Summary
Cadherins and catenins are thought to promote adhesion between pre and postsynaptic elements in the brain. Here we show a role for β-catenin in localizing the reserved pool of vesicles at presynaptic sites. Deletion of β-catenin in hippocampal pyramidal neurons in vivo resulted in a reduction in the number of reserved pool vesicles per synapse and an impaired response to prolonged repetitive stimulation. This corresponded to a dispersion of vesicles along the axon in cultured neurons. Interestingly, these effects are not due to β-catenin's involvement in cadherin-mediated adhesion or wnt signaling. Instead, β-catenin modulates vesicle localization via its PDZ binding domain to recruit PDZ proteins such as Veli to cadherin at synapses. This study defines a specific role for cadherins and catenins in synapse organization beyond their roles in mediating cell adhesion.
Introduction
Synapse formation is a well-concerted process that involves multiple stages. The initial synaptic contact is followed by a series of events including cell-cell adhesion and the appearance of specializations at regions of contact. Despite extensive studies of the adhesive interactions between pre- and postsynaptic membranes (Mizoguchi et al., 2002; Phillips et al., 2001; Polo-Parada et al., 2001), the mechanisms by which such interactions lead to the recruitment of synaptic proteins to pre- and postsynaptic terminals are not known. Moreover, while the timing of synaptogenic events has been described (Friedman et al., 2000), our understanding of the cellular mechanisms that orchestrate synapse assembly in the central nervous system (CNS) is limited.
Cadherins, a family of cell adhesion proteins found at adherens junctions, have been implicated in synapse formation (Ranscht, 2000; Serafini, 1997, 1999; Togashi et al., 2002). Classic cadherins mediate strong adhesion through interaction with β-catenin, which in turn associates with α-catenin and the actin cytoskeleton (Daniels et al., 2001; Ivanov et al., 2001). Recent literature has shown that cadherin-mediated cell junctions are signaling centers, not simply points of adhesion. For example, cadherins recruit and bind to several members of a second catenin family, the p120 catenins (p120ctn), which themselves link cadherin to a number of signaling pathways (Daniel and Reynolds, 1995; Reynolds et al., 1994; Shibamoto et al., 1995; Staddon et al., 1995). Members of the p120ctn family have been shown to activate Rac and inhibit Rho activity (Goodwin et al., 2003; Magie et al., 2002), to recruit the cytoplasmic tyrosine kinases Fer and Fyn (Piedra et al., 2003), and to contain PDZ binding motifs that recruit a variety of cytoplasmic PDZ motif-containing proteins (Izawa et al., 2002; Laura et al., 2002). Cadherins also recruit tyrosine kinases and phosphatases (Steinberg and McNutt, 1999), Arp2/3 complexes (Kovacs et al., 2002), PI-3 kinase (Pece et al., 1999), heterotrimeric GTPases (Meigs et al., 2001), and adaptor proteins (Xu et al., 1997; Yap and Kovacs, 2003). Similarly, in addition to its role linking cadherins to the cytoskeleton, β-catenin interacts with many signaling partners, including PI-3 kinase (Woodfield et al., 2001), IQGAP (Kuroda et al., 1996), APC, and several transcription factors (Barth et al., 1997). β-catenin also has a PDZ binding site at its carboxy terminus that has been shown to bind to Lin-7, the C. elegans homolog of Veli (Perego et al., 2000), and the membrane-associated guanylate kinase protein MAGI-1 or S-SCAM (Dobrosotskaya and James, 2000; Kawajiri et al., 2000; Nishimura et al., 2002), thereby linking cadherins to PDZ proteins as well as to the actin cytoskeleton. Thus, both cadherin and β-catenin interact with a variety of signaling proteins and may mediate multiple functions at synaptic junctions.
Cadherins and their associated catenins are expressed in the developing and adult CNS (Chauvet et al., 2003; Fannon and Colman, 1996; Montonen et al., 2001; Uchida et al., 1996) and are colocalized at both pre- and postsynaptic compartments prior to the assembly of a functional synapse. Indeed, N-cadherin and other components of the early active zone, including piccolo, bassoon, syntaxin, SNAP-25, and chromogranin B, are transported together in an active zone precursor vesicle and initiate synapse formation by establishing a potential active zone (Zhai et al., 2001). Subsequently, synaptic vesicle proteins such as synapsin are recruited presynaptically (Benson and Tanaka, 1998; Zhai et al., 2001), while SAP-90/PSD-95 and glutamate receptors are recruited postsynaptically (Coussen et al., 2002; Friedman et al., 2000).
The early pre- and postsynaptic localization of cadherin/catenin complexes suggests a role in synapse assembly. This is supported by a number of findings. First, anti-cadherin antibodies destabilize contacts between growth cones and their targets (Sanes and Yamagata, 1999) and disrupt subcellular localization of postsynaptic density elements (Honjo et al., 2000). Second, inhibition of cadherin-based adhesion between hippocampal neurons perturbs synapse formation and function (Togashi et al., 2002). Third, genetic analyses have shown that cadherins regulate synaptic vesicle accumulation in Drosophila (Iwai et al., 2002). In addition, β-catenin has been shown to promote synapse formation via its participation in the wnt signaling pathway (Hall et al., 2000).
The continued expression of cadherins and catenins at the synapse suggests a further role for these molecules in synapse plasticity. Indeed, synaptic activity can modulate the distribution of N-cadherin (Bozdagi et al., 2000; Shan et al., 2002) and β-catenin (Murase et al., 2002) and perturbations of classical cadherin function have been shown to affect hippocampal LTP (Manabe et al., 2000; Tanaka et al., 2000; Tang et al., 1998).
In the present study, we perturbed β-catenin function both in vivo and in vitro and examined the effects on synaptic development. Our results indicate that β-catenin plays a role in localizing synaptic vesicles at the presynaptic active zone by acting as a scaffolding protein to link cadherin clusters to PDZ domain-containing proteins via its PDZ binding motif. These results provide new insights into the molecular mechanisms by which the cadherin-catenin complex controls synapse formation.
Results
Conditional Ablation of the β-Catenin Gene in Hippocampal Pyramidal Neurons
To investigate the role of β-catenin in the maintenance of synaptic structure and function, we used the cre/loxP technology to specifically ablate the β-catenin gene in hippocampal pyramidal neurons following synapse formation. Mice containing loxP sites in the second and sixth introns of the β-catenin gene (Huelsken et al., 2001) were crossed to mice that express the cre gene under the control of CamKII regulatory elements (CamKIIcre) (Xu et al., 2000). The progeny were viable and fertile, and Nissl staining revealed no gross morphological defects in adult mutant mice (data not shown). To examine the pattern of CamIIKcre-controlled recombination, the CamIIKcre transgene was transferred to mice bearing the R26R reporter, a cre-inducible lacZ reporter (Soriano, 1999). Almost complete cre-mediated recombination was observed in the hippocampus, including CA1, CA2, and CA3 pyramidal neurons as well as dentate granule neurons (Figure 1A) and was initiated as early as P17 (see also Xu et al., 2000). Previous studies have shown that expression of this transgene is restricted to excitatory neurons that express endogenous CamKII (Xu et al., 2000).
Figure 1. Loss of β-Catenin in Hippocampal Pyramidal Neurons.
(A) X-gal staining in the hippocampus of adult mice heterozygous for the CamKcre transgene and the R26R lacZ reporter. Counterstaining with Fast Red. (Left panels) (B, D, F, and H) 50 µm coronal sections from adult controls; (right panels) (C, E, G, and I) 50 µm coronal sections from adult β-catenin mutants. (B and C) β-catenin expression is lost from most (approximately 80%) CA3 pyramidal neurons in mutant mice. (D–G) β-catenin expression is absent in CA1 pyramidal neurons and the SR of mutant mice. (H and I) Synapsin expression is similar in the SR of control and mutant mice. Antibodies: rabbit polyclonal anti-β-catenin (B–E), mouse monoclonal anti-β-catenin (F and G), mouse monoclonal anti-synaptophysin (H and I). Scale bar, 450 µm (A); 35 µm (B–E); 6.5 µm (F–I).
