Abstract
At present 75 species of Hypocrea have been identified in temperate Europe. Nineteen green-spored species and their Trichoderma asexual states are here described in detail. Extensive searches for Hypocrea teleomorphs in 14 European countries, with emphasis on Central Europe, yielded more than 620 specimens within five years. The morphology of fresh and dry stromata was studied. In addition, available types of species described from Europe were examined. Cultures were prepared from ascospores and used to study the morphology of cultures and anamorphs, to determine growth rates, and to extract DNA that was used for amplification and sequencing of three genetic markers. ITS was used for identification, while RNA polymerase II subunit b (rpb2) and translation elongation factor 1 alpha (tef1) were analyzed for phylogenetic reconstruction of the genus.
Several unexpected findings resulted from this project: 1) The previous view that only a small number of Trichoderma species form a teleomorph is erroneous. 2) All expectations concerning the number of species in Europe are by far exceeded. Seventy-five species of Hypocrea, two species of Protocrea, and Arachnocrea stipata, are herein identified in temperate Europe, based on the ITS identification routine using fresh material, on species described earlier without molecular data and on species recently described but not collected during this project. 3) Current data suggest that the biodiversity of Hypocrea / Trichoderma above soil exceeds the number of species isolated from soil. 4) The number of Trichoderma species forming hyaline conidia has been considered a small fraction. In Europe, 26 species of those forming teleomorphs produce hyaline conidia, while 42 green-conidial species are known. Three of the detected Hypocrea species do not form an anamorph in culture, while the anamorph is unknown in four species, because they have never been cultured.
This work is a preliminary account of Hypocrea and their Trichoderma anamorphs in Europe. Of the hyaline-spored species, H. minutispora is by far the most common species in Europe, while of the green-spored species this is H. strictipilosa.
General ecology of Hypocrea is discussed. Specific associations, either with host fungi or trees have been found, but the majority of species seems to be necrotrophic on diverse fungi on wood and bark.
The taxonomy of the genus will be treated in two parts. In this first part 19 species of Hypocrea with green ascospores, including six new teleomorph and five new anamorph species, are described in detail. All green-spored species belong to previously recognised clades, except H. spinulosa, which forms the new Spinulosa Clade with two additional new species, and H. fomiticola, which belongs to the Semiorbis Clade and forms effuse to large subpulvinate stromata on Fomes fomentarius, a trait new for species with green ascospores. Anamorph names are established prospectively in order to provide a basis for possible policy alterations towards their use for holomorphs.
Keywords: Arachnocrea, Ascomycetes, DNA barcode, Gliocladium, Hypocreales, ITS, morphology, phylogeny, Protocrea, rpb2, sequence analysis, systematics, tef1, Trichoderma
INTRODUCTION
The teleomorph genus Hypocrea - a brief history
Chaverri & Samuels (2003) treated the history of the genus Hypocrea/Trichoderma in detail. This section therefore serves to list important milestones and to summarise the most recent developments in the taxonomy and circumscription of the genus emphasising the teleomorph.
Hypocrea Fr. is the type genus of the family Hypocreaceae of the pyrenomycetous order Hypocreales. The genus was established by Fries (1825, p. 104) based on Sphaeria rufa Pers.: Fr. It is characterised by fleshy, internally light-coloured stromata with light-coloured perithecia in their upper part, with asci containing 8 uniseriately arranged, bicellular ascospores disarticulating in the ascus giving the ascus the appearance of being 16-spored. Most species were described in the 19th century. Little microscopic variability and poor descriptions may be the reasons why little progress was achieved in the taxonomy of Hypocrea during the 20th century. In Europe, Webster and collaborators (Webster 1964, Rifai & Webster 1966, Webster & Rifai 1968) restudied several species of Hypocrea and described H. psychrophila (Müller et al. 1972). Petrak (1940) described H. moravica and H. subalpina. In New Zealand, Dingley (1952, 1956, 1957) described eight new species and assigned their anamorphs to the single species Trichoderma viride. The most important revisional studies of Hypocrea in the pre-molecular era of the 20th century were those by Yoshimichi Doi (1966, 1969, 1972, 1978, 1982). He tried to untangle the taxonomic chaos by defining a generic concept with infrageneric groupings, based on type material and fresh specimens collected in Japan (Doi 1972) and later in South America (Doi 1975, 1976) and Indonesia (Samuels et al. 1990). His infrageneric classification of teleomorphs, however, is outdated and his taxa do not correlate with results of molecular phylogenetic analyses. However, Doi described nearly 50 new species (see e.g. Doi 1968, 1971, 1975, 1976, 1987, 2001, 2006, Doi et al. 2001), and included anamorph morphology in his descriptions. Because many of his cultures are lost it is difficult or impossible to verify the identity of many of his species using current methods. In many instances, Doi applied names of species described from Europe and North America to specimens collected in Japan, based on comparative morphology of teleomorphs. From what is known today, i.e. that morphology is often insufficient for identification, particularly if specimens from different continents are compared, it is likely that still many additional species may be recognised in Japan. Samuels and his collaborators extended the research to collections from North America and Australasia, including also specimens collected in Europe, while introducing molecular phylogenetic analyses into Hypocrea taxonomy (see e.g. Samuels et al. 1998, Dodd et al. 2002, 2003).
The genera Creopus (Link 1833) and Chromocrea (Seaver 1910) were both based on Hypocrea gelatinosa. Although widely used for species with green ascospores, neither Webster (1964) nor Doi (1972) accepted these generic names. Chaverri et al. (2001a, b, 2003a) and Chaverri & Samuels (2003) confirmed congenericity of these genera with Hypocrea based on gene phylogeny and monographed the green-spored species of Hypocrea, while describing many new species, including H. strictipilosa, the most common green-spored species of Hypocrea.
Lu et al. (2004) analysed an important group termed the Pachybasium core group herein of the former sect. Pachybasium, containing the most common hyaline-spored species in temperate regions, H. minutispora B.S. Lu, Fallah & Samuels.
Chamberlain et al. (2004) monographed the stipitate species of Hypocrea and synonymised the genera Podostroma and Podocrea with Hypocrea, mainly based on the Trichoderma anamorphs of H. leucopus and H. alutacea, but without using gene sequences. Gene phylogeny (Jaklitsch et al. 2008c) confirmed congenericity of Podostroma (type species P. leucopus P. Karst.) and Podocrea (type species P. alutacea (Pers.: Fr.) Lindau) with Hypocrea.
Jaklitsch et al. (2005, 2006a) described two new European species: Hypocrea voglmayrii, representing a clade of its own, also remarkable for its growth at 35 °C although found at altitudes above 1000 m, and Hypocrea crystalligena with a white-conidial anamorph, white crystals formed on the stroma surface and in culture, a species belonging to the Psychrophila clade despite its teleomorph morphology suggesting sect. Trichoderma.
Jaklitsch et al. (2006b) clarified and narrowly defined the morphological and phylogenetic concept of the type species of Hypocrea, H. rufa, and its anamorph, the type species of Trichoderma Pers., T. viride Pers. They described several new species, including H. viridescens, a species close to H. rufa, but distinguishable by anamorph morphology. All these species belong to the sect. Trichoderma as circumscribed below (see Phylogeny section).
Teleomorphs and anamorphs of the former sect. Hypocreanum (Bissett 1991a) and some similar species were treated by Overton et al. (2006a, b). These two papers clarified the concept of `well-known' species like H. citrina, H. pulvinata and H. sulphurea. However, several species remain to be assessed by molecular methods. Overton et al. (2006b) identified the type species of Protocrea Petch, P. farinosa (Berk. & Broome) Petch, as a species of Hypocrea, thus the genus became a synonym of Hypocrea. However, this synonymy was based on misidentified material. Jaklitsch et al. (2008b) redescribed the genus Protocrea based on P. farinosa as a genus with perithecia partially immersed in a subiculum and a white gliocladium-like anamorph. They clarified the phylogenetic position of Protocrea in the Hypocreaceae, and determined that Hypocrea pallida Ellis & Everh., whose phylogenetic position within the Hypocreaceae had been under debate since Rehner & Samuels (1994), belongs to Protocrea. They established the name Hypocrea decipiens for the species interpreted by Overton et al. (2006b) as H. farinosa.
The anamorph genus Trichoderma - taxonomic history
Hypocrea and Trichoderma are manifestations of the same organisms. Historically, they have been classified as different genera, therefore only those historical steps dealing mainly with anamorphs are treated here. Anamorphs of Hypocrea have been mainly classified in the genus Trichoderma, while a few species are still classified in other genera, like Gliocladium Corda or Stilbella Lindau (Rossman 2000, Seifert & Samuels 1997). Samuels (2006) gave a comprehensive chronological review of Trichoderma. At present the genus is conceived as saprobic, soil-inhabiting, fast growing, green or white moulds with hyaline phialides and 1-celled, green or hyaline conidia. However, conidiophores are difficult to define, and specific differences are difficult to quantify. This may be a reason why a conspicuous discrepancy exists between the number of names established in Hypocrea and those in Trichoderma. Persoon (1794) erected Trichoderma with three species, but only one of those was accepted until Rifai (1969) defined nine aggregate species. Based on this monograph, Bissett (1984, 1991a, b, Gams & Bissett 1998) subdivided the genus into five sections and recognised 27 species. The introduction of gene phylogeny in the nineties of the last century enabled researchers to identify and delimit species of Trichoderma more reliably. Since then the number of species has increased rapidly. First studies were based on ITS sequences (Kindermann et al. 1998, Lieckfeldt et al. 1998, Dodd et al. 2000). Later other genes were added, including those encoding 18S and 28S RNA, translation elongation factor 1-alpha (tef1), endochitinase (chi18-5 = ech42) (Lieckfeldt et al. 2000, Kullnig-Gradinger et al. 2002), rpb2 (Chaverri et al. 2003a), actin (act), and calmodulin (cal1) (Samuels et al. 2006a, Jaklitsch et al. 2008a). Several studies have consistently shown that tef1 introns provide the highest power of resolution within clades (Samuels et al. 2006, Jaklitsch et al. 2006a, b). From subsequent molecular analyses, it became clear that subgeneric sections of Trichoderma as defined by Bissett (1991a), particularly sect. Pachybasium, are phylogenetically heterogeneous (except for sect. Longibrachiatum), but they can be still used as descriptive terms for conidiophore morphology, and in part phylogenetically (sect. Longibrachiatum, sect. Hypocreanum).
Samuels (2006) and Druzhinina et al. (2006) reviewed the history and development of species concepts, recognition and identification, and importance of the genus in biological control. The latter determined that by 2006, Trichoderma encompassed at least 100 species characterised by molecular data.
One of the most complex species groups in Trichoderma is the former sect. Trichoderma. Samuels et al. (2006) described thirteen species of this section (`T. koningii'-like), 11 of them derived from Hypocrea teleomorphs, showing that the morphology of teleomorphs has little or no value in the differentiation of the species within sect. Trichoderma, but also their anamorphs are difficult to delimit based on morphology (see also Jaklitsch et al. 2006). Conidial morphology and growth rates remained the only phenotype traits useful in species delimitation in this group. This paper as well as the work treating the H. schweinitzii complex (Samuels et al. 1998) corroborates the view that species identification is impossible in certain groups of Hypocrea based on teleomorph morphology alone. The molecular analysis using ITS sequences alone also proved insufficient to distinguish species of this group.
An important approach to the identification of Hypocrea/Trichoderma species is the development of an oligonucleotide barcode (TrichOKEY, Druzhinina et al. 2005) and the respective application software available at www.isth.info. This allows identification of a majority of species within Hypocrea/Trichoderma, based on the combination of certain markers (anchors, hallmarks) of vouchered ITS1 and ITS2 sequences, obtained by a thorough analysis of these sequences compiled in a local database. The advantage of this system is that this database does not contain the substantial number of sequences in GenBank originating from wrongly identified species. In addition, the similarity search routine TrichoBLAST (Kopchinskiy et al. 2005) available on www.isth.info is based on ITS, tef1 and rpb2 sequences of vouchered specimens and provides more reliable identification of Hypocrea/Trichoderma than GenBank.
Worth mentioning is a trend to discover Trichoderma species in a specialised ecological niche, viz. species endophytic in terrestrial plants. Examples are Trichoderma taxi Chu L. Zhang, F.C. Lin & C.P. Kubicek endophytic in Taxus mairei (Zhang et al. 2007), or T. evansii Samuels, T. paucisporum and T. theobromicola in species of Cola, Lophira and Theobroma (Samuels et al. 2006b, Samuels & Ismaiel 2009).
Recently Degenkolb et al. (2006a, 2008a) introduced the analysis of secondary metabolites such as peptaibols and trichothecenes to support gene phylogeny of the new T. brevicompactum clade by chemotaxonomy.
Currently the complete genome sequences of three species, Trichoderma atroviride, T. reesei and T. virens are available (http://genome.jgi-psf.org/). They may facilitate the development of additional markers useful for phylogenetic analyses of Hypocrea / Trichoderma.
Although species of Trichoderma are usually addressed as important soil fungi, also in a quantitative sense, there is little information about the number of species detected based on modern identification techniques in larger geographic areas. Only a few local studies in soil have been published, e.g. in the Donau-Auen National Park near Vienna, Austria (Wuczkowski et al. 2003), the Nile valley of Egypt (Gherbawy et al. 2004), some places in China (Zhang et al. 2005), Tenerife (Zachow et al. 2008), Sardinia (Migheli et al. 2008), and two tumuli in Japan (Kiyuna et al. 2008). These studies did not detect more than 15 species (Migheli et al. 2008) in the examined regions. In addition, no screening above the soil level was undertaken.
Species concept
Purely morphological concepts of species delimitation in Hypocrea have serious limits and pose more questions than answers, despite the many names established in the genus. Particularly the teleomorph-anamorph relationship of Hypocrea/Trichoderma was unrecognised for a long time, despite the connection of H. rufa and T. viride illustrated by Tulasne & Tulasne (1865). Doi (1972) certainly improved the recognition of species by description of anamorphs that he cultured from ascospores, but he could, of course, not foresee the large number of species currently recognised. John Bissett and later Gary J. Samuels, who have been working intensely with emphasis on Trichoderma anamorphs, achieved substantial progress in recognition and delimitation of species. Samuels et al. (1998, 2006a) made convincingly clear, that a number of species within different groups of Hypocrea share the same teleomorph morphology, but form morphologically distinguishable anamorphs. Although Trichoderma anamorphs often show more recognisable interspecific phenotypic differences than their teleomorphs, the large and ever-increasing number of species makes recognition based on this cluster of phenotype traits difficult, particularly in the Viride and Harzianum clades, and the former sect. Pachybasium.
After the introduction and continuous application of molecular methods, particularly by Kubicek and his collaborators and the authors cited above, the species concept of Hypocrea / Trichoderma has changed dramatically. Species are now recognised as complex combinations of phylogenetic data, phenotypic data, including morphology of teleomorphs and/or anamorphs, growth rates, and to a lesser extent biogeographic and ecological data. Consequently, accurate identification relies on the comparison of gene sequences for most species. To determine phylogenetic relationships, the initially used ITS sequences are no longer considered useful due to low sequence variability and because some species pairs (e.g. H. sinuosa/H. cremea) have identical ITS sequences. Now generally genealogies of three or four genes are constructed and compared in order to meet the criteria of the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) concept (Taylor et al. 2000), but in complex groups (particularly sect. Trichoderma) tef1 intron 4 alone has proven to possess sufficient variability to resolve species.
Economic and social aspects of Hypocrea / Trichoderma
Hypocrea / Trichoderma is addressed by its anamorph name Trichoderma in the voluminous literature dealing with its importance in many aspects of human activities (Harman & Kubicek 1998). What makes species of Trichoderma important? One trait is the ability to produce a wide range of secondary metabolites (Sivasithamparam & Ghisalberti 1998, Degenkolb et al. 2008b); the second is the production of many enzymes that degrade a wide range of carbohydrates, such as cellulose, hemicelluloses, or chitin. Secondary metabolites have been identified as antibiotics active against fungi and bacteria, or toxins (e.g. peptaibols, gliotoxin). Cellulases, glucanases, pectinases, xylanases are used by fibre processing industries (e.g. production of bioethanol), in detergents (cellulases from T. reesei; also for biostoning), and for processing of animal feed. The combination of antibiotics and chitinases enable species of Trichoderma to attack other fungi. They are therefore used in biocontrol of phytopathogenic fungi (e.g. T. asperellum, T. atroviride, T. harzianum, and T. virens). Growth promotion of plants is another action of Trichoderma, as well as plant protection by Trichoderma endophytes, probably triggered by secondary metabolites like peptaibols, as reported by Harman et al. (2004), Hanada et al. (2008) or Degenkolb et al. (2006b). Attack by Trichoderma of other fungi has also negative effects, i.e. destruction of cultivated mushrooms (T. aggressivum, Samuels et al. 2002; T. pleuroticola, T. pleurotum, Park et al. 2006, Komon-Zelazowska et al. 2007). The ability of some species of Hypocrea/Trichoderma (T. longibrachiatum, H. orientalis) to grow at higher temperatures enables them to act as opportunistic pathogens of immune-compromised mammals including humans (see e.g. Kredics et al. 2003, Druzhinina et al. 2008).
A point supporting the importance of taxonomy and phylogeny of the genus is that it helps to explain and understand conflicting results and controversial reports from biocontrol and other applications. Negative and positive results reported for the same organism from similar sets of trials have been simply due to incorrect identification, i.e. trials had been carried out with different species. One good example is the name Trichoderma harzianum used for many different species (Kullnig et al. 2001), another Hypocrea rufa /Trichoderma viride (Jaklitsch et al. 2006b), cited in numerous papers and thought to be cosmopolitan. However, H. rufa is now more narrowly defined and determined to be temperate in distribution, thus many reports apply to different species of the sect. Trichoderma. Furthermore, thorough analysis of secondary metabolites (Degenkolb et al. 2008b) useful for chemotaxonomy (Degenkolb et al. 2008a) helps to predict biological properties of species and provides important or even crucial criteria in the selection of candidates for biological control of fungal pathogens. Clearly, mycotoxin (e.g. trichothecene)-producing Trichoderma species should not be used for such a purpose.
A note on nomenclature
Chaverri & Samuels (2003) and Samuels (2006) made suggestions to counteract Article 59 of the International Code of Botanical Nomenclature (McNeill et al. 2006) and abolish the use of the name Hypocrea for the holomorph in favour of Trichoderma. One generic name is in principle desirable for one organism. Phylogenetically the relationship of Hypocrea and Trichoderma is clear and a change to one name would only require new combinations. However, the adoption of a one species/one name system for all ascomycetes must be undertaken gradually. Difficulties in moving toward one name for one species include the numerous wrong anamorph/teleomorph relationships, as compiled in the Dictionary of the Fungi (Kirk et al. 2008). I want to point out also another thing: Morphological concepts attached to generic names facilitate access to mycology by helping to memorise the vast range of forms of fungi. Using anamorph names (mould) for the holomorph (stromata, ascomata) would entirely disrupt these concepts, cause chaos and contribute to the disrepute of the constantly decreasing number of taxonomists in mycology. In any case, I establish both teleomorph and anamorph names here in order to provide combinations in Trichoderma for a possible scenario in the future that may demand the use of Trichoderma for the holomorph.
Scope and objective of this project
Currently the IndexFungorum database (www.indexfungorum.org/Names/Names.asp) contains nearly 460 names in Hypocrea including infraspecific taxa. Most of these were described with short morphological diagnoses of teleomorphs in the 19th century, indicating either high variability in gross morphology or/and lack of comparison with earlier described species. More than 75 names have been determined by other mycologists to belong to other genera; many others are synonyms. Trichoderma anamorphs amount to ca. 150 names.
The introduction of gene phylogeny in the systematics of Hypocrea/Trichoderma has opened the way to safer species recognition. This facilitates a well-defined species circumscription and lead to a substantial proliferation of species numbers. Most of the names in Trichoderma are now clarified in terms of identity and species concept. In contrast, many names in Hypocrea are still obscure. Their reassessment and clarification is required for effective biodiversity studies and valid description of new species. No modern monograph of Hypocrea exists for any region including Europe. Molecular tools in combination with extensive fieldwork serve as an ideal basis for an assessment of the diversity of the genus in any geographical region.
This work was designed to study the biodiversity of the genus Hypocrea / Trichoderma forming teleomorphs in Europe, with emphasis on Central Europe. It is not a comprehensive classical monographic work based on extensive examination of herbarium specimens, but mainly constitutes the results of extensive, five-years collecting of Hypocrea teleomorphs, augmented by type studies. Accordingly, information on species distributions given in the individual descriptions is based on recently collected material. An attempt has been made to recollect all species described from Europe. In contrast to previous studies, morphological characteristics of fresh stromata and ecological factors such as specific fungus/host relationships are included in order to improve the morphological concept of teleomorphs as well as species definition and delimitation.
Strategy of species identification and recognition
Examination of type specimens of Hypocrea would not lead to a realistic recognition of species due to the conservation of morphological characteristics in teleomorphs combined with the lack of anamorphs and gene sequences. The same is true for fresh specimens, if only teleomorph morphology is studied. As pointed out by Samuels (2006), many species of Trichoderma and their Hypocrea teleomorphs are geographically limited, while others are widely distributed, and only a few are truly cosmopolitan (T. asperellum, T. harzianum). To assess the biodiversity for a given region, freshly collected specimens must be cultured and sequenced. Species previously described from that region should be recollected at or close to their original collection sites. Caution must be applied to the synonymy of names described from other regions based on morphology alone. Accordingly, a species is here regarded as new if it is phylogenetically distinct from all species with currently available molecular data and if there is no species matching its morphology, described from the same region.
Based on these prerequisites and the huge collection of gene sequences (www.isth.info/), the following strategy is used in this work: collect fresh Hypocrea teleomorphs, prepare cultures from ascospores, extract DNA from cultures and amplify and sequence ITS, and preliminarily identify species based on ITS using the TrichOKey routine (see above). Specimens yielding ITS sequences not recognised by TrichOKey are regarded as potentially new species. Subsequent sequencing of tef1, rpb2, (and chi18-5) are used to confirm or reject the new species postulate. In order to link a `new' species to an old name, a morphological comparison is made with species described from Europe, but unknown with regard to anamorph and DNA data. If these steps confirm the species as new, the respective specimens are described here as belonging to a new species. Some species described here as new may be conspecific with species previously described on a mere morphological basis, particularly from temperate zones of North America or Japan. However, to detect conspecificity with species described from other continents, similar studies must be performed in non-European regions.
MATERIALS AND METHODS
Isolates and specimens
Isolates including accession numbers of gene sequences investigated in this study are listed in Table 1. Isolates listed as C.P.K. are those maintained in the collection of the Institute of Chemical Engineering, Research Area Gene Technology and Applied Biochemistry, of the University of Technology Vienna. Representative isolates have been deposited at the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS). Specimens have been deposited in the Herbarium of the Institute of Botany, University of Vienna (WU), some at Kew (K). MTB in specimen descriptions means Messtischblatt, a topographic grid used for floristic mapping.
Table 1.
Isolates and accession numbers for rpb2 and tef1 sequences used in the phylogenetic trees, either determined in this study or retrieved from GenBank (NCBI) or (*) from the multiloci database of the ISTH webpage (www.isth.info/tools/blast/show_all_seq.php).
| Name part I | (Sub-) Continent1 | Strain | Accession rpb2 | Accession tef1 | Used in Fig. |
|---|---|---|---|---|---|
| Aphysiostroma stercorarium Barrasa et al. | E | ATCC62321 | EF469103 | -- | 1 |
| A. stercorarium | -- | CBS 148.85 | -- | FJ860607 | 1 |
| Hypocrea aeruginea Jaklitsch | E | CBS 120541 | FJ860516 | FJ860608 | 1, 2 |
| Trichoderma aggressivum Samuels & W. Gams | E | CBS 100525 | AF545541 | -- | 1 |
| H. alcalifuscescens Overton | E | TFC 181548 | DQ834462 | -- | 1 |
| H. alcalifuscescens | -- | TFC 00-36 | -- | FJ860610 | 1 |
| H. alni Jaklitsch | E | CBS 120633 | EU498349 | EU498312 | 1, 2 |
| H. alni | -- | C.P.K. 2494 | -- | EU498313 | 2 |
| H. alni | -- | C.P.K. 2854 | -- | EU498314 | 2 |
| H. alni | -- | C.P.K. 2858 | -- | EU498315 | 2 |
| H. alutacea (Pers.: Fr.) Tul. & C. Tul. | E | CBS 120535 | FJ179600 | FJ179567 | 1 |
| H. americana (Canham) Overton | NA | G.J.S. 92-93 | DQ835455 | -- | 1 |
| H. americana | -- | AFTOL-ID52 | -- | DQ471043 | 1 |
| T. arundinaceum Zafari, Gräfenhan & Samuels | Af, As, NA | ATCC 90237 | EU338326 | -- | 1 |
| T. asperellum Samuels, Lieckf. & Nirenberg | C | CBS 433.97 | EU248617 | -- | 1 |
| H. atrogelatinosa Dingley | Au | G.J.S. 95-159 | AF545508 | AF534603 | 1 |
| H. atroviridis Dodd, Lieckf. & Samuels | A, Au, E | CBS 119499 | FJ860518 | FJ860611 | 1 |
| H. aureoviridis Plowr. & Cooke | E | CBS 120536 | FJ179602 | -- | 1 |
| H. aureoviridis | -- | C.P.K. 2848 | -- | FJ860615 | 1, 2 |
| H. aureoviridis | -- | C.P.K. 2849 | -- | FJ860616 | 2 |
| H. aureoviridis | -- | C.P.K. 2857 | -- | FJ860617 | 2 |
| H. austrokoningii Samuels & Druzhin. | Au | CBS 247.63 | FJ442772 | -- | 1 |
| H. avellanea Rogerson & S.T. Carey | NA | CTR 77-155 | AF545562 | AY225857 | 1 |
| T. brevicompactum G.F. Kraus, C.P. Kubicek & W. Gams | C? | CBS 112443 | EU338319 | -- | 1 |
| T. brevicompactum | -- | TUBF 1076 | -- | ISTH* | 1 |
| H. brunneoviridis Jaklitsch | E | CBS 121130 | -- | EU498316 | 2 |
| H. brunneoviridis | -- | C.P.K. 2425 | -- | EU498317 | 2 |
| H. brunneoviridis | -- | CBS 120928 | EU498358 | EU498318 | 1, 2 |
| H. candida P. Chaverri & Samuels | CA | P.C. 59 | AY391899 | AY391962 | 1 |
| H. catoptron Berk. & Broome | Af, As | G.J.S. 02-76 | AY391900 | AY391963 | 1 |
| H. ceracea P. Chaverri & Samuels | NA | G.J.S. 88-28 | AY391901 | AY391964 | 1 |
| H. ceramica Ellis & Everh. | NA | CBS 114576 | FJ860531 | FJ860628 | 1, 2 |
| T. cerinum Bissett, C.P. Kubicek & Szakacs | As, E | CBS 120637 | FJ860532 | FJ860629 | 1, 2 |
| H. chlorospora Berk. & M.A. Curtis | CA, NA | G.J.S. 88-33 | AY391903 | AY391966 | 1 |
| H. chromosperma M.A. Curtis & Peck | NA | G.J.S. 94-68 | AY391913 | AY391974 | 1 |
| H. cinereoflava Samuels & Seifert | NA | G.J.S. 92-102 | DQ834461 | DQ834454 | 1 |
| H. cinnamomea P. Chaverri & Samuels | As, NA | G.J.S. 97-230 | AY391918 | -- | 1 |
| H. cinnamomea | -- | G.J.S. 97-237 | -- | AY391979 | 1 |
| H. citrina (Pers.: Fr.) Fr. | E, J, NA | C.P.K. 960 | FJ179603 | FJ860631 | 1 |
| H. costaricensis P. Chaverri & Samuels | CA | P.C. 21 | AY391921 | AY391980 | 1 |
| H. crassa P. Chaverri & Samuels | As, NA | G.J.S. 01-227 | AY481587 | -- | 1 |
| H. crassa | -- | DAOM 164916 | -- | AF534615 | 1 |
| H. cremea P. Chaverri & Samuels | Au, NA | G.J.S. 91-125 | AF545511 | AF534598 | 1 |
| H. crystalligena Jaklitsch | E | C.P.K. 1911 | DQ345348 | DQ345344 | 1 |
| H. cuneispora P. Chaverri & Samuels | NA | G.J.S. 91-93 | AF545512 | AF534600 | 1 |
| H. dacrymycella Cooke & Plowr. | E | WU 29044 | FJ860533 | FJ860633 | 1, 2 |
| H. danica Jaklitsch | E | CBS 121273 | FJ860534 | FJ860634 | 1, 2 |
| H. decipiens Jaklitsch, K. Põldmaa & Samuels | E, NA | G.J.S. 91-101 | DQ835520 | -- | 1 |
| H. decipiens | -- | CBS 121307 | -- | FJ860635 | 1 |
| H. delicatula Tul. & C. Tul. | E | CBS 120631 | FJ860535 | FJ860636 | 1 |
| H. dorotheae Samuels & Dodd | Au | G.J.S. 99-202 | EU248602 | -- | 1 |
| H. epimyces Jaklitsch | E | C.P.K. 1980 | EU498359 | EU498319 | 1, 2 |
| H. epimyces | -- | CBS 120534 | -- | EU498320 | 1, 2 |
| H. epimyces | -- | C.P.K. 2417 | -- | EU498321 | 2 |
| H. epimyces | -- | C.P.K. 2487 | -- | EU498322 | 2 |
| T. erinaceus Bissett, C.P. Kubicek & Szakacs | As | DIS 7 | EU248604 | -- | 1 |
| H. estonica P. Chaverri & Samuels | E | CBS 121556 | FJ860536 | FJ860637 | 1, 2 |
| H. estonica | -- | CBS 111147 | -- | FJ860638 | 2 |
| H. eucorticioides Overton | CA, SA | G.J.S. 99-61 | DQ835518 | DQ835474 | 1 |
| T. fertile Bissett | E, NA | DAOM 167070 | AF545545 | AF534617 | 1 |
| H. flaviconidia P. Chaverri, Druzhin. & Samuels | CA | G.J.S. 99-49 | EU883557 | -- | 1 |
| H. flaviconidia | -- | C.P.K. 455 | -- | AY665711 | 1 |
| H. fomiticola Jaklitsch | E | CBS 121136 | FJ860538 | FJ860639 | 1 |
| H. gelatinosa (Tode : Fr.) Fr. | E | C.P.K. 1618 | FJ179604 | FJ179569 | 1, 2 |
| T. hamatum (Bonord.) Bainier | C | DAOM 167057 | AF545548 | AF534620 | 1 |
| T. helicum Bissett, C.P. Kubicek & Szakacs | As | DAOM 230021 | DQ087239 | ISTH* | 1 |
| T. intricatum Samuels & Dodd | As, CA | G.J.S. 02-78 | EU241505 | -- | 1 |
| H. jecorina Berk. & Broome | PT | TUBF 363 | DQ087241 | -- | 1 |
| H. jecorina | -- | QM9414 | -- | Z23012 | 1 |
| H. koningii Lieckf., Samuels & W. Gams | E, NA | CBS 119500 | FJ860541 | -- | 1 |
| H. koningii | -- | C.P.K. 3564 | -- | FJ860642 | 1 |
| H. koningiopsis Samuels | A, (Af, E) | G.J.S. 93-20 | EU241506 | -- | 1 |
| H. leucopus (P. Karst.) H.L. Chamb. | E, NA | CBS 122499 | FJ179605 | FJ179571 | 1 |
| H. lixii Pat. | C | C.P.K. 1934 | FJ17960 | FJ179573 | 1, 2 |
| H. lixii | -- | C.P.K. 1935 | -- | EU498324 | 2 |
| H. lixii | -- | C.P.K. 1941 | -- | EU498325 | 2 |
| T. longibrachiatum Rifai | C? | CBS 816.68 | DQ087242 | EU401591 | 1 |
| H. longipilosa Jaklitsch | E, (NA) | CBS 120953 | FJ860542 | FJ860643 | 1, 2 |
| H. lutea (Tode) Petch | C? | CBS 121131 | FJ179609 | FJ860644 | 1 |
| H. megalocitrina Yoshim. Doi | J, NA | BEO 00-09 | AF545563 | AY225855 | 1 |
| H. melanomagna P. Chaverri & Samuels | Au | G.J.S. 99-153 | AY391926 | AY391985 | 1 |
| H. microcitrina Yoshim. Doi | J, NA | G.J.S. 91-61 | DQ835460 | DQ835478 | 1 |
| H. minutispora B.S. Lu, Fallah & Samuels | E, NA | CBS 121276 | FJ179610 | FJ179574 | 1 |
| H. moravica Petr. | E | C.P.K. 2489 | FJ860549 | -- | 1 |
| H. moravica | -- | C.P.K. 2411 | -- | FJ860650 | 1 |
| H. neorufa Samuels, Dodd & Lieckf. | E, NA | CBS 119498 | FJ860550 | FJ860653 | 1 |
| H. nigrovirens P. Chaverri & Samuels | CA | G.J.S. 99-64 | AF545518 | AF534582 | 1 |
| H. novaezelandiae Samuels & O. Petrini | Au | TR 175 | DQ857348 | -- | 1 |
| H. nybergiana T. Ulvinen & H.L. Chamb. | E | CBS 122500 | FJ179611 | FJ179575 | 1 |
| T. oblongisporum Bissett | NA | DAOM 167085 | AF545551 | AF534623 | 1 |
| H. ochroleuca Berk. & Ravenel | E, NA | CBS 119502 | FJ860556 | FJ860659 | 1 |
| T. ovalisporum Samuels & Schroers | SA | DIS 70A | FJ442742 | -- | 1 |
| H. pachybasioides Yoshim. Doi | C? | C.P.K. 2461 | FJ179613 | -- | 1 |
| H. pachybasioides | -- | C.P.K. 3131 | -- | FJ860661 | 1 |
| H. parapilulifera B.S. Lu, Druzhin. & Samuels | Au, E, NA | CBS 120921 | FJ179614 | FJ179578 | 1 |
| H. parepimyces Jaklitsch | E | CBS 122769 | FJ860562 | FJ860664 | 1, 2 |
| H. parepimyces | -- | CBS 122768 | -- | FJ860665 | 2 |
| H. parestonica Jaklitsch | E | C.P.K. 2427 | -- | FJ860666 | 2 |
| H. parestonica | -- | CBS 120636 | FJ860565 | FJ860667 | 1, 2 |
| H. parmastoi Overton | E | CBS 121139 | FJ860567 | FJ860668 | 1 |
| T. paucisporum Samuels, C. Suárez & K. Solis | SA | G.J.S. 01-13 | FJ150787 | -- | 1 |
| H. petersenii Samuels, Dodd & Schroers | E, NA | CBS 119507 | FJ860568 | FJ860670 | 1 |
| H. phyllostachydis P. Chaverri & Candoussau | E | CBS 114071 | FJ860570 | FJ860673 | 1, 2 |
| H. pilulifera J. Webster & Rifai | E | CBS 120927 | FJ179615 | FJ860674 | 1 |
| H. placentula Grove | E | CBS 121134 | FJ860572 | -- | 1 |
| H. placentula | -- | CBS 120924 | -- | FJ179580 | 1 |
| H. protopulvinata Yoshim. Doi | E, J, NA | C.P.K. 2434 | FJ860574 | FJ860677 | 1 |
| T. protrudens Samuels & P. Chaverri | As | DIS 119F | EU338322 | -- | 1 |
| H. pseudostraminea Yoshim. Doi | J, NA | G.J.S. 90-74 | DQ835454 | -- | 1 |
| H. pseudostraminea | -- | G.J.S. 95-189 | -- | DQ005521, DQ835446, DQ835480 combined | 1 |
| H. psychrophila E. Müll., Aebi & J. Webster | E | C.P.K. 1602 | FJ860575 | FJ860680 | 1 |
| T. pubescens Bissett | NA | DAOM 166162 | EU248613 | AF534624 | 1 |
| H. pulvinata Fuckel | E, NA | CBS 121279 | FJ860577 | FJ860683 | 1 |
| H. rodmanii Samuels & P. Chaverri | E, NA | C.P.K. 2852 | FJ860581 | FJ860688 | 1 |
| H. rogersonii Samuels | E, NA | CBS 119503 | FJ860583 | FJ860690 | 1 |
| T. rossicum Bissett, C.P. Kubicek & Szakacs | R | DAOM 230009 | DQ087240 | -- | 1 |
| H. rufa (Pers.: Fr.) Fr. | E, NA | CBS 119325 | EU711362 | DQ672615 | 1 |
| T. saturnisporum Hammill | C? | CBS 330.70 | DQ087243 | -- | 1 |
| T. scalesiae Samuels & H.C. Evans | SA | G.J.S. 03-74 | EU252007 | -- | 1 |
| H. schweinitzii (Fr.) Sacc. | E, NA | CBS 121275 | FJ860586 | -- | 1 |
| H. schweinitzii | -- | C.P.K. 2005 | -- | FJ860694 | 1 |
| H. semiorbis (Berk.) Sacc. | Au | DAOM 167636 | AF545522 | AF545568 | 1 |
| H. seppoi Jaklitsch | E | CBS 122498 | FJ179617 | FJ179581 | 1 |
| H. sinuosa P. Chaverri & Samuels | A, E | C.P.K. 1595 | FJ179619 | FJ860697 | 1, 2 |
| H. sinuosa | -- | C.P.K. 2008 | -- | FJ860698 | 2 |
| H. spinulosa Fuckel | E | CBS 121280 | FJ860589 | FJ860699 | 1, 2 |
| H. spinulosa | -- | CBS 121272 | FJ860590 | FJ860700 | 1, 2 |
| H. spinulosa | -- | CBS 311.50 | FJ860591 | -- | 1, 2 |
| T. spirale Bissett | C | DIS 311D | FJ442694 | -- | 1 |
| T. spirale | -- | DAOM 183974 | -- | AF534626 | 1 |
| H. stilbohypoxyli B.S. Lu & Samuels | A, Af, E | C.P.K. 1977 | FJ860592 | FJ860702 | 1 |
| H. straminea P. Chaverri & Samuels | As | G.J.S. 02-84 | AY391945 | AY391999 | 1 |
| H. strictipilosa P. Chaverri & Samuels | E, J, NA | C.P.K. 1601 | FJ860594 | FJ860704 | 1, 2 |
| H. strictipilosa | -- | C.P.K. 3135 | -- | FJ179583 | 2 |
| T. strigosum Bissett | A | DAOM 166121 | EU248605 | AF534629 | 1 |
| T. stromaticum Samuels & Pardo-Schulth. | SA | P.C. 209 | AF545539 | AF534613 | 1 |
| H. subalpina Petr. | E | CBS 119128 | FJ860595 | FJ860705 | 1 |
| H. sulawesensis Yoshim. Doi | As | G.J.S. 85-228 | AY391954 | AY392002 | 1 |
| H. sulphurea (Schwein.) Sacc. | E, NA | C.P.K. 1593 | FJ860599 | FJ860709 | 1 |
| H. surrotunda P. Chaverri & Samuels | NA | G.J.S. 88-73 | AF545540 | AF534594 | 1 |
| H. tawa Dingley | As, Au | G.J.S. 97-174 | AY391956 | AY392004 | 1 |
| H. thailandica P. Chaverri & Samuels | As | G.J.S. 97-61 | AY391957 | AY392005 | 1 |
| H. thelephoricola P. Chaverri & Samuels | E, NA | CBS 120925 | -- | FJ860711 | 1, 2 |
| H. thelephoricola | -- | C.P.K. 2480 | -- | FJ860712 | 2 |
| H. thelephoricola | -- | CBS 121138 | FJ860601 | -- | 1 |
| T. theobromicola Samuels & H.C. Evans | SA | DIS 85f | FJ007374 | -- | 1 |
| T. tomentosum Bissett | E, NA | DAOM 178713a | AF545557 | AF534630 | 1 |
| T. tomentosum | -- | C.P.K. 2563 | -- | FJ870906 | 2 |
| H. tremelloides (Schumach.) Fr. | E | CBS 120634 | FJ860602 | FJ860713 | 1 |
| H. victoriensis Overton | Au | G.J.S. 99-200 | DQ835517 | -- | 1 |
| H. victoriensis | -- | C.P.K. 3565 | -- | FJ860718 | 1 |
| H. virens P. Chaverri, Samuels & E.L. Stewart | NA, (E) | GLi39 | AF545558 | AF534631 | 1 |
| H. virescentiflava Speg. | CA, SA | P.C. 278 | AY391959 | AY392007 | 1 |
| H. viridescens Jaklitsch & Samuels | C | C.P.K. 2851 | FJ860606 | -- | 1 |
| H. viridescens | -- | C.P.K. 2046 | -- | DQ672608 | 1 |
| H. voglmayrii Jaklitsch | E | CBS 117711 | FJ179622 | DQ086146 | 1 |
| H. sp. 1 | E | CBS 119286 | FJ860517 | FJ860609 | 1 |
| H. sp. 2 | E | CBS 119284 | FJ860520 | FJ860613 | 1 |
| H. sp. 3 | E | CBS 122494 | FJ860525 | FJ860619 | 1 |
| H. sp. 4 | E | C.P.K. 2021 | FJ860526 | FJ860620 | 1 |
| H. sp. 5 | E | CBS 121133 | FJ860528 | FJ860622 | 1 |
| H. sp. 6 | E | C.P.K. 3127 | FJ860529 | FJ860625 | 1 |
| H. sp. 7 | E | CBS 120922 | FJ860587 | FJ860696 | 1 |
| H. sp. 8 | E | CBS 123828 | FJ860544 | FJ860646 | 1 |
| H. sp. 9 | E | CBS 120926 | FJ860540 | FJ860641 | 1 |
| H. sp. 10 | E | CBS 120537 | FJ860543 | FJ860645 | 1 |
| H. sp. 11 | E | C.P.K. 1896 | FJ860545 | -- | 1 |
| H. sp. 11 | -- | CBS 120632 | -- | FJ860649 | 1 |
| H. sp. 12 | E | C.P.K. 1900 | FJ860553 | -- | 1 |
| H. sp. 12 | -- | CBS 119506 | -- | FJ860657 | 1 |
| H. sp. 13 | E | CBS 120533 | FJ860559 | -- | 1 |
| H. sp. 13 | -- | CBS 122126 | -- | FJ860662 | 1 |
| H. sp. 14 | E | CBS 119283 | FJ860569 | FJ860672 | 1 |
| H. sp. 15 | E | CBS 119288 | FJ860578 | FJ860685 | 1 |
| H. sp. 16 | E | WU 29467 | FJ860585 | FJ860693 | 1 |
| H. sp. 17 | E | CBS 120929 | FJ860597 | FJ860707 | 1 |
| H. sp. 18 | E | CBS 120923 | FJ860605 | FJ860717 | 1 |
| Protocrea farinosa (Berk. & Broome) Petch | E | CBS 121551 | EU703935 | -- | 1 |
| P. farinosa | -- | C.P.K. 2472 | -- | EU703892 | 1 |
| P. pallida (Ellis & Everh.) Jaklitsch et al. | E, NA | CBS 121552 | EU703944 | -- | 1 |
|
P. pallida |
--
|
CBS 299.78 |
--
|
EU703900
|
1 |
A. Americas; CA, NA, SA. Central-, North-, South America; Af. Africa; As. Asia; Au. Australasia; C, C?. (probably) cosmopolitan; E. Europe; J. Japan; R. Russia.
Ascospore isolates were prepared in the following way from fresh specimens of Hypocrea stromata: parts of stromata were crushed in sterile distilled water. The resulting suspension was transferred to cornmeal agar plates (Sigma, St. Louis, Missouri) supplemented with 2 % (w/v) D(+)-glucose-monohydrate (CMD), and 1 % (v/v) of an aqueous solution of 0.2 % (w/v) streptomycin sulfate (Sigma) and 0.2 % (w/v) neomycin sulfate (Sigma). Plates were incubated overnight at 25 °C. Few germinated ascospores or a part of an ascus were transferred to fresh plates of CMD, using a thin platinum wire. The plates were sealed with Parafilm (Pechiney, Chicago, Illinois) and incubated at 25 °C. The cultures obtained were maintained at 15 °C and parts frozen and kept at -80 °C.
Growth characterisation
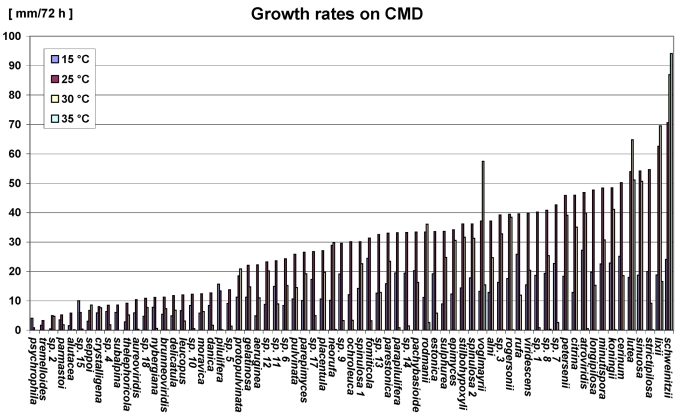
Growth rates, the optimum temperature of growth, and colony characteristics were determined on three different media at four different temperatures. The strains were pregrown on CMD, or MEA (2 % malt extract, 2 % agar-agar, both from Merck) where noted, until they reached a diameter of 55–65 mm. Agar plugs 0.5 cm diam were then cut from the margin of the colonies and transferred to fresh medium, 1.0–1.5 mm from the edge of the 9-cm-diam Petri dish with the mycelium facing down on the surface of the agar. CMD, PDA (potato dextrose agar, Merck, Darmstadt, Germany) and low nutrient agar (SNA, Nirenberg 1976; pH adjusted to 5.5) were used. The tests were performed at 15 °C (with alternating 12 h weak UV light (Philips TL-D/08 blacklight blue) and 12 h darkness), 25 °C (with alternating 12 h cool white fluorescent light and 12 h darkness), and 30 °C and 35 °C (both in darkness). The different lighting conditions were due to a simultaneous use of incubators to culture for anamorph morphology. For growth at 25 °C, the Petri dishes were sealed with Parafilm to avoid drying out of the agar caused by the ventilator of the cooling incubator (MIR 153, Sanyo, Gunma, Japan). The maximum colony radius was measured once daily for at least 7 d or until the plates were entirely covered with mycelium. The growth rate was calculated by linear regression of log t versus log r (t = time of incubation, r = radius measured from the edge of the agar plug), using only measurements from the phase where the logarithmic increase of the colony radius was linear over log t. The data given are ranges obtained from 3–6 experiments for all media and temperatures, except species with stipitate stromata, H. alutacea, H. nybergiana and H. seppoi, where only single experiments were carried out.
In addition, the plates were examined daily under the compound microscope at low magnification (10× objective), and the time of first appearance of conidia, autolytic behaviour of marginal hyphae, coilings in surface hyphae, presence of chlamydospores, formation of pigments and odour, and the colony appearance were noted.
