis was observed in Tg PDAPP mice[7], but its significance in this mouse model of AD remains unclear. In contrast to the improvements in spatial learning and memory observed in Tg PDAPP(D664A)B254 mice with respect to Tg PDAPP mice across all ages tested, astrogliosis was unchanged in brains from Tg PDAPP(D664A)B254 mice at 13 mo (Figure 6b). Although no causal relationships can be inferred from these observations, our results argue that increases in numbers of reactive astrocytes do not have a deleterious effect in spatial learning and memory in this mouse model of AD, and suggest that the astrocytic proliferative response may respond to increases in the levels of expression of the hAPP transgene and/or to proportional increases in Aβ production and deposition (Figure 6a). Moreover, our results can be interpreted as suggesting that an increase in the number of reactive astrocytes may have no role, or potentially a beneficial effect, in this mouse model of AD.
To summarize, the results of the present study confirm our previous findings[7,32] and indicate that the cleavage of hAPP at Asp664 by caspases (or possibly by a non-caspase protease or proteases) does not overtly affect Aβ production and deposition nor astrogliosis, but is a critical step in the development of behavioral abnormalities in FAD-hAPP transgenic mice. Therefore, Aβ-mediated learning and memory deficits in the PDAPP mouse model of AD may involve APP cleavage at Asp664. The results of the present study also lend support to a recently proposed model of AD, in which Aβ binds to and oligomerizes APP[2,19,21,22,34,35], leading to cleavage at Asp664 and cytotoxicity[21,22]. Our results do not exclude the possibility that the Asp664->Ala mutation affects the AD phenotype not by preventing cleavage at Asp664 but rather by destroying, or conversely stabilizing, an as-yet-uncharacterized protein-protein interaction at APP’s C-terminus; however, in either case, the mediation of Aβ toxicity in vivo by APP via an intracytoplasmic mechanism (be it cleavage or modulation of protein-protein interaction, or both) is supported by the current observations.
The C-terminal cleavage of APP by caspases truncates APP aminoterminally to sequences that are required for its interaction with motor proteins, components of the stress response, and transcriptional transactivators[5,10–12,14,17,18,25]. Cleavage of APP by transiently activated caspases at neuronal terminals may therefore disrupt its interaction with several different protein complexes and thus alter the normal processing, turnover or function of the molecule. Thus, it is possible that protective mechanisms that partially compensate for Aβ toxicity may be upregulated when the C-terminal cleavage of APP is precluded. If upregulation of pathways that result in resistance to Aβ toxicity have a role in preventing the development of AD-like deficits in Tg PDAPP(D664A) mice, the appearance of a non-significant trend to poorer performance in older PDAPP(D664A) mice suggests that these pathways might be down-regulated with increasing age. Alternatively, it is conceivable that cognitive function in Tg PDAPP(D664A) mice can withstand ongoing low-levels of Aβ-mediated damage until late in life, when the accumulated amyloid or the consequences of prior Aβ-mediated damage may result in a trend to emergent cognitive abnormalities.
The results presented here point to a key role for the C-terminal cleavage of APP (or alternatively, a protein-protein interaction requiring Asp664) in the development of late functional AD-like deficits in a transgenic mouse model. Furthermore, our data indicate that the intracytoplasmic domain of APP may play an important role in the pathogenesis of AD, and suggest that the cleavage of APP at Asp664 may represent a therapeutic target.
