Abstract
Humans rely on interaural level differences (ILDs) to determine the location of sound sources, particularly for high-frequency sounds. Previously, ILD-discrimination performance with a 4-kHz pure tone was reported to improve with multi-hour training. Here the effect of the same training regimen on ILD discrimination with a 4-kHz tone sinusoidally amplitude modulated (SAM) at 0.3 kHz was examined. Ten of the 16 trained listeners improved more than untrained controls, demonstrating training-induced learning. However, compared to the learning previously obtained with the 4-kHz pure tone, learning with the SAM tone was less predictable based on starting performance, took longer to complete, and was characterized by specificity to stimulus type (SAM vs pure tones) rather than stimulus frequency. These differences demonstrate an influence of amplitude modulation on learning of ILD discrimination. This influence suggests that the auditory system makes use of amplitude envelope information in determining ILD-discrimination performance, a form of interaction between time and level processing in the binaural system.
INTRODUCTION
Many auditory skills improve with practice even in adults (for review, see Watson, 1980; Irvine and Wright, 2005; Wright and Zhang, 2006, 2009), raising the possibility that training programs can be used to treat individuals with hearing disorders and to create new hearing expertise. To help establish the principles of auditory learning, we have been examining how basic auditory skills in normal-hearing adults improve with training and how the improvements generalize to untrained conditions. They also use these learning patterns to make inferences about the neural processes underlying the trained skills (also see Karni and Sagi, 1991; Ahissar and Hochstein, 2004). Of interest here is how listeners learn to better detect changes in two primary sound-localization cues, interaural level differences (ILDs) and interaural time differences (ITDs). Sensitivity to these cues allows us to register the source of a sound in space as well as to separate concurrent sounds from different sources.
Human sensitivity to ILDs and ITDs represents an exquisite example of neural computation by the central nervous system through which information that is not available at the sensory periphery is extracted (Hafter, 1984). ILDs arise because a sound is attenuated by the head and torso during its transmission so that the sound level at the ear closer to the source is larger than that at the ear farther from the source. The ILD magnitude depends on the stimulus frequency as well as the stimulus source location. Because at low frequencies the sound wave largely “bends around” the head and torso and therefore undergoes little attenuation, ILDs are considered to function best at high frequencies. ITDs are created because it takes the sound less time to travel to the closer ear than to the farther ear. The magnitude of the ITD is almost solely determined by the difference in the sound-transmission length to the two ears. For pure tones, ITDs can only serve as an effective cue to sound source location at low frequencies (<∼1.5 kHz). However, they are effective at high frequencies for sounds with low-frequency amplitude envelopes.
Though ILD and ITD sensitivities have been extensively studied with both physiological and psychophysical methods, examinations of the change in such sensitivity with experience have only recently begun (Wright and Fitzgerald, 2001; Rowan and Lutman, 2006, 2007; Zhang and Wright, 2007). We previously observed learning on ILD, but not ITD, discrimination in normal-hearing adults induced by multiple-day training, suggesting a type of modifiability that influences level but not timing sensitivity in the binaural system. In these tasks, listeners discriminated sounds that differed only in their ILD or ITD value. Following convention, we term the task ILD or ITD discrimination based on the cue manipulated (e.g., see Stern et al., 1983; Koehnke et al., 1986; Yost and Dye, 1988). In their initial investigation, we trained one group of listeners for multiple days on ILD discrimination with a high-frequency (4 kHz) pure tone and another group on ITD discrimination with a low-frequency (0.5 kHz) pure tone (Wright and Fitzgerald, 2001). Training-induced learning (defined as significantly more improvement in trained listeners than untrained controls) was observed for ILD but not for ITD discrimination, and the ILD learning did not generalize to the ITD condition. The we subsequently tested whether the distinct learning patterns observed in their initial investigation resulted from the difference between the stimulus frequencies (4 vs 0.5 kHz) by training another group of listeners, using the same regimen as before, on ITD discrimination with a 4-kHz tone sinusoidally amplitude modulated (SAM) at 0.3 kHz (Zhang and Wright, 2007). The SAM tone was used because humans are not sensitive to ITDs in pure tones in the high-frequency region. There was no training-induced improvement on this 4-kHz SAM ITD condition, similar to the training results for ITD discrimination with the 0.5-kHz pure tone but different from those for ILD discrimination with the 4-kHz pure tone. Thus, the learning pattern varies with the cue manipulated, instead of with the stimulus frequency region used (Zhang and Wright, 2007). Taken together, these results demonstrate that our multi-day training regimen can differentially modify the neural processes governing performance on interaural discrimination when different cues are manipulated.
Taking advantage of this demonstration, here we used the same training regimen to investigate whether the timing characteristics of the stimulus influence ILD processing in terms of its modifiability. Specifically, they asked whether amplitude modulating the trained stimulus results in a different learning pattern on ILD discrimination from that previously obtained with the 4-kHz pure tone (Wright and Fitzgerald, 2001). While the binaural system is best known for the microsecond level of time calculation shown by ITD sensitivity, impressive temporal fidelity in the range of a few milliseconds is also present in the neural circuits traditionally considered as the ILD pathway (for review, see Tollin, 2003). Tollin (2003) suggested that such high temporal fidelity in ILD processing allows temporal variations in the stimuli arriving at each ear to be accurately represented and compared in a time-locked manner over short time intervals. However, to date, behavioral demonstrations that the timing characteristics of the stimulus influence ILD processing are lacking. Here, we compared ILD processing, in terms of its modifiability, between two stimuli with different timing characteristics: a 4-kHz pure tone [data from Wright and Fitzgerald (2001)] and a 4-kHz SAM tone (the present experiment). Toward this end, they trained a new group of listeners on ILD discrimination with the SAM tone using the same training regimen as in the previous ILD-discrimination training experiment with the pure tone. The amplitude modulation (AM) rate of the SAM tone was 0.3 kHz (a period of 3.3 ms), a rate that likely taxes the temporal fidelity in ILD processing. Thus, ILD performance with this stimulus could potentially benefit from improved temporal processing. A difference in ILD-discrimination learning patterns between the SAM tone and the pure tone would indicate an influence of AM on the neural processes governing ILD-discrimination performance.
MATERIALS AND METHODS
Listeners
Thirty-two normal-hearing human adults (19 women) between the ages of 18 and 36 years (average of 22.5 years) participated in the experiment. All were paid for their participation. None of the listeners had previous experience in any psychoacoustic experiment.
Experimental organization
The experiment consisted of a pretest session, nine ∼1-h training sessions, and a post-test session conducted on consecutive days except weekends. Half of the listeners (n=16), referred to as the trained listeners, participated in all of the sessions. The other half (n=16), referred to as controls, only participated in the pre- and post-test sessions. In each training session, listeners obtained 12 threshold estimates (see Sec. 2D) on a single condition, referred to as the trained condition. In the pre- and post-tests, they obtained five threshold estimates on each of six conditions, one trained and five related untrained conditions. The condition order was randomized across listeners but fixed for each listener between the pre- and post-tests.