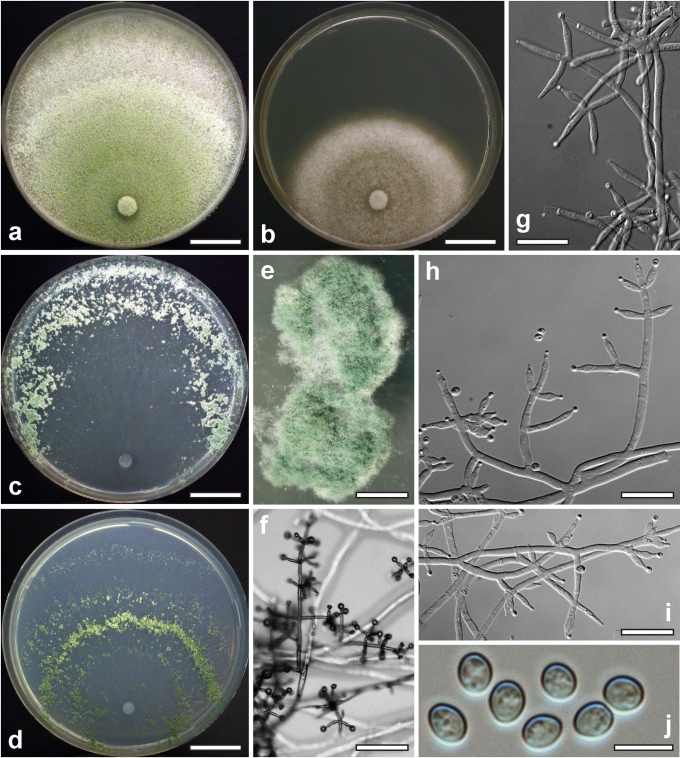
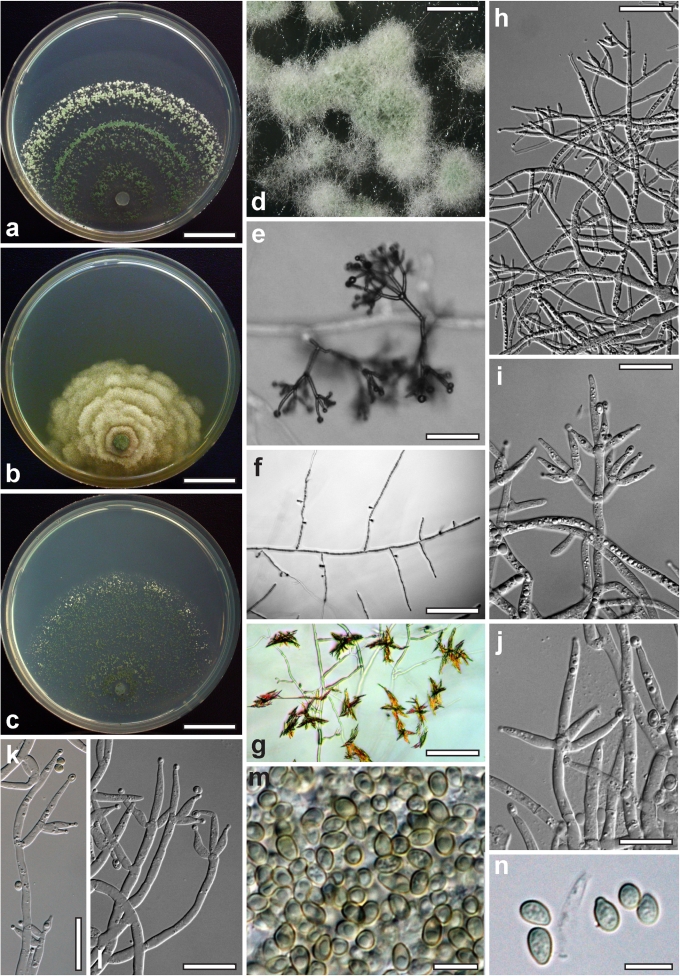
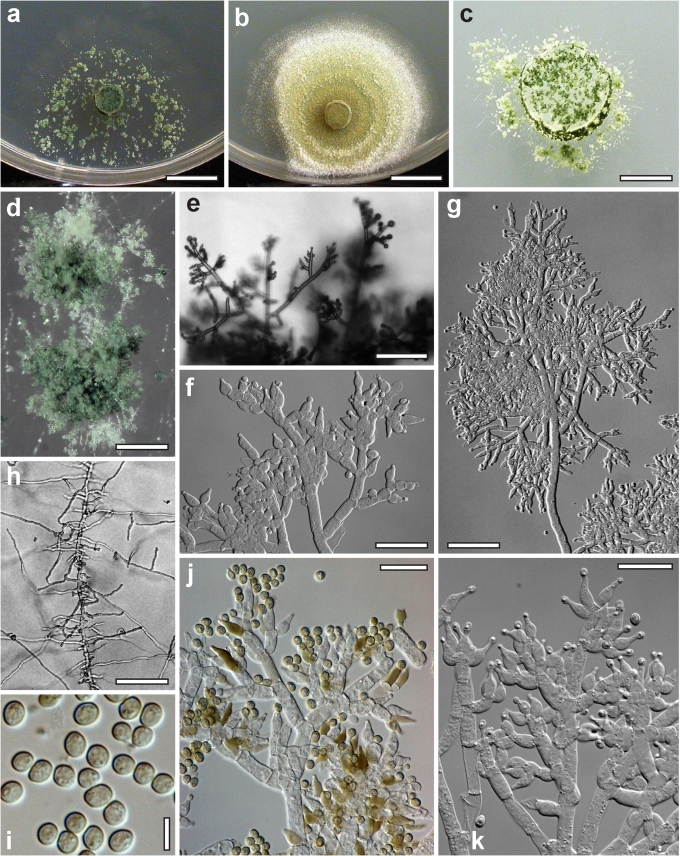
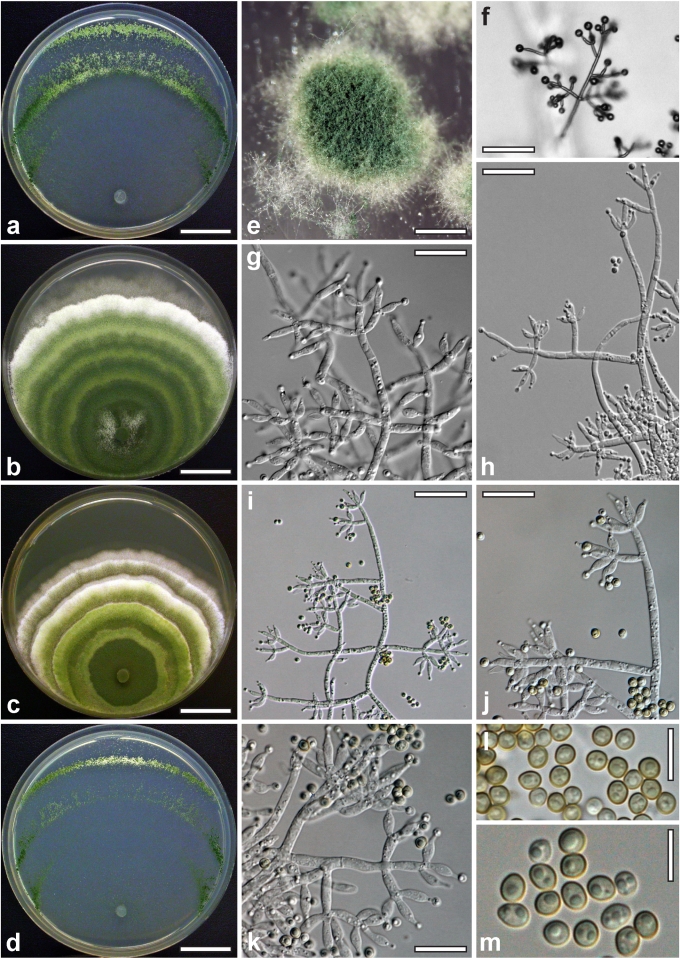
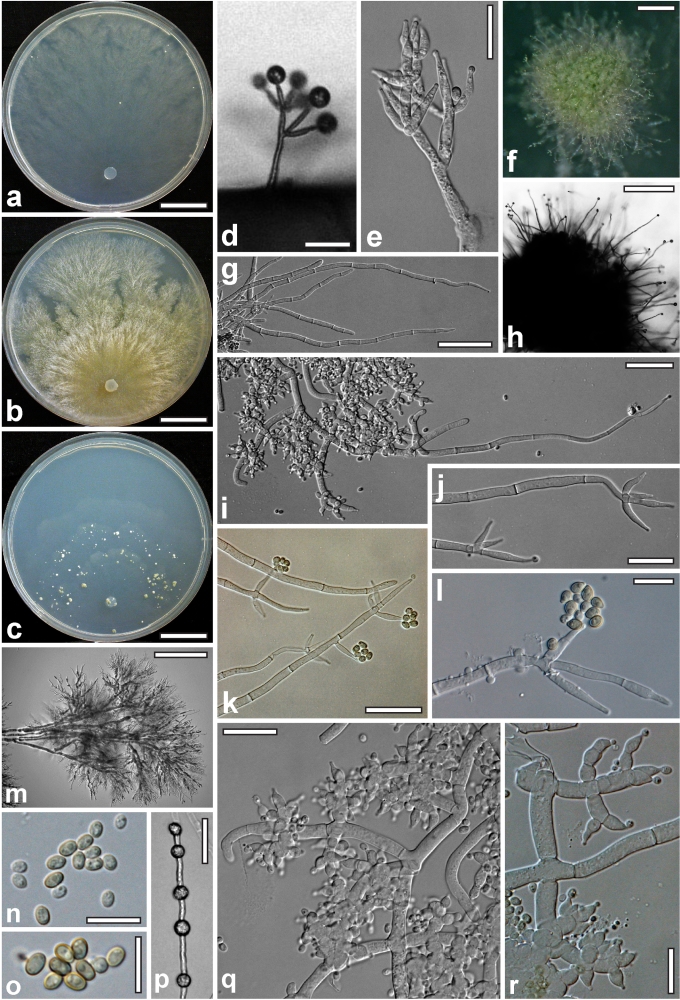
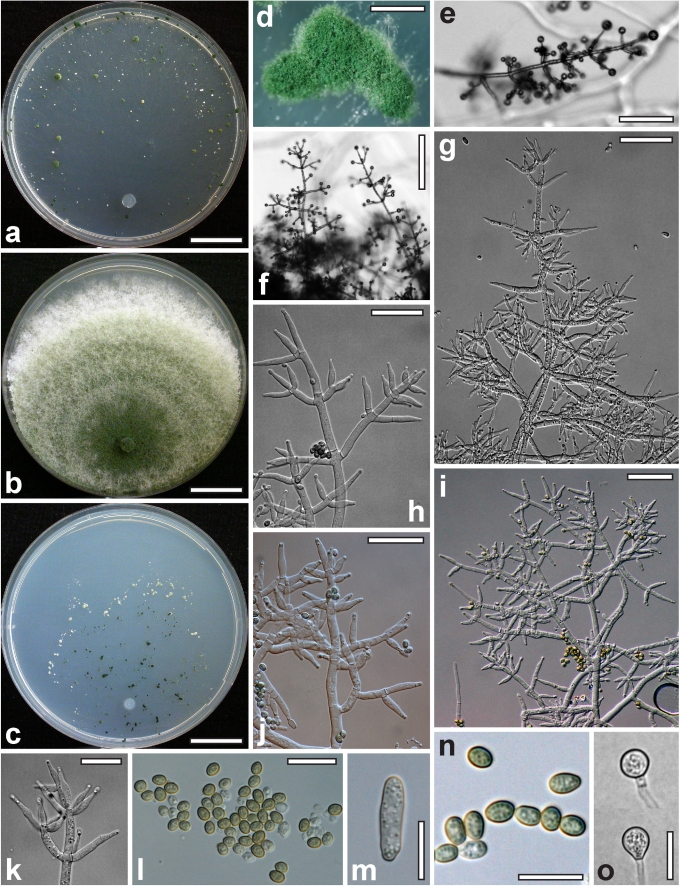
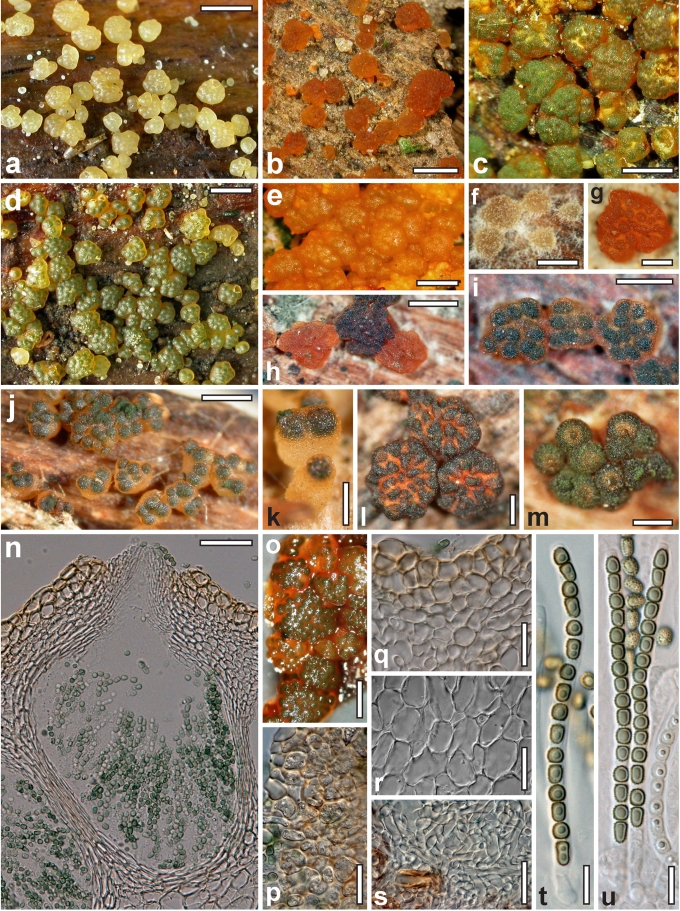
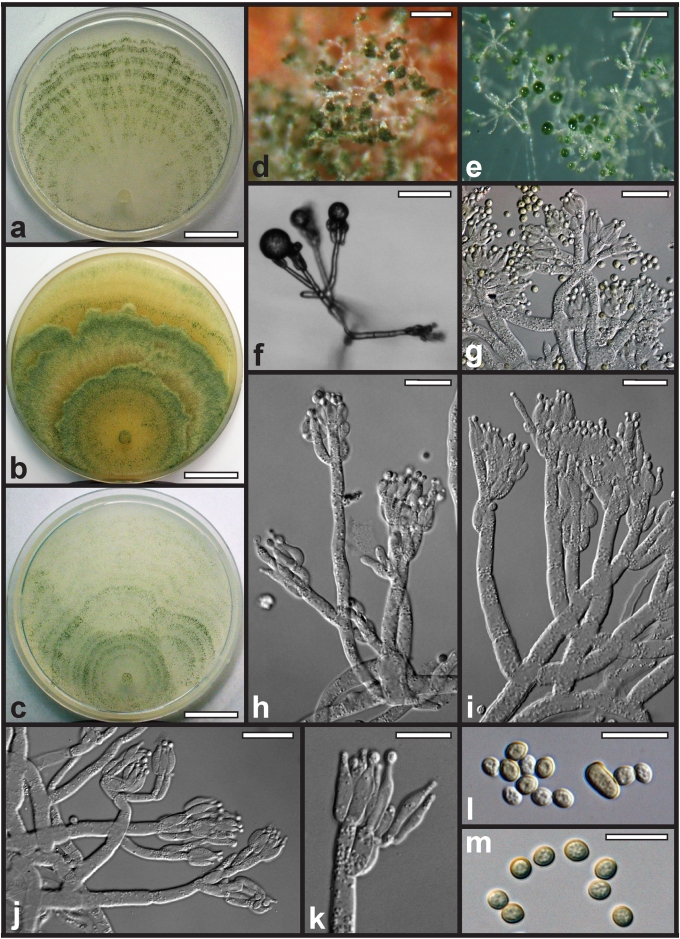
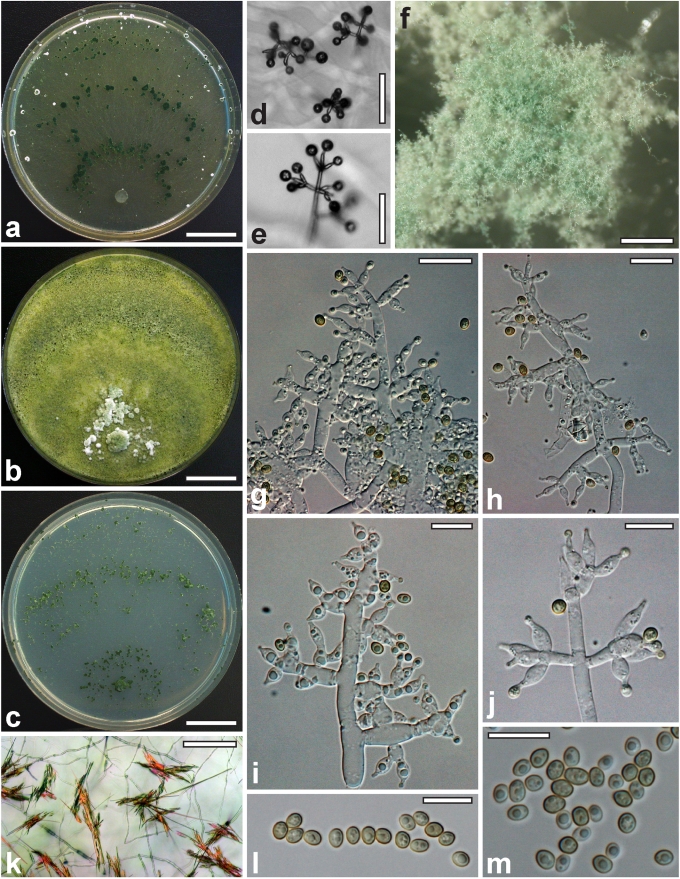
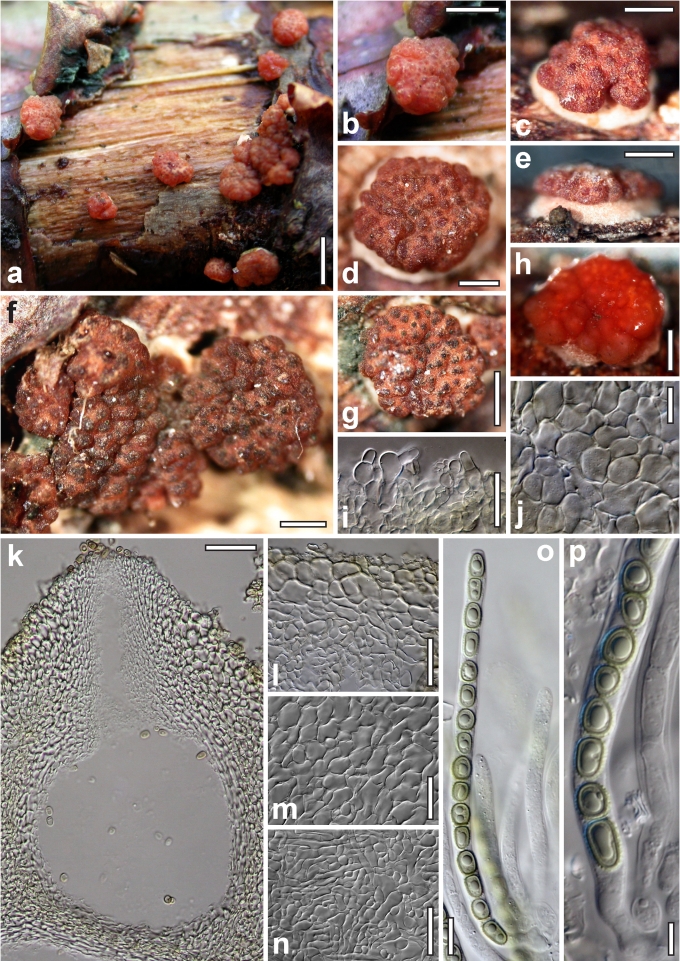
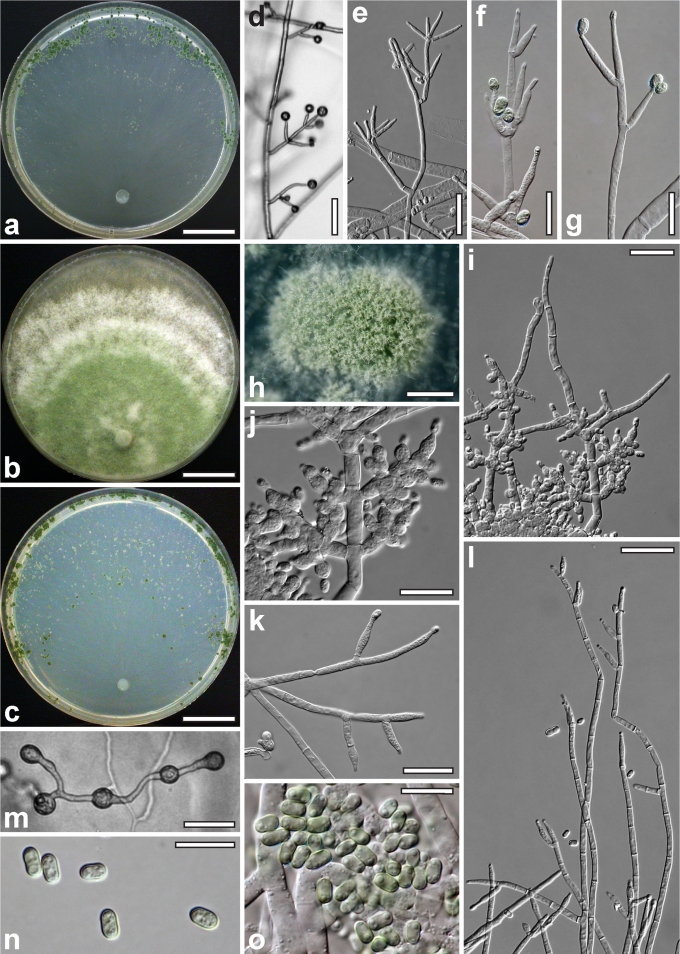
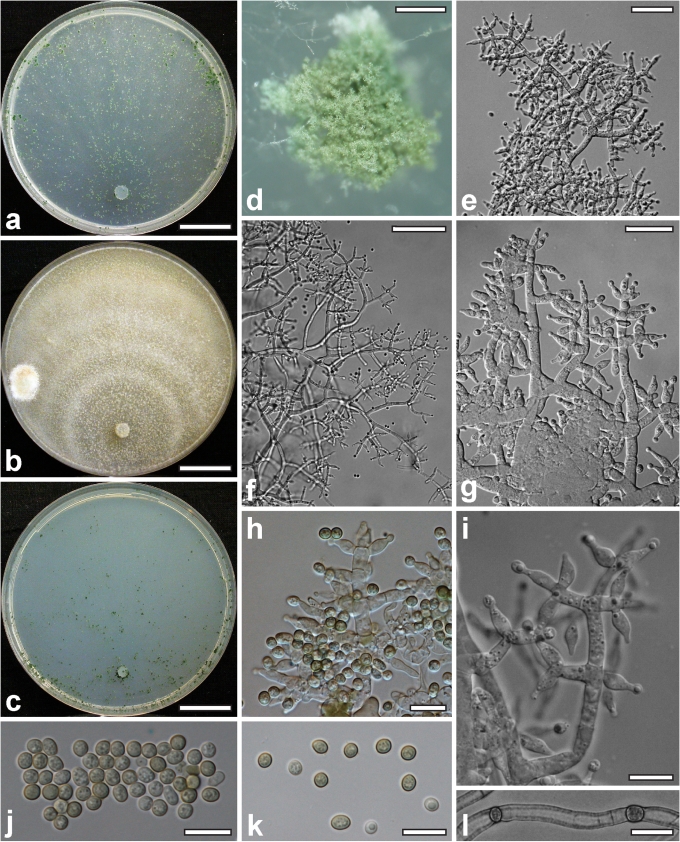
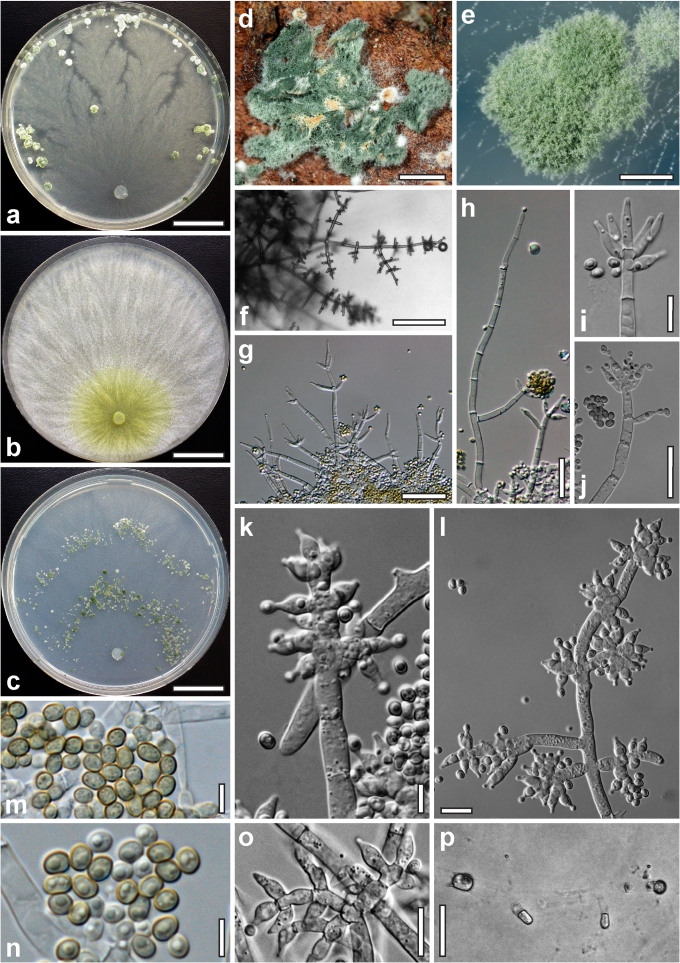
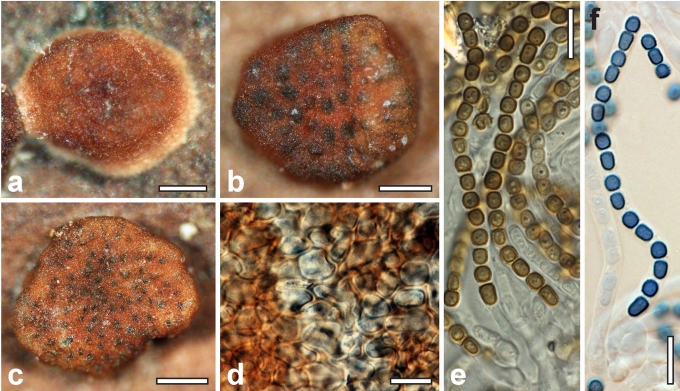
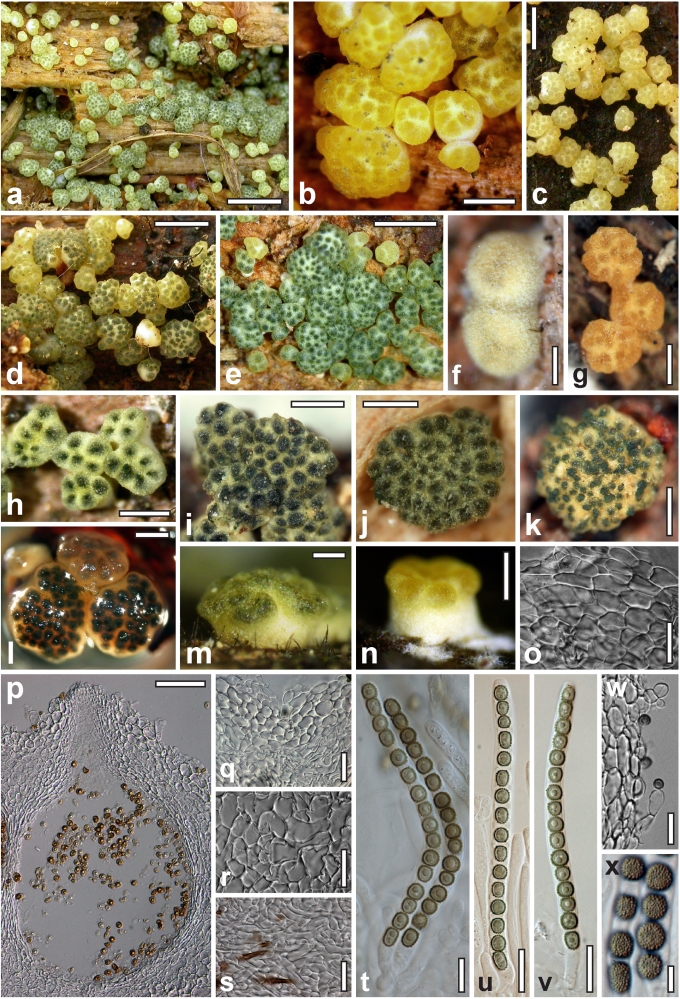
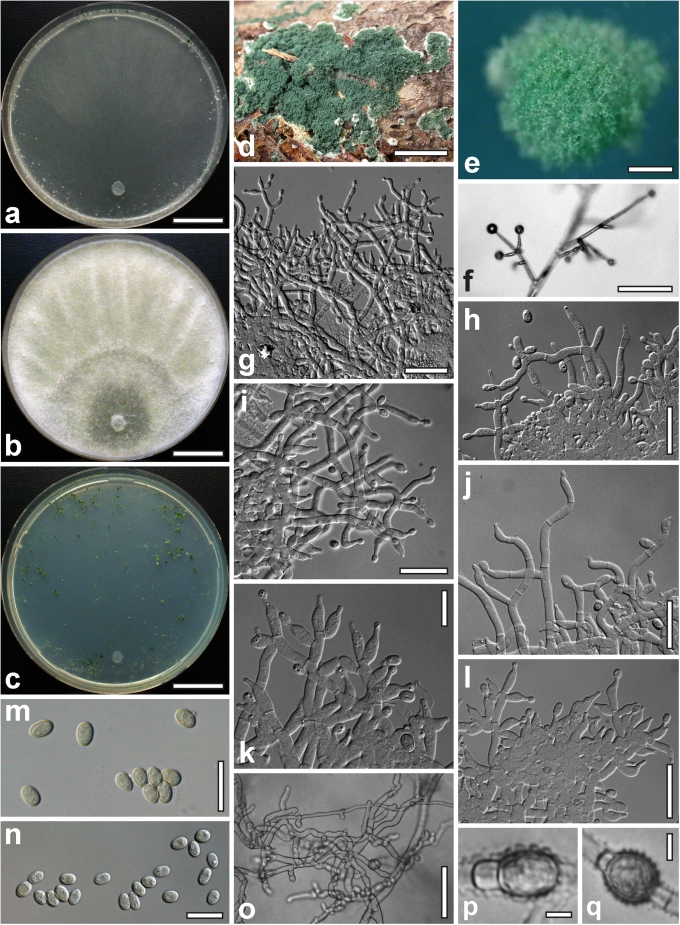
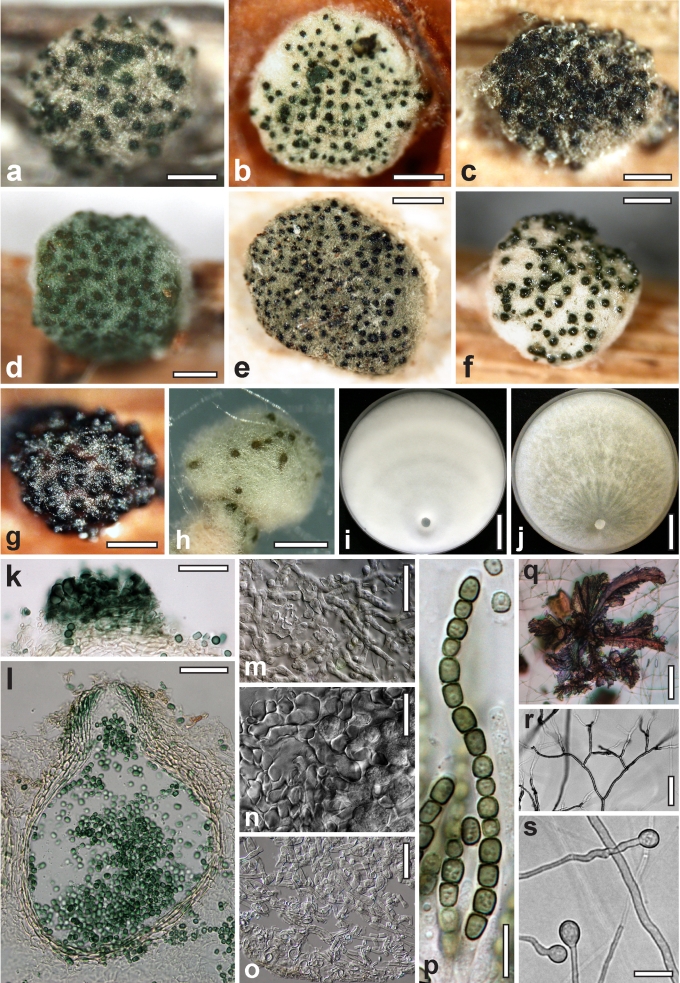
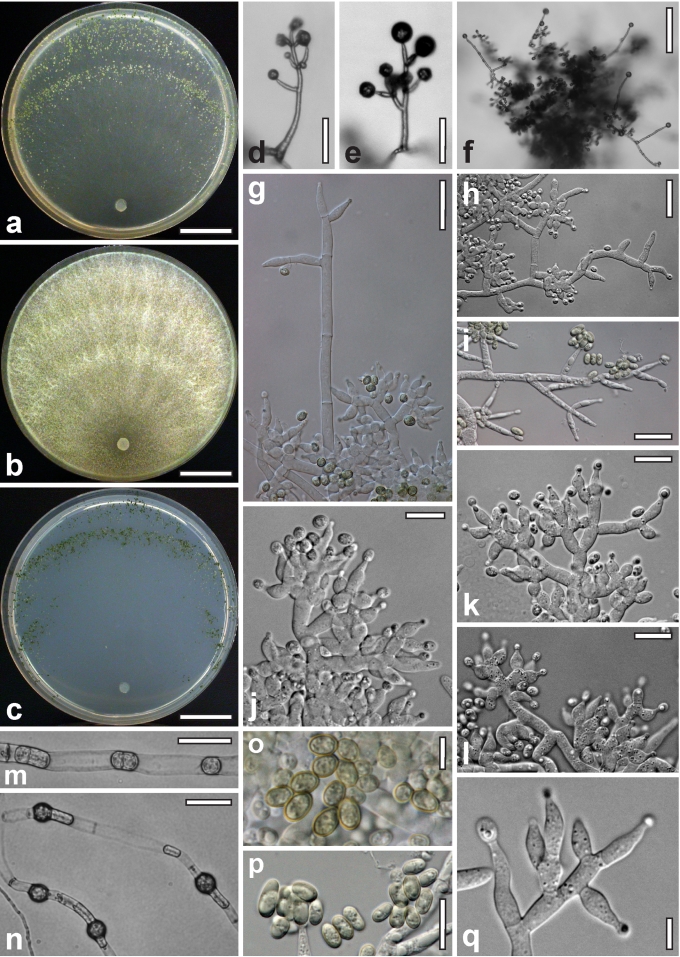
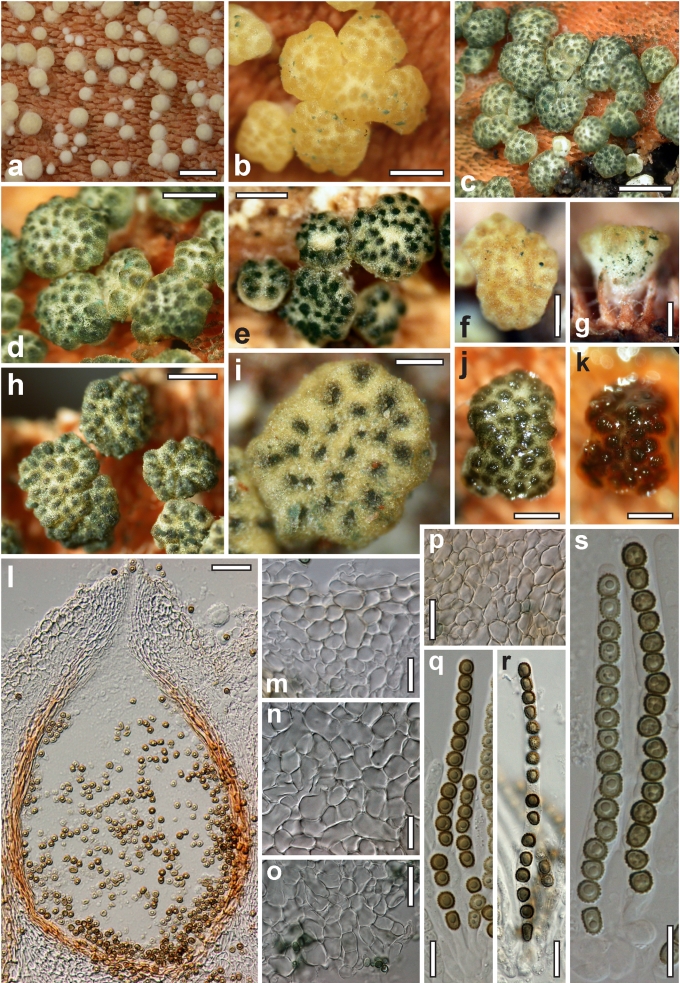
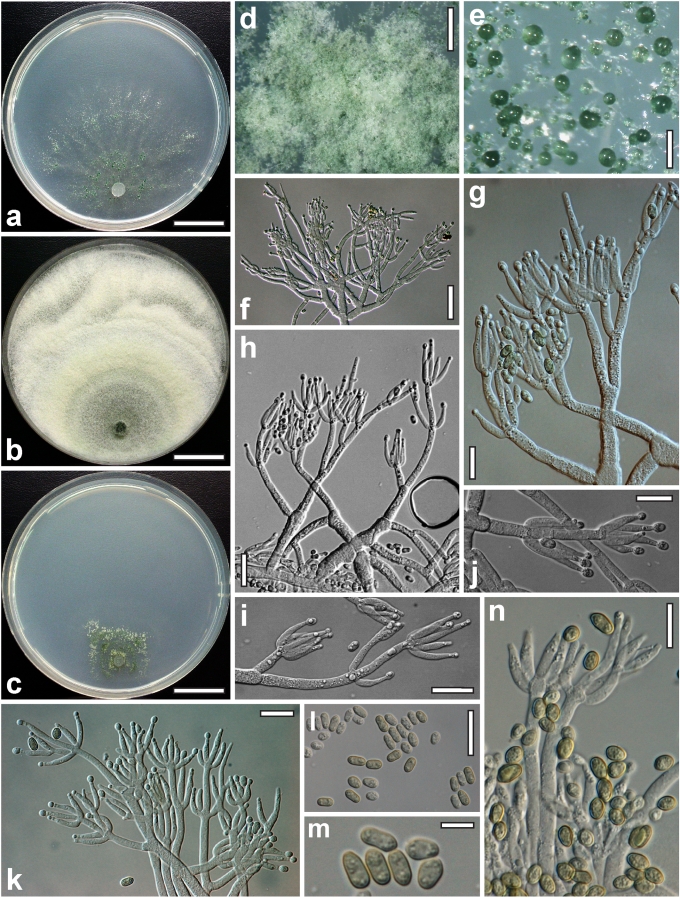
Morphological observations
Conidiation structures were examined, measured and photographed on a compound microscope from cultures grown on CMD, SNA, or PDA or MEA in certain cases, on the plates under low magnification and after mounting in 3 % KOH. The following characters were measured: length of conidia, width of conidia, length of phialides, width of phialides at the base, width of phialides at the widest point. The size of chlamydospores was measured by examining colonies grown on CMD or SNA under the conditions described above using the 40× objective of a compound microscope.
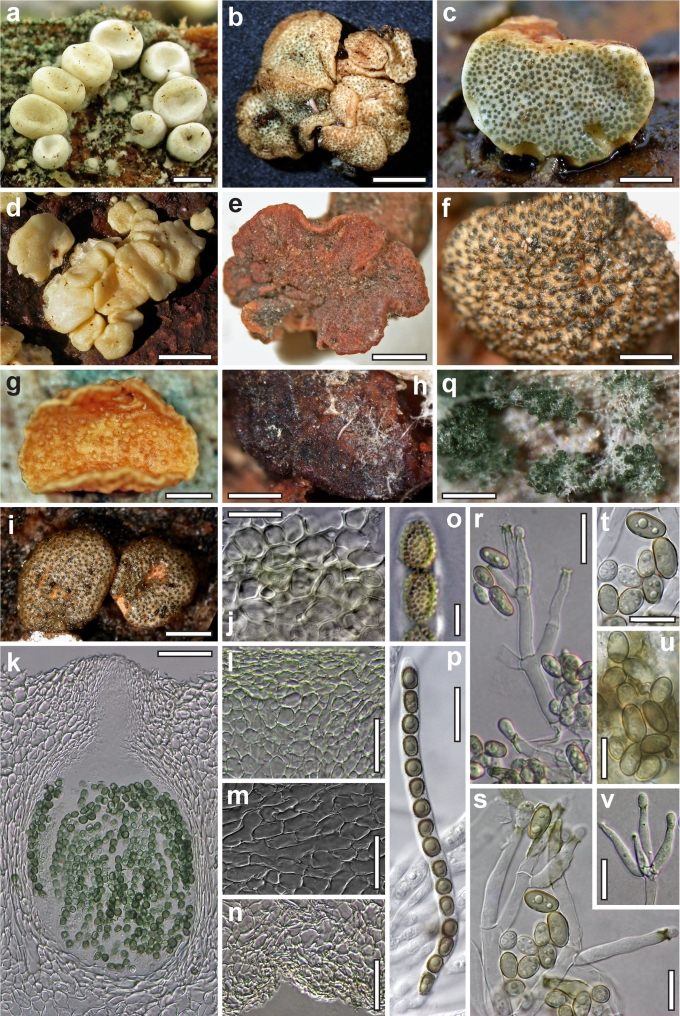
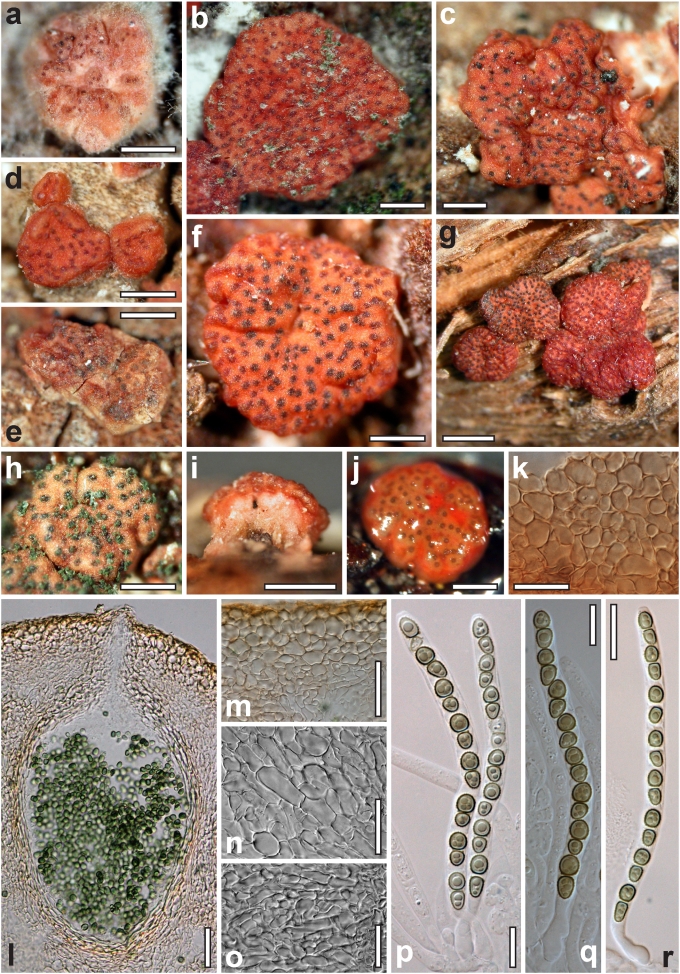
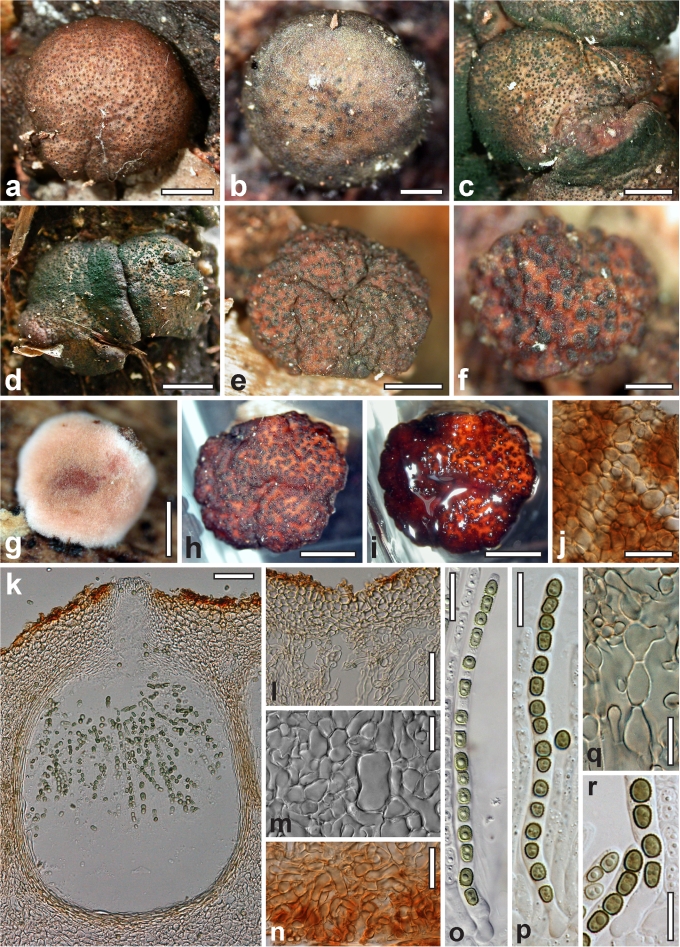
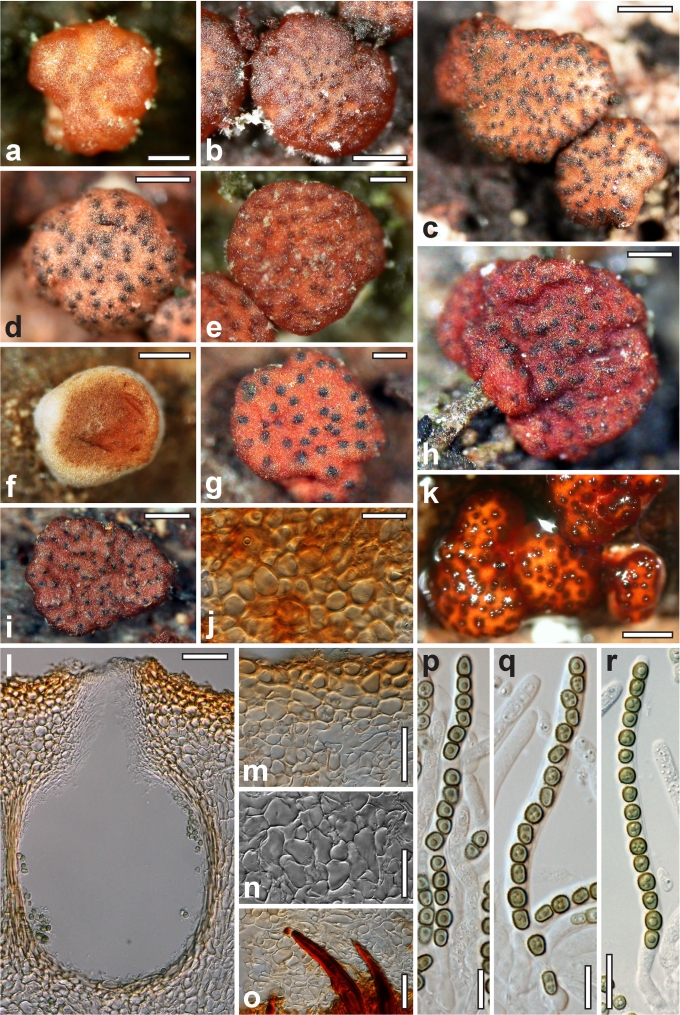
Dry stromata of Hypocrea were rehydrated overnight with water vapour in a closed glass chamber at room temperature, treated briefly with 3 % KOH to observe colour changes, embedded in Tissue-Tek O.C.T. Compound 4583 (Sakura Finetek Europe B.V., Zoeterwoude, The Netherlands) and sectioned at a thickness of 10–12 μm with a freezing microtome. Sections were measured and photographed in lactic acid, or 3 % KOH where noted.
Whenever possible, gross morphology including colour and approximate sizes of fresh stromata were determined in the field in order to estimate changes caused by drying. In dry stromata the diameter, height, colour and shape of the stromata were determined. In sections the following teleomorph characteristics were evaluated: perithecium shape, length and width; colour, width of perithecium wall; length and diameter of ostioles; thickness and structure of the surface region (cortex), structure and size of cells of the subcortical (between the perithecia and the cortex), subperithecial (below the perithecia) and basal regions of the stroma. Length and width of asci and distal and proximal ascospore cells were measured in separate preparations in 3 % KOH (or water or lactic acid where noted). Measurements are reported as maxima and minima in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses. Nomarski differential interference contrast (DIC) was used for observations and measurements. Images were recorded with the Nikon Coolpix 4500 or DS-U2 digital cameras. Measurements were carried out directly through microscope oculars or using the NIS-Elements D (v. 2.2 or 3.0) software. Colours were determined with Methuen's Handbook of Colour (Kornerup & Wanscher 1981). Colour terms are not strictly followed, therefore also the codes are cited.
DNA extraction, PCR amplifications and sequencing
Mycelium for DNA extraction was grown on PDA or MEA covered by sterile cellophane. Genomic DNA was extracted using the Plant DNeasy Mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions, using approximately 150 ± 50 mg fresh mycelium. A region of nuclear DNA, containing the ITS1 and 2 regions, was amplified by PCR using the primer combinations SR6R and LR1 (White et al. 1990). A 1.3 kb fragment of the tef1 gene encoding translation elongation factor 1 alpha was amplified using the primer pair EF1728F (Chaverri & Samuels 2003) and TEF1LLErev (Jaklitsch et al. 2005). This fragment includes the fourth and the fifth intron and a part of the last large exon (6). A 1 kb fragment of RNA Polymerase II subunit B (rpb2) was amplified using the primer pair fRPB2-5f and fRPB2-7cr (Liu et al. 1999). PCR products were either purified using the QIAquick Kit (Qiagen) according to the manufacturer's instructions, or by an enzymatic PCR cleanup (Werle et al. 1994). For the latter, 20 μL PCR reactions were digested with 10 units Exonuclease I (Fermentas, St. Leon-Rot, Germany) and 2 units Calf Intestine Alkaline Phosphatase (Fermentas) for 45 min at 37 °C, followed by an enzyme deactivation step at 85 °C for 15 min. DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, UK) and an automated DNA sequencer (ABI Genetic Analyzers, Applied Biosystems) with the same primers as in PCR or with the internal primers 5'-CCGTGA(T/C)TTCATCAAGAACATG-3' and 5'-TTGGCAGTGTCCATCTTGTTG-3' for tef1, and 5'-GATCAA(C/T)(A/C)GAGG(C/T)ATGGA(G/A)GT-3' and 5'-GA (G/A)AAGATTTT(G/A)AA(C/T)TC(C/T)TG(G/A)T-3' for rpb2. All sequences used in this study are listed in Table 1.
Molecular phylogenetic analyses
To identify isolates of Hypocrea/Trichoderma, ITS sequences were subjected to the TrichOKey routine (Druzhinina et al. 2005), sequences of rpb2 and tef1 to TrichoBLAST (Kopchinskiy et al. 2005). Both tools are implemented on www.ISTH.info. In addition, the NCBI Nucleotide Blast Search was employed to check for the most recent accessions.
To show the phylogeny of the genus including nearly all known species that form Hypocrea teleomorphs in Europe, an extensive matrix containing rpb2 sequences of 135 taxa of Hypocrea/Trichoderma was constructed, using both representative sequences from this work and from GenBank. To increase the number of variable sites in the phylogenetic analysis, the rpb2 data matrix was combined with a matrix of sequences representing a part of tef1-exon 6 of all species available (122 taxa), including two sequences from the ISTH multiloci database. Due to sequence variations within H. spinulosa sequences of three isolates of this species were included. Protocrea farinosa and P. pallida were selected as outgroup taxa. Sequences were aligned with ClustalX v.1.81 (Thompson et al. 1997) and visually refined in Genedoc 2.6 (Nicholas et al. 1997). Alternative alignments done with the online version of Muscle (www.ebi.ac.uk/Tools/muscle/) resulted in the same phylogenetic trees (not shown). The individual interleaved NEXUS files were produced with ClustalX and manually combined. After the exclusion of leading and trailing gap regions and tef1 introns, 892 characters for rpb2 and 637 characters for tef1 were included in the phylogenetic analyses. Maximum parsimony (MP) analyses of the combined DNA matrix was performed with PAUP* v.4.0 b10 (Swofford 2002), using 10,000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, COLLAPSE=MAXBRLEN, steepest descent option not in effect). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data. MP bootstrap analysis with 1000 replicates was performed in the same way, but using 10 rounds of random sequence addition and subsequent TBR branch swapping during each bootstrap replicate. The Bayesian approach to phylogenetic reconstruction (Rannala & Yang 1996, Yang & Rannala 1997) was implemented with MrBayes 3.0B4 (Huelsenbeck & Ronquist 2001). Modeltest 3.6 (Posada & Crandall 1998) was used to compare the likelihood of different nested models of DNA substitution and to select the best-fit model for the two individual data sets under the Akaike Information Criterion (TVM+I+G for rpb2, GTR+I+G for tef1). The rpb2 and tef1 datasets were partitioned for the Bayesian analysis. Three parallel runs of four incrementally heated simultaneous Markov chains were performed over 1 and 3 million generations from which every 100th tree was sampled in each run. The trees before apparent stationary probability distribution of the cold chain were discarded. A 90 % majority rule consensus of the remaining trees was computed to obtain estimates for the probabilities that groups are monophyletic based on the sequence data (posterior probabilities). Statistical support of nodes of the resulting phylogenetic tree is indicated for three range combinations: MP bootstrap values (BS) = 70–100 and Bayesian posterior probabilities (PP) = 95–100, BS < 70 and PP = 95–100, and BS > 70 and PP < 95.
Along with the taxonomic treatment of European species with green ascospores a phylogenetic tree containing these species and the North American H. ceramica (sensu Chaverri & Samuels 2003) and two Trichoderma species of the Harzianum Clade was calculated, using tef1 introns 4 and 5 and exon 6. Hypocrea fomiticola, located in the Semiorbis Clade (see Fig. 1), was excluded from the analyses due to non-alignability of its tef1 introns with the dataset of other green-spored species. For visualisation as a rectangular phylogram (Fig. 2) the resulting tree is rooted with H. phyllostachydis, a species not clustered with other green-spored taxa in the combined tree (Fig. 1). The same methods as described for the combined analysis were used, apart from the substitution model (HKY+I+G) used for the Bayesian analysis. Statistical support of nodes of the resulting phylogenetic tree is indicated for the ranges BS = 89–100 and PP = 95–100, and BS < 89 and PP = 91–93. The phylogenetic trees were deposited in the Treebase database (www.treebase.org/) and are available under the study accession number S2425 (matrix accession numbers M4598, M4599).
Fig. 1.
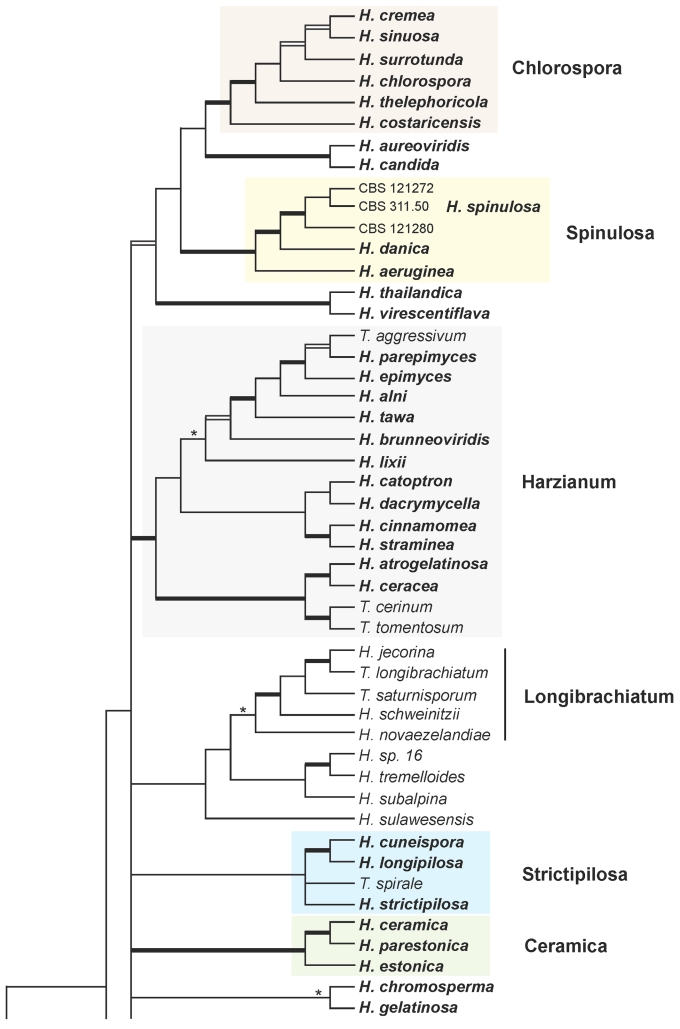
Strict consensus tree of length 5952 resulting from a maximum parsimony (MP) analysis of 1529 characters of the combined rpb2 - tef1 exon alignment of 135 species of Hypocrea/Trichoderma. Broad black lines represent nodes with MP bootstrap values (BS) = 70–100 and Bayesian posterior probabilities (PP) = 95–100, black boxes stand for nodes with BS < 70 and PP = 95–100, and asterisks (*) for nodes with BS > 70 and PP < 95. For strain and accession numbers see Table I. Names of Hypocrea species with green ascospores are indicated in bold. Major clades containing green-spored species are underlain with colour, clade names are indicated on the right.
Fig. 2.
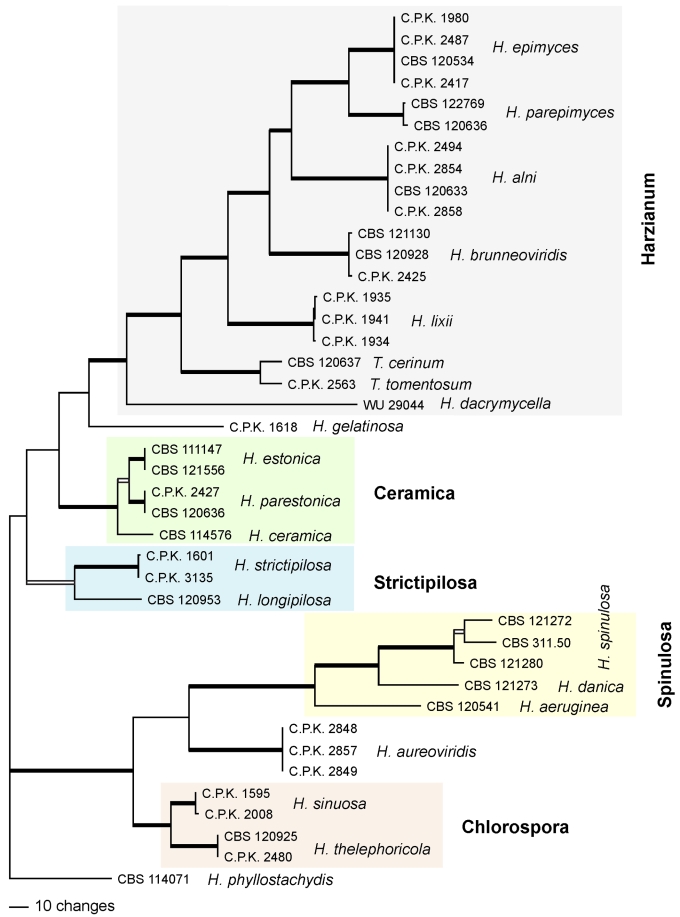
One of 2 phylograms of length 1218 resulting from a MP and Bayesian analysis of 1293 characters of the tef1 (introns and exon) alignment. The major clades containing European species of Hypocrea with green ascospores are represented. MP bootstrap values of 89–100 % and Bayesian posterior probabilities of 95–100 % are indicated by broad black lines, BS < 89 and PP = 91–93 by black boxes. Indicated numbers are strain numbers. Clades are underlain with colour, clade names are given in bold.
RESULTS AND DISCUSSION
Biodiversity, species numbers
In a first attempt to assess the biodiversity of Hypocrea in Europe emphasis was laid on Central Europe, in particular Austria, because most European climatic zones are represented in this country, Pannonian in the East, oceanic and continental in the West and North, illyrian in the South, (sub-)alpine in the Alps, and diverse microclimates. Later the search was extended to other countries.
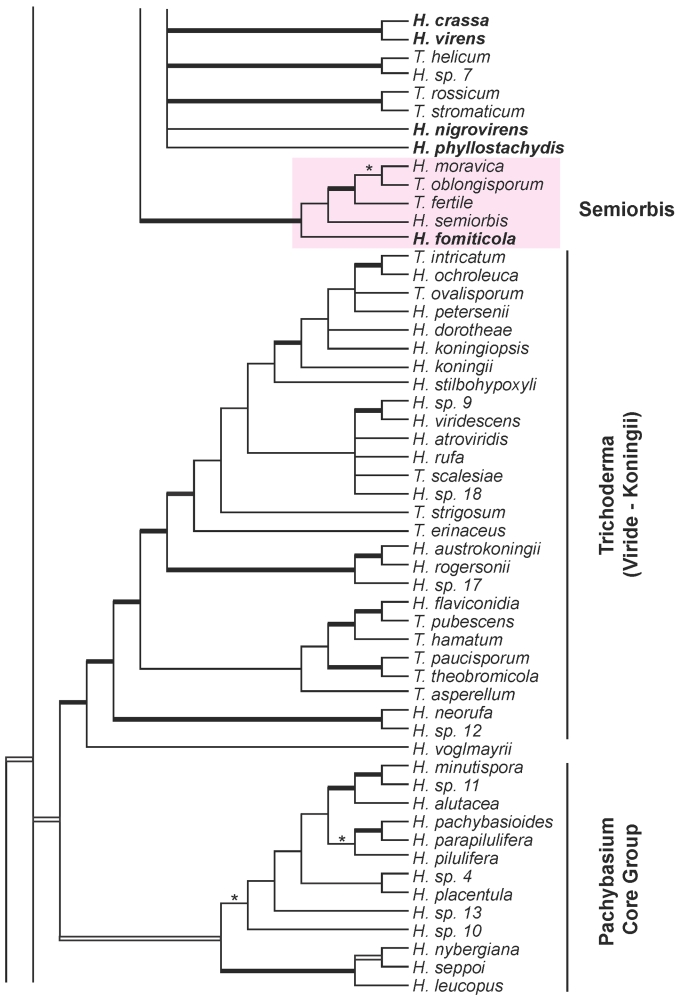
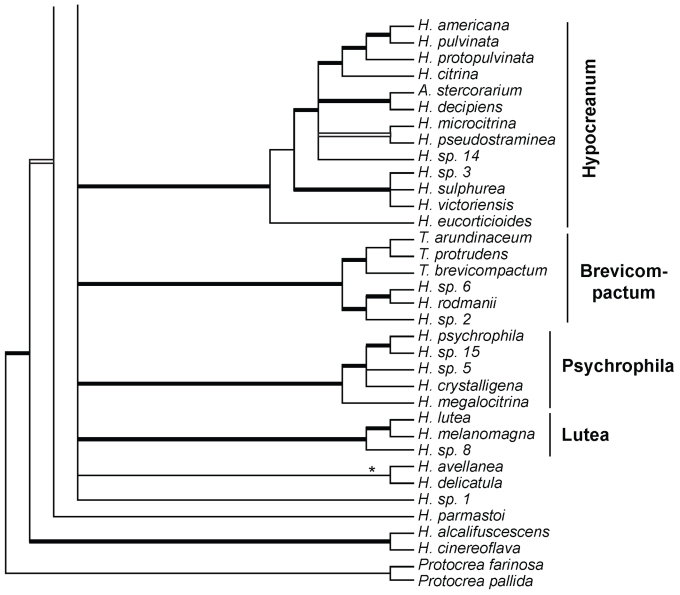
More than 620 specimens containing Hypocrea teleomorphs were collected in 14 European countries in the years 2003–2008. This number includes specimens sent by several mycologists. Several hundred isolates were prepared including cultures from associated anamorphs in case of immature specimens. Identification of material freshly collected even in a small geographic area made clear that any initial expectations concerning species of Trichoderma forming teleomorphs had been dramatically underrated. Expectations had been mostly based on experience attained by workers dealing with the soil mycoflora, but also on specimens found in some European herbaria. Seventy-five species of Hypocrea, two species of Protocrea and Arachnocrea stipata have been identified in Europe. These include species described previously without molecular data and those recently described but not found during the project. Protocrea was recently redescribed (Jaklitsch et al. 2008) and will not be treated further. For descriptions of Arachnocrea stipata see Moravec (1956a), Dennis (1981), or Rossman et al. (1999). Thirty-seven Hypocrea species were undescribed in an early phase of the project. Of these, Samuels and collaborators (Lu & Samuels 2003, Samuels et al. 2006, Degenkolb et al. 2008a) described four (H. petersenii, H. rodmanii, H. rogersonii, H. stilbohypoxyli), Overton and collaborators (2006b) described two (H. alcalifuscescens, H. parmastoi), and Jaklitsch and collaborators (2005, 2006a, b, 2008a, b, c) described seven (H. alni, H. brunneoviridis, H. crystalligena, H. decipiens, H. seppoi, H. viridescens, H. voglmayrii). The residual new species will be described in two parts. This part contains all species having green ascospores. Those species having hyaline ascospores will be treated in a second publication. Trichoderma states are described and given scientific names. Species to be described as new in the second publication are coded here with numerals (Hypocrea sp. 1, 2, etc.).