Acknowledgments
We thank Dr. Mari Golub, University of California, Davis for critically reading the manuscript and Molly Susag for administrative assistance. Supported in part by NIHNS045093 to DB, Alzheimer’s Association NIRG 04-1054 to VG and IIRG 06-27717 to DB, and an award from S.D. Bechtel, Jr., to V.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burger C, Lopez MC, Feller JA, Baker HV, Muzyczka N, Mandel RJ. Changes in transcription within the ca1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol Learn Mem. 2007;87:21–41. doi: 10.1016/j.nlm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Chen CD, Oh SY, Hinman JD, Abraham CR. Visualization of app dimerization and app-notch2 heterodimerization in living cells using bimolecular fluorescence complementation. J Neurochem. 2006;97:30–43. doi: 10.1111/j.1471-4159.2006.03705.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of alzheimer’s disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 4.Crawley JN. What’s wrong with my mouse? New York: Wiley-Liss; 2000. p. 329. [Google Scholar]
- 5.Galvan VBS, Spilman P, Gorostiza OF, Peel A, Crippen D, Sidhu G, Ichijo H, Bredesen DE. Interaction of ask1 and the b-amyloid precursor protein in a stress-signaling complex. Neurobiol Dis. 2007 doi: 10.1016/j.nbd.2007.06.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvan V, Chen S, Lu D, Logvinova A, Goldsmith P, Koo EH, Bredesen DE. Caspase cleavage of members of the amyloid precursor family of proteins. J Neurochem. 2002;82:283–294. doi: 10.1046/j.1471-4159.2002.00970.x. [DOI] [PubMed] [Google Scholar]
- 7.Galvan V, Gorostiza OF, Banwait S, Ataie M, Logvinova AV, Sitaraman S, Carlson E, Sagi SA, Chevallier N, Jin K, Greenberg DA, Bredesen DE. Reversal of alzheimer’s-like pathology and behavior in human app transgenic mice by mutation of asp664. Proc Natl Acad Sci U S A. 2006;103:7130–7135. doi: 10.1073/pnas.0509695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of alzheimer’s amyloid-beta precursor protein and amyloidogenic a beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 9.Hell K, Saleh M, Crescenzo GD, O’Connor-McCourt MD, Nicholson DW. Substrate cleavage by caspases generates protein fragments with smac/diablo-like activities. Cell Death Differ. 2003;10:1234–1239. doi: 10.1038/sj.cdd.4401298. [DOI] [PubMed] [Google Scholar]
- 10.Ho CS, Marinescu V, Steinhilb ML, Gaut JR, Turner RS, Stuenkel EL. Synergistic effects of munc18a and x11 proteins on amyloid precursor protein metabolism. J Biol Chem. 2002;277:27021–27028. doi: 10.1074/jbc.M201823200. [DOI] [PubMed] [Google Scholar]
- 11.Homayouni R, Rice DS, Sheldon M, Curran T. Disabled-1 binds to the cytoplasmic domain of amyloid precursor-like protein 1. J Neurosci. 1999;19:7507–7515. doi: 10.1523/JNEUROSCI.19-17-07507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999;19:5179–5188. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inomata H, Nakamura Y, Hayakawa A, Takata H, Suzuki T, Miyazawa K, Kitamura N. A scaffold protein jip-1b enhances amyloid precursor protein phosphorylation by jnk and its association with kinesin light chain 1. J Biol Chem. 2003;278:22946–22955. doi: 10.1074/jbc.M212160200. [DOI] [PubMed] [Google Scholar]
- 15.Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV. Environmental enrichment mitigates cognitive deficits in a mouse model of alzheimer’s disease. J Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janus C. Search strategies used by app transgenic mice during navigation in the morris water maze. Learn Mem. 2004;11:337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-i. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 18.Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. Ethe intracellular domain of the beta-amyloid precursor protein is stabilized by fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001;276:40288–40292. doi: 10.1074/jbc.C100447200. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzo A, Yuan M, Zhang Z, Paganetti PA, Sturchler-Pierrat C, Staufenbiel M, Mautino J, Vigo FS, Sommer B, Yankner BA. Amyloid beta interacts with the amyloid precursor protein: A potential toxic mechanism in alzheimer’s disease. Nat Neurosci. 2000;3:460–464. doi: 10.1038/74833. [DOI] [PubMed] [Google Scholar]