Task and conditions
For all stimulus conditions, the listeners were asked to discriminate between two sounds, presented through headphones, that differed only in their ILD or ITD value. Discrimination ability was measured in a two-interval-forced-choice procedure. In each trial, stimuli were presented in two visually marked 300-ms observation intervals that were separated by a 660-ms silent period. In one interval randomly chosen on each trial, a standard stimulus was presented. In the other interval, a signal stimulus was presented that differed from the standard stimulus only by a variable ΔILD or ΔITD that always favored the right ear (Wright and Fitzgerald, 2001; Zhang and Wright, 2007). The listeners reported which interval they perceived as containing the signal stimulus by pressing a key on a computer keyboard. Visual feedback was provided after each response throughout the entire experiment. Before starting each condition in the pretest, listeners were presented with samples of the standard and signal stimuli that, as they reported verbally or by pointing, produced distinct lateral positions. These samples were also provided before each 60-trial block throughout the experiment.
Here, we report data from seven stimulus conditions, one trained and six untrained. The trained condition, ILD discrimination with a 4-kHz tone SAM at 0.3 kHz and a 0-dB standard ILD, was included in the pre- and post-tests of all listeners. Each of the six untrained conditions included in this report was tested in only half of the listeners (eight trained listeners and eight controls per condition). These untrained conditions differed from the trained one either only in the standard ILD (6 vs 0 dB), the carrier frequency (6 vs 4 kHz), the modulation rate (0.15 vs 0.3 kHz), the stimulus type [pure tone (4 or 0.3 kHz) vs SAM tone], or the cue manipulated (ITD vs ILD). Four other untrained conditions were tested for a purpose unrelated to the current experiment and thus are not described here.
Procedure
From each 60-trial block, a discrimination threshold for ILD or ITD was estimated using a 3-down, 1-up adaptive procedure. The ΔILD or ΔITD value was decreased after every three consecutive correct responses and increased after each incorrect response (Levitt, 1971). The signal values at which the direction of change switched from decreasing to increasing or from increasing to decreasing were denoted as reversals. When there were seven or more reversals within a block, we discarded the first three reversals (if the total number of reversals was odd) or four reversals (if the total number of reversals was even) and averaged the remaining reversals to estimate the ΔILD or ΔITD value required for 79% correct responses (threshold). When there were fewer than seven reversals, performance on that block was marked as “insufficient reversals.” In the ILD conditions, the starting value of the ΔILD was 6 dB, and the step size was 0.5 dB until the third reversal and 0.25 dB thereafter. In the ITD condition, the starting value of ΔITD was 1 μs, forcing the listeners to guess on the first trial (see also Wright and Fitzgerald, 2001; Zhang and Wright, 2007). The step size was multiplications or divisions by 100.2 until the third reversal and by 100.05 thereafter (Saberi, 1995). The minimum value was 0 dB for ΔILD and 1 μs for ΔITD. The maximum value was 650 μs for ΔITD, approximately the maximum naturally occurring time delay between the two ears in humans (e.g., Feddersen et al., 1957; Kuhn and Guernsey, 1983). There was no maximum for ΔILD. We chose these parameters for the adaptive algorithms to be consistent with their previous experiments on interaural discrimination learning (Wright and Fitzgerald, 2001; Zhang and Wright, 2007). The differences in the parameters between the ILD and ITD conditions do not appear to influence the effectiveness of threshold estimation (Zhang and Wright, 2007).
Consistent with our previous experiment (Zhang and Wright, 2007) for the ITD-discrimination condition with the trained SAM tone, a substantial proportion (36%) of blocks failed to yield valid threshold estimates. Because omitting these blocks can lead to underestimation of ITD discrimination thresholds (Zhang and Wright, 2007), we reevaluated performance in the invalid blocks following the approach used in that report. Briefly, for tracks that yielded fewer than seven reversals (and hence were marked as insufficient reversals), they estimated thresholds based on the last four reversals when there were six total reversals and excluded the tracks yielding fewer than six reversals in total. When a track yielded a threshold estimate greater than 650 μs by calling for nominal ΔITD values exceeding 650 μs, they replaced the estimate with 650 μs.
Stimulus generation
The SAM tones were synthesized by sinusoidally modulating the amplitude of a sinusoidal carrier to 100% depth. In all conditions, the stimuli to both ears started and ended simultaneously, and each stimulus had a total duration of 300 ms, including 10-ms rise∕fall cosine ramps. For pure tones, the starting phase of the right-ear stimulus was randomized across intervals. For SAM tones, the starting phases of both the carrier and modulation waveforms to the right ear were randomized across intervals and were independent of each other. The nominal stimulus level was 50 dB SPL both for the pure tones and for SAM tones before modulation. This level was low enough to avoid the influence of combination products in the SAM-tone stimuli (Plomp, 1965). In the ILD conditions, the sound level was 50 dB SPL minus 0.5 times the desired ILD for the left-ear stimulus, and 50 dB SPL plus 0.5 times the desired ILD for the right-ear stimulus. There was no time or phase difference between the two ears. In the ITD condition, the desired ongoing ITD was set by delaying the starting phases of both the carrier and the modulator of the left-ear stimulus relative to those of the right-ear stimulus. There was no level difference between the two ears.
We used a digital-signal processing board (Tucker-Davis Technologies in Gainesville, Florida, AP2) to generate all stimuli. The stimuli to each ear were then delivered through separate 16-bit digital-to-analog converters (TDT DD1), anti-aliasing filters (8.5-kHz low-pass, TDT FT5), and programmable attenuators (TDT PA4). Finally, the stimuli were sent through a headphone buffer (TDT HB6) to headphones with circumaural cushions (Sennheiser, HD265). Listeners were seated in a double-walled sound-attenuating booth.