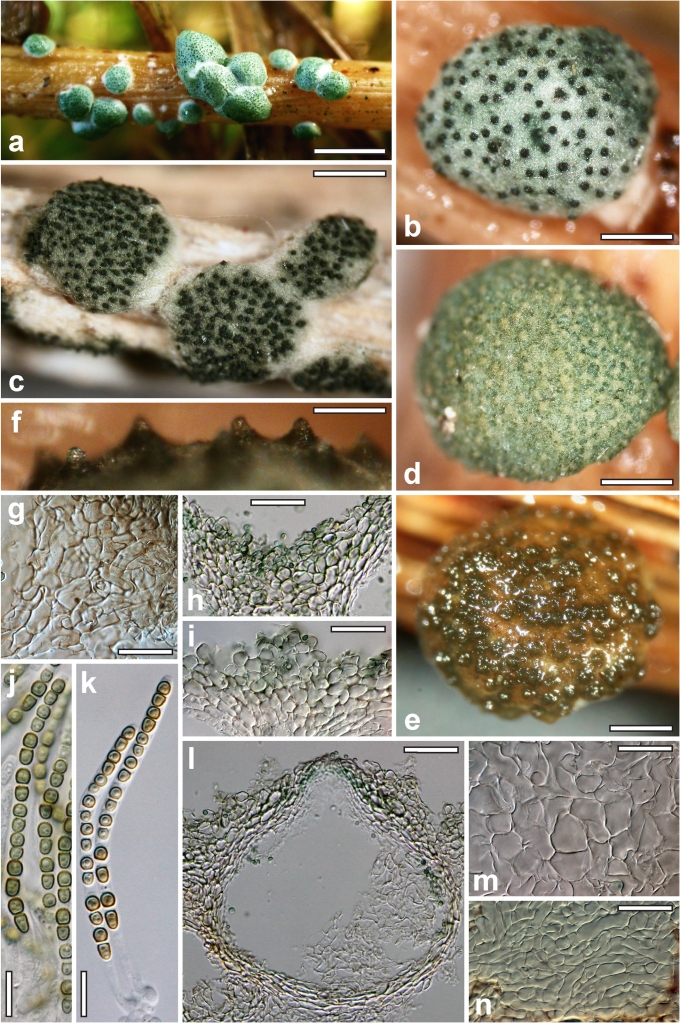
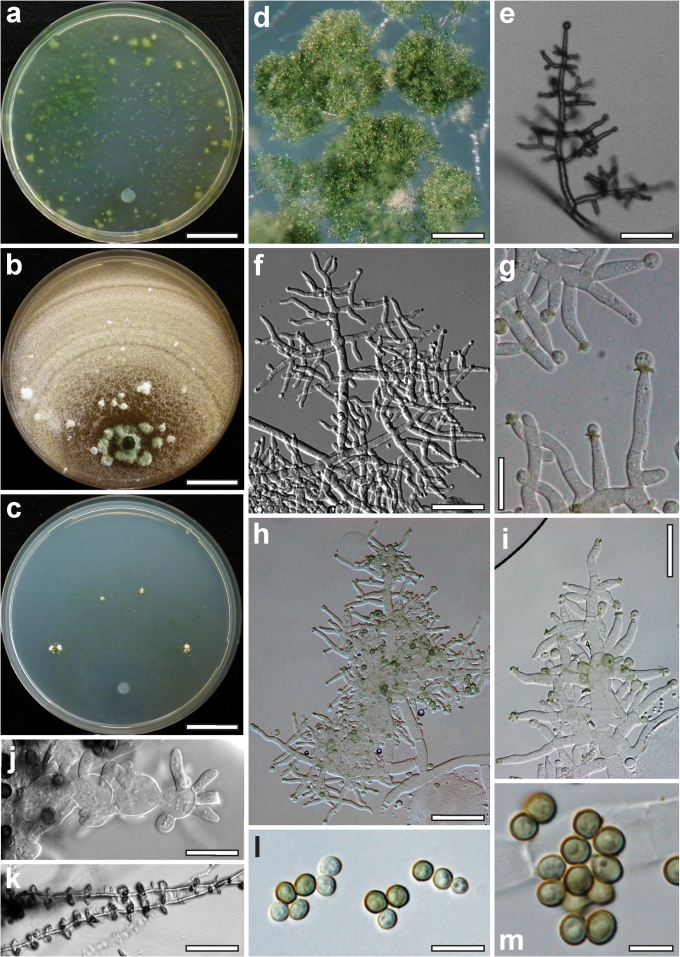
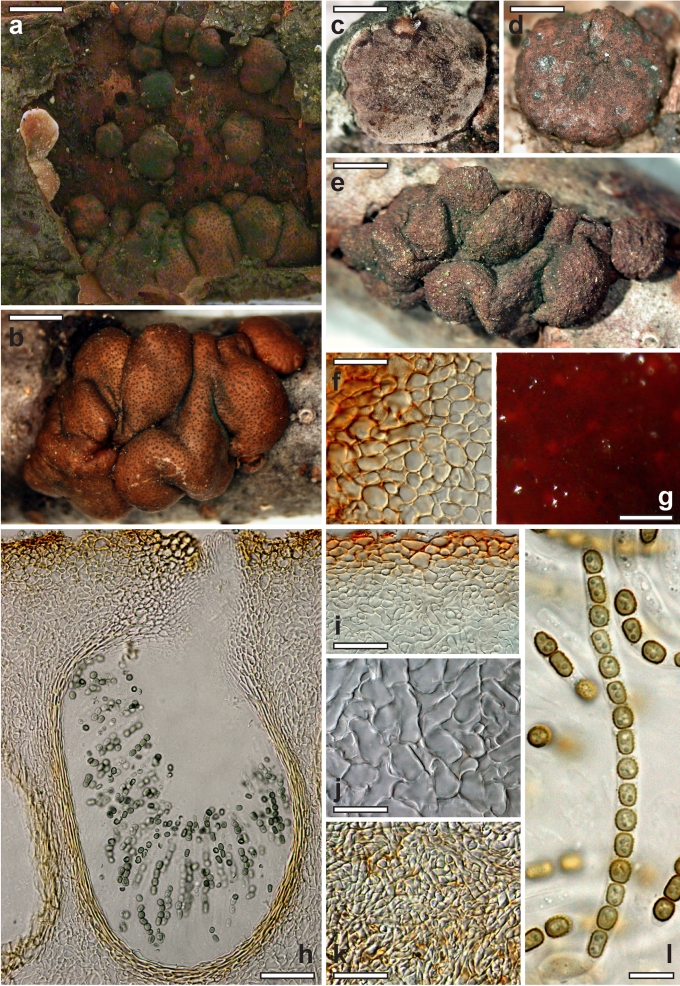
Nineteen species of Hypocrea detected in Europe have green ascospores. They are here described in detail. Previously undescribed taxa include four new holomorphs, two new teleomorphs and the anamorph of Hypocrea dacrymycella. The teleomorph of Trichoderma longipile Bissett, H. longipilosa, is reported for the first time from Sweden. Notes on the morphological distinction of taxa are given after each description (see Taxonomy section). All green-spored species belong to previously recognised clades, except H. spinulosa, which constitutes the new Spinulosa Clade with two additional new species (H. aeruginea and H. danica), and H. fomiticola, which belongs to the Semiorbis Clade and forms effuse to large subpulvinate stromata on Fomes fomentarius, a trait new to species with green ascospores.
As far as available, all type specimens of European species without recent treatment have been examined. Results are incorporated in the individual species descriptions here. A list of doubtful or excluded names will be published in the second publication. Three species described in the 19th century from England, H. argillacea W. Phillips & Plowr., H. splendens W. Phillips & Plowr. and H. strobilina W. Phillips & Plowr., probably represent good species of the genus and are included in the number of species given above. Unfortunately, they have not been recollected despite two extensive searches in England, thus no molecular data are available for these species and their phylogenetic position is still unknown. Two species, Hypocrea dacrymycella and a new one with hyaline ascospores (H. sp. 16, see Fig. 1), do not grow in culture. Gene sequences of these species were determined using DNA extracted from stromata.
This work is a preliminary biota of Trichoderma forming teleomorphs in Europe. Biodiversity is here used basically in the sense of species numbers. It is currently premature and meaningless to apply community analysis and calculate biodiversity measures such as the Shannon index (see e.g. Zachow et al. 2008). This would require more extensive sampling. Based on collecting of Hypocrea teleomorphs for five years, abundance and species composition in a given region cannot be predicted. Each year of collecting yielded different species and species clusters. To illustrate this, an area of 0.3 square kilometres in Mauerbach (near Vienna, Austria) serves as a reference of a biodiversity hotspot. Over five years 17 Hypocrea species were collected in this area. During 2003–2008, 3–5 excursions were made each year in which one (in 2003) to 13 (in 2005) species were found each year. Although the epitype of Hypocrea gelatinosa is from this area, this species was not seen there in 2003 and 2004, while it appeared several times in 2005 and 2006. This is just an example showing that the frequency of occurrence based on the number of specimens depends significantly on the time-span of collecting, among other variables. Based on all specimens collected during this five year study, 15 species were only collected once, while 17 species (including Protocrea farinosa) were collected more than 10 times. The most common species in Europe is the hyaline-spored Hypocrea minutispora (64 specimens plus numerous additional observations) followed by the green-spored H. strictipilosa (48 specimens). Hypocrea gelatinosa and H. crystalligena were collected more than 30 times, H. lixii and H. viridescens were collected more than 20 times. Species common in Europe may be uncommon locally. As an example, H. pachybasioides replaces the otherwise common H. minutispora around the lake Ammersee (Bavaria, Germany) nearly completely. Species not or rarely found during the collection period need not necessarily be rare. Hypocrea tremelloides had been regarded a rare species, but in September 2007 it was collected five times within 8 d in various areas of England.
The high number of species detected in this work suggest that the biodiversity of Hypocrea/Trichoderma on and above the litter layer exceeds the number of species isolated from soil, even in the absence of an estimate of clonal Trichoderma species that may be isolated from various sources above ground. In fact, generally Trichoderma anamorphs are considerably more common on plant material than Hypocrea teleomorphs. There is, however, no information on how this relates to species numbers.
Life cycle, contaminations and reliability of ascospore isolations
Numerous field observations in Central Europe indicate that the Trichoderma anamorph develops before the Hypocrea teleomorph is formed, with some overlap in time. Usually young / immature stromata are associated with numerous conidiophores. The latter often grow even on the top of young stromata. Less commonly they are found on overmature stromata, suggesting that a complete anamorph–teleomorph–anamorph life cycle may take place under optimum conditions. However, such conditions are also optimal for other species, implying that Trichoderma anamorphs (and teleomorphs) of two or three species may be present on the same stick of wood. Collecting of a specimen may result in the distribution of conidia of all present species over the whole specimen. Hence, caution must be applied in the ascospore isolation procedure, considering the difficult distinction of the minute germinated ascospore cells and conidia under a 10× objective. Resulting cultures and gene sequences have to be evaluated critically with respect to the plausibility of identification given the teleomorphic source material.
More than one isolate of a species improve confidence in correct isolation considerably. Sequences obtained from several specimens of a single species collected in Europe are identical within nearly all species, while sequences of isolates from other continents that group into one species in phylogenetic analyses, may deviate in a variable number of nucleotides. The latter may be explained by intermediate stages in allopatric speciation. In addition, contamination may occur in laboratories where other species of the same genus are processed. In any case, experience in morphology is an essential precondition for correct isolation, particularly when new species may only be based on a single specimen/isolate. Species with slow germination of ascospores and slow growth may turn out especially problematic. Frequent contaminants like T. harzianum (sensu lato) and T. atroviride grow rapidly and sometimes make ascospore isolation from slow-growing species impossible. In this study, T. atroviride, T. cerinum, T. harzianum, T. koningii, and T. tomentosum appeared as contaminants of various teleomorphs. Examples of slowly germinating species are H. alutacea, H. subalpina, and H. tremelloides. In addition, teleomorphs of several species may be found together, e.g. H. lixii has been found with stromata of H. gelatinosa, H. minutispora or H. viridescens; H. minutispora with H. gelatinosa, H. rogersonii, H. sinuosa, H. strictipilosa, or H. sp. 12; H. schweinitzii with H. minutispora or H. viridescens; H. moravica with H. thelephoricola and H. sp. 4.
Macro-ecology of Hypocrea in Europe
Hypocrea/Trichoderma has been usually characterised as a genus of saprotrophs that degrade organic materials of both natural and xenobiotic origins (Klein & Eveleigh 1998). Rossman (1996) suggested that Hypocreales on rotting wood may be necrotrophic on fungal hyphae in the wood. I am following this view, because virtually all specimens of Hypocrea on wood and/or bark contain other fungi, mainly anamorphic fungi, basidio- and ascomycetes, mostly in overmature condition. The powerful complex of enzymes produced by Hypocrea / Trichoderma may allow the fungus to exhibit both ways of nutrition at the same time; saprotrophic nutrition on wood by the action of cellulases and hemicellulases, and necrotrophic exploitation of fungal hosts on or in wood by chitinases after killing them with antibiotics. Some species of Hypocrea, which form stromata on fungi (e.g. H. thelephoricola on Steccherinum ochraceum), do this not only on their fructifications (e.g. basidiomes) but also in close proximity on wood. This observation suggests the exploitation of vegetative hyphae of the fungal host. Another finding may indicate a predominant growth or stroma formation on other fungi. In Central Europe Hypocrea stromata appear late in the year, mostly from July, i.e. after their potential fungal hosts have already passed the optimum phase of their life cycle. Only few species have been found early in the year, e.g. H. sulphurea on fresh Exidia basidiomes and H. minutispora on hard wood of Fagus from the end of May on. Trichoderma anamorphs appear slightly earlier in spring. Later occurrence of stromata, however, may alternatively only indicate that the formation of the teleomorph requires higher temperatures, i.e. more energy than their anamorphs. Possibly H. minutispora is one of the exceptional species that grow primarily on wood. Indications for this may be the short time this species needs for stroma development, on hard, little degraded and often barely moist wood. The ability to use hard wood may also be the reason why H. minutispora is the most common species in Europe and other temperate regions.
Eight species of Hypocrea and 13 % of the collected specimens were found specifically on other fungi, 2 % (6 species) on gramineous or herbaceous hosts, 2 % (4 species) on soil and forest debris, and 83 % (57 species) on wood and bark of trees and shrubs. Of the latter 9 % of the specimens were found on wood of coniferous trees. Of the specimens found on wood and bark, 34 % were collected on Fagus sylvatica. This result is certainly biased by the high frequency of this tree in Central Europe, but numerous observations clearly show a marked preference of Hypocrea for the Fagales, in terms of both species numbers and abundance of teleomorph material. Tendencies concerning the decomposition state of wood were observed: Most species of Hypocrea with hyaline ascospores occur on wood dead for a short time (approximately 1–2 years), while there is a marked tendency of green-spored species to colonise well-rotted, often crumbly wood.
Few species of Hypocrea in Europe can be characterised by specific host associations, viz. those growing on other fungi: Hypocrea estonica and H. parestonica (Hymenochaete spp.), H. fomiticola (Fomes fomentarius), H. protopulvinata (Fomitopsis pinicola), H. pulvinata (Fomitopsis pinicola, Piptoporus betulinus), H. sulphurea (Exidia spp.), H. thelephoricola (Steccherinum ochraceum), H. sp. 14 (Phellinus). Many other species overgrow fungi (e.g. H. lixii on polypores, this and other species also on stromata of pyrenomycetes and corticiaceous basidiomycetes), but they also occur on wood and bark without obvious association with a fungus. Some species seem to be specifically associated with certain trees or shrubs: H. alni (Alnus glutinosa, rarely Betula pendula), H. dacrymycella and H. subalpina (conifers), H. psychrophila and an undescribed species (Rhododendron spp.), H. sp. 16 (Sambucus), or H. voglmayrii (Alnus alnobetula, rarely A. incana). Species on gramineous plants are probably not host-specific, but none of them except H. pilulifera has been found on wood and bark. Only the holotype of the latter species is known from a non-woody host (Juncus). No species of Hypocrea are known to be obligate biotrophs, although H. dacrymycella may be a candidate for this, because neither ascospores nor conidia germinated on artificial media.
Habitats that provide high yields in Hypocrea teleomorphs are difficult to characterise. Mixed debris-rich forests are usually most promising. Main substrates are dead branches and twigs lying on the ground of forests, and fungi growing on them. Human action such as tree felling or chopping off branches enhances the availability of such substrates. Virtually all Hypocrea specimens collected on conifers are from cut branches or logs. Other factors that determine stroma formation relate to long-term retention of humidity. An example is the development of microclimates by leaves covering branches on the ground. Exceptions are H. lutea, H. schweinitzii and H. voglmayrii, which occur on exposed branches. This finding correlates with growth of these species at higher temperatures (35 °C). Another related factor is soil composition. Calcareous soils dry out quickly, therefore substantially more specimens are found in areas characterised by acidic soil, particularly during dry periods. Non-homogeneous soil distribution causes non-homogeneous moisture distribution, e.g. a small area of loamy ground within a forest may produce many Hypocrea teleomorphs, while none may appear in the remaining part of the forest. This also means that climatic parameters given for a region offer little information about putative occurrence of Hypocrea. The extent and persistence of humidity appear to be the climatic factors that are most critical in stroma formation in Hypocrea, offering apparently only a narrow window of optimum conditions. Collecting in Europe in recent years clearly indicated that longer periods, which are too wet or too dry, are correlated with a poor yield of Hypocrea teleomorphs. Trichoderma anamorphs develop under wet and dry conditions, although drought may also result in apparent absence of these fungi.
Ambient temperature also affects Hypocrea fruiting. Generally, the number of species collected is positively correlated with temperature, but often the number of stromata per specimen decreases at temperatures higher than 30 °C. Some species, e.g. H. lixii, appear more frequently in warm periods. On the other hand, Hypocrea stromata occur until late in the year, i.e. until frost prevents growth and stroma formation. In England H. aureoviridis and H. minutispora were collected even until February.
High altitudes (1000–2000 m, montane and (sub-) alpine zones) in Central Europe are characterised by low temperatures and specialised plant communities. Some species have only been found in these habitats: H. psychrophila, H. voglmayrii, H. sp. 15. In addition, H. subalpina is also primarily found in these zones, but it occurs on the same host (Picea abies) at sea level in Estonia. This suggests that, rather than the altitude, climatic conditions of Northern Europe similar to those in the Austrian Alps are the critical factor determining fruiting of these species, given that the same hosts are present in these regions.
Finally, the lifetime of Hypocrea stromata is limited under humid conditions by growth of hyphomycetes on them, even before maturation, e.g. frequently observed with Hypocrea rufa on logs stored in shady places. In hot weather, stromata are frequently attacked by mites and insect larvae, most conspicuously by larvae of a Mycetophagidae, possibly a species of Triphyllus Latr. (will be shown in the second publication), frequently and conspicuously found feeding on stromata of H. rogersonii, but also observed with H. gelatinosa, H. lixii, H. sinuosa, and H. strictipilosa.
Phylogeny and generic limits
GCPSR (Taylor et al. 2000) is currently the standard of species recognition in fungi. The applicability of this concept is, however, limited by the strongly varying power of resolution among phylogenetic markers used to delimit the large number of taxa within Hypocrea / Trichoderma. As determined previously (see e.g. Samuels et al. 2006a), ITS is not suitable for a phylogenetic reconstruction of Hypocrea/Trichoderma, genus-wide or of large clades, due to a low number of variable sites and long insertions in certain species, while tef1 introns can only be aligned within single clades or certain groups of clades due to their high variability. Sequences of chi18-5 are useful but only available for a limited number of species. Rpb2 sequences exhibit intermediate variability and a corresponding power of resolution, and this is therefore currently the main marker available for most species of Hypocrea/Trichoderma to construct a genus-wide phylogenetic tree. The tef1 exon 6 shows less variability among species than rpb2, but provides additional characters useful to enlarge the data matrix of rpb2 sequences.
The combined analysis of rpb2 and tef1-exon sequences (1529 characters, of which 550 are parsimony-informative) of 135 species (137 isolates) of Hypocrea/Trichoderma resulted in 173 trees (best score = 5952). The strict consensus tree of this tree set (Fig. 1) contains 72 species of Hypocrea/Trichoderma occurring in Europe as teleomorphs or holomorphs, and 11 solely as anamorphs. Clustering of species in clades as recognised previously (Chaverri & Samuels 2003, Jaklitsch et al. 2008a, Degenkolb et al. 2008a) is reaffirmed. Clade or sectional names are adopted except for the following groupings: a) the new Spinulosa Clade with three European species; b) a substantially enlarged `Pachybasium Core Group' with 13 European species, which comprises the earlier Pilulifera (Chaverri & Samuels 2003), Pachybasioides (Jaklitsch et al. 2008a) or Minutisporum (Degenkolb et al. 2008a) clades and all European species formerly classified in Podostroma P. Karst. (see also Jaklitsch et al. 2008c); c) a substantially enlarged Megalocitrina clade now more appropriately named Psychrophila clade, because it comprises mainly 4 European species similar to H. psychrophila in teleomorph and/or anamorph traits; and d) sect. Trichoderma (see also Jaklitsch et al. 2006b), with 14 species occurring in Europe, is re-instated as a phylogenetic entity. After the exclusion of T. aureoviride and the T. harzianum group (sometimes regarded as members of this section; cf. Bissett & Gams 1998), this section is conceived as a phylogenetically homogeneous group, with H. voglmayrii at its base. The section replaces the Viride, Rufa and Koningii clades that have been used in varying circumscriptions. Sect. Trichoderma is not fully resolved; tef1 introns are necessary for this purpose (cf. Samuels et al. 2006a; Jaklitsch et al. 2006b).
European species with green ascospores are distributed among several clades (Chlorospora, Spinulosa, Harzianum, Strictipilosa, and Ceramica) and in several well-supported species pairs within the large grouping at the top of the tree (Fig. 1), with varying statistical support. The only exception is H. fomiticola, which clusters with hyaline-spored species in the Semiorbis Clade. Tef1 introns of this species cannot be aligned with those of other green-spored species; therefore, H. fomiticola was excluded from the subsequent analysis. To test whether the topology of the combined tree with respect to the green-spored Hypocrea species could be confirmed and its statistical support be improved by tef1 introns, a phylogenetic tree was constructed using a tef1 fragment containing 1293 characters, of which 355 are parsimony-informative. The (unrooted) analysis resulted in two trees (best score = 1218), which only differ in the relative position of two isolates of H. lixii. One of these trees is shown in Fig. 2. The grouping of the Chlorospora clade with H. aureoviridis and the Spinulosa Clade, is strongly supported. Hypocrea aeruginea and H. danica are supported in both trees as species separate from H. spinulosa, while the isolate CBS 121280 is not supported as a species distinct from H. spinulosa. The Strictipilosa Clade is supported by the Bayesian, but not by the MP analysis; H. longipilosa is distinct from H. strictipilosa in both trees. This clade received good support in a combined MP and Bayesian analysis of tef1 and chi18-5 sequences of a reduced dataset (due to lack of chi18-5 sequences; data not shown). The Ceramica Clade is supported in both analyses and H. parestonica is separated from H. estonica, while grouping within the clade is variable. The Harzianum Clade, including H. dacrymycella, is highly supported in both analyses, and the new species H. parepimyces is clearly separated from all European species with similar teleomorph traits (H. epimyces, H. brunneoviridis and H. alni).
The genus Hypocrea as it is circumscribed here follows Overton et al. (2006b) with respect to an inclusion of species with entirely `prosenchymatous stromata', i.e. subicula. On phylogenetic grounds (see Fig. 1), H. alcalifuscescens, H. cinereoflava, and perhaps H. parmastoi could possibly be excluded from Hypocrea, although there are no convincing morphological and/or ecological arguments for this. In fact, teleomorphs of H. avellanea and H. delicatula have teleomorphs morphologically similar to these species, i.e. perithecia partially immersed in a subiculum. Except for H. cinereoflava (anamorph Stilbella flavipes (Peck) Seifert, see Seifert & Samuels 1997), all these species have verticillium-like anamorphs, not gliocladium-like, as in Protocrea, which is recognised as a genus well separated from Hypocrea (Jaklitsch et al. 2008b).
Morphology
This study confirms earlier conclusions that species identification based on morphology of the teleomorph is only possible for a limited number of species. For most, the morphology of the anamorph is necessary, for others there is no certainty without gene sequences. Interestingly, gross morphology of fresh stromata is more diagnostic than microscopic characters, and provides often sufficient information for a preliminary identification. Micromorphology usually does not add much taxonomic information, but in individual cases characteristics such as reaction of stromata to KOH, composition of stroma tissues, and ascospore characters allow easy identification. Previous studies do not account for traits of fresh stromata or changes in morphology upon drying. Herbarium specimens are often difficult to interpret. One of the most serious hindrances in identification based on stromata is that many, even unrelated, species share nearly identical teleomorphic characters, i.e. little interspecific variability, while many species show a high intraspecific variability, even within populations/specimens. The best examples supporting this are teleomorphs of Trichoderma sect. Trichoderma and the Pachybasium core group (see Phylogeny section) centered around H. minutispora and H. pachybasioides. Generally, differences seen in fresh stromata are no longer evident in dry stromata.
Important for the recognition of species is the combination of phenotype and genotype data. Consistent differences in ITS sequences, even in only few nucleotides, indicate a new species. Hypocrea brunneoviridis and H. parepimyces are good examples to illustrate this finding. Due to the poor growth of the first isolate of H. brunneoviridis it was regarded a `defective mutant', and its ITS sequence was ignored. It was neglected until the isolate of a second specimen exhibiting the same traits was discovered. However, the nearly gliocladium-like conidiation and slow growth was found to differ significantly from all other species with similar teleomorphs. Eventually rpb2 and tef1 sequences supported the recognition as a new species. Stromata of H. parepimyces strongly resembled those of H. brunneoviridis, but growth was unlimited and rapid, conidiation was similar to that of H. epimyces, whereas the concentric conidiation zones on PDA remained white or turned pale greenish. This latter finding was recognised as the most striking and diagnostic trait of H. parepimyces, apart from differences in gene sequences. This clearly shows that cultures and anamorphs provide additional information increasing confidence in identification. However, many differences among species are difficult to quantify, particularly because of the large number of species in the genus.
The number of Trichoderma species forming hyaline conidia (white in mass) has been considered a small fraction (Lu et al. 2004). An explanation for this may be the selection criteria for the recognition of Trichoderma (green conidia and fast growth) applied in isolations from soil. In Europe, the species forming hyaline conidia have substantially increased in number, as they are now adding up to 26 versus 42 green-conidial species. Hyaline conidia are mostly found in sect. Hypocreanum, in the Pachybasium Core Group and some lone lineages, while all species of sect. Trichoderma and all species with green ascospores form green conidia. However, three of the detected species do not form an anamorph, while in four species the anamorph is still unknown, because they have not been cultured.
Definitions and taxonomic value of morphological traits
The following definitions are given here to explain what is meant by the terms used in phenotype descriptions.
Teleomorph
Asci: are not a diagnostic trait, their size is largely dependent on ascospore size; the length of the stipe is included in the measurements. Asci appear considerably wider in thin KOH mounts shortly before drying out.
Ascospores: vary little in size, but some individual species can be delimited using this character. Green ascospores turn brown in KOH, and appear often too brown in photographs taken from mounts in KOH; the colour in KOH is also dependent on the thickness of mounts, i.e. in thinner mounts ascospores appear more distinctly brown.
Ostiolar dots/regions/areas: are measured in dry stromata in this study. They include the ostiolar opening and a part of the surrounding cortex of the stroma, which appears darkened using a hand lens. Sometimes perithecial contours are parts of ostiolar dots. Ostiolar dots are typically inconspicuous in teleomorphs of Trichoderma sect. Trichoderma. True ostioles, i.e. the ostiolar openings, are colourless, generally minute, inconspicuous or invisible. They are best seen under strong magnification after re-wetting of stromata. The apical width of ostioles in section refers more correctly to the ostiolar width at the stroma surface; ostioles may be attenuated at the true apex; the width includes the wall of the ostioles, except where noted. Ostiolar dots are of limited value in species delimitation, although typical, fresh material of certain species can often easily be identified using this character: e.g. fine, plane dots densely set in large numbers as in H. strictipilosa vs. large, projecting dots in small numbers as in H. sinuosa.
Perithecia: are immersed in a single layer in the upper part of the stroma. The perithecial height measured in median section includes the apex located at 1/3–1/2(–2/3) of the lower part of the ostiolar canal. Perithecia appear larger in crush mounts than in sections. There is considerable variation in the size of perithecia, but ranges are overlapping, therefore not taxonomically useful. The perithecial wall may be colourless or pigmented or change its colour in KOH (see e.g. H. thelephoricola vs. H. sinuosa), i.e. sometimes it is taxonomically useful.
Stroma: is a more or less pseudoparenchymatous fungal tissue containing usually more than one perithecium; highly conserved in Hypocrea, i.e. not particularly informative in many species. Stroma is here used in a broad sense, i.e. including also entirely prosenchymatous (`hyphal') stromata, more correctly described as subicula that form usually widely effused mats. Such forms are rare in Hypocrea.
Stromata, fresh/dry, immature/mature: Stromata in nature may be fresh, dry, or re-wetted. They may be immature, mature or overmature. Their appearance is often strongly dependent on climate conditions. Fresh stromata are soft. Their sizes as determined in the field are only estimates. They often shrink considerably upon drying. Other authors have described dry stromata only. The mature state of stromata may be indicated by green, white or yellow, floccose, granular or filiform ascospore deposits. These should not be mistaken for white or green floccules caused by conidiophores of the anamorph often present on immature, sometimes also (over-)mature stromata.
Stromata, colours: are variable and difficult to define, but are useful for preliminary identification of species. Colour changes upon drying of fresh stromata can be diagnostic. Drying in nature poses difficulties, e.g. originally yellow stromata may be already reddish brown at the time of collection. In addition, the presence of only one developmental state of stromata in a specimen makes even preliminary identification difficult. Usually some uncertainty remains, particularly in species with yellow, pulvinate stromata, or reddish brown to brown, pulvinate stromata. Yellow colours are difficult to capture with digital cameras correctly. Images taken through the stereo-microscope are often too red or too white, depending on the white balance method used.
Stromatic tissues: are studied in vertical section. The morphology of the several parts of the stroma may be diagnostic, particularly the cortical tissue, e.g. pseudoparenchymatous in H. aeruginea, prosenchymatous in H. danica and H. spinulosa.
Cultures
Autolytic activity: is a semi-quantitative estimation of usually circular colourless excretions at tips or less commonly at septa of mainly superficially growing hyphae; often they are accompanied by death of hyphal segments. In some species, autolytic excretions and dying hyphae become yellow, reddish or brown.
Coilings: are circular-oriented parts of aerial hyphae or vegetative hyphae on the agar surface. These structures are absent to abundant, usually more abundant on PDA (sometimes on SNA) than on other media.
Colour of colony reverse: was determined on white background. Usually no or little discoloration of CMD and SNA is caused by growth of Hypocrea/Trichoderma, with few exceptions, e.g. green colour by H. aeruginea. Pigments are more frequently formed on PDA; mostly yellow, brown, less commonly reddish colours are noted. However, formation may vary among isolates and may be absent in old cultures.
Growth media: are selected for reasons of compatibility with studies by G.J. Samuels.
CMD: provides good growth, long-term vitality, usually good conidiation, but conidiation often degenerates and may be absent after several subsequent transfers.
PDA: provides good growth, usually abundant mycelium, but conidiation is usually effuse and ill defined, often remaining colourless or white in green-conidial species. However, this medium often provides diagnostic macroscopic growth/conidiation patterns. SNA: although often comparable with CMD, it yields cultures of low biomass, with hyphae degenerating soon. However, the main advantage of SNA over CMD is a more reliable and transfer-resistant conidiation. Conidiation morphology on SNA and CMD is usually in accordance with that found in nature.
Growth plates / colonies: centre of the colony = area around the inoculation plug (called plug in descriptions); proximal area = area behind the plug, i.e. short growth distance to the margin of the Petri dish; distal areas = opposite proximal areas, i.e. long growth distance to the margin of the Petri dish; middle of the colony = centre of the plate. Lateral areas = areas close to the lateral margins of the Petri dish. Margin stated without further specification denotes the distal colony margin.
Hyphae, primary: are the first hyphae leaving the inoculation plug, establishing the principal hyphal network. These are often conspicuously wider than secondary hyphae.
Hyphae, secondary: are branches originating from primary hyphae; these are usually narrower than or of similar size as primary hyphae. Relative differences in hyphal width are characteristic for many species. The value of this trait is limited by the large number of Hypocrea / Trichoderma species.
Odour of colonies: is of limited use for identification. Coconut-like odour on CMD and PDA, caused by the antifungal antibiotic 6-pentyl-α-pyrone, is typical for some species of sect. Trichoderma. Other odours may be formed on PDA, but experience is required for their detection, because odours are difficult to quantify and most people are neither able to perceive specific odours nor link them to known odours.
Conidiation
The following scenarios are found on CMD at 25 °C in Trichoderma:
Pustulate conidiation only, i.e. complex branching in tufts, pustules, shrubs, or granules
-
Pustulate conidiation preceded by effuse conidiation
- Effuse conidiation on conidiophores with less complex branching than in pustules, but otherwise similar to pustulate conidiation, with phialides and conidia being in the same range or phialides tending to be slightly longer and narrower than in pustules
- Effuse conidiation on simple, mostly verticillium-like, conidiophores different from pustulate conidiation in shape, size and arrangement of phialides, terminal branches and/or conidia (`synanamorph'); see e.g. Chaverri & Samuels 2003)
Effuse conidiation only, often starting shortly after the onset of growth (typical for T. sect. Hypocreanum).
Conidial heads, wet vs. dry: wet heads occur in many species, often they can be only seen on plates with the lid attached by parafilm, and removal of the lid may result in immediate drying of heads.
Conidiophores/trees, regular: means a tree-like or more or less pyramidal shape (such as Picea abies), attenuated upwards, branches mostly paired and increasing in length from the top down, branches substituted by phialides at and near the top. The term trees is mostly used for side branches or ends of conidiophores having this shape as is common in Trichoderma. In pustules, conidiophores or trees are referred to the terminal branching system on the periphery of the pustule.
Conidiophore terminology:
Acremonium-like: is the least complex, short, effuse type of conidiation in Trichoderma. The conidiophores consist of a stipe of one to a few cells, with one or few phialides originating directly from this stipe on a single level. This conidiation structure is typical for sect. Hypocreanum, where conidiation is initially acremonium-like but usually develops into a verticillium-like structure bearing long phialides.
Verticillium-like: unbranched or little branched erect conidiophores with verticils or whorls of distinctly divergent phialides on several levels; phialides within a whorl originate on the same level around the main axis. Contrary to true Verticillium, the term is used here also for conidiophores with secondary branches having similar arrangements.
Gliocladium-like: unbranched or more or less intensely branched conidiophores with penicilli of densely packed and appressed, more or less parallel, phialides at the top.
Pachybasium-like: conidiophores typically densely branched and stout, with small ampulliform or lageniform phialides. Part of the conidiophore often ending in simple or branched, sterile or fertile elongations.
Trichoderma-like: is difficult to circumscribe, used by many authors, but generally not in this study due to the lack of a strict borderline to verticillium-like. It comprises narrow, flexible and richly branched conidiophores that may be irregular, with distinct curvatures or terminally pyramidal with branches often at right angles. The phialides are typically lageniform, in irregular clusters, often bent, sometimes repetitive. Frequently, verticillium-like structures are also present on trichoderma-like conidiophores.
Effuse conidiation (synanamorphs): conidiation without formation of distinct, macroscopically visible units. They are usually made up of more or less evenly distributed simple erect conidiophores, macroscopically only visible if densely arranged and forming green conidia. Effuse conidiation may also occur in macroscopically visible, downy or hairy areas on long aerial hyphae. Conidia of effuse conidiation are typically formed in wet heads.
Pustules: are in their extreme form distinct dense opaque conidiation structures, often pulvinate with circular outline, originating on a single stipe, a thick aerial hypha from which primary branches emerge. Pustules may grade into transparent, loose structures, i.e. tufts.
Shrubs, granules: are small conidiation units (usually larger single conidiophores) spread on the agar like sand (granules), often scarcely visible macroscopically; 0.2–0.5 mm diam. They may constitute the final stage of conidiation or develop between effuse and pustulate conidiation.
Tufts, also called `fluffy tufts': are conidiation structures appearing macroscopically as loose, cottony, often confluent masses or aggregations of conidiophores with variable outlines, usually not circular. In some species pustules develop from tufts.
Phenotype of the Hypocrea teleomorph
Stromata solitary, gregarious or densely crowded, flat and widely effuse, or clavate, upright and stipitate, extending several centimeters, or pulvinate, turbinate, lenticular or discoidal and one to few millimeters diam; often shrinking and/or changing colour upon drying; entirely or partly attached to the host. Outline circular, angular, elongate to irregular, sometimes undulate. Surface convex, flat or concave, glabrous or velutinous, smooth, uneven, tubercular or rugose, often floccose by conidiophores when young, often covered by white, yellow or green spore deposits when mature. Uneven, tubercular or rugose appearance of surface and presence of spore deposits strongly enhanced by drying. Margin sterile or fertile, attached or free, often elevated above host level, with sterile sides becoming exposed. Sides vertical or attenuated downward, smooth or covered by mycelium, particularly at the base of the stroma. Ostioles minute, typically invisible on the stroma surface. Plane or convex dots on the stroma surface comprising ostiolar openings, surrounding cortical tissue and sometimes perithecial contours, often conspicuous, but typically inconspicuous in teleomorphs of T. sect. Trichoderma. Perithecia entirely immersed in a single upper layer, perithecial contours inconspicuous or prominent. Stroma colour variable, usually light or bright, changing in 3 % KOH or not. Entostroma soft-textured, white or pale brownish or rosy inside.
Stroma development: typically anamorph formed first, followed by the appearance of white mycelium, becoming compacted and variably pigmented, perithecial dots appearing, pigment intensifying or changing, finally surface covered by spore deposits. Often a subeffuse to effuse young stage observed, followed by an `effluent' development, i.e. breaking up into several individual stromata. Effluent stroma development seen also in culture (H. spinulosa, on MEA; not shown).
Stroma anatomy: ostioles cylindrical or conical, plane with the surface or slightly projecting, periphysate, sometimes surrounded at the apex by specialised hyaline cells. Perithecia usually crowded, flask-shaped, ellipsoidal or globose. Peridium hyaline or pigmented, changing colour in KOH or not, typically thickened around the ostiole, at the sides consisting of narrow, compressed, thick-walled, refractive cells, the latter larger and more isodiametric at the base and around the ostiole. Sometimes undifferentiated hyphae (typically on immature stromata) or differentiated hairs (typically on mature stromata) present on the stroma surface. Stroma tissue consisting of 1–4 recognisable regions: cortical tissue typically pseudoparenchymatous, i.e. a textura angularis of thin- or thick-walled, isodiametric or oblong, hyaline or evenly or unevenly pigmented cells, less commonly prosenchymatous, of smooth or encrusted hyphae; changing colour in KOH or not. Subcortical tissue when differentiated from cortex, a t. angularis of typically thin-walled, (sub-)hyaline cells, frequently intermixed with (sub-)hyaline hyphae, or consisting exclusively of narrow hyphae. Subperithecial tissue typically a t. angularis-epidermoidea of (sub-)hyaline cells variable in shape and size, or mixed with or consisting exclusively of wide hyphae. Base similar to the subperithecial tissue, but often of cells tending to be smaller than those directly below the perithecia, or frequently mixed with or consisting exclusively of typically thick-walled hyphae. Asci cylindrical, less commonly subclavate in the upper part due to oblique orientation of ascospores, usually with a short stipe, base often thickened, croziers present or not; apex thickened to 1–1.5(–2) μm, with or without an inconspicuous ring, inamyloid, non cyanophilic; asci containing 8 bicellular ascospores in uniseriate arrangement. Ascospores disarticulating within the asci into a distal (= upper) and a proximal (= lower) cell, hyaline and often yellow or orange when overmature, or green in water and lactic acid, becoming brown in KOH; multiguttulate when vital, with 1–2 guttules per cell when dead. Cells finely (verruculose) or coarsely (verrucose) warted, dimorphic (= of different shape), less commonly monomorphic, distal cell typically (sub-)globose, less commonly ellipsoidal or wedge-shaped, proximal cell typically oblong or wedge-shaped, less commonly ellipsoidal or subglobose, typically narrower and often longer than distal cell. Ascospore cells never acute. Contact area often distinctly flattened, at least when young.
Phenotype of cultures and anamorphs
Optimal growth at (20–)25 °C for most species. Mycelium typically covering a 90 mm diam Petri dish within 1–2 wk, with few exceptions. On CMD and SNA colony hyaline, thin; surface glabrous, downy or floccose by aerial hyphae; mycelium and/or conidiation sometimes forming concentric zones. Conidiation becoming visible as white or (yellowish) green granules, tufts or pustules. Autolytic activity and coilings absent to conspicuous. Diffusing pigments and specific odours typically not formed on SNA, but sometimes formed on CMD. Chlamydospores formed in most species, particularly on SNA, terminal at the ends of hyphae or intercalary within hyphal cells. On PDA colony typically dense, aerial hyphae often forming strands and thick mats, reaching the cover of the Petri dish in certain species. Autolytic activity and coilings variable. On PDA diffusing pigments and odours more frequently present than on CMD or SNA. Conidiation on PDA effuse in most species, on surface and aerial hyphae, remaining white or turning green. Chlamydospores usually not determinable due to the dense mycelium.
Organisation of conidiation varying with species, media, temperature and light conditions, effuse or in tufts or pustules (or smaller units) or combinations of these. Effuse conidiation usually spreading from the inoculation plug on surface and aerial hyphae, on mostly short and simple, acremonium- verticillium- or sometimes gliocladium-like conidiophores, with conidia formed in heads of clear liquid, colourless or green, depending on the conidial colour, on narrowly lageniform or subulate phialides. Pustulate conidiation in shrubs, granules, tufts or pustules, with or without sterile or fertile elongations projecting from the pustule surface. Pustules arising from a thick stipe with thick walls swelling on the outer side in 3 % KOH. Primary asymmetric branches emerging from the stipe, further branching usually difficult to assess, irregularly oriented and often forming a dense reticulum within the pustule or forming radially arranged main axes. Conidiophores in pustules trichoderma-, pachybasium-, or gliocladium-like. Right-angled paired branches common in many species. Phialides solitary or more commonly in whorls of 2–6; lageniform, subulate or ampulliform, thickened at one position or not, straight, curved or sinuous, often tending to be longer and narrower when solitary, sometimes percurrently proliferating, rarely branched at the apex. Conidia hyaline (white in mass), yellow-green or green, globose, subglobose, oval, ellipsoidal or oblong to cylindrical, smooth, less commonly verruculose or verrucose, with or without minute guttules; abscission scar typically indistinct.
Growth rates
The system for growth rate determination applied here was developed by G.J. Samuels (see e.g. Samuels et al. 2006a). He applied this system to as many isolates of each species as possible using subtle differences in growth rate on PDA and/or SNA to aid in species delimitation. In the current work, growth rates of only one or two representative isolates per species were determined. CMD was used in addition and emphasis was placed on details of culture morphology and development. Inclusion of higher numbers of isolates per species would result in wider ranges of growth rates for a given species, apart from slightly wider morphological variation. Therefore, only conspicuous differences in growth rate are here interpreted as significant in species delimitation. Fig. 3 shows growth rates of 67 species of Hypocrea/Trichoderma on CMD, given as mm colony radius after 72 h at four temperatures, arranged by length reached at 25 °C. As can be seen in Fig. 3, a wide range of growth rates has been determined for the genus, from a virtual absence of growth at 25 °C in H. psychrophila, and a growth rate < 5 mm in H. tremelloides, to ca. 70 mm in H. schweinitzii (anam. T. citrinoviride Bissett). Only five species (H. lixii, H. lutea, H. rodmanii, H. schweinitzii and H. voglmayrii) grew at 35 °C, reaching 2.6 mm in H. rodmanii to 94 mm in H. schweinitzii, while the growth of 0.5 mm by H. atroviridis is regarded as negligible. Within the range tested, H. schweinitzii shows even a growth optimum at 35 °C. On the other hand, H. psychrophila has a growth optimum below 25 °C, and grew and sporulated even at 4–6 °C (not shown). Also Hypocrea pilulifera and H. sp. 15 grew faster at 15 °C than at 25 °C. Growth at 30 °C has been found to be most variable and poor in many isolates.
Fig. 3.
Growth rates of 68 species of Hypocrea/Trichoderma on CMD, given as mm per 72 h at 4 temperatures, arranged by 25 °C. Values are means of (1–)3–6 experiments with 1–2 representative isolates per species; values for stipitate species are from single experiments. H. spinulosa 1 = CBS 121280, H. spinulosa 2 = CBS 121272. T. cerinum = CBS 120637.
Among the green-spored species of Hypocrea significant differences in growth rates were detected: species pairs with similar morphology like H. sinuosa/H. thelephoricola, and H. danica/H. spinulosa (2 isolates) can be easily separated on growth rate. Also H. aeruginea grew significantly more slowly than H. spinulosa; H. aureoviridis and H. gelatinosa were significantly slower than H. sinuosa and H. strictipilosa, while the latter pair cannot be separated using this character. Hypocrea brunneoviridis grew significantly more slowly than H. parepimyces, H. epimyces and H. alni, while a separation of H. parepimyces from the latter two species using growth rates is not possible.
Due to the availability of only four incubators it was decided to omit 20 °C, but this temperature, common in temperate areas, may be optimal for growth and conidiation of many species. Optimum growth temperatures determined here to be 15 or 25 °C, may therefore in fact be 20 °C for some species.
Initially experiments were carried out with different brands of media. Conspicuous differences were found between brands of the same medium (e.g. PDA from Merck and Difco) in both growth rates and extent of conidiation, and variation was even found between different batches of the same brand (CMA from Sigma). Therefore standardisation of growth rate experiments with respect of growth media and possibly also other conditions, e.g. temperature variation around an adjusted value in incubators, mode of preculturing, time between experiments with the same isolate, etc., is difficult and results can probably only be effectively interpreted within one laboratory. This may be problematic when trying to identify species of Trichoderma, particularly within sect. Trichoderma, with growth rates determined in a different laboratory. Consequently, also descriptions of culture and anamorph morphology and development should only be compared under the same growth conditions. Similar issues are known in the identification of Penicillium species and a method for the comparison of growth media was published (Clemmensen et al. 2007, Suhr et al. 2002).
TAXONOMY
The green-spored species of Hypocrea in Europe
The following key provides a basis for species identification using morphological and ecological traits. Ideally, fresh stromata should be used. Some species have to be cultured for identification. All green-spored species with anamorphs have green conidia on CMD or SNA. Further notes that may help to distinguish taxa on a morphological basis are given after each description. This key can only be used for species identification in Europe. It is not transferable to other continents. In addition, it is important to note that additional undescribed green-spored species may occur in Europe.
Key to the species of Hypocrea with green ascospores occurring in Europe
1. On gramineous or herbaceous hosts............................................................................................................................................................2 1. On wood and bark or fungi............................................................................................................................................................................5
2. Stromata brown, ostiolar dots brown to black; on bamboo (Phyllostachys) in southern France....................................H. phyllostachydis 2. Stromata white or green; ostiolar dots green to black...................................................................................................................................3
3. Stromatal cortex green, pseudoparenchymatous (cellular); anamorph green-conidial.............................................................H. aeruginea 3. Stromatal cortex white or green, prosenchymatous (hyphal); no anamorph formed in culture.....................................................................4
4. On Chelidonium, Juncus, Molinia and other hosts; good growth at 30 °C...............................................................................H. spinulosa 4. On Calamagrostis in Denmark; poor growth at 30 °C.....................................................................................................................H. danica
5. Stromata green to black, lenticular, pulvinate to irregular, on wood, bark and various fungi, particularly polypores............................H. lixii 5. Stroma colour different, on similar hosts.......................................................................................................................................................6
6. Fresh stromata with red or reddish- (to orange-) brown colours...................................................................................................................7 6. Fresh stromata with white, yellow (to orange) colours, brown only when overmature................................................................................13
7. On and around basidiomes of Hymenochaete spp.......................................................................................................................................8 7. On wood and bark.........................................................................................................................................................................................9
8. Stromata bright raspberry-red when young, sometimes fading to yellowish when old, Northern Europe...................................H. estonica 8. Stromata dull red when young, Central Europe......................................................................................................................H. parestonica
9. Perithecial contours conspicuous, i.e. stromatal surface tubercular; fresh stromata incarnate to reddish, KOH–; distal ascospore cell 5–8 × 4–5.5 μm......................................................................................................................................H. longipilosa 9. Perithecial contours inconspicuous or invisible in fresh stromata, i.e. stromatal surface smooth; stromata reddish brown or orange-brown, KOH+; distal ascospore cell smaller........................................................................................10
10. In wet, grassy or boggy habitats, often along brooks; on Alnus glutinosa (rarely Betula); stromata pulvinate, dark reddish brown; ostiolar dots inconspicuous; conidiation on PDA pale green..............................................................................................................H. alni 10. In mixed, debris-rich woodland.................................................................................................................................................................. 11
11. Stromata discoidal, turbinate or substipitate; conidiation on PDA in conspicuous green concentric zones.............................H. epimyces 11. Stromata pulvinate.....................................................................................................................................................................................12
12. Stromata reddish brown; growth of cultures limited on CMD, PDA and SNA; conidia in mass dark green, conidiophores (almost) gliocladium-like..........................................................................................................................H. brunneoviridis 12. Stromata more orange-brown; growth in culture unlimited; concentric conidiation zones on PDA white or at most pale green.....................................................................................H. parepimyces
13. Stromata effuse to subpulvinate, large; on and around basidiomes of Fomes fomentarius; distal ascospore cell 5–8 × 4–5.5 μm......................................................................................................................................H. fomiticola 13. Stromata pulvinate, turbinate or discoidal; not on Fomes; distal ascospore cell smaller except in H. dacrymycella................................14
14. Surface of fresh stromata distinctly tubercular..........................................................................................................................................15 14. Surface of fresh stromata smooth, not tubercular.....................................................................................................................................17
15. Stromata translucent, appearing waxy to gelatinous; when young colourless, yellow or orange; black when mature and dry; anamorph gliocladium-like.........................................................................................................H. gelatinosa 15. Stromata not appearing waxy to gelatinous; anamorph variable..............................................................................................................16
16. On and around basidiomes of Steccherinum ochraceum; perithecial walls orange in KOH; distal ascospore cell 3.3–5.2 × 3.0–4.7 μm.....................................................................................................................H. thelephoricola 16. On wood and bark, widespread; perithecial walls colourless in KOH; distal ascospore cell 4.0–6.5 × 3.5–5.8 μm...................H. sinuosa
17. Stromata discoidal or turbinate to pulvinate; on wood of conifers (Pinus, Picea); distal ascospore cell 5–9 × 4.5–9 μm................................................................................................................................H. dacrymycella 17. Stromata pulvinate or lenticular; on wood of various trees, distal ascospore cell smaller........................................................................18
18. Ascospore cells monomorphic except in ascus base; stromata golden-yellow to orange, covered by whitish flakes when young; ostiolar dots variable, Northern Europe and Atlantic region...................................H. aureoviridis 18. Ascospore cells dimorphic; stromata white to yellowish, usually without conspicuous flakes; typically with numerous, fine, densely disposed ostiolar dots; widespread and common....................................................H. strictipilosa
DESCRIPTION OF THE SPECIES
Hypocrea aeruginea Jaklitsch, sp. nov. MycoBank MB513177. Fig. 4.
Fig. 4.
Teleomorph of Hypocrea aeruginea (WU 29031). a, b, d. Fresh stromata (a, b. mature, d. immature). c. Dry mature stromata. e. Mature stroma in 3 % KOH after reconstitution in water. f. Projecting ostioles (stroma reconstituted in water). g. Stroma surface in face view. h, i. Cortical tissue in section (with perithecial walls in lateral parts). j, k. Asci with ascospores. l. Perithecium in section. m. Subperithecial tissue in section. n. Base of stroma in section. Scale bars: a = 2 mm. b, e = 0.4 mm. c = 0.5 mm. d = 0.2 mm. f = 0.1 mm. g, m = 15 μm. h = 30 μm. i = 25 μm. j, k = 10 μm. l = 35 μm. n = 20 μm.
Anamorph: Trichoderma aerugineum Jaklitsch, sp. nov. MycoBank MB513178. Fig. 5.
Fig. 5.
Cultures and anamorph of Hypocrea aeruginea (CBS 120541). a–c. Cultures at 25 °C (a. on CMD, 42 d. b. on PDA, 21 d. c. on SNA, 21 d). d. Conidiation tufts (15 °C, 14 d). e, f, h, i. Conidiophores (e. on aerial hypha on growth plate, 25 °C, 8 d; f. 15 °C, 14 d; h, i. 25 °C, 7 d). g. Phialides (25 °C, 7 d). j. Thickened cells with pegs associated with stromata. k. Hyphae with pegs (`barbed wire-like') in agar. l, m. Conidia (14 d, 15 °C). d–m. On SNA. Scale bars: a–c = 20 mm. d = 0.4 mm. e = 35 μm. f = 25 μm. g, l = 10 μm. h = 30 μm. i, j = 15 μm. k = 50 μm. m = 5 μm.
Teleomorphosis similis Hypocreae spinulosae, differt cortice pseudoparenchymatoso et praesentia anamorphosis. Occurrit in culmis Graminearum. Anamorphosis Trichoderma aerugineum. Conidiophora in agaro SNA effuse distributa et in caespitibus irregularibus. Phialides lageniformes vel subulatae, (7–)9–16(–23) × (2.4–)2.7–3.5(–4) μm, collari viridi lateraliter projecto praeditae. Conidia subglobosa, ovalia vel ellipsoidea, viridia, glabra ad verruculosa, (3.0–)3.5–4.5(–5.5) × (2.7–)3–4(–5) μm.
Etymology: referring to the bluish green stroma colour.
Stromata when fresh 0.5–2 mm diam, to ca. 1 mm thick, solitary, gregarious, or aggregated in small numbers, pulvinate or turbinate, attached to the host by hyphae, easily detached; sides often attenuated downward, surrounded at the base by white cottony mycelium when young. Surface smooth, ostiolar dots convex to conical and projecting to ca. 60 μm, first colourless, later brownish to pale olive, finally green to black. Lower side white, smooth, flat or curved upward. Overall colour light bluish green, darker green when wet, surface and spore powder dark green to black when mature or old.
Stromata when dry (0.4–)0.6–1.4(–1.8) × (0.3–)0.5–1.3(–1.7) mm, 0.2–0.5 mm thick (n=14), pulvinate or turbinate, surface smooth; sides white, compact, smooth, glabrous or with white mycelium. Ostiolar dots (35–)40–70(–79) μm (n=14) diam, densely disposed, light green when immature, dark green to black when mature, often covered by spore tendrils. Spore powder dark green.
Stroma anatomy: Ostioles (37–)43–53(–60) μm long, projecting (6–)11–28(–38) μm, (26–)30–43(–52) μm wide at the apex (n=30), periphyses 1.5–3 μm wide; hyaline inside, lined outside by cortical cells. Perithecia (110–)140–190(–215) × (90–)108–152(–170) μm (n=30), flask-shaped or globose, immersed, often projecting to a third of their height; peridium (12–)16–26(–31) μm (n=30) wide at the base, (8–)10–18(–22) μm (n=30) at the sides, yellow, in upper part of perithecia merging with and indiscernible from cortical tissue. Discontinuous areas of stroma surface covered by hyaline, brownish to green hyphae (2.0–)2.5–4.0(–6.0) μm (n=33) wide, apparently degenerating with age. Surface of mature stromata with sparse `hairs' (8–)13–26(–38) × (4–)5–8(–9) μm (n=30), of solitary, upright cortical cells or 2–3 elongate to cylindrical cells, smooth, rarely verruculose. Cortical layer (19–)25–43(–51) μm (n=30) thick, a t. angularis of isodiametric to oblong, thin-walled cells (3–) 6–13(–17) × (3–)4–8(–11) μm (n=66) in face view and in vertical section, uppermost layer distinctly (brownish) green, gradually lighter downward, yellow-green to hyaline. Subcortical tissue not differentiated. Subperithecial tissue a hyaline t. angularis–epidermoidea of thin-walled cells, (5–)7–17(–22) × 4–10(–14) μm (n=30), at sides and base interspersed with hyaline to greenish brown hyphae (1.5–)3.0–6.5(–9.0) μm (n=30) wide. Asci 60–70(–78) × (3.3–)3.5–4.2(–4.5) μm, stipe (6–)9–15(–19) μm long (n=30); croziers present. Ascospores green, brown in KOH and thin preparations, verruculose, cells dimorphic with little difference in size, distal cell (2.7–)3.0–4.0(–4.6) × (2.5–)2.7–3.4(–4.0) μm, l/w (0.9–)1.0–1.2(–1.4) (n=40), (sub-)globose; proximal cell (2.7–)3.0–4.5(–6.2) × (2.0–)2.4–3.0(–3.5) μm, l/w (1–)1.2–1.6(–1.8) (n=40), plump wedge-shaped to oblong, or subglobose. Size strongly dependent on stage of development. Nearly black dry stromata turning green with yellowish brown tone upon reconstitution in water; sterile sides pale green. No distinct colour change noted in 3 % KOH, but yellowish brown perithecial walls becoming more distinct.
Cultures and anamorph: Optimum growth at 25 °C on all media, no growth at 35 °C. On CMD after 72 h 2–8 mm at 15 °C, 20–23 mm at 25 °C, 9–13 mm at 30 °C; mycelium covering the plate after 11 d at 25 °C. Colony hyaline, thin, circular; margin diffuse or well-defined. Mycelium loose, hyphae narrow, sinuous, with highly variable orientation; little on agar surface; white spots of condensed mycelium appearing, numerous fine pegs formed on submerged hyphae, spots growing and becoming (yellowish) green, 28CD5–8 to 28E7–8. Green pigment diffusing into the agar, spreading across the entire plate upon extended incubation (e.g. 6 wk) at 15 °C. Aerial hyphae scant. Conidiation noted from 6–8 d, green after 2 wk; effuse, on short conidiophores erect on surface hyphae, mainly on white to green spots and on aerial hyphae along the margin, sinuous or short-helical, dry; also developing within the agar. Autolytic excretions absent or inconspicuous, no coilings seen. No distinct odour noted. Chlamydospores noted after 2 wk, uncommon, mainly intercalary, globose. At 15 °C no conidiation noted within 2 wk. At 30 °C conidiation noted after 4–5 d, effuse; colony becoming yellow-green, 29CD5–6, from the proximal margin. Chlamydospores frequent, large, (9–)14–25(–30) × (7–)13–24(–29) μm, l/w 1.0–1.2(–1.6) (n=33), intercalary, rarely terminal, globose or angular, rarely fusoid or pyriform, with smooth to wavy surface.
On PDA after 72 h 4–6 mm at 15 °C, 16–17 mm at 25 °C, 14–16 mm at 30 °C; mycelium covering the plate after 19–21 d at 25 °C. Colony circular, dense, finely zonate, margin well defined, surface for the most part becoming downy to densely hairy due to aerial hyphae. Aerial hyphae abundant, of two kinds, a) short, erect and wide, b) long, narrow, loosely disposed above short ones. White spots or flat tufts appearing on and around the plug, growing to ca. 5 mm diam, confluent to a continuum around the plug and turning green, 25–26E4–6, in subsequent experiments paler, 26C3, 26–27B3; mat of aerial hyphae becoming greyish greenish. Autolytic excretions common in marginal hyphae, no coilings seen. Reverse rosy-orange to orange-brown 5–6B4, 5C5, later dull brownish, 6CD5–8, darker and dull brown in the centre, 6E7–8; reverse of tufts turning black. No distinct odour noted. Conidiation noted from 3–4 d, green after 4 d, abundant, starting on and around the plug, on short, erect, delicate, straight to sinuous conidiophores on surface hyphae of flat central spots or tufts; also on aerial hyphae, with long sinuous phialides and short branches. At 15 °C colony finely zonate, surface turning green 28CD4–6 from the centre, downy to farinose; reverse pale orange to dull orange-brown, 5B4–5, 5CD5–6. Conidiation effuse, abundant on numerous, short, delicate conidiophores, sessile and on barbed-wire like aerial hyphae. At 30 °C colony irregular, dense, downy, margin ill-defined; white tufts formed on and around the plug, spreading across the colony and becoming greenish in the centre. Reverse dull orange-brown, 5AB4–5, blackish green spots below tufts. Conidiation sessile, short, central. Autolytic excretions abundant, minute.