- 20.Lu DC, Rabizadeh S, Chandra S, Shayya RF, Ellerby LM, Ye X, Salvesen GS, Koo EH, Bredesen DE. A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat Med. 2000;6:397–404. doi: 10.1038/74656. [DOI] [PubMed] [Google Scholar]
- 21.Lu DC, Shaked GM, Masliah E, Bredesen DE, Koo EH. Amyloid beta protein toxicity mediated by the formation of amyloid-beta protein precursor complexes. Ann Neurol. 2003;54:781–789. doi: 10.1002/ana.10761. [DOI] [PubMed] [Google Scholar]
- 22.Lu DC, Soriano S, Bredesen DE, Koo EH. Caspase cleavage of the amyloid precursor protein modulates amyloid beta-protein toxicity. J Neurochem. 2003;87:733–741. doi: 10.1046/j.1471-4159.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 23.Madeira A, Pommet JM, Prochiantz A, Allinquant B. Set protein (taf1beta, i2pp2a) is involved in neuronal apoptosis induced by an amyloid precursor protein cytoplasmic subdomain. Faseb J. 2005;19:1905–1907. doi: 10.1096/fj.05-3839fje. [DOI] [PubMed] [Google Scholar]
- 24.Markowska AL, Savonenko AV. Protective effect of practice on cognition during aging: Implications for predictive characteristics of performance and efficacy of practice. Neurobiol Learn Mem. 2002;78:294–320. doi: 10.1006/nlme.2002.4064. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda S, Yasukawa T, Homma Y, Ito Y, Niikura T, Hiraki T, Hirai S, Ohno S, Kita Y, Kawasumi M, Kouyama K, Yamamoto T, Kyriakis JM, Nishimoto IM. C-jun n-terminal kinase (jnk)-interacting protein-1b/islet-brain-1 scaffolds alzheimer’s amyloid precursor protein with jnk. J Neurosci. 2001;21:6597–6607. doi: 10.1523/JNEUROSCI.21-17-06597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 29.Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci U S A. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechnick RN, Chesnokova VM, Kariagina A, Price S, Bresee CJ, Poland RE. Reduced immobility in the forced swim test in mice with a targeted deletion of the leukemia inhibitory factor (lif) gene. Neuropsychopharmacology. 2004;29:770–776. doi: 10.1038/sj.npp.1300402. [DOI] [PubMed] [Google Scholar]
- 31.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 32.Saganich MJ, Schroeder BE, Galvan V, Bredesen DE, Koo EH, Heinemann SF. Deficits in synaptic transmission and learning in amyloid precursor protein (app) transgenic mice require c-terminal cleavage of app. J Neurosci. 2006;26:13428–13436. doi: 10.1523/JNEUROSCI.4180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarmeas N, Stern Y. Cognitive reserve: Implications for diagnosis and prevention of alzheimer’s disease. Curr Neurol Neurosci Rep. 2004;4:374–380. doi: 10.1007/s11910-004-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaked GM, Kummer MP, Lu DC, Galvan V, Bredesen DE, Koo EH. Abeta induces cell death by direct interaction with its cognate extracellular domain on app (app 597–624) Faseb J. 2006;20:1254–1256. doi: 10.1096/fj.05-5032fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soba P, Eggert S, Wagner K, Zentgraf H, Siehl K, Kreger S, Lower A, Langer A, Merdes G, Paro R, Masters CL, Muller U, Kins S, Beyreuther K. Homo-and heterodimerization of app family members promotes intercellular adhesion. Embo J. 2005;24:3624–3634. doi: 10.1038/sj.emboj.7600824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soriano S, Lu DC, Chandra S, Pietrzik CU, Koo EH. The amyloidogenic pathway of amyloid precursor protein (app) is independent of its cleavage by caspases. J Biol Chem. 2001;276:29045–29050. doi: 10.1074/jbc.M102456200. [DOI] [PubMed] [Google Scholar]
- 37.Streit WJ. Microglia and alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- 38.Tecott LH, Nestler EJ. Neurobehavioral assessment in the information age. Nat Neurosci. 2004;7:462–466. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- 39.Tesco G, Koh YH, Tanzi RE. Caspase activation increases beta-amyloid generation independently of caspase cleavage of the beta-amyloid precursor protein (app) J Biol Chem. 2003;278:46074–46080. doi: 10.1074/jbc.M307809200. [DOI] [PubMed] [Google Scholar]
- 40.Venero C, Tilling T, Hermans-Borgmeyer I, Herrero AI, Schachner M, Sandi C. Water maze learning and forebrain mrna expression of the neural cell adhesion molecule l1. J Neurosci Res. 2004;75:172–181. doi: 10.1002/jnr.10857. [DOI] [PubMed] [Google Scholar]
- 41.Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP. Spatial memory and learning in transgenic mice: Fact or artifact? News Physiol Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]