RESULTS
Learning during the training sessions
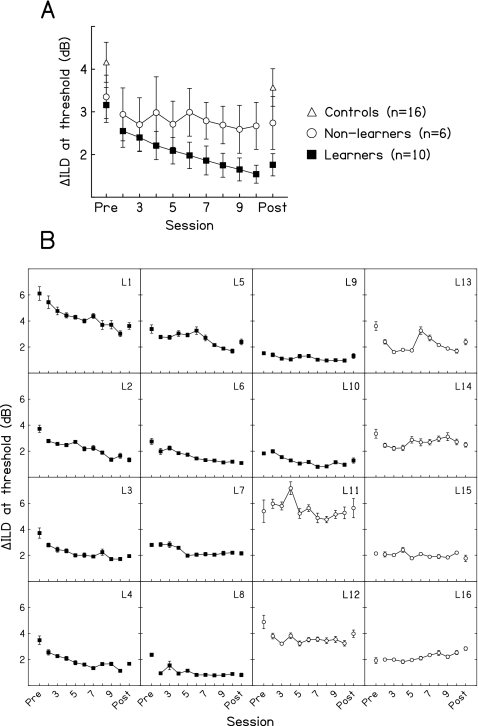
Through multi-hour training, performance on the trained condition (ILD discrimination with a 4-kHz carrier SAM at 0.3 kHz and a 0-dB standard ILD) improved significantly in the majority of the trained listeners (Fig. 1). As a group, the trained listeners showed a significant decrease in their daily mean thresholds across the nine training sessions (not shown), as indicated by a repeated-measures one-way analysis of variance (ANOVA) (p<0.001) and a negative slope of a regression line fitted over training sessions (slope=−0.123 dB∕session, p=0.004). However, the effect of training varied markedly across individual listeners. We identified an individual listener as having learned across training sessions only if that listener’s performance met all of the following three criteria: (1) a significant one-way ANOVA on thresholds across training days without repeated measures, (2) a significant linear regression of thresholds fitted over training days, and (3) a negative slope of the regression. Alpha was set at 0.05 in all of the analyses. Using these criteria, the 16 trained listeners fell into two groups according to their performance during training. Ten out of the 16 listeners improved through training and are referred to as learners [Fig. 1b, L1–L10, ANOVA: all p≤0.012, regression: all p≤0.007 and all slopes<0 dB∕session]. The other six listeners did not meet the criteria and are referred to as non-learners [Fig. 1b, L11–L16]. Among the six non-learners, one failed all three criteria (all p values≥0.20, slope=0.785 dB∕session), one failed both the criteria for ANOVA and for significant regression (all p values>0.24), three did not show a significant regression (all p values≥0.38), and one did not have a significant ANOVA (p=0.23). The mean thresholds of the learners decreased steadily across all training sessions [Fig. 1a, filled squares, regression: slope=−0.117 dB∕session, p=0.007], while those of the non-learners remained at approximately the same level throughout training (open circles, regression: slope=−0.009 dB∕session, p=0.894). Interestingly, the learners and non-learners had similar pretest thresholds (t test, p=0.777), so whether or not a listener would improve through training could not be predicted from starting performance. Because of their distinct patterns of behavior during the training sessions, we conducted the following analyses separately for the learners and non-learners (also see Wright et al., 1997; Karmarkar and Buonomano, 2003).
Figure 1.
Performance on the trained condition. (A) The mean ILD-discrimination thresholds for the 10 learners (filled squares), 6 non-learners (open circles), and 16 controls (open triangles) from the pretest, training sessions, and post-test. (B) Individual data for the ten learners (filled squares) and six non-learners (open circles). Error bars represent ±1 standard error (A) across or (B) within listeners.
Learning between the pre- and post-tests
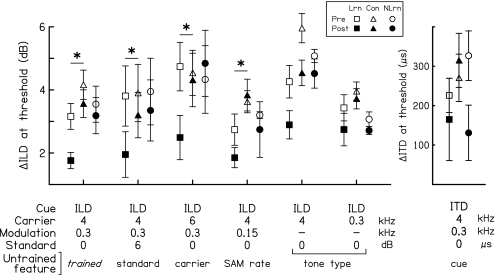
On the trained condition, the learners improved significantly more than the controls between the pre- and post-tests (Fig. 2, the condition on the far left). The thresholds of the controls (triangles) decreased significantly between the pre-(open symbols) and post-tests (filled symbols), as revealed by a paired t test (p=0.013). However, the learners (squares) showed an even greater improvement, as indicated by a significant group by session interaction in a two group (learners vs controls) by two session (pretest vs post-test) ANOVA (p=0.022). Confirming this result, the pretest thresholds of the two groups did not differ significantly (independent t test, p=0.151), but the post-test thresholds were significantly lower in the learners than in the controls (independent t test, p=0.006). It is worth noting that, though not significantly different, the average pretest threshold of the learners (3.16 dB) was 1 dB lower than that of the controls (4.16 dB). Group differences in starting performance have been observed frequently in perceptual learning investigations without consistent patterns or clearly identifiable causes (e.g., trained listeners started worse than controls: Wright and Fitzgerald, 2001; Fitzgerald and Wright, 2005; the opposite pattern: Mossbridge et al., 2006). In the present case, the lower starting thresholds in the learners than in the controls actually strengthens the claimed effect of training because lower starting thresholds are associated with smaller training-induced improvements on this task (see below).
Figure 2.
Pre- and post-test performance on the trained and untrained conditions. The mean discrimination thresholds of the learners (squares), the non-learners (circles), and the controls (triangles) for both the pretest (open symbols) and post-test (filled symbols) in the six ILD conditions (left panel) and one ITD condition (right panel). The error bars represent ±1 standard error across listeners. Conditions are denoted on the abscissa by the interaural cue manipulated, the carrier frequency, the modulation rate, and the standard cue value. The trained condition is at the far left. For each untrained condition, the untrained feature is marked. Asterisks indicate that there was a significant difference in the amount of improvement from the pretest to the post-test between the learners and controls [p<0.05 for the group by time interaction in a two group (learners vs controls) by two time (pretest vs posttest) ANOVA].
The learners also improved more than the controls on the untrained ILD-discrimination conditions with SAM stimuli but not on those with pure-tone stimuli or on the untrained ITD-discrimination condition. The controls (Fig. 2, triangles) showed significant learning between the pre-(open symbols) and post-tests (filled symbols) only on two of the six untrained conditions: the 6-dB standard ILD and the 4-kHz pure-tone conditions (paired t tests, p≤0.038; for other conditions, p≥0.3). Compared to the controls, the learners (squares) improved significantly more on all of the untrained ILD SAM conditions tested (Fig. 3, second to fourth condition from the left), including those with the untrained standard ILD (6 dB; ANOVA group by session interaction: p=0.024), the untrained carrier (6 kHz; p<0.001), and the untrained modulation rate (0.15 kHz; p=0.086, but p=0.039 according to an analysis of covariance that took into account the difference in pretest threshold between the two groups). For the remaining three untrained conditions (right three conditions), there was no difference between the learners and controls (all p values≥0.304).
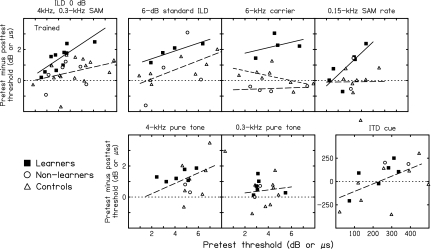
Figure 3.
The relationship between the pretest threshold and the amount of improvement. Pretest thresholds (abscissa) and pretest minus post-test thresholds (ordinate) for individual learners (filled squares), non-learners (open circles), and controls (open triangles) are shown for each of the seven tested conditions (panels). On each condition, the number of the regression lines fitted is based upon the results of between-group comparisons (see text): one line (all listeners, dashed lines), two lines (learners, solid lines; controls and non-learners, dashed lines), and three lines (learners, solid lines; non-learners, dashed lines; controls, short-dashed lines). The dotted lines represent zero improvement.
Unlike the learners, the non-learners (Fig. 2, circles) did not improve more than controls (triangles) between the pre-(open symbols) and post-tests (filled symbols) on any condition, trained or untrained (p=0.038 for the ILD 6-kHz carrier condition, but with more improvement in the controls than non-learners; p≥0.202 for the other conditions). Note, however, that on four out of the six untrained conditions, the analyses were based on only two non-learners and thus should be viewed as tentative. For example, the two non-learners both happened to improve (though neither significantly, independent-sample t tests, p≥0.25) between the pre- and post-tests on the ITD condition (Fig. 3, bottom right panel, circles) and appeared to show a trend of learning more than controls. However, considering the marked variability in the improvement of controls (Fig. 3, bottom right panel, triangles), the sample size of the non-learners was too small to support any specific conclusion.
Finally, the relationship between the amount of learning and pretest threshold varied across conditions, as well as across different listener groups. To examine the influence of pretest threshold on the amount of learning, we fitted regression lines to the amount of threshold improvement between the pre- and post-tests over the pretest threshold across individual listeners (Fig. 3). Based on the between-group comparisons reported above, on each condition, a separate line was fitted to each statistically different listener group. For the trained condition (Fig. 3, top left panel), because the statistical analyses revealed no difference between the non-learners (open circles) and controls (open triangles) but a difference between the learners (filled squares) and controls, we fitted a separate regression line for learners and one common line for the other two groups. The slope of the regression line for the learners was significantly different from zero (slope: 0.48; p=0.006; solid line), indicating that the magnitude of the improvement increased with increasing pretest threshold. In contrast, the slope of the regression line for controls and non-learners did not differ significantly from zero (p=0.104; dashed line), indicating that the amount of improvement was approximately independent of the pretest value. For the untrained conditions, the magnitude of improvement tended to increase with increasing pretest threshold in general (p≤0.085), except in the cases of the 6-kHz carrier condition, the 0.3-kHz pure-tone condition, and the controls and non-learners in the 0.15-kHz SAM rate condition (p≥0.168).
DISCUSSION
The primary purpose of the present experiment was to examine the extent to which AM influences ILD-discrimination learning. Here, we compare the current ILD-discrimination training results obtained with the SAM-tone (4-kHz carrier, 0.3-kHz AM) to those previously obtained with a pure tone (4 kHz) using the same training regimen (Wright and Fitzgerald, 2001) and discuss the implications of the differences between these results in terms of the neural processes governing ILD-discrimination ability. Before doing so, we first briefly discuss the lack of learning in a subset of the trained listeners (non-learners).
Non-learners
In the current experiment, a considerable portion of the trained listeners (6 out of 16, or 37%) failed to improve across training sessions. The lack of learning in a subset of trained listeners is not unusual. Large individual differences in improvement magnitude have been frequently observed in perceptual learning (e.g., Nagarajan et al., 1998; Irvine et al., 2000; Delhommeau et al., 2002). In the auditory-learning investigations in which whether or not an individual listener improved over training was determined, the probability of learning across individual listeners varies considerably across tasks [e.g., 0%–50% for ITD discrimination (Wright and Fitzgerald, 2001; Rowan and Lutman, 2007; Zhang and Wright, 2007), 42%–79% for temporal-interval discrimination (Wright et al., 1997; Karmarkar and Buonomano, 2003), 67%–86% for temporal-order discrimination (Mossbridge et al., 2006; Mossbridge et al., 2008), and 100% for SAM rate discrimination (Fitzgerald and Wright, 2005)] as well as across stimulus conditions for the same task (e.g., Karmarkar and Buonomano, 2003). To date, there is no systematic investigation into the cause of such individual differences in the ability to improve perceptually. In most cases, the listeners who failed to learn tended to have good starting performance, suggesting a ceiling effect for learning. However, in the current experiment, a ceiling effect could not explain the lack of learning in the non-learners because whether or not a listener improved through training could not be predicted from the starting performance. This homogeneity of starting performance of the learners and the non-learners also suggests that the non-learners did not fail to learn due to general factors that are likely to result in systematic changes in performance, such as an inability to maintain attention, confusion about the task, or the use of different strategies to solve the task (also see Sec. 4B2 for further discussion). An absence of learning that could not be predicted from starting performance can also be seen on an auditory temporal-interval discrimination task in the individual-learning figure [Karmarkar and Buonomano, 2003, Fig. 1b]. Future experiments are needed to determine whether the lack of learning in individuals who have room to improve results from a general inability to improve perceptually or from task-dependent defects in cognitive or perceptual plasticity.
Differences between ILD-discrimination learning with pure and SAM tones
Learning patterns
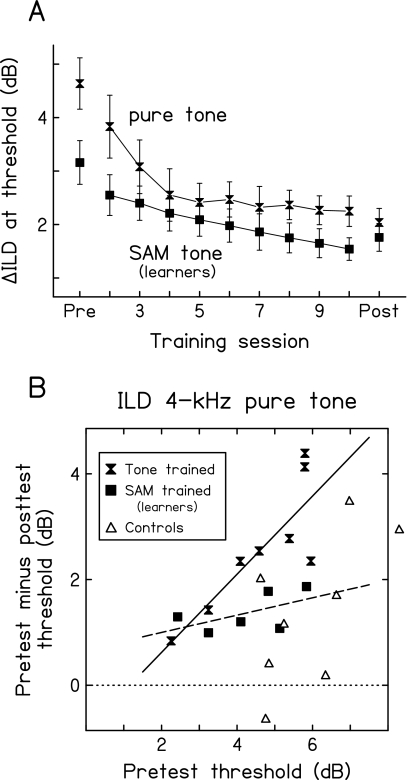
While multi-hour training yielded significant improvement on ILD discrimination both with pure and SAM tones, the results differed between the two cases in the predictability, rate, and generalization pattern of that learning. First, whether or not a listener learned could be predicted from the starting performance when ILD discrimination was trained with the pure tone, but not with the SAM tone. With the pure tone, two out of eight trained listeners did not improve. These two listeners had starting thresholds among the best. With the SAM tone, however, as mentioned above, the six non-learners could not be distinguished from the learners based on pretest thresholds (Fig. 3, top left panel, open circles). Second, in those who learned, the rate of learning appeared to be slower with the SAM tone than with the pure tone, based on a two group (SAM trained vs pure-tone trained) by nine training session ANOVA (group by session interaction: p=0.049). With the pure tone [Fig. 4a, hourglasses, data from Wright and Fitzgerald, 2001], performance improved rapidly in the first few sessions and leveled off in the following sessions, while with the SAM tone (squares), performance continued to improve at a constant rate throughout all of the nine sessions. Third, ILD-discrimination learning generalized across stimulus frequencies following training with the SAM tone, but not with the pure tone. With the pure tone, learning did not generalize from the trained 4-kHz tone to untrained tones at 6 or 0.5 kHz, while with the SAM tone, learning generalized from the trained 4-kHz carrier to the untrained 6-kHz carrier.
Figure 4.
Comparison between the current data and those previously obtained for ILD-discrimination training with a 4-kHz tone (Wright and Fitzgerald, 2001). (A) The mean ILD-discrimination thresholds across the test and training sessions for the present learners (n=6 of 8) trained with a 4-kHz tone SAM at 0.3 kHz (SAM tone, filled squares) and the previous listeners (n=8) trained with a 4-kHz tone (pure tone, filled hourglasses). (B) The amount of improvement (ordinate) on the ILD 4-kHz pure-tone condition plotted against the pretest threshold (abscissa) for each of the present SAM-trained learners (filled squares, n=6 of 8), the present controls (open triangles, n=8), and the previous listeners trained with the 4-kHz tone (filled triangles, n=8). Regression lines were fitted separately for the present learners (dashed line) and the previous listeners (solid line). Zero improvement is denoted by the dotted line.