On SNA after 72 h 2–5 mm at 15 °C, 10–13 mm at 25 °C, 7–9 mm at 30 °C; growth terminated before mycelium covering the plate (examined for 4 wk) at 25 °C. Colony hyaline, circular, with little mycelium on agar surface; central surface hyphae degenerating soon. Margin diffuse; broad loose marginal or distal zone hairy to fluffy due to abundant, long aerial hyphae to several mm high; becoming dark green, 26E4–6, 27–28DE4–6. Irregular, green, dense spots with minute shrubs or small roundish pustules to 0.7 mm diam formed. Whitish to greenish, translucent stromata to 5 mm diam formed, surrounded by barbed wire-like hyphae. Autolytic excretions inconspicuous, no coilings seen. No pigment, no distinct odour noted. Chlamydospores rare, noted after 4 wk (also after 1 wk at 30 °C). Conidiation noted from (4–)6 d, green after 8–9 d, mostly effuse, also submerged in the agar. Conidiophores numerous, in rows on surface hyphae, concentrated in a broad, concentric, green, hairy to powdery zone, and ascending several mm on aerial hyphae in the hairy marginal zone, 50–200(–300) μm long, simple, broad, often wider at the apex, with paired or unpaired branches often in right angles. Conidia formed in small numbers in wet or mostly dry heads to 20 μm. Continuous transition from simple conidiophores to complex shrubs and small pustules, mostly to 350 μm diam, noted. Shrubs and small pustules with short, sterile, straight to sinuous elongations to 180 μm long; surrounded by submerged hyphae with numerous fine pegs (barbed-wire like). Shrubs and small pustules formed on a stipe branched into several main axes with mostly unpaired conidiophores in dense fascicles. Stipe, main axes and conidiophores 6–9 μm wide at the base, attenuated upwards to 2.5–3 μm within a short distance. Conidiophores broad at the apex or often regular, i.e. with phialides at the top, followed by 1-celled branches, and, often paired branches increasing in length with distance from the top. Commonly several conidiophores convergent, resulting in complex, dense, globose or irregular structures. Phialides originating on cells (2–)2.5–4(–5) μm wide, mostly solitary or in whorls of 2–3; when in larger numbers, not originating at a common point, but slightly shifted against each other. Phialides (7–)9–16(–23) × (2.4–)2.7–3.5(–4) μm, l/w (2.3–)3–5(–6.8), (1.7–)2.3–3.0(–3.5) μm wide at the base (n=35), lageniform to subulate, with green, laterally projecting collarette, some entirely green, straight, curved or sinuous, without conspicuous thickening, typically thickest at or near the base. Conidia (3.0–)3.5–4.5(–5.5) × (2.7–)3–4(–5) μm, l/w (1.0–)1.1–1.2(–1.3) (n=40), subglobose or oval (or ellipsoidal), deeply green, smooth, at 15 °C with slightly uneven surface; with some minute guttules; scar indistinct, less commonly distinctly truncate. `Stromata' (after 21 d) 0.3–1.7 mm diam, covered by conidiophores, sterile, consisting of aggregated hyaline hyphae with short cylindrical appendages or pegs. Many cells of stromatal hyphae distinctly swollen, globose, ellipsoidal or oblong, (5–)9–22(–30) × (5–)8–18(–28) μm, l/w 1.0–1.3(–1.8) (n=30); some densely aggregated, pseudoparenchymatous, often resembling udders in shape; pegs cylindrical, straight or sinuous, (4–)6–11(–14) × 2.5–4.0(–5.0) μm, l/w (1.3–)2.0–3.6(–4.3) (n=30), 1-celled, separated from hyphae or thickened cells by a septum. At 15 °C colony dense; surface becoming granular, downy at the margin; marginal zone becoming green. Conidiation on long aerial hyphae and in green tufts 0.3–0.8(–1) mm diam, concentrically arranged along the margin. At 30 °C aerial hyphae abundant, collapsing and forming fluffy, cottony floccules; mycelium condensed into white spots in the centre; floccules and spots becoming greenish. Conidiation mainly on long aerial hyphae.
On MEA mycelium covering the plate after 2–3 wk; colony circular, finely zonate, with numerous aerial hyphae forming a white, later green, cottony mat. Centre becoming yellow. Conidiation starting in the centre, abundant, effuse, ascending on aerial hyphae, whitish, becoming yellow, finally green.
Habitat: on dead stalks of Calamagrostis epigejos.
Distribution: Europe, Germany, only known from type locality.
Holotype: Germany, Hessen, Landkreis Fulda, Gersfeld, Rhön, Rotes Moor (between Gersfeld and Wüstensachsen), from parking place Moordorf at B278 into the moor, elev. 810 m, 50°28'06” N, 09°58'23” E, on dead standing or lying stalks and leaves of Calamagrostis epigejos, soc. remnants of a setose brown fungus, 29 Aug. 2006, W. Jaklitsch & H. Voglmayr (W.J. 2960), WU 29031, ex-type culture CBS 120541 = C.P.K. 2456. Holotype of Trichoderma aerugineum isolated from WU 29031 and deposited as a dry culture with the holotype of H. aeruginea as WU 29031a.
Notes: Although superficially resembling H. spinulosa, this species is distinct, easily distinguished from the latter by the pseudoparenchymatous cortex and the formation of an anamorph. The peculiar green pigment formed by H. aeruginea on CMD is unique among all Hypocrea/Trichoderma isolates. Hypocrea aeruginea formed stromata on oatmeal agar (CBS, pers. comm.).
Hypocrea alni Jaklitsch, Mycologia 100: 799 (2008). MycoBank MB512089. Fig. 6.
Fig. 6.
Teleomorph of Hypocrea alni. a, b. Fresh stromata (all mature except rosy immature stromata on the left in a). c–e. Dry stromata (c. immature, velutinous, d, e. mature). f. Stroma surface in face view. g. Surface of mature stroma in 3 % KOH after reconstitution in water. h. Perithecium in section. i. Cortical and subcortical tissue in section. j. Subperithecial tissue in section. k. Base of stroma in section. l. Ascus with ascospores. a, c, f–k. WU 28224, b, e. WU 28226, d. WU 28232, l. WU 28225. Scale bars: a = 2 mm. b, e = 1.4 mm. c, d = 1 mm. f = 20 μm. g = 100 μm. h = 35 μm. i = 30 μm. j = 15 μm. k = 25 μm. l = 10 μm.
Anamorph: Trichoderma alni Jaklitsch, Mycologia 100: 799 (2008). MycoBank MB512095. Fig. 7.
Fig. 7.
Cultures and anamorph of Hypocrea alni (CBS 120633). a, b. Cultures on PDA after 7 d (a. 25 °C, b. 30 °C). c. Culture on CMD (25 °C, 7 d). d. Culture on SNA (25 °C, 7 d). e. Conidiation tufts (CMD, 25 °C, 17 d). f, g. Conidiophores of effuse conidiation (25 °C, f. SNA, on growth plate, 3 d. g. CMD, mounted, 4 d). h, i. Conidiophores from tufts (CMD, 25 °C, 7 d). j. Conidia (CMD, 25 °C, 10 d). Scale bars: a–d = 19 mm. e = 1 mm. f = 50 μm. g, h = 20 μm. i = 25 μm. j = 5 μm.
Stromata when fresh 1–8(–12) mm diam, 1–3(–5) mm thick, solitary, gregarious or aggregated in small groups, sometimes erumpent through bark fissures, pulvinate to nearly semiglobose, or undulate; broadly attached. Margin free and often wrinkled. Perithecia entirely immersed, surface smooth, tubercular to rugose, with a whitish downy covering layer or flakes when young. Ostiolar dots numerous, typically hardly visible, brown, turning green to nearly black due to spore powder. Colour reddish grey, grey-brown to light reddish brown when young, becoming dark reddish brown, 9E7–8, often covered by dark green spore powder.
Stromata when dry 0.5–6.0 × 0.5–4.7 mm, 0.3–2.8 mm thick (n=90); pulvinate to semiglobose, sometimes discoidal with sunken centre, undulate or coarsely folded. Outline variable, circular, oblong to irregular. Margin thick, rounded, typically free. Sides straight and vertical or attenuated downward, velutinous, whitish to light grey or light brown when young; rarely with white basal mycelial margin. Surface when young smooth or covered with whitish or greenish flakes of Trichoderma conidiophores; surface when mature smooth and iridescent or finely to coarsely tubercular. Ostiolar dots (15–) 35–70(–94) μm (n=80) diam, inconspicuous, typically visible only under strong magnification, numerous, densely disposed, plane, dark brown to black or dark green from spore powder. Colour when young grey to grey-brown (ca. 5DE3); when mature or old dark (grey to reddish) brown, 5–7F2–4, 7F3–8, 8F3–5 to 9EF4–6, to black; cream or brownish inside. Consistency of stromata hard. Spore powder dark green, effuse, granular to pustulate. In 3 % KOH stromata dark blood-red, ostiolar openings distinct, hyaline.
Stroma anatomy: Ostioles (69–)74–97(–110) μm long, plane or projecting to 10 μm, (17–)20–30(–36) μm wide at the apex (n=30), apical cells parallel, cylindrical, 1–3 μm wide, ends broadly rounded. Perithecia (180–)210–260(–290) × (110–)120–200(–260) μm (n=30), globose to flask-shaped, crowded, 8–9 per mm of stroma length. Peridium (14–)17–25(–29) μm wide at the base, (5–)9–17(–21) μm at sides (n=30), yellow to nearly orange. Cortical layer (17–)19–36(–46) μm (n=30) thick, a t. angularis of thick-walled, orange-brown to yellow, angular to oblong cells (5–)7–14(–18) × (3.5–)5.0–8.5(–12.0) μm (n=60) in face view and in vertical section; covered by a thin, non-continuous layer of an orange, amorphous substance and collapsed or compressed cells, arranged in minute flakes. Cortical cells downwards gradually paler yellow, merging into the hyaline subcortical t. angularis to t. epidermoidea of thin-walled, roundish to oblong cells (3.5–)4.5–11(–17) × (2.0–)3.5–6.5(–7.0) μm (n=30) and hyaline hyphae (2.5–)3.5–5.5(–6.0) μm (n=32) wide, descending parallel between perithecia. Subperithecial tissue consisting of a hyaline t. epidermoidea of variably thick-walled cells (5–)10–33(–53) × (3–)7–16(–20) μm (n=30), including some hyphal elements. Basal tissue hyaline to yellowish brown, consisting of a dense t. intricata of thick-walled hyphae (2.5–)3.0–5.0(–6.0) μm (n=33) wide. No hairs on mature stromata, no stipe seen. Asci (67–)80–96(–113) × (4.0–)4.5–5.5(–6.5) μm, stipe (2–)7–30(–50) μm long (n=80). Ascospores green, brown in KOH, verrucose, warts to ca. 0.5 μm diam, cells dimorphic, distal cell (3.7–)4.0–5.0(–6.0) × (3.0–)3.5–4.0(–4.5) μm, l/w (1.0–)1.1–1.3(–1.5) (n=90), (sub-)globose to oblong or wedge-shaped, proximal cell (3.5–)4.5–6.0(–7.2) × (2.7–)3.0–3.5(–4.0) μm, l/w (1.0–)1.3–1.9(–2.4) (n=90), oblong to wedge-shaped or subglobose; some asci only with 4 ascospores.
Cultures and anamorph: Optimum growth at 25 °C on all media; no growth at 35 °C. On CMD after 72 h 10–13 mm at 15 °C, 35–40 mm at 25 °C, 23–25 mm at 30 °C. Mycelium loose, covering the plate after 5 d at 25 °C.; hyphae narrow, radial. One or several broad concentric zones of conidiation appearing, starting at the distal margin of the colony. Surface becoming downy, developing fine white floccules, fluffy tufts or pustules to 3 mm diam, confluent to amorphous masses to 10(–15) mm diam. Aerial hyphae frequent, particularly at the colony margin, erect, richly branched, several mm high, becoming fertile. Autolytic activity absent or inconspicuous. Coilings frequent. No diffusing pigment, no distinct odour noted. Chlamydospores noted only after 2 wk at 30 °C. Conidiation at 25 °C noted after 2 d, first effuse, starting around the plug, spreading across the colony, later in variable cottony fluffy tufts or pustules, generally with a hairy aspect due to superposed long fertile aerial hyphae; after 5 d slowly becoming matt grey-green 26–27D4, 27E4–5. Effuse conidiation: conidiophores loosely disposed, verticillium-like, little branched, 2–4 μm wide; short, erect, or on aerial hyphae to 1–2 mm long and high, generally asymmetrical. Stipe thick-walled, to 1 mm long and 4–5 μm wide, attenuated to 2 μm toward ends; bearing unpaired branches. Branches to 250 μm long and (2.0–)2.5–3.5(–4.5) μm wide; with 1–2-fold further loose asymmetrical branching, terminating in broad or pyramidal side branches with numerous, terminal, minute, wet conidial heads, 20 μm diam, greenish under the stereo-microscope. Side branches short, simple, of a 1–3 celled axis and paired or unpaired, typically distinctly divergent, often right-angled branches successively shorter toward the tip of the axis, bearing phialides solitary or divergent in whorls of mostly three, often with two paired phialides directly below terminal whorl, arising from cells 1.5–4.0(–4.5) μm wide; cell below the phialide often slightly thickened. Phialides (8–)9–15(–18) × (2.2–)2.5–3.0(–3.5) μm, l/w (2.5–)3.0–5.6(–7.0), (1.5–)1.7–2.5(–2.7) μm wide at the base (n=30), lageniform, mostly thickened above the middle, straight and symmetrical, less commonly slightly curved and inequilateral. Conidia 3.0–4.0(–4.2) × (2.2–)2.5–3.0(–3.2) μm, l/w 1.1–1.3(–1.4) (n=32), green, smooth, subglobose to oval or ellipsoidal, with few fine guttules, scar indistinct. Pustulate conidiation (after 7–10 d): tufts/pustules arising from a stipe 4–7 μm wide, asymmetrically branched to form a loose reticulum of irregularly oriented narrow conidiophores, becoming dense within pustule upon formation of conidia; periphery remaining loose, sometimes with straight to sinuous, sterile to fertile elongations 70–130(–150) μm long. Right-angled paired branches uncommon; short, usually 1-celled branches of minute terminal side branches often paired, bearing phialides solitary or in whorls of 2–3(–5), most commonly in cruciform whorls of three. Phialides (6–)7–11(–13) × (2.2–)2.5–3.5(–4.5) μm, l/w (1.7–)2.5–4.2(–5.6), (1.2–)1.5–2.0(–2.2) μm wide at the base (n=32), lageniform to ampulliform, thickened at or above the middle, often attenuated at the base, particularly narrow when solitary. Conidia (2.2–)2.5–3.5(–4.7) × (2.2–)2.5–2.7(–3.0) μm, l/w 1.0–1.4(–1.8) (n=32), green, mostly subglobose to oval, sometimes ellipsoidal, smooth, with few minute guttules. At 15 °C fluffy tufts arranged in a broad downy, distal zone, remaining white. At 30 °C colony zonate; coilings numerous; conidiation effuse and in greenish granules on the proximal margin and tufts in a broad distal zone.
On PDA after 72 h 10–13 mm at 15 °C, 27–32 mm at 25 °C, 17–22 mm at 30 °C; mycelium covering the plate after 6–7 d at 25 °C. Colony indistinctly zonate. Mycelium dense, opaque, thick; hyphae wide, sinuous and often forming strands on the margin. Margin thin, ill defined. Aerial hyphae numerous, several mm high, without a distinct orientation, adhering in strands, forming a dense white downy, fluffy or floccose mat, with large drops of fluid in aged cultures. Autolytic activity absent or inconspicuous. Coilings frequent. No diffusing pigment, no distinct odour noted. Conidiation noted after 2–3 d, effuse, starting around the plug on short aerial hyphae, spreading on lower levels of mats of aerial hyphae across the plate; becoming pale to matt grey-green 27CE3–5 to 26DE4 after 5–7 d in a proximal to central area and often in several additional, indistinctly separated, flat, velutinous, concentric zones. At 15 °C fluffy tufts formed in the centre, becoming only pale greenish in proximal areas. At 30 °C hyphae becoming yellow, conidiation scant, colourless. Autolytic excretions numerous, yellowish brown. Coilings numerous. Reverse becoming (grey-)yellow to orange, 4A5–6, 4B4–8, or 5B5–6.
On SNA after 72 h 10–13 mm at 15 °C, 30–32 mm at 25 °C, 11–13 mm at 30 °C; mycelium covering the plate after ca. 6 d at 25 °C. Colony similar to CMD; mycelium loose, hyphae more curly, marginal surface hyphae wide. Margin ill defined. Indistinctly separated, downy concentric zones with numerous, long, erect aerial hyphae, appearing in distal parts of the colony; developing ill defined fluffy tufts to 2.5 mm diam, confluent to 5 mm, becoming diffuse pale green 27C3–4, 27E3–6 after 4 d, starting in proximal areas of the growth plate; often margin of tufts remaining white. Autolytic activity absent or inconspicuous, stronger at 30 °C. Coilings conspicuous, numerous at all temperatures. No diffusing pigment, no distinct odour noted. Chlamydospores noted after 2 wk at 30 °C, (4.5–)6–10(–13) × (3.5–)5.5–9.0(–11) μm, l/w (0.8–)0.9–1.3(–1.7) (n=31), globose, pyriform or angular, smooth, terminal and intercalary. Conidiation similar to CMD, noted after 2 d. At 15 °C conidiation effuse and in amorphous greenish tufts. At 30 °C conidiation scant, simple, effuse.
Habitat: On wood and bark of Alnus glutinosa, in one instance found on Betula pendula; in wet habitats, often along rivers.
Distribution: Europe, collected in Austria, The Netherlands, Ukraine and the UK.
Holotype: United Kingdom, Derbyshire, Peak District National Park, Baslow, Longshaw Country Park, 53°18'20” N, 01°36'18” W, elev. 350 m, on partly decorticated branches of Alnus glutinosa, 1–4 cm thick, holomorph, 11 Sep. 2004, W. Jaklitsch & H. Voglmayr (W.J. 2701), WU 28224, ex-type culture CBS 120633 = C.P.K. 1982. Holotype of Trichoderma alni isolated from WU 28224 and deposited as a dry culture with the holotype of H. alni as WU 28224a.
Other specimens examined: Austria, Niederösterreich, Mödling, Wienerwald, Kaltenleutgeben, along brook Dürre Liesing between Am Brand and Stangau, MTB (,Messtischblatt`) 7862/4, 48°06`45“N, 16°08`43“E, elev. 450 m, on partly decorticated branch of Alnus glutinosa, on wood, bark and effete immersed pyrenomycete, soc. Steccherinum ochraceum, Hypocrea moravica; holomorph, 22 Oct. 2006, H. Voglmayr & W. Jaklitsch, W.J. 3027 (WU 28225, culture C.P.K. 2494). Italy, Sardinia, central western coastal area close to Capo Mannu, elev. 239 m, 40°04'06” N, 8°32'15” E, anamorph isolated from soil sample, Oct. 2004, Q. Migheli (culture C.P.K. 2657 = UNISS 10-16). Netherlands, Utrecht, De Uithof, small mixed deciduous forest between sport park Olympos, botanical garden and motorway crossing Rijnsweerd, elev. 0 m, on corticated branches of Alnus glutinosa 0.5–2 cm thick, on wood and bark, holomorph, anamorph partly distorting and overgrowing basidiomata of Macrotyphula cf. contorta, 18 Nov. 2006, H. Voglmayr, W.J. 3044 (WU 28226, culture C.P.K. 2854). Utrecht, between Botanical Garden and University at Leuvenlaan, elev. 0 m, on branch of Alnus glutinosa, holomorph, 2 Dec. 2006, H. Voglmayr, W.J. 3051 (WU 28227, culture C.P.K. 2858). Ukraine, Poltava oblast, Mirgorod area, close to Velikiye Sorochintsy, 800 m W from the river Psyol, flood lands alder forest, 49°58'14” N, 33°59'03” E, elev. ca. 100 m, on branches of Alnus glutinosa, 18 Aug. 2007, A. Akulov, AS 2488 (WU 28232, culture C.P.K. 3154). United Kingdom, Lancashire, Ribble Valley, Clitheroe, north from Dunsop Bridge, 53°56'47” N, 02°32'24” W, elev. ca. 300 m, on 2–3 cm thick branch of Alnus glutinosa, holomorph, anamorph soc. initials of Macrotyphula cf. contorta basidiomata, 6 Sep. 2007, W. Jaklitsch & H. Voglmayr, W.J. 3140 (WU 28229, culture C.P.K. 3141); north from and close to Bashal Eaves, 53°53'52” N, 02°28'31” W, elev. 200 m, on corticated twigs of Alnus glutinosa 2–3 cm thick in wet grass, holomorph, 7 Sep. 2007, W. Jaklitsch & H. Voglmayr, W.J. 3141 (WU 28230, culture C.P.K. 3142). Norfolk, North Wootton, close to Castle Rising, 52°47'15” N, 00°26'55” E, elev. ca. 10 m, on corticated twig of Betula pendula 1–2.5 cm thick in wet Sphagnum, holomorph, 9 Sep. 2007, W. Jaklitsch & H. Voglmayr, W.J. 3145 (WU 28231, culture C.P.K. 3145). North Yorkshire, Thornton le Dale, Dalby Forest, Forest Drive, shortly after Low Dalby at Dalby Beck, 54°16'58” N, 00°41'21” W, elev. 150 m, on thin corticated twigs of Alnus glutinosa in wet grass, mainly anamorph, 5 Sep. 2007, W. Jaklitsch & H. Voglmayr, W.J. 3138 (WU 28228, culture C.P.K. 3139).
Hypocrea aureoviridis Plowr. & Cooke, Grevillea 8: 104 (1880). Fig. 8.
Fig. 8.
Teleomorph of Hypocrea aureoviridis. a, b. Fresh stromata (a. immature, b. mature). c–h. Dry stromata (c, e, f, h. mature with varying amounts of white scurf; d, g. immature, d. half dry). i. Mature stroma in 3 % KOH after reconstitution in water. j. Perithecium in section. k. Cortical and subcortical tissue in section. l. Stroma surface in face view. m. Subperithecial tissue in section. n. Base of stroma in section. o, p. Asci with ascospores. a–c, h. WU 29035. d, f, g, i–n. WU 29033. e, p. WU 29034. o. WU 29036. Scale bars: a, h. 0.5 mm. b, c, e, f. 0.7 mm. d, g. 1 mm. i. 1.3 mm. j, k, n. 25 μm. l. 20 μm. m. 30 μm. o, p. 10 μm.
= Chromocrea aureoviridis (Plowr. & Cooke) Petch, Trans. Br. mycol. Soc. 21: 293 (1938).
Anamorph: Trichoderma aureoviride Rifai, Mycol. Pap. 116: 34 (1969). Fig. 9.
Fig. 9.
Cultures and anamorph of Hypocrea aureoviridis. a–c. Cultures at 25 °C (a. on CMD, after 14 d. b. on PDA, after 21 d. c. on SNA, after 21 d). d. Conidiation tufts on CMD (25 °C, 9 d). e. Conidiophores/shrubs of effuse conidiation on CMD (25 °C, 9 d). f. Surface hyphae on SNA (25 °C, 4 d). g. Crystals in agar (CMD, 15 °C, 7 d). h, i. Conidiophores from pustules. j–l. Phialides. m, n. Conidia. h–n. CMD, 25 °C, 7 d. a–d, f, g, m, n. CBS 120536. e, h–l. C.P.K. 2848. Scale bars: a–c = 19 mm. d = 0.8 mm. e = 65 μm. f = 0.2 mm. g = 0.15 mm. h = 30 μm. i, k, l = 15 μm. j = 10 μm. m, n = 5 μm.
Stromata when fresh 1–10 mm diam, to ca. 1–1.5 mm thick, discoidal when immature, pulvinate when mature, centrally attached; margin free, sharp when immature, later thick and rounded; surface smooth; colour first white, becoming pale yellow with a white scurf on the surface, finally golden yellow with ostiolar dots becoming dark green to black. Stromata after reconstitution in water olive, 4EF4–5, flakes less conspicuous, slightly darker brown in 3 % KOH, drying black.
Stromata when dry (0.5–)1.2–2.8(–5) × (0.5–)1.1–2.4(–3.8) mm, (0.1–)0.3–0.5(–0.8) mm thick (n=100), solitary, gregarious or aggregated in small numbers, sessile, discoidal, (flat) pulvinate to lentiform; broadly attached, but often large part of stroma free with narrowly rounded margins remaining close to the wood surface. Outline circular or with lobed margin. Surface flat, convex or with depressed centre, smooth to slightly tubercular, first finely downy, later usually densely covered by whitish flakes (more conspicuous than on fresh stromata), sides and base sometimes with white mycelium or flakes when young. Ostiolar dots (24–)43–97(–165) μm (n=130) diam, densely disposed, first indistinct, diffuse, slightly darker than stroma surface, orange to olive, later distinct, minute between surface flakes or large black spots when flakes absent, broad, plane, umbilicate or convex, becoming dark green to black due to ascospores; ostiolar openings glassy, hyaline, ca. 15–25 μm diam. Colour yellow to dull orange, 4A2–4, 5AB3–5(–6), 6AB4–6, to dull orange-brown or greyish orange 7CD4 when immature; macroscopically difficult to determine when mature, colour impression much dependent on background, of green to black ostiolar dots on an orange background, resulting in pale or dull greyish green, greyish brown to olive, 1CD2–3(–4), 2E3–4, 2B3, 3E3, 29–30CD3, 28–30EF3–4, 4BD3–4(–5), 5E4–6, 5CD3–5; sometimes dark green, brown or partly black due to the dull dark green to black spore deposits. Associated anamorph light green, powdery.
Stroma anatomy: Ostioles (48–)55–68(–78) μm long, plane or projecting to 10(–20) μm, (16–)21–35(–47) μm wide at the apex inside (n=30); hyaline, vertical apical cells inconspicuous, 1–4 μm wide. Perithecia (130–)160–195(–220) × (75–)100–150(–214) μm (n=30), flask-shaped to globose at the stroma margin, crowded, ca. 8–9 per mm; peridium (10–)12–20(–25) μm (n=30) wide at the base, (5–)8–14(–17) μm (n=30) at the sides, yellow-orange. Cortical layer (23–)25–42(–68) μm (n=30) thick, a t. angularis of pale orange, thin-walled, isodiametric to oblong, angular cells (4–) 7–15(–20) × (4–)6–11(–14) μm in face view and in vertical section, merging with the peridium at the ostioles. Surface with amorphous material or/and groups of collapsed cells. Hairs on mature stromata (10–)15–30(–40) × 3–6(–8) μm (n=30), seen on sides and free lower surface as remnants of hyaline hyphae or as elongations of the basal t. intricata, cylindrical, rarely submoniliform, 1 to several-celled, ends broadly rounded, smooth or rough. Subcortical tissue scant, a t. intricata of hyaline hyphae (2–)3–5(–6) μm (n=30) wide. Subperithecial tissue a dense t. epidermoidea of variable thin-walled hyaline cells (7–)9–27(–41) × (6–)7–11(–13) μm (n=32); at the base merging into a dense t. intricata of thin-walled brownish hyphae (2.5–)3–5(–7) μm (n=32) wide, mainly at the point of attachment; penetrating into the wood. Asci (60–)66–90(–135) × (4.0–)4.7–5.8(–6.8) μm, stipe (0–)4–19(–33) μm long (n=90). Ascospores (yellowish) green, turning brown in KOH, distinctly verrucose, cells monomorphic, (sub-)globose, distal cell (3.0–)3.3–4.0(–4.8) × (3.0–)3.4–4.0(–4.4) μm, l/w (0.8–)0.9–1.1(–1.2) (n=120), proximal cell (2.8–)3.2–4.0(–5.0) × (3.0–)3.3–4.0(–4.3) μm, l/w (0.8–)0.9–1.1(–1.3) (n=120); contact area often flattened. Ascospore basal in the ascus (and sometimes adjacent ascospore) dimorphic, distal part (sub-)globose (to wedge-shaped), (2.8–)3.3–4.2(–5.0) × (2.5–) 3.0–3.5(–4.0) μm, l/w (1.0–)1.1–1.3(–1.4) (n=50), proximal part oblong (to wedge-shaped), (3.4–)4.2–5.5(–6.5) × (2.2–)2.5–3.2 (–3.7) μm, l/w (1.3–)1.4–2.0(–2.6) (n=50); occasionally also cells of other ascospores slightly dimorphic.
Cultures and anamorph: Growth slow, optimum growth at 25 °C on CMD and SNA, on PDA similar at 15 and 25 °C; at 30 °C only as short hyphae, dying soon; no growth at 35 °C. On CMD after 72 h 5–7 mm at 15 °C, 10–11 mm at 25 °C, 0.2–0.5 mm at 30 °C; mycelium covering the plate after ca. 3 wk at 25 °C. Mycelium loose, hyphae narrow, appearing rigid, right-angled branches frequent. Aerial hyphae inconspicuous, erect, becoming fertile. No autolytic excretions seen, coilings absent to moderate. Agar becoming diffuse dull yellow to brownish, 3A2–3, 4A3. No distinct odour noted. Conidiation noted after 1–2 d, green after ca. 5 d, starting on solitary sessile phialides or minute simple conidiophores or shrubs around the plug, spreading across the plate. Shrubs formed on surface hyphae, erect, 100–250 μm long, simple, typically widened apically to form broom- or fan-shaped structures; originating on thick-walled stipes to 13 μm wide, often moniliform. Conidiophores asymmetric, in right angles or not, width as in pustules. Phialides solitary or in whorls of 2–3(–4), strongly inclined upwards, narrowly lageniform to subulate. Conidia formed in wet heads to 40 μm diam. Tufts or pustules formed in concentric rings or randomly distributed, first white, turning green from within (26E4–8, 27DE5–6, 26–28F7–8, on yellow background), often with persistent white margin; growing to 0.3–1.5(–2) mm diam, confluent in lines. Tufts/pustules usually fluffy but opaque, with conidiation denser within; arising on thick-walled, sometimes moniliform stipes 7–9(–14) μm wide, asymmetrically branched into primary branches 5–6.5 μm wide, with some thickened, globose branching points to ca. 10 μm wide. Conidiophores (1.5–)2–3(–4) μm wide, flexible, often curved; no main axes discernible; right angles common, branching asymmetrical. Short side branches sometimes structurally similar to shrubs, but margins of pustules usually with simple regular trees. Elongations on young pustules long, straight or slightly sinuous, becoming fertile and inconspicuous. Phialides solitary or in whorls of 2–3(–5–6), sometimes cruciform; originating on cells 1.5–3 μm wide. Conidia formed in numerous minute dry heads mostly < 10 μm diam. Phialides (5–)8–15(–20) × 2.0–2.5(–3.0) μm, l/w (1.8–) 3.4–6.6(–9.5), (1.0–)1.5–2.2(–2.5) μm wide at the base (n=43), narrowly lageniform, rarely ampulliform, straight or curved, thickest in the middle, base thin, neck gradually attenuated and often long. Conidia (2.7–)3.0–3.8(–4.8) × (2.0–)2.3–2.6(–3.0) μm, l/w (1.1–) 1.3–1.6(–1.9) (n=64), green, smooth, subglobose or ellipsoidal, often attenuated toward one end, finely guttulate or with 1–2 larger guttules; scar indistinct or narrowly truncate. Chlamydospores noted after 2–3 wk, (5–)6–10(–12) × (5–)6–8.5(–10) μm, l/w (0.9–)1.0–1.3(–1.7) (n=35), infrequent, (sub-)globose, ellipsoidal or fusoid, thick-walled, intercalary and terminal in thin hyphae. At 15 °C colony irregular, resembling ice crystals, becoming yellow 3A3–4 to 4A4; numerous yellow crystals formed within agar; conidiation in minute whitish to green shrubs or tufts spreading from the plug.
On PDA after 72 h 3.5–5 mm at 15 °C, 4–5 mm at 25 °C, 0–0.2 mm at 30 °C; mycelium not reaching the distal margin or covering the plate after > 1 mo at 25 °C. Hyphae as on CMD, colony irregular to lobed, dense; margin loose and hyaline; surface whitish to yellowish downy. Aerial hyphae numerous, branched, without a distinct orientation, forming strands, becoming fertile. Autolytic activity inconspicuous, coilings rare to moderate; odour none to slightly mushroomy; agar becoming discoloured from the plug, dull yellow to yellowish- or light brown, 3–4A4, 4B4–8, 5CD5–8, 5EF6–8, yellow pigment diffusing into the agar. Conidiation noted after 2 d, green after ca. 3 wk, starting around the plug on minute shrubs, spreading across the plate on aerial hyphae, becoming dense and fluffy; also fan-shaped tufts formed; surface on and around the plug becoming dark green. At 15 °C colony irregular, dense, yellow to brown 4A4–5, 6DE7–8, with numerous yellow crystals in the agar also formed beyond colony margin. Conidiation in shrubs spreading from the plug, colourless, greenish only in the stereo-microscope.
On SNA after 72 h 5–6 mm at 15 °C, 8–10 mm at 25 °C, 0–0.2 mm at 30 °C; mycelium covering the plate after > 1 mo at 25 °C. Colony irregular, hyphae appearing rigid, right angles common. Aerial hyphae inconspicuous, erect, becoming fertile. No autolytic activity, coilings, pigment, distinct odour and chlamydospores noted. Conidiation noted after 1–2 d, green after ca. 5 d, starting in minute broom- or pine-like, apically widened shrubs with heads to 50 μm, followed by whitish granules around the plug, spreading across the colony, growing to small dense tufts or pustules 0.3–1.5 mm, turning green 27D5–6, 28F7–8, 28E6–7, often forming indistinct and confluent concentric rings; pustules with numerous dry minute heads on regular trees and short straight fertile elongations (as in T. strictipile). At 15 °C colony irregular, turning yellowish (1A4); numerous yellow crystals within agar, surface indistinctly granular to hairy; conidiation scant, on simple conidiophores loosely disposed across the colony, tufts rare, becoming greenish.
Habitat: on strongly decayed, crumbly wood of deciduous trees, often green from algae.
Distribution: North Europe and Atlantic region of Europe, common in the UK and the Netherlands.
Lectotype: England, Norfolk, North Wootton, on wood, Aug. 1875. C.B. Plowright (ex herb. M.C. Cooke), K(M) 161972. The specimen contains a piece of wood of Corylus avellana with three immature stromata, and a small envelope containing a piece of Quercus wood with one incomplete overmature stroma. Rifai & Webster (1966) designated the material on Quercus as lectotype, and according to these authors, Plowright collected the material on Corylus at the same place in 1879. Due to the bad condition of the lectotype the following specimen is designated here as the epitype of Hypocrea aureoviridis: England, Norfolk, Thetford, Thetford National Forest Park, close to Lynford, MTB 3530/1, 52°28'54” N, 00°41'01” E, elev. 30 m, on decorticated, well-rotted hardwood, 3–4 cm thick, soc Eutypa sp., 13 Sep. 2004, W. Jaklitsch & H. Voglmayr (W.J. 2708), K(M) 162235, ex-epitype culture CBS 120536 = C.P.K. 1907).
Other specimens examined: Germany, Sachsen-Anhalt, Landkreis Aschersleben-Staßfurt, Staßfurt, Horst, MTB 4135/1, 51°52'00” N, 11°35'00” E, elev. 30 m, on branch of Sambucus nigra 9 cm thick, on wood, 7 Nov. 2003, R. Geiter, W.J. 2489 (WU 29032, culture C.P.K. 1610). Netherlands, Utrecht, west from the CBS, at a ditch north from the Botanical Garden, elev. 0 m, on partly corticated branches of Salix caprea 1–5 cm thick, soc. green algae, Corticiaceae and mould, 11 Nov. 2006, H. Voglmayr, W.J. 3033 (WU 29034, culture C.P.K. 2848). At the CBS, beside the guest house at a water channel, elev. 0 m, on branches of Acer campestre, Quercus robur, and Rubus fructicosus 1–3 cm thick, on/soc. green algae, Eutypa sp., holomorph, 13 Nov. 2006, H. Voglmayr, W.J. 3034 (WU 29035, culture C.P.K. 2849). Oud Amelisweerd, between Utrecht and Bunnik, elev. 0 m, on partly decorticated branch of Fagus sylvatica 10 cm thick, soc. green algae, 25 Nov. 2006, H. Voglmayr, W.J. 3049 (WU 29036, culture C.P.K. 2857). De Uithof, small mixed deciduous forest between sport park Olympos, botanical garden and motorway crossing Rijnsweerd, elev. 0 m, on branch of Fraxinus excelsior, soc. green algae, 28 Nov. 2006, H. Voglmayr, W.J. 3050 (WU 29037). Sweden, Skåne, Allerum, Hittarp, on branch of Sambucus nigra, 6 Feb. 1995, S.-Å. Hanson 18297; Gärdslöv, 0.8 km north of Tärnö, on deciduous wood, 3 Dec. 2003, S.-Å. Hanson 2003-321. Raus, Örby, on branch of Populus sp., 30 Oct. 1993, S.-Å. Hanson 10705. United Kingdom, Essex, Debden, Spinney Wood, on branches of Ulmus glabra, 18 Feb. 2007, K. Robinson, comm. P. Wilberforce (WU 29039); Hertfordshire, Benington, Combs Wood, on branch of Quercus robur, 21 Feb. 2007, K. Robinson, comm. P. Wilberforce, W.J. 3071 (WU 29038); Stevenage, Box Wood, on branch of Fraxinus excelsior, 21 Aug. 2007, K. Robinson (WU 29525). Norfolk, Dersingham, Snettisham, close to Lodge Hill Farm, 52°52'54” N, 00°28'07” E, elev. 10 m, on decorticated branch of Fraxinus excelsior 8 cm thick, on wood, 9 Sep. 2007, W. Jaklitsch & H. Voglmayr, W.J. 3146 (WU 29040).
Notes: Hypocrea aureoviridis, originally described from England, is so far only known from Europe, with a marked northern Atlantic distribution, not uncommon in England and the Netherlands, not rare along the Atlantic coast of France (C. Lechat, pers. comm.); the most southern record in Germany seen is from Staßfurt. This species is often mixed up with H. strictipilosa. Stromata are inconspicuous when mature due to their greyish green colour, but immature stromata are conspicuous due to the golden yellow colour when fresh, orange when dry. Also conspicuous whitish flakes on the stroma surface, best seen on dry immature stromata, characterise this species, as well as the monomorphic ascospores (except the spore basal in the ascus). Conidiophores of pustules are reminiscent of sect. Trichoderma, particularly T. viride, due to narrow branches and lack of main axes. In isolates from the Netherlands, pustules on CMD were less evenly distributed than in British isolates. Stromata are often found in a dry state. Information and images of fresh stromata were contributed by the collector H. Voglmayr.
Hypocrea brunneoviridis Jaklitsch, Mycologia 100: 805 (2008). MycoBank MB512097. Fig. 10.
Fig. 10.
Teleomorph of Hypocrea brunneoviridis. a–c, e. Fresh stromata (a. stroma initial, b. stroma initial with partial pigmentation, c. immature, e. mature). d, h. Dry stromata (d. immature. h. mature). f. Stroma surface in face view. g. Surface of mature stroma in 3 % KOH after reconstitution in water. i. Perithecium in section. j. Cortical and subcortical tissue in section. k. Subperithecial tissue in section. l. Base of stroma in section. m, n. Asci with ascospores. a–c, e, n. WU 28235; d, f–m. WU 28233. Scale bars: a = 0.4 mm. b, e, g, h = 0.7 mm. c, d = 1 mm. f = 15 μm. i = 35 μm. j, l = 25 μm. k = 30 μm. m, n = 10 μm.
Anamorph: Trichoderma brunneoviride Jaklitsch, Mycologia 100: 805 (2008). MycoBank MB512098. Fig. 11.
Fig. 11.
Cultures and anamorph of Hypocrea brunneoviridis. a–c. Cultures after 7 d at 25 °C (a. On CMD. b. On PDA. c. On SNA). d. Granular pustules (after 7 d). e. Conidiophores on growth plate (after 12 d). f, g, j, k. Conidiophores (after 4–5 d). h. Hyphae with pegs. i. Conidia. f, g, i, j. On MEA, at 25 °C. d, e, h, k. On CMD, at 25 °C. a–d, h, k. CBS 121130. e. C.P.K. 2425. f, g, i, j. CBS 120928. Scale bars: a, b = 10 mm. c = 2.6 mm. d = 0.5 mm. e = 70 μm. f, k = 20 μm. g = 35 μm. h = 0.1 mm. i = 5 μm. j = 15 μm.
Stromata when fresh 1–10 mm diam, to 3 mm thick, solitary, gregarious or aggregated in small numbers (2–3), thickly pulvinate. Outline circular or angular. Margin wavy. Surface smooth. Ostiolar dots distinct, numerous, densely disposed, finely papillate, becoming green. Development and colour: first appearing as white mycelium, compacting, then centre becoming brownish or rosy to reddish brown with olive margin or entirely rosy, grey-orange or reddish brown 6B4 to 8E5–8; later dark reddish brown, often with olive tones, finally dark brown 7F3–8; blackening or green by ejected spore powder; inside cream to light brown or with dark brown spots; often intense (grey-)green, 26DE4–6, Trichoderma on and around immature rosy stromata.
Stromata when dry 1–9 × 1–6 mm, 0.4–2.5 mm thick (n=60); typically pulvinate to semiglobose; button-like (short cylindrical) to discoidal with sunken centre when young or when old and collapsed; sessile, broadly attached. Outline circular to oblong, sometimes irregular, with undulate margins. Margin thickly rounded, free. Sides rounded or straight, covered with short hairs, whitish, olive, becoming smooth and brown; with whitish to cream mycelial margin at the base when young. Surface granulose by slightly projecting ostioles and/or perithecia, rugose or smooth. Ostiolar dots (24–)37–67(–87) μm (n=75) diam, numerous, densely disposed, minute; first pale, diffuse and plane, later convex to papillate; finally dark brown, dark green or black due to ascospores; sometimes visible only under strong magnification. Colour first rosy brownish, brown-orange or light (yellow-)brown, 5–6CD4–5, 5–6E5–8, when young, (reddish) brown, 7E4–6, 7–8E6–8 to mostly dark (reddish) brown, 7–8F4–8, 9F6–8, to nearly black when mature or old. Stromata often covered by dark green spore deposits; inside yellowish to brownish or light wood coloured, particularly when old. Associated anamorph effuse, powdery and dark green (26F4–8). Dry stromata after reconstitution with water brick red, becoming reddish brown and slowly turning black in 3 % KOH.
Stroma anatomy: Ostioles (64–)69–90(–106) μm long, umbilicate, plane or projecting to 10(–20) μm, (16–)20–36(–52) μm wide at the apex (n=30). Perithecia (130–)160–230(–290) × (90–)110–180(–245) μm (n=30), 8–10 per mm length of stroma, globose to flask-shaped or ellipsoidal, often laterally compressed. Peridium (12–)14–22(–30) μm (n=30) wide at the base, (5–)11–19(–23) μm (n=30) wide at the sides, yellow. Surface wavy, smooth; no hairs seen. Cortical layer (18–)20–35(–46) μm (n=30) thick, around the whole stroma except the area of attachment, cells at sides more hyphal; of a yellow- to orange-brown t. angularis of thin-walled, isodiametric to oblong cells (4.5–)7–13(–17) × (3.5–)5–8(–10) μm (n=60) in face view and in vertical section, compressed at the surface. Subcortical tissue of a t. angularis to t. epidermoidea of subhyaline cells (3–) 5–13(–17) × (2–)4–8(–10) μm (n=30), mixed with hyaline hyphae (2.5–)3.5–6.0(–8.5) μm (n=30) wide. Subperithecial tissue of a t. epidermoidea to t. angularis of (sub-)hyaline, angular to oblong cells (7–)12–30(–43) × (5–)9–16(–21) μm (n=30); at the base of the stroma slightly smaller and mixed with thick-walled, hyaline to yellowish hyphae (2.5–)3.5–6.5(–8.5) μm (n=30) wide, penetrating the wood. Asci (70–)80–102(–113) × (4.0–)4.5–5.0(–6.0) μm, stipe (1–)8–20(–30) μm (n=45). Ascospores yellowish green, verrucose; cells dimorphic, distal cell (3.0–)3.5–4.7(–5.7) × (2.5–)3.5–4.0(–4.5) μm (n=75), l/w (0.9–) 1.1–1.3(–1.6) (n=75), subglobose, less commonly wedge-shaped, proximal cell (3.5–)4.5–6.0(–7.0) × (2.5–)3.0–3.5(–4.0) μm, l/w (1.2–)1.3–1.8(–2.3) (n=75), oblong or wedge-shaped; proximal cell in ascus base often larger.
Cultures and anamorph: Mycelium covering the plate only on MEA; precultures therefore on MEA. Optimum growth at 25 °C on all media, no growth at 35 °C. On CMD after 72 h 4–6 mm at 15 °C, 4–15 mm at 25 °C, 3–11 mm at 30 °C. Colony circular to irregular. Margin often ill defined, e.g. torn into teeth. Margin of the plug becoming green, followed by the formation of white granules or pustules to 1.5–2 mm diam, with powdery to granulose surface, spreading across the colony, becoming dark green, 28EF5–8, 27F4–8, 27E4–6, to nearly black, aggregating to 3 mm. Marginal hyphae forming numerous short pegs, 15–70(–220) μm long, before termination of growth. Aerial hyphae absent to scant. No autolytic excretions, no coilings seen. No diffusing pigment, no distinct odour noted. No chlamydospores, only thickenings in hyphae seen. Conidiation noted after 1–3 d, starting on and around the plug in short minute shrubs with wet heads to 40(–70) μm diam, spreading, growing to pustules, becoming dry and green after 4–6 d. Pustules first loose, arising from a stipe 7–8(–11) μm wide, with radial, steeply ascending branches. Main branches 4–6 μm wide, 3–4 μm toward ends. Thick clusters formed within tufts, often with marginal branches still sterile. Branching generally asymmetrical, except for terminal branches. Terminal branches straight, narrowly pyramidal, with lower branches often paired and in right angles, others strongly inclined upward, bearing divergent phialides on several levels in whorls of 2–5, often strongly inclined upward to nearly parallel, originating on cells (3.0–)3.5–4.5(–5.0) μm wide. Phialides (5.5–)7.0–11.0(–13.5) × (3.0–)3.5–4.5(–5.0) μm, l/w (1.3–)1.8–2.8(–4.0), (1.5–)2.0–3.0(–3.5) μm wide at the base (n=63), lageniform, plump, often longer and narrower in terminal position. Conidia (3.2–)3.5–4.5(–5.5) × (2.5–)3.0–3.5(–3.7) μm, l/w (1.1–)1.2–1.4(–1.6) (n=63), green, smooth, subglobose to ellipsoidal or oval, with minute guttules and minute, flat, often indistinct scar. At 15 °C conidiation in small shrubs or pustules, dark green 27E5–6, 26–27F5–8, confluent to a continuum around the plug. At 30 °C hyphae finely submoniliform, conidiation absent or in small shrubs or green pustules.
On MEA conidiation in several flat, (yellow-)green concentric zones; conidiophores in dense lawns or confluent, erect, bearing numerous, minute, wet heads to 20(–30) μm diam; main axes (stipes) 6–9 μm wide, attenuated upward to (3–)4–5 μm, forked basally, or asymmetrically branched on few (max. four) levels, bearing one or several terminal side branches 0.2–0.3 mm diam, short and broad or pyramidal, often with paired branches. Solitary conidiophores to 0.8 mm long; generally strongly inclined upward, side branches to 150 μm long, some oriented downward; phialides arising in whorls of 2–5 on often slightly thickened cells (3.5–) 4.0–5.0(–5.5) μm, divergent, but usually strongly curved upward, lageniform to ampulliform, thickened below or in the middle, becoming green when old. Phialides and conidia as described on CMD (measurements combined).
On PDA after 72 h 4–6 mm at 15 °C, 4–10 mm at 25 °C, 1–9 mm at 30 °C. Colony either conspicuously irregular with growth stopping after few days, or first circular, compact, dense, flat, white around the plug, and margin well defined. Often growth discontinued, e.g. laterally, resulting in irregular outlines. Marginal surface hyphae finely submoniliform. Aerial hyphae inconspicuous. No autolytic excretions seen. Coilings frequent. Reverse grey-yellow 3–4C4–5, light to grey- or golden-yellow 4AC5–6 to (yellow-)brown 5–6E7–8. No distinct odour noted. Conidiation noted after 1–2 d, green after ca. 3 d, starting around the plug, spreading across the colony, on short simple erect conidiophores forming dense lawns; concentrated in several alternating, grey-green and (olive-)yellow, concentric zones, 29CD4–8, 28CD5–6, 3–4CD4–6. Surface of zones becoming powdery and eventually turning dull olive to (yellow-, olive-)brown, 3DE4–6, 4E6–8, 5E4–5. Sometimes stromata formed after ca. 3 wk. Stromata pulvinate, to 10 mm diam, several mm thick, green and velutinous due to conidiation on their surface, dull yellow-brown 5EF6–8, hard and compact inside, developing perithecia but remaining sterile. At 15 °C conidiation on terminal branches of more regular shape than at 25 °C, amassing in a floccose central zone, becoming grey-green 29–30CD4–5 to olive-brown 4CD3–4. Central surface dotted with numerous orange-brown drops, 200–400(–800) μm diam. Reverse pale yellow 2–3A3, grey-yellow 4B4–6, to yellow-brown 5E7–8. At 30 °C conidiation effuse, on small, densely disposed shrubs, not becoming green.
On SNA after 72 h 2–5 mm at 15 °C, 3–12 mm at 25 °C, 2–5 mm at 30 °C. Colony dense, mycelium radial. Margin ill defined, torn into teeth; marginal hyphae forming pegs. Aerial hyphae lacking. No autolytic excretions seen. No coilings seen. No diffusing pigment, no distinct odour noted. Conidiation noted after 2–3 d, green after 3–6 d, starting in shrubs around the margin of the plug, aggregating to a matt green 28E4–7 continuum. In addition fine dark green, finally 28F5–8, pustules spreading across the colony and growing to 2 mm. Conidia becoming compacted into numerous wet drops to 100(–200) μm diam. Conidiation also noted submerged in the agar in the centre of the colony. Chlamydospores (5–)6–10(–11) × (5–)6–8 μm, l/w (0.9–)1.0–1.4(–1.5) (n=20), infrequent, in marginal hyphae of the colony, (sub-)globose, terminal, rarely intercalary. At 15 °C conidiation in numerous confluent, green 27–28E4–6, flat tufts or fine pustules to 1 mm diam. At 30 °C conidiation in a green continuum around the plug and in green shrubs to 1 mm diam with wet heads.
Habitat: on well-rotted wood and bark of deciduous trees, collected on Alnus incana and Fagus sylvatica.
Distribution: Europe (Austria, Germany).
Holotype: Germany, Bavaria, Unterfranken, Landkreis Haßberge, Haßfurt, close to Mariaburghausen, left roadside heading from Knetzgau to Haßfurt, MTB 5929/3, 50°00'33” N, 10°31'10” E, elev. 270 m, on partly decorticated branches of Fagus sylvatica 3–5 cm thick, on wood, bark and effuse basidiomycete, soc. and on stromata of Hypoxylon fragiforme, soc. Laxitextum bicolor, Pycnoporus cinnabarinus, rhizomorphs, effete pyrenomycete, Calosphaeria sp. in bark, holomorph, 4 Aug. 2004, H. Voglmayr & W. Jaklitsch (W.J. 2571), WU 28233, ex-type culture CBS 121130 = C.P.K. 2014. Holotype of Trichoderma brunneoviride isolated from WU 28233 and deposited as a dry culture with the holotype of H. brunneoviridis as WU 28233a.
Other specimens examined: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Zabrde, MTB 9452/4, 46°33'00” N, 14°25'17” E, elev. 550 m, on decorticated branch of Fagus sylvatica 4–5 cm thick, on wood, soc. Polyporus brumalis, farinose Corticiaceae and moss, holomorph, teleomorph overmature, 29 Oct. 2005, W. Jaklitsch & H. Voglmayr, W.J. 2870 (WU 28234, culture C.P.K. 2425). Steiermark, Bruck/Mur, Gußwerk, Rotmoos bei Weichselboden, beside dried brook at south side of the Rotmoos, MTB 8356/2, 47°40'56“N 15°09'25“E, elev. 690 m, on corticated log of Alnus incana 10 cm thick, holomorph, 27 Sep. 2006, H. Voglmayr, W.J. 2992 (WU 28235, culture CBS 120928 = C.P.K. 2477).
Hypocrea dacrymycella Cooke & Plowr., Grevillea 12: 100 (1884). Fig. 12a–o.
Fig. 12.
Hypocrea dacrymycella / T. dacrymycellum. a–p. Teleomorph. a–d. Fresh stromata (a, d. immature; b. partly mature; c. mature). e–h. Dry stromata (g. immature; e, h. partly mature; f. mature). i. Stroma reconstituted in water. j. Cortex in face view (in 3 % KOH). k. Perithecium. l. Cortical and subcortical tissue. m. Subperithecial tissue. n. Base. k–n in vertical section. o. Ascospore showing verrucae. p. Ascus with ascospores. q–v. Anamorph associated with stromata. q. Habit, with conidial heads. r, s. Conidiophores. t, u. Conidia. v. Whorl of four phialides. a–c, f, i–n. WU 29043. d. WU 29041. e. H. viscidula lectotype K 133498. g, o. W.J. 1243. h. H. dacrymycella holotype K 114743. p. WU 29042, q–t, v WU 29042a. u. C. velenovskyi holotype. Scale bars: a, c, e, i = 1.3 mm. b = 2.2 mm. d = 4.7 mm. f, g = 0.7 mm. h = 1 mm. j, p = 20 μm. k = 45 μm. l = 25 μm. m, n = 35 μm. o = 5 μm.
≡ Hypocrea viscidula W. Phillips & Plowr., Grevillea 13: 79 (1885).
≡ Creopus velenovskyi Z. Moravec, Česká Mykol. 10: 88 (1956).
≡ Hypocrea velenovskyi (Z. Moravec) P. Chaverri & Samuels, Stud. Mycol. 48: 99 (2003).
[= Hypocrea cupularis (Fr.) Sacc. sensu Winter (1887; as a dubious species), sensu Migula (1913), and sensu Petch (1938, p. 293, as Chromocrea cupularis (Fr.) Petch).
= Sphaeria cupularis Fr., Linnaea 1830: 539].
Anamorph: Trichoderma dacrymycellum Jaklitsch, sp. nov. MycoBank MB513179. Fig. 12q–v.
Anamorphosis T. dacrymycellum, stromatis consociata. Incrementum in agaris nunquam observatum. Phialides anguste lageniformes vel subulatae, (13)15–24(–31) × (3.5–)4.0–5.3(–6.3) μm, viridescentes. Conidia oblonga ad ovoidea vel ellipsoidea, viridia, glabra, (6.2–)7.0–10.5(–13) × (3.8–)4.5–5.8(–7.7) μm.
Stromata when fresh 2–10(–12) mm diam and 1–3(–4) mm thick, single, gregarious or densely aggregated in fascicles of 2–10, first short cylindrical or button-shaped, becoming discoidal, turbinate to distinctly cupulate, centrally attached, often on a short stipe; sometimes flat pulvinate with a convex surface, particularly when growing on cut areas. Outline mostly circular, in fascicles often distorted by mutual pressure, sometimes ellipsoidal or oblong. Surface glabrous, smooth, centrally depressed to concave or flat; margin free, first obtuse, later acute, revolute or projecting upwards, often undulate to lobed; sterile sides smooth. Colour white to pale yellow, 1A2–3 to 2A3(–4) when young and immature, becoming green with yellow margin upon maturation; ostiolar areas slightly projecting yellowish dots or spots, slightly darker than stroma surface, green when mature.
Stromata when dry (0.8–)2–5(–7) × (0.8–)1.5–4(–5.4) mm, (0.4–)0.6–2.3(–4.5) mm thick (n=45). First appearing as white mycelium, becoming compacted to small white buttons or beakers, with white radiating mycelium on the outside and around the base; then usually discoidal to turbinate, sometimes stipitate, centrally narrowly attached, easily detached. Margin first obtuse, becoming acute, usually widely projecting over thick short stipe, partly irregularly distorted-convolute. Surface mostly concave to even, verruculose by slightly projecting ostioles/perithecia, sometimes somewhat floccose-scaly between ostiolar areas. More rarely stromata flat pulvinate with slightly convex surface; immature stromata appearing somewhat gelatinous, particularly at sterile sides. Ostiolar dots (47–)75–146(–180) μm (n=80) diam, numerous, densely disposed, reddish brown in immature stromata, slightly darker than surrounding stroma surface, becoming green to nearly black by ascospores when mature, sometimes covered by conical or globose masses of ascospores. Colour when dry and immature white, pale yellow, (1–)2A2–3, 3A3–5, dull yellow to orange, 4A3–7, 5BC5–6; a mixture of yellow to orange ground colour plus green ostioles when mature, resulting in dull greyish olive, 3–4E3–5, 4E6–8, 4F3–6, to green 28–29EF5–8, 27E4–6, or nearly black; partly mature stromata 4B4–5, 3C4–5, 4C5; base green to black due to adhering conidia. In old herbarium material surface appearing somewhat glassy or waxy, with darker colours, immature from yellow 4A5–6, 5B5–6, ochre to rust, 6BC4–5, dark and dull orange-, reddish brown, 8EF5–8, mature greyish brown to nearly black, 7F3–4 to 8EF3–4(–5), margin lighter, orange-brown. Reconstituted mature stromata larger and thicker by 30–50 %, dull green, ca. 29–30E3–5, becoming brown after addition of 3 % KOH.
Stroma anatomy: Ostioles (47–)70–125(–150) μm (n=35) long, (25–)29–41(–45) μm (n=20) wide at the apex, plane with the surface or projecting to 25 μm, conical or cylindrical, lined with a palisade of oblong hyaline cells narrowly clavate at the apex and to 4 μm thick. Perithecia (175–)190–380(–440) × (135–)160–235(–265) μm (n=35), globose or flask-shaped; peridium (14–)16–23 (–25) μm (n=20) wide at the base, (5–)10–19(–22) μm (n=20) at the sides, gradually merging with the subcortical tissue at the apex. Cortical layer (26–)30–46(–63) μm (n=30) thick, glabrous, pale yellowish, appearing somewhat gelatinous at sides, of a distinct coarse t. angularis, cells compressed and walls to 1.5(–2) μm thick, the uppermost thin layer nearly amorphous due to strong compression of cells. Surface cells (5–)7–16(–29) × (4–)5–12(–19) μm (n=120) in face view and vertical section, somewhat glassy, gradually merging into the hyaline subcortical tissue. Subcortical tissue a t. angularis of isodiametric cells (6–)8–26(–42) × (4–)6–14(–20) μm (n=30), oblong between perithecia; cells elongate between perithecia. Subperithecial tissue a dense t. epidermoidea of thin-walled, angular, oblong or lobed cells (4–)10–36(–70) × (4–)8–16(–24) μm (n=60), cells more regularly angular directly below perithecia, more hyphal at the base, hyaline in fresh material, yellowish in herbarium material. Asci (109–)122–146(–163) × (6.0–)6.5–8.0(–9.5) μm (n=41), stipe (5–)8–22(–37) μm (n=30) long. Ascospores green, (greenish-, olive-) brown in herbarium specimens, coarsely tuberculate, verrucae numerous, variable, to 0.8(–1.5) μm long; cells dimorphic, distal cell (5.0–)6.3–8.0(–9.2) × (4.4–)5.5–7.5(–9.0) μm, l/w (0.9–)1.0–1.2(–1.7) (n=148), (sub-) globose to wedge-shaped; proximal cell (5.2–)6.5–9.0(–12) × (4.3–)5.0–6.8(–8.5) μm, l/w (0.7–)1.0–1.5(–2.1) (n=148), (sub-) globose, oblong or wedge-shaped; often both cells similar, but proximal cell typically narrower.
Anamorph in vivo: Mostly immature stromata often accompanied by widely effused, bright to dark green colonies, 25E5–6 to 25–26F5–8, 26F5–8, sometimes black around the bases of stromata, particularly in old herbarium specimens. Phialides formed in verticillium-like to nearly gliocladium-like whorls, i.e. divergent or directed upwards in steep angles, sometimes two in parallel. Phialides large, lageniform or subulate, (13–)15–24(–31) μm long, (3.5–)4.0–5.3(–6.3) μm wide and (2.7–)3.2–4.3(–4.8) μm wide at the base (n=30); thickest below the middle, becoming green in age; with funnel-shaped, often green collarette. Conidia (6.2–)7.0–10.5(–13) × (3.8–)4.5–5.8(–7.7) μm, l/w (1.1–)1.4–1.9 (–2.6) (n=120), olive to greenish brown, deep green in mass, oblong, also oval or ellipsoidal, smooth, eguttulate or with few tiny guttules; formed in compact heads.
Cultures: Despite several attempts, neither ascospores nor conidia germinated on CMD, MEA, PDA, and SNA, even after addition of vitamins or autoclaved aqueous extracts of wood/bark of Picea abies.
Habitat: in small to moderate numbers on wood of conifers, also on cut areas (more pulvinate forms), less commonly on bark; often below loosely attached bark of logs stored at shady roadsides, logs kept moist by large herbs such as Petasites covering them.
Distribution: Europe, collected in Austria, Czech Republic, Germany, UK; uncommon.
Typification: Holotype: United Kingdom, Norfolk, Brandon, on Scotch fir (= Pinus sylvestris), 7 Nov. 1881, C.B. Plowright (K 114743). This specimen is in poor condition. The stromata are largely immature, but one large stroma with mature ascospores, and also typical conidia were found. The large ascospores and conidia as well as typical immature stromata and occurrence on pine permit unequivocal identification. The type material of H. viscidula was collected at the same day at the same location as the holotype of H. dacrymycella. It is present in K in three envelopes as K 114742, K 133497 and K 133498. Conclusions by Chaverri & Samuels (2003, p. 107) were drawn from K 114742, which contains only immature stromata. However, both K 133497 and K 133498 contain mature stromata. H. viscidula is clearly conspecific with H. dacrymycella. K 133498 is here designated as the lectotype of H. viscidula. An epitype of Hypocrea dacrymycella is designated here due to the poor condition of the holotype: Austria, Kärnten, Völkermarkt, Eisenkappel, ca. 2 km after the town heading to Seebergsattel, MTB 9553/3, 46° 26' 16”N, 14° 33' 40”E, elev. 780 m, on partly decorticated, cut log of Picea abies 38 cm thick, stored at the roadside on the ground between herbs, stromata on wood below easily detached bark, holomorph, 17 Aug. 2004, W. Jaklitsch (W.J. 2602), WU 29042. Holotype of Trichoderma dacrymycellum: part of WU 29042, deposited with the epitype as WU 29042a.
Other specimens examined: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Schwarzgupf, MTB 9452/4, 46° 32' 11”N, 14° 25' 13”E, elev. 640 m, mainly decorticated cut log segments of Picea abies >10 cm thick, in pile on the ground, stromata on wood, soc. Actidium nitidum, 24 Oct. 1998, W. Jaklitsch, W.J. 1243. Osttirol, Lienz, Hopfgarten in Defereggen, Dölach, between the transformer and beverage depot, MTB 9041/3, 46° 55' 23”N, 12° 32' 41”E, elev. 990 m, cut log of Picea abies, 20 cm thick, stored at shady roadside, partly in water, stromata on wood, breaking through wet bark on underside of log, immature, soc. Nemania sp., 4 Sep. 2003, W. Jaklitsch, W.J. 2375 (WU 29041). Vorarlberg, Feldkirch, Laternsertal, Laterns, close to a bridge shortly before the village coming from Bonacker, MTB 8724/1, 47° 15' 53”N, 09° 43' 11”E, elev. 1020 m, on partly decorticated cut logs of Picea abies 15–50 cm thick, in piles, stromata on wood, very moist, below loose bark, few on bark, also on cutting areas, holomorph, soc. white mould on stromata, 1 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2662 (WU 29043). Czech Republic, Central Bohemia, Mnichovice near Prague; on Picea abies in cavities of a stump, holomorph; Nov. 1934; J. Velenovský 29/1947 (holotype of Creopus velenovskyi, PRM 153288). Germany, Baden Württemberg, Freiburg, Landkreis Breisgau-Hochschwarzwald, St. Märgen, parking place Holzschlag, MTB 8014/2, 47° 59`53“N, 08° 05`03“E, elev. 620 m, on partly decorticated cut logs of Picea abies 60–70 cm thick, in a pile, stromata on wood, holomorph, teleomorph immature, 2 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2669 (WU 29044). Berlin, Grunewald, on the base of a log of Pinus sylvestris, holomorph, 1891, P. Hennings, F 61484 and F 61485 (S), cited in Rehm: Ascomyceten 1445, as Hypocrea cupularis. United Kingdom, Norfolk, Thetford, Thetford National Forest Park, near and north of the town, MTB 35-30/3, 52° 26' 28”N, 00° 44' 01”E, elev. 30 m, cut log segments of Pinus sylvestris 17 cm thick, on grassy ground, stromata on bark, upper side of log, holomorph, teleomorph immature, soc. unidentified Corticiaceae, 13 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2705 (WU 29045). Yorkshire, Dalby Forest, White Cliff Rigg, SE86Y5, on decorticated wood of Pinus sylvestris, 28 Sep. 1992, C.R. Stephenson, det. B. Spooner (K 20824, as Chromocrea cupularis).
Notes: Hypocrea dacrymycella is characterised by the occurrence on conifers in Europe, large discoidal stromata, large ascospores, and the largest green conidia currently known in Trichoderma. Its anamorph has not been described previously. Although not assessed by molecular methods, the close association of the Trichoderma with stromata and the large sizes of phialides and conidia provide convincing evidence for an anamorph-teleomorph relationship. The distinctive conidia were seen in nine specimens including all type materials examined, thus also significantly support the conclusions regarding the synonymies recognised here. They also aid to identify H. dacrymycella.
Stromata are often found in immature stages, suggesting slow development and maturation. Pulvinate stromata, mostly found on cut surfaces, are sometimes similar to those of H. aureoviridis, which can be distinguished by much smaller, monomorphic ascospore cells.
Petch (1938) recognised the conspecificity of H. dacrymycella and H. viscidula, but listed Chromocrea cupularis (Fr.) Petch as the valid name for this species. However, although the teleomorph was described as cupulate, the only authentic specimen from Herb. E. Fries (UPS 133487) labelled Hypocrea cupularis, does not exhibit discoidal or cupulate stromata and is not conspecific with H. dacrymycella. Although the specimen is in good condition, unequivocal identification is difficult. Chaverri & Samuels (2003) interpreted it as H. gelatinosa. Hypocrea velenovskyi is clearly conspecific with H. dacrymycella, based on all traits characterising this species, including conidia.
Hypocrea danica Jaklitsch, sp. nov. MycoBank MB513180. Fig. 13.
Fig. 13.
Hypocrea danica (WU 29046, CBS 121273). a–d. Fresh stromata (a–c. mature; d. immature; b. side view). e. Dry mature stromata. f. Mature stroma reconstituted in water. g. Stroma of f after addition of 3 % KOH). h. Ostiole in lateral view. i, j. Cultures at 25 °C after 7 d (i. on PDA. j. on SNA). k. Perithecium. l. Cortical and subcortical tissue. m. Subperithecial tissue. n. Base. k–n in vertical section. o, p. Asci with ascospores (p. after prolonged incubation in KOH). Scale bars: a, d–g = 0.4 mm. b, c = 0.3 mm. h = 15 μm. i, j, n = 20 μm. k–m = 25 μm. o, p = 10 μm.
Anamorph not formed.
Hypocreae spinulosae similis, differt genetice et in incremento tardiore in agaris CMD, PDA et SNA, praesertim conspicue in 30 °C. Anamorphosis absens.
Etymology: due to its occurrence in Denmark.
Stromata when fresh 0.5–2 mm diam, 0.4–0.7 mm thick; solitary to gregarious, short cylindrical to turbinate; base and sides often with white cottony mycelium; attached to the substrate only by some hyphae; easily detached. Surface first matt, finely silky, later smooth and glabrous, light green to turquoise, mainly 26BC3–4. Ostioles dark green, slightly projecting. Lower side plane to strongly curved inside, smooth, white.
Stromata when dry 0.4–1.7 × 0.4–1.2 mm, 0.1–0.4 mm thick (n=10); flat pulvinate, with white mycelium on margins and light green, smooth to silky surface when young; or pulvinate to subturbinate (sides attenuated downwards), lacking marginal mycelium, with dark green, tubercular or fissured surface when mature or old. Ostiolar dots black, convex, (23–)30–50(–80) μm (n=30) diam. Spore powder dark green. Stromata after reconstitution in water larger, pulvinate, smooth (papyraceous), pale green, turning macroscopically black (brown to olive plus black ostioles in the stereo-microscope) in 3 % KOH.
Stroma anatomy: Ostioles (53–)59–74(–81) μm long, hyaline internal part projecting to (10–)17–36(–50) μm, close to apex (14–) 18–27(–31) μm wide (n=30), to ca. 55 μm wide including wall; conical, hyaline inside, (sub-)apically surrounded by greenish cells to ca. 6 wide; periphyses narrow, apically convergent, cylindrical, some with beak-like ends 1–2 μm wide. Perithecia (118–)135–170(–185) × (70–)85–125(–170) μm (n=30), flask-shaped, also globose at stroma sides. Peridium (6–)11–17(–21) μm thick (n=60) at the base and sides, subhyaline to yellowish. Layer above and between perithecia (10–)15–32(–41) μm (n=30) thick, hyaline to pale green at upper and lateral surface, a t. intricata of thin-walled hyphae (1.5–)2–5(–7) μm (n=30) wide; surface smooth, plane to wavy. Subperithecial tissue a hyaline, ill-defined t. angularis–epidermoidea of variable, thin-walled cells (5–)7–22(–35) × (3–) 6–14(–17) μm (n=35), interspersed with few wide hyphae. Base a loose t. intricata of thick-walled (1 μm) hyphae (3–)4–8(–11) μm (n=30) wide; lowest layer dense, hyphae variably oriented, therefore also appearing cellular in section. Asci (57–)63–75(–86) × (4.0–)4.3–5.0(–5.8) μm, stipe (3–)4–11(–19) μm long (n=30). Ascospores green, becoming slowly bright rust- to ochre brown in KOH, nearly smooth, cells dimorphic, but with little difference, distal cell (3.0–)3.7–4.5(–5.0) × (2.5–)3.0–4.0(–4.4) μm, l/w 1.0–1.3(–1.6) (n=30), (sub-)globose, proximal cell (3.2–)3.5–4.5(–5.0) × (2.5–)2.8–3.3(–3.5) μm, l/w (1.1–)1.2–1.5 (n=30), oblong to wedge-shaped.
Cultures: Optimum growth at 25 °C on all media, little growth at 30 °C accompanied by death of hyphae within few days, no growth at 35 °C. No anamorph formed. On CMD after 72 h 7–14 mm at 15 °C, 10–19 mm at 25 °C, 1–4 mm at 30 °C; mycelium covering the plate after 11–13 d at 25 °C. Colony circular, margin well defined, dense, in the centre hyphae loose, soon dying, becoming empty; distal part indistinctly zonate; surface hyphae radial, wide, septa distinct. Local condensation of mycelium in the centre, spreading, colony becoming mottled by small irregular spots. Minute globose, intercalary chlamydospores appearing around hyphal aggregations. Aerial hyphae loosely disposed, long and high towards the colony margin. Autolytic excretions inconspicuous, frequent and minute at 30 °C, coilings inconspicuous. No pigment, no distinct odour noted.
On PDA after 72 h 5–6 mm at 15 °C, 22–23 mm at 25 °C, 1–3 mm at 30 °C; mycelium covering the plate after 7–8 d at 25 °C. Colony zonate, of a broad dense centre and a loose outer zone; hyphae wide. Aerial hyphae abundant, dichotomously branched, with many large drops and connectives, loosely cottony, whitish; growing up to the lid of the Petri dish; some descending and joining the agar in richly branched anchoring points. Colony margin diffuse or irregularly shaped due to aerial hyphae. No distinct odour, no diffusing pigment noted, reverse pale yellowish 3–4B3, 4A3. Autolytic excretions inconspicuous, more frequent in contact points of agar surface and aerial hyphae, abundant and minute at 30 °C. No coilings noted.
On SNA after 72 h 3–5 mm at 15 °C, 8–18 mm at 25 °C, 0.2–1.5 mm at 30 °C; mycelium covering the plate after 8–9 d at 25 °C. Colony hyaline, margin diffuse, mycelium loose, irregularly arranged. Aerial hyphae frequent, dichotomously branched, long, ascending to the lid of the Petri dish, growing faster than hyphae on and within agar; propagation of the colony therefore mainly by aerial hyphae. Autolytic excretions inconspicuous, more frequent in contact points of agar surface and aerial hyphae, slightly more frequent at 30 °C, no coilings noted. No pigment, no distinct odour noted. No stromata formed. Chlamydospores (6–)10–22(–34) × (4–)5–12(–16) μm, l/w 1.1–2.8(–4.7) (n=35) after 21 d at 25 °C, infrequent, globose, intercalary.
Habitat: on dead stems and sheaths of Calamagrostis epigejos.
Distribution: Denmark, only known from type locality.
Holotype: Denmark, Nordjylland, Tranum, meadow at Vestkystvejen, close to the crossing with Strandvejen, 57°08'32” N, 09°26'28” E, elev. 10 m, on dead, standing and lying stems and sheaths of Calamagrostis epigejos, 25 Aug. 2006, H. Voglmayr & W. Jaklitsch (W.J. 2945), WU 29046, ex-type culture CBS 121273 = C.P.K. 2448).
Notes: The only detectable phenotypic differences of H. danica from H. spinulosa are a more intense green stroma colour, a slower growth rate and only little growth at 30 °C in H. danica. In addition, no stroma formation in culture was observed in H. danica. H. aeruginea differs in having a pseudoparenchymatous cortex and the formation of a Trichoderma anamorph.
Hypocrea epimyces Sacc. & Pat., Tabulae Analyticae Fungorum 4: 175, fig. 387 (1891). Fig. 14.
Fig. 14.
Teleomorph of Hypocrea epimyces. a, g, h. Fresh stromata (a. immature, g, h. mature). b. Envelope of the holotype (drawing by Saccardo). c, e, f, j. Dry stromata (c. immature. e. mature, stipitate. f, j. mature). d. Mature stroma in 3 % KOH after reconstitution in water. i. Base of stroma in section. k. Perithecium in section. l. Stroma surface in face view. m. Cortical and subcortical tissue in section. n. Subperithecial tissue in section. o, p. Asci with ascospores. a, d–g, i, k–p. WU 28237. b, f. holotype. c. WU 28236; h, j. WU 28239. Scale bars: a, d, g = 1.2 mm. c = 1.7 mm. e = 0.8 mm. f = 0.5 mm. h = 2 mm. i, l, n = 25 μm. j = 1.4 mm. k = 35 μm. m = 30 μm. o, p = 10 μm.
≡ Hypocrea vinosa Pat. 1881, Rev. Mycol. Toulouse III, fasc. 12:11 (1881) [non Cooke, Grevillea 8: 65 (1879)].
Anamorph: Trichoderma epimyces Jaklitsch, Mycologia 100: 808 (2008). MycoBank MB512096. Fig. 15.
Fig. 15.
Cultures and anamorph of Hypocrea epimyces. a–d. Cultures after 7 d (a. on CMD, 25 °C. b. on PDA, 25 °C. c. on PDA, 30 °C. d. on SNA, 25 °C). e. Pustule (9 d). f. Conidiophore of effuse conidiation on growth plate (3 d). g–k. Conidiophores from pustules. l, m. Conidia. e–m. CMD, 25 °C. a–f, l, m. CBS 120534. g–k. C.P.K. 2487. Scale bars: a–d = 20 mm. e = 0.5 mm. f = 50 μm. g, j = 15 μm. h, i = 20 μm. k = 10 μm. l, m = 5 μm.
Stromata when fresh 1–14 mm diam, 1–3 mm thick, solitary, gregarious or aggregated in small numbers, flat pulvinate, lenticular, discoidal to turbinate, sometimes undulate, attenuated to a dark base, centrally attached. Margin or large part of stroma free, sometimes lobed. Surface essentially smooth, with numerous, densely disposed, slightly darker reddish brown ostiolar dots becoming green when mature. Colour first white, centre becoming rosy, pale brick-red, grey-orange, -red or incarnate, often mixed with a yellowish ground tone, 6AB4, 6B5–6, 6C5–7, 7B5–6, 8B4–5; turning brown-orange, reddish to olive brown at maturation, 5CD4–5, 9C6–7, 9D7–8; dark green by spore powder or with black spots when old.
Stromata when dry 1–13 × 1–10 mm, 0.5–2.8 mm (n=43) thick, flat pulvinate, discoidal, lenticular to placentiform with rounded margin, sessile or turbinate with sharp margin and short, thick stipe attenuated downward. Outline circular or somewhat elongate to irregular. Margin free, projecting to 1 mm over short central stipe, often undulate to crenate. Sides and stipe often covered with floccose, cream, rust, grey, brown to nearly black mycelium to the base. Surface often flat or concave, smooth to finely but distinctly granulose to rugose or tubercular. Ostiolar dots (24–)32–56(–71) μm (n=43) diam, numerous, minute, conspicuously densely (ca. 8/mm) disposed, circular, flat or convex, first reddish brown, later dark to black, with circular perforation. Colour matt red, grey-red, brown-orange to light (reddish) brown, 8CD4–8, 7CD6–7, when immature, later dark reddish brown, 7–8E5–8, 8F5–8, 9E5–6, sometimes matt red or rosy with olive tone 7–8CD3–5; (over)mature stromata dark reddish brown with black spots, or dark brown 7F4, 8F4–6, often covered by dark green 25F7–8 spore powder. In 3 % KOH stromata dark reddish brown with finely papillate, hyaline ostioles. Associated anamorph first white, becoming green, with white margin, finally dark green 26F5–8, thickly pulvinate to effuse, powdery.
Stroma anatomy: Ostioles (47–)55–72(–80) μm long, plane, rarely projecting to 12(–28) μm, (10–)13–25(–40) μm wide at the apex (n=30). Perithecia (115–)170–230(–260) × (100–)120–180(–240) μm (n=30), ellipsoidal or subglobose, numerous, usually crowded; peridium (10–)12–18(–22) μm (n=30) wide at the base, (9–)10–14(–16) μm (n=30) at the sides, yellowish. Cortical layer (16–)22–34(–38) μm (n=36) thick, a t. angularis of thin-walled, isodiametric angular cells (5–)7–14(–20) × (3–)5–10(–13) μm (n=65) in face view and in vertical section; reddish brown in water, orange in lactic acid, extending around whole stroma except area of attachment; with a thin layer of amorphous material on the surface. No hairs seen. Subcortical tissue of a (sub-)hyaline t. epidermoidea of thin-walled cells (3–)4–10(–15) × (2.5–)3.0–6.5(–9.0) μm (n=30), mixed with hyaline hyphae (2–)3–5(–6) μm (n=30) wide. Subperithecial tissue of a dense hyaline t. epidermoidea of thin-walled, globose, angular, lobed or elongate cells (4–)8–30(–55) × (4–)5–17(–24) μm (n=32). Stipe-like base penetrating into the wood, orange-brown, inhomogeneously pigmented, dense; of globose to angular cells 4–10(–16) × 3–6(–10) μm (n=30), mixed with pigmented hyphae (2.5–)4–5(–6) μm (n=30) wide. Asci (65–)75–100(–117) × (4.2–)5.0–6.0(–6.5) μm, stipe (2–)5–17(–28) μm long (n=46). Ascospores green, becoming brown in KOH, distinctly verrucose; cells dimorphic, distal cell (3.5–)4.0–5.0(–5.5) × (2.7–)3.2–4.0 (–4.7) μm, l/w (1.0–)1.1–1.3(–1.6) (n=60), (sub-)globose to plump wedge-shaped, proximal cell (3.5–)4.2–5.5(–6.2) × (2.7–)3.0–3.7 (–4.2) μm, l/w (1.1–)1.3–1.7(–1.8) (n=60), oblong, wedge-shaped to subglobose; sometimes orientation inverted.
Cultures and anamorph: Optimum growth at 25 °C on all media, no growth at 35 °C. On CMD after 72 h 12–13 mm at 15 °C, 33–35 mm at 25 °C, 30–32 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Hyphae narrow, loosely disposed. Aerial hyphae inconspicuous, becoming fertile. Autolytic excretions rare. No coilings noted. No diffusing pigment, no distinct odour noted. Chlamydospores infrequent, noted after 8–9 d. Conidiation at 25 °C first noted after 1 d around the plug, effuse, macroscopically invisible, on phialides sessile on surface hyphae, or on short, erect conidiophores spreading across the colony to the distal margin, loosely disposed, denser in downy zones. Short conidiophores simple or of a stipe with short side branches, to ca. 150 μm long, asymmetric at the base. Branches often paired on higher levels, often strongly inclined upward, unbranched or with one further branch. Terminal branches of 1 to few cells, with solitary or 2–3 divergent or nearly parallel phialides; in distal and lateral areas of the colony also on aerial hyphae to 0.6 mm long and 6–9 μm wide, bearing short side branches to 100(–200) μm long and corresponding to short sessile conidiophores. Phialides (5–)9–15 (–17) × (2.5–)2.7–3.5(–4.0) μm, l/w (1.9–)3.0–4.8(–5.4), (1.5–)2.0–3.0(–3.5) μm wide at the base (n=35), lageniform to subulate, often curved or sinuous, slightly thickened on varying levels, sometimes base strongly attenuated. Conidia held in minute wet heads to 30(–40) μm diam, green after 4–6 d, finally collapsing, conidia becoming dry. Pustulate conidiation at 25 °C noted after 6–7 d when effuse conidiation degenerated/collapsed for the greatest part. Pustules 0.5–1.5 mm diam, circular, with granular to fluffy surface, confluent, intermingled with conidiophores of effuse conidiation, gregarious, often densely aggregated to nearly continuous at the distal margin; becoming dark green 26–27F7–8 from the ends of the concentric zones; constructed of a transparent reticulum with narrow branches on a stipe 5–7(–9) μm wide, with verrucose walls to nearly 2 μm thick. Branching mostly asymmetrical, right angles common, straight fertile elongations to 350 μm. Conidiophores mostly 4–6 μm wide, 2–3 μm terminally; sometimes with thickened cells to 12 μm diam. Terminal branches generally short, ill defined or symmetric, with mostly 1-celled branches, slightly wider downward; with phialides solitary or divergent in whorls of (2–)3–4(–5), mostly 3, often steeply inclined upward, originating on cells 1.5–3.5 μm wide. Conidia held in numerous, minute, initially wet heads to 20 μm diam. Phialides (6–)7–10(–12) × (2.0–)2.5–3.0(–3.5) μm, l/w (2.0–)2.5–3.7(–4.7), (1.0–)1.5–2.2(–2.7) μm wide at the base (n=68), lageniform to ampulliform, often inequilateral and curved upward, to sigmoid, sometimes repetitive; thickenings variable, neck sometimes long and thin. Conidia (2.7–)3.0–3.7(–4.2) × (2.2–)2.7–3.0(–3.5) μm, l/w (1.0–)1.1–1.3(–1.4) (n=100), yellowish green, smooth, subglobose to oval, less commonly ellipsoidal; with 1 or several guttules, scar indistinct. At 15 °C development slow, conidiation effuse and dense in fluffy tufts at distal and lateral margins, becoming tardily green. At 30 °C conidiation effuse and in loose fluffy tufts or green confluent micropustules at the proximal margin, and in few powdery concentric zones, becoming dark green 28EF7–8.
On PDA after 72 h 10–11 mm at 15 °C, 20–26 mm at 25 °C, 20–23 mm at 30 °C; mycelium covering the plate after 7–8 d at 25 °C. Colony more or less circular with hyaline wavy margin. Mycelium densely interwoven, with little difference of width among hyphae. Aerial hyphae abundant, forming a dense mat several mm high, densely branched, with many strands and drops, radial only at the margin. Numerous dense conidiation nests overlain by loose long aerial hyphae, causing a densely granular central surface. Centre becoming homogeneously light green. Subsequently several thick, padded, velvety, alternating green and white concentric zones formed, finally all zones green of various shades, 27E4–6, 26F5–6, 27F6–8. Autolytic excretions inconspicuous. No coilings seen. No diffusing pigment, no distinct odour noted. Conidiation at 25 °C noted after 1 d, green after 2–3 d, starting around the plug on short aerial hyphae, later on densely disposed, short micropustules and long aerial hyphae ascending several mm; spreading across the colony. Conidia formed in numerous minute heads on short pyramidal branches. At 15 °C colony indistinctly zonate, downy. Conidiation loose in densely disposed microtufts and on long aerial hyphae, becoming only slightly greenish in the centre, or remaining hyaline. At 30 °C colony with distinct, convex, green concentric zones. Conidiation abundant.
On SNA after 72 h 11–13 mm at 15 °C, 30–32 mm at 25 °C, 22–23 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony similar to CMD, mycelium more curly; primary hyphae thick and prominent on surface. Long and high aerial hyphae at the colony margin becoming fertile. Autolytic excretions rare. Coilings moderate to frequent. No pigment, no distinct odour noted. Chlamydospores (4–)5–8(–10) × (3–)4–6(–8) μm, l/w (0.9–)1.0–1.5(–2.1) (n=45), noted after 7 d, numerous, but loosely disposed; variable, subglobose to pyriform when terminal, ellipsoidal to angular when intercalary. Conidiation noted after 1 d, first effuse, more abundant than on CMD; sessile or on short and simple conidiophores with 1–3 phialides, starting around the plug, spreading across the entire colony, concentrated in several downy to finely granular or powdery concentric zones; becoming greenish after 4–6 d. Pustulate conidiation appearing as small granules or tufts of ca. 0.5 mm diam in the concentric zones and the centre; confluent to 2.5 mm. Conidia gathering in large green drops to 250 μm diam, denser and darker green (26–27F7–8) at lateral ends of the concentric zones. At 15 °C colony not or only indistinctly zonate. Conidiation effuse, with heads to ca. 25 μm, and in few tufts to 1 mm diam, with long, fertile, straight to sinuous elongations. At 30 °C colony circular, with several alternating broad and narrow zones. Autolytic activity and coilings conspicuous. Chlamydospores numerous, distinctly more than at 25 °C, globose to angular, terminal and intercalary. Conidiation effuse. On oatmeal agar stromata are formed (CBS 120534; CBS, pers. comm.).
Habitat: On medium to well-decomposed wood; typically on decorticated branches deeply submerged in leaf litter, usually in association with other fungi; holotype on a Phellinus basidiome, uncommon.
Distribution: Europe, collected in Austria, Germany, France.
Holotype: France, Jura, Poligny, on Polyporus (Phellinus) nigricans, Jul. 1881, N. Patouillard (PAD). Epitype: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Zabrde, MTB 9452/4, 46°32`58“N, 14°25`11“E, elev. 565 m, on decorticated branch of Fagus sylvatica 4 cm thick, on wood, soc. Tomentella sp, unidentified Corticiaceae, Nemania serpens, Tubeufia cerea (on effete black pyrenomycete) and old Bertia moriformis, holomorph, 19 Aug. 2004, W. Jaklitsch (W.J. 2608), WU 28237, ex-epitype culture CBS 120534 = C.P.K. 1981. Holotype of Trichoderma epimyces isolated from WU 28237 and deposited as a dry culture with the epitype of H. epimyces as WU 28237a.
Other specimens examined: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Aussicht, MTB 9452/4, 46°32'50” N, 14°25'00” E, elev. 600 m, on decorticated branches of Fagus sylvatica, on strongly decomposed wood, some soc./on Steccherinum fimbriatum and soc./on a brown Corticiaceae, holomorph, 14 Oct. 2006, W. Jaklitsch, W.J. 3019 (WU 28239, culture C.P.K. 2487). Niederösterreich, Wien-Umgebung, Mauerbach, Friedhofstrasse, MTB 7763/1, 48°15'16” N, 16°10'33” E, elev. 350 m, on decorticated branch of Fagus sylvatica 4–5 cm thick, covered with leaves, soc./on orange Corticiaceae and rhizomorphs, ozonium; holomorph, teleomorph immature, maturation after incubation at RT, 10 Sep. 2005, W. Jaklitsch, W.J. 2849 (WU 28238, culture C.P.K. 2417, from conidia). Germany, Bavaria, Oberbayern, Landkreis Eichstätt, Altmühltal, 2–3 km after Pfahldorf towards Eichstätt, MTB 7033/4, 48°57`00“N, 11°18`20“E, elev. 540 m, on branch of Fagus sylvatica 4 cm thick, on wood, soc./on ozonium, Armillaria rhizomorphs, Phanerochaete sanguinea, effete pyrenomycetes, holomorph, 5 Aug. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2576 (WU 28236, culture C.P.K. 1980).
Hypocrea estonica P. Chaverri & Samuels, Mycologia 95: 1119 (2003). Fig. 16.
Fig. 16.
Teleomorph of Hypocrea estonica. a–d. Fresh stromata (a, b, d. immature, a. initial stage, b. with conidiophores on top, c. mature). e–i. Dry stromata (e. overmature. f, h, i. mature. g. immature/mature mixed. i. side view). j. Mature stroma in 3 % KOH after reconstitution in water. k. Stroma surface in face view. l. Perithecium in section. m. Cortical and subcortical tissue in section. n. Subperithecial tissue in section. o. Base of stroma in section. p–r. Asci with ascospores. a–d, j–o, r. WU 29049. e. holotype BPI 744577. f, g, i. specimen from UPS. h, p, q. WU 29048. Scale bars: a, b = 0.6 mm. c–f, h–j = 0.4 mm. g = 1 mm. k, r = 15 μm. l–o = 25 μm. p, q = 10 μm.
Anamorph: Trichoderma estonicum P. Chaverri & Samuels, Stud. Mycol. 48: 66 (2003). Fig. 17.
Fig. 17.
Cultures and anamorph of Hypocrea estonica. All at 25 °C. a–c. Cultures (a. on CMD, 14 d. b. on PDA, 7 d. c. on SNA, 14 d). d, e. Conidiophores of effuse conidiation on CMD after 3 d (d. on agar plug, e. from the colony surface around the plug). f, h. Pustules (CMD, 10-11 d. h. elongations highlighted). g, k, j, l. Elongations (CMD, 10-11 d. g. simple, k. with phialides along its length, j. terminal parts with 3–4 phialides, l. terminal phialides and conidia). i. Conidiophore from pustules with elongation (CMD, 10 d). m. Marginal patch of hyphae (PDA, 7 d). n, o. Conidia (CMD, 10 d). p. Chlamydospores (SNA, 14 d). q. Part of conidiophore from pustule (CMD, 10 d). r. Phialides from pustule (CMD, 10 d). a–c, f, l, m, p. C.P.K. 3149. d. ex-type culture CBS 111147. e, g-j, n, o, q, r. CBS 121556. Scale bars: a–c = 20 mm. d, i, k, p = 25 μm. e, j, q = 15 μm. f, h, m = 0.25 mm. g = 45 μm. l, n, o, r = 10 μm.
Stromata when fresh 1–3 mm diam, 0.5–1 mm thick, pulvinate, surface smooth, with fine, distinct, black ostiolar dots when mature. Colour bright `raspberry red`or greyish red 7B4–6 when young, darker red 10AB5–7 or reddish brown when mature. Associated Trichoderma light green.
Stromata when dry (0.3–)0.7–2.0(–3.2) × (0.2–)0.6–1.5(–2.4) mm, (0.15–)0.2–0.5(–0.7) mm thick (n=80); mostly solitary to gregarious or aggregated in small numbers; (flat) pulvinate, turbinate or discoidal, broadly or narrowly attached; base sometimes with white mycelium. Outline variable, circular, angular, lobed or oblong. Margin free, acute or rounded, sometimes with projecting perithecia; sides vertical or attenuated downwards, whitish, rosy or orange. Surface iridescent, smooth, in older stromata tubercular or rugose. Ostiolar dots (31–)40–72(–118) μm (n=110) diam, plane or convex, initially large and diffuse, becoming small, distinct and well defined, circular-elongate, densely disposed; dark red to brown when immature, dark green to black upon maturation. Colour and development: starting as white mycelium, compacting, initials rosy (plus yellowish tones) 7A4–5, 8A3–4, with pruinose surface; when immature rosy between dark red ostiolar dots, often with white margin, resulting in raspberry-, greyish- or orange-red colours 9B5–6, 8D6–8, 9D5–8, 10AB(4–)5; ostiolar dots becoming dark green to black, but surface paler with maturation and in age, brownish red, yellow-red or yellow-orange, 9BC6–8, 8AB5–6, i.e. colour fading with age, except for dark areas covered by dark green spore powder. Mature stromata after reconstitution thicker, more pulvinate, with smooth surface, orange-brown; ostiolar dots black, convex; stroma sides concolorous with surface; not changing colour in 3 % KOH.
Stroma anatomy: Ostioles (57–)63–80(–88) μm long, projecting to 8(–20) μm, (20–)26–38(–45) μm wide at the apex (n=30), long cylindrical to conical. Perithecia (137–)165–205(–215) × (60–)100–155(–170) μm (n=30), ellipsoidal, flask-shaped or globose. Peridium (15–)17–24(–29) μm (n=30) wide at the base, (7–)12–17(–19) μm (n=30) at the sides, yellowish. Cortical layer around whole stroma except area of attachment, (17–)20–29(–32) μm (n=30) thick, a subhyaline to pale yellowish t. angularis of thin-walled isodiametric cells (4–)6–13(–17 × (3–)4–8(–10) μm (n=63) in face view and in vertical section, mostly below a thin, yellow, superficial, amorphous layer of compressed cells and hyphae (1.5–)3–5(–6) μm (n=30) wide. Subcortical tissue a hyaline t. angularis of thin-walled cells (3–)4–9(–11) × (2.5–)3–5(–6.5) μm (n=30), and hyphae (2.0–) 2.5–5.0(–8.0) μm (n=30) wide between perithecia. Subperithecial tissue a (sub-)hyaline t. angularis-epidermoidea of variable thin-walled cells (5–)8–22(–29) × (5–)6–12(–13) μm (n=30), denser towards base, of smaller cells mixed with thick-walled hyaline hyphae (4–)5–8(–10) μm (n=30) wide. Asci (75–)88–103(–111) × (4.8–)5.0–6.7(–8.0) μm, stipe (4–)10–21(–33) μm long (n=60). Ascospores yellowish-, olive-, brown-green, distinctly warted; cells dimorphic, distal cell (3.2–)4.2–5.3(–6.5) × (3.0–)3.7–4.5(–5.5) μm, l/w (0.9–)1.0–1.3(–1.5) (n=124), (sub-)globose to wedge-shaped; proximal cell (3.5–)4.5–6.0(–6.8) × (2.8–)3.2–4.0(–5.0) μm, l/w (1.0–)1.2–1.6(–2) (n=124), wedge-shaped or oblong, often longer in basal ascospore.
Anamorph on natural substrate effuse, light (bluish) green; conidia (2.5–)2.8–3.5(–4.5) × 2.2–2.6 μm, l/w (1.1–)1.2–1.5(–1.7) (n=30), ellipsoidal, green, smooth.
Cultures and anamorph: Optimum growth at 25 °C on CMD and PDA, at 15–25 °C on SNA; no growth at 35 °C. At 30 °C little growth, hyphae autolysing and dying soon. On CMD after 72 h 18–20 mm at 15 °C, 31–35 mm at 25 °C, 4.5–7 mm at 30 °C; mycelium covering the plate after 6–7 d at 25 °C. Colony dense, not zonate, margin ill-defined to lobate. Aerial hyphae scant. No coilings seen, autolytic excretions rare, minute, but abundant at 30 °C. No pigment, no distinct odour noted. Chlamydospores absent or rare. Conidiation in two forms, first effuse, after 2–4 d, later in fine pustules to ca. 1 mm diam, appearing at the distal margin, becoming macroscopically (pale) green after (10–)12–14 d. Effuse conidiation starting on and around the plug, spreading across the colony in some isolates. Conidiophores emerging from surface hyphae, 50–100(–250–450) μm long, erect, verticillium-like, but phialides and branches steep, nearly gliocladium-like. Structure simple, phialides or short branches emerging on 1–2(–4) levels, mostly unpaired, from a main thick-walled axis to 4.5 μm wide. Branches 1–3 celled, 2–3.5 μm wide, often with thickened cells. Phialides solitary or in whorls of 2–4, divergent, but often strongly curved upwards. Conidia formed in wet heads <30 μm diam, greenish in the stereo-microscope. Phialides (8–)11–17(–21) × (2.3–)2.4–3.1 (–3.7) μm, l/w (3.0–)3.8–6.5(–8.8), (1.3–)1.8–2.5(–2.8) μm wide at the base (n=36), lageniform or subulate, symmetrical or strongly curved. Conidia (3.2–)3.5–4.7(–5.6) × (2.2–)2.7–3.2(–3.5) μm, l/w (1.2–)1.3–1.5(–1.8) (n=70), green, smooth, finely multiguttulate; shape variable, ellipsoidal, oblong, subglobose or drop-like due to prominent scar.
Pustulate conidiation after 10–11 d: Pustules formed along the margin of the colony, light green, with numerous straight elongations; 1–1.3 mm diam including elongations, outline circular. Elongations straight to sinuous, projecting to 80–330(–450) μm, 4–5.5 μm wide at the base, attenuated to 2–2.5 μm terminally, smooth to verruculose, straight to sinuous, unbranched or less commonly with 1–3 long, widely disposed, asymmetric, nearly right-angled branches; with 1–3(–4) terminal phialides on cells 1.5–3.5 μm wide; conidia formed in minute wet heads <20 μm diam. Phialides on elongations (6.5–)9–17(–25) × (2.0–)2.3–3.0(–3.5) μm, l/w (2.5–) 3.3–6.7(–10.5), (1.5–)1.8–2.5(–3.0) μm wide at the base (n=60), lageniform to subulate, often asymmetric, curved upwards or sinuous in terminal whorls, thickest in variable positions, neck often long and abruptly narrowed. Pustules formed on stipes to 9–10 μm wide, with thick walls swelling outside. Primary branches 5–6.5 μm wide; branching points often thickened; further branching dense and difficult to assess, with formation of conidia starting central in the pustule, but after conidiation on elongations. Conidiophores within pustules and on bases of elongations pachybasium-like, dense, short, stout, 3–5 μm wide, with paired or unpaired branches in right angles or slightly inclined upwards; phialides divergent in whorls of 3–5(–6), formed on slightly thickened cells 3.5–5.0(–6) μm wide. Phialides (3.5–)4–8(–11.5) × (2.5–)3.0–3.5(–4.0) μm, l/w (1.1–)1.2–2.7(–4.6), (1.3–)1.8–2.6(–3.2) μm wide at the base (n=63), lageniform or ampulliform with short necks on bases of elongations and within pustules, lageniform with long narrow necks on the periphery of pustules; thickest mostly at or below the middle, often inequilateral, green when old. Conidia (2.7–)3.2–4.5(–6.3) × (2.0–)2.3–3.0(–3.3) μm, l/w (1.1–)1.3–1.7(–2.6) (n=63), ellipsoidal, oblong or drop-like, (yellowish) green, thick-walled, smooth, with several fine guttules, scar indistinct or prominent, short truncate; some conidia formed on long phialides on elongations cylindrical. At 15 °C colony homogeneous, not zonate, conidiation in pale green pustules to 3 mm diam with sterile, sinuous to helical elongations.
On PDA after 72 h 18–20 mm at 15 °C, 29–31 mm at 25 °C, 2–5 mm at 30 °C; mycelium covering the plate after 7–8 d at 25 °C. Colony circular, dense, forming two zones, a dense circular centre becoming dull yellow to brownish, and a looser whitish(-yellowish) lobate marginal zone; surface hyphae wide, with conspicuously radial orientation, partly agglutinating to strands. Aerial hyphae along strands of surface hyphae numerous, short, otherwise long and high, loosely disposed in the centre, but abundant and dense outside centre, radially oriented on lower levels, aggregating to form thick strands, appearing as stellate teeth. Aerial hyphae thin and irregularly oriented at higher levels and collapsing into floccules. Reverse cream to yellow-brown 3A4–5, 4A3–5, 4B4. Coilings frequent at the colony margin; autolytic excretions infrequent, mainly from coilings, minute but abundant at 30 °C. No distinct odour noted. Conidiation noted after 3–5 d, effuse, poorly developed on upper levels of aerial hyphae, not becoming green. At 15 °C little conidiation in whitish central tufts, not becoming green, no pigment formed, growth stopping before covering the plate entirely.
On SNA after 72 h 12–18 mm at 15 °C, 11–17 mm at 25 °C, 1.5–5 mm at 30 °C; mycelium covering the plate after ca. 3 wk at 25 °C. Colony hyaline, thin, margin ill-defined to lobate, subsequently several irregular concentric growth zones formed. White pustules appearing around the plug and in distal zones, becoming pale (yellowish) green 29–30–1CD4–6 to dull olive 1–3D4–6. Aerial hyphae scant. Autolytic excretions minute, frequent, abundant at 30 °C; coilings rare. No pigment, no distinct odour noted. Conidiation noted after 5–6 d in white pustules predominantly arranged in distal areas of the colony; pustules to 1–3 mm diam, roundish-oblong, confluent, macroscopically green after 8 d, with straight to sinuous fertile elongations to ca. 450 μm long, bearing minute wet conidial heads <30 μm diam. Chlamydospores (6–)7–11(–17) × (4–)6–10 μm, l/w 0.8–1.5(–2) (n=30), seen after 1 wk, conspicuously abundant after 10 d, measured after 15d at 25 °C, intercalary and terminal, globose to irregular, sometimes 2–3 celled. At 15 °C conidiation pachybasium-like, greenish, in pustules to 3 mm diam with long, straight, mostly sterile elongations.
Habitat: on and around basidiomes of Hymenochaete spp.
Distribution: Northern Europe (Estonia, Sweden, United Kingdom).
Holotype: Estonia, Valgamaa, Vaabina, material overmature, on Hymenochaete tabacina, soc. effete ?Helminthosphaeria sp., 20 Apr. 1996, K. Põldmaa (BPI 744577).
Other specimens examined: Sweden, Scania, Skurup parish, 300 meters northwest of the castle Svaneholm in the Skurup parish in Scania, on Hymenochaete tabacina on Salix sp., holomorph, teleomorph immature, 14 Aug. 2006, I. Mansson, S-A. Hanson 06-472 (WU 29047, culture (from conidia) C.P.K. 2484). Same locality, 23 Dec. 2006, holomorph, soc. Bertia moriformis, S-A. Hanson 06-727 (WU 29048, culture CBS 121556 = C.P.K. 2868). Uppland, Fibysjön, Vänge par., Fiby urskog, Hymenochaete tabacina / Alnus glutinosa, also on surrounding wood, holomorph, 18 Sep. 1973, S. Ryman (UPS). United Kingdom, Hertfordshire, Waterford, Waterford Heath, Mole Wood, 51°48'42” N, 0°05'22” W, elev. 70 m, on and around basidiomata of Hymenochaete corrugata on cut branches, 10–12 cm thick, of Corylus avellana, also in moss, holomorph, 12 Sep. 2007, W. Jaklitsch, K. Robinson, H. Voglmayr, W.J. 3156 (WU 29049, culture C.P.K. 3149).
Notes: Hypocrea estonica is morphologically indistinguishable from the North American H. ceramica sensu Chaverri & Samuels 2003. Both species occur on Hymenochaete spp., while the holotype of H. ceramica was collected on a different aphyllophoraceous fungus on Juniperus sp. and is characterised by large, hairy, dull reddish brown stromata with only inconspicuous ostiolar dots. H. ceramica sensu Chaverri & Samuels 2003 may therefore not be conspecific with the holotype of H. ceramica. Hypocrea estonica was originally described as having yellow stromata. This may be explained by a fading of red colours with age, and old/overmature stromata may appear pale yellow-orange. In fact, a re-examination of the holotype revealed overmature stromata devoid of asci, but still most with pale rosy to pale yellowish red colours. All recently collected specimens have bright raspberry red colours. They all agree in gene sequences with the ex-type culture CBS 111147 of H. estonica.
In addition, the Central-European counterpart of H. estonica, H. parestonica, is difficult to distinguish morphologically from H. estonica. Apart from some differences in stroma colour, the following differences were noted: The cortical cells are paler in H. estonica than in H. parestonica, and hyphae on the stroma surface are homogeneously disposed, not in fascicles, although some areas may be glabrous. For differences in culture and anamorph characteristics among these species see the notes under H. parestonica.
Hypocrea fomiticola Jaklitsch, sp. nov. MycoBank MB513183. Fig. 18.
Fig. 18.
(Page 51). Teleomorph of Hypocrea fomiticola. a–i. Fresh stromata (a. habit on hymenium of the host. b. mature, with immature secondary stromata. c, f, g, i. mature. d. initial stage. e, h. immature). j, n. Parts of dry mature stromata. k. Vertical section of fresh mature stroma. l. Stroma surface in face view. m. Mature stroma reconstituted in water. o. Perithecium in section. p. Cortical and subcortical tissue in section. q. Subperithecial tissue in section. r. Base in section. s, t. Asci with ascospores. u. Ascospores. a, c, e, g, i–m, o–r, u. WU 29050. b. WU 29051. n, t. S. pallida b viridis (F 65918). d, f, h, s. WU 29052. Scale bars: a = 20 mm. b, k = 1.5 mm. c–e, g = 3 mm. f, h = 1 mm. i, m = 0.5 mm. j, n = 0.3 mm. l, p, r–t = 15 μm. o = 60 μm. q = 25 μm. u = 10 μm.
Anamorph: Trichoderma fomiticola Jaklitsch, sp. nov. MycoBank MB513184. Fig. 19.
Fig. 19.
Cultures and anamorph of Hypocrea fomiticola (CBS 121136). a–c. Cultures at 25 °C (a. on CMD, 14 d. b. on PDA, 7 d. c. on SNA, 14 d). d. Confluent pustules (25 °C, 9 d). e, f. Conidiophores on pustule margins (SNA, 15 °C, 7 d). g–j. Conidiophores (g. 15 °C, 14 d. h–j. 25 °C, 7 d). k. Phialides (15 °C, 14 d). l–n. Conidia (15 °C, 13–14 d). o. Chlamydospores (25 °C, 14 d). d, g–o. On CMD. Scale bars: a–c = 19 mm. d = 0.8 mm. e, f = 60 μm. g = 35 μm. h–j = 25 μm. k, l, o = 15 μm. m, n = 10 μm.
Stromata in et prope basidiomatibus Fomitis fomentarii, late pulvinata vel effusa, lutea, brunnea ad viridia. Asci cylindrici, (95–)108–135(–160) × (5.0–)5.5–6.5(–7.2) μm. Ascosporae bicellulares, ad septum disarticulatae, verrucosae, virides, brunneae in KOH, pars distalis subglobosa, ellipsoidea vel cuneata, (4.0–)5.0–6.7(–8.2) × (3.7–)4.3–5.0(–5.5) μm, pars proxima oblonga vel subglobosa, (4.0–)5.0–7.0(–9.3) × (2.5–)3.5–4.4(–5.0) μm. Anamorphosis Trichoderma fomiticola. Conidiophora in agaris CMD et SNA in pustulis. Phialides lageniformes vel subulatae, (6–)8–18(–28) × (2.5–)3.0–3.7(–4.0) μm. Conidia ellipsoidea, ovoidea vel oblonga, (3.3–)3.5–6.5 (–10.2) × (2.5–)2.8–3.4(–4.0) μm, viridia, glabra.
Etymology: fomiticola means dwelling on Fomes.
Stromata when fresh 2–135 × 2–70 mm, 0.5–5 mm thick, widely effuse, thin, virtually lacking a subperithecial tissue, or thick with a massive subperithecial tissue difficult to distinguish from host tissue, effluent, i.e. disintegrating into densely aggregated broadly pulvinate parts, broadly attached; marginal parts lobed, becoming detached. Surface velutinous or farinose when young, with emerging ostioles splitting the uppermost whitish layer into flakes; smooth, white to yellowish between projecting ostioles when mature; condensed and shiny when old. Margin rounded, thick, partly whitish, edge partly free. Ostiolar areas large, up to ca. 160 μm diam, papillate, convex to conical or semiglobose, pale brown, dark reddish brown to dark green or nearly black due to green ascospores. Stroma colour inside yellowish to pale wood-coloured; surface yellow(-brown), 4AB3–5 to 5CD5–6 (wet) when young/immature, then brown to reddish brown 4D4, 4E4–6; when mature dull olive, (1–) 3–4CD4–5, 1E3–6, blackish green when old; during drying surface strongly darkening, more green 25F5–8 due to ejected ascospores. Secondary, white to yellowish, broadly pulvinate stromata formed on the stroma surface. Associated anamorph dark green.
Stromata when dry effuse, undulate, or cracked, irregularly broadly pulvinate, continuous parts 6–55 × 6–28 mm, 0.3–2(–4) mm thick (n=65); broadly attached, margin rounded and adherent, rarely sharp and free to a large extent. Surface coarsely wavy to tuberculate and finely granular due to projecting ostiolar dots, glassy when old. Ostiolar areas (40–)50–86(–125) μm (n=65) diam, papillate and surface cracked around them, first yellowish- or reddish brown with pale minute circular perforations when immature, later green or black due to maturation of ascospores. Stroma colour and development: white mycelium becoming yellow 4–5A3–4, compacting, later yellow or with a whitish covering between dark ostiolar dots, resulting in dull yellow-, golden-brown, 5BD(E)4–6 (–6CD7–8), becoming olive- or grey-green, 5E3–8, 5F5–8, 4E4–5, to black due to ejected dark green to black spore powder; younger parts dull olive-green, 2–3DEF3–5. Pale brownish inside. Stromata after reconstitution in water dull yellow with olive-brown dots to ca. 100 μm diam; dots distinctly papillate to semiglobose, or diffuse and only slightly projecting; in 3 % KOH stromata reddish- to dark brown, peridium red.
Stroma anatomy: Ostioles (110–)128–160(–173) μm long, projecting (11–)20–45(–68) μm, (47–)54–85(–108) μm wide at the apex (n=30), with a dense hyaline apical palisade of narrow, apically rounded, cylindrical cells and some clavate cells 7–11 μm wide. Perithecia (230–)265–345(–400) × (110–)140–210(–245) μm (n=30), large, crowded or not, ca. 5 per mm, flask-shaped to narrowly ellipsoidal; peridium (15–)18–26(–32) μm (n=30) wide at the base and (7–)11–19(–22) μm (n=30) at the sides, yellow (similar to cortex). Surface viscid, with numerous ascospores adhering to it, and covered by hyaline to yellowish hyphae 2–5 μm wide, at least when young; hyphae collapsing. Cortical layer (7–)8–17(–22) μm (n=30) thick; thin, yellow, amorphous, no distinct cells discernible in section, in face view a t. angularis of yellowish brown, globose to isodiametric angular cells (3.5–)5–8(–10) × (3–) 4–6.5(–7.5) μm (n=30) below hyphal covering. Subcortical tissue of vertically oriented yellowish hyphae, short-celled and irregularly oriented toward cortex; cells (4–)5–10(–13) × (3–)4–6(–6.5) μm (n=30). Subperithecial tissue conspicuously variable in thickness, mainly of wide vertical hyphae, appearing as a t. epidermoidea to nearly t. porrecta of thin-walled cells (6–)8–31(–64) × (5–)6–11(–16) μm (n=31), yellowish, paler yellow to hyaline towards base; basal cells smaller and more thick-walled, (4–)5–11(–18) × (3–)4–8(–10) μm (n=30), inhomogeneously ochre-yellow, mixed with some hyphae (4–)5–8(–10) μm (n=30) wide. Asci (95–)108–135(–160) × (5.0–)5.5–6.5(–7.2) μm, stipe (6–)13–30(–48) μm long (n=70). Ascospores green, becoming brown in KOH, with flattened contact areas, distinctly verrucose; cells dimorphic, distal cell (4.0–)5.0–6.7(–8.2) × (3.7–)4.3–5.0(–5.5) μm, l/w (1.0–)1.1–1.4(–1.7) (n=140), (sub-)globose, ellipsoidal or wedge-shaped, proximal part (4.0–)5.0–7.0(–9.3) × (2.5–)3.5–4.4(–5.0) μm, l/w (1.1–)1.3–1.8(–2.5) (n=140), oblong, slightly attenuated towards base, or subglobose.
Cultures and anamorph: Optimum growth at 25 °C on all media, limited growth at 30 °C on all media (curly hyphae with broom-like branching, soon autolysing and dying), no growth at 35 °C. On CMD after 72 h 22–27 mm at 15 °C, 28–34 mm at 25 °C, 2–4 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony outline irregular, margin wavy to lobed. Mycelium conspicuously loose, primary hyphae wide (> 10 μm), secondary hyphae thin. Aerial hyphae and autolytic activity nearly absent; coilings moderate. No pigment, no distinct odour noted. Conidiation noted after 1–2 d, starting on short shrubs or microtufts close to the plug and the proximal margin, strongly branched, asymmetric, with short, symmetric terminal trees; mostly dry, also with few wet heads < 20 μm diam. Shrubs spreading across the plate, growing to pustules. Pustules to ca. 2.5 mm diam, compact, opaque, hemispherical, becoming dark green 27–29EF5–8 after 3–4 d at 25 °C. Formation of conidia starting within pustules. Main axes forming broad trees due to upward inclination of long lower side branches. Main axes bearing side branches paired or unpaired, in right angles to strongly curved upwards; side branches close to top short, 1-celled, with a terminal whorl of phialides, others bearing short trees or few solitary phialides or whorls of phialides along their length. Terminal trees projecting as fertile elongations to ca. 0.3 mm; right angles frequent; finally degenerating, with conidia adhering in chains. Structure more characteristic at 15 °C: tufts or pustules loose, with hairy surface due to radially arranged projecting fertile trees; of a stipe ca. 5.5–6.5 μm wide, with thickenings to 8–9 μm wide, forming primary branches asymmetrically. Primary branches (main axes) forming long regular trees projecting to ca. 1.3 mm from the tuft core. Main axes loosely branched, branches shorter upwards, variable, often in right angles on lower levels, mostly strongly inclined upwards on higher levels. Also side branches forming regular trees. Branches in trees narrow, paired or not. Width of conidiophores mostly 3–5.5 μm, slightly narrowed towards ends. Phialides formed on cells 2–3.5(–4) μm wide, solitary or in whorls of 2–5(–6), divergent. Conidia formed in minute wet or dry heads to 30 μm diam. Phialides (6–)8–18(–28) × (2.5–)3.0–3.7(–4.0) μm, l/w (1.5–)2.6–5.4(–7.6), (1.6–)2–3(–3.5) μm wide at the base (n=65), variable, lageniform or subulate, with thickenings in or below the middle, straight to strongly curved upwards or sigmoid, some nearly ampulliform on the same conidiophore. Conidia (3.3–) 3.5–6.5(–10.2) × (2.5–)2.8–3.4(–4.0) μm, l/w (1.2–)1.3–1.9(–2.8) (n=75), intensely green, smooth, ellipsoidal to oval to oblong, rarely cylindrical and to 14.5 × 4 μm, finely multiguttulate, scar indistinct. Chlamydospores noted after 2–3 d, (7.5–)9–12(–14) × (7–)8–10(–12) μm, l/w (0.9–)1.0–1.2(–1.3) (n=30), globose to pyriform, smooth, thick-walled, abundant, terminal, rarely intercalary. At 15 °C colony crystal-like with fan-shaped radial lobes or streaks; mycelium very loose; conidiation in loose green pustules to ca. 4 mm diam.
On PDA after 72 h 12–14 mm at 15 °C, 25–27 mm at 25 °C, 1.5–2 mm at 30 °C; mycelium covering the plate after 7–8 d at 25 °C. Colony dense, covered by thick, loose, white tomentum of irregularly oriented aerial hyphae several mm high (later collapsing), except for a narrow, thin, hyaline margin and flat central area. Whitish tufts formed around the plug, irregularly disposed, confluent, turning green; also conidiation on aerial hyphae turning green, resulting in dull greyish green convex zones, 26–27F3–8. Autolytic activity and coilings nearly absent. No pigment, no distinct odour noted. Conidiation noted after 2 d, green after 3–4 d at 25 °C. At 15 °C central conidiation tufts forming a pale green, 27DE3–4 to 28DE4–6, fluffy continuum, later dull green 26F4–5, followed by light and dark green, 26–27F4–8, hairy concentric zones.
On SNA after 72 h 15–17 mm at 15 °C, 14–19 mm at 25 °C, 1–2.5 mm at 30 °C; mycelium covering the plate after >3 wk at 25 °C. Colony irregularly lobed, resembling ice crystals. Mycelium loose, with conspicuous difference of width between oldest and youngest hyphae. Aerial hyphae inconspicuous. Autolytic activity moderate, coilings frequent. No pigment, no distinct odour noted. Chlamydospores noted after 3–5 d. Conidiation noted after 1–2 d, starting in central shrubs and high up on aerial hyphae, mostly dry, also in wet heads to 30 μm diam, later in fluffy to compact dense tufts or pustules with variable outline to 1.5 mm diam irregularly distributed across the entire colony, confluent to ca. 5 mm diam, first white, becoming green after 3–4 d at 25 °C, finally dark green to nearly black, 27–29F5–8. At 15 °C pustules to 3 mm diam, confluent to 4.5 mm long, with long trees projecting to 1.5 mm from pustules, similar to CMD.
Habitat: on and around basidiomes of Fomes fomentarius on Fagus sylvatica in virgin forests.
Distribution: Austria.
Holotype: Austria, Niederösterreich, Lilienfeld, Sankt Aegyd am Neuwalde, Lahnsattel, at forest road, MTB 8259/1, 47°46'25“N 15°31'24“E, elev. 960 m, on a basidiome of Fomes fomentarius (hymenium) on a trunk of Fagus sylvatica 35 cm diam on the ground, 27 Sep. 2006, H. Voglmayr (W.J. 2989), WU 29050, ex-type culture CBS 121136 = C.P.K. 2475. Holotype of Trichoderma fomiticola isolated from WU 29050 and deposited as a dry culture with the holotype of H. fomiticola as WU 29050a.
Other specimens examined: Austria, Niederösterreich, Lunz am See, Rothwald, Kleiner Urwald, MTB 8256/2, elev. ca. 1000 m, on mycelium of Fomes fomentarius adjacent to basidiomes on a trunk of Fagus sylvatica on the ground, on bark, soc. moss, 20 Aug. 2007, A. Urban, W.J. 3125 (WU 29051, culture C.P.K. 3137). Großer Urwald, MTB 8256/2, elev. ca. 1000 m, on hymenium and attachment area of a basidiome of Fomes fomentarius, soc. white mould, holomorph, 10 Oct. 2007, A. Urban & G. Koller, W.J. 3179 (WU 29052).
Notes: This species is the only green-spored species of Hypocrea currently known to form effused stromata. The formation of secondary stromata on old stromata is another peculiar characteristic of H. fomiticola. Ascospores are larger than in all other green-spored species occurring in Europe except H. dacrymycella. A specimen labelled Sphaeria pallida b viridis, ex herb E. Fries (S, F 65918), communicated by K. Põldmaa, was examined. It agrees in all morphological respects with H. fomiticola.
Hypocrea gelatinosa (Tode: Fr.) Fr., Summa Veg. Scand. p. 383 (1849). Fig. 20.
Fig. 20.
Teleomorph of Hypocrea gelatinosa. a–e, j, k. Fresh stromata (a, b, e. immature, b. partly dried in nature. c, d, j, k. mature. k. uni-, biperitheciate). f–i, l, m. Dry stromata (f. stroma initials with subiculum. g. immature. h. immature, mature and anamorph. i, l, m. mature. m. reduced stroma with free perithecia). n. Perithecium in section. o. Mature stromata in 3 % KOH after reconstitution in water. p. Stroma surface in face view. q. Cortical and subcortical tissue in section. r. Subperithecial tissue in section. s. Base in section. t, u. Asci with ascospores. a, d. WU 29061. b, c. WU 29054. e. WU 29053. f. WU 29065. g, t. WU 29060. h, n–s. WU 29081. i. WU 29062. j, k. WU 29055. l, u. WU 29066. m. WU 29074. Scale bars: a, b, d, e = 1 mm. c, h, o = 0.7 mm. f, g, l, m = 0.3 mm. i, j = 0.5 mm. k = 0.2 mm. n = 40 μm. p, q = 20 μm. r, s = 25 μm. t, u = 10 μm.
≡ Sphaeria gelatinosa Tode, Fungi Mecklenb. 2: 48 (1791): Fries, Syst. Mycol. 2: 336. (1823).
≡ Creopus gelatinosus (Tode: Fr) Link, Handb. Erkenn. Gew. 3: 349 (1833).
≡ Chromocrea gelatinosa (Tode: Fr.) Seaver, Mycologia 2: 58 (1910).
= Hypocrea moriformis Cooke & Massee, Grevillea 12: 3 (1888).
Anamorph: Trichoderma gelatinosum P. Chaverri & Samuels, Stud. Mycol. 48: 68 (2003). Fig. 21.
Fig. 21.
Cultures and anamorph of Hypocrea gelatinosa. a–c. Cultures after 14 d at 25 °C (a. on CMD. b. on PDA. c. on SNA). d. Anamorph (conidial heads) on natural substrate. e. Wet conidial heads on CMD (25 °C, 9 d, stereo-microscope,). f. Small conidiation shrub on SNA (25 °C, 7 d, compound microscope, 10x objective). g–j. Conidiophores (9–11 d). k. Phialides (9 d). l, m. Conidia (9–11 d). g–m. On CMD at 25 °C. a–c, f. C.P.K. 1618. d. WU 29073. e, i–l. CBS 121550. g, h, m. CBS 121555. Scale bars: a–c = 20 mm. d, e = 150 μm. f = 50 μm. g, j = 20 μm. h, i = 15 μm. k–m = 10 μm.
Stromata when fresh 0.3–2 mm diam, 0.3–1 mm thick, gregarious to densely aggregated, (flat) pulvinate, soft, narrowly or broadly attached, easily detached, with 1–50 perithecia per stroma, waxy, glassy or gelatinous, translucent, shiny, Dacrymyces stillatus-like. Outline circular or angular; surface granular and mulberry-like due to projecting perithecia. Ostioles invisible. Colour when immature either nearly colourless, pale yellowish, pale greenish yellow, amber or pale to deep (yellow-, brown-)orange, (3A3), 4A2–5, 5A4–5, 6C7–8, turning grey to green from within upon maturation; dark (greyish) green with an orange background when mature, 27F4–6, 29–30F7–8, 25F3–4.
Stromata when dry (0.2–)0.5–1(–1.3) mm diam, 0.2–0.6 mm thick (n=80); irregularly pulvinate, turbinate, or discoidal, compact or with more or less free perithecia on an orange hypostroma; sometimes confluent and condensed into a continuum with indistinct separating lines; broadly attached; translucent, glassy, waxy, appearing gelatinous. Outline roundish, angular or oblong. Margin free, variable, plump or sharp when immature. Sides vertical or attenuated downward, smooth, yellow, orange, dull reddish or brown. Surface plane to convex, granular or often conspicuously tubercular by projecting perithecia; perithecial dots (47–)90–180 (–280) μm (n=140) wide, first concolorous with surrounding stroma, turning greenish to black; perithecial outlines sometimes conical with tips pointing to the centre. Ostioles typically invisible, less commonly seen as minute dots or distinctly papillate to conical. Colour generally more intense and darker (orange) when dry. When immature stroma and (slightly projecting) perithecia of same lightness and colour, homogeneous, varying in colour from yellow over light to dark orange, greyish orange, to brown-red or dark reddish brown, 4A4–5, 5AB4–5, mostly 5–7(–8)AB6–8; 6CD7–8, 8CD7–8, 9C6–8, 7–8E6–8; changing to olive 3E4–6, and finally black (on a dark orange background) when mature. Spore powder intense to dark green. Base, mostly of young stromata, sometimes covered and/or surrounded by white mycelium. Mycelium of thick hyphae, belonging to the anamorph, sometimes also seen as white to greenish floccules with conidial heads on stroma surface. Anamorph on natural substrate otherwise effuse, powdery, light to medium green, of white thick conidiophores and distinct green gliocladium-like heads. Mature stroma after reconstitution in water black (dark green on orange background under magnification), gelatinous, swollen, with convex perithecia. No change in KOH noted, only orange colour more prominent.
Stroma anatomy: Ostioles (65–)76–98(–105) μm long, projecting to 18(–26) μm, (22–)34–56(–72) μm wide at the apex (n=30), with clavate to globose hyaline cells 5–8 μm wide lining the apex. Perithecia (175–)200–260(–280) × (80–)140–225(–260) μm (n=30), globose or flask-shaped, projecting solitary or in groups with the covering cortex. Peridium (13–)16–24(–27) μm (n=30) thick at the base, (9–)12–20(–26) μm (n=30) at the sides, hyaline or yellowish, merging with the subcortical t. angularis in the upper part; cells coarse at the base. Surface smooth, cortical layer (15–) 20–30(–37) μm (n=30) thick, around whole stroma except area of attachment, a t. angularis of 1–3 layers of thick-walled (1–1.5 μm), angular, yellow cells (4–)9–18(–28) × (3–)6–14(–18) μm (n=60) in face view and in vertical section. Subcortical tissue a hyaline t. angularis of thin-walled cells (4–)5–12(–18) × (3–)4–8(–10) μm (n=30). Subperithecial tissue a hyaline t. angularis of thick-walled cells (10–)18–34(–39) × (7–)11–21(–26) μm (n=30). Base a dense t. intricata of hyaline, thick-walled hyphae (3–)4–7(–9) μm (n=30) wide, with variable orientation, also penetrating the substrate. Asci (75–)85–108(–120) × (4.5–)4.8–5.6(–6.0) μm; stipe (7–)12–22(–29) μm long (n=70). Ascospores green, verrucose, warts roundish, to 0.5 μm long; cells dimorphic, distal cell (3.3–)4.0–4.8(–5.4) × (3.2–) 3.5–4.3(–5.0) μm, l/w (0.9–)1.0–1.2(–1.5) (n=110), subglobose (to wedge-shaped), proximal cell (3.4–)4.3–5.7(–6.7) × (2.5–)3.0–3.7(–4.2) μm, l/w (1.1–)1.3–1.7(–2.2) (n=110), oblong to wedge-shaped or subglobose; basal cell sometimes elongate.
Cultures and anamorph: optimum growth at 25 °C on all media; no growth at 35 °C. On CMD after 72 h 9–12 mm at 15 °C, 21–23 mm at 25 °C, 12–17 mm at 30 °C; mycelium covering the plate after 2 wk at 25 °C. Mycelium loose, radially arranged, with numerous short narrow terminal hyphae; becoming indistinctly zonate, later finely zonate by several concentric, green (27F7–8), farinose conidiation zones with conidiophores arranged in radial lines. Aerial hyphae loosely distributed, long, mainly along margins, becoming fertile. No autolytic excretions noted; coilings absent or rare and minute. No chlamydospores seen. Agar colourless or becoming dull or greenish yellow in age. No distinct odour noted. Conidiation effuse, noted around the plug after 1–2 d, spreading across the plate, macroscopically green from 6–7 d, becoming concentrated in greenish, farinose, concentric zones. Conidiophores erect on surface hyphae, from simple, mononematous with 1–2 conidial heads and to 150(–200) μm long, to complex shrubs to 0.5 mm high. Short conidiophores also branching off in right angles from some aerial hyphae to 1 mm long. Conidiophore branching asymmetric, variable, right-angled at lower levels, to steeply inclined upward with increasing height; terminal branches often parallel and symmetrically branched. Branches thick, (6–)8–10 μm wide and thick-walled at low levels including stipe, attenuated upwards to 4–6 μm. Metulae (2.0–)2.5–4.0(–4.5) μm wide, bearing phialides solitary or more commonly in parallel, gliocladium-like whorls of 2–6. Conidia formed in clear watery drops to ca. 50 μm diam, to 100(–200) μm diam when old, bursting and releasing dry conidia; only conidia green. Phialides (5–)7–12(–16) × (2.4–)3.0–3.8(–5) μm, l/w (1.5–)2.1–3.7(–4.8), (1.1–)1.5–2.5(–3.7) μm wide at the base (n=60), lageniform, mostly distinctly inequilateral, symmetric in centres of whorls, thickest at or above the middle, green when old. Conidia (3.2–)3.5–4.5(–6.5) × (2.8–)3.0–3.4(–3.6) μm, l/w 1.1–1.3(–1.8) (n=70), oval (or ellipsoidal, subglobose, few oblong or cylindrical), first hyaline, turning green, smooth, thick-walled, finely multiguttulate; abscission scar indistinct or narrowly truncate.
On PDA after 72 h 8–10 mm at 15 °C, 18–20 mm at 25 °C, 9–12 mm at 30 °C; mycelium covering the plate after 10–14 d at 25 °C. Colony circular with wavy margin; mycelium of coarsely radial arrangement, hyphae thin, densely interwoven; several broad irregular concentric zones formed. Surface first whitish downy to farinose, later green 26D3–4, 27D4–5, punctate or farinose by numerous conidial heads to 150 μm. Aerial hyphae frequent, mainly radially oriented, to 2 mm high, forming strands, becoming fertile. Autolytic excretions inconspicuous to frequent at the margin, common at 15 °C; coilings uncommon, frequent at 15 and 30 °C. Odour indistinct. Reverse of yellow concentric zones, 3A3, 3AB4–6, later olive to green 3C6–7, 30EF7–8, 4E7–8, 1E5–8. Conidiation noted after 2 d, green from 5–6 d, effuse, mostly dry, spreading across the entire colony, dense. Conidiophores forming microtufts with more complex branching than on CMD, gliocladium-like, first short, later ascending on long aerial hyphae. At 15 °C colony indistinctly zonate; surface whitish downy by dense conidiation not becoming green within 2 wk; conidial heads only faintly greenish in the stereo-microscope; agar becoming yellow to orange 4A7–8, 5AB7–8. At 30 °C colony with variable outlines, finely zonate, surface whitish farinose, reverse becoming pigmented from the plug, yellow, orange, 3A4–5, 4A4–8, 5AB7–8, 6B7–8; conidiation scant, colourless.
On SNA after 72 h 5–10 mm at 15 °C, 10–16 mm at 25 °C, 7–8 mm at 30 °C; mycelium covering the plate after 2 wk at 25 °C. Colony hyaline, thin, shiny; mycelium dense, radially arranged, with numerous short fine terminal hyphae; becoming finely zonate, with first downy, later powdery or farinose surface, green 27E4–8. Aerial hyphae numerous and to 4 mm high along the margin, becoming fertile. Autolytic excretions and coilings common, inconspicuous. No diffusing pigment, no distinct odour noted. Conidiation noted after 1–2 d, spreading from the plug, effuse, green after 6 d, numerous gliocladium-like heads to 100 μm diam on conidiophores as described on CMD, but with more complex branching. Chlamydospores seen after 1–2 wk, (5–)6–11(–13) × (3–)5–8(–9) μm, l/w 0.9–1.5(–2) (n=42), globose, oval, broadly ellipsoidal or pyriform, terminal and intercalary, smooth to verruculose. At 30 °C conidiation scant, colourless.
Habitat: on medium to well decayed wood, also on bark and overgrowing various fungi.
Distribution: Europe (Austria, France, Germany, Netherlands, Slovenia, Ukraine, United Kingdom).
Holotype: Germany, Mecklenburg; on rotten wood (holotype: Tab. 123 a–d, 124 a–f in Tode 1791); no material preserved.
Epitype: Austria, Niederösterreich, Wien-Umgebung, Mauerbach, Friedhofstrasse, MTB 7763/1, 48°15`16“N, 16°10`11“E, elev. 320 m, on decorticated branch of Carpinus betulus, soc. immature teleomorph of Trichoderma cerinum, 17 Oct. 1998, W. Jaklitsch (W.J. 1231), BPI 747556, ex-epitype culture CBS 114246.
Other specimens examined: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Gupf (Writze), MTB 9452/2, 46°33'05” N, 14°27'15” E, elev. 730 m, on decorticated branch of Corylus avellana 5–6 cm thick, on wood, holomorph, soc. Steccherinum fimbriatum and other Corticiaceae, 29 Oct. 2005, H. Voglmayr & W. Jaklitsch, W.J. 2875 (WU 29074). Same village, at brook Tumpfi, MTB 9452/4, 46°32'35” N, 14°25'32” E, elev. 565 m, on branch of Salix cinerea, soc. Hymenochaete tabacina, 13 Oct. 2006, W. Jaklitsch, W.J. 3017 (WU 29078, culture CBS 121555 = C.P.K. 2486). Niederösterreich, Hardegg, beech forest near Felling, MTB 7161/1, 48°51'47” N, 15°49'58” E, elev. 480 m, on decorticated branch of Fagus sylvatica, 6–7 cm thick, on wood, 21 Jul. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2533 (WU 29053, culture C.P.K. 1618). Melk, Weitental, Leiben, at Hofmühle, MTB 7757/2, 48°14'51” N, 15°17'23” E, elev. 270 m, on decorticated branch of Carpinus betulus 5–6 cm thick, on wood, soc. Kirschsteiniothelia aethiops, Steccherinum ochraceum, resupinate basidiomycete, 25 Jul. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2536 (WU 29054, culture CBS 121550 = C.P.K. 1918). Loosdorf, Dunkelsteiner Wald, 0.7 km south from Umbach, MTB 7758/4, 48°14'04” N, 15°25'48” E, elev. 370 m, on decorticated branches of Fagus sylvatica 4–7 cm thick, on wood and thin Corticiaceae, soc. Dacrymyces stillatus, holomorph, 5 Oct. 2004, W. Jaklitsch, W.J. 2767 (WU 29069, culture C.P.K. 1932). Weins, eastern access, close to road to Persenbeug, MTB 7756/3, 48°12'00” N, 15°02'39” E, elev. 290 m, on branch of Carpinus betulus 4–5 cm thick, on dark wood, soc. Hypocrea lixii, Mollisia sp. and rust rhizomorphs, 25 Jul. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2546 (WU 29055). Wien-Umgebung, Mauerbach, Friedhofstrasse, MTB 7763/1, 48°15'18” N, 16°10'12” E, elev. 330 m, on branch of Carpinus betulus 8 cm thick, on light and dark wood, holomorph, 13 Aug. 2005, W. Jaklitsch, W.J. 2826 (WU 29071); same locality, all on Carpinus betulus: 30 Sep. 2006, W. Jaklitsch, W.J. 2997 (WU 29076); 7 Oct. 2006, W. Jaklitsch & H. Voglmayr, W.J. 3001 (WU 29077). Holomorph, 14 Oct. 2007, W. Jaklitsch, W.J. 3180 (WU 29081). Pressbaum, Rekawinkel, forest path south of the train station, MTB 7862/1, 48°10'45” N, 16°01'59” E, elev. 360 m, on decorticated branch of Fagus sylvatica 6 cm thick, on wood, 20 Aug. 2005, W. Jaklitsch, W.J. 2828 (WU 29072). Oberösterreich, Grieskirchen, Natternbach, mixed forest at Gaisbuchen, MTB 7548/3, 48°24'39” N, 13°41'40” E, elev. 580 m, on decorticated branch of Fagus sylvatica 6–8 cm thick, on wood, soc. hyphomycetes, 1 Aug. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2551 (WU 29056, culture C.P.K. 1919). Steiermark, Leibnitz, Eichberg-Trautenburg, MTB 9358/2, elev. ca. 400 m, on branch of Carpinus betulus, soc. Kirschsteiniothelia aethiops, 19 Sep. 1996, W. Jaklitsch, W.J. 949. Vienna, 19th district, Hermannskogel, close to Fischerhaus, MTB 7763/2, 48°15'59” N, 16°18'02” E, elev. 360 m, on branch of Carpinus betulus, 13 Oct. 1996, W. Jaklitsch, W.J. 976. Vorarlberg, Bludenz, Nenzing, Rabenstein, at Beschling, MTB 8824/1, 47°11'28” N, 09°40'09” E, elev. 675 m, on decorticated branch of Fagus sylvatica 4–5 cm thick, on wood, 29 Aug. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2629 (WU 29061, culture C.P.K. 1924). Feldkirch, Rankweil, behind the hospital LKH Valduna, MTB 8723/2, 47°15'40” N, 09°39'00” E, elev. 510 m, on partly decorticated branch of Fagus sylvatica 3–4 cm thick, on wood, bark and Ascodichaena rugosa, soc. Steccherinum fimbriatum, holomorph, 31 Aug. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2640 (WU 29062, culture C.P.K. 1925). France, Lorraine, Vosges, Col de la Schlucht, Route des Cretes, Gazon du Faing, Foret des Hospices de Nancy, MTB 788/3, 48°07'24” N, 07°04'11” E, elev. 1000 m, on decorticated branch of Fagus sylvatica 5 cm thick, on wood, soc. Hypocrea minutispora, Stereum rugosum, holomorph, 04 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2674 (WU 29064, culture C.P.K. 1927). Germany, Bavaria, Oberbayern, Eichstätt, Ried, on left side shortly after the junction to Tagmersheim MTB 7132/3, 48°49'39” N, 11°03'08” E, elev. 420 m, on decorticated cut branch of Fagus sylvatica 4 cm thick, on wood, 6 Aug. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2577 (WU 29059, culture C.P.K. 1922). Starnberg, Pähl, Pähler Schlucht, MTB 8033/3, 47°54'38” N, 11°11'50” E, elev. 650 m, on decorticated branches of Fagus sylvatica 5–10 cm thick, on wood and white Corticiaceae, soc. long-necked coelomycete, 7 Aug. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2584 (WU 29060, culture C.P.K. 1923). Tutzing, Erling, Hartschimmel area, MTB 8033/1, 47°56'36” N, 11°11'02” E, elev. 700 m, on decorticated branch of Fagus sylvatica 5 cm thick, on wood, holomorph, 03 Sep. 2005, W. Jaklitsch, W.J. 2835 (WU 29073, culture from conidia C.P.K. 2412). Traunstein, Trostberg, Stadtwald, WSW from the hospital, MTB 7941/3, 48°02'N 12°31'18” E, elev. 540 m, on decorticated branch of Fagus sylvatica 12 cm thick, on wood, soc. Corticiaceae, 10 Jul. 2005, T. Lohmeyer, W.J. 2817 (WU 29070, culture C.P.K. 1933). Unterfranken, Haßfurt, Jesserndorf, at junction to road Ebern/Haßfurt, MTB 5930/1, 50°05'38” N, 10°40'28” E, elev. 350 m, on decorticated branch of Fagus sylvatica 6 cm thick, on wood, holomorph, soc. Chaetosphaeria pygmaea/myriocarpa, 4 Aug. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2558 (WU 29057, culture C.P.K. 1920). Haßfurt, close to Mariaburghausen, left roadside heading from Knetzgau to Haßfurt, MTB 5929/3, 50°00'33” N, 10°31'10” E, elev. 270 m, on decorticated branch of Fagus sylvatica 3–5 cm thick, on wood, also on Quercus (not harvested, immature), 4 Aug. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2560 (WU 29058, culture C.P.K. 1921). Niedersachsen, Landkreis Goslar, Seesen, Kurpark, 51°53'02” N, 10°12'33” E, elev. 240 m, on branch of Betula pendula, holomorph, 27 Aug. 2006, W. Jaklitsch & H. Voglmayr, W.J. 2953 (WU 29075). Nordrhein-Westfalen, Köln, Landkreis Düren, Hürtgenwald, Zerkall, Nordeifel, MTB 5304/2, 50°42'00” N, 06°27'00” E, elev. 190 m, on decorticated branch of ?Alnus sp., overmature, 31 Aug. 2004, B. Wergen, comm. K. Siepe, W.J. 2648 (WU 29063, culture C.P.K. 1926). Netherlands, Utrecht, west from the CBS building, elev. 0 m, on strongly decayed branch of Salix caprea, soc. moss, 17 Nov. 2006, H. Voglmayr, W.J. 3043 (WU 29079). Sweden, Kropp, Björka skog, V Delen, on branch of Carpinus betulus, 18 Jul. 2007, S-Å. Hanson 2007-221. Ukraine, Kyiv, Goloseevo forest near National Agrarian Academy of Ukraine, on branch of Carpinus betulus, 15 Nov. 2006, A. Akulov, AS 2110 (WU 29080). United Kingdom, Cambridgeshire, Cambridge, Wandlebury Estate, 52°09'27” N, 00°10'57” W, elev. 50 m, on branch of Fagus sylvatica 9 cm thick, on wood, holomorph, 12 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2703 (WU 29066, culture C.P.K. 1929). Norfolk, Emilys Wood, near Brandon, MTB 35-31/2, 52°28'08” N, 00°38'20” E, elev. 20 m, on decorticated branch of Fagus sylvatica 5–7 cm thick, on wood, holomorph, 13 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2711 (WU 29068, culture C.P.K. 1931). Thetford National Forest Park, near and north of the town, MTB 35-30/4, 52°26'26” N, 00°43'55” E, elev. 30 m, on cut branch of Fagus sylvatica 9–11 cm thick, on wood and bark, soc. Xylaria hypoxylon, old Quaternaria quaternata, old Hypoxylon fragiforme, Eutypa spinosa, Corticiaceae, holomorph, 13 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2706 (WU 29067, culture C.P.K. 1930). Yorkshire, Sheffield, Hollow Meadows, near the Methodist Church, 53°23'23” N, 01°38'35” W, elev. 400 m, on partly decorticated branch of Fagus sylvatica 4–6 cm thick, on wood and bark, holomorph, 11 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2700 (WU 29065, culture C.P.K. 1928).
Notes: Hypocrea gelatinosa is so far only known from Europe. It is the only green-spored species with waxy to gelatinous stromata on this continent. Due to the small size and dark colour of mature stromata this species is infrequently collected, although it is common, albeit rare in certain years. Immature stromata resemble basidiomata of Dacrymyces stillatus, but differ by a mulberry-like surface due to prominent perithecia. The waxy, glassy, translucent, pale yellowish to orange, immature stromata devoid of a contrast in lightness and colour between perithecia and stroma are characteristic for this species. The bright orange colour seems to be positively correlated with hot and dry climate. For additional descriptions see Webster (1964) and Chaverri & Samuels (2003). Based on an examination of the holotype (K 130985), Hypocrea moriformis Cooke & Massee (Grevillea 17: 3 (1888)), described from Carlisle, England, is clearly conspecific with H. gelatinosa. The North American Hypocrea ceracea P. Chaverri & Samuels (2003) is similar, but differs in shorter phialides and the more pachybasium-like conidiophores. It has not been found in Europe. Also H. nigrovirens P. Chaverri & Samuels (Chaverri et al. Samuels 2001b), described from Costa Rica, may show some superficial similarity. Waxy stromata are also typical for the hyaline-spored species H. sp. 16 and H. tremelloides. Similar gliocladium-like conidiophores that form green conidia are currently known in several other species (Chaverri & Samuels 2003).
Hypocrea lixii Pat., Rev. Mycol. Toulouse 13: 138 (1891). Fig. 22.
Fig. 22.
(Page 59) Teleomorph of Hypocrea lixii. a–f. Fresh stromata (a–d. immature, e, f. mature). g–j. Dry stromata (g. immature. h–j. mature). k. Stroma side with hairs in section. l. Perithecium in section. m. Stroma surface in face view. n. Cortical and subcortical tissue in section. o. Subperithecial tissue in section. p. Base of stroma in section. q, r. Asci with ascospores. a, i, j. WU 29095. b, d. WU 29098. c. WU 29088. e, f. WU 29101. g. WU 29084. h, k–q. WU 29082. r. WU 29089. Scale bars: a = 2.8 mm. b, d–f, j = 1 mm. c, g = 0.4 mm. h, i = 1.5 mm. k, l, n, p = 30 μm. m, q, r = 10 μm. o = 50 μm.
= Hypocrea lentiformis Rehm, Hedwigia 37: 193 (1898) fide Chaverri & Samuels (2003).
= Chromocrea nigricans Imai, Trans. Sapporo Nat. Hist. Soc. 14: 102 (1935).
≡ Hypocrea nigricans (Imai) Yoshim. Doi, Bull. Natl. Sci. Mus. Tokyo 15: 732 (1972).
= Hypocrea nigricans f. octospora Yoshim. Doi, Bull. Natl. Sci. Mus. Tokyo 15: 734 (1972).
Anamorph: Trichoderma harzianum Rifai, Mycol. Pap. 116: 38 (1969). Fig. 23.
Fig. 23.
Cultures and anamorph of Hypocrea lixii. a–c. Cultures after 7 d at 25 °C (a. on CMD. b. on PDA. c. on SNA). d, e. Effuse conidiation (25 °C, 3d). f. Fluffy tuft (25 °C, 5 d). g–j. Conidiophores (25 °C, 5 d). k. Crystals in agar (35 °C, 7 d). l, m. Conidia (25 °C, 5 d). d–m. On CMD. a–c, f–m. CBS 120630. d. C.P.K. 1599. e. C.P.K. 2389. Scale bars: a–c = 19 mm. d, e = 40 μm. f, k = 0.3 mm. g, h = 15 μm. i, j, l, m = 10 μm.
Stromata when fresh 0.5–5(–7) mm diam, 0.5–1.5(–2.8) mm thick, considerably thicker than dry, solitary, gregarious to densely aggregated, often in large numbers; discoidal, lenticular, turbinate to (flat) pulvinate. Outline circular to irregular when old. Margin thick, rounded, often free; sides often vertical and downy. Surface plane to convex, downy to smooth when young, granulose when old; ostiolar dots indistinct, plane or convex, brownish, 20–50 μm diam. Colour first white becoming pale green and downy, or incarnate to rosy, greenish brown, greyish green, becoming olive- to dark green upon maturation, 28DF4–5, 30E5–6, 26–27E6–8, 25–26F2–4, 27F3–5, 25F6–7, blackening with age; often rosy- to greyish brown inside.
Stromata when dry (0.4–)1–3(–6) × (0.3–)0.8–2.5(–5.6) mm, (0.2–)0.3–0.8(–1.7) mm thick (n=212); starting as white mycelium, compacting, forming minute discs or short cylinders, greenish, olive, often with rosy to light brown tones, often with green anamorph floccules on the surface; surface downy, smooth to slightly tubercular or rugose, with white radiating base mycelium; later becoming lenticular, discoidal and often depressed in the centre, to flat pulvinate or flat turbinate or irregularly lobed or tubercular when (over-)mature. Outline circular, angular to irregular. Base sometimes with white to greenish mycelium. Margin or edge usually free, thick, rounded, or often sharp when sides vertical, sometimes crenate, sometimes widely free and stromata only centrally attached. Sides vertical or attenuated downwards, often downy, covered with white, olive, rust, greyish to nearly black hairs. Surface flat or concave, less commonly convex, smooth, finely granulose or rugose to strongly tubercular when (over-)mature. Ostioles usually invisible, only rarely seen as minute convex brownish to black dots (16–)22–41(–63) μm (n=80), hyaline after rehydration. Colour when immature/young white with green or rosy to light brown centre, yellowish, brown with green margin, or pale bluish green 25B4 to 25CD4–5, green, to dark (olive-)green 25F3–8 or 1F1–4, grey, greyish brown to nearly black. Colour when mature macroscopically mostly dark green, grey, to black, sometimes dark brown, greyish brown to greyish green 1CD3–5, 1–2EF3–8, 28–30F3–8; no change in 3 % KOH; inside often rosy to dull brown, often glassy or waxy. Ejected ascospores often condensed into dark green to black pustules on surface.
Stroma anatomy: Ostioles (50–)60–80(–87) μm long, plane or projecting to 17(–22) μm, (17–)20–35(–48) μm wide at the apex (n=30), lined with a palisade of narrow hyaline cylindrical cells 2–3 μm wide at the apex. Perithecia (145–)175–240(–280) × (82–) 120–170(–200) μm (n=30), globose, flask-shaped or ellipsoidal, densely disposed, ca. 6 per mm, peridium (11–)13–18(–20) μm (n=30) wide at the base, (7–)9–16(–18) μm (n=30) at the sides, dull (yellowish) green, merging with subcortical tissue around ostioles. Cortical layer (14–)16–30(–47) μm (n=30) thick, encasing entire stroma except the area of attachment, a coarse green to greenish brown t. angularis of few layers of angular to oblong thin-walled cells (4–)6–12(–18) × (3–)5–8(–12) μm (n=64) in face view and in vertical section; sometimes stroma projecting above perithecia to ca. 80 μm, causing a wavy or tuberculate surface. Cortex at the sides towards base merging into a t. intricata with projecting ends of hyphae forming lateral hairs (8–)11–37(–68) × (3.5–)4.0–6.5(–9.5) μm (n=30), cylindrical to submoniliform; i.e. subperithecial tissue surrounded by a t. intricata laterally and at the base, hyaline, partly brown. Subcortical tissue a t. angularis of (sub)hyaline cells (3–)4–9(–13) × (2.5–)3–6(–8.5) μm (n=33) below the cortex, downwards changing into a t. intricata of hyaline thin-walled hyphae (1.5–)2.5–4.5(–6) μm (n=33) wide. Subperithecial tissue a dense t. epidermoidea to t. angularis of thin-walled hyaline cells (6–)9–30(–43) × (4–)7–17(–23) μm (n=30) of variable shape, becoming brownish with age, smaller downwards. Base a dense t. intricata of hyphae (2.5–)3.0–7.0(–9.5) μm (n=32) wide, often appearing as globose cells due to variable orientation of hyphae. Asci (65–)77–98(–117) × (4.4–)4.8–5.7(–6.8) μm, stipe (2–)7–18(–30) μm long (n=130). Ascospores green, becoming brown in KOH, verrucose; cells dimorphic but often similar, distal cell (3.3–) 3.8–4.6(–6.0) × (2.8–)3.5–4.2(–5.0) μm, l/w (0.9–)1.0–1.2(–1.5) (n=252), subglobose, oblong or ellipsoidal, proximal cell (3.3–)4.0–5.2(–7.0) × (2.5–)3.0–3.7(–4.4) μm, l/w (1.0–)1.2–1.6(–2) (n=252), oblong, wedge-shaped or subglobose.
Cultures and anamorph: Optimum growth at 30 °C on all media, growing also at 35 °C. On CMD after 72 h 18–20 mm at 15 °C, 62–63 mm at 25 °C, 68–71 mm at 30 °C, 12–20 mm at 35 °C; mycelium covering the plate after 4 d at 25 °C. Mycelium loose, primary surface hyphae thick (to ca. 15 μm). Aerial hyphae inconspicuous, short, irregularly distributed, or long in distal areas of the colony, becoming fertile. Agar becoming yellowish (3A3–4, later dull yellowish brown 4B5–6) from the plug, often pigment appearing as yellow crystals in various areas of the colony, often dissolving again. No autolytic activity noted at 25 °C, but conspicuous at higher temperatures. No coilings, no distinct odour noted. Chlamydospores noted after 5–14 d, infrequent, more frequent at 35 °C. Conidiation noted after 2 d on whitish floccules in a broad concentric zone close to the plug, growing to thick fluffy tufts, becoming macroscopically green after 3–4 d, often few additional, ill-defined, concentric zones of tufts developing in distal areas; accompanied by effuse conidiation on simple conidiophores not structurally differing from those in tufts except from phialides tending to be slightly longer and narrower. Conidia first formed within pustules, forming minute wet heads < 20 μm diam becoming dry. Tufts or ill-defined pustules 0.5–3 mm diam, confluent to irregular masses to 7 mm long; structure first loose, becoming compacted and opaque due to dense conidiation; surface granulose to hairy, loose, medium green, collapsing from top, becoming dark green 27F7–8 to nearly black. Tufts/pustules arising on a stipe branched into a basal net opening into long main axes (to ca. 300 μm), at the base bearing mostly 2–3 celled branches, often right-angled or slightly inclined upwards, cells long (30–40 μm), becoming shorter and 1-celled towards ends, paired or not, followed by phialides and often 1 solitary terminal phialide. Stipe and main axes 5–7 μm wide, attenuated upwards to 2.5–4 μm, origin of phialides ca. (1.5–)2.5–4(–5.5) μm wide; sometimes branching points thickened to 6–7 μm. Side branches and ends of main axes to ca. 100 μm long, base to 7 μm wide, branches paired, mostly 1-celled, longer downwards, bearing phialides solitary or divergent in whorls of 2–4(–5), often arising from thickened cells similar in shape to phialides. Elongations in young pustules ca. 100–200 μm long, straight or slightly sinuous and sterile, with mostly unpaired branches on various levels, becoming fertile and inconspicuous. Phialides (5–)5.5–7.5(–8.5) × (2.4–)2.7–3.4(–3.7) μm, l/w (1.5–)1.7–2.6(–3), 1.5–2.2(–2.5) μm wide at the base (n=32), ampulliform or lageniform with distinct thickening mostly in or above the middle, often with long narrow beak and narrow base. Conidia (3.0–)3.4–3.8(–4.0) × (2.5–)2.7–3.0 μm, l/w 1.2–1.3(–1.5) (n=32), (yellowish) green, smooth, (subglobose-)ovoid(-ellipsoidal), with few (often one large plus several smaller) guttules, scar indistinct. At 15 °C yellow crystals often more conspicuous, also in lines along hyphae, fluffy tufts in concentric zones becoming green 27E5–6. At 30 °C pustules dark green 27F7–8. At 35 °C numerous large crystals to ca. 0.7 mm long and macroscopically visible as fine yellowish brown granules, hyphae becoming yellow, dying soon; conidiation effuse to finely granular, autolytic excretions numerous, colony becoming bright yellow 3A4–7, fading into dull yellowish brown.
On PDA after 72 h 14–16 mm at 15 °C, 50–61 mm at 25 °C, 60–63 mm at 30 °C, 16–18 mm at 35 °C; mycelium covering the plate after 3–5 d at 25 °C. Colony dense, compact, with wide primary surface hyphae, developing few yellow to green (27DE3–4) concentric zones, usually ill-defined, diffuse, indistinctly separated, or lacking; numerous long aerial hyphae forming a white cottony tomentum often with radial strands, large connectives, coilings and drops, rendering the colony opaque, becoming green starting at the plug, condensing into broad floccules or fluffy tufts or pustules mainly in the centre; autolytic activity and coilings inconspicuous, no distinct odour noted; pigmentation of agar lacking, slightly yellowish or yellow, 3A5, 3B5–6, 4A4–7, starting from the plug, mixed with translucent olive to green colour of conidiation, turning dull (yellowish) brown, 4D5–6(–8), 4C7, 5C5(–8). Conidiation noted after 2d, macroscopically green after 3–5 d, effuse, dense, starting around the plug on aerial hyphae, conidia formed in minute wet heads on short symmetric trees. At 15 °C conidiation sparse, remaining white or becoming faintly greenish. At 30 °C conidiation abundant, effuse, green, with several yellow and green thick convex zones, reverse yellow to yellowish brown in various tones, 3B5–6, 4A5–6, 5CD5–6. At 35 °C colony raised above agar surface, forming several thick yellow to green concentric zones, reverse becoming bright yellow 3A6–7 to 4A5–7, turning yellowish- or orange-brown 4B6–8 to 5C7–8; conidiation abundant, effuse.
On SNA after 72 h 15–18 mm at 15 °C, 45–50 mm at 25 °C, 50–55 mm at 30 °C, 9–15 mm at 35 °C; mycelium covering the plate after ca. 5 d at 25 °C. Colony thin, hyaline, denser than on CMD, secondary hyphae thin, numerous, with many short ends, marginal surface hyphae conspicuously wide, mycelium soon degenerating. Aerial hyphae inconspicuous, autolytic excretions scant, frequent at 35 °C; coilings rare. No distinct odour noted. Agar colourless to faintly diffuse yellowish 3A3. Chlamydospores noted after 4–5, frequent but loosely disposed, terminal and intercalary. Conidiation noted after 1–2 d, starting in white fluffy tufts spreading from centre, forming concentric zones, compacting to pustules to 3 mm diam, aggregating to 10 mm. Conidiophores regular, phialides curved upwards, some long straight to slightly sinuous sterile to fertile elongations present, becoming macroscopically green after 3 d, finally dark green 27F7–8, degenerating, collapsing. At 15 °C margin ill-defined, numerous yellowish crystals noted; conidiation in white to greenish confluent fluffy tufts in concentric zones, often forming a large continuum in the centre. At 30 °C conidiation in green 27F4–8 confluent pustules with long sterile to fertile elongations, forming amorphous masses in indistinct concentric zones; needle-like yellow crystals noted; chlamydospores moderate, minute. At 35 °C colony ill-defined, dense, hyphae narrow, little mycelium on surface, numerous needle-like crystals; conidiation sparse to moderate, autolytic excretions numerous, agar pale yellowish. Chlamydospores (35 °C, 9 d) (4–)5–11(–15) × (3–)4–7(–10) μm, l/w (0.8–)0.9–2.1(–3.3) (n=60), globose to pyriform, oblong and angular in wide hyphae, numerous, terminal and intercalary.
Habitat: on wood, bark and fungi.
Distribution: possibly cosmopolitan; common in Europe (Austria, Denmark, Germany, France, United Kingdom), particularly in warmer periods.
Holotype: Papua New Guinea, On hymenium of Ganoderma pourii; Jul. 1891; Lix (FH). Epitype: Thailand, Saraburi Province: Khao Yai National Park, Wang Jumpee Trail; on hymenium of Ganoderma sp.; 31 Jul. 1997, K. Põldmaa, P. Chaverri & G.J. Samuels #8233 (BPI 745654, ex-epitype culture G.J.S. 97-96 = ATCC MYA-2478 = CBS 110080).
Specimens examined: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Drau-Auen, near road to Dullach, MTB 9452/1, 46°32'51” N, 14°24'31” E, elev. 410 m, on Phellinus punctatus/Salix sp., on wood, bark and hymenium of the Phellinus, holomorph, 30 Oct. 2005, H. Voglmayr & W. Jaklitsch, W.J. 2878, WU 29100, culture C.P.K. 3116. Niederösterreich, Gänserndorf, Thayatal, Marchegg, at Gscheibter Berg, south from the train station of Marchegg, MTB 7767/4, 48°14'37” N, 16°55'19” E, elev. 160 m, on branch of Sambucus nigra, holomorph, soc. ?Trichoderma cerinum, 1 Nov. 2004, H. Voglmayr, W.J. 2786, WU 29097, culture C.P.K. 1941. Melk, Weins, eastern access, beside road to Persenbeug, MTB 7756/3, 48°12'00” N, 15°02'39” E, elev. 290 m, on branch of Fagus sylvatica 4–5 cm thick, on wood, soc. Hypocrea gelatinosa, immature, culture from conidia, 25 Jul. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2545, WU 29088, culture C.P.K. 1934. Mödling, Gießhübl, Wassergspreng, side of forest road; MTB 7963/1, 48°05'47“N, 16°12'15“E, elev. 380 m, on decorticated branch of ?Fraxinus excelsior 7–8 cm diam, 17 Sep. 2006, H. Voglmayr, W.J. 2974, WU 29101, culture C.P.K. 2465. Wien-Umgebung, Mauerbach, Friedhofstrasse, MTB 7763/1, 48°15'20” N, 16°10'13” E, elev. 330 m, on branch of Carpinus betulus, holomorph, 5 Oct. 2002, W. Jaklitsch, W.J. 1994. Same area, 48°15'15” N, 16°10'33” E, elev. 360 m, on partly decorticated branch of Fagus sylvatica 4–5 cm thick, on wood and bark, soc. Lasiosphaeria strigosa and effete pyrenomycetes, holomorph, 10 Sep. 2005, W. Jaklitsch, W.J. 2848, WU 29099. Oberösterreich, Grieskirchen, Neukirchen am Walde, Leithen, MTB 7648/2, 48°22'18” N, 13°47'09” E, elev. 400 m, on broken log of Carpinus betulus 15 cm thick, on wood, holomorph, teleomorph immature, culture from conidia, 16 Aug. 2003, H. Voglmayr, W.J. 2337, WU 29086, culture C.P.K. 2389. Schärding, St. Willibald, riverine forest at Aichet, MTB 7648/1, 48°21'17” N, 13°41'01” E, elev. 400 m, on branch of Alnus glutinosa, holomorph, 30 Jul. 2005, H. Voglmayr, W.J. 2823, WU 29098. Steiermark, Graz-Umgebung, Sankt Radegund bei Graz, Kickenheim, downwards from the bridge along the brook, MTB 8859/1, 47°10'16” N, 15°30'41” E, elev. 520 m, on decorticated branch of Alnus glutinosa, 4 cm thick, on wood and on/soc. resupinate polypore, soc. effete pyrenomycete, 20 Aug. 2004, W. Jaklitsch, W.J. 2614, WU 29091, culture, C.P.K. 1936. Mariatrost, Wenisbucherstraße (close to crossing with Himmelreichweg), MTB 8858/4, 47°06'47” N, 15°29'03” E, elev. 470 m, on partly decorticated branch of Fagus sylvatica 3 cm thick, on wood and below loose bark, soc. Neorehmia ceratophora, holomorph, teleomorph immature, culture from conidia, 8 Aug. 2003, H. Voglmayr & W. Jaklitsch, W.J. 2317, WU 29083, C.P.K. 1596. Same locality, on branch of Corylus avellana 4–6 cm thick, on wood, on/soc. Hymenochaete cinnamomea, soc. Hypoxylon fuscum and other effete pyrenomycetes, holomorph, 8 Aug. 2003, H. Voglmayr & W. Jaklitsch, W.J. 2322, WU 29084, C.P.K. 1599. Weiz, Laßnitzthal, opposite Arboretum Gundl across the road, MTB 8959/2, 47°04'17” N, 15°38'38” E, elev. 420 m, on decorticated branch of Carpinus betulus 4–5 cm thick, on moist naked wood, soc. Kirschsteiniothelia aethiops, Arcyria incarnata, 8 Aug. 2003, H. Voglmayr & W. Jaklitsch, W.J. 2324, WU 29085. Tirol, Imst, Stams, Stamser Eichenwald, MTB 8731/2, 47°16'32” N, 10°58'30” E, elev. 645 m, on branch of Quercus robur, on bark, soc. Durella commutata, effete pyrenomycetes and rhizomorphs; holomorph, teleomorph immature, culture from conidia, 1 Sep. 2003, W. Jaklitsch, W.J. 2347, WU 29087, culture C.P.K. 2391. Vienna, 23rd district, Maurer Wald, MTB 7863/1, 48°08'57” N, 16°14'50” E, elev. 360 m, on branch of Ulmus sp., holomorph, 03 Oct. 1998, W. Jaklitsch, W.J. 1222. Denmark, Langeland, Skovsgård Gods, Konabbe Skov, on hymenium of old Fomes fomentarius on the ground in old beech forest, 16 Sep. 2006, T. Læssøe, TL-12743, WU 29102, culture C.P.K. 2466. France, Moselle, Lorraine, Pont a Mousson, halfway to highway between Nancy/Metz, 48°55'26” N, 06°05'55” E, elev. 200 m, on decorticated branch of Fagus sylvatica 5–6 cm thick, on wood, soc. Hypocrea minutispora and H. viridescens, immature, culture from conidia, 5 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2681, WU 29093, culture C.P.K. 1937. Germany, Bavaria, Starnberg, Tutzing, Erling, Hartschimmel-Gelände, Goaslweide, MTB 8033/1, 47°56`35“N, 11°11`02“E, elev. 735 m, on decorticated branch of Quercus robur on the ground, soc. effete pyrenomycete, 6 Jul. 2003, P. Karasch, W.J. 2274, WU 29082, culture CBS 120630, C.P.K. 939. Pähl, Pähler Schlucht, MTB 8033/3, 47°54'38” N, 11°11'50” E, elev. 650 m, on branch of Fagus sylvatica 10 cm thick, on wood, 7 Aug. 2004, W. Jaklitsch, H. Voglmayr & P. Karasch, W.J. 2585, WU 29089, culture C.P.K. 1935. United Kingdom, Buckinghamshire, Slough, Burnham Beeches, 51°33'17” N, 00°38'18” W, elev. ca. 30 m, on decorticated branch of Fagus sylvatica 9 cm thick, on wood, holomorph, 15 Sep. 2004, W. Jaklitsch, W.J. 2716, WU 29094, culture C.P.K. 1938. North East London, Epping Forest, between Robin Hood Roundabout and Hill Wood, 51°39'15” N, 00°02'13” E, elev. 40 m, on decorticated branch of Fagus sylvatica 4–5 cm thick, on wood and a resupinate polypore, soc. Hypocrea viridescens, Diatrype decorticata, Ascocoryne sp. (anamorph), holomorph, 16 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2722, WU 29095, culture C.P.K. 1939 and at Wake Valley Pond and Sunshine Plain, 51°40'13” N, 00°03'14” E, elev. 30 m, on partly decorticated branches of a large tree of Fagus sylvatica 8–18 cm thick on the ground, on wood, bark and Trametes versicolor, soc. Pleurotus pulmonarius, Tomentella sp., holomorph, 16 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2726, WU 29096, culture C.P.K. 1940.
Notes: This species is common in Europe, particularly during warmer periods. Although usually recognisable by sight due to the green-coloured stromata, immature stromata may be confused with H. schweinitzii. Discoidal or lenticular stromata are also formed by H. epimyces and H. parepimyces, but both have reddish- or orange-brown colours and conspicuous ostiolar dots.
The Harzianum clade is phylogenetically exceedingly complex, and Hypocrea lixii is a species aggregate rather than a well-defined species. Chaverri & Samuels (2002) established the teleomorph/anamorph relationship of H. lixii and T. harzianum sensu stricto, but Chaverri et al. (2003b) showed by a four-gene phylogeny of the T. harzianum/H. lixii complex, that the ex-neotype of T. harzianum, strain CBS 226.95, did not cluster with the ex-epitype of H. lixii, strain G.J.S. 97-96. In addition, Druzhinina et al. (2009) rejected the teleomorph/anamorph relationship after a thorough analysis involving numerous strains. The anamorphs of the different H. lixii lineages are morphologically difficult to distinguish from each other, from T. harzianum s. stricto, but also from other species of the Harzianum clade such as T. aggressivum. Due to these difficulties in recognising phenotypic differences no formal taxonomic implications have been published yet. To do this, more isolates from'H. lixii' teleomorphs are needed on a worldwide basis.
Hypocrea longipilosa Jaklitsch, sp. nov. MycoBank MB513185. Fig. 24.
Fig. 24.
Teleomorph of Hypocrea longipilosa (WU 29106). a, b. Fresh stromata (a. habit, b. immature). c–g. Dry stromata (c–e. immature. e. side view. f, g. mature). h. Mature stromata in 3 % KOH after reconstitution in water. i. Projecting cells (`hairs') on stroma side. j. Stroma surface in face view. k. Perithecium in section. l. Cortical and subcortical tissue in section. m. Subperithecial tissue in section. n. Base of stroma in section. o. Ascus with ascospores. p. Ascospores in ascus base. Scale bars: a = 1.5 mm. b, h = 1 mm. c–f = 0.4 mm. g = 0.8 mm. i, j, o, p = 10 μm. k = 35 μm. l–n = 20 μm.
Anamorph: Trichoderma longipile Bissett (as T. longipilis), Can. J. Bot. 69(11): 2395 (1992) [1991]. Fig. 25.
Fig. 25.
Cultures and anamorph of Hypocrea longipilosa (CBS 120953). a–c. Cultures after 14 d at 25 °C (a. on CMD. b. on PDA. c. on SNA). d–g. Conidiophores of effuse conidiation (SNA, 25 °C, 5 d; d. on aerial hypha, e–g. from agar surface). h. Conidiation pustule. i, j. Conidiophores from pustules (i. with short sterile elongations). k, l. Fertile elongations (k. terminal phialides). m. Chlamydospores (CMD, 30 °C, 6 d). n, o. Conidia. h–l, n, o. On SNA, 25 °C, 7 d. Scale bars: a–c = 20 mm. d, e, l = 30 μm. f, g, j = 15 μm. h = 0.3 mm. i, k, m = 20 μm. n, o = 10 μm.
Stromata solitaria vel gregaria, incarnata vel rubra, pulvinata, superficie tuberculata, 1–3.5 mm diam. Asci cylindrici, (94–)108–133(–145) × (5.7–)6.4–7.2(–7.5) μm. Ascosporae bicellulares, luteo-virides, verrucosae, ad septum disarticulatae, pars distalis subglobosa vel cuneata, (5.0–)5.5–7.3(–8.4) × (3.8–)4.2–5.0(–5.6) μm, pars proxima oblonga vel cuneata, (5.0–)5.5–7.5(–8.4) × (3.4–)3.6–4.5(–5.0) μm. Anamorphosis Trichoderma longipile Bissett.
Stromata when fresh 1–3.5 mm diam, to 1.5 mm thick, solitary, gregarious or aggregated in small numbers, pulvinate, sides attenuated to a short stout and white stipe, surface tubercular to moriform, perithecial contours visible; shrinking considerably upon drying; reddish, brownish to nearly orange-brown, 7C7–8, partly 8D7–8, ostiolar areas diffuse dots slightly darker than stroma surface.
Stromata when dry (0.8–)1–2(–2.5) × (0.7–)0.8–1.6(–2.2) mm, 0.3–0.6(–0.8) mm thick (n=16); irregularly pulvinate, often on broad whitish to cream downy base, broadly attached. Outline circular, angular or oblong. Surface conspicuously coarsely tubercular due to distinctly prominent perithecial protuberances, velutinous between perithecia when young; perithecia in small numbers, sometimes only few perithecia on a reduced crustose hypostroma. Ostiolar areas (47–)60–108(–134) μm (n=30) diam, indistinct or greyish green convex upper part of perithecia, irregularly spaced. Colour difficult to define, macroscopically (reddish) brown, 8–9F5–8 to 9E5–6, incarnate (pale reddish) in the stereo-microscope; with rosy, reddish to orange down between perithecia. Size of stromata increased by ca. 35 % after reconstitution with water; colour incarnate to light (orange-)red, unchanged by 3 % KOH. Associated anamorph dull green, 26–27F5–8, hairy, with white straight to sinuous elongations; conidia yellowish green, cylindrical.
Stroma anatomy: Ostioles (65–)77–110(–134) μm long, projecting to 20(–35) μm, (30–)37–54(–60) μm wide at the apex (n=25); margin lined with a palisade of hyaline cylindrical cells, narrowly clavate at the apex and 2–3.5(–5) μm wide. Perithecia (115–) 160–240(–260) × (110–)130–210(–270) μm (n=25), globose or flask-shaped; peridium (8–)14–22(–25) μm (n=50) wide at the base and at the sides, hyaline to pale yellowish. Cortical layer (17–) 20–35(–37) μm (n=30) thick, present around entire stroma except area of attachment, (orange-)yellow to yellow-brown t. angularis of isodiametric to oblong angular cells (5–)7–15(–20) × (4–)5–11(–15) μm (n=60) in face view and in vertical section, walls to ca. 1 μm thick; rosy to red in KOH. Hairs on mature stromata (4–)5–11(–16) × (2.5–)3.0–4.5(–5.0) μm (n=30), 1–3 celled outgrowths mostly of subperithecial hyphae, often of a narrow cylindrical terminal cell on a broad (sub-)globose base, rare or collapsed on upper surface, more frequent on sides. Subcortical tissue a dense hyaline to yellowish t. angularis to t. epidermoidea of thin-walled cells (3–)4–11(–13) × (2.5–)3–8(–9.5) μm (n=30), mixed with few thin-walled hyphae 2.5–4 μm wide. Subperithecial tissue a t. angularis to t. epidermoidea of thin-walled hyaline to yellowish, globose, angular to lobed cells (5–)9–25(–34) × (4–)6–14(–18) μm (n=33), smaller towards base. Base of a dense t. epidermoidea mixed with hyaline hyphae (1.5–)2.5–5.5(–7.5) μm (n=30) wide. Asci (94–)108–133 (–145) × (5.7–)6.4–7.2(–7.5) μm, stipe (11–)12–26(–35) μm long (n=30). Ascospores yellow-green, densely verrucose, verrucae to ca. 0.5 μm wide; cells dimorphic, but often similar in size, distal cell (5.0–)5.5–7.3(–8.4) × (3.8–)4.2–5.0(–5.6) μm, l/w (1.1–)1.2–1.6 (–1.9) (n=30), subglobose to wedge-shaped, proximal cell (5.0–) 5.5–7.5(–8.4) × (3.4–)3.6–4.5(–5.0) μm, l/w (1.2–)1.4–1.9(–2.1) (n=30), oblong to wedge-shaped.
Cultures and anamorph: Optimum growth at 25 °C on all media, slightly slower on PDA than on CMD and SNA; no growth at 35 °C. On CMD after 72 h 19–20 mm at 15 °C, 47–48 mm at 25 °C, 12–19 mm at 30 °C; mycelium covering the plate after 4–5 d at 25 °C. Colony homogeneous, mycelium radially oriented, with numerous fine curly ends, primary hyphae thick, particularly close to the margin. Aerial hyphae frequent in distal areas of the colony, long and high to 1–2 mm. Autolytic activity absent, coilings absent or rare. No pigment, no distinct odour noted. Conidiation starting after 2–3 d, similar to but less than on SNA; first effuse, macroscopically invisible, mostly in distal regions of the colony; of long solitary phialides on surface hyphae or erect aerial hyphae with few phialides strongly inclined upwards and minute conidial heads soon becoming dry; later white loosely branched tufts appearing in 1(–2) broad concentric zone at the distal margin, becoming macroscopically green (finally 27–28F6–8) after 6–7 d and growing to 1.5 mm diam, confluent to 3 mm, with straight to sinuous sterile elongations to 350 μm long, becoming fertile. Chlamydospores infrequent, noted after 4–5 d. At 15 °C pustules remaining white. At 30 °C conidiation scant, effuse, colourless; chlamydospores abundant, noted after 2–3 d.
On PDA after 72 h 15–17 mm at 15 °C, 35–38 mm at 25 °C, 9–11 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony dense, flat, surface shiny, mycelium of thick radial hyphae and thin secondary hyphae forming a dense mesh; numerous conspicuous thick, parallel, finely sinuous hyphae forming the colony margin. Aerial hyphae numerous, short, erect, strongly branched, forming a dense lawn giving the surface a farinose to downy appearance; longer and loosely distributed in distal regions of the colony. Centre and concentric zones becoming fluffy and white. Centre later turning light green; green colour spreading from the centre across the plate, light or dull, 26–27CD3–4, zonation becoming indistinct. Autolytic activity inconspicuous, coilings absent or rare. No pigment on the reverse, no distinct odour noted. Conidiation starting after 2 d, effuse, mostly on short aerial hyphae in the centre spreading across denser areas, remaining effuse; long straight sterile elongations noted; turning macroscopically green after 6–7 d. At 15 °C poor growth, colony angular or irregular, surface finely granular due to submoniliform surface hyphae, marginal hyphae with hyperbranching forming pegs and brushes, little effuse conidiation. At 30 °C centre of the colony becoming thick and yellow, with radial folds, margin hyaline, thin, irregular, broom-like; autolytic activity conspicuous, conidiation scant.
On SNA after 72 h 17–18 mm at 15 °C, 42–44 mm at 25 °C, 6–11 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony and mycelium similar to CMD, thick primary hyphae dominant, radial, margin wavy; broad marginal zone becoming downy. Loose fluffy tufts appearing mainly laterally and in few indistinct distal concentric zones including the distal margin, white, becoming green, growing to 2 mm diam, confluent to 10 mm; mycelium degenerating. Aerial hyphae numerous on the entire colony except centre, long, ascending several mm, becoming fertile. Autolytic activity inconspicuous, coilings moderate to common. No pigment, no distinct odour noted. Conidiation seen after 1–2 d, first around the plug, effuse, scant, solitary phialides on surface hyphae or on short conidiophores, more on long high aerial hyphae in one or several broad distal concentric zones; soon followed by the formation of loose fluffy tufts or pustules, lateral or in a broad distal concentric zone; with long straight sterile elongations, becoming fertile, heads dry, macroscopically green after 6–7 d at 25 °C, 27DE3–4 to 27–28F6–8. Effuse conidiation (25 °C, 5 d): sessile on surface hyphae or on aerial hyphae to ca. 2 mm long, as solitary phialides or as simple conidiophores (20–)30–80(–150) μm long and 2.5–3.5 μm wide, 1–2 celled and unbranched, or scarcely and mostly asymmetrically branched bearing phialides solitary or in whorls of 2–4, each with a minute wet conidial head < 20 (rarely to 50) μm diam. Phialides generally distinctly inclined or curved upwards, unpaired, less commonly paired or cruciform, arising from cells to 5 μm wide, often wider than conidiophore. Phialides (8.5–)11–20(–23.5) × (2.2–)2.7–3.7(–4.5) μm, l/w (2–)3.3–6.4(–7.3), 2.0–2.8(–3.4) μm wide at the base (n=30), subulate or lageniform, rarely ampulliform, thickest at or slightly above the base. Conidia (3.8–)4.2–8.2(–12) × (3.0–)3.3–4.5(–5.5) μm, l/w 1.2–2.0(–2.7) (n=30), variable, oval to oblong, often laterally constricted, hyaline to dilute green, smooth, scar often distinct, with few minute guttules. Pustulate conidiation (25 °C, 7 d) first a loose transparent net with long trees and sterile elongations. Elongations numerous, straight to sinuous, rarely helical, generally slightly inclined upwards, 100–200(–450) μm long, 3–5 μm wide, attenuated to 2–3 μm terminally, appearing verrucose under low magnification but smooth in the microscope, unbranched or with loosely arranged, mostly unpaired branches, becoming fertile, bearing 1(–2) terminal phialides and minute dry heads < 15 μm diam. Phialides (4–)10–17(–21) × (2.0–)2.4–3.2(–4.3) μm, l/w (2.1–)3.3–6.6(–8.3), (1.6–)2.0–2.6(–3.0) μm wide at the base (n=32), subulate or lageniform, sometimes enlarged in the middle, mostly solitary. Conidiophores within pustules 3–6.5 μm wide, trees with mostly unpaired, long, several-celled, nearly right-angled branches at lower levels, upwards shorter and tending to be paired more frequently, terminal branches 1-celled and with a whorl of 2–3 phialides, slightly inclined upwards; branches often with 2 paired phialides on several levels shortly after each other, followed by 2–3 terminal phialides, resulting in dense conidiation. Phialides (3.5–)4.3–6.4(–8.0) × (3.0–)3.4–4.3(–4.8) μm, l/w (1.1–)1.2–1.6(–1.8), (2.0–)2.4–3.2(–3.7) μm wide at the base (n=30), short and thick, ampulliform or subglobose, arising from cells 3–4 μm wide, sometimes 1 or 2 cells below phialides thickened (submoniliform). Conidia (3.9–)4.2–6.4(–7.7) × (2.5–)2.6–3.4(–4.0) μm, l/w (1.3–) 1.5–2.1(–2.5) (n=30), bright green, smooth, cylindrical or oblong, often slightly constricted laterally, thick-walled, often 1 or a group of minute guttules close to each end. Pustules often superimposed by long aerial hyphae with effuse conidiation usually degenerated when pustulate conidiation develops. Chlamydospores (25 °C, after 16 d) smooth, eguttulate, (4.5–)6.5–9(–11) × (3.5–)6–8(–9) μm, l/w 1.0–1.2(–1.3) (n=31), globose to pyriform when terminal, (5.5–)7–12(–13) × (3.5–)4.5–7 μm, l/w (1.3–)1.4–1.9(–2.2) (n=15), often angular, when intercalary; first noted around tufts from 5–7 d, spreading across entire plate, numerous, much more abundant than on CMD. At 15 °C conidiation effuse and pustulate, white to greenish, chlamydospores numerous. At 30 °C little growth, hyphae forming pegs or hyperbranching, dying soon; autolytic activity conspicuous, no conidiation seen, chlamydospores noted from 3 d.
Habitat: teleomorph on a branch of Corylus avellana on moist grassy ground; anamorph isolated from wood of Populus and Ulmus and soil.
Distribution: Canada (anamorph), Europe. Teleomorph only known from the type locality in Sweden.
Holotype: Sweden, Uppsala Län, 3–4 km north from Österbybruck, close to the road to Forsmark, MTB 4373/4, 60°14'28” N, 17°55'43” E, elev. 40 m, on corticated branch of Corylus avellana 2.5 cm thick, on wood and bark, soc. Cosmospora purtonii, Melanomma pulvis-pyrius, Diatrypella verruciformis, and a discomycete, 5 Oct. 2003, W. Jaklitsch (W.J. 2437), WU 29106, culture CBS 120953 = C.P.K. 981.
Notes: This species is based on a single specimen containing only a few stromata. The incarnate colour of stromata, the distinctly tubercular surface and large green ascospores are characteristic. The anamorph was originally isolated from wood of Populus and Ulmus and from soil in Canada (Bissett 1991b). T. oblongisporum Bissett differs in unbranched and straighter elongations. The cylindrical, often laterally pinched conidia, formed on minute ampulliform phialides in pustules, have not been seen in anamorphs of any other Hypocrea species known from Europe. Teleomorphs of H. estonica and H. parestonica show some superficial similarities with H. longipilosa. They occur on species of Hymenochaete and differ e.g. in smooth stroma surface, smaller ascospores and slightly shorter and ellipsoidal conidia. In addition, the growth patterns on PDA are distinct.
Hypocrea parepimyces Jaklitsch, sp. nov. MycoBank MB513186. Fig. 26.
Fig. 26.
Teleomorph of Hypocrea parepimyces. a–d. Fresh stromata (a. immature, b. young, partly mature, c, d. mature, d. discoidal). e–g. Dry stromata (e, f. mature, g. stroma initial). h. Mature stroma reconstituted in water. i. Stroma of h after addition of 3 % KOH. j. Stroma surface in face view. k. Perithecium in section. l. Cortical and subcortical tissue in section. m. Subperithecial tissue in section. n. Base of stroma in section. o, p. Asci with ascospores. q. Submoniliform subcortical hypha. r. Ascospores. a–e, h–o, q. WU 29107. f, g, p, r. WU 29108. Scale bars: a, h, i = 1.5 mm. b, f, g = 0.5 mm. c, e = 1 mm. d = 2.5 mm. j, m, n = 20 μm. k, l = 40 μm. o–q = 15 μm. r = 10 μm.
Anamorph: Trichoderma parepimyces Jaklitsch, sp. nov. MycoBank MB513187. Fig. 27.
Fig. 27.
Cultures and anamorph of Hypocrea parepimyces. a–c. Cultures after 14 d at 25 °C (a. on CMD. b. on PDA. c. on SNA). d. Conidiation pustule. e–i. Conidiophores from pustules. j, k. Conidia. l. Chlamydospores (SNA, 25 °C, 17 d). d–k. On CMD, at 25 °C, after 10–13 d. a–c, f, i, k, l. CBS 122768. d, e, g, h, j. CBS 122769. Scale bars: a–c = 20 mm. d = 0.2 mm. e = 30 μm. f = 60 μm. g, l = 20 μm. h–k = 10 μm.
Teleomorphosis similis Hypocreae brunneoviridis et H. epimycetis. Stromata pulvinata vel discoidea, 2–10 mm diam, ad 3 mm alta, aurantio-brunnea ubi matura et sicca. Asci cylindrici, (90–)100–120(–130) × (4.7–)5–6(–6.5) μm. Ascosporae bicellulares, virides, verruculosae, ad septum disarticulatae, pars distalis subglobosa, (3.5–)4.3–5.4(–7) × (3.3–)3.5–4.5(–4.8) μm, pars proxima oblonga vel cuneata, (4.0–)4.5–6.0(–7.5) × (2.8–)3.2–4.0(–4.5) μm. Anamorphosis Trichoderma parepimyces. Similis Trichodermatis epimycetis, differt in absentia conidiophororum effusorum et in coloniis zonatis in PDA non vel tarde et pallide viridescentibus. Phialides lageniformes, (5–)6–10(–16) × (2.7–)3.0–3.7(–4.3) μm. Conidia in CMD et SNA viridia, subglobosa vel ellipsoidea, glabra, (3.0–)3.3–4.2(–6.0) × (2.5–)2.8–3.3(–4.2) μm.
Stromata when fresh (2–)3–10 mm diam, to 2.5(–3) mm thick, on wood/below bark, solitary, gregarious to densely aggregated in convolutes, lentiform, turbinate/substipitate or pulvinate; smooth, small and pale or dark (olive-)green when young, later incarnate or dark reddish brown, more orange-brown in the stereo-microscope, with numerous small, slightly projecting, dark (green) ostiolar dots. Spore powder deeply green. Associated anamorph effuse, powdery, light to dark green.
Stromata when dry (0.9–)1.5–4.5(–7) × (0.7–)1.3–3.7(–5.2) mm, 0.6–1.2(–1.7) mm thick (n=40), lentiform, discoidal, pulvinate, coarsely turbinate (discoidal with a narrowed base), or irregularly undulate, broadly or narrowly attached; mostly lenticular and dark brown-olive when young. Outline circular, oblong or irregular. Margin free, rounded or sharp, also lobed. Sides straight vertical or attenuated downward into a thick cylindrical stipe, glabrous, concolorous with stroma surface or frequently covered by rust, dark brown or grey to black mycelium, sometimes transient into white mycelium on wood. Surface flat, convex or concave, smooth when young, finely tubercular to rugose when mature. Ostiolar dots (30–)45–95(–160) μm (n=60) diam, generally distinct, mostly slightly papillate, dark red when immature, becoming black. Colour macroscopically dull dark brown 7F4, 8F4–6, dark reddish brown 9–10F6–8, to black, under magnification first reddish brown and partly green, and sometimes covered by green conidiophores, later orange-brown plus black dots. Spore deposits dark green 25F7–8 or darker. Stromata after reconstitution in water flat pulvinate, surface smooth, orange plus dark reddish brown to nearly black, broadly convex, ostiolar dots. After addition of 3 % KOH first more orange and ostiolar dots dark reddish brown; ostioles minute, hyaline; surface slowly becoming red, macroscopically black. Cortical pigment inhomogeneous, orange plus fine dark red particles.
Stroma anatomy: Ostioles (84–)90–120(–140) μm long, plane or projecting to 20(–35) μm, (24–)33–55(–67) μm wide at the apex (n=30); apical cells inconspicuous. Perithecia (210–)250–325 (–350) × (140–)175–257(–310) μm (n=30), globose or flask-shaped, peridium (15–)16–23(–28) μm (n=30) wide at the base, (13–)15–20(–28) μm (n=30) at the sides, yellow. Cortical layer (22–)28–47 (–58) μm (n=30) thick, present around whole stroma except area of attachment, of a thin, orange-brown, amorphous layer of collapsed-compressed cells on the surface, inhomogeneous, of flat flakes; below this layer a t. angularis of yellow or subhyaline, thick-walled (1 μm), isodiametric cells (5–)7–14(–18) × (3–)5–9(–13) μm (n=70) in face view and in vertical section. Cortex at lower sides and base merging into brown cylindrical hyphae, projecting part (10–)13–36(–56) × (4–)5–8(–9) μm (n=30); some also scattered on upper parts. Subcortical tissue a (sub-)hyaline t. angularis to t. globulosa of thin-walled cells (4–)5–9(–15) × (3–)4–6(–8) μm (n=35), interspersed by or nearly exclusively of thin-walled (sub-)hyaline hyphae (2–)3–5(–6) μm (n=30) wide, with numerous inflated, globose or clavate (moniliform) cells (4.5–)6–9(–12) μm (n=30) wide. Subperithecial tissue a hyaline t. angularis-epidermoidea of thick-walled cells (7–) 10–30(–48) × (5–)7–15(–24) μm (n=30), smaller in the base and (orange-)brown with inhomogeneous pigment distribution, mixed with hyphae (3–)4–8(–11) μm (n=30) wide. Asci (90–)100–120(–130) × (4.7–)5–6(–6.5) μm, stipe (6–)11–22(–30) μm long (n=60). Ascospores (olive-)green, becoming brown in KOH, verruculose; cells dimorphic, distal cell (3.5–)4.3–5.4(–7.0) × (3.3–)3.5–4.5(–4.8) μm, l/w (1–)1.1–1.4(–1.6) (n=60), (sub-)globose (to oblong or cuneate), proximal cell (4.0–)4.5–6.0(–7.5) × (2.8–)3.2–4.0(–4.5) μm, l/w (1.1–)1.3–1.8(–2.5) (n=60), oblong to plump wedge-shaped (or subglobose).
Cultures and anamorph: Optimum growth at 25 °C on all media; no growth at 35 °C. On CMD after 72 h 9–11 mm at 15 °C, 25–27 mm at 25 °C, 17–20 mm at 30 °C; mycelium covering the plate after 1 wk at 25 °C. Colony circular, dense with loose centre and little mycelium on agar surface, surface appearing radially fan-shaped, not or indistinctly zonate; margin wavy. Pustules appearing behind the plug and in distal areas, spreading in a lawn on the entire plate, concentrated along the margin and in the middle; first white, becoming dark green with a yellow component, 28–29EF5–8. Aerial hyphae nearly none. No autolytic excretions seen, coilings inconspicuous. No pigment, no distinct odour noted. No chlamydospores seen. Conidiation noted after 7–8 d at the proximal margin of the plate in shrubs/pustules with oblong or irregular outline, 0.3–1.5 mm diam, green after 10 d. No effuse conidiation seen. Pustules dry, transparent, with discontinuous surface, granular by densely disposed, individually projecting conidiophores. Conidiation developing from within, pustules often green within while still white outside; ends first sterile, becoming fertile, no elongations seen. Pustules formed on a stipe of variable length, asymmetrically branched into primary branches. Both stipe and primary branches thick-walled and 6–11(–12) μm wide, outer wall swelling in KOH. Further branching often paired and in right angles or slightly inclined upward, forming a loose reticulum with frequently thickened, globose branching points to 7–9 μm wide. Reticulum terminating in numerous, mostly symmetric conidiophores to 150 μm long, mostly narrower towards apex. Axes of conidiophores straight to distinctly sinuous, 3–5 μm wide, with short, 1–3 celled, terminal branches (2.0–)2.5–4.5 μm wide, often in right angles, paired or not. Phialides solitary or in whorls of mostly 2–3(–4, –5 in terminal whorls) on slightly thickened cells 2.5–4.5 μm wide, divergent, sometimes repetitive, i.e. cell below phialide thickened; forming conidia in large numbers. Branches and phialides becoming green in age. Phialides (5–)6–10(–16) × (2.7–)3.0–3.7(–4.3) μm, l/w 1.7–3.2(–5.4), (1.7–)2.0–2.7(–3.2) μm wide at the base (n=60), lageniform, straight or sinuous, equi- or inequilateral, widest median, neck variable, often long and distinct. Conidia (3.0–)3.3–4.2(–6.0) × (2.5–)2.8–3.3(–4.2) μm, l/w 1.1–1.4(–1.8) (n=67), subglobose or ellipsoidal, few oblong with median constriction, green, smooth, with minute guttules, scar indistinct; eventually adhering in chains.
On PDA after 72 h 6–8 mm at 15 °C, 22–24 mm at 25 °C, 19–21 mm at 30 °C; mycelium covering the plate after 1 wk at 25 °C. Colony circular, dense, concentrically zonate, of several broad, flat and hairy zones and narrow convex white zones; margin thick and coarsely wavy; marginal hyphae sinuous. Surface finely condensed, becoming floccose by collapsing aerial hyphae and conidiation; sometimes white mycelial spots with large stromata of pseudoparenchymatous brown tissue and moniliform hyphae formed, remaining sterile. Aerial hyphae numerous but loosely disposed, without defined orientation, denser along the margin, concentrated, longer, several mm high and forming thick strands in white narrow zones. Conidiation noted after 2–4 d, effuse, starting around the plug, spreading, of numerous conidiophores to 200 μm long, on high levels of aerial hyphae, concentrated in narrow white zones. Later conidiation also in crowded small pustules in narrow concentric zones; remaining white or becoming tardily and faintly yellow-greenish, 27CE4–5. Autolytic excretions inconspicuous, no coilings seen. No diffusing pigment formed, reverse dull yellowish 3AB3–4, 4B4, 3C4–6, finally brown 5–6CD7–8. Odour indistinct or slightly mushroomy.
On SNA after 72 h 7–8 mm at 15 °C, 24–26 mm at 25 °C, 16–18 mm at 30 °C; mycelium covering the plate after 1 wk at 25 °C. Colony hyaline, circular, dense, silky, indistinctly zonate, margin wavy; central surface hyphae soon empty, mycelium appearing loose; outer part denser, radially fan-shaped, white pustules to 1.5 mm diam appearing at the proximal margin and in an ill-defined concentric zone, finally across the entire colony, becoming dark green, 28–29EF5–8, 27F5–8. Conidiation noted after 5–6 d, green after 7–8 d. Aerial hyphae scant, short. Autolytic excretions inconspicuous, yellow; no coilings seen. No pigment, no distinct odour noted. Chlamydospores (6–)7–10(–13) × (3.5–) 5–9(–11) μm, l/w (0.8–)1.0–1.7(–2.3) (n=30), noted after 2 wk, uncommon, intercalary, rarely terminal, subglobose, ellipsoidal, rectangular, or pyriform, smooth, sometimes 2–celled.
Habitat: on branches of Fagus sylvatica.
Distribution: Europe (Austria).
Holotype: Austria, Niederösterreich, Mödling, close to Sulzwiese at Richardshof, MTB 7963/2, 48°03'48” N, 16°16'12” E, elev. 400 m, on decorticated branch of Fagus sylvatica 5–6 cm thick in leaf litter, holomorph, soc. effete Hypoxylon sp. and rhizomorphs, 9 Oct. 2005, H. Voglmayr (W.J. 2861), WU 29107, ex-type culture CBS 122769 = C.P.K. 2421. Holotype of Trichoderma parepimyces isolated from WU 29107 and deposited as a dry culture with the holotype of H. parepimyces as WU 29107a.
Other specimens examined: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Trieblacher Weg, close to the Bauhof, at forest margin/roadside, MTB 9452/4, 46°32'31” N, 14°25'45” E, elev. 580 m, on partly decorticated branch of Fagus sylvatica 2 cm thick, in leaf litter, on wood, soc. rhizomorphs, effete pyrenomycete, Corticiaceae, Ascodichaena rugosa on bark, holomorph, 10 Jul. 2007, W. Jaklitsch, W.J. 3121 (WU 29108, culture CBS 122768 = C.P.K. 3130). Niederösterreich, Baden, Heiligenkreuz, Siegenfeld, NE slope of the Schaberriegel, MTB 7963/3, elev. 380 m, on branch of Fagus sylvatica 10–15 cm thick, covered by leaves, soc. Trichoderma tomentosum, 14 Sep. 2008, H. Voglmayr, W.J. 3215 (WU 29399, culture C.P.K. 2562).
Notes: Hypocrea parepimyces is a species of the Harzianum complex difficult to recognise. Except for more orange-brown colours, stromata of this species are virtually indistinguishable from those of H. brunneoviridis, while they share also elements of H. epimyces (discoidal stromata) and H. lixii (olive colours in young stromata). The ostiolar dots in H. parepimyces are generally more distinct than in these species. Trichoderma parepimyces is similar to T. epimyces, trichoderma- to pachybasium-like on CMD, but no effuse conidiation has been seen. Small pustules randomly distributed over the entire growth plate differ from all species mentioned above, including T. alni with larger pustules disposed more or less in concentric zones. The formation of concentric zones on PDA is reminiscent of T. epimyces, but the conidiation remains white or turns only tardily and faintly greenish (a diagnostic trait).
Hypocrea parestonica Jaklitsch, sp. nov. MycoBank MB513188. Fig. 28.
Fig. 28.
Teleomorph of Hypocrea parestonica. a–d. Fresh stromata (a, b. immature, c, d. mature). e–i. Dry stromata (e. immature, f. stroma initial, g–i. mature). j. Stroma surface in face view. k. Mature stroma in 3 % KOH after reconstitution in water. l. Perithecium in section. m. Cortical and subcortical tissue in section. n. Subperithecial tissue in section. o. Base of stroma in section showing two setae of the host. p–r. Asci with ascospores. a–d, g, i–o, q. 29110. e, r. WU 29111. f, h, p. WU 29109. Scale bars: a, e, g, h = 0.2 mm. b–d, f, i = 0.4 mm. j, m, r = 15 μm. k = 0.6 mm. l, o = 30 μm. n = 20 μm. p, q = 10 μm.
Anamorph: Trichoderma parestonicum Jaklitsch, sp. nov. MycoBank MB513189. Fig. 29.
Fig. 29.
Cultures and anamorph of Hypocrea parestonica. a–c. Cultures (a. CMD, 21 d, 15 °C. b. PDA, 7 d, 25 °C. c. SNA, 24 d, 15 °C). d. Anamorph on Hymenochaete tabacina. e. Conidiation pustule (SNA, 15 °C, 21 d). f. Conidiophore on the periphery of young pustule (SNA, 15 °C, 8 d). g–j. Elongations (10–13 d. i, j. phialides). k, l. Conidiophores from pustules (10 d). m, n. Conidia (10–13 d). o. Phialides (13 d). p. Chlamydospores (SNA, 25 °C, 14 d). g–o. CMD, 25 °C. a–c, e–i, k, l, n, p. C.P.K. 2427. d. WU 29109. j, m, o. CBS 120636. Scale bars: a–c = 20 mm. d = 1 mm. e = 0.6 mm. f = 90 μm. g, h = 30 μm. i, l, o = 10 μm. j, p = 20 μm. k, m, n = 5 μm.
Teleomorphosis similis Hypocreae estonicae, differt in stromatibus siccis perrubris. Stromata typice in basidiomatibus Hymenochaetae tabacinae, solitaria vel gregaria, pulvinata vel turbinata, 1–3 mm diam, 0.5–1.5 mm alta, rubra. Asci cylindrici, (80–) 88–104(–120) × (5.0–)5.3–6.2(–7.0) μm. Ascosporae bicellulares, verruculosae, virides, ad septum disarticulatae, pars distalis subglobosa vel cuneata, (3.7–) 4.3–5.0(–6.0) × (3.0–)4.0–4.5(–5.2) μm, pars proxima oblonga vel cuneata vel subglobosa, (4.0–)4.3–5.5(–6.5) × (3.0–)3.3–4.0(–4.5) μm. Anamorphosis Trichoderma parestonicum. Conidiophora effusa absentia vel rara. Pustulae cum elongationibus fertilibus. Phialides in elongationibus lageniformes vel subulatae, (7–)9–17(–26) × (2.3–)2.5–3.5(–4.0) μm. Phialides in pustulis lageniformes vel ampulliformes, (4.5–)5.5–7.5(–8.5) × (2.7–)3.0–3.7(–4.0) μm. Conidia luteo-viridia, ellipsoidea, glabra, (3.0–)3.4–4.3(–5.4) × (2.3–)2.5–3.0(–3.5) μm.
Stromata when fresh 1–3 mm diam, 0.5–1.5 mm thick, usually in large numbers; solitary, gregarious or aggregated in small numbers, pulvinate or turbinate; outline circular to irregular, margin free. Surface smooth, iridescent, appearing finely downy when young. Ostiolar dots inconspicuous, broad, reddish brown and only slightly darker than stroma surface when immature, broad dull green to black spots when mature, plane or slightly projecting. Colour light reddish brown when young, later dull reddish to reddish brown 7B5, 8CD5–6, 9CD5–8.
Stromata when dry (0.4–)0.8–1.7(–2.4) × (0.3–)0.7–1.5(–2.0) mm, 0.2–0.5(–0.8) mm thick (n=65), macroscopically difficult to see, flat pulvinate to turbinate or depressed in the centre (discoidal), broad or narrowly attached with constricted base. Outline circular, angular or lobed. Margin free, thick, rounded or acute, sometimes elevated. Sterile sides or constricted bases when visible straight or attenuated downwards, covered with white or rust hyphae when young, later glabrous and orange or reddish brown. Surface flat, convex or concave, first with anamorph floccules, later smooth, rugose or finely tubercular, sometimes with rust hairs. Ostiolar dots (30–)38–72(–118) μm (n=90) diam, generally inconspicuous, flat to slightly convex, hardly visible, poorly delimited or appearing as fine dots under strong magnification, dark reddish brown when immature, dark green to black when mature. Colour difficult to assess due to limited macroscopic visibility, first white, becoming rosy plus yellow from the centre, later dull red to dark orange-brown or reddish brown 9DE6–8, 8CF5–8 or darker; older stromata sometimes paler, more orange, 7B5–6 or rust to dull orange-red, 7–8CD7–8, homogeneous between black dots. Sometimes stroma interior brownish when mature or old. Spore powder dark green to black. After reconstitution in water stroma surface more convex, smooth, margin thick, round, wavy to angular, ostiolar dots more distinct, more convex. No colour change in 3 % KOH, ostiolar dots larger, dark brown.
Stroma anatomy: Ostioles (60–)76–97(–103) μm long, projecting to 18(–23) μm, (28–)35–52(–66) μm wide at the apex (n=30), with cylindrical to clavate apical cells 2–4 μm wide; ostiolar margin indistinct. Perithecia (165–)200–260(–280) × (130–)140–195(–237) μm (n=30), subglobose or flask-shaped, crowded or not, 4–7 per mm, peridium (15–)18–26(–30) μm (n=30) wide at the base, (6–)12–20(–22) μm (n=30) at the sides. Cortical layer (17–)20–34(–44) μm (n=30) thick, a bright yellow to orange t. angularis of distinct, thick-walled, isodiametric, globose to angular cells (5–)6–13(–14) × (4–)5–10(–14) μm (n=33) in face view and (4–)5–10(–13) × (3–)4–7(–8) μm (n=33) in vertical section; lateral cortex with hyphal outgrowths towards base; upper surface covered by spots of amorphous matter and yellowish, cylindrical, smooth hairs (9–)11–20(–23) × (2.3–)2.5–3.2(–3.6) μm (n=10), often collapsed and agglutinated in fascicles. Subcortical tissue a subhyaline to pale yellowish t. angularis of thin-walled cells (4.5–)7–14(–17) × (4–)5–9(–12) μm (n=33), interspersed by thin-walled (sub-)hyaline hyphae (2–)3–7(–9) μm (n=30) wide. Subperithecial tissue a hyaline to pale yellowish t. angularis-epidermoidea of variable thin-walled cells (5–)8–19(–24) × (4–)5–10(–13) μm (n=30), penetrating the fungal host. Asci (80–)88–104(–120) × (5.0–)5.3–6.2(–7.0) μm, stipe (4–)11–23(–36) μm long (n=70); often containing only 6 or 7 mature ascospores. Ascospores olive-green, coarsely tubercular; cells dimorphic, distal cell (3.7–)4.3–5.0(–6.0) × (3.0–)4.0–4.5(–5.2) μm, l/w (0.9–)1.0–1.2(–1.4) (n=90), (sub-)globose, in apical position often wedge-shaped, proximal cell (4.0–)4.3–5.5(–6.5) × (3.0–)3.3–4.0(–4.5) μm, l/w (1.0–)1.2–1.5(–2.0) (n=90), oblong to wedge-shaped, or subglobose.
Anamorph on natural substrate pulvinate or effuse, few mm to 2 cm diam, light to medium green, also with yellowish spots, hairy.
Cultures and anamorph: Optimum growth at 25 °C on all media; limited growth at 30 °C on all media; no growth at 35 °C. On CMD after 72 h 15–17 mm at 15 °C, 28–37 mm at 25 °C, 15–30 mm at 30 °C; mycelium covering the plate after 6–10 d at 25 °C. Colony hyaline, of loose mycelium with notable difference in width among hyphae, not zonate; margin ill-defined, lobed; central hyphae dying soon, appearing empty, white spots of clumped mycelium formed within agar, white tufts to ca. 1.5 mm diam appearing mainly at the proximal and distal margins of the colony, irregularly disposed, becoming pale, finally dark green. Aerial hyphae nearly lacking. No autolytic excretions noted, coilings absent or rare. No distinct odour, no pigment noted. Chlamydospores noted after 2–4 wk, rare, minute, globose. Effuse conidiation lacking or seen as scant short conidiophores with minute conidial heads on and around the plug. Conidiation in tufts/pustules noted after 11–17 d (after 6–7 d at 30 °C), green after (1–)2–3 wk. Pustules/tufts irregularly distributed, of irregular outlines, with granulose to velvety surface, often white margins, and typically loose texture, 0.3–1.5 mm diam, aggregating to 4 mm diam, with elongations bearing minute green terminal heads, projecting from the entire surface. Various green and yellow colour tones noted within pustules, conidia formed from within pustule, first hyaline to yellow, becoming green. Structure of pustules variable, of a loose reticulum of short, irregularly arranged or long, radially arranged and straight primary axes to 0.8 mm long; with numerous unpaired branches mostly in right angles, forming short conidiophores to 100 μm long with densely disposed clusters of short terminal side branches in right angles to the axes, bearing whorls of (2–)4–6 phialides and numerous minute dry conidial heads. Conidiophores and origins of phialides 3–5 μm wide. Phialides in pustules and on bases of elongations (4.5–)5.5–7.5(–8.5) × (2.7–) 3.0–3.7(–4.0) μm, l/w (1.4–)1.6–2.3(–2.7), (2.0–)2.2–3.0(–3.4) μm wide at the base (n=60), plump lageniform to ampulliform, often with narrow neck and base and conspicuous thickening below or in the middle, often inequilateral and curved upwards. Conidia (3.0–) 3.4–4.3(–5.4) × (2.3–)2.5–3.0(–3.5) μm, l/w (1.1–)1.2–1.5(–1.8) (n=63), yellow-green, smooth, ellipsoidal, less commonly oblong or oval, thick-walled, with few minute guttules, scar indistinct or distinct and flat/truncate. Elongations straight to sinuous, (30–)50–250(–300) μm long, first sterile, becoming fertile, with phialides or short branches on 1–3 apical levels. Terminal phialides solitary or in whorls of 2–5(–6) in steep angles, bearing wet conidial heads to 15 μm diam. Also along elongations some loosely disposed, mostly solitary, lageniform phialides or 1-celled, typically unpaired, right-angled branches noted. Phialides on elongations (7–)9–17(–26) × (2.3–)2.5–3.5(–4.0) μm, l/w (2.0–)2.8–6.1(–9.4), (1.8–)2.0–2.7(–3.3) μm wide at the base (n=67), lageniform or subulate, straight or curved upwards, less commonly sigmoid, without conspicuous thickenings. In younger pustules less difference in shape of phialides between elongations and in pustules noted; i.e. phialides becoming thicker/plumper in pustules with age. At 15 °C colony mottled and strongly lobate; conidiation in broad roundish pustules to ca. 5 mm diam and 1 mm high, confluent to 11 mm, becoming yellowish green 28D4–6, with fertile elongations to 350 μm long. At 30 °C colony with ill-defined margin, conidiation scant in small densely aggregated tufts remaining hyaline, reverse pale citrine 1–2A2–3 below tufts.
On PDA after 72 h 14–16 mm at 15 °C, 32–36 mm at 25 °C, 7–20 mm at 30 °C; mycelium covering the plate after 6–7 d at 25 °C. Colony circular, dense, mycelium of wide primary hyphae and numerous short, narrow, densely intertwined secondary hyphae; margin well-defined. Centre flat, with little aerial hyphae, turning yellow 3A4–5. Residual colony becoming thick due to abundant aerial hyphae, forming a radial, centrally stellate, surface structure. Dense radial patches formed by short, narrow, sessile aerial hyphae, superposed by long and wide, radially arranged aerial hyphae with numerous thin side branches; later aggregating to strands with coilings and numerous drops. Indistinctly separated, concentric zones formed, becoming yellow from the centre; surface becoming floccose due to collapsed aerial hyphae. Later minute greenish micropustules formed in the centre, residual surface dull yellowish greenish, floccose with irregular conidiation tufts; also white, fertile, irregularly disposed spots and tufts formed, sometimes also light brown sterile stromata 5 mm diam, >1 mm high after ca. 3 wk. Conidiation effuse, first noted after 4–5 d at the proximal margin and at the beginning of the white stellate mat of aerial hyphae, 6–8 mm from the plug; becoming greenish after 3 wk or remaining white. Conidiophores sessile and on aerial hyphae, minute, simple, verticillium- to pachybasium-like, thick, with paired branching and ampulliform phialides, ascending on aerial hyphae and spreading across the colony. Autolytic excretions inconspicuous, minute and frequent at 30 °C, coilings inconspicuous. Reverse bright yellow, 2A4, 3A4–6, around the plug, colour spreading, becoming dull yellow 4B4–5 to brown in aged cultures. Odour indistinct, mushroomy or sour. At 15 °C more irregular growth; colony dense, with ill-defined, white, hairy margin; hyphae becoming submoniliform. Aerial hyphae forming a white tomentum. Conidiation effuse, concentric, starting 5–6 mm from the plug, spreading, remaining colourless. Coilings in aerial hyphae conspicuous. Reverse yellowish 4A3–4. At 30 °C colony irregular, thick, dense, whitish downy, growth soon stopping, surface and reverse becoming bright yellow 3A5–8; autolytic excretions numerous, minute, brownish. Odour must-like. No conidiation seen.
On SNA after 72 h 11–14 mm at 15 °C, 25–34 mm at 25 °C, 5–13 mm at 30 °C; mycelium covering the plate after 6–9 d at 25 °C. Colony hyaline, similar to CMD, but margin whitish due to densely intertwined, reticulate hyphae; margin ill-defined to lobate, surface hyphae soon dead, appearing empty; colony therefore becoming conspicuously transparent. White pustules mainly formed on lateral lobes, later irregularly distributed across the entire colony, concentrated in 2(–3) irregular concentric zones in distal areas of the plate, becoming (yellowish) green, 28CD5–6, 28EF5–8, 27DE4–6, reaching 2–4 mm diam, confluent to 6–7 mm length. Aerial hyphae inconspicuous, long and more frequent at the margins. Conidiation noted after 6–8 d in small shrubs and in pustules with straight to sinuous elongations, pachybasium-like, green after 8–10 d. Autolytic excretions inconspicuous, more frequent at 30 °C, coilings inconspicuous. No pigment, no distinct odour noted. Chlamydospores noted after 4–7 d, globose, angular or oblong, (3.5–)4.5–8(–10) × (3.5–)4–6(–7) μm, l/w 0.9–1.6(–2.1) (n=30), spreading from the centre, abundant, terminal and intercalary. At 15 °C colony similar to CMD but denser, with pustules smaller and more numerous than on CMD. Conidiation abundant, pachybasium-like in green, 28CE4–6, 28F6–8, 27DF4–6, pustules with fertile elongations, arranged in broad concentric zones; conidia adhering in chains with age. No autolytic excretions, no coilings, no pigment, no distinct odour noted. Chlamydospores frequent, minute. At 30 °C growth rate soon decreasing; conidiation effuse, scant; autolytic excretions moderate, minute, brownish; chlamydospores common.
Habitat: on and around basidiomes of Hymenochaete tabacina.
Distribution: Central Europe (Austria, Germany).
Holotype: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Tumpfi, MTB 9452/4, 46°32`35“N, 14°25`32“E, elev. 565 m, on Hymenochaete tabacina/Salix cinerea and Corylus avellana, twigs 1–2 cm thick, soc. Steccherinum ochraceum, effete Lophiostoma sp., 25 Sep. 2006, H. Voglmayr & W. Jaklitsch (W.J. 2987), WU 29110, ex-type culture CBS 120636 = C.P.K. 2474. Holotype of T. parestonicum isolated from WU 29110 and deposited as a dry culture with the holotype of H. parestonica as WU 29110a.
Other specimens examined: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Gupf (Writze), MTB 9452/2, 46°33'04” N, 14°27'11” E, elev. 730 m, on Hymenochaete tabacina/Corylus avellana, branches 9–10 cm thick, holomorph, soc. Hypoxylon fuscum, 29 Oct. 2005, H. Voglmayr & W. Jaklitsch, W.J. 2874 (WU 29109, culture C.P.K. 2427). Germany, BW, N v. Schwäbisch Hall, NSG “Kupfermoor”, elev. 375 m, on Hymenochaete tabacina/Salix cinerea and bark around basidiomata, holomorph, soc. Rhizodiscina lignyota, moss, lichens, white rhizomorphs, 8 Sep. 2007, L. Krieglsteiner (WU 29111, culture C.P.K. 3167).
Notes: Hypocrea parestonica is the Central-European sister species of the North-European H. estonica and the North-American H. ceramica (sensu Chaverri & Samuels 2003), morphologically hardly distinguishable from the latter two, although immature fresh stromata of H. parestonica have a less bright and iridescent surface. Immature stromata are also reminiscent of H. tremelloides. Dry mature stromata are in average (particularly in WU 29110) darker and deeper red or more dull reddish brown than those of H. estonica. Dull stroma colours are more in accord with the holotype of H. ceramica. On CMD (at 25 °C) T. parestonicum differs from T. estonicum in scant or nearly lacking effuse conidiation. In both species pustules are formed late, in T. estonicum they are pale yellowish green or pale green, in T. parestonicum medium green, while in T. ceramicum (CBS 114576) pustules appear already after four days, become dark green and form distinct concentric rings on CMD. In addition, colony morphology on PDA differs among these species.
Hypocrea phyllostachydis Chaverri & Candoussau, Mycol. Prog. 3: 33 (2004). Fig. 30.
Fig. 30.
Teleomorph of Hypocrea phyllostachydis (holotype BPI 802617). a–c. Dry stromata (a. immature, b, c. mature). d. Stroma surface in face view. e, f. Asci with ascospores (f. in cotton blue/lactic acid added after examination in 3 % KOH). Scale bars: a = 0.15 mm. b, c = 0.3 mm. d–f = 10 μm.
Anamorph: Trichoderma phyllostachydis Chaverri & Samuels, Stud. Mycol. 48: 80 (2003).
For descriptions and illustrations see Chaverri et al. 2004 and Chaverri & Samuels 2003.
A short redescription of stromata based on re-examination of the holotype is given here.
Stromata when dry (0.4–)0.5–1.2(–1.4) × (0.3–)0.5–1(–1.2) mm, 0.2–0.5 mm thick (n=16), solitary or gregarious, broadly attached, pulvinate or button-like, more or less circular in outline; with yellowish base mycelium, and orange-brown, downy surface when young. When mature edge free; sides vertical, concolorous with the surface or with concolorous hairs. Surface flat, smooth and finely velutinous, with scattered, plane or convex, circular or diffuse ostiolar dots, first light brown and only slightly darker than stroma surface, later black. Fertile part sometimes laterally projecting over the base. Stroma colour macroscopically dark brown to black, honey-coloured, light to dark brown or cinnamon-brown in the stereo-microscope.
Stroma anatomy: Cortical tissue a t. angularis of thick-walled, yellow cells (5–)7–11(–14) × (3–)5–8(–10) μm (n=30) in face view and (6–)7–15(–24) × (4–)6–10(–14) μm (n=35) in section, below an amorphous layer of collapsed cells/hyphae in lactic acid; pigment inhomogeneously distributed. Cortical tissue and perithecial wall reddish orange in KOH. Subcortical tissue similar, but containing some hyphae and cells hyaline. Subperithecial tissue a t. angularis of thin-walled hyaline cells. Asci (70–)74–81(–84) × (3.5–)4.0–4.5(–4.6) μm, stipe (7–)10–18(–20) μm long (n=20). Ascospores (olive-) green, brown in KOH, blue in cotton blue/lactic acid, thick-walled, verruculose; cells dimorphic, distal cell (3.0–)3.2–3.6(–3.8) × (2.9–) 3.1–3.4(–3.6) μm, l/w (0.9–)1.0–1.1(–1.3) (n=30), (sub-)globose, proximal cell (3.3–)3.5–4.3(–5.0) × (2.5–)2.7–3.0 μm, l/w 1.2–1.6(–1.9) (n=30), oblong to nearly wedge-shaped or subglobose.
Habitat: on decaying culms of the bamboo Phyllostachys bambusoides.
Distribution: France.
Holotype: France. Pyrénées Atlantiques, Parc Du Chateau D'Uhart Mixe, 20 km south of Sauveterre, near Spanish frontier; on Phyllostachys bambusoides; 26 Sep. 1992; F. Candoussau & J.P. Chaumeton F.C. 249 (BPI 802617; ex-type culture G.J.S. 92-123 = ATCC MYA-3066 = CBS 114071 = DAOM 232101; holotype of Trichoderma phyllostachydis: dry culture BPI 843665).
Other known specimen (not examined): France. Pyrénées Atlantiques: Osseran, Sauveterre, Bambous d'Osseran; on Phyllostachys bambusoides; 23 Aug. 1992; J.P. Chaumeton FC 249 (BPI 802861; culture: G.J.S. 92-81).
Notes: Hypocrea phyllostachydis is the only green-spored species of Hypocrea known to occur on bamboo in Europe.
Hypocrea sinuosa P. Chaverri & Samuels, Stud. Mycol. 48: 81 (2003). Fig. 31.
Fig. 31.
Teleomorph of Hypocrea sinuosa. a–e. Fresh stromata (a. habit, b, c. immature, d, e. mature). f–k. Dry stromata (f. stroma initial, g. immature, h–k. mature). l. Mature stroma in 3 % KOH after reconstitution in water. m, n. Dry stromata in side view (m. mature, n. immature). o. Stroma surface in face view. p. Perithecium in section (in 3 % KOH!). q. Cortical and subcortical tissue in section. r. Subperithecial tissue in section. s. Base of stroma in section. t–v. Asci with ascospores. w. Stroma surface with two hairs (and ascospore cells). x. Ascospores showing verrucae. a–f, j, t. WU 29114. g. WU 29123. h, l–s, u, w, x. WU 29112. i, v. WU 29116. k. WU 29122. Scale bars: a = 2.5 mm. b, g, j, l = 0.5 mm. c–e = 1.5 mm. f, m, n = 0.2 mm. h, i, k = 0.4 mm. o, q, s, u–w = 15 μm. p = 40 μm. r = 25 μm. t = 10 μm. x = 5 μm.
Anamorph: Trichoderma sinuosum P. Chaverri & Samuels, Stud. Mycol. 48: 81 (2003). Fig. 32.
Fig. 32.
Cultures and anamorph of Hypocrea sinuosa. a–c. Cultures at 25 °C after 14 d (a. On CMD. b. On PDA. c. On SNA). d. Anamorph on natural substrate. e. Conidiation tuft (SNA, 7 d). f. Conidiophore of effuse conidiation on aerial hypha (SNA, 8 d). g–l. Conidiophores from pustules (SNA, 7-9 d). m, n. Conidia (SNA, 7–9 d). o. Mycelial densification below pustule (CMD, 14 d). p, q. Chlamydospores (SNA, 11 d). e–q. All at 25 °C. a–c, o. CBS 121129. d. WU 29120. e, h, k–m. C.P.K. 3151. f, g, i, j, n, p, q. C.P.K. 1595. Scale bars: a–c = 20 mm. d = 1.6 mm. e = 0.2 mm. f, g = 30 μm. h–j, l = 20 μm. k, m, n = 10 μm. o = 90 μm. p, q = 5 μm.
Stromata when fresh 0.5–2.5 mm diam, 0.5–1.2 mm thick, solitary, gregarious or densely crowded, discrete/not confluent, pulvinate or turbinate; outline circular; surface convex to flat; perithecial contours prominent and bright yellow at least when young, later green by ascospores, on white to yellowish surface. Resulting colour yellow 1A4–5, later light yellow-green 29CD5–6, green 27D4–6 when mature.
Stromata when dry (0.3–)0.4–1.2(–2) × (0.2–)0.3–1.1(–1.7) mm, 0.2–0.5(–0.9) mm thick (n=90); mostly turbinate or short stipitate, more pulvinate when young, with 1 to ca. 50 perithecia per stroma; sometimes 2–3 stromata on a common, broadly attached basal stroma. Outline circular or angular. Surface plane or convex, uneven to tubercular due to perithecial mounds, finely whitish floccose or covered by green anamorph floccules when young. Margin free, elevated, wavy due to projecting perithecia; fertile part often projecting over sterile sides. Sides attenuated downward or vertical, white or yellow, smooth or with white mycelium at the base. Perithecia distinctly prominent, visible as large, diffuse, convex dots (31–)72–163(–235) μm (n=120) diam, less prominent when mature, with surface smoother or concealed by abundant dark green spore powder. Ostioles typically invisible. Colour and development: first white mycelium, becoming compacted into light yellow, short cylindrical buttons without dots, with perithecial dots bright yellow, 1–2A2–4, 3A3–6, 4A3–4; becoming olive, dull greenish yellow (green on yellow background), 3B4–6, 2C4–5, 1B3–4, green 25E4–6, dark green with olive tone, 27–30F5–8, 29–30E5–8, 1EF5–8, 1–2B4(5), finally black when covered by spore powder. Perithecia typically more intensely or darker (yellow or yellow-brown) coloured than the white to pale yellow stroma surface; more orange when developed in hot weather and eaten by mites. No colour change noted after addition of 3 % KOH. Anamorph effuse, powdery, dark green 25F6–8.
Stroma anatomy: Ostioles (60–)66–83(–92) μm long, plane with the surface or projecting to 28(–42) μm, (28–)30–51(–62) μm wide at the apex (n=31), conical, apical cells sometimes clavate and to 5 μm wide. Perithecia (195–)220–275(–300) × (115–)150–212(–250) μm (n=31), flask-shaped or globose, crowded, 6–7 per mm stroma. Peridium 18–28(–36) μm (n=31) wide at the base, (7–)11–18(–21) μm (n=31) at the sides, hyaline to pale yellowish in lactic acid and KOH. Cortical tissue present only on fertile layer, (15–)18–30(–40) μm (n=30) thick, a t. angularis of thin-walled, (sub-)hyaline, isodiametric or oblong cells (4–)8–17(–24) × (3–)5–12(–16) μm (n=30) in face view and (4–)6–16(–21) × (3–)5–8(–9) μm (n=30) in vertical section. Surface of mature stroma not smooth due to loose hyaline cells and undifferentiated hyphae or hairs. Hairs (9–)13–26(–40) × (3–)4–7(–8) μm (n=30), scant, 1–4 celled, with constricted septa. Subcortical tissue when present, a t. angularis of thin-walled, hyaline cells (4–)6–13(–18) × (3–)4–8(–11) μm (n=30), variably intermixed with thick-walled hyaline hyphae (2–)3–8(–13) μm (n=30) wide. Subperithecial tissue a t. angularis of variable, thick-walled hyaline cells (5–)9–34(–57) × (4–)7–17(–26) μm (n=30), not covered by cortex at sides, but often terminating in e.g. fascicular hyphae causing a floccose surface at lower levels of the stroma. Base of small subperithecial cells and thick-walled hyphae (2.5–)3.5–5.5(–7.0) μm (n=30) wide. Asci (87–)95–113(–128) × (5.5–)6–7(–8) μm, stipe (4–)9–18(–32) μm long (n=60). Ascospores dull green to olive, brown in KOH, distinctly verrucose, warts to 0.5 μm long; cells dimorphic but similar in size, distal cell (4.0–)4.8–5.6(–6.5) × (3.5–)4.5–5.3(–5.8) μm, l/w (0.9–)1.0–1.1(–1.3) (n=120), (sub-)globose, proximal cell (3.8–)4.8–6.2(–8.4) × (3.3–)4.0–4.7(–5.3) μm, l/w (0.9–)1.1–1.5(–2.1) (n=120), oblong, plump wedge-shaped or subglobose; contact area often distinctly flattened.
Cultures and anamorph: Optimum growth at 25–30 °C on all media; no growth at 35 °C. On CMD after 72 h 11–20 mm at 15 °C, 48–61 mm at 25 °C, 25–52 mm at 30 °C; mycelium covering the plate after 4 d at 25 °C. Colony circular, homogeneous, not zonate, mycelium loose; primary, radially orientated hyphae conspicuously thick (>10 μm), little on the agar surface. Aerial hyphae nearly lacking. Minute but characteristic spots of strongly branched, densely intertwined, thin hyphae formed within agar prior to the formation of tufts/pustules directly above them. Tufts to 1.5 mm diam, fluffy, becoming green 27F7–8. Conidiation noted after (5–)7–14 d, green after 9–14 d. Effuse conidiation on aerial hyphae lacking or scant, of few scattered minute wet conidial heads on long narrow phialides. Pustulate conidiation mostly along the margin of the colony, dry, dense, on sinuous conidiophores; conidia finally adhering in chains. Chlamydospores infrequent, noted after 2 wk or lacking. Autolytic excretions inconspicuous, sometimes abundant at 15 °C; no coilings seen. No pigment, no distinct odour noted. At 15 °C conidiation in green, 26D3–4, pustules; effuse conidiation slightly better developed than at 25 °C, acremonium- to verticillium-like, conidia formed in wet green heads to 30 μm diam.
On PDA after 72 h 9–14 mm at 15 °C, 41–45 mm at 25 °C, 28–47 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony circular, compact, margin somewhat wavy. Thick surface hyphae radially oriented, forming strands toward the margin, densely cemented within the colony; surface partly and indistinctly zonate by aerial hyphae. Aerial hyphae abundant, of two kinds, i.e. numerous, short spinulose, in dense lawns, superposed by long, narrow, loosely disposed hyphae forming strands, ascending to and becoming fertile to the lid of the Petri dish in the colony centre; resulting white to yellowish cottony tomentum finally collapsing, becoming floccose. Conidiation noted after 2–6 d, effuse, first sessile, simple, with long phialides, spreading from the centre across the colony and ascending on long aerial hyphae, also in some pustules 0.5–1 mm diam appearing along (distal) margin; colourless, typically not becoming green, only greenish in the stereo-microscope. Autolytic excretions inconspicuous, but often abundant at 15 and/or 30 °C; no coilings seen. No diffusing pigment noted, reverse whitish to yellowish 3A3, 4B4, 2B4–5. No distinct odour noted.
On SNA after 72 h 10–19 mm at 15 °C, 32–48 mm at 25 °C, (3–)24–40 mm at 30 °C; mycelium covering the plate after 5–8 d at 25 °C. Colony circular, homogeneous, not zonate, margin somewhat wavy. Hyphae curly, loosely disposed, nearly exclusively thick primary hyphae on the agar surface. Aerial hyphae scant, sometimes frequent along the colony margin. Mycelium degenerating and irregularly disposed pustules to 1.5 mm diam appearing mainly on the distal colony margin, often in a broad belt; first white, becoming green 27–28E6–7. Autolytic excretions often abundant at all temperatures; coilings lacking or frequent at the margins. No pigment, no distinct odour noted. Conidiation noted after 5–6 d, green after 6–8 d, first effuse, scant, sessile or on aerial hyphae, soon on tufts/pustules, degenerating soon. Pustules fluffy with hairy or velvety surface, formed on stipes 10–14 μm wide at the base (somewhat compressed in mounts), and 7–9 μm wide close to the pustule; interior ill-defined, branching loose, asymmetric, typically no main axis and no well-defined conidiophores notable. Conidiophores 3–5 μm wide, often with thickenings to 7(–9) μm; ends 2.5–3 μm wide, typically distinctly sinuous and projecting as short hairs to ca. 100 μm from pustule surface. Branches and phialides in right angles or inclined slightly upwards, typically unpaired with some tendency to be paired toward conidiophore ends. Phialides solitary or in whorls of 2–3, sometimes cruciform, formed on cells 2.5–5 μm wide, sometimes repetitive. Conidia formed in wet heads to 20 μm diam. Phialides (5.5–)7.5–12(–16) × (2.5–)3.0–3.5(–4.0) μm, l/w (1.6–)2.2–3.9(–5.5), (1.2–)1.8–2.7(–3.2) μm wide at the base (n=60), lageniform, less commonly ampulliform, often inequilateral, curved or sinuous, neck often long and abruptly constricted, sometimes base long and thin, thickening indistinct, variable, mostly (sub-)median. Conidia (3.5–)4.3–5.0(–6.0) × (2.8–)3.0–3.5(–4.2) μm, l/w (1.1–)1.3–1.6(–1.9) (n=60), ellipsoidal, green, smooth, with fine or no guttules, scar indistinct. Chlamydospores (7–)8–12(–13) × (6–)7–10(–11) μm, l/w (0.9–)1.0–1.4(–1.8) (n=30), noted after 6–10 d, abundant (also at 30 °C), globose or ellipsoidal, verrucose (including adjacent hyphal cells), thick-walled, intercalary and terminal, sometimes 2–3 celled.
Habitat: on strongly decomposed wood and bark of deciduous and coniferous trees, often accompanied by a long-necked coelomycete, various other fungi, algae and moss.
Distribution: North America; Europe (Austria, Czech Republic, France, Germany, Netherlands, Ukraine, UK), widely distributed and locally common.
Holotype: USA, New York, Green County, Diamond Notch, 74°10' W, 42°10' N, elev. 500 m; on well-rotten decorticated wood; 26 Sep. 1998; P. Chaverri, G.J. Samuels, H.L. Chamberlain (BPI 843649; ex-type culture P.C. 8 = CBS 114247 = DAOM 232839. holotype of T. sinuosum: dry culture BPI 843666).
Other specimens examined: Austria, Niederösterreich, Melk, Leiben, Weitental, at Hofmühle, MTB 7757/2, 48°14'51” N, 15°17'23” E, elev. 270 m, on decorticated branch of Carpinus betulus 3–5 cm thick, on wood, soc. Exidia glandulosa, 25 Jul. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2537 (WU 29115, culture C.P.K. 2006). Melk, Weins, eastern access, at road to Persenbeug, MTB 7756/3, 48°12'00” N, 15°02'39” E, elev. 290 m, on branch of Fagus sylvatica 3 cm thick, on black wood, overgrowing leaves, soc. Propolis versicolor, Diatrype flavovirens, hyphomycetes, a myxomycete, holomorph, 25 Jul. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2543 (WU 29116, culture C.P.K. 2007). Wien Umgebung, Mauerbach, Friedhofstrasse, MTB 7763/1, 48°15'18” N, 16°10'12” E, elev. 325 m, on decorticated branch and log of Carpinus betulus 8–20 cm thick, on wood, leaves and Armillaria rhizomorphs, soc. Hypoxylon howeianum, H. rubiginosum, effete ?Chaetosphaeria sp., 18 Jul. 2004, W. Jaklitsch, W.J. 2528 (WU 29114, culture CBS 121129 = C.P.K. 1612). Ten additional records from the same area. Mauerbach, Hainbuch, MTB 7763/1, 48°17'07” N, 16°09'57” E, elev. 320 m, on branches of Fagus sylvatica 8–16 cm thick, on wood, soc. white mould, effete Eutypa lata, 30 Sep. 2006, W. Jaklitsch, W.J. 2995 (WU 29123, culture C.P.K. 3120). Pressbaum, Rekawinkel, forest path south from the train station, MTB 7862/1, 48°10'46” N, 16°01'55” E, elev. 370 m, on corticated branches of Fagus sylvatica 8–12 cm thick, on bark, wood, overgrowing leaves, Corticiaceae and Hypoxylon fragiforme, soc. Phlebiella vaga, Corticiaceae, hyphomycetes, Armillaria rhizomorphs, Stereum hirsutum and Tulasnella violea, holomorph, 18 Oct. 2003, H. Voglmayr & W. Jaklitsch, W.J. 2469+2470 (WU 29113, cultures C.P.K. 992, C.P.K. 2404). Oberösterreich, Schärding, St. Willibald, Oberantlang, Antlinger Berg (Hochstraß) near Siegl, MTB 7648/1, 48°23'01” N, 13°42'09” E, elev. 460 m, on decorticated branch of Fraxinus excelsior, on wood, soc. moss, 29 Jul. 2004, H. Voglmayr, W.J. 2549 (WU 29117, culture C.P.K. 2008). Steiermark, Leoben, Hieflau, Hartelsgraben (Gesäuse), MTB 8454/1, 47°35'29” N, 14°42'24” E, elev. 520 m, on branches of Fagus sylvatica 4–10 cm thick on the ground, on strongly decomposed wood, bark and leaves, soc. H. minutispora, effete Annulohypoxylon cohaerens on bark, black pyrenomycetes and hyphomycetes, holomorph, 7 Aug. 2003, H. Voglmayr & W. Jaklitsch, W.J. 2313 (WU 29112, culture C.P.K. 1595). Vorarlberg, Feldkirch, Rankweil, behind the hospital LKH Valduna, MTB 8723/2, 47°15'40” N, 09°39'00” E, elev. 510 m, on decorticated branch of Abies alba 5 cm thick, on wood, holomorph, 31 Aug. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2642 (WU 29118, culture C.P.K. 2009). Czech Republic, Čižov, walking path from Hardegg, Lower Austria, 48°51'34” N, 15°51'53” E, elev. 330 m, on trunk of Carpinus betulus 18 cm thick, on blackened wood, 19 Jul. 2008, H. Voglmayr & W. Jaklitsch & O. Sükösd, W.J. 3202 (WU 29125). France, Lorraine, Département Meurthe-et-Moselle, Héming, between Étang du Stock and Maizieres de Vic, MTB 727/3, 48°43'35” N, 06°54'07” E, elev. 180 m, on partly decorticated branches of Carpinus betulus 5–10 cm thick, on wood, bark and moss, soc. immature Hypocrea minutispora, Hypoxylon fuscum, holomorph, 05 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2678 (WU 29120, culture C.P.K. 2011). Pont a Mousson, halfway between the town and motorway Nancy/Metz, 48°55'26” N, 06°05'55” E, elev. 200 m, on partly decorticated branches of Fagus sylvatica 4–8 cm thick, on wood and bark, soc. Datronia mollis, long-necked coelomycete, white mould, holomorph, 5 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2683 (WU 29121, culture C.P.K. 2012). Germany, Baden Württemberg, Freiburg, Landkreis Breisgau-Hochschwarzwald, St. Märgen, Holzschlag, MTB 8014/2, 47°59`53“N, 08°05`03“E, elev. 620 m, on cut log segment of Abies alba 70 cm thick, on wood, side and cutting area, soc. black coelomycete, algae, moss, 2 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2668 (WU 29119, culture C.P.K. 2010). Netherlands, Gelderland, Ede, Otterlo, National park De Hoge Veluwe, near entrance Otterlo, MTB 380/4, 52°06'10” N, 05°47'24” E, elev. 30 m, on little decorticated branch of Fagus sylvatica 5–7 cm thick, on bark and Picea needles, soc. Hypoxylon fragiforme, Lopadostoma turgidum, holomorph, 18 Sep. 2004, W. Jaklitsch & W. Gams & H. Voglmayr, W.J. 2727 (WU 29122, culture C.P.K. 2013). Ukraine, Kharkov, Zadonetskoe forestry, Gomolshansky National Nature Park, Zmiev area, on decorticated branch of Pinus sylvestris, 25 Jun. 2006, A. Akulov A.S. 1926 (culture C.P.K. 2863). Vicinity of V.N. Karasin National University Biological Station, on branch of Quercus robur, 7 Jul. 2007, O. Prilutsky, comm. A. Akulov, A.S. 2305. United Kingdom, Hampshire, Lyndhurst, New Forest, Whitley Wood, 50°50'50” N, 1°34'50” W, elev. 30 m, on branch of Fagus sylvatica 3 cm thick, holomorph, 14 Sep. 2007, W. Jaklitsch & H. Voglmayr, W.J. 3162 (WU 29124, culture C.P.K. 3151).
Notes: This species is easily identified when fresh due to the citrine colour and prominent perithecia. In hot weather and often when eaten by mites, the stroma colour becomes rather orange than yellow. Hypocrea thelephoricola is similar, but occurs only on and around basidiomata of Steccherinum ochraceum; it differs also in smaller ascospores, in perithecial walls turning orange in KOH, while the ostiole remains hyaline, and in a gliocladium-like anamorph. Generally immature stromata are more diagnostic than dry ones. When mature or old, particularly after repeated drying and re-wetting, perithecia are less prominent, making the distinction from H. strictipilosa difficult, sometimes even from H. gelatinosa. The similar North American H. chlorospora has not been found in Europe. Effuse conidiation in T. sinuosum on CMD and SNA is rare and scant. It was therefore not described in detail, but only observed on growth plates, where it appeared to be reminiscent of sect. Hypocreanum, as e.g. in H. sulphurea. A characteristic trait in CMD cultures is the conspicuous mycelial densifications developing prior to conidiation pustules to form their bases. Verrucose chlamydospores have not been seen in any other species from Europe.
Hypocrea spinulosa Fuckel, Jahrb. Nassau. Ver. Naturk. 23–24: 184 (1870) [1869–70]. Fig. 33.
Fig. 33.
Hypocrea spinulosa. a–e. Dry stromata (a–c, e. mature, d. immature). f. Mature stroma reconstituted in water. g. Mature stroma in 3 % KOH after reconstitution in water. h. Stroma on SNA (25 °C, 27 d). i, j. Cultures on PDA (25 °C, 7 d). k. Ostiole in side view. l. Perithecium in section. m. Cortical tissue in section from stroma side. n. Subperithecial tissue in section (from stroma on MEA). o. Base of stroma in section. p. Ascus with ascospores. q. Crystal in CMD (25 °C, 29 d). r. Aerial hyphae re-connecting with agar (SNA, 25 °C, 4 d). s. Chlamydospores (SNA, 15 °C, 10 d). a, h, i, n, q. WU 29127 (CBS 121280). b, f, g, j–l, p, r, s. WU 29128 (CBS 121272). c. holotype. d. WU 29126. e, m, o. H. moliniae lectotype M 0124358. Scale bars: a, b = 0.3 mm. c, d, q = 0.2 mm. e–h = 0.4 mm. i, j = 19 mm. k, l = 30 μm. m, o = 15 μm. n, s = 20 μm. p = 10 μm. r = 0.1 mm.
≡ Chromocrea spinulosa (Fuckel) Petch ex Brooks & Mathieson, Trans. Brit. Mycol. Soc. 33: 350 (1950).
≡ Creopus spinulosus (Fuckel) J. Moravec, Česká Mykol. 10: 88 (1956).
(?) = H. moliniae Pass., Erb. critt. Ital., Ser. 2: no. 1077 (1881).
Anamorph: not observed.
Stromata when fresh 0.5–2(–3) mm diam, 0.5–1 mm thick, solitary, gregarious or aggregated in groups of 2–3, pulvinate, with roundish to elongate outline, often surrounded by white mycelium. Surface smooth except for convex to conical, green to black ostiolar dots. Colour white or yellowish green when young, later typically light or greyish green.
Stromata when dry (0.3–)0.5–1.2(–2.0) × (0.2–)0.4–1.0(–1.5) mm, 0.2–0.4(–0.7) mm thick (n=41); discoidal, flat cylindrical, or flat pulvinate, broadly attached, only by hyphae, becoming easily detached from the substrate; with ca. 10–100 perithecia per stroma; outline circular or oblong. Surface velvety or hairy, smooth when young, uneven when mature due to slightly or distinctly projecting, convex or conical, densely disposed, ostioles (28–)36–67(–94) μm (n=84) diam. Sides whitish or greenish and often covered at the base or entire height by ample white to yellowish, cottony mycelium, at least when young. Colour of stroma surface whitish, pale/dull yellowish-, greyish-, brownish green or light green, ostioles olive to brownish when immature, later dark green or black; resulting macroscopic colour dark green to black. Development: first white mycelial tufts, compacting and becoming greenish from the centre or remaining white before the appearance of ostioles. Spore deposits green to black. Stromata (WU 29128) after reconstitution in water cylindrical or turbinate, considerably thicker than dry, white to pale green, with dark green, conical ostioles hyaline in their centres; after addition of 3 % KOH slowly becoming dilute yellowish brown, finally dark brown to black.
Stroma anatomy: Ostioles (37–)44–59(–72) μm long, plane or projecting to 40(–60) μm, broadly conical, inner part (12–)20–30(–37) μm wide at the apex (n=40), with hyaline convergent periphyses and similar parallel apical cells 2–4 μm wide; ostioles (40–)48–70(–82) μm (n=40) wide at the apex including margin; marginal cells bright green (like ascospores), thick-walled, isodiametric to oblong, angular, sometimes globose and to ca. 9 μm wide. Perithecia (92–)135–190(–210) × (82–)110–160(–200) μm (n=40), globose, less commonly ellipsoidal or flask-shaped, gradually merging into ostioles at the top; upper part sometimes projecting beyond superficial hyphal layer. Peridium (7–)11–17(–22) μm (n=80) wide at the base and sides, hyaline or pale yellowish. Cortical layer (15–) 20–44(–65) μm (n=60) thick, a t. intricata of narrow, thin-walled, greenish to brownish hyphae (1.0–)1.5–3.5(–5.0) μm (n=62) wide. Subcortical tissue absent or a loose t. intricata of hyaline hyphae (2.5–)3.5–7(–10) μm (n=61) wide, fraying out into the distinctly narrower cortical hyphae. Subperithecial tissue a hyaline t. globulosa-angularis-epidermoidea of thick hyphae and globose and angular cells (5–)6–27(–45) × (3–)5–14(–18) μm (n=60), denser and more hyphal at the base. Hyphae on stroma sides and base (except area of attachment) corresponding to cortical hyphae. Asci (60–)68–83(–98) × (3.8–)4.0–5.2(–6.5) μm, stipe (2–)6–12(–17) μm long (n=75). Ascospores bright or dull yellowish green, brown in KOH, verruculose or spinulose; cells dimorphic but often with little difference, distal cell (2.7–)3.5–4.8(–5.7) × (2.5–)3.0–4.0(–4.7) μm, l/w (0.9–)1.0–1.4(–1.7) (n=105), subglobose, ellipsoidal or angular, proximal cell (2.8–)3.4–5.0(–6.4) × (2.2–)2.7–3.4(–4.3) μm, l/w (1.0–)1.1–1.6(–2.4) (n=105), oblong, angular, plump wedge-shaped or subglobose.
Cultures: Optimum growth at 25 °C on all media; no growth at 35 °C. On CMD after 72 h 13–19 mm at 15 °C, 29–38 mm at 25 °C, 20–33 mm at 30 °C; mycelium covering the plate after 6–7 d at 25 °C. Colony hyaline, thin, circular with wavy or ill-defined margin, not zonate; mycelium loose or dense, radially oriented, with conspicuous difference in width among surface hyphae; prominent marginal hyphae sinuous; little mycelium on the surface; hyphae in the centre degenerating soon, becoming empty. Aerial hyphae only notable on the margin, or sometimes some long aerial hyphae within the colony collapsing to form floccules. Autolytic excretions absent or scant, no coilings seen. No pigment, no distinct odour noted. No conidiation seen. Chlamydospores noted after 5 d, or absent. Sometimes formation of large crystals noted in the agar after ca. 3 wk.
On PDA after 72 h 11–15 mm at 15 °C, 27–36 mm at 25 °C, 15–26 mm at 30 °C; mycelium covering the plate after 5–8 d at 25 °C. Colony circular, margin well-defined, dense; surface becoming covered by abundant, long and thin aerial hyphae, ascending for several mm, forming a dense white mat, radially oriented at the margin. In CBS 121272 hyphae distinctly wavy, centre stellate and flat, otherwise surface with a dense white mat of numerous, long aerial hyphae, dichotomously branched, ascending to the lid of the Petri dish, partly descending and re-connecting with agar, finally collapsing, forming flakes/tufts. Autolytic excretions and coilings absent or scant, autolysis more common in ends of aerial hyphae meeting the agar surface. Surface white, reverse yellowish 4A3–4, 4B4; no diffusing pigment, no distinct odour noted. No conidiation, no stromata seen. At 15 °C hyphae distinctly sinuous in both isolates, stellate structure of central surface (CBS 121272) more pronounced. At 30 °C colony dense, opaque, becoming covered by a thick, zonate, white mat; in CBS 121272 aerial hyphae growing faster than hyphae inside agar, growing to the lid, some descending and reuniting with the agar, forming an ill-defined, fluffy margin and a mottled, cottony colony surface.
On SNA after 72 h 7–13 mm at 15 °C, 22–41 mm at 25 °C, 10–31 mm at 30 °C; mycelium covering the plate after 5–8 d at 25 °C. Colony hyaline, circular, dense, hyphae degenerating soon from the centre, appearing empty. Aerial hyphae numerous, long, several mm high, loosely disposed, dichotomously branched, collapsing, forming tufts. In CBS 121272 colony irregularly lobed, with mottled and hairy surface; growth mainly by numerous, long, dichotomously branched aerial hyphae first ascending to the lid of the Petri dish, later descending and uniting with agar surface with characteristic shrub-like terminal branches. Autolytic excretions and coilings absent or inconspicuous. No pigment, no distinct odour noted. No conidiation seen. Chlamydospores noted after (4–)6–8 d, numerous on the entire plate, terminal and intercalary, large, smooth, in CBS 121280 (25 °C, 10 d) (7–)9–22(–39) × (4.5–) 6–10(–12) μm, l/w 0.9–3.2(–6) (n=35), variable, mostly oblong, ellipsoidal or irregular (intercalary), (sub-)globose or pyriform when terminal, some 2-celled; in CBS 121272 (15 °C, 10 d) (7–)9–15(–17) × (7–)9–12(–14) μm, l/w 0.9–1.3(–1.7) (n=30), (sub-)globose, oval, few oblong or ellipsoidal. Sometimes (CBS 121280) stromata formed after ca. 2 wk. Stromata 0.6–1.1 mm (n=6) diam, to ca. 1 mm thick, aggregated in a group at the distal margin; first appearing as white cottony tufts, becoming partly greenish and developing irregularly disposed, first brownish and translucent, later green, convex or subconical ostioles (40–)50–71(–80) μm (n=19) diam; surface hairy. No asci and ascospores seen. At 30 °C colony irregularly lobed.
On MEA (CBS 121280, examined after 14–17 d at 25 °C) sometimes stromata formed in a white mat of aerial hyphae within 2 wk. Stromata 5–7 × 4–6 mm (n=5), to ca. 2 mm high, two smaller stromata formed by disintegration of a larger one (effluent development!), irregularly pulvinate; outline oblong-roundish, thick, margin wavy. Colour whitish to grey-brown, 4D2, 5–6CD2, or grey-green. Surface tubercular, compact velvety-hairy, whitish, with thin-walled, terminally clavate hyphae 2.5–4 μm wide. Ostioles (24–) 38–70(–80) μm (n=30) diam, irregularly disposed on the stroma surface, amber, light or olive-brown, green only by spore deposits, plane or convex, broad, with flat apex, cylindrical vertical apical cells 1.5–2.5 μm wide inside, and partly globose, hyaline cells 2–6 μm wide outside. Peridium ochre, amber or brown. Subperithecial tissue a t. globosa-angularis-epidermoidea of cells (6–)11–25(–32) × (5–)9–15(–18) μm (n=30), as in the holotypes of H. spinulosa and H. moliniae. Asci (63–)68–78(–83) × 4–5 μm, stipe (8–)9–20(–24) μm long (n=12). Ascospores bright green, verrucose; cells with flat contact areas, dimorphic, distal cell (3.0–)3.5–4.5(–5.0) × (2.7–)3.0–3.5(–4.0) μm, l/w (1.0–)1.1–1.4(–1.7) (n=30), wedge- or mitra-shaped or subglobose, proximal cell (3.5–)3.7–4.5(–5.0) × 2.5–3.0(–3.5) μm, l/w (1.2–)1.3–1.6(–1.8) (n=30), oblong to wedge-shaped.
Habitat: on standing or lying stalks and leaves of various gramineous hosts (see British checklist http://194.203.77.76/fieldmycology/GBCHKLST/gbchklst.asp), Juncus spp. and herbaceous plants (e.g. Chelidonium, Plantago) in moist grassland with tall stands of grass. Early stages of stromata found also on partially living, green stalks.
Distribution: Europe (Austria, Denmark, Germany, Italy, United Kingdom).
Holotype: Germany, Hessen, Rheingau, Oestrich-Winkel, Mühlberg, on decaying stems of Chelidonium majus, soc effete black pyrenomycete; May (no year given), Fuckel (G).
Other specimens examined: Austria, Niederösterreich, Zwettl, Altmelon, Meloner Au, meadow at forest margin, MTB 7555/2, 48°27'35” N, 14°57'04” E, elev. 880 m, on stems of Juncus effusus and Festuca sp., 7 Sep. 2006, H. Voglmayr & W. Jaklitsch, W.J. 2972 (WU 29128, culture CBS 121272 = C.P.K. 2464). Denmark, Sjælland, Bregnebjerg, Boserup Skov, open exposed area next to mixed deciduous forest and an apple plantation, on culms of Plantago lanceolata and unidentified, partly green, grass, 3 Sep. 2006, T. Læssøe, (WU 29128, culture CBS 121280 = C.P.K. 2463). Germany, Niedersachsen, Landkreis Soltau-Fallingbostel, Soltau, Großes Moor, entering from Wardböhmen, 52°51`09“N, 09°56`28“E, elev. 70 m, on stems of Juncus effusus, 27 Aug. 2006, H. Voglmayr & W. Jaklitsch, W.J. 2951 (WU 29126); culture preparation failed; identity not absolutely certain. Italy, Parma province, in a forest near Collecchio; on piled culms of Molinia coerulea. G. Passerini [Erb. Critt. Ital. Ser. II, exs. 1077]. lectotype of H. moliniae Pass. (M!) here designated. United Kingdom, isol. J. Mathiesen, det. D.G. Catcheside, Oct. 1950; (culture CBS 311.50 = C.P.K. 1510; `albino mutant' of CBS 310.50); only culture seen.
Notes: Stromata of H. spinulosa may be mistaken for those of H. aeruginea or H. danica. While H. aeruginea is easily distinguished due to its pseudoparenchymatous cortex and the formation of a green Trichoderma anamorph, H. danica differs only by slower growth rates, limited growth at 30 °C, and gene sequences.
The type material of H. moliniae Pass. agrees perfectly with the concept of H. spinulosa. However, in lack of gene sequences a synonymy is not certain. The material in K (K 137609) consists of a single stroma, therefore the material in M (M 0124358) is here designated as the lectotype of H. moliniae. The two isolates of freshly collected H. spinulosa show slight differences in gene sequences and in the following phenotypic traits: stromata in WU 29128 are white rather than green, the subperithecial tissue is an ill-defined t. angularis, while globose cells are dominant in the types of H. spinulosa and H. moliniae and in stromata of CBS 121280 (from WU 29127) formed in MEA cultures. The latter finding may be related to the age of stromata. Also, colonies differ from CBS 121280 in the abundance and organisation of aerial hyphae, and no stroma formation has been seen in CBS 121272 (from WU 29128). An interesting finding is that H. spinulosa does not form an anamorph, but fertile stromata are formed on rich media such as MEA or OA, less commonly on SNA. H. spinulosa is one of the few examples in this genus where mating type studies were carried out (Mathieson 1952). Further notes and descriptions of H. spinulosa can be found also in Petch (1938), Brooks & Mathieson (1950), Moravec (1956b), and Engel & Hanff (1986).
Hypocrea strictipilosa P. Chaverri & Samuels, Mycologia 95: 1128 (2003). Fig. 34.
Fig. 34.
Teleomorph of Hypocrea strictipilosa. a–e. Fresh stromata (a. immature/mature mixed, b, d. mature, c. immature, e. mature, side view). f–k. Dry stromata (f. immature, g–k. mature). l. Mature stroma reconstituted in water. m. Mature stroma in 3 % KOH after reconstitution in water. n. Perithecium in section. o. Cortical and subcortical tissue in section. p. Subperithecial tissue in section. q. Base of stroma in section. r. Stroma surface in face view. s. Ascospores in ascus base. t–v. Asci with ascospores. a, c. WU 29143, b. WU 29131, d. WU 29153, e, g. WU 29139, h. WU 29150, f. WU 29132, i, m–r. WU 29161, j, t. WU 29156, k, v. WU 29148, l. WU 29152, s, u. WU 29162. Scale bars: a, c = 2 mm. b = 1.6 mm. d = 1.2 mm. e, h, j–l = 0.4 mm. f, g, i, m = 0.3 mm. n, p = 25 μm. o, q, r, t–v = 15 μm. s = 10 μm.
= Hypocrea aureoviridis f. macrospora Yoshim. Doi, Bull. Natl. Sci. Mus. 14: 728 (1972), fide Chaverri & Samuels (2003).
Anamorph: Trichoderma strictipile Bissett, Can. J. Bot. 69: 2410 (1991) (as `strictipilis'). Fig. 35.
Fig. 35.
Cultures and anamorph of Hypocrea strictipilosa. a–c. Cultures at 25 °C after 14 d (a. on CMD. b. on PDA. c. on SNA). d, e. Conidiophores of effuse conidiation on growth plate (7 d). f. Conidiation tuft with elongations on growth plate (7 d). g–k. Conidiophores from pustules (8–9 d; i. from small shrub, intermediate effuse/pustulate). l. Ampulliform phialides (9 d). m, n. Chlamydospores (16 d). o, p. Conidia (8–9 d). q. Lageniform phialides on elongation (9 d). d–q. At 25 °C. d, e, g–l, o–q. On CMD. f, m, n. On SNA. a–c, f, h, j, k–n, q. C.P.K. 1616. d, e. C.P.K. 2398. g, o. C.P.K. 2433. i, p. C.P.K. 3135. Scale bars: a–c = 20 mm. d = 30 μm. e, g–i, m, n = 20 μm. f = 0.1 mm. j–l, p = 10 μm. o, q = 5 μm.
Stromata when fresh (0.7–)1–3(–4) mm diam, 0.5–1(–1.5) mm thick, (flat) pulvinate or lenticular, less commonly turbinate, basal part often like a broad stipe and pale orange; centrally attached; surface smooth, with numerous fine but distinct ostiolar dots; outline roundish; sides sterile, white. Colour white to pale yellowish when young, then yellow 3A3, 3B4–5, 4A2–5; ostiolar dots first pale brownish, becoming olive to dark green upon maturation on a white or yellow (to orange or brownish) background; resulting colour olive, greyish yellow or shades of green 2C6–7, 2B6, 3DE6–7, 4B5–6, 28D4–5, 25B2–3, 1DE6–8, 30D5–7, 26EF7–8, dark green to black due to ascospores when old, sometimes finally (reddish) brown 7E5–6, or 5CD6–7.
Stromata when dry (0.4–)0.6–1.8(–4.2) × (0.3–)0.6–1.6(–2.8) mm, (0.2–)0.3–0.6(–1.1) mm thick (n=100); mostly gregarious or aggregated, often in large lawns; (flat) pulvinate, discoidal or turbinate with circular or angular outline, broadly attached, often with white radiating base mycelium when young. Margin mostly free, rounded or sharp, fertile part sometimes slightly projecting over sterile sides; sterile sides either lacking or vertical or attenuated downward, smooth, often orange. Surface flat, slightly convex or concave, smooth and often covered by conidiophores when young, later finely tubercular or rugose and covered by ample dark green spore deposits when mature; less commonly covered by white scurf when young, penetrated by ostioles in round angular or elongate fissures. Ostiolar dots (24–)47–95(–157) μm (n=200) diam, typically numerous and densely arranged, plane or convex; large, diffuse and pale brownish to ochre when young, but small, well-defined and dark green to black when mature. Perithecia only rarely projecting. Stroma surface between ostiolar dots white to yellowish, less commonly orange. Orange tones present mostly in old, overmature or repeatedly dried stromata. Overall colour from white, pale or greyish yellow to greyish orange, 3B3, 4–5AB3–4 when immature, later brown-orange, olive or dull green, mostly 3–4CE4–6, but also 1–5CD4–5, 5E4–6, 29–30DE4–6, 1DE4–6, 28–30F4–8, 1–3F4–8, finally dark green 27F4–8, to black. Development: white mycelial tufts compacting on a white radiating mycelium to white or pale yellowish buttons, becoming pale cream-yellowish, forming numerous yellowish to pale brownish ostiolar dots; becoming pulvinate or turbinate with more well-defined dots, darkening due to maturing ascospores. Stromata after reconstitution in water flat pulvinate, lentiform to turbinate; white with brown perithecia when immature, brownish green when mature; ostiolar dots convex, shiny, dark brown, green or olive; after addition of KOH brown; true ostioles minute, hyaline.
Anamorph in association with mainly young stromata effuse, powdery, dark green 25E4–6, 25–26F5–8; on bark more pulvinate and hairy than on wood.
Stroma anatomy: Ostioles (47–)55–66(–70) μm long, plane or projecting to 20(–30) μm, (23–)29–46(–61) μm wide at the apex (n=30); wall yellow, no differentiated cells seen. Perithecia (135–) 165–210(–235) × (88–)115–170(–190) μm (n=30), flask-shaped or globose. Peridium (5–)10–17(–20) μm (n=60) wide at the base and sides, yellow, orange-yellow in KOH. Surface smooth. Cortical layer thin, (12–)14–22(–24) μm (n=30) thick, well-discernible, a t. angularis of light yellow (orange-yellow in KOH), thick-walled (1 μm), isodiametric to oblong cells (3–)6–14(–24) × (2.5–)4–8(–11) μm (n=60) in face view and in vertical section. Subcortical tissue a hyaline t. angularis–epidermoidea of thin-walled angular cells (4–) 5–10(–15) × (2.5–)3.5–6.5(–8.5) μm (n=30) and hyphae (2.5–)3–5(–7) μm (n=30) wide. Subperithecial tissue a hyaline t. angularis–epidermoidea of thin-walled cells (6–)10–25(–36) × (5–)6–15(–24) μm (n=30), smaller and thick-walled toward base, at the base (attachment area) dense and intermingled with thick-walled hyaline hyphae (2–)3–5(–7) μm (n=30) wide. Asci (90–)100–120(–136) × (5.0–)5.5–6.5(–7.7) μm, stipe (6–)10–23(–36) μm long (n=100). Ascospores (yellowish) green, verruculose; cells dimorphic, distal cell (4.0–)4.7–5.5(–6.5) × (3.7–)4.4–5.0(–5.4) μm, l/w (0.9–)1.0–1.2(–1.5) (n=180), (sub-)globose or wedge-shaped, proximal cell (4.5–)5.0–6.5(–8.0) × (3.2–)3.7–4.3(–5.0) μm, l/w (1.0–)1.2–1.7(–2.2) (n=180), oblong or wedge-shaped, less commonly subglobose, often slightly longer in ascus base.
Cultures and anamorph: Optimum growth at 25 °C on all media; no growth at 35 °C. On CMD after 72 h 19–21 mm at 15 °C, 52–56 mm at 25 °C, 9–10 mm at 30 °C; mycelium covering the plate after 4 d at 25 °C. Colony hyaline, thin, more or less circular, with loose mycelium and thick primary marginal surface hyphae and numerous thin secondary hyphae, homogeneous, later with few inconspicuous, slightly downy, concentric zones. Aerial hyphae scant or inconspicuous, typically lacking in the centre, more frequent and long in distal and marginal areas of the colony. No autolytic excretions, no coilings seen. No diffusing pigment, no distinct odour noted. Chlamydospores noted after 3 wk, uncommon. Conidiation noted after 2 d, green after 6–14 d, first effuse, short, loosely arranged outside the centre, becoming concentrated and appearing on long aerial hyphae in several slightly downy concentric zones; verticillium-like, conidia formed in minute wet heads. Later conidiation in minute granules, shrubs or pustules 0.2–1 mm diam with or without straight elongations, formed in zones of effuse conidiation, mostly concentrated in distal areas of the plate, turning dark green 28E7–8, 27–28F7–8. Shrubs/pustules after 8–9 d mostly 0.2–0.5 mm diam and height, first loose, becoming dense to opaque due to dense conidiation within pustules; formed on thick-walled stipe to 10 μm wide at the base; further branching asymmetric, primary branches (bases of main axes) 7–9 μm wide, thick-walled, quickly narrower upwards, mostly 4–5 μm wide, terminal branches and origins of phialides 2.5–4.0(–4.5) μm wide. Main axes straight or sinuous, mostly ill-defined, terminating in short (ca. 50 μm) fertile conidiophores, often with few short paired branches in right angles or slightly inclined upward, and solitary phialides on upper levels; or terminating in straight, first sterile elongations projecting 100–200 μm, becoming fertile. Phialides solitary or in whorls of 2(–3) on elongations, solitary or in whorls of 2–3(–5) on conidiophores, divergent but often steeply inclined. Conidia formed in minute wet or dry heads <30 μm diam. Phialides (4–)5–15(–23) × (2.7–)3.0–4.0(–4.5) μm, l/w (1.2–)1.6–4.1(–6.2), (1.7–)2.0–3.0(–3.5) μm wide at the base (n=95), lageniform and inequilateral or ampulliform and symmetric or with bent neck; often both shapes present on the same conidiophore in various positions; thickest mostly below the middle; sometimes longer and more narrowly lageniform to subulate on elongations. Conidia (3.4–)3.8–6.0(–8.5) × (2.5–)3.0–3.5(–4.5) μm, l/w 1.2–1.8(–2.4) (n=93), ellipsoidal, less commonly oblong or oval, green, smooth, multiguttulate or with few large guttules, scar indistinct. Considerable intraspecific variation of conidial size (e.g. length of 3.5–4.5(–5.2) μm in an isolate), but no significant difference in size noted between pustule interior and elongations, albeit with some tendency of longer and more oblong conidia formed on elongations. At 15 °C conidiation effuse, pustules formed late. At 30 °C slow and limited growth of narrow helical hyphae; no conidiation noted.
On PDA after 72 h 14–18 mm at 15 °C, 40–46 mm at 25 °C, 18–24 mm at 30 °C; mycelium covering the plate after 5 d at 25 °C. Mycelium of curved frizzy hyphae with conspicuous differences in width, marginal hyphae parallel, aggregating to radial strands. Colony circular, dense, becoming zonate; concentric zones thick, white, hairy due to abundant long aerial hyphae, ascending several mm, forming strands, coilings and large drops. Autolytic excretions inconspicuous, coilings infrequent except in aerial hyphae. No diffusing pigment noted, reverse at most greyish yellow 4BC4, odour none or slightly mushroomy. Conidiation noted after 1–2 d, remaining colourless or becoming greyish green 28CD5–6 after 2 wk, effuse, simple, irregularly verticillium-like; first short, spreading from the centre, later at low to median levels on long aerial hyphae; conidia formed in numerous minute wet heads on phialides solitary or in whorls of 2–3. At 15 °C concentric zones better developed than at 25 °C, with white farinose surface due to fluffy tufts becoming greenish from the centre. Conidiation on fluffy tufts and effuse on aerial hyphae. At 30 °C colony irregular, zonate; conidiation effuse.
On SNA after 72 h 18–21 mm at 15 °C, 42–46 mm at 25 °C, 3–4 mm at 30 °C; mycelium covering the plate after 5 d at 25 °C. Colony similar to CMD, homogeneous. Long aerial hyphae only frequent in distal areas of the colony. Autolytic excretions and coilings inconspicuous. No pigment, no distinct odour noted. Conidiation noted after 2 d, green after 5–6 d, first effuse, irregularly acremonium- to verticillium-like, short and on aerial hyphae, and in small dry pustules appearing in few concentric zones. Pustules 0.3–1.5 mm diam, with often inconspicuous, first sterile, later fertile straight elongations; becoming dark green 28D5–6, 27F7–8. Chlamydospores 5–13(–24) × (4–)5–9(–12) μm, l/w 0.8–1.8(–3.1) (n=45), noted after 1 wk, numerous, globose or oval when terminal, more angular and oblong or ellipsoidal when intercalary, some 2–3 celled. At 15 °C conidiation effuse and sometimes in large aggregates of green pustules. At 30 °C little growth, no conidiation seen.
Habitat: on (medium to) well-rotted wood of deciduous and coniferous trees; generally associated with other fungi and/or moss.
Distribution: Europe, North America, the most common green-spored species in north-temperate zones.
Holotype: Canada, Quebec, Montreal; on rotting log; 20 Sep. 1979, G.P. White (DAOM 172827, ex-type culture DAOM 172827) (not examined).
Specimens examined: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, close to the village (,Tumpfi'), MTB 9452/4, 46°32'37” N, 14°25'35” E, elev. 565 m, on branch of Corylus avellana, on/soc. Corticiaceae, 25 Sep. 2006, H. Voglmayr & W. Jaklitsch, W.J. 2988 (WU 29158); Zabrde, MTB 9452/4, 46°33'00” N, 14°25'17” E, elev. 550 m, on broken corticated tree tops of Picea abies 2–3 cm thick, on bark, soc. Corticiaceae, Bertia moriformis, holomorph, 29 Oct. 2005, H. Voglmayr & W. Jaklitsch, W.J. 2871 (WU 29154); same place, on tree tops of Picea abies and Fagus sylvatica 2-4 cm thick, soc. Bertia moriformis, Exidia sp., 3 Jul. 2007, W. Jaklitsch, W.J. 3108 (WU 29160). Villach Land, Nötsch im Gailtal, Kerschdorf, MTB 9347/3, 46°37`15“N, 13°33`50“E, elev. 860 m, on decorticated branch of Alnus incana, 13 Oct. 2002, W. Jaklitsch, W.J. 2002. Völkermarkt, Gallizien, shortly after Vellach heading to Sittersdorf, MTB 9453/1, 46°34'09” N, 14°31'39” E, elev. 440 m, on branch of Quercus petraea, mostly on wood, soc. Corticiaceae, rhizomorphs, 11 Jul. 2007, W. Jaklitsch, W.J. 3123 (WU 29161). Niederösterreich, Hollabrunn, Hardegg, beech forest shortly before Felling in direction Riegersburg, MTB 7161/1, 48°51'47” N, 15°49'49” E, elev. 480 m, on decorticated branch of Fagus sylvatica 4 cm thick, on wood and various dark hyphomycetes, soc. ?Ascocodinaea corticiorum, Mollisia sp., 21 Jul. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2535 (WU 29139, culture C.P.K. 1616). St. Pölten Land, Michelbach, ditch at a brook at Mayerhöfen, MTB 7860/4, 48°07'16” N, 15°45'51” E, elev. 410 m, on decorticated branch of Fraxinus excelsior 5 cm thick, on wood and old Hypoxylon sp., soc. Polydesmia pruinosa, holomorph, 18 Oct. 2003, W. Jaklitsch, H. Voglmayr & W. Klofac, W.J. 2478 (WU 29137, culture C.P.K. 1606). Wien-Umgebung, Mauerbach, Friedhofstrasse, MTB 7763/1, 48°15'20” N, 16°10'13” E, elev. 330 m, on branch of Carpinus betulus, holomorph, 5 Oct. 2002, W. Jaklitsch, W.J. 1992. Same place, on branch of Carpinus betulus, on/soc. yellowish Corticiaceae, 17 Jul. 2005, W. Jaklitsch, W.J. 2818, WU 29153, culture C.P.K. 2037. Same place, on branches of Fagus sylvatica and Carpinus betulus 4–5 cm thick, mostly overmature, 7 Oct. 2006, W. Jaklitsch & H. Voglmayr, W.J. 3004 (WU 29159, culture CBS 121137 = C.P.K. 2780). Same place, 48°15'24” N, 16°10'15” E, on branch of Carpinus betulus, soc Cordana pauciseptata and Tubeufia cerea, 14 Oct. 2007, W. Jaklitsch, W.J. 3181. Oberösterreich, Grieskirchen, Neukirchen am Walde, Leithen, MTB 7648/2, 48°22'25” E 13°47'00”, elev. 400 m, on branch of Carpinus betulus 3–4 cm thick, on wood, holomorph, 16 Aug. 2003, H. Voglmayr, W.J. 2336 (WU 29129, culture C.P.K. 1601). Schärding, St. Willibald, eastern margin of Großer Salletwald, mixed forest ca. 25 years old, MTB 7648/3, 48°21'04“N, 13°42'12“E, elev. 660 m, on branch of Fagus sylvatica 7–8 cm thick, on decorticated wood and brown Corticiaceae, holomorph, 8 Sep. 2003, H. Voglmayr, W.J. 2390 (WU 29130, culture C.P.K. 955). Czech Republic, Bohemian Switzerland, Mezní Louka, Kozí Hrbet/Ponova Louka, MTB 5151/2, 50°53'05” N, 14°19'27” E, elev. 320 m, on decorticated branch of Fagus sylvatica 5–6 cm thick, on wood and effete Eutypa lata, soc. Mollisia sp., Nemania sp., holomorph, 19 Sep. 2003, J. Holec & W. Jaklitsch, W.J. 2399 (WU 29131, culture C.P.K. 962). Southern Bohemia, Pohorská Ves, ,wet birch wood' south from the road Malonty - Pohorská Ves, MTB 7353/2, 48°40'01” N, 14°37'49” E, elev. 730 m, on branches of Alnus glutinosa 3–6 cm thick, on wood, bark and resupinate polypore, holomorph, 25 Sep. 2003, W. Jaklitsch, W.J. 2424+2425 (WU 29132, cultures C.P.K. 975, C.P.K. 976). Same collection data, on wood and on/soc. Corticiaceae, soc. hyphomycetes, Mollisia sp., W.J. 2426 (WU 29133, culture C.P.K. 977). Denmark, Soenderjylland, Rise Skov, between Roedekro and Aabenraa, 55°03'34” N, 09°22'01” E, elev. 70 m, on partly decorticated branch of Corylus avellana 7 cm thick, on/ soc. corticiaceous fungi and effete Hypoxylon sp., 23 Aug. 2006, H. Voglmayr & W. Jaklitsch, W.J. 2936 (WU 29156). Estonia, Tartu County, Vara Community, Alatskivi forestry, on decaying wood, soc. hyphomycetes, mostly immature, holomorph, 7 Oct. 2005, K. Põldmaa, TAA(M) 17956 (WU 29155, culture C.P.K. 2433). France, Lorraine, Moselle, Héming, between Étang du Stock and Maizieres de Vic, Parc Lorraine, Département de La Moselle, MTB 727/3, 48°43'35” N, 06°54'07” E, elev. 180 m, on branch of Carpinus betulus 4 cm thick, on wood and old Corticiaceae, soc. Hypocrea minutispora, effete pyrenomycete, Mollisia sp. and Melogramma campylosporum in bark, 5 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2679 (WU 29148, culture C.P.K. 2032). Germany, Baden Württemberg, Tübingen-Pfrondorf, 48°32`00“N, 09°03`00“E, elev. 350 m, on branch of Fagus sylvatica, on dark wood, soc. long-necked black coelomycete, 18 Oct. 2004, E. Weber, H.-O. Baral & K. Siepe, W.J. 2788 (WU 29152, culture C.P.K. 2020). Bavaria, Oberbayern, Landkreis Eichstätt, Altmühltal, 2–3 km after Pfahldorf in direction Eichstätt, MTB 7033/4, 48°57'00” N, 11°18'20” E, elev. 540 m, on decorticated branch of Fagus sylvatica 6–7 cm thick, on wood, 5 Aug. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2575 (WU 29145, culture C.P.K. 2029). Oberfranken, Coburg, Zilgendorf, forest south/southeast of the village, MTB 5831/2, 50°09'54” N, 10°58'26” E, elev. 450 m, on mostly decorticated branches of Fagus sylvatica 4–6 cm thick, on wood, soc. Nemania serpens, holomorph, 4 Aug. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2557 (WU 29140, culture C.P.K. 2023). Unterfranken, Landkreis Haßberge, Eltmann, 1.8 km before Unterschleichach in direction Fabrikschleichach, MTB 6029/2, 49°57`07“N, 10°38`00“E, elev. 360 m, on decorticated branch of Quercus petraea 7 cm thick, on wood, soc. moss, 5 Aug. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2572 (WU 29144, culture C.P.K. 2028); Landkreis Haßberge, close to Mariaburghausen, left roadside heading from Knetzgau to Haßfurt, MTB 5929/3, 50°00'33” N, 10°31'10” E, elev. 270 m, on partly corticated branches of Tilia cordata 5–6 cm thick, on bark, soc. Hypocrea minutispora, Peniophora rufomarginata and white rhizomorphs, holomorph, 4 Aug. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2564+2565 (WU 29141, cultures C.P.K. 2024, C.P.K. 2025). Same collection data, on mainly corticated branch of Pinus sylvestris 6–7 cm thick, on wood and bark, W.J. 2566 (WU 29142, culture C.P.K. 2026). Same collection data, on decorticated branches of Fagus sylvatica and Quercus petraea 4–6 cm thick, on wood, soc. effete ?Chaetosphaeria sp., W.J. 2567 (WU 29143, culture C.P.K. 2027). Hessen, Landkreis Fulda, Gersfeld, Rhön, Rotes Moor (between Gersfeld and Wüstensachsen), from parking place Moordorf at B278 before entering the moor, 50°27'35” N, 09°58'59” E, elev. 810 m, on branch of Betula pubescens ssp. carpatica, 29 Aug. 2006, H. Voglmayr & W. Jaklitsch, W.J. 2958 (WU 29157). Rheingau, Oestrich-Winkel, between Johannisberg and Stephanshausen, MTB 5913/4, 50°01'12” N, 07°57'45” E, elev. 270 m, on partly decorticated branch of Carpinus betulus 2 cm thick, on inner bark, 21 Sep. 2004, W. Jaklitsch & H. Voglmayr, W.J. 2740 (WU 29150, culture C.P.K. 2034). Rheinland-Pfalz, Eifel, Landkreis Daun, Gemündener Maar, MTB 587/1, 50°10'46” N, 06°50'10” E, elev. 400 m, on partly decorticated branches of Carpinus betulus 4–9 cm thick, on wood (and bark), soc. Byssocorticium pulchrum, Bertia moriformis, holomorph, 21 Sep. 2004, H. Voglmayr & W. Jaklitsch, W.J. 2739 (WU 29149, culture C.P.K. 2033). Italy, Tuscany, Appenines, Arezzo, Badia Prataglia, Parco Nationale Foreste Casentinesi, Reserve Integrale “Sasso Frattino”, 43°47'00” N, 11°52'00” E, elev. >500 m, on branches of Fagus sylvatica, on dark wood, 8 Oct. 2004, A. Akulov (CWU 1114, WU 29151, culture C.P.K. 2035). Sweden, Uppsala Län, 3–4 km north from Österbybruck, close to the road to Forsmark, MTB 4373/4, 60°14'18” N, 17°55'49” E, elev. 40 m, on cut tree top, partly corticated, of Picea abies 6–7 cm thick, on wood and bark, 5 Oct. 2003, W. Jaklitsch, W.J. 2438 (WU 29134, culture C.P.K. 2394). Same area, 60°14'11” N, 17°55'41” E, on cut tree top of Picea abies 6 cm thick, on wood, soc. moss, Phlebiella vaga, white Corticiaceae, 5 Oct. 2003, W. Jaklitsch, W.J. 2440 (WU 29135, culture C.P.K. 2396). Sunnersta, forest opposite the virgin forest Vardsätra Naturpark across the road, MTB 3871/2, 59°47'23” N, 17°37'53” E, elev. 15 m, on branch of Populus tremula, on wood, holomorph, 8 Oct. 2003, W. Jaklitsch, W.J. 2452 (WU 29136, culture C.P.K. 2398). Ukraine, Kharkov district, Zmiev, National nature park Gomolshanskie lesa, 49°42' N, 36°22' E, elev. 100 m, on decorticated branch of Quercus robur, on wood and Exidia glandulosa, 24 Jun. 2004, A. Akulov (WU 29138, culture C.P.K. 1611). Same area, on branch of Quercus robur, on wood and Hypoxylon sp., 22 Jul. 2004, A. Akulov (WU 29146, culture C.P.K. 2030). Same area, on branch of Quercus robur, 12 Jul. 2007, A. Akulov, A.S. 2443 (WU 29162, culture C.P.K. 3135). Krym, Avtonomna Respublika, Yalta, Yalta Mountain Forest Natural Reserve, Mountain Crimea, 44°30' N, 34°10' E, elev. >400 m, on branch of Fagus sylvatica, on wood, 4 Jul. 2004, A. Bereznitsky, comm. A. Akulov (WU 29147, culture C.P.K. 2031). Poltava district, Mirgorod area, close to Velikiye Sorochintsy, 800 m W from the river Psyol, on branch of Alnus glutinosa, soc. Mollisia sp., 18 Aug. 2007, A. Akulov, A.S. 2493 (WU 29163). United Kingdom, Hertfordshire, Bayford, Great Groves Wood, on decorticated branch of Carpinus betulus, 7 Oct. 2007, K. Robinson (WU 29164). Stevenage, Box Wood, on decorticated branches of Carpinus betulus and Quercus robur, 4 Dec. 2007, K. Robinson (WU 29165). Numerous additional specimens observed but not harvested.
Notes: Hypocrea strictipilosa is common. It is usually identified as H. gelatinosa, but the latter has a waxy or gelatinous appearance of stromata and large projecting perithecia, particularly when fresh. Diagnostic for fresh stromata of H. strictipilosa are the flat, smooth, not waxy, stroma surface, that is less pigmented than in species of the chlorospora group (H. sinuosa, H. thelephoricola), and the numerous fine ostiolar dots. The colour of mature stromata is generally difficult to determine. Orange or brown colours and uneven, sometimes nearly gelatinous, stroma surface are found in old, overmature stromata and result from hot and dry weather or repeated drying/re-wetting. Only rarely stromata show a whitish scurf on their surface and an orange background colour. Such forms may be similar to H. aureoviridis, but the latter can easily be distinguished by its monomorphic ascospores. Considerable intraspecific variation has been noted in the anamorph. Generally, longer and more subulate phialides are noted in isolates, which form only simple conidiophores and small shrubs rather than well-defined pustules.
Hypocrea thelephoricola P. Chaverri & Samuels, Stud. Mycol. 48: 96 (2003). Fig. 36.
Fig. 36.
Teleomorph of Hypocrea thelephoricola. a–e. Fresh stromata (a. stroma initials, b. immature, c–e. mature). f–i. Dry stromata (f, g. immature, g. side view. h, i. mature). j. Mature stroma reconstituted in water. k. Mature stroma in 3 % KOH after reconstitution in water. l. Perithecium in section (in 3 % KOH!). m. Cortical and subcortical tissue in section. n. Subperithecial tissue in section. o. Base of stroma with hairs in section. p. Stroma surface in face view. q–s. Asci with ascospores. a–d, f–h, j–r. WU 29166. e, s. WU 29168. i. WU 29169. Scale bars: a, d = 0.6 mm. b, e, h = 0.5 mm. c = 1 mm. f, j, k = 0.4 mm. g, i = 0.2 mm. l, o = 25 μm. m = 15 μm. n, p = 20 μm. q–s = 10 μm.
Anamorph: Trichoderma thelephoricola P. Chaverri & Samuels, Stud. Mycol. 48: 96 (2003). Fig. 37.
Fig. 37.
Cultures and anamorph of Hypocrea thelephoricola. a–c. Cultures at 25 °C after 14 d (a. on CMD. b. on PDA. c. on SNA). d. Conidiation tufts (SNA, 25 °C, 5 d). e. Wet conidial heads on growth plate (SNA, 25 °C, 5 d). f–k. Conidiophores. l, m. Conidia. n. Conidia and phialides. f–n. from CMD at 25 °C after 6–7 d. a–e, g, h, i, j, l, m. CBS 120925. f, k, n. C.P.K. 2493. Scale bars: a–c = 20 mm. d = 0.5 mm. e = 0.2 mm. f = 30 μm. g, j, n = 10 μm. h, i, k, l = 15 μm. m = 5 μm.
Stromata when fresh 0.5–1.5 mm diam, to ca. 1 mm thick, turbinate or pulvinate; first white, then with bright yellow, projecting perithecia, the latter becoming green on white to pale yellowish stroma surface; ostiolar/perithecial dots numerous and densely disposed when mature, often covered by green spore deposits. Associated anamorph effuse, medium to dark green.
Stromata when dry (0.3–)0.5–1(–1.3) × (0.2–)0.4–0.9(–1.2) mm, (0.2–)0.3–0.6(–0.8) mm thick (n=60); scattered, gregarious or aggregated in large lawns, typically turbinate on fresh basidiomata of the host, or pulvinate on wood and bark, sometimes cylindrical. Attachment area on aculei of the host punctiform, but broad on flat host areas. Outline circular, oblong, often angular or coarsely crenate by laterally projecting perithecia. Margin free, often sharp, elevated and laterally projecting beyond sterile sides/base. Sides attenuated downward or vertical, white (-yellowish-greenish), smooth or powdery; sometimes with white base mycelium. Surface smooth or uneven due to perithecial dots, glabrous or sometimes covered by whitish, finely farinose scurf becoming penetrated by ostioles and flaking off. Perithecial dots (40–)60–128(–180) μm (n=90) wide, numerous and densely disposed, first diffuse, later distinct and distinctly projecting, convex, circular to elongate, usually less projecting or plane and surface more smooth when mature or old. Ostiolar openings only rarely visible as minute, plane or conical, light or dark centres of perithecial dots. Colour pale to bright yellow 1–3A2–5, 4A3–4 when immature; later olive to dark green perithecial dots on a white or yellow (to nearly orange) stroma surface, resulting in greyish yellow 4B4–5, 3–4CD4–5, or various green tones dependent of maturation and combination with immature stromata, 1CD4–6 to 30CD4–6, 2F5–8, later 28–30E5–6, 1–4E4–5(–6), black when old. Development: white tufts compacting to small buttons, growing and turning yellow by perithecia, becoming more warm yellow, olive to green upon maturation. Ascospore deposits dark green to black. Stromata after reconstitution in water and subsequent addition of 3 % KOH (reddish) brown (due to orange-red peridium).
Stroma anatomy: Ostioles (41–)52–74(–90) μm long, plane or projecting to 16(–26) μm, (19–)28–43(–54) μm wide at the apex (n=30); with hyaline marginal cells, sometimes clavate or subglobose, to 5 μm wide at the apex. Perithecia (148–)180–240(–270) × (55–)115–190(–215) μm (n=30), crowded, 4–7 per mm stroma, flask-shaped or globose. Peridium (5–)11–18(–26) μm (n=60) wide at the base and sides, yellow. Peridium orange in KOH except for the hyaline ostiolar area. Cortical layer (15–)16–33(–49) μm (n=30) thick, a pale yellowish textura angularis of thin-walled, isodiametric to oblong cells (4–)8–19(–33) × (3–)5–10(–14) μm (n=60) in face view and in vertical section, ill-separated from subcortical tissue, fraying out into hairs on stroma sides. Surface smooth, with scant hyphal outgrowths; hairs common at sides and base, (13–)16–30(–40) × (4–)5–8(–10) μm (n=30) hyaline, 1–4 celled, thin-walled, variable, cylindrical or moniliform with globose basal cells. Subcortical tissue a hyaline t. angularis of thin-walled cells (4–)5–11(–18) × (3–)4–7(–10) μm (n=30), intermingled with some hyaline, thin-walled hyphae (2–)3–6(–6.5) μm (n=30) wide. Subperithecial tissue a hyaline t. angularis of thin-walled cells (6–)11–26(–34) × (5–)8–15(–18) μm (n=30), more hyphal at sides and base. Asci (74–)83–107(–123) × (5.0–)5.3–6.3(–7.0) μm, stipe (3–)8–21(–35) μm long (n=60). Ascospores olive or dull green, (green-, orange-) brown in KOH, verrucose, verrucae to 0.5 μm long; cells dimorphic, plump, distal cell (3.3–)3.8–4.6(–5.2) × (3.0–)3.5–4.3(–4.7) μm, l/w (0.7–)1.0–1.1(–1.4) (n=120), (sub-) globose, proximal cell (3.0–)4.0–5.2(–6.4) × (2.6–)3.2–3.8(–4.2) μm, l/w (0.8–)1.1–1.5(–2.2) (n=120), oblong, wedge-shaped or subglobose.
Cultures and anamorph: Optimum growth at 25 °C on all media; no growth at 35 °C. On CMD after 72 h 2–4 mm at 15 °C, 8–10 mm at 25 °C, 5–6 mm at 30 °C; mycelium covering the plate after 3–4 wk at 25 °C. Colony hyaline, thin, radially fan-shaped, with irregular, cobweb- or ice crystal-like outline and inconspicuous alternate narrow/wide zones; mycelium with radially varying density, hyphae narrow, submoniliform. Surface farinose or granulose from the centre and becoming green by conidiation. Aerial hyphae inconspicuous, becoming fertile. Autolytic excretions and coilings absent. No pigment, no distinct odour noted. No chlamydospores seen. Conidiation noted after 1–2 d, green after 5 d, (sub-) gliocladium-like, short, spreading in a lawn from the centre across the entire colony, effuse to pustulate with continuous transition from simple, erect, unbranched conidiophores with a single gliocladium-like head to 130 μm diam, over most commonly asymmetrically branched shrubs with several heads, to macroscopically distinct, small pustules 0.4–0.8 mm diam, turning dark green 27E5–6, 26–28F5–8, some coalescing, forming groups to 2–3(–5) mm long. Conidiophores/shrubs/ pustules with loose architecture and asymmetric branching, on a stipe 7–11 μm wide, with thick walls swelling in KOH and verrucose outside. Primary branches arising from the stipe similar, mostly unpaired and 4–6 μm wide, terminating in more or less verticillate whorls of branches on 1 or few levels, bearing short, divergent, 1-celled branches. Terminal branches (1.5–)2.0–2.5(–4.0) μm wide, usually strongly curved and more or less parallel, straight or sinuous. Phialides in whorls of 2–5(–6), less commonly solitary, divergent, parallel in terminal position on conidiophores, originating on cells 2.0–4.5 μm wide; cell below phialide often thickened. Conidia formed in wet, gliocladium-like, green heads becoming dry, finally sometimes adhering in chains. Phialides (7.5–)10–15(–20) × (2.2–)2.5–3.3(–4.0) μm, l/w (2.5–)3.3–5.2(–7.3), (1.4–)1.8–2.5(–3) μm wide at the base (n=65), lageniform to subulate, equilateral in the middle of a whorl, otherwise inequilateral, curved, thickest at variable position, green when old. Conidia (4.0–)4.3–6.0(–8.8) × (2.8–)3.0–3.6(–4.2) μm, l/w (1.2–)1.3–1.8(–2.1) (n=60), yellow-green, subglobose when young, ellipsoidal or oblong when mature, smooth, with no or some fine guttules, scar indistinct or truncate. At 15 °C colony irregularly lobate, becoming green (granules) around the plug, granules spreading radially along lobes, growing and aggregating to 3.5 mm diam, dark green 27F5–8; effuse conidiation spreading from the plug, forming conidia in wet heads to 120(–170) μm diam. At 30 °C colony distinctly and finely zonate, with surface in the centre and at the zone margins becoming farinose. Conidiation in shrubs to 0.5 mm diam spreading from the centre, mostly dry, becoming green, forming aggregates.
On PDA after 72 h 4–5 mm at 15 °C, 14–15 mm at 25 °C, 2–6 mm at 30 °C; mycelium covering the plate after 11–12 d at 25 °C. Colony circular, dense, developing alternating broad and narrow zones, surface white to yellowish downy by numerous long aerial hyphae. Autolytic excretions and coilings scant. No diffusing pigment noted, reverse yellowish, 3–4AB3–4. Odour none or slightly mushroomy. Conidiation noted after 2 d, green after 4 d, effuse, (nearly) gliocladium-like, conidiophores asymmetrically branched, short, phialides steep, some parallel, conidia formed in wet heads to 50 μm diam, ascending on aerial hyphae. At 15 °C colony of irregular outline, dense, densely farinose, whitish-yellowish-green, conidiation effuse. At 30 °C colony circular, dense, surface zonate, white farinose, reverse yellow 3–4A3–4 to dull yellowish brown 5CD5–8. Conidiation effuse, poor.
On SNA after 72 h 1–3 mm at 15 °C, 4–5 mm at 25 °C, 0–1 mm at 30 °C; mycelium covering the plate after ca. 5 wk at 25 °C. Colony hyaline, of irregular outline, dense, indistinctly zonate, downy, with submoniliform marginal hyphae; growth within agar faster than on surface; mycelium degenerating soon. Aerial hyphae common, becoming fertile. Autolytic excretions and coilings scant. No pigment, no distinct odour noted. No chlamydospores seen. Conidiation noted after 2–3 d, green after 4 d, first in small pustules concentrated around the plug, confluent, becoming dark green, 28F5–8. Additional granules or minute pustules spreading more or less radially across the colony; more complex and higher than on CMD, green, 27DE3–6, 28DE4–7, 29E4–8. Conidia formed in wet heads to ca. 130 μm diam, soon becoming dry in larger shrubs. At 15 °C little growth, conidiation abundant, green, conidia formed in large wet heads to 200(–350) μm diam. At 30 °C colony circular, dense, zonate, surface granulose; conidiation more verticillium-like with low conidial yield, pale greenish.
On MEA the plate becoming entirely covered by mycelium after less than 10 d, with yellow and green conidiation zones.
Habitat: on and around basidiomata of Steccherinum ochraceum on wood and bark.
Distribution: North America; Europe (Austria).
Holotype: USA, Maryland, Washington County, South Mountain State Park, Raven Rock Hollow; on hymenium of Steccherinum ochraceum, and well-rotted wood around basidiomata, soc. Nemania sp. on wood, 4 Sep. 1995; G.J. Samuels, H.-J. Schroers (BPI 737702; ex-type culture of T. thelephoricola: G.J.S. 95-135 = CBS 114237 = DAOM 232843, dry culture BPI 843671).
Other specimens examined: Austria, Niederösterreich, Bruck/Leitha, Mannersdorf am Leithagebirge, Schweingraben, MTB 8065/2, 47°58`20“N, 16°37`41“E, elev. 240 m, on hymenium of Steccherinum ochraceum/Carpinus betulus, holomorph, good material, 5 Sep. 2005, H. Voglmayr, W.J. 2845 (WU 29166, culture CBS 120925 = C.P.K. 2415). Wien-Umgebung, Mauerbach, Friedhofstrasse, MTB 7763/1, 48°15'16” N, 16°10'12” E, elev. 320 m, on Steccherinum ochraceum/Carpinus betulus, branches 6 cm diam, also on well-rotted wood close to the basidiomata, and soc./partly on an effete Nemania sp., soc. rhizomorphs, leaves; holomorph, 30 Sep. 2006, W. Jaklitsch, W.J. 2999 (WU 29167, culture C.P.K. 2480). Same area, 48°15'22” N, 16°10'14” E, elev. 330 m, on Steccherinum ochraceum/Quercus petraea, also on bark and on/soc. Corticiaceae, soc. hyphomycetes, effete pyrenomycete, 7 Oct. 2006, W. Jaklitsch & H. Voglmayr, W.J. 3005 (WU 29168, culture CBS 121138 = C.P.K. 2482). Kaltenleutgeben, along brook Dürre Liesing between Am Brand and Stangau, MTB 7862/4, 48°06'45” N, 16°08'43” E, elev. 450 m, on Steccherinum ochraceum/Alnus glutinosa, twigs 1–4 cm diam, and on bark, soc. Bisporella citrina, Hypocrea sp. 4 and H. moravica, holomorph, 22 Oct. 2006, W. Jaklitsch & H. