Abstract
The aim of the present study was to investigate the effects of 4-hydroxy-2-nonenal (4-HNE) on tube formation by human bone marrow endothelial cells (HBMEC). We found that 4-HNE at physiologically-achievable concentrations (5 and 10 μM) inhibited the formation of tubes. Western blot analysis revealed that inhibition of tube formation by 4-HNE was associated by increased expression of chondromodulin-I (CHM-I), a protein with well known anti-angiogenic properties. Cell viability assays showed that 4-HNE at concentrations of 10 μM or less did not cause HBMEC cell death. Luciferase reporter assays did not show any inducing effect of 4-HNE on the promoter activity of human CHM-I gene indicating that post-transcriptional or post-translational modifications may account for the up-regulation of CHM-I. Collectively, the results of the present study show for the first time that 4-HNE inhibits tube formation by HBMECs indicating a potential anti-angiogenic activity of 4-HNE. This inhibition occurs at least in part via 4-HNE-induced CHM-I protein expression.
Keywords: 4-HNE, chondromodulin-I, tube formation assay, angiogenesis, endothelial cells, ALDH3A1
Introduction
The mammalian cornea, located on the anterior surface of the eye, is an avascular tissue that serves as a protective barrier between the environment and the internal ocular structures [1]. The protective role of cornea is attributed mainly to abundant, water-soluble proteins known as corneal crystallins [2]. One of the most important corneal crystallins is the aldehyde dehydrogenase 3A1 (ALDH3A1) [1]. Our recent studies have shown that Aldh3a1(−/−) knockout mice exhibit increased levels of 4-hydroxy-2-nonenal (4-HNE) in their lens and cornea [3]. 4-HNE is a α, β-unsaturated aldehyde produced within cells as a byproduct of lipid peroxidation [4]. ALDH3A1 oxidizes 4-HNE with high specificity [5]. Thus, the increased levels of 4-HNE observed in Aldh3a1(−/−) transgenic knockout mice have been attributed to the lack of ALDH3A1 protein [6].
The physiological concentration of 4-HNE in the human plasma ranges between 0.3 and 1.0 μM; however, under conditions of oxidative stress these levels can increase to 10 μM – 5 mM [4]. 4-HNE is a highly reactive molecule that can form adducts with proteins and result in their degradation [7]. In addition to its cytotoxic role, 4-HNE has been proposed to act as a second messenger in signaling pathways induced by reactive oxygen species (ROS) [8]. It has also been shown that 4-HNE regulates gene expression via interaction with transcription factors, membrane receptors and transcription repressors [9]. Several studies have shown that 4-HNE affects cellular functions (e.g. cellular proliferation, differentiation, transformation and apoptosis) through cell signaling pathways [8]. Angiogenesis, the multistep process by which new vessels arise from pre-existing vasculature, may also be influenced by 4-HNE. However, its reported effects are conflicting, with some studies describing inhibition of angiogenesis [10; 11]and others promotion [12; 13]. A few mechanisms by which 4-HNE affects angiogenesis have been proposed [10] but, in general, these mechanisms remain to be defined.
Interestingly, the increased 4-HNE levels in cornea of Aldh3a1(−/−) knockout mice were accompanied by increased expression of chondromodulin-I (CHM-I) protein [6]. CHM-I, known for its anti-angiogenic activity, is expressed in abundance in fetal cartilage and also can be found in other mammalian avascular tissues such as cardiac valves and cornea [14]. It is a single transmembrane protein (317 amino acids) that is processed post-translationally by the cleavage of the C-terminal intracellular fragment by furin protease, generating a mature 120-amino acid glycoprotein [14].
Angiogenesis plays a central role in physiological processes, such as embryogenesis and wound healing, and in several pathological conditions including carcinogenesis, rheumatoid arthritis, liver fibrosis, proliferative retinopathy and corneal neovascularization [15]. Accordingly, the elucidation of the effects of 4-HNE on angiogenesis and the mechanisms by which it exerts these effects has important pathophysiologic and therapeutic implications. In the present study, we investigated the effects of 4-HNE on the tube formation, a marker of angiogenesis, and the expression of CHM-I in human endothelial cells.
