Summary
To test the molecular nature of the NGF receptor responsible for the ability of NGF to rescue septal cholinergic neurons following axotomy, we infused polyclonal antibodies that act as specific agonists of trkA (RTA) into the lateral ventricle of fimbria–fornix lesioned animals. Rats receiving chronic intraventricular infusions of RTA showed significantly more low affinity NGF receptor immunoreactive (p75NGFR-IR) neurons on the lesioned side than did control animals 2 weeks following unilateral fimbria–fornix lesion. RTA also initiated cholinergic sprouting. Infusions of RTA in combination with an antibody that blocks p75NGFR (REX) did not reduce the cell savings effect observed with RTA alone. However, animals infused with RTA plus REX demonstrated significantly less sprouting. These findings suggest that antibody-induced trkA activation is sufficient to mediate NGF-promoted survival of axotomized cholinergic neurons in vivo.
Introduction
The nerve growth factor (NGF) family of neurotrophins is present in limited amounts in the central nervous system (CNS) and is known to function in neural development, differentiation, and survival (for reviews, see Barde, 1989; Thoenen, 1991). Central to understanding mechanisms mediating neurotrophin signal transduction is the elucidation of the molecular nature of functional neurotrophin receptors. NGF, the best-characterized neurotrophin, recognizes at least two classes of cell surface receptors (Meakin and Shooter, 1991), a fast-dissociating, low affinity NGF receptor (p75NGFR) and a slow-dissociating, high affinity NGF receptor (tyrosine receptor kinase A [trkA]). p75NGFR binds NGF and all other known members of the neurotrophin family (Rodriguez-Tebar et al., 1990; Ernfors et al., 1990) but does not possess intrinsic tyrosine kinase activity (Radeke et al., 1987). Specificity in neurotrophin binding is conferred by the Trk family of tyrosine kinases.
The human trk proto-oncogene encodes a 140,000 dalton glycoprotein, designated TrkA (Martin-Zanca et al., 1989), which acts as a functional NGF receptor (Kaplan et al., 1991a; Klein et al., 1991). TrkA possesses protein tyrosine kinase activity (Martin-Zanca et al., 1989; Meakin and Shooter, 1991) that is activated by picomolar (i.e., biologically relevant) quantities of NGF (Kaplan et al., 1991b; Klein et al., 1991; Jing et al., 1992). Although it is generally agreed that TrkA binding is necessary to generate functional responses to NGF, there exists a great deal of controversy as to whether TrkA activation is sufficient, and, despite intense investigation, the receptor mechanism by which the specific effects of NGF are mediated is unresolved.
Typical biological responses to NGF correlate with the presence of high affinity receptors for NGF (Green et al., 1986; Weskamp and Reichardt, 1991), and TrkA expression appears necessary for the generation of high affinity binding sites (Klein et al., 1991; Meakin and Shooter, 1991; Radeke et al., 1987; Weskamp and Reichardt, 1991) as well as biological responsiveness to NGF. For example, mutant pheochromocytoma (PC12) cell lines expressing significantly reduced levels of TrkA are deficient in high affinity NGF binding and unresponsive to NGF, but when transfected with trkA, they demonstrate NGF responsiveness by neurite outgrowth, cellular hypertrophy, and survival in serum-free medium (Loeb et al., 1991). Additionally, MAH cells, an immortalized sympathoadrenal progenitor cell line, transfected with trkA, respond to NGF application with neurite extension and proliferation (Verdi et al., 1994a), and ectopic TrkA expression is responsible for meiotic maturation in Xenopus oocytes (Nebreda et al., 1991) as well as survival in NGF-independent sensory neurons (Allsopp et al., 1993). Furthermore, chimeric receptors constructed from the extracellular domain of tumor necrosis factor (TNF) receptor and cytoplasmic TrkA elicit survival and differentiation of PC12 cells upon application of exogenous TNF (Rovelli et al., 1993), and trkA-deficient mice show marked loss of NGF-responsive sensory and sympathetic neurons (Smeyne et al., 1994).
Although mounting evidence suggests that the presence of TrkA is required to generate high affinity binding sites and mediate cellular responses to NGF, the relative contribution of p75NGFR is undetermined. Some investigators demonstrated that coexpression of p75NGFR and TrkA was required to yield both classes of receptors (Hempstead et al., 1991; Benedetti et al., 1993; Berg et al., 1991) and increased the association rate of NGF, resulting in higher affinity NGF binding than that of TrkA alone (Mahadeo et al., 1994). Furthermore, p75NGFR was necessary for NGF responsiveness as assayed by c-fos transcription and tyrosine phosphorylation (Berg et al., 1991; Hempstead et al., 1991). These data assessing the relative contribution of p75NGFR to functional NGF receptors led to a model proposing that a receptor complex consisting of both p75NGFR and TrkA is responsible for the biological effects of NGF (Bothwell, 1991). However, others postulate that p75NGFR may serve as a loading mechanism, increasing the association rate of NGF for TrkA (Jing et al., 1992; Ibañez et al., 1993), or that p75NGFR modifies the ligand specificity of TrkA (Benedetti et al., 1993; Barker and Shooter, 1994; Hantzopoulos et al., 1994).
Although experiments to determine the relative importance of p75NGFR and TrkA in NGF signal transduction have been conducted by expressing these receptors ectopically in nonneuronal cells, few studies have attempted to discern which receptor or receptor complex is necessary to mediate NGF-promoted responses in neurons in vitro (Allsopp et al., 1993; Barrett and Bartlett, 1994; Clary et al., 1994). Targeted mutation of NGF receptors by homologous recombination has provided the only clues elucidating this question in neural populations in vivo (Lee et al., 1992; Smeyne et al., 1994).
The most prominent cells in the CNS that express TrkA are NGF-responsive basal forebrain cholinergic neurons. Among the important functions of NGF on this population isthe abilityto rescue septal cholinergic neurons from axotomy when applied exogenously (Hefti, 1986; Williams et al., 1986; Kromer, 1987; Gage et al., 1988). NGF infusions have also been demonstrated to regulate choline acetyltransferase (ChAT) expression (Hefti et al., 1984, 1985) and the expression of NGF receptors (Gage et al., 1989; Higgins et al., 1989; Holtzman et al., 1992) and to induce sprouting of cholinergic terminals (Gage et al., 1988).
To discern the molecular nature of the functional NGF receptor in vivo, we infused polyclonal antibodies directed against the entire extracellular domain of rat TrkA (RTA) into unilaterally fimbria–fornix (FF)–lesioned rats. Since immunoglobulin G (IgG) fractions of RTA specifically bind TrkA and function as agonists to TrkA in vitro, stimulating receptor activation and autophosphorylation as well as cell survival and neurite outgrowth in PC12 cells and sympathetic neurons (Clary et al., 1994), we hypothesized that infusions of RTA would mimic the effects of NGF and rescue cholinergic neurons following FF transection. Here, we report the effects of chronic intracerebroventricular (ICV) infusions of RTA on septal cholinergic neurons axotomized by FF lesion and provide experimental evidence that TrkA activation is a sufficient condition for NGF-mediated rescue effects on septal cholinergic neurons in vivo.
Results
RTA Specificity
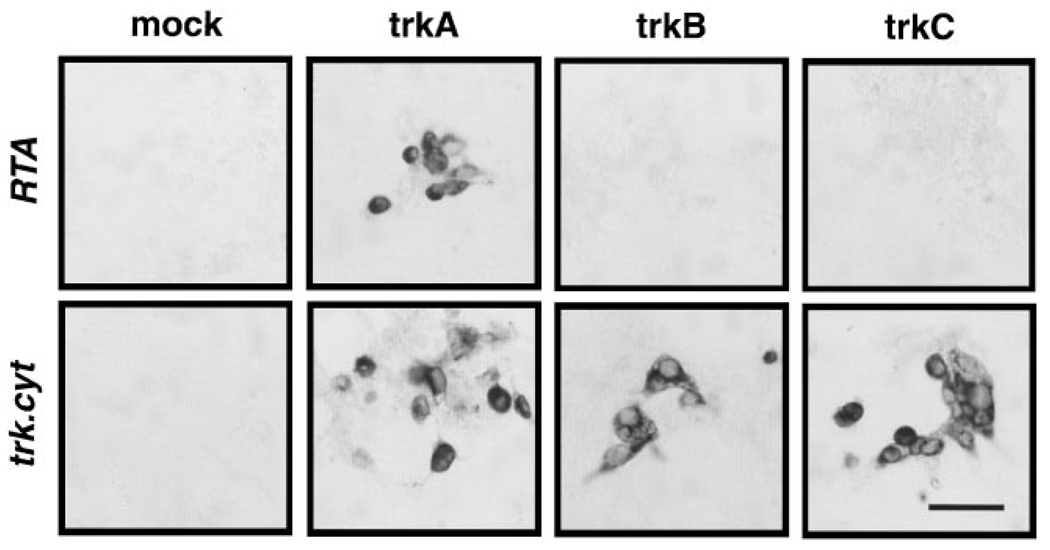
The RTA antibody was raised against a recombinant truncated form of the rat TrkA receptor expressed in baculovirus-infected Sf900 cells. The resulting IgG has been shown to bind the TrkA receptor specifically, but not the TrkB or TrkC receptors, by immunoprecipitation and immunoblotting analysis (Clary et al., 1994). However, as in vivo infusions present the antibody to the receptors in a cellular context, we sought to test whether we could detect any interaction of the RTA IgG with the TrkB or TrkC receptors as expressed in cells. We transiently transfected the cell line HEK293 with plasmids directing expression of rat TrkA, rat TrkB, or rat TrkC and probed each transfection with either the RTA antibody or an antibody that recognizes all Trk receptors (trk.cyt). Strong expression of all three Trk receptors was observed (Figure 1), but no cross-reaction of the RTA antibody with TrkB or TrkC was detected. From this result, taken together with the earlier biochemical characterization, we conclude that the binding, and therefore the activity, of the RTA antibody is specific for the TrkA receptor.
Figure 1. Specificity of the RTA Antisera.
Human embryo kidney 293 cells were mock transfected or transfected With expression plasmids encoding rat TrkA, TrkB, or TrkC. The cultures were subsequently stained with either the RTA antibody oran antibody recognizing the cytoplasmic tails of all three Trk receptors (trk.cyt).
Scale bar, 50 µm.
Surgical Procedures and Experimental Design
Young adult female Fisher 344 rats received unilateral aspirative lesions of the FF and continuous infusions of the following solutions: RTA (α-TrkA), RTA plus REX (α-TrkA plus α-p75NGFR), NGF, control infusions of CSF, REX, or rabbit serum (RS), or rats received FF lesions alone. Two weeks following lesion and pump implantation, animals were sacrificed and evaluated histologically both for lesion completeness and for antibody diffusion to the medial septum (MS).
Penetration of RTA and Antibody Specificity In Vivo
Coronal sections through the septum were immunostained to visualize the extent of diffusion of infused antibodies (RTA, REX, and RS). Anti-rabbit antibodies recognized the polyclonal rabbit TrkA antibody and showed the tissue distribution of RTA in the parenchyma 2 weeks following chronic infusion (Figure 2A). Visual inspection showed that antibody penetration of RTA and other antibody infusates (RTA plus REX, REX, and RS; data not shown) was adequate to reach the cells of interest, the MS cholinergic neurons. Moreover, detection of the distribution of RTA revealed that RTA was localized to cells (presumably cholinergic cells) of the basal forebrain (Figure 2B) and the striatum (Figures 2C and 2D), populations that express TrkA (Holtzman et al., 1992; Gibbs and Pfaff, 1994). Although cholinergic neurons of the basal forebrain express p75NGFR in addition to TrkA, cholinergic neurons in the striatum only express TrkA (Holtzman et al., 1992). We interpret the localization of the antibody to these cellular populations as evidence of the specificity of RTA in vivo.
Figure 2. Diffusion and Localization of Infused RTA.
Overview of a representative coronal section at low power immunostained with an anti-rabbit antibody that highlighted the distribution of infused RTA in vivo (A). RTA was localized to cells in the basal forebrain (B) and striatum (C, D). (B) and (D) are enlarged areas of (A) and (C), respectively.
Scale bar, 50 µm in (B) and (D), 100 µm in (C).
Histological Appearance of Cholinergic Cells within the FF-Lesioned Septum
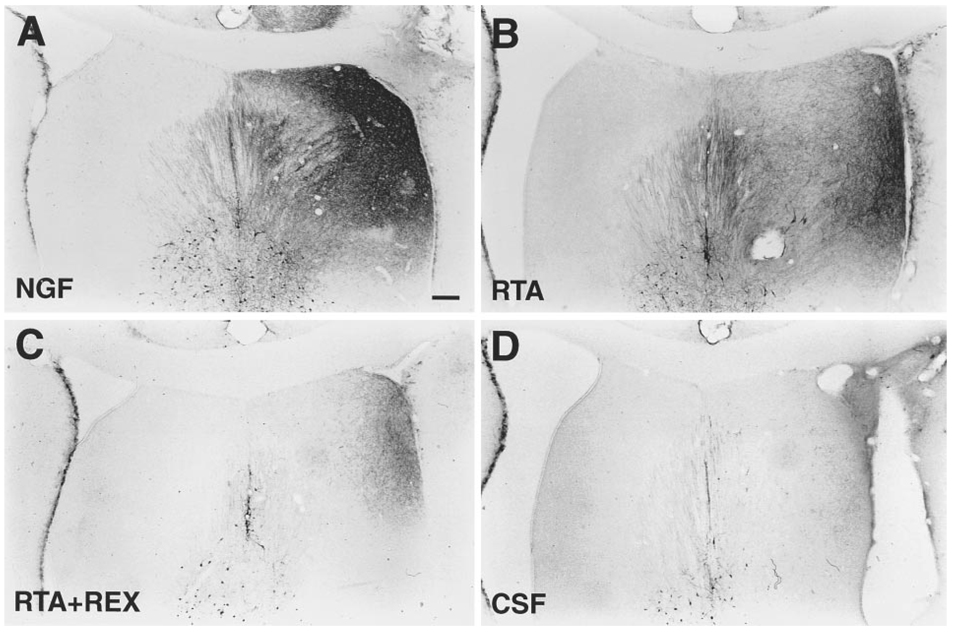
Those animals demonstrating complete transection of the FF upon visual inspection and adequate penetration of the antibody were included in the quantitation of cholinergic MS neurons. No obvious differences in the contralateral septum were observed 2 weeks following FF lesion. However, coronal sections through the MS revealed the loss of p75NGFR–immunoreactive(IR) cells ipsilateral to the lesion (right side) in control animals (Figure 3B). Remaining neurons on the lesioned side of control animals appeared shrunken and faintly immunoreactive. Animals infused with NGF (Figure 3A), RTA (Figure 3C), or RTA in combination with REX (data not shown), in contrast, exhibited considerable cell savings on the ipsilateral side when compared with lesioned control animals. p75NGFR–IR neurons on the ipsilateral side were not noticeably smaller or less immunoreactive than those on the contralateral side in NGF-infused animals and appeared only slightly so in animals infused with RTA or RTA in combination with REX. We also observed a sprouting response in the dorsolateral quadrant of the septum in animals infused with NGF, RTA alone (Figures 3A and 3C), and, to a lesser extent, in animals infused with RTA in combination with REX.
Figure 3. p75NGFR-IR MS Neurons Following FF Lesion.
Coronal sections through the MS immunostained with antibodies directed against p75NGFR show MS neurons 2 weeks following unilateral FF lesion (right side) in NGF- (A), CSF-(B), or RTA-infused animals (C). Characteristic sprouting of p75NGFR–IR processes is observed in NGF- and RTA-infused animals (A and C).
Scale bar, 200 µm.
Septal Cell Savings Following ICV Infusions of RTA and RTA plus REX
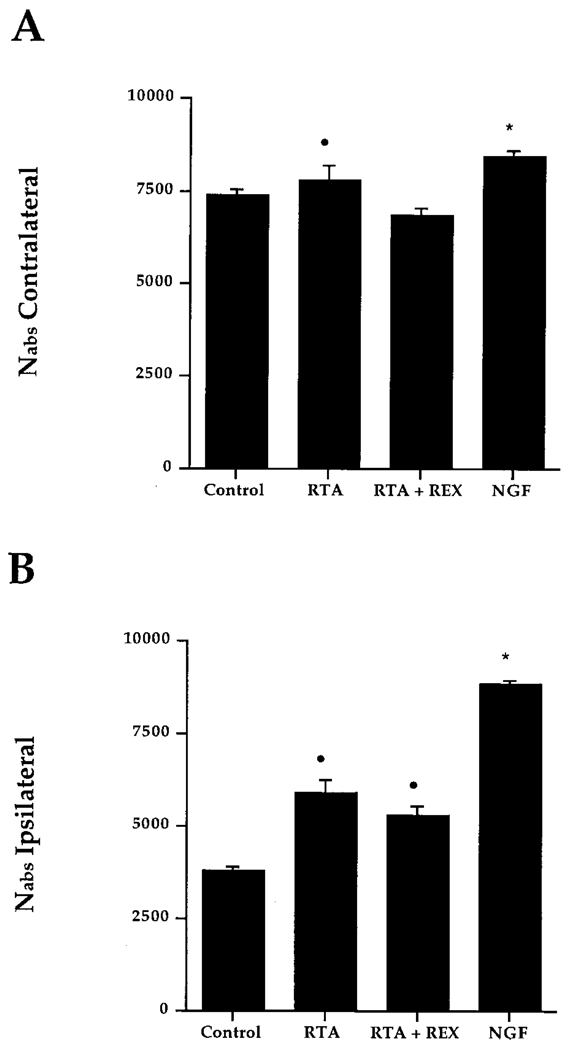
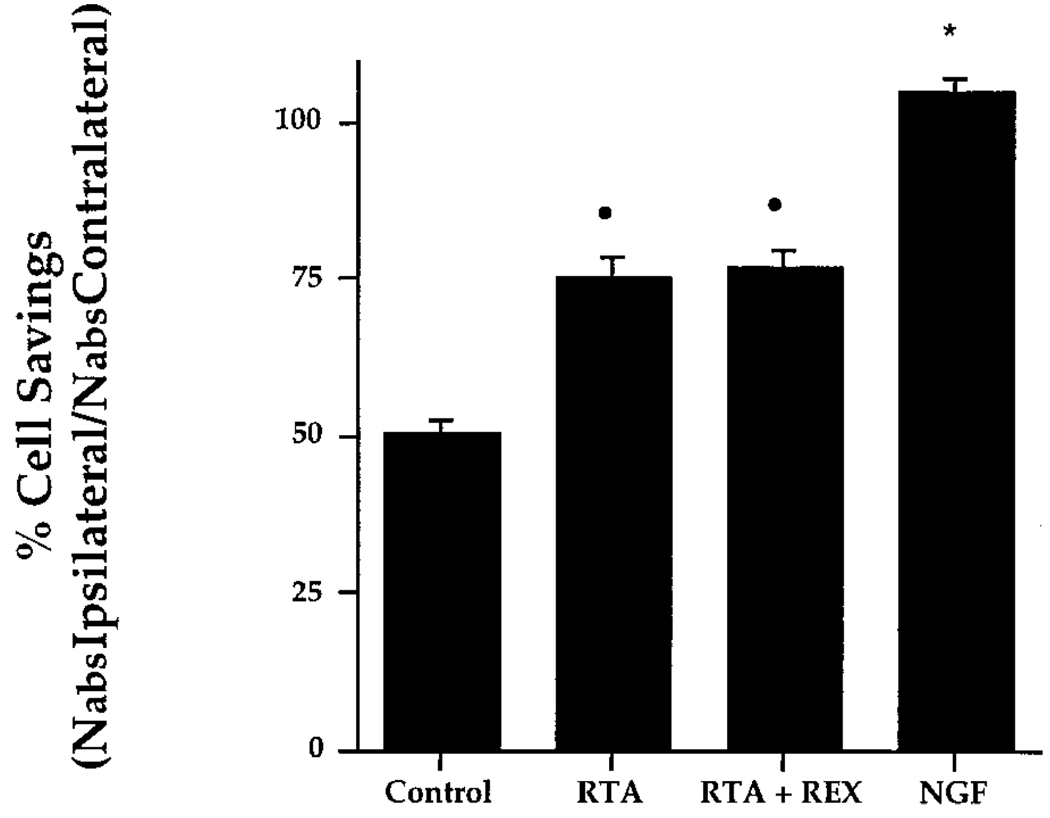
Quantification of p75NGFR–IR neurons in the MS of control animals (intact, CSF, lesion only, preimmune serum, or REX) by unbiased stereology revealed no differences in the number of cells present on the contralateral side, allowing us to combine these groups into an overall control group. By the same rationale, consistent ipsilateral cell loss allowed us to pool individual control groups. MS cell number for the combined control group was then compared with that of experimental groups and NGF-infused animals. NGF-treated animals served as our positive control. Experimental groups included animals infused with RTA (1.0 mg/ml) and animals infused with RTA (1.0 mg/ml) along with REX (0.1 mg/ml). REX binds p75NGFR and has shown no biological effects other than inhibition of NGF binding to p75NGFR (Weskamp and Reichardt, 1991). This group was included to eliminate any binding to the p75NGFR or possible interaction of p75NGFR with TrkA via dimerization. Comparison of the total number of MS neurons(Nabs)revealed small, but significant, differences in the number of cells contralateral to the lesion. Significantly more neurons were observed on the contralateral side in animals infused with NGF when compared with controls or RTA plus REX–infused animals (Figure 4A; p < 0.01). Nabs for RTA-infused animals was significantly different from RTA plus REX–infused animals (p < 0.05), but not different from that of NGF-infused animals (p < 0.01). Quantification of the p75NGFR–IR cell number in the ipsilateral MS confirmed that chronic infusion of RTA or RTA in combination with REX was effective in rescuing p75NGFR–IR neurons ipsilateral to the lesion (Figure 4B). When expressed as percent cell savings (Nabs ipsilateral/Nabs contralateral), 75.3% ± 3.4% of the p75NGFR–IR neurons were present on the ipsilateral side as compared with the contralateral side in animals infused with RTA (Figure 5). This result was significantly different from control animals (50.7% ± 1.9%). Infusion of RTA in combination with REX did not reduce the cell savings effect seen with RTA alone (77.1% ± 2.6%). NGF rescued a significantly greater proportion of neurons on the ipsilateral side (104.9% ± 2.3%).
Figure 4. Absolute Number of p75NGFR–IR Neurons within the MS 2 Weeks Following FF Lesion.
(A), contralateral (intact) side; (B), ipsilateral (lesioned) side. Bars represent SEM.
(A) Dot indicates difference from RTA plus REX group (p < 0.05); asterisk indicates differences from control and RTA plus REX groups (p < 0.01).
(B) Dot indicates difference from control group (p < 0.01); asterisk indicates differences from control, RTA, and RTA plus REX groups (p < 0.01).
Figure 5. Percent of p75NGFR–IR Cell Savings in the MS.
Percentage is calculated as (Nabs ipsilateral/Nabs contralateral) × 100. Bars represent SEM. Dot indicates differences from control group (p < 0.01); asterisk indicates differences from control, RTA, and RTA plus REX groups (p < 0.01).
Sprouting of p75NGFR–IR Fibers
Chronic ICV infusions of NGF induced the sprouting of a fine network of p75NGFR–IR processes in the dorsolateral quadrant of the septum (Figure 6A), as do infusions of RTA (Figure 6B) and, to a much lesser extent, RTA infused along with REX (Figure 6C). Densitometric quantitation of p75NGFR–IR sprouting demonstrated no significant differences in the amount of sprouting in individual control groups and allowed them to be combined into an overall control group. Animals infused with RTA demonstrated a significant amount of sprouting when compared with control animals or animals infused with RTA in combination with REX (Figure 7), whereas NGF-infused animals showed significantly more sprouting than both experimental and control groups. RTA plus REX–infused animals did not differ from controls.
Figure 6. p75NGFR-IR Sprouting Following FF Lesion.
Representative coronal sections through the septum show p75NGFR–IR processes in the dorsolateral quadrant of the septum 2 weeks following FF lesion in NGF- (A), RTA- (B), RTA plus REX– (C), and CSF-infused animals (D).
Scale bar, 200 µm.
Figure 7. Quantification of the Density of p75NGFR–IR Fibers in the Dorsolateral Quadrant of the Septum.
Bars represent SEM. Dot indicates differences from control group (p < 0.01) and RTA plus REX group (p < 0.05); asterisk indicates differences from control, RTA, and RTA plus REX groups (p < 0.01).
Discussion
To resolve whether TrkA activation is sufficient to mediate signal transduction, we infused IgG fractions of antibodies directed against the extracellular domain of TrkA ICV following FF lesions. IgG fractions of RTA bind TrkA initiating the biological effects of NGF in vitro, stimulating receptor activation and autophosphorylation, as well as cell survival and neurite outgrowth in PC12 cells and sympathetic neurons (Clary et al., 1994). The data presented here are the first evidence that selective activation of TrkA is sufficient to rescue septal cholinergic neurons from axotomy, mimicking the biological effects of NGF in vivo. In addition, the fact that infusions of REX in combination with RTA block sprouting suggests a role for p75NGFR in cholinergic sprouting.
RTA Infusions Rescue Septal Cholinergic Neurons Following Axotomy
Using unbiased stereology, we estimate that approximately 15,000 cholinergic neurons comprise the MS. Extensive evidence indicates that, following FF lesion, roughly half of these degenerate and die within 2 weeks (Arimatsu et al., 1988; Montero and Hefti, 1988; Tuszynski et al., 1990). However, some studies suggest that not all of these cells die, but that some persist in a dysfunctional state for extended periods of time (Hagg et al., 1988; Naumann et al., 1992). Chronic infusions of RTA alone or in combination with REX were effective in supporting approximately 75% of the total cholinergic population following axotomy, whereas approximately half of the lesioned cholinergic population degenerated in control animals.
ICV infusion of NGF increased the total number of neurons by a small but significant amount on the contralateral, or unlesioned, side of the MS, an effect that has not previously been described. The fact that NGF is known to up-regulate cholinergic markers such as ChAT, p75NGFR, and TrkA (Hefti et al., 1985; Gage et al., 1989; Higgins et al., 1989; Holtzman et al., 1992) suggests that NGF up-regulates p75NGFR expression in the contralateral septum. Because most reports concerning cell savings express their results only as a ratio (percent ipsilateral/contralateral), small differences in cell number ipsilateral or contralateral to the lesion are not likely to be noticed.
Estimation of p75NGFR–IR Neuronal Number by Unbiased Stereology
Cholinergic cell number in the MS was analyzed by the disector procedure (Sterio, 1984). We chose p75NGFR as a cholinergic marker for several reasons. First, p75NGFR expression overlaps with ChAT expression in approximately 90% of MS neurons (Batchelor et al., 1989) and with TrkA in over 90% of MS neurons (Gibbs and Pfaff, 1994), thus providing an accurate approximation of cholinergic neurons. Second, the antibody used to detect p75NGFR is a monoclonal antibody, and the secondary antibody used to identify it does not bind the polyclonal antibody infused and distributed throughout the septum, which obscures the cells and boundaries of the MS. Third, the neuropil is more distinct when immunostained with p75NGFR than with ChAT, so a more accurate volume of the MS nucleus can be defined. For these reasons, MS boundaries can be consistently defined, allowing for objective criteria for inclusion of structures (see Experimental Procedures).
Is NGF-Independent TrkA Activation Sufficient to Mediate the Cell Survival Effects of NGF In Vivo?
The number of p75NGFR–IR MS neurons present 2 weeks following FF lesion in RTA- and RTA plus REX–infused animals is consistent with the view that NGF binding to both p75NGFR and TrkA is not necessary to mediate cholinergic cell savings. We determined that there exists a population of cholinergic neurons that require only TrkA activation to remain viable after axotomy, since RTA infusions rescue cholinergic neurons following axotomy, and infusions of REX with RTA do not diminish this effect. Although the cross-linking of TrkA by RTA might be subtly different in vivo versus in vitro (e.g., by binding TrkA monomers or homodimers),we predict that RTA functions similarly in vivo and that TrkA stimulation by RTA-induced oligomerization of TrkA mediates signal transduction, supporting cholinergic cell survival.
Emerging evidence argues that TrkA activation alone sufficiently mediates the biological effects of NGF. Although antibodies directed against p75NGFR (REX) completely block NGF binding to p75NGFR, they do not prevent high affinity receptor binding of NGF in PC12 cells. Neither do they inhibit NGF-dependent survival of sensory neurons, NGF-induced neurite outgrowth, or rapid phosphorylation of phospholipase C-γ1 (PLCγ1; Vetter et al., 1991). Additionally, oligomerization of TrkA with antibody-induced cross-linking is sufficient to mimic NGF responsiveness in PC12 and sympathetic neurons in the absence of NGF (Clary et al., 1994). Other reports also indicate that p75NGFR is not a necessary component of the signal-transducing receptor complex, suggesting rather a homodimerization of TrkA to mediate NGF signaling (Radeke and Feinstein, 1991; Jing et al., 1992). Importantly, a mutant NGF molecule that binds TrkA, but not p75NGFR, retains biological activity, promoting NGF-dependent survival and differentiation (Ibañez et al., 1992). The failure to detect direct associations between p75NGFR and TrkA by immunoprecipitation or cross-linking strengthens the suggestion that a receptor complex consisting of both components is not the prevalent mechanism for NGF signal transduction.
As expected, NGF rescued approximately 100% of lesioned cholinergic neurons. When compared with NGF, infused at 100 µg/ml (a dose that has previously been demonstrated to rescue all cholinergic neurons axotomized by FF lesion; Williams et al., 1986), RTA was apparently not completely effective at rescuing cholinergic neurons or initiating p75NGFR–IR sprouting. There are several possible explanations for this result: first, RTA is not as effective a ligand as NGF; second, the concentration of RTA that reached cholinergic neurons was insufficient to bind and activate fully all functional TrkA receptors; third, some p75NGFR–IR septal cholinergic neurons do not express TrkA and therefore are not rescued by RTA; fourth, concomitant p75NGFR and TrkA activation is required to rescue all cholinergic cells affected by the lesion; or some combination of these possibilities.
Differences in ligand efficacy could explain differences in cell savings observed between NGF and RTA infusion in vivo. When compared with NGF, RTA is not as effective at supporting the survival of sympathetic neurons in vitro, and NGF addition does not restore the maximal level of survival supported by NGF alone (Clary et al., 1994). This finding indicates that RTA binding to TrkA is not as efficacious as binding of the endogenous ligand (NGF). Moreover, many factors, in addition to receptor binding affinities, such as endocytosis and trafficking of the receptor, could easily vary between RTA and NGF as well as between the in vivo and the in vitro situation.
It is possible that 100% cell savings were not observed with RTA infusions because the amount of RTA that reached the MS cholinergic neurons was insufficient to activate TrkA fully and induce signal transduction. We infused RTA at a concentration of 1.0 mg/ml, the highest dose practically available to us, but we do not know whether this dose saturates, binding all available TrkA receptors. Traditionally, antibodies are believed to have limited access to brain parenchyma via ICV infusion. The molecular weight of RTA is approximately six times that of the human β NGF infused (150,000 daltons versus 26,000 daltons), which alone could account for differences in penetration. Although we determined that RTA reached septal cholinergic neurons, because staining intensity using peroxidase immunohistochemistry is not linear, we were unable to assess accurately the relative amounts of antibody that penetrated the tissue. One animal, inadvertently infused intraseptally (allowing the antibody greater access to MS neurons), displayed the highest proportion of cell savings (86%). This finding suggests that greater antibody access to the cholinergic cell population increases the proportion of cell savings and that diffusion of RTA is the limiting factor in this experiment. It is important to note that doses of NGF lower than 100 µg/ml are not nearly as effective in saving cholinergic neurons following axotomy (Barnett et al., 1990), and we chose a saturating dose of NGF (100 µg/ml) to ensure 100% cell savings as our positive control.
Alternatively, rescue of some cholinergic cells following FF lesion may require p75NGFR activation, alone or in combination with TrkA activation. Overlapping distribution of p75NGFR and TrkA in MS neurons (> 90%), as indicated by in situ hybridization or immunocytochemical studies (Gibbs and Pfaff, 1994), suggests that only a very small population might utilize different receptor signaling mechanisms. Other investigators, however, have stressed the importance of a specific ratio of p75NGFR-to-TrkA expression (10:1; Hempstead et al., 1991), the in vivo detection of which is beyond the limits of immunohistochemical or in situ hybridization methods. Still others hold that p75NGFR increases the association rate of NGF for TrkA (Jing et al., 1992; Ibañez et al., 1993) or modifies the ligand specificity of TrkA (Benedetti et al., 1993; Clary et al., 1994), and the fact that REX blocks some high affinity binding sites in vitro indicates that some high affinity sites may be p75NGFR-dependent (Weskamp and Reichardt, 1991). The failure to rescue all cholinergic cells by RTA or RTA plus REX infusions following axotomy, as well as the decreased sprouting response we observed in RTA plus REX–infused animals, are consistent with these interpretations.
RTA Infusions Initiate Sprouting
Infusions of RTA significantly induce sprouting of p75NGFR–IR fibers in the dorsolateral quadrant of the septum, a characteristic of NGF infusions following FF lesions (Gage et al., 1988). Although a common feature in NGF-infused animals, this sprouting event has been poorly described in the literature and is not necessarily a tropic (or directional) phenomenon. Nevertheless, it is interesting that RTA-infused animals exhibit this biological response along with NGF-infused animals and indicates that TrkA activation is able to mimic other responses to NGF in vivo in addition to supporting cholinergic cell survival. RTA plus REX–infused animals, however, did not show significant p75NGFR–IR sprouting when compared with control animals. This finding may suggest a role for p75NGFR in mediating cholinergic sprouting in vivo and also indicates that the biological effects of NGF and the respective receptor mechanisms that mediate them may be dissociated.
Is p75NGFR Involved in NGF Signal Transduction In Vivo?
NGF binds p75NGFR with low affinity (∼10-9), and some reports indicate that the presence of p75NGFR, along with TrkA, is necessary to mediate signal transduction (Hempstead et al., 1991; Berg et al., 1991). A variety of studies support the proposed importance of p75NGFR in NGF signaling. When expressed in PC12 cells, chimeric receptors comprised of the extracellular ligand-binding domain of the human epidermal growth factor (EGF) receptor that were fused to transmembrane and cytoplasmic domains of p75NGFR resulted in neurite outgrowth and nuclear signaling in response to EGF application (Yan et al., 1991). In MAH cells, ectopic p75NGFR expression in excess amounts enhanced NGF-induced autophosphorylation of TrkA on tyrosine residues when compared with cells expressing TrkA alone (Verdi et al., 1994b). Biologically, disruption of the p75NGFR gene in homozygous mice resulted in decreased sensory innervation of the footpad (Lee et al., 1992, 1994), and trigeminal sensory neurons derived from these animals showed reduced responsiveness to NGF (Davies et al., 1993), indicating that p75NGFR increases the sensitivity of neurons toward NGF. p75NGFR may also mediate an apoptotic signal in NGF-responsive neurons during development (Rabizadeh et al., 1993; Barrett and Bartlett, 1994).
Our data suggest that at least a portion of cholinergic neurons do not require p75NGFR binding to initiate rescue following FF lesion. RTA specifically activates TrkA, and, when it is infused in combination with REX, which has been demonstrated to block but not activate p75NGFR, there is no reduction in the cell savings effect. Addition of REX does, however, result in significantly less sprouting, indicating a role for p75NGFR in this response to NGF. We expect that REX infusion oligomerizes p75NGFR extracellularly, inhibiting interaction of both the extracellular and intracellular domains of p75NGFR with itself or with TrkA. Inhibition of p75NGFR interaction by REX could account for the reduced sprouting response we see in RTA plus REX–infused animals. Additionally, these data suggest that the biological effects of NGF may be dissociated, mediated, or both by different receptor(s) or receptor complexes. It is also possible that infusion of REX obscures immunoreactivity to p75NGFR by binding receptors that might otherwise be bound during immunohistochemical recognition. However, the fact that we do not see a reduction in MS Nabs in animals infused with REX does not support this interpretation.
NGF infusions in FF-lesioned animals with targeted mutations in p75NGFR or trkA will shed more light on the relative contributions of p75NGFR and TrkA to this interesting phenomenon. Introduction of mutated neurotrophins that bind only one class of NGF receptor has been fruitful in vitro (Ibañez et al., 1992) and may also further elucidate the NGF receptor(s) necessary to confer signal transduction in vivo. Understanding the mechanism by which NGF signal transduction is further modulated in different biological responses (e.g., survival versus sprouting) also promises to provide an interesting area of study.
Conclusions
This report provides the first in vivo experimental evidence that activation of TrkA alone is sufficient to rescue cholinergic neurons following axotomy. Cell savings observed in the absence of exogenously applied NGF and in the presence of blocked p75NGFR strongly suggest that signaling via TrkA alone is sufficient to activate the NGF-dependent survival responses of septal cholinergic neurons in vivo. The use of a selective trkA agonist that mimics at least some of the action of NGF in vivo may prove useful for further elucidation of the mechanism by which related members of the neurotrophin family function and suggests possible mechanisms for therapeutic intervention in specific neurotrophic interactions implicated in human degenerative diseases.
Experimental Procedures
Development and Specificity of RTA Antibody
Preparation of the RTA antibody has been described (Clary et al., 1994). In brief, the DNA sequence encoding the extracellular domain of the rat TrkA receptor was generated by polymerase chain reaction (PCR) and inserted into a baculovirus transfer vector. A recombinant baculovirus expressing the extracellular domain of rat TrkA was isolated and purified; Sf900 cells were infected with the virus, and the recombinant extracellular domain of TrkA was purified from the medium by lentil lectin and anion exchange chromatography. Rabbits were immunized with the recombinant protein, and RTA IgG was prepared from their serum as described (Clary et al., 1994). RTA has been shown to recognize TrkA, but not TrkB or TrkC, by immunoblot analysis and immunoprecipitation. Preparation of the REX IgG recognizing the extracellular domain of p75NGFR was done with a similar protocol and has been described (Weskamp and Reichardt, 1991).
To test the cellular specificity of the RTA antibody, HEK293 cells were transiently transfected with the rat TrkA, rat TrkB, or rat TrkC cDNA in the vector CDM8 by the lipofectamine method (GIBCO BRL) and stained with 2 µg/ml of either the RTA or trk.cyt IgG. The trk.cyt antibody is directed to the cytoplasmic tail of TrkA and recognizes all three Trk receptors (Clary et al., 1994).
Surgical and Histological Procedures
Female Fischer 344 rats (150–200 gm body weight) were anesthetized with a mixture of ketamine (75 mg/kg), acepromazine (0.75 mg/kg), and rompun (4 mg/kg) and placed in a Kopf stereotaxic apparatus for all surgical procedures. Adult female Fisher 344 rats (n = 29) were implanted with ICV cannulae ipsilateral to the lesion with minipump implantation followed immediately by unilateral FF transection. Complete unilateral aspirative lesions of the FF and supracallosal striae were made under visual inspection as previously described (Gage et al., 1983). Aspirative lesions, conducted with the aid of a surgical microscope, were made on the right side of the brain ipsilateral to the cannula implantation site. Osmotic minipumps (Alzet) and attached cannulae (Small Parts, Incorporated) were filled with polyclonal rabbit antibodies to rat TrkA (RTA; 1.0 mg/ml; Clary et al., 1994) (n = 7), RTA (1.0 mg/ml) in combination with polyclonal antibodies to p75NGFR (REX; 0.1 mg/ml; Weskamp and Reichardt, 1991) (n = 5), or human β NGF (100 µg/ml; Barnett et al., 1990) (n = 5). Control animals consisted of rats that received FF lesions only (n = 2) or those that received infusates of preimmune rabbit serum (RS; 1.0 mg/ml) (n = 4), artificial cerebrospinal fluid (CSF [pH 7.4]; Na, 150 mM; K, 3.0 mM; Ca, 1.4 mM; Mg, 0.8 mM; P, 1.0 mM; Cl, 155 mM) (n = 3), or REX (0.1 mg/ml) (n = 3). Normal unlesioned animals (n = 4) were also included in the control population. Solutions were continuously infused at a rate of 0.5 µl/hr for 2 weeks, at which time animals were sacrificed.
Rats were anesthetized (as described above) and transcardially perfused with 50 ml of saline followed by 250 ml of ice-cold 4% paraformaldehyde at 2 weeks following FF transection and pump implantation. Sections were processed for immunohistochemistry by the following procedure. Endogenous peroxidases were first blocked with 0.6% H2O2; then sections were permeabilized with 0.25% Triton X-100 and blocked with 10% horse serum (p75NGFR) or 10% goat serum (antibody localization). Sections from a one-in-six series were incubated with anti-mouse p75NGFR monoclonal antibody diluted 1:100. Sections were also processed for peroxidase immunocytochemistry with biotinylated rabbit anti-goat antibodies to assess tissue distribution of RTA, REX, and RS following chronic ICV infusion. The antigen–antibody complex was reacted with horse anti-mouse or goat anti-rabbit secondary antibody coupled to biotin. Staining was developed by the avidin–biotin method (Vector Laboratories) with nickel intensification using 3,3-diaminobenzidine as the chromogen.
Analysis and Quantification
Only those rats exhibiting accurate ICV cannula placement, infusate penetration into the MS, and complete and discrete FF lesions were chosen for quantitative analysis (n = 26). Cell numbers were determined by quantitative stereology as previously described (Peterson et al., 1994). For the purposes of this paper, the rostral–caudal anatomical boundaries of the MS were defined as ∼+ 0.70 mm to −0.26 mm (as described by Paxinos and Watson) to include the MS and vertical limb of the diagonal band of Broca (vdbB) from where it can be distinguished from the horizontal limb of the diagonal band of Broca (hdbB) to just prior to the crossing of the anterior commisure. We defined the MS as this area because both the MS nucleus and the vdbB are affected by the lesion, and in practice, the boundary distinction between the vdbB and hdbB is more distinct than the more artificial boundary between the MS and vdbB. The lateral boundaries included the MS cells that were interspersed within the neuropil of the MS nucleus.
Cavalieri Estimator of Reference Volume
The volume of the MS was determined by the method of Cavalieri (Michel and Cruz-Orive, 1988) as previously described (Peterson et al., 1994; Peterson and Jones, 1993). In brief, a point-counting grid printed on an acetate sheet was placed over the video monitor upon which the entire MS was displayed from a low power objective (4×). The area around each point was calibrated with a stage micrometer. By knowing the distance (d) between sections and multiplying that by the area per point (Ap), we used each point as a volume probe. The total volume (Vref) of the MS was determined by counting the number of points (Q) overlying the MS in semiserial (one-in-six) sections and multiplying that sum (ΣQ) by the volume associated with each point by the formula Vref = (ΣQ)(d)(Ap).
Optical Disector Estimator of Neuron Number
The numerical density of p75NGFR–IR MS neurons in a known volume was determined by the unbiased optical dissector method (Sterio, 1984), which has been previously described (Peterson et al., 1994). Briefly, a one-in-six series of coronal sections was observed on an Olympus Vanox AH2 microscope with a high-numerical-aperture objective (40×). An unbiased counting frame was printed on an acetate sheet and placed on a high resolution monitor screen on which the video signal from the microscope was displayed. Calibration of focusing depth (z-travel) was accomplished with an electronic microcator (Heidenhain model MT12).
Neuronal estimates were compiled for each side of the MS. Each section was positioned so that the counting frame overlaid the dorsal tip of the septum on one side of the midline. The counting frame was applied to that region and every second region in a lateral direction until the lateral border of the MS was reached, whereafter the counting frame was returned to the midline. Those cells that fell directly on the midline were excluded by positioning the counting frame 40 µm from the actual midline. The number of neurons that fell in the frame was counted according to the rules for the optical disector (Sterio, 1984; Peterson et al., 1994), yielding a number per known volume, or numerical density value (Nv), which is averaged for the entire sampled region. Using the Vref obtained above, we calculated the total number of p75NGFR–IR MS neurons by multiplying the mean number per known sample volume by the total volume of the region. This value was not a density measurement but an estimation of the total or absolute number (Nabs) of neurons present. This approach to quantitation is not biased by neuronal size, shape, or distribution, or by volumetric changes in the containing structure due to either experimental or artifactual effects.
Quantification of p75NGFR–IR Sprouting
p75NGFR–IR sprouting in the dorsolateral quadrant of the septum was analyzed with National Institutes of Health (NIH) Image 1.55b5 (available through anonymous file transfer protocol from zippy.nimh.nih.gov). Five sections immunostained with p75NGFR from a one-in-six series through the MS were projected from an Olympus Vanox AH2 microscope with a low-numerical-aperture objective (4×) and a photo lens of 2.5. The ipsilateral septum was displayed on a high resolution monitor screen via a video signal from the microscope. The area displayed approximated the ipsilateral dorsal septum extending from the corpus callosum to the base of the lateral ventricle and from the midline to the center of the lateral ventricle. The image was captured onto a Macintosh Quadra 700 running NIH Image 1.55b5 and saved to an optical disk. Sections were analyzed offline by a macro sequence in the Image program to ensure that a constant density threshold value was applied to each section. A box corresponding to approximately 300 × 300 µm (90,000 µm2) was then overlaid over identical areas of the tissue by way of a macro sequence, and four areas per section were systematically sampled in the dorsolateral septum. All pixels in the sampled areas above the set threshold were considered to represent sprouting fibers. Twenty measurements of total pixels above threshold were thus saved for each animal and summed to obtain the amount of total p75NGFR–IR sprouting per animal.
Statistical Analyses
A multiway analysis of variance was performed to test for significance between groups. Fisher posthoc comparisons yielded statistical significance when indicated. All statistical analyses were performed by use of analyses of variance with 95% and 99% confidence intervals.
Acknowledgments
We gratefully thank Daniel Peterson for methodological guidance, and Mark Tuszynski and Steven Forbes for surgical assistance.
This work was supported by NIH grants 5T32 AG00216, AG06088, AG08514, and NS28121.
References
- Allsopp TE, Robinson M, Wyatt S, Davies AM. Ectopic trkA expression mediates a NGF survival response in NGF-independent sensory neurons but not in parasympathetic neurons. J. Cell Biol. 1993;123:1555–1566. doi: 10.1083/jcb.123.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimatsu Y, Miyamoto M, Tsukui H, Hatanaka H. Nerve growth factor promotes survival of retrogradely labeled hippocampus projecting neurons in the rat basal forebrain in vitro. Dev. Brain Res. 1988;45:297–301. doi: 10.1016/0165-3806(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Barde Y-A. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNGFR reduces NGF binding to trkA on PC12 cells. Neuron. 1994;13:203–215. doi: 10.1016/0896-6273(94)90470-7. [DOI] [PubMed] [Google Scholar]
- Barnett J, Baecker P, Routledge-Ward C, Bursztyn-Pettegrew H, Chow J, Nguyen B, Bach C, Chan H, Tuszynski MH, Yoshida K, Rubalcava R, Gage FH. Human β nerve growth factor obtained from a baculovirus expression system has in vitro and in vivo neurotrophic activity. Exp. Neurol. 1990;110:11–24. doi: 10.1016/0014-4886(90)90047-v. [DOI] [PubMed] [Google Scholar]
- Barrett GL, Bartlett PF. The p75 nerve growth factor receptor mediates survival or death depending on the stage of sensory neuron development. Proc. Natl. Acad. Sci. USA. 1994;91:6501–6505. doi: 10.1073/pnas.91.14.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Armstrong DM, Blaker SN, Gage FH. Nerve growth factor receptor and choline acetyltransferase colocalization in neurons within the rat forebrain: response to fimbria-fornix transection. J. Comp. Neurol. 1989;284:187–204. doi: 10.1002/cne.902840204. [DOI] [PubMed] [Google Scholar]
- Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc. Natl. Acad. Sci. USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg MM, Sternberg DW, Hempstead BL, Chao MV. The low-affinity p75 nerve growth factor (NGF) receptor mediates NGF-induced tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA. 1991;88:7106–7110. doi: 10.1073/pnas.88.16.7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Keeping track of neurotrophin receptors. Cell. 1991;65:915–918. doi: 10.1016/0092-8674(91)90540-f. [DOI] [PubMed] [Google Scholar]
- Clary DO, Weskamp G, Austin LR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol. Biol. Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Lee K, Jaenisch R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron. 1993;11:565–574. doi: 10.1016/0896-6273(93)90069-4. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Gage FH, Bjorklund A, Stenevi U. Reinnervation of the partially deafferented hippocampus by compensatory collateral sprouting from spared cholinergic and noradrenergic afferents. Brain Res. 1983;268:27–37. doi: 10.1016/0006-8993(83)90387-6. [DOI] [PubMed] [Google Scholar]
- Gage FH, Armstrong DM, Williams LR, Varon S. Morphological response of axotomized septal neurons to nerve growth factor. J. Comp. Neurol. 1988;269:147–155. doi: 10.1002/cne.902690112. [DOI] [PubMed] [Google Scholar]
- Gage FH, Batchelor P, Chen KS, Chin D, Higgins GA, Koh S, Deputy S, Rosenberg MB, Fischer W, Bjorklund A. NGF receptor re-expression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron. 1989;2:1177–1187. doi: 10.1016/0896-6273(89)90184-0. [DOI] [PubMed] [Google Scholar]
- Gibbs RE, Pfaff DW. In situ hybridization detection of trkA mRNA in brain: distribution, colocalization with p75NGFR and up-regulation by nerve growth factor. J. Comp. Neurol. 1994;341:324–339. doi: 10.1002/cne.903410304. [DOI] [PubMed] [Google Scholar]
- Green SH, Rydel RE, Connolly JL, Greene LA. PC12 mutants that possess low-but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J. Cell. Biol. 1986;102:830–843. doi: 10.1083/jcb.102.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T, Manthorpe M, Vahlsing HL, Varon S. Delayed treatment with nerve growth factor reverses the apparent loss of cholinergic neurons after brain damage. Exp. Neurol. 1988;101:303–312. doi: 10.1016/0014-4886(88)90013-1. [DOI] [PubMed] [Google Scholar]
- Hantzopoulos PA, Suri C, Glass DJ, Goldfarb MP, Yancopoulos GD. The low affinity NGF receptor, p75, can collaborate with each of the trks to potentiate functional responses to the neurotrophins. Neuron. 1994;13:187–201. doi: 10.1016/0896-6273(94)90469-3. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J. Neurosci. 1986;8:2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F, Dravid A, Hartikka J. Chronic intraventricular injections of nerve growth factor elevate hippocampal choline acetyltransferase activity in adult rats with partial septo-hippocampal lesions. Brain Res. 1984;293:305–311. doi: 10.1016/0006-8993(84)91237-x. [DOI] [PubMed] [Google Scholar]
- Hefti F, Hartikka J, Eckenstein F, Gnahn H, Heumann R, Schwab M. Nerve growth factor increases choline acetyltransferase but not survival or fiber outgrowth of cultured fetal septal cholinergic neurons. Neurosci. 1985;14:55–68. doi: 10.1016/0306-4522(85)90163-0. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Koh S, Chen KS, Gage FH. NGF induction of NGF receptor gene expression and cholinergic neuronal hypertrophy within the basal forebrain of the adult rat. Neuron. 1989;3:247–256. doi: 10.1016/0896-6273(89)90038-x. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Li Y, Parada LF, Kinsman S, Chen C, Valletta JS, Zhou J, Long JB, Mobley WC. p140trk mRNA marks NGF-responsive forebrain neurons: evidence that trk gene expression is induced by NGF. Neuron. 1992;9:465–478. doi: 10.1016/0896-6273(92)90184-f. [DOI] [PubMed] [Google Scholar]
- Ibañez CF, Ebendal T, Barbany G, Murray-Rust J, Blundell TL, Persson H. Disruption of the low affinity receptor-binding site in NGF allows neuronal survival and differentiation by binding to the trk gene product. Cell. 1992;69:329–341. doi: 10.1016/0092-8674(92)90413-7. [DOI] [PubMed] [Google Scholar]
- Ibañez CF, Ilag LL, Murray-Rust J, Persson H. An extended surface of binding to Trk tyrosine kinase receptors in NGF and BDNF allows the engineering of a multifunctional pan-neurotrophin. EMBO J. 1993;12:2281–2293. doi: 10.1002/j.1460-2075.1993.tb05882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Tapley P, Barbacid M. Nerve growth factor mediates signal transduction through trk homodimer receptors. Neuron. 1992;9:1067–1079. doi: 10.1016/0896-6273(92)90066-m. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Hampstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991a;252:554–557. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zanca DM, Parada LF. Tyrosine phosphorylation of and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991b;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Klein R, Jing S, Nanduri V, O’Rourke E, Barbacid M. The trk proto-oncogene encodesa receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Kromer L. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987;235:214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Lee K, Li E, Huber J, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lee K, Bachman K, Landis S, Jaenisch R. Dependence on p75 for innervation of some sympathetic targets. Science. 1994;263:1447–1449. doi: 10.1126/science.8128229. [DOI] [PubMed] [Google Scholar]
- Loeb DM, Maragos J, Martin-Zanca D, Chao MV, Parada LF, Greene LA. The trk proto-oncogene rescues NGF responsiveness in mutant NGF-nonresponsive PC12 cell lines. Cell. 1991;66:961–966. doi: 10.1016/0092-8674(91)90441-z. [DOI] [PubMed] [Google Scholar]
- Mahadeo D, Kaplan L, Chao MV, Hempstead BL. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. J. Biol. Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol. Cell. Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. Molecular investigation of the high-affinity nerve growth factor receptor. Neuron. 1991;6:153–163. doi: 10.1016/0896-6273(91)90130-r. [DOI] [PubMed] [Google Scholar]
- Michel RP, Cruz-Orive LM. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J. Microsc. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Montero CN, Hefti F. Rescue of lesioned septal cholinergic neurons by nerve growth factor: specificity and requirement for chronic treatment. J. Neurosci. 1988;8:2986–2999. doi: 10.1523/JNEUROSCI.08-08-02986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann T, Peterson GM, Frotscher M. Fine structure of rat septohippocampal neurons: II. A time course analysis following axotomy. J. Comp. Neurol. 1992;325:219–242. doi: 10.1002/cne.903250207. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Martin-Zanca D, Kaplan DR, Parada LF, Santos E. Induction by NGF of meiotic maturation of Xenopus oocytes expressing the trk proto-oncogene product. Science. 1991;252:558–561. doi: 10.1126/science.1850550. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Second Edition. Harcourt Brace Jovanovich, Publishers, San Diego: Academic Press, Inc; 1986. [Google Scholar]
- Peterson DA, Jones DG. Determination of neuronal number and process surface area: a stereological approach to in vitro quantitation. J. Neurosci. Methods. 1993;46:107–120. doi: 10.1016/0165-0270(93)90146-i. [DOI] [PubMed] [Google Scholar]
- Peterson DA, Lucidi-Phillipi CA, Eagle KL, Gage FH. Perforant path damage results in progressive neuronal death and somal atrophy in layer II of entorhinal cortex and functional impairment with increasing postdamage age. J. Neurosci. 1994;14:6872–6885. doi: 10.1523/JNEUROSCI.14-11-06872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabizadeh S, Oh J, Zhong L, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Radeke ML, Feinstein SC. Analytical purification of the slow, high affinity NGF receptor: identification of a novel 135 kd polypeptide. Neuron. 1991;7:141–150. doi: 10.1016/0896-6273(91)90082-b. [DOI] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tebar A, Dechant G, Barde Y-A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Rovelli G, Heller RA, Canossa M, Shooter EM. Chimeric tumor necrosis factor-TrkA receptors reveal that ligand-dependent activation of the TrkA tyrosine kinase is sufficient for differentiation and survival of PC12 cells. Proc. Natl. Acad. Sci. USA. 1993;90:8717–8721. doi: 10.1073/pnas.90.18.8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Armstrong DA, Gage FH. Basal forebrain cell loss following fimbria/fornix transection. Brain Res. 1990;508:241–248. doi: 10.1016/0006-8993(90)90402-w. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Ip N, Yancopolous GD, Anderson DJ. Expression of trk in MAH cells lacking the p75 low-affinity nerve growth factor receptor is sufficient to permit nerve growth factor-induced differentiation to postmitotic neurons. Proc. Natl. Acad. Sci. USA. 1994a;91:3949–3953. doi: 10.1073/pnas.91.9.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdi JM, Birren SJ, Ibañez CF, Persson H, Kaplan DR, Benedetti M, Chao MV, Anderson DJ. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 1994b;12:733–745. doi: 10.1016/0896-6273(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Vetter ML, Martin-Zanca D, Parada LF, Bishop JM, Kaplan DR. Nerve growth factor rapidly stimulates tyrosine phosphorylation of phospholipase C-γ1 by a kinase activity associated with the product of the trk proto-oncogene. Proc. Natl. Acad. Sci. USA. 1991;88:5650–5654. doi: 10.1073/pnas.88.13.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Williams LW, Varon S, Peterson GM, Wictorin K, Fischer W, Bjorklund A, Gage FH. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc. Natl. Acad. Sci. USA. 1986;83:9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Schlessinger J, Chao MV. Chimeric NGF-EGF receptors define domains responsible for neuronal differentiation. Science. 1991;252:561–563. doi: 10.1126/science.1850551. [DOI] [PubMed] [Google Scholar]