β-catenin is normally expressed in cell bodies of CA3 (Figure 1B) and CA1 pyramidal neurons (Figure 1D) and exhibits a punctate pattern in the stratum radiatum (SR) as previously reported (Uchida et al., 1996) (Figure 1D). Analysis of β-catenin expression in mutant slices revealed that approximately 80% of CA3 pyramidal neurons were negative for β-catenin (Figure 1C). In contrast, β-catenin immunoreactivity was completely absent in CA1 pyramidal neurons and in the SR of mutant animals (Figures 1E and 1G).
Absence of β-catenin did not appear to affect the expression of a number of different synapse-specific markers, including synaptophysin (Figures 1H and 1I), synapsin, PSD-95, and NR2A (data not shown).
Alterations in Synapse Number, Vesicles per Synapse, and Synaptic Responses in β-Catenin Mutant Mice
To more rigorously examine the synaptic effects of β-catenin ablation, we used electron microscopy (EM) to analyze the morphology of synapses in the SR of adult control and mutant mice. In the mutants, we observed a 25% increase in the number of excitatory, spine synapses (Figures 2A, 2B, arrowheads and 2D), and a 3-fold increase in perforated synapses (Figures 2C and 2E), with no significant changes in the average length of the postsynaptic density (data not shown). Furthermore, there was a 40% reduction in the undocked pool of vesicles per synapse, whereas the number of docked vesicles located less than one vesicle diameter from the presynaptic membrane was similar in both mutant and control sections (Figures 2A, 2B, and 2F). The reduction in the number of vesicles per synapse in mutant mice was not merely a redistribution of vesicles over a larger number of synapses. Indeed, in wild-type animals 3917.34 vesicles were associated with synapses in a 700 µm2 area as compared to 2886.81 vesicles in mutants. As Western blot analysis revealed no changes in synaptophysin and synaptotagmin protein expression (data not shown), the decreased number of vesicles associated with synapses in mutant mice is very likely due to mislocalization of vesicles as opposed to an overall reduction in vesicles.
Figure 2. Morphological and Functional Analyses of Hippocampal CA1 Synapses.
(A–C) Electron microscope images of synapses in the SR of adult control (A) and β-catenin mutant (B and C) mice. (D–F) Synapses measured were those in which a welldefined postsynaptic density was observed (arrowheads). An area representing 700 µm2 of SR per animal was used for quantification of synapse density. The number of docked vesicles and undocked vesicles (vesicle cluster) was counted in at least 150 synapses per genotype. n = 4 per genotype. (G–L) Effects of β-catenin deletion on excitatory synaptic transmission in hippocampal CA1 synapses. Schaeffer collaterals were stimulated, and fEPSPs as well as fiber volleys were recorded in CA1 area of adult hippocampal slices. Open circles: wt; n = 5 animals. Filled triangles: MT; n = 4–6 animals. Data from multiple recordings of the same genotype were pooled and expressed as mean ± SEM. (G) Fiber volley amplitudes at different stimulation intensities. n = 13 for wt slices, and n = 10 for MT slices. (H) Input-output curves for basal synaptic transmission. The mean fEPSP slopes were plotted against fiber volley amplitude. The fiber volley amplitudes were binned, and corresponding EPSP slopes were averaged between slices. n = 13 for wt slices, and n = 10 for MT slices. (I) Paired-pulse facilitation (PPF) at low stimulation intensity (approximately 12.5% of maximal strength). Two stimuli were applied at various interpulse intervals (IPI, 10–100 ms). The ratios of the second and first EPSP slopes were calculated, and mean values are plotted against different IPIs. n = 9 for wt slices, and n = 8 for MT slices. (J) Posttetanic potentiation (PTP). A train of tetanic stimulation (100 Hz, 1 s, indicated by arrow) was applied to the CA1 synapses in the presence of the NMDA receptor antagonist Apv (100 µM), and EPSP slopes were plotted against time (4 min before and 10 min after the tetanus). n = 15 for wt slices, and n = 12 for MT slices. (K) Normal synaptic responses to a brief, high-frequency stimulation (HFS, 100 Hz, 40 pulses, in the presence of the NMDA receptor antagonist Apv, 100 µM) at CA1 synapses. n = 18 for wt slices, and n = 17 for MT slices. Inset: examples of EPSPs elicited by the HFS. (L) Reduced synaptic responses to prolonged repetitive stimulation (14 Hz, 80 pulses, Apv, 100 µM) in MT (n = 21 slices) compared to wt (n = 18 slices). Inset: superimposed EPSPs at 20th and 80th pulses. Asterisks denote significant differences from controls (p < 0.05). wt, wild-type; MT, β-catenin mutant mice. Scale bar, 250 nm (A and B); 190 nm (C).
To examine the physiological consequences of these morphological differences, electrophysiological tests were performed on hippocampal CA1 synapses. First, we measured the amplitude of fibervolley, which reflects the numberof activated inputs (fibers), at different stimulation intensities. The amplitudes of fibervolley in wild-type and β-catenin mutant slices were almost identical over a wide range of stimulation intensities, suggesting that ablation of β-catenin does not affect the number of afferent axons (Figure 2G). Next, we compared basal synaptic transmission by measuring the input-output relation between the fiber volley amplitude and the fEPSP slope. The synaptic response was higher in β-catenin mutant slices compared to wild-type slices when stimulus intensities were low, but this difference diminished at higher stimulation intensities, possibly due to saturation of the postsynaptic response (Figure 2H). The increase in basal transmission at low stimulation intensities could be due to changes in the numberof presynaptic release sites (N), the probability of quantal release (Pr), and/or the postsynaptic quantal response (Q). The increase in the number of spine synapses revealed by electron microscopy (Figure 2D) is consistent with the increase in “N.” The maximal fEPSP responses in wild-type and β-catenin mutant slices were the same (data not shown), suggesting that there was no change in “Q.” We next measured paired-pulse facilitation (PPF), a form of short-term plasticity frequently used to infer the changes in “Pr” (Zucker, 1989). Figure 2I shows that PPF at a low stimulation was almost identical in wild-type and mutant slices. Consistent with the lack of change in “Pr,” we did not detect any differences between genotypes in posttetanic potentiation (PTP), the enhanced transmitter release observed shortly after the termination of a tetanic stimulation (Figure 2J). Taken together, these results raise the possibility that the increase in basal transmission seen in β-catenin mutant synapses is due to a change in “N” but not “Q” or “Pr.”
EM analysis did not reveal any obvious differences in the number of docked vesicles (Figure 2F), which are thought to be the morphological correlate of the readily releasable pool (RRP) of quanta (Cabin et al., 2002; Dobrunz and Stevens, 1997; Larkman et al., 1997; Zucker, 1989). To confirm that β-catenin does not affect RRP, we determined normalized synaptic responses to a brief, high-frequency repetitive stimulation (HFS, 100 Hz, 40 pulses), a protocol known to primarily deplete the RRP. As shown in Figure 2K, the EPSP slopes exhibited a continuous decline over time, indicative of a gradual depletion of docked vesicles with no obvious differences between wild-type and mutant slices. The results of electrophysiological and EM analyses suggest that the number of readily releasable quanta per synapse is not regulated by β-catenin.
In contrast, EM analysis revealed a marked reduction in the number of non-docked vesicles, which are thought to correspond to the reserved pool of quanta (Figure 2F). To investigate the physiological consequences of the morphological changes, we recorded synaptic responses to a prolonged repetitive low-frequency stimulation train (PRS; 14 Hz, 300 pulses), typically used to determine the size of the reserve pool of vesicles (Cabin et al., 2002). During PRS at the CA1 synapses, the depletion of RRP of vesicles is replenished by the reserved pool of vesicles. The depletion is usually faster than the replenishment, leading to a gradual decline of the EPSP slopes (Figure 2L). In β-catenin mutant slices, the decline was much faster than that in wild-type slices (Figure 2L). These results together with EM analysis showing that mutant synapses have fewer non-docked vesicles suggest that a major function of β-catenin is to control the size of the reserve vesicle pool, possibly by regulating the localization of synaptic vesicles to the nerve terminals.
Presynaptic Defects in Cultured Hippocampal Neurons Lacking β-Catenin
To better understand the role of β-catenin at the synapse, we cultured hippocampal neurons from mice homozygous for the β-catenin flox allele and transfected them with a cre expresssion vector to excise β-catenin. Synaptophysin-GFP was cotransfected into neurons to mark the localization of synaptic vesicles. Synaptic vesicle marker proteins have been widely used as GFP fusion proteins to mark presynaptic sites in vertebrates (Ahmari et al., 2000; Saegusa et al., 2002; Sankaranarayanan and Ryan, 2001; Wang et al., 2002). Synaptophysin-GFP expression was punctate in wild-type rat hippocampal cultures and colocalized with two endogenous synaptic vesicle proteins, synaptotagmin (Figures 3A–3C) and synapsin (data not shown), as well as with N-cadherin (data not shown). Synaptophysin-GFP also colocalized with the early presynaptic marker protein bassoon in β-catenin flox neurons not transfected with cre (Figures 3D–3F). These data indicate that synaptophysin-GFP is a reliable marker for synaptic vesicle localization.
Figure 3. Presynaptic Assembly Is Affected in Cultured Hippocampal Neurons Lacking β-Catenin.
(A–C) Confocal images of rat hippocampal neurons transfected with synaptophysin-GFP (A) show colocalization with synaptotagmin ([B and C], arrows). (D–R) Confocal images of hippocampal neurons cultured from homozygous β-catenin flox mice. (D–F) Neurons transfected with synaptophysin-GFP (D) and immunostained with anti-Bassoon (E) show colocalization of synaptophysin-GFP and Bassoon puncta ([E and F], arrows). (G–O) Neurons expressing cre exhibit a diffuse pattern of synaptophysin-GFP expression (G, J, and M). Endogenous synaptotagmin is also diffusely expressed in neurons lacking β-catenin (H and I), whereas Bassoon ([K and L], arrows) and N-cadherin ([N and O], arrows) expression remains punctate. (P–R) PSD-95-GFP expression is normal and colocalizes with synaptophysin in neurons lacking β-catenin. Asterisks indicate immunopositive puncta on cells not transfected with synaptophysin-GFP or PSD-95-GFP. (S) Diffusion of synaptic vesicles was quantified by measuring the average length of the major axis of synaptophysin-GFP fluorescence in cells expressing synaptophysin-GFP alone or coexpressing the cre recombinase. (T) The density of Bassoon puncta in cells expressing synaptophysin-GFP or synaptophysin-GFP plus cre was quantified and expressed as the number of puncta per 10 µm neurite length ± SE. (U) The size of PSD-95-GFP fluorescence was quantified in cells expressing PSD-95-GFP alone or coexpressing the cre recombinase. Student’s t tests were performed, and asterisks indicate p values of <0.05. Scale bar, 10 µm.
Expression of cre recombinase in β-catenin flox neurons efficiently decreased β-catenin protein levels (Supplemental Figure S1 at http://www.neuron.org/cgi/content/full/40/4/719/DC1). Coexpression of synaptophysin-GFP and cre resulted in a more diffuse pattern of synaptophysin-GFP expression along the axon, indicating that loss of β-catenin inhibits the synaptic localization of vesicles (Figures 3G, 3J, and 3M). Diffuse expression of endogenous synaptotagmin (Figures 3G–3I) and synapsin (data not shown) was also seen following ablation of β-catenin. Interestingly, bassoon (Figures 3J–3L) and N-cadherin (Figures 3M–3O) expression was still punctate in these cells, suggesting that β-catenin is involved in some but not all steps of presynaptic assembly. The magnitude of synaptophysin-GFP fluorescence diffusion was measured by quantifying the length of the major axis of each puncta, which was 45% greater in neurons lacking β-catenin (Figure 3S). The intensity of synaptophysin-GFP fluorescence per puncta was also significantly decreased in neurons lacking β-catenin, suggesting that synaptophysin-GFP was indeed more diffusely expressed along the neuron and not upregulated overall (data not shown). These results together with the finding that the number of synaptic vesicles per synapse is reduced in β-catenin mutant mice in vivo suggest that β-catenin controls the localization and clustering of synaptic vesicles.
In vivo analysis revealed an increase in the number of synapses in the SR. To examine whether β-catenin plays a direct role in controlling synapse number, we counted the density of bassoon immunoreactive puncta in wild-type cells or those lacking β-catenin. To our surprise, no significant differences were observed in the density of bassoon-positive puncta (Figure 3T). It is possible that the increase in synapse number seen in vivo may reflect a compensatory response to β-catenin deletion.
To determine whether loss of β-catenin affects postsynaptic sites, neurons were transfected with PSD-95-GFP, a known marker of postsynaptic densities (Craven et al., 1999; El-Husseini et al., 2000). Deletion of β-catenin did not elicit observable effects on the shape and distribution of PSD-95-GFP puncta (Figure 3P), and quantitative analysis confirmed that ablation of β-catenin did not alter the size of PSD-95-GFP puncta (Figure 3U). In addition, PSD-95-GFP puncta colocalized with endogenous synaptophysin in wild-type axons synapsing onto mutant cells, indicating that PSD-95 localizes at synapses even in the absence of β-catenin (Figures 3P–3R). Our data does not exclude the possibility that other postsynaptic proteins are affected by β-catenin ablation.
We next examined the pattern of synaptophysin-GFP expression over time using time-lapse imaging. When hippocampal neurons derived from β-catenin flox mice were transfected with synaptophysin-GFP alone, the resultant fluorescent boutons were stable over at least 30 min (Figure 4A) as described previously for VAMP-GFP expression (Ahmari et al., 2000). Interestingly, in cells lacking β-catenin, the localization of synaptophysin-GFP was not stable; rather, the fluorescence was diffusely yet unevenly distributed and moved along the axon rapidly and erratically overtime compared to cells expressing β-catenin (Figures 4B and 4C and Supplemental Movie S1 at http://www.neuron.org/cgi/content/full/40/4/719/DC1). This random movement was unlike the more directed movements of the small, discrete VAMP-GFP transport packets observed by Ahmari et al. (2000), which were also observed in our wild-type and mutant cells.
Figure 4. Localization of Synaptophysin-GFP Fluorescence Is More Dynamic over Time in Neurons Lacking β-Catenin.
Time-lapse confocal images of synaptophysin-GFP in β-catenin flox cultures in the absence of cre (A) or in cells expressing cre (B). Arrows mark dramatic changes in synaptophysin-GFP localization over time. Scale bar, 5 µm.
Critical Role of PDZ Binding Domain of β-Catenin in Presynaptic Vesicle Localization
β-catenin consists of three domains: an NH2-terminal domain that interacts with α-catenin, a central domain of 12 armadillo repeats that binds to the cytoplasmic tail of cadherins and to LEF/TCF transcription factors (Daniels et al., 2001; Ivanov et al., 2001), and a COOH-terminal domain that mediates interactions with transcriptional regulators and contains a PDZ binding motif (Perego et al., 2000) (Figure 5A). To further investigate the mechanism by which β-catenin regulates synaptic vesicle localization, β-catenin deletion mutants lacking single domains and designed to inhibit distinct functions of endogenous β-catenin were expressed in primary hippocampal cultures. The β-catΔN construct is known to prevent strong cell-cell adhesion by inhibiting association of cadherins with the actin cytoskeleton (Funayama et al., 1995), and the β-catΔC and β-catΔPDZ constructs are predicted to uncouple proteins that bind to these regions from cadherin clusters (Perego et al., 2000). β-catΔARM both prevents strong cell-cell adhesion as well as uncouples PDZ proteins from cadherin clusters. Expression of all constructs was confirmed by immunohistochemistry using a polyclonal β-catenin antibody (data not shown).
Figure 5. The PDZ Binding Domain of β-Catenin Is Important for Synaptic Vesicle Localization.
(A) Protein binding regions and distinct domains of β-catenin. (B) The average length of the major axis of synaptophysin-GFP fluorescence was measured in cells expressing synaptophysin-GFP alone or coexpressing synaptophysin-GFP plus the indicated β-catenin. (C–L) Confocal images of rat hippocampal cultures. A punctate pattern of synaptophysin-GFP expression is observed in cells expressing synaptophysin-GFP alone (C) or coexpressing g-catAN (D). Synaptophysin-GFP expression is more diffuse in cells expressing β-catΔARM (E), β-catΔPDZ (F), and β-catΔAC (I). (F–H) Expression of β-catΔPDZ results in diffuse immunostaining of synaptotagmin that coincides with that of synaptophysin-GFP. (IΔK) Although expression of β-catΔC results in a diffuse pattern of synaptophysin-GFP staining, the pattern of bassoon immunostaining remains punctate. (L and M) Neurons expressing GFP plus either β-catΔC (L) or β-catΔPDZ (M) and immunostained with anti-synaptophysin. Note the punctate localization of synaptophysin in wild-type cells making contacts with neurons in which β-catenin function is inhibited. Asterisks indicate p values of <0.05 calculated using Student’s t tests (compared to synaptophysin-GFP alone). Scale bar, 10 µm (C–K); 3 µm (L and M).
Rat hippocampal cells expressing synaptophysin-GFP plus β-catΔN displayed a punctate pattern of fluorescence similar to that seen in cells expressing synaptophysin-GFP alone (Figures 5C and 5D). Thus, neither strong cadherin-mediated cell-cell adhesion nor the recruitment of α-catenin to cadherin clusters is required for the discrete localization of synaptic vesicles at presumptive synapses. In contrast, expression of β-catΔ ARM, β-catΔPDZ, and β-catΔC, each of which inhibits the recruitment of PDZ proteins to cadherin clusters, resulted in a more diffuse expression of synaptophysin-GFP along the axon (Figures 5E, 5F, and 5I). This diffusion was confirmed by immunolabeling with synaptotagmin (Figures 5G and 5H) and synapsin (data not shown). Interestingly, the magnitude of synaptophysin-GFP diffusion along the axon was similar in cells expressing β-catΔC and β-catΔPDZ to those lacking β-catenin (Figures 3P and 5B), indicating that the main role of β-catenin in presynaptic assembly is to act as a scaffold to link cadherins with PDZ proteins.
There is considerable remodeling and de novo formation of synapses at the time the above experiments were performed (10–12DIV) (Papa et al., 1995; Ziv and Smith, 1996); however, to ensure β-catenin’s role in synaptic development, we assayed earlier time points. Cells cotransfected with β-catΔC or β-catΔPDZ at 5DIV and examined at 7DIV exhibited a significant dispersion of synaptophysin-GFP fluorescence along the axon (2.06 µm ± 0.2,1.83 µm ± 0.1, p < 0.001 for both) compared to cells transfected with synaptophysin-GFP alone (1.08 µm ± 0.05, n = at least 170 puncta).
Consistent with ourfinding that the pattern of bassoon expression is not disrupted in neurons lacking β-catenin (Figures 3K and 3L), expression of β-catΔC did not affect either the punctate pattern of bassoon labeling (Figures 5J and 5K) or its density per 10 µm neurite length (data not shown). Moreover, expression of β-catΔPDZ had no effect on the overall morphology of PSD-95-GFP clusters and their colocalization with synaptic markers such as bassoon and N-cadherin (data not shown). These data suggest that the interaction of cadherins with PDZ proteins selectively regulates synaptic localization of vesicles without affecting the assembly and localization of early pre- and postsynaptic components.
To examine possible transsynaptic effects of mutant β-catenin expression, neurons were transfected with either β-catΔC or β-catΔPDZ plus GFP to identify transfected cells and cultures immunostained using a synaptophysin antibody. Synaptophysin expression in wild-type neurons making contacts with postsynaptic neurons expressing mutant β-catenin was discrete and punctate similar to that seen in wild-type synapses (Figures 5L and 5M). Thus, alteration of β-catenin function in postsynaptic neurons does not perturb the presynaptic clustering and localization of vesicles.
To confirm the importance of the PDZ binding domain of β-catenin in vesicle localization, we coexpressed fulllength β-catenin, β-catΔN, or β-catΔPDZ together with cre in β-catenin flox neurons. Expression of full-length β-catenin and β-catΔN significantly rescued the diffuse vesicle phenotype seen following ablation of β-catenin, although the rescue was only partial in both cases, most probably due to the levels of protein expressed (Figures 6C, 6D, and 6F). In marked contrast, expression of β-catΔPDZ in cells lacking β-catenin had no effect on the delocalization of synaptophysin-GFP (Figures 6E and 6F).
Figure 6. Rescue of Synaptic Vesicle Phenotype in Neurons Lacking β-Catenin.
(A–E) Confocal images of hippocampal neurons cultured from homozygous β-catenin flox mice, transfected with synaptophysin-GFP, cre, and mutant β-catenin. Expression of full-length β-catenin and β-catΔN restores the punctate pattern of synaptophysin-GFP staining (C and D), reflected by the decreased average length of synaptophysin-GFP fluorescence along the axon compared to cells expressing cre alone (F). Expression of β-catΔPDZ does not affect synaptophysin-GFP expression following β-catenin excision (E), and the average length of synaptophysin-GFP fluorescence is similar to that in cells expressing cre alone (F). Scale bar, 10 µm.
PDZ Protein Function in Synaptic Localization of Vesicles
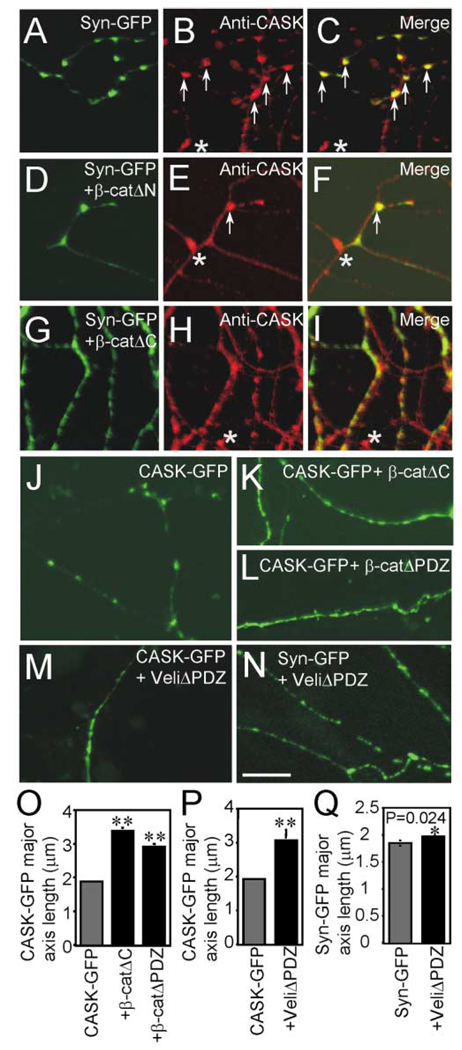
The data presented above suggest that β-catΔC and β-catΔPDZ perturb vesicle localization by preventing the recruitment of PDZ domain-containing proteins to cadherin clusters. To date, the PDZ binding domain of β-catenin has been shown to bind to only two PDZ proteins, MAGI-1 (also called S-SCAM) (Dobrosotskaya and James, 2000; Nishimura et al., 2002) and Veli, the mammalian homolog of the C. elegans protein Lin-7 (Perego et al., 2000). Since the role for S-SCAM in neurons has thus far been limited to postsynaptic sites (Hirao et al., 1998; Iida et al., 2002; Mok et al., 2002; Nishimura et al., 2002) and the phenotypes we observed were mainly presynaptic, we focused on Veli, which forms tight complexes with CASK and Mint1 (Butz et al., 1998) or with CASK and Caskin1 (Tabuchi et al., 2002). The expression pattern of CASK was examined using immunohistochemistry (Figures 7A–7I) or a CASK-GFP construct (Figures 7J–7M). Inhibition of the interaction between PDZ proteins and β-catenin by β-catAC and β-catΔPDZ resulted in a significant diffusion of CASK-GFP fluorescence (Figures 7K, 7L, and 7O). This was in marked contrast to CASK-GFP expression in the wild-type cells, which was punctate (Figure 7J) and localized appropriately to synapses, as seen bycolocalization with synaptophysin and bassoon (data not shown).
Figure 7. PDZ Protein Function in Synaptic Localization of Vesicles.
(A–N) Confocal images of rat hippocampal cultures. (A–I) Neurons transfected with synaptophysin-GFP alone (A) or cotransfected with β-catΔN (D) or β-catΔC (G) were immunostained with anti-CASK (B, E, and H). Synaptophysin-GFP and CASK were colocalized in cells expressing synaptophysin-GFP alone ([C], arrows) or coexpressing β-catΔN ([F], arrows). CASK immunoreactivity was more diffusely expressed in cells expressing β-catΔC (H and I). Asterisks indicate CASK-positive puncta on cells not transfected with synaptophysin-GFP. CASK-GFP expression is punctate in cells expressing CASK-GFP alone (J) but is more diffuse in cells coexpressing either β-catΔC (K) or β-catΔPDZ (L). Expression of VelitΔPDZ results in a dramatic diffusion of CASK-GFP (M) but not of synaptophysin-GFP (N).(O–Q) Diffusion of CASK-GFP or synaptophysin-GFP was quantified by measuring the average length of the major axis of fluorescent puncta. Student’s t tests were performed, and asterisks indicate a p value of <0.05. Scale bar, 10 µm.
β-catenin’s ability to recruit PDZ proteins coincides with its ability to cluster synaptic vesicles, suggesting that β-catenin functions to recruit PDZ domain-containing proteins such as Veli/CASK/Mint1or Veli/CASK/Caskin1 to cadherin clusters which may in turn “capture” or retain synaptic vesicles at discrete sites. To examine whether these complexes are directly involved in the clustering of synaptic vesicles, we perturbed the localization of these proteins at the synapse by expressing a mutant Veli construct that lacks the PDZ domain and cannot bind to β-catenin (Perego et al., 2000) but can still bind to CASK (Butz et al., 1998). Expression of VeliΔPDZ resulted in a 2-fold diffusion of CASK-GFP expression similar to that seen following expression of β-catΔC and β-catΔPDZ (Figures 7M and 7P). Despite this, coexpression of VeliΔPDZ with synaptophysin-GFP resulted in a minor (yet statistically significant) diffusion of synaptophysin-GFP. Importantly, this diffusion was significantly smaller than that seen in cells lacking β-catenin or expressing β-catΔC and β-catΔPDZ (Figures 7N and 7Q). Thus, there must be other binding partners of β-catenin that mediate its promotion of synaptic vesicle localization.
Discussion
While substantial evidence suggests that cell adhesion molecules are involved in synapse development and plasticity (Ranscht, 2000), their mechanisms of action at the synapse remain largely unknown. In this study, we have focused on the role of cadherins and catenins in synapse assembly. Previous work had implicated a role for cadherins in synapse development (Togashi et al., 2002); however, cadherins have been shown to associate with several different catenins, including β-catenin and members of the p120ctn family, and to activate several different signaling pathways. Thus the mechanism(s) through which cadherin binding regulates synapse formation has been unclear. We now show a role for β-catenin in presynaptic assembly independent of its role as a linker to the F-actin cytoskeleton.
We have perturbed β-catenin function in vivo and in vitro to modulate the function of all classic cadherins expressed in hippocampal neurons. We found that β-catenin is important for appropriate localization of the reserved pool of synaptic vesicles, which is necessary during sustained repetitive synaptic transmission. Further, we demonstrate that the PDZ binding domain of β-catenin plays a critical role in the mobilization and clustering of vesicles in presynaptic terminals. Finally, we show that although the PDZ binding domain of β-catenin is important for the localization of Veli and CASK, which are most likely associated with either Mint1 or Caskin1, to synapses, these complexes may not play a major role in vesicle localization. To our knowledge, this study reveals for the first time an important presynaptic function of β-catenin in controlling the size of the reserved vesicle pool in nerve terminals. By revealing the critical role of the PDZ binding domain of β-catenin, our findings provide insight into how the cadherin-catenin system functions during synaptogenesis.
Role of β-Catenin in the Localization of Undocked Vesicle Clusters
Functionally, synaptic vesicles in the nerve terminal can be divided into three interconnected pools: the readily releasable pool (RRP), the reserved pool, and the resting pool (Sudhof, 2000). Morphological analysis at the electron microscopy level, however, can only distinguish docked vesicles (those that are attached to presynaptic membranes) from undocked vesicles. Substantial evidence suggests that docked vesicles correspond to those in the RRP, while undocked vesicles are those in the reserved/resting pool. Electron microscopy of β-catenin mutant brains revealed a selective reduction of undocked vesicles by about 40% per synapse, with no change in the number of docked vesicles. These observations were further substantiated by electrophysiological recordings. Wild-type and mutant β-catenin synapses exhibited almost identical synaptic responses to a brief HFS, which is known to selectively deplete vesicles from the RRP. In contrast, mutant β-catenin synapses responded poorly to prolonged repetitive stimulation, which is designed to mobilize and deplete the reserved/resting pool. These results suggest that β-catenin functions to control the size of the reserved/resting pool of vesicles.
While in vivo studies are critical to reveal the physiological role of β-catenin in hippocampal synapses, in vitro approaches are better suited to investigate how β-catenin works mechanistically. To identify the localization of synaptic vesicles, we transfected cultured hippocampal neurons with synaptophysin-GFP. It should be noted that docked vesicles account for a very minor proportion of total vesicles in a presynaptic terminal; consequently, puncta size is indicative of the distribution of undocked vesicles. Using this technique, we provide some insights into the role of β-catenin during presynaptic development. First, we show that β-catenin regulates the localization/trafficking of vesicles to presynaptic sites. Indeed, synaptophysin-GFP is diffusely, yet unevenly, expressed along the axon in hippocampal neurons lacking β-catenin. Moreover, time-lapse imaging reveals that vesicle localization along the axon is much more dynamic in the absence of β-catenin due to a loss of instructive signaling that localizes vesicles to appropriate and discrete positions. Second, we show that β-catenin’s control of vesicle clustering is independent of the initial steps in the assembly of nascent presynaptic terminals (Zhai et al., 2001) and that bassoon and N-cadherin remain punctate in neurons lacking β-catenin. Finally, we show that β-catenin’s effects are cell-autonomous and that perturbation of β-catenin function postsynaptically does not affect the localization of synaptic vesicles presynaptically.
β-catenin is involved in (1) tethering cadherin to the actin cytoskeleton, (2) recruiting PDZ proteins, and (3) participating in wnt-induced transcription events. We show that recruitment of PDZ proteins to cadherin sites is the primary mechanism by which β-catenin regulates the reserved pool of synaptic vesicles. Indeed, expression of β-catΔPDZ results in the mislocalization of synaptophysin-GFP similar to that seen when β-catenin is eliminated. Further, expression of this construct in cells lacking β-catenin does not rescue the vesicle phenotype. PDZ proteins such as the Drosophila protein scribbled have been implicated in the regulation of synaptic vesicle localization. Interestingly, scrib mutants displayed an increased number of synaptic vesicles in the reserved vesicle pool (Roche et al., 2002) similar to that seen in Drosophila N-cadherin mutants (Iwai et al., 2002). The identity of the PDZ protein(s) that binds to β-catenin and specifically regulates vesicle localization is still not known; however, we show that Veli and the proteins that complex with it, such as CASK, Mint1, and Caskin1, are not essential mediators of this presynaptic function. Consistent with this, deletion of Mint1 in excitatory hippocampal neurons does not affect synaptic function, possibly due to the presence of Mint2 in these cells (Ho et al., 2003).
The cadherin-actin cytoskeleton link appears to be dispensable for vesicle clustering, as shown by punctate expression of synaptophysin-GFP in β-catΔN-expressing cells and the ability of this construct to rescue the vesicle phenotype in cells lacking β-catenin. This is consistent with a recent report showing that synaptic vesicle localization is normal in hippocampal neurons cultured from αN-catenin null mice (Togashi et al., 2002). Our data leaves open the possibility that β-catenin regulates the stability of F-actin at the presynaptic compartment (Colicos et al., 2001).
Finally, while wnt signaling has been shown to promote synaptic differentiation in the cerebellum (Hall et al., 2000), we show that this pathway is not involved in the presynaptic action of β-catenin in hippocampal neurons. Full length β-catenin as well as all mutant constructs used in the present study are able to promote wnt signaling (Funayama et al., 1995; Sehgal et al., 1997); however, expression of β-catΔC and β-catΔPDZ (but not full-length β-catenin or β-catΔN) results in the dispersion of synaptophysin-GFP signals.
The Role of β-Catenin in the Regulation of Synapse Numbe
Ablation of β-catenin in vivo resulted in a small but significant increase in the number of synapses as seen by electron microscopy. This increase was substantiated by input-output curves from mutant brain slices showing an increase in the synaptic response at a lower range of stimulation intensities. As the density of synapses in vitro was similar in wild-type and β-catenin mutant cells, we postulate that increases in the density of synapse seen in vivo may be due to compensatory changes brought about by decreases in synaptic efficacy. Indeed, previous studies have shown that decreased activation of mature hippocampal synapses results in an increase in the numberof spines to compensate for lost synaptic activity (Kirov and Harris, 1999). Alternatively, cultured hippocampal neurons may not have the microenvironment necessary to mediate the effect of β-catenin on synapse number.
Another interesting morphological effect of targeting β-catenin in the hippocampus was a 3-fold increase in the density of perforated or split synapses. An increase in split synapses is often seen under conditions mediating synaptic plasticity (Buchs and Muller, 1996; Jones et al., 1997; Lynch et al., 1994; Robinson and Kolb, 1997; Toni et al., 2001). Though controversial, it has been postulated that perforated synapses may be an intermediary step during synaptic plasticity, resulting in the generation of new synapses (Colicos et al., 2001; Sorra et al., 1998; Toni et al., 2001). Thus, the increase in perforated synapses may be a prelude to the increase in total synapse number.
Our results indicate that the main function of β-catenin in synapse development is to regulate the formation of the reserved/resting pool in the presynaptic compartment. We propose a model whereby interaction of cadherin molecules in trans results in localization of β-catenin at synaptic sites, setting up nucleation sites for the further recruitment of synaptic PDZ proteins and other scaffold proteins. Trafficking of early synaptic proteins such as bassoon and N-cadherin to the presynaptic membrane through large dense-core vesicles is β-catenin independent. Subsequent localization of PDZ proteins and synaptic vesicles at the synapse, however, is dependent on β-catenin. Thus, β-catenin may directly recruit synaptic vesicles to the synapse via its interaction with PDZ proteins or may act to retain vesicles at these sites following recruitment by other proteins, such as neurexins/neuroligins and synCAM. Indeed, the localization of cadherin/catenin complexes in sites flanking the active zones suggests that this complex may be involved in defining the borders of the synapse.
Experimental Procedures
Mouse Strains
To generate mice lacking β-catenin in excitatory neurons in the hippocampus, mice with two loxP-flanked β-catenin alleles (β-catenin flox/flox) were crossed with mice in which CamKII regulatory elements control cre recombinase expression (CamKcre). In all experiments, conditional mutants (β-catenin flox/flox; CamKcre/+) were compared to β-catenin flox/flox littermates.
To analyze the distribution of cre-mediated recombination, we generated mice heterozygous for both the CamKcre transgene and the R26R reporter.
For mouse hippocampal cultures, mice homozygous for the floxed β-catenin allele were bred and litters sacrificed at P0. Animal procedures were approved by the U.C.S.F. Committee on Animal Research.
Recombinant DNA
Full-length mouse β-catenin, β-catFL (gift from R. Kemler), was inserted into pCDNA3 at the BamHI site. β-catΔN (lacking amino acids 1–149), β-catΔC (lacking amino acids 687–781), β-catΔPDZ (lacking amino acids 771–781), and VeliΔPDZ (lacking amino acids) were generated using standard PCR.
The PSD-95-GFP and CASK-GFP constructs were gifts from D. Bredt; synaptophysin-GFP was a generous gift from T. Nakata.
Neuronal Cultures
Rat hippocampi from E18 fetal rats were prepared as previously described (Xie et al., 2000) and plated at a density of 130 cells/mm2. Neurons were transfected using the Qiagen Effectene transfection kit at 10DIV or as indicated.
Immunohistochemistry and X-Gal Staining
Adult males (P30-P60) were deeply anesthetized and perfused with PBS (pH 7.4), followed by 4% paraformaldehyde in PBS and a series of sucrose-PBS solutions (15%–30%). Whole brains were sliced into 50 µm thick coronal sections on a vibratome. Histochemical detection of β-galactosidase in adult coronal brain slices was performed as previously described (Xu et al., 2000). Immunohistochemistry on free-floating sections was performed as previously described (Rico et al., 2002). Neuron cultures were fixed in 4% paraformaldehyde/4% sucrose for 10 min, permeabilized in 0.1% Triton-X for 10 min, and then blocked in 10% goat serum for 1 hr at room temperature. Primary antibodies were applied in 1% goat serum overnight at 4°C, and secondary antibodies were applied in 1% goat serum for 1 hr at room temperature. Primary antibodies: mouse anti-β-catenin (Transduction Labs, Lexington, KY, and Zymed, San Francisco, CA), rabbit anti-β-catenin (Kypta et al., 1996), mouse anti-synapsin I (Chemicon, Temecula, CA), mouse anti-synaptophysin (Sigma, Saint Louis, MO), mouse anti-synaptotagmin 1 (Matthew et al., 1981), mouse anti-PSD-95 (ABR, Golden, CO), rabbit anti-NR2B (Upstate Biotechnology, Lake Placid, NY), mouse anti-CASK (Transduction Labs, Franklin Lakes, NJ), rabbit anti-N-cadherin (gift from D. Colman, Mount Sinai School of Medicine), mouse anti-Bassoon (Stressgen Biotechnologies, Victoria, British Columbia). Secondary antibodies: Alexa 488 and Texas red-conjugated goat anti-mouse or anti-rabbit (Molecular Probes, Eugene, OR). Littermates were processed in parallel in each experimental group (n = 3).
Electron Microscopy
Adult male mice were perfused with PBS (pH 7.4) followed by 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The heads were removed and stored overnight at 4°C. Following dissection and postfixation for 1 hr in 1 % osmium tetroxide, 0.1 M phosphate buffer, the hippocampi were dehydrated using a series of ethanol dilutions and were flat-embedded in an Epon-Araldite mixture. Ultrathin sections were cut and stained with uranyl acetate and lead citrate. The pyramidal cell layer was identified in ultrathin sections, and in each animal, the area representing five grids directly underlying the pyramidal cell layer was used for analyses. Ten electron micrographs from each grid were examined without knowledge of genotype. Spine synapses were identified by an electron dense region associated with at least five vesicles presynaptically and that lacked cellular organelles or contained a spine apparatus postsynaptically. Only vesicles within 550 nm from the presynaptic active were counted. Final magnification of micrographs, 50,000×.
Slice Preparation and Electrophysiology
Recordings from transverse hippocampal slices (400 µm) prepared from adult (P30-P60) mutant and control male mice were obtained as described previously (Pozzo-Miller et al., 1999).
Image Analysis and Quantification
Transfected hippocampal neurons were imaged using a Zeiss-Axiovert 200M deconvolution microscope (63×/1.4 Oil Plan-Apochromat) modified for SlideBook (3.0.10.5) software by Intelligent Imaging Innovations (Denver, CO). All images were captured with the same exposure time. The selected images were exported to Adobe Photoshop (Adobe Systems Inc, San Jose, CA) and/or NIH Image.
To quantify the localization of GFP fusion proteins, images were imported into NIH image where puncta were identified and analyzed at a threshold of 55 and minimum pixel size of 10. At least 500 puncta were measured per condition, and 7–20 cells from at least three separate cultures were analyzed.
To quantify the density of Bassoon puncta at least 400 µm total neurite length per condition from at least three separate cultures was analyzed.
For time-lapse analysis, transfected neurons were imaged every 30 s using a Zeiss LSM 5 Pascal microscope (63×/1.4 Oil Plan-Apochromat) and the corresponding LSM 5 Pascal Software.
Supplementary Material
Acknowledgments
We thank D. Rogers, B. Rico, and C. McBain for helpful comments on the manuscript; and D. Bredt, D. Colman, and T. Nakata for reagents. This work was supported by the Howard Hughes Medical Institute and a Human Frontiers Science Project Fellowship (to S.X.B.). J.H. and W.B. are supported by the Volkswagen-Stiftung.
References
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr. Opin. Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J. Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Buchs PA, Muller D. Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc. Natl. Acad. Sci. USA. 1996;93:8040–8045. doi: 10.1073/pnas.93.15.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz S, Okamoto M, Sudhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet N, Prieto M, Fabre C, Noren NK, Privat A. Distribution of p120 catenin during rat brain development: potential role in regulation of cadherin-mediated adhesion and actin cytoskeleton organization. Mol. Cell. Neurosci. 2003;22:467–486. doi: 10.1016/s1044-7431(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Colicos MA, Collins BE, Sailor MJ, Goda Y. Remodeling of synaptic actin induced by photoconductive stimulation. Cell. 2001;107:605–616. doi: 10.1016/s0092-8674(01)00579-7. [DOI] [PubMed] [Google Scholar]
- Coussen F, Normand E, Marchal C, Costet P, Choquet D, Lambert M, Mege RM, Mulle C. Recruitment of the kainate receptor subunit glutamate receptor 6 by cadherin/catenin complexes. J. Neurosci. 2002;22:6426–6436. doi: 10.1523/JNEUROSCI.22-15-06426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol. Cell. Biol. 1995;15:4819–4824. doi: 10.1128/mcb.15.9.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Eklof Spink K, Weis WI. beta-catenin: molecular plasticity and drug design. Trends Biochem. Sci. 2001;26:672–678. doi: 10.1016/s0968-0004(01)01952-1. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya IY, James GL. MAGI-1 interacts with beta-catenin and is associated with cell-cell adhesion structures. Biochem. Biophys. Res. Commun. 2000;270:903–909. doi: 10.1006/bbrc.2000.2471. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J. Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin M, Kovacs EM, Thoreson MA, Reynolds AB, Yap AS. Minimal mutation of the cytoplasmic tail inhibits the ability of E-cadherin to activate Rac but not phosphatidylinositol 3-kinase: direct evidence for a role for cadherin-activated Rac signaling in adhesion and contact formation. J. Biol. Chem. 2003;278:20533–20539. doi: 10.1074/jbc.M213171200. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Hirao K, Hata Y, Ide N, Takeuchi M, Irie M, Yao I, Deguchi M, Toyoda A, Sudhof TC, Takai Y. A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. J. Biol. Chem. 1998;273:21105–21110. doi: 10.1074/jbc.273.33.21105. [DOI] [PubMed] [Google Scholar]
- Ho A, Morishita W, Hammer RE, Malenka RC, Sudhof TC. A role for Mints in transmitter release: Mint 1 knockout mice exhibit impaired GABAergic synaptic transmission. Proc. Natl. Acad. Sci. USA. 2003;100:1409–1414. doi: 10.1073/pnas.252774899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo Y, Nakagawa S, Takeichi M. Blockade of cadherin-6B activity perturbs the distribution of PSD-95 family proteins in retinal neurones. Genes Cells. 2000;5:309–318. doi: 10.1046/j.1365-2443.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Iida J, Nishimura W, Yao I, Hata Y. Synaptic localization of membrane-associated guanylate kinase-interacting protein mediated by the pleckstrin homology domain. Eur. J. Neurosci. 2002;15:1493–1498. doi: 10.1046/j.1460-9568.2002.01987.x. [DOI] [PubMed] [Google Scholar]
- Ivanov DB, Philippova MP, Tkachuk VA. Structure and functions of classical cadherins. Biochemistry (Mosc.) 2001;66:1174–1186. doi: 10.1023/a:1012445316415. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Hirota Y, Ozaki K, Okano H, Takeichi M, Uemura T. DN-cadherin is required for spatial arrangement of nerve terminals and ultrastructural organization of synapses. Mol. Cell. Neurosci. 2002;19:375–388. doi: 10.1006/mcne.2001.1081. [DOI] [PubMed] [Google Scholar]
- Izawa I, Nishizawa M, Tomono Y, Ohtakara K, Takahashi T, Inagaki M. ERBIN associates with p0071, an armadillo protein, at cell-cell junctions of epithelial cells. Genes Cells. 2002;7:475–485. doi: 10.1046/j.1365-2443.2002.00533.x. [DOI] [PubMed] [Google Scholar]
- Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol. Learn. Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- Kawajiri A, Itoh N, Fukata M, Nakagawa M, Yamaga M, Iwamatsu A, Kaibuchi K. Identification of a novel beta-catenin-interacting protein. Biochem. Biophys. Res. Commun. 2000;273:712–717. doi: 10.1006/bbrc.2000.3002. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Harris KM. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nat. Neurosci. 1999;2:878–883. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complexto direct actin assembly in nascent adhesive contacts. Curr. Biol. 2002;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J. Biol. Chem. 1996;271:23363–23367. doi: 10.1074/jbc.271.38.23363. [DOI] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J. Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman AU, Jack JJ, Stratford KJ. Quantal analysis of excitatory synapses in rat hippocampal CA1 in vitro during low-frequency depression. J. Physiol. 1997;505:457–471. doi: 10.1111/j.1469-7793.1997.457bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laura RP, Witt AS, Held HA, Gerstner R, Deshayes K, Koehler MF, Kosik KS, Sidhu SS, Lasky LA. The Erbin PDZ domain binds with high affinity and specificity to the carboxyl termini of delta-catenin and ARVCF. J. Biol. Chem. 2002;277:12906–12914. doi: 10.1074/jbc.M200818200. [DOI] [PubMed] [Google Scholar]