Materials and methods
Cell culture conditions and reagents
HBMECs were cultured as described previously [16]. In all experiments, cells were maintained in a humidified incubator containing 5% carbon dioxide in air at 37° C. All tissue culture media, supplements, growth factors, assay reagents and buffers were purchased from Gibco®/Invitrogen (Carlsbad, CA, USA) unless otherwise specified. 4-HNE solution (10 mg/ml in 100% ethanol) was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). For all treatments, 4-HNE was diluted in phosphate buffered saline (PBS) at pH 7.4. The maximum concentration of ethanol in cell culture medium was 0.06% (v/v).
Tube formation assay
Tube formation was performed as described elsewhere [17]. Briefly, 150 μl of an extracellular matrix solution (Matrigel™, BD Biosciences, Franklin Lakes, NJ, USA) was added to each well of a 6-well plate and allowed to solidify for at least 30 min at 37°C. Afterwards, HBMECs were plated (1 × 105 cells/well) on the surface of the matrigel and treated with 4-HNE (1, 5 and 10 μM) or vehicle (0.06% ethanol in PBS). Cells were incubated for 16 hrs and then the effect of 4-HNE on tubular morphogenesis was documented microscopically and photographed. Each experiment was repeated at least 3 times.
Western blot analysis for CHM-I protein in 4-HNE-treated HBMECs
HBMECs (1.5 × 106 cells per plate) were seeded in 100 mm culture plates, allowed to adhere for 24 h and then were exposed to 5 or 10 μM 4-HNE for 16 h. Control cells were exposed to medium containing an equivalent volume of vehicle (0.06% ethanol in PBS). At the end of the treatment period, cells were extracted in cell lysis buffer as described previously [18]. Protein concentrations in cell lysates were measured using a kit for the bicinchoninic acid method (Pierce, Rockford, IL, USA), according to the manufacturer’s instructions. Cell lysates were then subjected to SDS/PAGE and immunoblotted according to previously described methods [18]. Relevant proteins were identified by incubation with rabbit anti-human pre-mature CHM-I (1:2500) or mouse anti-human mature CHM-I (1:20000; R&D Systems, Minneapolis, MN, USA) for 1h at room temperature. Membranes were incubated with either goat anti-rabbit IgG (Calbiochem, Gibbstown, NJ, USA) or rabbit anti-mouse IgG (Sigma, St Louis, MO, USA) G-conjugated horseradish peroxidase secondary antibodies (1:5000) for 30 min at room temperature. Labeled protein bands were detected by enhanced chemiluminescence (PerkinElmer, Waltham, MA, USA). All membranes were reprobed for β-actin to permit loading correction. Densitometry data were normalized to β-actin and analyzed using the Quantity One software (Bio-Rad, Ca, USA). Each experiment was repeated 3 times.
Cell viability assays
Three different assays, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), the protein-staining sulforodamine B (SRB) and the lactate dehydrogenase (LDH), were used to assess the effect of 4-HNE on the viability of HBMECs. In all three assays, cells (1.5 × 104 per well) were seeded into 96-well culture plates and allowed to adhere for 24 h. After attachment, the cells were treated for 16 h with 4-HNE (1, 5, 10, 20 or 40 μM) or vehicle (0.06% ethanol in PBS; negative control) or 2% Triton X-100 (positive control). The MTT assay was conducted according to the protocol of Mosmann [19]. The protein-staining sulforodamine B (SRB) assay was carried out according to the protocol described by Vichai et al. [20]. In both assays, cell survival was defined as the absorbance and expressed as a percentage of that found in the vehicle controls. LDH release assay was performed using the CytoTox-One™ kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Each cell viability assay was conducted in triplicate, and data are presented as the means ± SD from three independent experiments.