Another interesting difference between ILD-discrimination learning with the pure and SAM tones was that learning in one case (the SAM tone) did not generalize to the other (the pure tone). In the present experiment, the SAM-trained learners did not improve more than controls on ILD discrimination with untrained pure tones, either at the trained carrier frequency (4 kHz) or at the trained modulation rate (0.3 kHz), suggesting a failure of generalization that is attributable to stimulus type (SAM vs pure tone) rather than stimulus frequency. Strengthening this conclusion, for the ILD 4-kHz pure-tone condition, these learners, just like controls, improved significantly less than the listeners trained with the 4-kHz pure tone itself [Fig. 4b, bottom panel; pure-tone trained data from Wright and Fitzgerald, 2001], according to a two group (SAM trained vs pure-tone trained) by two session (pretest vs posttest) ANOVA (group by session interaction, p=0.034). Thus, the current learners did not fail to generalize their learning to the pure tone at the trained carrier frequency because they had no room to improve.
These different learning and generalization patterns for ILD discrimination with the SAM and the pure tones suggest that the current multi-hour training regimen differentially influenced the neural system in the two cases. First, the neural processes affected by training with the SAM tone and the pure tone appear to have different characteristics. In the pure-tone case, the affected neural process seems to influence perception of different ILD values in a frequency-specific manner and to be readily modifiable unless its output is already near optimal. In the SAM-tone case, the modified neural process seems to influence ILD-discrimination performance in a stimulus-type-specific manner, responding to amplitude modulated tones regardless of location, carrier frequency, and modulation rate but not to pure tones even at the same frequency as the trained SAM tone. These different characteristics could result from either different neural circuitries being modified or from different modifications in the same neural circuitry. Further, the differences in the predictability and time course of learning observed with the two stimulus types also suggest different characteristics of the modifications themselves. With the pure tone, the modification took place readily with training, improving ILD sensitivity except in listeners with the best starting performance, and was completed relatively rapidly within a few training sessions. In contrast, with the SAM tone, the modification occurred with a probability of approximately 60%, independent of the initial performance, and proceeded at a nearly constant rate for more than ten days. These different characteristics of modifications hint at different types of neural changes resulting from training with the SAM tone and the pure tone.
Note that the characteristics described do not specify the physiological loci of the neural modifications incurred by training. Specificity of perceptual learning to stimulus features has often been taken as evidence that modifications occur in the early stages of sensory processing (Karni and Sagi, 1991; Poggio et al., 1992; Ahissar and Hochstein, 2004; Fahle, 2004). Supporting this idea, neural changes accompanying learning have been identified in primary sensory cortices (Furmanski et al., 2004; Clapp et al., 2005; Li et al., 2008; Pourtois et al., 2008). However, it has also been proposed that changes in later stages of neural processing could also result in perceptual improvements that are specific to certain stimulus features through the reweighting of sensory information from different channels based on the task demand (Mollon and Danilova, 1996). Indeed, neural changes during perceptual learning have also been identified beyond the sensory cortices, including the associative (Law and Gold, 2008) and frontal (Krigolson et al., 2009) cortices. Uniting the two lines of thought, there is increasing evidence that perceptual learning involves synergistic, dynamic responses of multiple systems. For example, perceptual training has been reported to induce changes spanning sensory, motor, associative, and cognitive systems (Vaina et al., 1998; Schiltz et al., 2001; Sigman et al., 2005; van Wassenhove and Nagarajan, 2007). Further, the neural modifications are dynamic in that different sites can be affected at different time points in training (Karni et al., 1998; Petersen et al., 1998; Atienza et al., 2002; Gottselig et al., 2004) and changes at some loci can be reversed when learning is complete (Vaina et al., 1998; Yotsumoto et al., 2008).
Possible explanations
The differences in ILD-discrimination learning observed in the pure- and SAM-tone experiments appear to be attributable to the differences in the amplitude envelope shape, rather than in the pitch, overall stimulus level, or incongruence between the two cues in these two stimulus types. Though the pure and SAM tones used in training differed in perceived pitch, this difference does not appear to account for the different ILD-discrimination learning patterns with these two stimulus types. The pitch of a pure tone corresponds well with the stimulus frequency, while that for a SAM tone is close to the modulation rate and changes little with the carrier frequency (for an overview, see Moore, 1997). Therefore, the two trained stimuli used in the present and previous ILD-discrimination training experiments, though they shared the same central frequency (4 kHz), differed widely in pitch (4 kHz for the pure tone and ∼0.3 kHz for the SAM tone). Thus, the different learning patterns for the pure and SAM tones might be attributed to the differences in their pitches. In the pure-tone experiment, the frequency specificity of ILD-discrimination learning can be readily translated into pitch specificity. However, in the SAM-tone experiment, learning was generalized to both an untrained carrier frequency (with a pitch similar to the trained one) and an untrained SAM rate (and hence an untrained pitch), but not to pure tones at either the trained carrier frequency (with an untrained pitch) or the trained SAM rate (with a pitch similar to the trained one). Thus, learning patterns differed between the two stimulus types even when the pitches were taken into consideration.
Similarly, it appears that the different learning patterns did not result from the different stimulus levels that were used in the pure- and SAM-tone experiments. The nominal stimulus level was 70 dB SPL (sound pressure level) in the previous pure-tone ILD training experiment (Wright and Fitzgerald, 2001) but was only 50 dB SPL in the present SAM-ILD training experiment (to avoid combination products, see Sec. 2). This difference conceivably could result in different patterns of modification by the same training paradigm because overall stimulus level has been reported to influence the responses of ILD sensitive neurons along the ascending ILD pathway (Semple and Kitzes, 1987; Irvine and Gago, 1990; Irvine et al., 1996; Park et al., 2004). However, this account cannot readily explain the lack of generalization from the trained SAM tone to the pure tones in the current experiment, in which all of the stimuli were presented at the same level.
Another difference between the two stimulus types, the potential conflict between the ILD and ITD cues, also seems unlikely to be the cause of the different learning patterns observed in the two experiments. In natural listening environments, ILD and ITD values co-vary with the sound source location and thus are congruent with each other. However, in the ILD- and ITD-discrimination tasks that have been used to separately investigate ILD and ITD sensitivity, the ILD and ITD values are manipulated independently, creating unnatural situations in which the two cues may not indicate the same sound source location. While there was cue incongruence in the ILD-discrimination training with both the SAM and the pure tones, the influence of this incongruence on performance may have differed due to the difference in human sensitivity to ITDs in the two types of stimuli. For the 4-kHz pure tone used in the previous training experiment, cue incongruence was unlikely to have influenced performance because listeners are not sensitive to ITDs in high-frequency pure tones and therefore might have solved the task using only the ILD cue. Thus, the performance improvement may faithfully reflect improved ILD sensitivity. In contrast, for the 4-kHz SAM tone in the current experiment, in which the perceived sound position varies with both ITDs and ILDs, incongruence between the two cues might have caused the perceived sound image to be diffused or sometimes even split (e.g., Hafter and Jeffress, 1968). Thus, multiple strategies of solving the task might have been available to the listeners during training with the SAM tone and consequently could have influenced the learning pattern. For example, the non-learners might have been prevented from learning by confusion caused by the different locations indicated by the two cues, and the learners, instead of having learned the ILD cue itself, might have learned to ignore the ITD cue or even to use image diffuseness to solve the task. If so, the performance improvement with the SAM tone would reflect an improved ability of the neural system to exclude conflicting information or to make use of the diffuseness of the sound image rather than reflect better ILD sensitivity. However, the our previous training on ITD discrimination with the same SAM tone, which presumably presented the same level of cue conflict, yielded no learning in any of the nine trained listeners (Zhang and Wright, 2007). Thus, the current learning pattern for ILD discrimination with the SAM tone differs both from that of ILD discrimination with a pure tone at the same frequency (a stimulus for which listeners are sensitive only to ILDs and hence are unlikely to be influenced by cue incongruence) and from that of ITD discrimination with the same SAM tone (a stimulus for which listeners are sensitive to both cues and hence are likely subject to possible influences of cue incongruence). This observation suggests that the effect of the current training regimen varies both with the cue manipulated (ILD vs ITD) and with the stimulus type employed (SAM vs pure tone), instead of with the presence or absence of conflicting cues.
We instead suggest that the different learning patterns of ILD discrimination with pure and SAM tones reflect the influence of AM on ILD sensitivity. While it is possible to explain these different patterns by assuming that there are separate channels of ILD processing for pure tones and AM sounds, we do not favor this view for two reasons. First, to date, they are aware of no report of ILD sensitive neurons that are activated by AM stimuli but not by pure tones, or vice versa. Second, because most naturally occurring sounds are amplitude modulated, it seems unlikely that separate neural resources should be dedicated to pure tones. Rather, we propose that the temporal fluctuations in the amplitude envelope of high-frequency stimuli play an active role in ILD processing. Current ILD-processing models typically imply that these fluctuations are smoothed out and have little influence on ILD sensitivity (e.g., the level-meter model by Hartmann and Constan, 2002). However, this assumption has not been thoroughly tested, leaving open the possibility that envelope fluctuations do influence ILD encoding. Given this possibility, the improvements on ILD discrimination in the SAM-tone experiment may have resulted from modifications in the extraction of the amplitude envelope itself and∕or in the transmission of the extracted envelope to ILD encoding. This proposal appears feasible based on neurophysiological data. In many high-frequency, ILD-responsive neurons in the brainstem, fluctuations in the stimulus amplitude envelope are faithfully preserved, or even enhanced (Joris and Yin, 1995), and the brainstem nuclei that are typically thought to play a crucial role in ILD processing are also regarded as contributing to AM processing (Brugge et al., 1993; Joris et al., 2004). In contrast, we suggest that the improvements in the pure-tone experiment resulted from modifications in processes unrelated to AM processing. Supporting this idea, the specificity of the pure-tone learning to the stimulus frequency but not to the standard ILD value is consistent with reports that ILD sensitive neurons at several stages of ILD processing, including the lateral superior olive, inferior colliculus, and primary auditory cortex, respond in a frequency-specific manner to a broad range of ILD values (for review, see Ehret and Romand, 1997; Park et al., 2004). Note that the proposed modifications for both stimulus types could have occurred either in quite early stages of ILD processing or in later stages that adjust or interpret the output of the initial encoding stages. The latter possibility is particularly plausible for the SAM-tone case because the affected neural process distinguishes between SAM tones and pure tones even at the same frequency, a feature that is more often demonstrated by late than early stages of auditory processing.
One specific manner in which AM processing might affect ILD sensitivity is through the adjustment of the strength of envelope phase-locking in ILD encoding. High-frequency ILDs are initially computed by brainstem neurons that cannot phase lock to the fine structure of high-frequency sounds but can do so to low-frequency AM of these sounds (up to around 0.5 kHz AM, Joris and Yin, 1995). If training with the SAM tone, through either bottom-up or top-down mechanisms, were to strengthen phase-locking to the peaks of the fluctuating amplitude envelope, where the difference in the sound level from the two ears is largest, ILD sensitivity would be enhanced. This benefit would not be present for pure tones. According to this scenario, though the modifications induced by ILD-discrimination training with the SAM tone may be present in neural circuitry that is activated by both AM sounds and pure tones, behavioral benefits would occur only for AM sounds, as we observed in the present experiment.
Differences between ILD- and ITD-discrimination learning
The current results, when added to the previous ones obtained with the same training regimen for ILD and ITD discriminations with different stimuli (Wright and Fitzgerald, 2001; Zhang and Wright, 2007), confirm the notion that learning of lateralization was determined more by the cue manipulated than by any other aspect of the stimulus. For ITD discrimination, after the ∼2 h pretest, listeners did not benefit from further multi-hour training with either a pure tone or a SAM tone (0.5-kHz tone, Wright and Fitzgerald, 2001; 4-kHz tone SAM at 0.3 kHz, Zhang and Wright, 2007). In contrast, the multi-hour training on ILD discrimination yielded significant additional learning in the majority of the trained listeners with both stimulus types (4-kHz tone, Wright and Fitzgerald, 2001; 4-kHz tone SAM at 0.3 kHz, the present experiment). In other words, under the current training paradigm, with both pure and SAM tones, ITD discrimination performance reached asymptote by the end of the ∼2 h pretest (Wright and Fitzgerald, 2001; Zhang and Wright, 2007), while ILD performance continued to improve in most listeners (Wright and Fitzgerald, 2001 and the current experiment), though with a longer time course for the SAM than the pure tone. Notably, this distinction between ILD and ITD discriminations held even when the same standard stimulus (the SAM tone) was used during training. It remains to be resolved whether the lack of learning in a subset of the listeners trained on ILD discrimination with the SAM tone (the current experiment) resulted from the same cause as the lack of learning on ITD discrimination with both stimulus types (Wright and Fitzgerald, 2001; Zhang and Wright, 2007). The distinct effects of multi-hour training on ILD and ITD discriminations are consistent with the idea of differential neural processing of ILDs and ITDs in humans. It appears that under the current training regimen, the neural mechanisms that determine ITD discrimination sensitivity are less modifiable in long term than those underlying ILD-discrimination sensitivity and that this difference in modifiability lies between the two cues rather than between different frequency regions or different amplitude envelope shapes.
CONCLUSIONS
We examined the effect of multi-hour training on ILD discrimination with a 4-kHz tone SAM at 0.3 kHz. Ten out of the 16 trained listeners improved more than untrained controls. Compared to previous results of ILD-discrimination training with a 4-kHz pure tone (Wright and Fitzgerald, 2001), learning with the SAM tone showed less predictability of occurrence based on starting performance, a longer time course, and specificity to stimulus type (amplitude modulated tones vs pure tones) rather than stimulus frequency. Among several other possibilities, we suggest that the differences between the two ILD-discrimination training results indicate that the sound-localization system has the ability to access amplitude envelope information in a sound and use that information to improve ILD representation. This ability, if confirmed, would represent an under investigated type of interaction between temporal and level processing in the binaural system.
Acknowledgments
We thank Rodrigo Cadiz for technical support and Karen Banai, Julia Huyck, Nicole Marrone, Julia Mossbridge, Jeanette Ortiz, and Andrew Sabin for helpful comments on previous drafts of this paper. This work is supported by NIH∕NIDCD and The Hugh Knowles Center for Clinical and Basic Science in Hearing and Its Disorders.
References
- Ahissar, M., and Hochstein, S. (2004). “The reverse hierarchy theory of visual perceptual learning,” Trends Cogn. Sci. 8, 457–464. 10.1016/j.tics.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Atienza, M., Cantero, J. L., and Dominguez-Marin, E. (2002). “The time course of neural changes underlying auditory perceptual learning,” Learn. Memory 9, 138–150. 10.1101/lm.46502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge, J. F., Blatchley, B., and Kudoh, M. (1993). “Encoding of amplitude-modulated tones by neurons of the inferior colliculus of the kitten,” Brain Res. 615, 199–217. 10.1016/0006-8993(93)90030-Q [DOI] [PubMed] [Google Scholar]
- Clapp, W. C., Kirk, I. J., Hamm, J. P., Shepherd, D., and Teyler, T. J. (2005). “Induction of LTP in the human auditory cortex by sensory stimulation,” Eur. J. Neurosci. 22, 1135–1140. 10.1111/j.1460-9568.2005.04293.x [DOI] [PubMed] [Google Scholar]
- Delhommeau, K., Micheyl, C., Jouvent, R., and Collet, L. (2002). “Transfer of learning across durations and ears in auditory frequency discrimination,” Percept. Psychophys. 64, 426–436. [DOI] [PubMed] [Google Scholar]
- Ehret, G., and Romand, R. (1997). The Central Auditory System (Oxford University Press, New York: ). [Google Scholar]
- Fahle, M. (2004). “Perceptual learning: A case for early selection,” J. Visualization 4, 879–890. 10.1167/4.10.4 [DOI] [PubMed] [Google Scholar]
- Feddersen, W. E., Sandel, T. T., Teas, D. C., and Jeffress, L. A. (1957). “Localization of high-frequency tones,” J. Acoust. Soc. Am. 29, 988–991. 10.1121/1.1909356 [DOI] [Google Scholar]
- Fitzgerald, M. B., and Wright, B. A. (2005). “A perceptual learning investigation of the pitch elicited by amplitude-modulated noise,” J. Acoust. Soc. Am. 118, 3794–3803. 10.1121/1.2074687 [DOI] [PubMed] [Google Scholar]
- Furmanski, C. S., Schluppeck, D., and Engel, S. A. (2004). “Learning strengthens the response of primary visual cortex to simple patterns,” Curr. Biol. 14, 573–578. 10.1016/j.cub.2004.03.032 [DOI] [PubMed] [Google Scholar]
- Gottselig, J. M., Brandeis, D., Hofer-Tinguely, G., Borbely, A. A., and Achermann, P. (2004). “Human central auditory plasticity associated with tone sequence learning,” Learn. Memory 11, 162–171. 10.1101/lm.63304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafter, E. R. (1984). “Spatial hearing and the duplex theory: How viable is the model?,” in Dynamic Aspects of Neucortical Function, edited by Edelman G. M., Gall W. E., and Cowan W. M. (Neurosciences Research Foundation, John Wiley and Sons, New York: ), pp. 425–448. [Google Scholar]
- Hafter, E. R., and Jeffress, L. A. (1968). “Two-image lateralization of tones and clicks,” J. Acoust. Soc. Am. 44, 563–569. 10.1121/1.1911121 [DOI] [PubMed] [Google Scholar]
- Hartmann, W. M., and Constan, Z. A. (2002). “Interaural level differences and the level-meter model,” J. Acoust. Soc. Am. 112, 1037–1045. 10.1121/1.1500759 [DOI] [PubMed] [Google Scholar]
- Irvine, D. R., and Gago, G. (1990). “Binaural interaction in high-frequency neurons in inferior colliculus of the cat: Effects of variations in sound pressure level on sensitivity to interaural intensity differences,” J. Neurophysiol. 63, 570–591. [DOI] [PubMed] [Google Scholar]
- Irvine, D. R., Martin, R. L., Klimkeit, E., and Smith, R. (2000). “Specificity of perceptual learning in a frequency discrimination task,” J. Acoust. Soc. Am. 108, 2964–2968. 10.1121/1.1323465 [DOI] [PubMed] [Google Scholar]
- Irvine, D. R., Rajan, R., and Aitkin, L. M. (1996). “Sensitivity to interaural intensity differences of neurons in primary auditory cortex of the cat. I. Types of sensitivity and effects of variations in sound pressure level,” J. Neurophysiol. 75, 75–96. [DOI] [PubMed] [Google Scholar]
- Irvine, D. R., and Wright, B. A. (2005). “Plasticity of spectral processing,” Int. Rev. Neurobiol. 70, 435–472. 10.1016/S0074-7742(05)70013-1 [DOI] [PubMed] [Google Scholar]
- Joris, P. X., Schreiner, C. E., and Rees, A. (2004). “Neural processing of amplitude-modulated sounds,” Physiol. Rev. 84, 541–577. 10.1152/physrev.00029.2003 [DOI] [PubMed] [Google Scholar]
- Joris, P. X., and Yin, T. C. T. (1995). “Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences,” J. Neurophysiol. 73, 1043–1062. [DOI] [PubMed] [Google Scholar]
- Karmarkar, U. R., and Buonomano, D. V. (2003). “Temporal specificity of perceptual learning in an auditory discrimination task,” Learn. Memory 10, 141–147. 10.1101/lm.55503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, A., Meyer, G., Rey-Hipolito, C., Jezzard, P., Adams, M. M., Turner, R., and Ungerleider, L. G. (1998). “The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex,” Proc. Natl. Acad. Sci. U.S.A. 95, 861–868. 10.1073/pnas.95.3.861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni, A., and Sagi, D. (1991). “Where practice makes perfect in texture discrimination: Evidence for primary visual cortex plasticity,” Proc. Natl. Acad. Sci. U.S.A. 88, 4966–4970. 10.1073/pnas.88.11.4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehnke, J., Colburn, H. S., and Durlach, N. I. (1986). “Performance in several binaural-interaction experiments,” J. Acoust. Soc. Am. 79, 1558–1562. 10.1121/1.393682 [DOI] [PubMed] [Google Scholar]
- Krigolson, O. E., Pierce, L. J., Holroyd, C. B., and Tanaka, J. W. (2009). “Learning to become an expert: Reinforcement learning and the acquisition of perceptual expertise,” J. Cogn. Neurosci. 21, 1834–1841 [DOI] [PubMed] [Google Scholar]
- Kuhn, G. F., and Guernsey, R. M. (1983). “Sound pressure distribution about the human head and torso,” J. Acoust. Soc. Am. 73, 95–105. 10.1121/1.388727 [DOI] [PubMed] [Google Scholar]