- Lynch MA, Voss KL, Rodriguez J, Bliss TV. Increase in synaptic vesicle proteins accompanies long-term potentiation in the dentate gyrus. Neuroscience. 1994;60:1–5. doi: 10.1016/0306-4522(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- Manabe T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, Yamamoto M, Yoda H, Miyakawa T, Takeichi M, Chisaka O. Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol. Cell. Neurosci. 2000;15:534–546. doi: 10.1006/mcne.2000.0849. [DOI] [PubMed] [Google Scholar]
- Matthew WD, Tsavaler L, Reichardt LF. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J. Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs TE, Fields TA, McKee DD, Casey PJ. Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta-catenin release. Proc. Natl. Acad. Sci. USA. 2001;98:519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, Honda T, Kiyohara Y, Heo K, Higashi M, et al. Nectin: an adhesion molecule involved in formation of synapses. J. Cell Biol. 2002;156:555–565. doi: 10.1083/jcb.200103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok H, Shin H, Kim S, Lee JR, Yoon J, Kim E. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J. Neurosci. 2002;22:5253–5258. doi: 10.1523/JNEUROSCI.22-13-05253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montonen O, Aho M, Uitto J, Aho S. Tissue distribution and cell type-specific expression of p120ctn isoforms. J. Histochem. Cytochem. 2001;49:1487–1496. doi: 10.1177/002215540104901202. [DOI] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- Nishimura W, Yao I, Iida J, Tanaka N, Hata Y. Interaction of synaptic scaffolding molecule and Beta-catenin. J. Neurosci. 2002;22:757–765. doi: 10.1523/JNEUROSCI.22-03-00757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa M, Bundman MC, Greenberger V, Segal M. Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. J. Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J. Biol. Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Massari S, Longhi R, Pietrini G. Mammalian LIN-7 PDZ proteins associate with beta-catenin at the cell-cell junctions of epithelia and neurons. EMBO J. 2000;19:3978–3989. doi: 10.1093/emboj/19.15.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, et al. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol. Cell. Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Parada L, Bose CM, Landmesser LT. Alterations in transmission, vesicle dynamics, and transmitter release machinery at NCAM-deficient neuromuscular junctions. Neuron. 2001;32:815–828. doi: 10.1016/s0896-6273(01)00521-9. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J. Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B. Cadherins: molecular codes for axon guidance and synapse formation. Int. J. Dev. Neurosci. 2000;18:643–651. doi: 10.1016/s0736-5748(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol. Cell. Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat. Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche JP, Packard MC, Moeckel-Cole S, Budnik V. Regulation of synaptic plasticity and synaptic vesicle dynamics by the PDZ protein Scribble. J. Neurosci. 2002;22:6471–6479. doi: 10.1523/JNEUROSCI.22-15-06471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa C, Fukuda M, Mikoshiba K. Synaptotagmin V is targeted to dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells. J. Biol. Chem. 2002;277:24499–24505. doi: 10.1074/jbc.M202767200. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Formation of lamina-specific synaptic connections. Curr. Opin. Neurobiol. 1999;9:79–87. doi: 10.1016/s0959-4388(99)80010-5. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat. Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Sehgal RN, Gumbiner BM, Reichardt LF. Antagonism of cell adhesion by an alpha-catenin mutant, and of the Wnt-signaling pathway by alpha-catenin in Xenopus embryos. J. Cell Biol. 1997;139:1033–1046. doi: 10.1083/jcb.139.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T. An old friend in a new home: cadherins at the synapse. Trends Neurosci. 1997;20:322–323. doi: 10.1016/s0166-2236(97)01126-0. [DOI] [PubMed] [Google Scholar]
- Serafini T. Finding a partner in a crowd: neuronal diversity and synaptogenesis. Cell. 1999;98:133–136. doi: 10.1016/s0092-8674(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Shan W, Yoshida M, Wu XR, Huntley GW, Colman DR. Neural (N-) cadherin, a synaptic adhesion molecule, is induced in hippocampal mossy fiber axonal sprouts by seizure. J. Neurosci. Res. 2002;69:292–304. doi: 10.1002/jnr.10305. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N, Takeichi M, et al. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J. Cell Biol. 1995;128:949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized IacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Fiala JC, Harris KM. Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. J. Comp. Neurol. 1998;398:225–240. [PubMed] [Google Scholar]
- Staddon JM, Smales C, Schulze C, Esch FS, Rubin LL. p120, a p120-related protein (p100), and the cadherin/catenin complex. J. Cell Biol. 1995;130:369–381. doi: 10.1083/jcb.130.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS, McNutt PM. Cadherins and their connections: adhesion junctions have broader functions. Curr. Opin. Cell Biol. 1999;11:554–560. doi: 10.1016/s0955-0674(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle revisited. Neuron. 2000;28:317–320. doi: 10.1016/s0896-6273(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Biederer T, Butz S, Sudhof TC. CASK participates in alternative tripartite complexes in which Mint 1 competes for binding with caskin 1, a novel CASK-binding protein. J. Neurosci. 2002;22:4264–4273. doi: 10.1523/JNEUROSCI.22-11-04264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Shan W, Phillips GR, Arndt K, Bozdagi O, Shapiro L, Huntley GW, Benson DL, Colman DR. Molecular modification of N-cadherin in response to synaptic activity. Neuron. 2000;25:93–107. doi: 10.1016/s0896-6273(00)80874-0. [DOI] [PubMed] [Google Scholar]
- Tang L, Hung CP, Schuman EM. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J. Neurosci. 2001;21:6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J. Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Yang F, He XP, Je HS, Zhou JZ, Eckermann K, Kawamura D, Feng L, Shen L, Lu B. Regulation of neuromuscular synapse development by glial cell line-derived neurotrophic factor and neurturin. J. Biol. Chem. 2002;277:10614–10625. doi: 10.1074/jbc.M106116200. [DOI] [PubMed] [Google Scholar]
- Woodfield RJ, Hodgkin MN, Akhtar N, Morse MA, Fuller KJ, Saqib K, Thompson NT, Wakelam MJ. The p85 subunit of phosphoinositide 3-kinase is associated with beta-catenin in the cadherin-based adhesion complex. Biochem. J. 2001;360:335–344. doi: 10.1042/0264-6021:3600335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Markesbery WR, Lovell MA. Survival of hippocampal and cortical neurons in a mixture of MEM + and B27-supplemented neurobasal medium. Free Radic. Biol. Med. 2000;28:665–672. doi: 10.1016/s0891-5849(99)00268-3. [DOI] [PubMed] [Google Scholar]
- Xu Y, Guo DF, Davidson M, Inagami T, Carpenter G. Interaction of the adaptor protein Shc and the adhesion molecule cadherin. J. Biol. Chem. 1997;272:13463–13466. doi: 10.1074/jbc.272.21.13463. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J. Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J. Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu. Rev. Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.