Luciferase assay
Constructs consisting of pGVB2 vectors containing three different (−832/+87; −446/+87; −297/+87) 5′-flanking regions of the human CHM-I gene, fused to the firefly luciferase gene, were used to determine whether 4-HNE treatment affects the promoter activity of CHM-I gene. These constructs were a generous gift from Dr. Toshimi Michigami [21]. As an endogenous control, the TR vector containing the Renilla luciferase gene was used. Briefly, HBMECs were seeded (1.25 × 105 per well) in a 24-well plate. Cells were cotransfected in antibiotic-free medium with 0.5 μg pGVB2 vector containing the luciferase reporter gene along with the promoter regions of human CHM-I gene, and 5 ng TR vector containing the Renilla luciferase gene. For each transient transfection, 2 μl of the FuGENE® HD transfection reagent (Roche, IN, USA) was used. Twenty-four h after transient transfection, the cells were treated for 16 h with 4-HNE (5 or 10 μM) or vehicle (0.06% ethanol in PBS). The firefly luciferase activity was determined by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions and expressed as relative light units normalized to the luciferase activity of the Renilla luciferase (i.e. the values of firefly luciferase activity were divided by the values of Renilla luciferase activity). Each set of experiments was conducted in triplicate and are presented as means ± SD from three independent experiments.
Statistical analysis
To identify significant differences, a one-way ANOVA was applied followed by Dunnett’s post-hoc test for multiple pair wise comparisons. Differences were considered significant at p<0.05.
Results
4-HNE inhibits in vitro tube formation of HBMEs
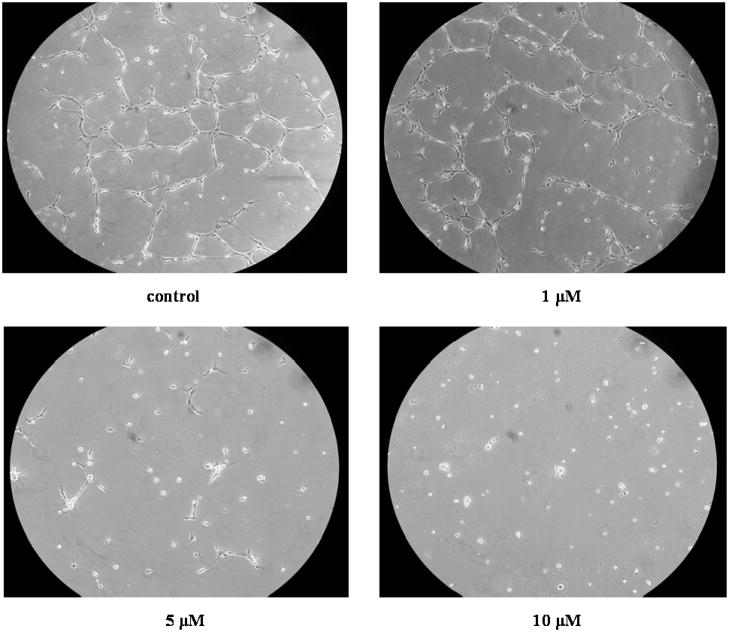
The tube formation assay was used to examine the effect of 4-HNE on angiogenesis in vitro (Fig. 1). At concentrations of 5 and 10 μM, 4-HNE clearly inhibited the formation of tubes.
Fig. 1.
Representative pictures of formation of tube-like structures by HBMECs. Cells were plated onto Matrigel and treated with vehicle control (0.06% ethanol in PBS) or different concentrations (1, 5 and 10 μM) of 4-HNE for 16 h. After treatment, cells were visualized microscopically and photographed at magnification 100X.