- Law, C. T., and Gold, J. I. (2008). “Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area,” Nat. Neurosci. 11, 505–513. 10.1038/nn2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Li, W., Piech, V., and Gilbert, C. D. (2008). “Learning to link visual contours,” Neuron 57, 442–451. 10.1016/j.neuron.2007.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollon, J. D., and Danilova, M. V. (1996). “Three remarks on perceptual learning,” Spatial Vis. 10, 51–58. 10.1163/156856896X00051 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J. (1997). An Introduction to the Psychology of Hearing (Academic, London: ). [Google Scholar]
- Mossbridge, J. A., Fitzgerald, M. B., O’Connor, E. S., and Wright, B. A. (2006). “Perceptual-learning evidence for separate processing of asynchrony and order tasks,” J. Neurosci. 26, 12708–12716. 10.1523/JNEUROSCI.2254-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossbridge, J. A., Scissors, B. N., and Wright, B. A. (2008). “Learning and generalization on asynchrony and order tasks at sound offset: Implications for underlying neural circuitry,” Learn. Memory 15, 13–20. 10.1101/lm.573608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan, S. S., Blake, D. T., Wright, B. A., Byl, N., and Merzenich, M. M. (1998). “Practice-related improvements in somatosensory interval discrimination are temporally specific but generalize across skin location, hemisphere, and modality,” J. Neurosci. 18, 1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, T. J., Klug, A., Holinstat, M., and Grothe, B. “Interaural level difference processing in the lateral superior olive and the inferior colliculus,” J. Neurophysiol. 92, 289–301 (2004). 10.1152/jn.00961.2003 [DOI] [PubMed] [Google Scholar]
- Petersen, S. E., van Mier, H., Fiez, J. A., and Raichle, M. E. (1998). “The effects of practice on the functional anatomy of task performance,” Proc. Natl. Acad. Sci. U.S.A. 95, 853–860. 10.1073/pnas.95.3.853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp, R. (1965). “Detectability threshold for combination tones,” J. Acoust. Soc. Am. 37, 1110–1123. 10.1121/1.1909532 [DOI] [PubMed] [Google Scholar]
- Pourtois, G., Rauss, K. S., Vuilleumier, P., and Schwartz, S. (2008). “Effects of perceptual learning on primary visual cortex activity in humans,” Vision Res. 48, 55–62. 10.1016/j.visres.2007.10.027 [DOI] [PubMed] [Google Scholar]
- Rowan, D., and Lutman, M. E. (2006). “Learning to discriminate interaural time differences: An exploratory study with amplitude-modulated stimuli,” Int. J. Audiol. 45, 513–520. 10.1080/14992020600801434 [DOI] [PubMed] [Google Scholar]
- Rowan, D., and Lutman, M. E. (2007). “Learning to discriminate interaural time differences at low and high frequencies,” Int. J. Audiol. 46, 585–594. [DOI] [PubMed] [Google Scholar]
- Saberi, K. (1995). “Some considerations on the use of adaptive methods for estimating interaural-delay thresholds,” J. Acoust. Soc. Am. 98, 1803–1806. 10.1121/1.413379 [DOI] [PubMed] [Google Scholar]
- Schiltz, C., Bodart, J. M., Michel, C., and Crommelinck, M. (2001). “A pet study of human skill learning: Changes in brain activity related to learning an orientation discrimination task,” Cortex 37, 243–265. 10.1016/S0010-9452(08)70571-9 [DOI] [PubMed] [Google Scholar]
- Semple, M. N., and Kitzes, L. M. (1987). “Binaural processing of sound pressure level in the inferior colliculus,” J. Neurophysiol. 57, 1130–1147. [DOI] [PubMed] [Google Scholar]
- Sigman, M., Pan, H., Yang, Y., Stern, E., Silbersweig, D., and Gilbert, C. D. (2005). “Top-down reorganization of activity in the visual pathway after learning a shape identification task,” Neuron 46, 823–835. 10.1016/j.neuron.2005.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, R. M.Jr., Slocum, J. E., and Phillips, M. S. (1983). “Interaural time and amplitude discrimination in noise,” J. Acoust. Soc. Am. 73, 1714–1722. 10.1121/1.389395 [DOI] [PubMed] [Google Scholar]
- Tollin, D. J. (2003). “The lateral superior olive: A functional role in sound source localization,” Neuroscientist 9, 127–143. 10.1177/1073858403252228 [DOI] [PubMed] [Google Scholar]
- Vaina, L. M., Belliveau, J. W., des Roziers, E. B., and Zeffiro, T. A. (1998). “Neural systems underlying learning and representation of global motion,” Proc. Natl. Acad. Sci. U.S.A. 95, 12657–12662. 10.1073/pnas.95.21.12657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wassenhove, V., and Nagarajan, S. S. (2007). “Auditory cortical plasticity in learning to discriminate modulation rate,” J. Neurosci. 27, 2663–2672. 10.1523/JNEUROSCI.4844-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, C. S. (1980). “Time course of auditory perceptual learning,” Ann. Otol. Rhinol. Laryngol. Suppl. 89, 96–102. [PubMed] [Google Scholar]
- Wright, B. A., Buonomano, D. V., Mahncke, H. W., and Merzenich, M. M. (1997). “Learning and generalization of auditory temporal-interval discrimination in humans,” J. Neurosci. 17, 3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, B. A., and Fitzgerald, M. B. (2001). “Different patterns of human discrimination learning for two interaural cues to sound-source location,” Proc. Natl. Acad. Sci. U.S.A. 98, 12307–12312. 10.1073/pnas.211220498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, B. A., and Zhang, Y. (2006). “A review of learning with normal and altered sound-localization cues in human adults,” Int. J. Audiol. 45, 92–98. 10.1080/14992020600783004 [DOI] [PubMed] [Google Scholar]
- Wright, B. A., and Zhang, Y. (2009). “Insights into human auditory processing gained from perceptual learning,” in The Cognitive Neurosciences IV, edited by Gazzaniga M. S. (The MIT Press, Cambridge, Mass.). [Google Scholar]
- Yost, W. A., and Dye, R. H.Jr., (1988). “Discrimination of interaural differences of level as a function of frequency,” J. Acoust. Soc. Am. 83, 1846–1851. 10.1121/1.396520 [DOI] [PubMed] [Google Scholar]
- Yotsumoto, Y., Watanabe, T., and Sasaki, Y. (2008). “Different dynamics of performance and brain activation in the time course of perceptual learning,” Neuron 57, 827–833. 10.1016/j.neuron.2008.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., and Wright, B. A. (2007). “Similar patterns of learning and performance variability for human discrimination of interaural time differences at high and low frequencies,” J. Acoust. Soc. Am. 121, 2207–2216. 10.1121/1.2434758 [DOI] [PubMed] [Google Scholar]