Effects of 4-HNE on viability of HBMECs
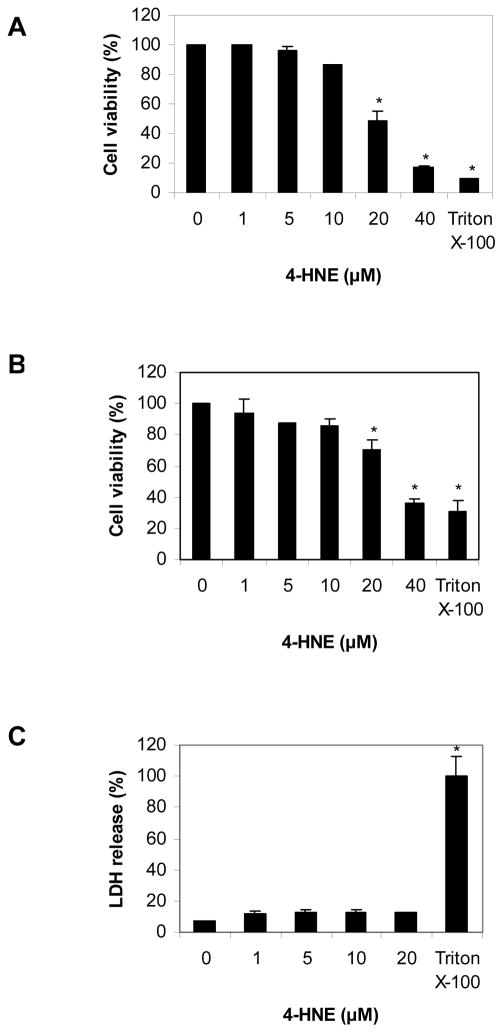
Three different cell viability assays, MTT, SRB and LDH, were used in order to assess the effect of 4-HNE on the viability of HBMEC cells. Each one of these assays represents a different endpoint. Specifically MTT assay measures the activity of mitochondrial dehydrogenase; SRB assay measures the total cellular protein level; LDH assay measures the membrane integrity based on cellular release of lactate dehydrogenase enzyme. In the MTT assay, cell viability was reduced in a concentration-dependent manner by concentrations of 4-HNE exceeding 10 μM (Fig. 2A). A similar result was obtained using the SRB assay (Fig. 2B). However, in the LDH assay, there was not any statistically significant difference in the release of LDH enzyme between the control cells and the cells treated with 4-HNE. (Fig. 2C). When examined microscopically, the morphology of cells treated with 4-HNE at concentrations ≥20 μM was characteristic of cell apoptosis (i.e., circular cells with membrane blebbing) and completely different than that observed for cells treated with Triton X-100 (i.e., cell necrosis) (data not shown).
Fig. 2.
Cell viability assays: (A) MTT assay; (B) SRB assay; (C) LDH assay. HBMECs were treated with vehicle control (0.06% ethanol in PBS) or different concentrations of 4-HNE for 16 h. 2% Triton X-100 was used as positive control. Values are mean ± S.D. from three independent experiments carried out in triplicate. *p<0.05 when compared with the vehicle control. Statistical analysis was by ANOVA followed by Dunnett’s post-hoc test.
4-HNE induces the expression of human CHM-I protein in HBMECs
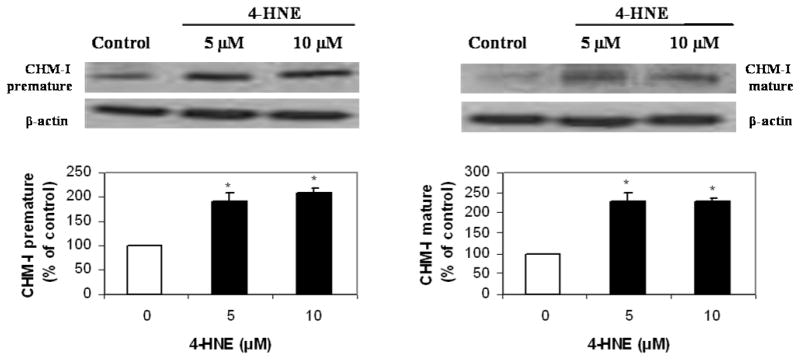
Since the tube formation assay showed that 4-HNE inhibited formation of tubes by HBMECs, the effect of 4-HNE was examined on the expression of the anti-angiogenic protein CHM-I. Two forms of CHM-I protein have been identified [14], the precursor (37 kDA) and the mature (25 kDa) protein. Western blot analyses were carried out for both forms. There was a 2-fold increase in the expression of both forms of CHM-I protein in the HBMECs treated by tube-inhibiting concentrations of 4-HNE, namely 5 and 10 μM (Fig. 3).
Fig. 3.
Representative Western blots show expression of both pre-mature and mature CHM-I protein forms following 16 h treatment with vehicle control (0.06% ethanol in PBS) or 4-HNE at concentrations of 5 or 10 μM. The same membrane was reprobed for β-actin to permit loading correction. Densitometric quantification for CHM-I is reported. Values represent the mean ± S.D. values (n = 3). *p<0.05 when compared with the vehicle control. Statistical analysis was by ANOVA followed by Dunnett’s test.
Effects of 4-HNE on the promoter activity of human CHM-I gene
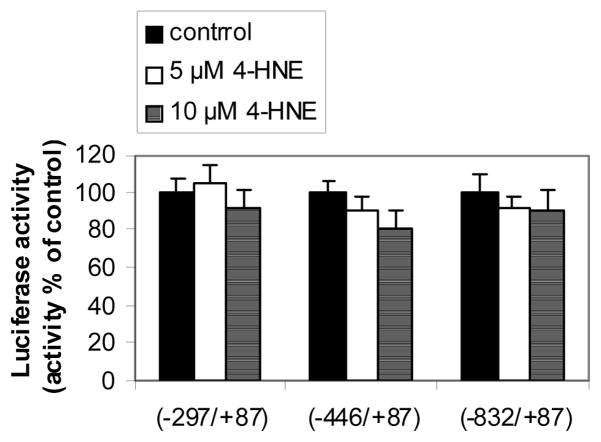
Using constructs containing three different regions (−832/+87; −446/+87; −297/+87) of the promoter of human CHM-I gene fused to a luciferase gene, the influence of 4-HNE on the promoter activity of the human CHM-I gene in HBMECs was examined. Concentrations of 4-HNE shown to induce CHM-I protein (i.e., 5 and 10 μM) (Fig. 3) did not affect any of these promoter regions (Fig. 4).
Fig. 4.
Luciferase assay. To determine whether 4-HNE affects the expression of CHM-1 gene by interacting with its promoter region, constructs consisting of pGVB2 vectors containing three different (−832/+87; −446/+87; −297/+87) 5′-flanking regions of the human CHM-I gene fused to the firefly luciferase gene were used. Transiently-transfected cells were incubated with vehicle control (0.06% ethanol in PBS) or 4-HNE (5 or 10 μM) for 16 h. Values are mean ± S.D. from three independent experiments carried out in triplicate.
Discussion
The identification of key molecules, as well as the understanding of mechanisms involved in the regulation of angiogenesis, may lead to the development of drugs to be used in various pathologies for angiogenesis inhibition or stimulation [15]. 4-HNE, a molecule exhibiting important biological properties [8; 9], has been shown to affect angiogenesis [10; 12], although the exact effect is not clear. In the present study, the possible effects of 4-HNE on angiogenesis were investigated using an in vitro assay, tube formation by HBMECs. The results showed that 4-HNE inhibited tube formation at concentrations achieved physiologically (i.e. 5μM or 10 μM) [4]. Three different cell viability assays (MTT, SRB and LDH) were used for assessing the effects of 4-HNE on HBMECs. None of these assays showed any effect at lower concentrations of 4-HNE (≤ 10 μM) on the viability of HBMECs. This would argue against the inhibition of tube formation by HBMECs being due to an increase in cell death. Interestingly, higher concentrations of 4-HNE (≥ 20 μM) reduced cell viability as measured by MTT and SRB assays, but failed to affect LDH release. This could be explained by the fact that MTT and SRB assays detect both cell apoptosis and cell necrosis, while the LDH assay detect mainly cell necrosis since it is based on the disintegration of cell membrane for the release of LDH enzyme. Consistent with this proposal are a number of studies that have shown that 4-HNE causes mainly cell apoptosis [9]. The LDH results in the present study are similar to those reported in studies using different cells [22].
The inhibition of tube formation observed may be attributed to the increased expression of both forms (pre-cursor and mature) of CHM-I, an anti-angiogenic protein. CHM-I has been found to inhibit angiogenesis in human umbilical vein endothelial cells [23], in cardiac valves of mice [24] and in human retinal vascular endothelial cells [25]. It has been suggested that CHM-I inhibits angiogenesis by inhibiting DNA synthesis and proliferation of endothelial cells [23; 26]. The increased expression of CHM-I has been associated with decreased activity or expression of angiogenic factors. For example, CHM-I is deposited in the interterritorial matrix of cartilage. Although cartilage includes also many angiogenic factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2) and transforming growth factor (TGF)-beta, CHM-I seems to impede the activity of these angiogenic factors (although the exact mechanism is not known) and therefore inhibits angiogenesis in the cartilage matrix [27]. In another study, the normally avascular aortic valves of CHM-I knockout mice showed increased VEGF expression and angiogenesis [28]. Accordingly, the observed inhibitory effect of 4-HNE on tube formation by HBMEC cells may be attributed to the increased levels of CHM-I.
In order to further explore the mechanism(s) by which 4-HNE induced CHM-I expression in HBMECs, the effect of 4-HNE on the promoter activity of CHM-I gene was examined using a transient transfection assay. Concentrations of 4-HNE that induced CHM-I protein expression (5 and 10 μM) did not affect the tested regions (−832/+87; −446/+87; −297/+87) of the CHM-I promoter. Therefore, the up-regulation of the expression of CHM-I protein may be due to activation of other promoter regions of the CHM-I gene. To address this possibility, a bioinformatic analysis using the TFM-Explorer (Sequoia-Inria, France; http://bioinfo.lifl.fr/TFM) software was conducted to find putative transcription factors that could bind to the CHM-I gene promoter region ranging from −3000 bp to +87 bp. Two of the putative transcription factors, namely CREB (binding at −1079/−932 site) and c-Fos (binding at −2060/−1706 site) have been reported to be activated by 4-HNE [29; 30]. c-Fos and Jun proteins form the heterodimer activator protein-1 (AP-1) that regulates many cellular processes, including angiogenesis [31]. Unfortunately, these promoter regions were not examined in the present study; however we intent do this in future studies.
Up-regulation of CHM-I expression by 4-HNE could also have occurred through a posttranscriptional mechanism resulting in increased stability of CHM-I mRNA. For example, 4-HNE has been shown to affect c-myc mRNA levels through a post-transcriptional mechanism [32]. The increase in expression of both forms (precursor and mature) of CHM-I protein by 4-HNE in the present study is consistent with 4-HNE acting not only at a transcriptional level to regulate CHM-I protein, but also at a post-translational level by increasing the cleavage of the premature form by furin protease. Interestingly, 4-HNE induces the expression of transforming growth factor beta 1, a protein that is also cleaved by furin protease [33].
In conclusion, the results of the present study showed that 4-HNE acts as an inhibitor of tube formation. The inhibition of tube formation may involve, at least in part, the up-regulation of the anti-angiogenic protein CHM-I. Consequently, the results suggest that 4-HNE may exert anti-angiogenic activity. Inhibition of angiogenesis has been implicated as a pathophysiological protective mechanism against several diseases, such as cancer, rheumatoid arthritis, atherosclerosis and corneal neovascularization [15]. The potential anti-angiogenetic effects of 4-HNE observed in the present study, support the notion that 4-HNE at physiological concentrations can function as a good stress, or ‘eustress’, by providing protective effects [34]. The up-regulation of CHM-I by 4-HNE reported here may also explain the upregulation of the Chm-I gene in the cornea of Aldh3a1(−/−) mice characterized by increased 4-HNE levels [6]. Since the cornea of Aldh3a1(−/−) mice do not exhibit any angiogenesis [35], it is likely that the upregulation of Chm-I gene in Aldh3a1–null mice may be a compensatory event preventing angiogenesis in these mice. That may imply a possible role of corneal ALDH3A1 and perhaps other corneal crystallins in maintaining the avascularity of the cornea. This is currently under investigation in our laboratory.
Acknowledgments
We thank Dr. Yuji Hiraki for providing us with the antihuman pre-mature CHM-I, Dr. Toshimi Michigami for luciferace constructs of the CHM-I promoter and Dr. Babette Weksler for the HBMEC cells. We also thank Drs. David Thompson and Ying Chen for critical reading and discussion of this manuscript. This work was supported by National Institutes of Health (NIH) grants EY11490 (VV) and ES09554 (DR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Estey T, Piatigorsky J, Lassen N, Vasiliou V. ALDH3A1: a corneal crystallin with diverse functions. Exp Eye Res. 2007;84:3–12. doi: 10.1016/j.exer.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Piatigorsky J. Review: A case for corneal crystallins. J Ocul Pharmacol Ther. 2000;16:173–180. doi: 10.1089/jop.2000.16.173. [DOI] [PubMed] [Google Scholar]
- 3.Lassen N, Bateman JB, Estey T, Kuszak JR, Nees DW, Piatigorsky J, Duester G, Day BJ, Huang J, Hines LM, Vasiliou V. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knock-out mice. J Biol Chem. 2007;282:25668–25676. doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 5.Pappa A, Estey T, Manzer R, Brown D, Vasiliou V. Human aldehyde dehydrogenase 3A1 (ALDH3A1): biochemical characterization and immunohistochemical localization in the cornea. Biochem J. 2003;376:615–623. doi: 10.1042/BJ20030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasiliou V, Davis J, Nees D, Kuszak JR, Lassen N, Orlicky D, Piatigorsky J. Aldh3a1(−/−) knockout mice reveal novel functions of corneal crystallins. Invest Ophthalmol Vis Sci. 2007;48:4932. E-Abstract. [Google Scholar]
- 7.Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, Chaudhary P, Awasthi S. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic Biol Med. 2008;45:111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisker O, Onizuka S, Banyard J, Komiyama T, Becker CM, Achilles EG, Barnes CM, O’Reilly MS, Folkman J, Pirie-Shepherd SR. Generation of multiple angiogenesis inhibitors by human pancreatic cancer. Cancer Res. 2001;61:7298–7304. [PubMed] [Google Scholar]
- 11.Lim Y, Phung AD, Corbacho AM, Aung HH, Maioli E, Reznick AZ, Cross CE, Davis PA, Valacchi G. Modulation of cutaneous wound healing by ozone: differences between young and aged mice. Toxicol Lett. 2006;160:127–134. doi: 10.1016/j.toxlet.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur J Pharmacol. 2003;458:283–289. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]
- 13.Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, Siemann D, Vieweg J. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol. 2008;181:346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- 14.Shukunami C, Hiraki Y. Chondromodulin-I and tenomodulin: the negative control of angiogenesis in connective tissue. Curr Pharm Des. 2007;13:2101–2112. doi: 10.2174/138161207781039751. [DOI] [PubMed] [Google Scholar]
- 15.Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008;73:751–762. doi: 10.1134/s0006297908070031. [DOI] [PubMed] [Google Scholar]
- 16.Schweitzer KM, Vicart P, Delouis C, Paulin D, Drager AM, Langenhuijsen MM, Weksler BB. Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest. 1997;76:25–36. [PubMed] [Google Scholar]
- 17.Miura S, Emoto M, Matsuo Y, Kawarabayashi T, Saku K. Carcinosarcoma-induced endothelial cells tube formation through KDR/Flk-1 is blocked by TNP-470. Cancer Lett. 2004;203:45–50. doi: 10.1016/j.canlet.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Lassen N, Estey T, Tanguay RL, Pappa A, Reimers MJ, Vasiliou V. Molecular cloning, baculovirus expression, and tissue distribution of the zebrafish aldehyde dehydrogenase 2. Drug Metab Dispos. 2005;33:649–656. doi: 10.1124/dmd.104.002964. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 21.Yanagihara I, Yamagata M, Sakai N, Shukunami C, Kurahashi H, Yamazaki M, Michigami T, Hiraki Y, Ozono K. Genomic organization of the human chondromodulin-1 gene containing a promoter region that confers the expression of reporter gene in chondrogenic ATDC5 cells. J Bone Miner Res. 2000;15:421–429. doi: 10.1359/jbmr.2000.15.3.421. [DOI] [PubMed] [Google Scholar]
- 22.Peng ZF, Koh CH, Li QT, Manikandan J, Melendez AJ, Tang SY, Halliwell B, Cheung NS. Deciphering the mechanism of HNE-induced apoptosis in cultured murine cortical neurons: transcriptional responses and cellular pathways. Neuropharmacology. 2007;53:687–698. doi: 10.1016/j.neuropharm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Hiraki Y, Kono T, Sato M, Shukunami C, Kondo J. Inhibition of DNA synthesis and tube morphogenesis of cultured vascular endothelial cells by chondromodulin-I. FEBS Lett. 1997;415:321–324. doi: 10.1016/s0014-5793(97)01151-4. [DOI] [PubMed] [Google Scholar]
- 24.Hakuno D, Fukuda K. Role of anti-angiogenic factor chondromodulin-I for maintaining cardiac valvular function. Clin Calcium. 2007;17:361–372. [PubMed] [Google Scholar]
- 25.Funaki H, Sawaguchi S, Yaoeda K, Koyama Y, Yaoita E, Funaki S, Shirakashi M, Oshima Y, Shukunami C, Hiraki Y, Abe H, Yamamoto T. Expression and localization of angiogenic inhibitory factor, chondromodulin-I, in adult rat eye. Invest Ophthalmol Vis Sci. 2001;42:1193–1200. [PubMed] [Google Scholar]
- 26.Hiraki Y, Inoue H, Iyama K, Kamizono A, Ochiai M, Shukunami C, Iijima S, Suzuki F, Kondo J. Identification of chondromodulin I as a novel endothelial cell growth inhibitor. Purification and its localization in the avascular zone of epiphyseal cartilage. J Biol Chem. 1997;272:32419–32426. doi: 10.1074/jbc.272.51.32419. [DOI] [PubMed] [Google Scholar]
- 27.Kusafuka K, Hiraki Y, Shukunami C, Kayano T, Takemura T. Cartilage-specific matrix protein, chondromodulin-I (ChM-I), is a strong angio-inhibitor in endochondral ossification of human neonatal vertebral tissues in vivo: relationship with angiogenic factors in the cartilage. Acta Histochem. 2002;104:167–175. doi: 10.1078/0065-1281-00642. [DOI] [PubMed] [Google Scholar]
- 28.Hakuno D, Kimura N, Yoshioka M, Fukuda K. Molecular mechanisms underlying the onset of degenerative aortic valve disease. J Mol Med. 2008 doi: 10.1007/s00109-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 29.Kreuzer T, Grube R, Wutte A, Zarkovic N, Schaur RJ. 4-Hydroxynonenal modifies the effects of serum growth factors on the expression of the c-fos proto-oncogene and the proliferation of HeLa carcinoma cells. Free Radic Biol Med. 1998;25:42–49. doi: 10.1016/s0891-5849(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 30.Pugazhenthi S, Phansalkar K, Audesirk G, West A, Cabell L. Differential regulation of c-jun and CREB by acrolein and 4-hydroxynonenal. Free Radic Biol Med. 2006;40:21–34. doi: 10.1016/j.freeradbiomed.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 31.Jochum W, Passegue E, Wagner EF. AP-1 in mouse development and tumorigenesis. Oncogene. 2001;20:2401–2412. doi: 10.1038/sj.onc.1204389. [DOI] [PubMed] [Google Scholar]
- 32.Fazio VM, Barrera G, Martinotti S, Farace MG, Giglioni B, Frati L, Manzari V, Dianzani MU. 4-Hydroxynonenal, a product of cellular lipid peroxidation, which modulates c-myc and globin gene expression in K562 erythroleukemic cells. Cancer Res. 1992;52:4866–4871. [PubMed] [Google Scholar]
- 33.Chiarpotto E, Castello L, Leonarduzzi G, Biasi AF, Poli G. Role of 4-hydroxy-2,3-nonenal in the pathogenesis of fibrosis. Biofactors. 2005;24:229–236. doi: 10.1002/biof.5520240127. [DOI] [PubMed] [Google Scholar]
- 34.Chen ZH, Niki E. 4-hydroxynonenal (4-HNE) has been widely accepted as an inducer of oxidative stress. Is this the whole truth about it or can 4-HNE also exert protective effects? IUBMB Life. 2006;58:372–373. doi: 10.1080/15216540600686896. [DOI] [PubMed] [Google Scholar]
- 35.Nees DW, Wawrousek EF, Robison WG, Jr, Piatigorsky J. Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice. Mol Cell Biol. 2002;22:849–855. doi: 10.1128/MCB.22.3.849-855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]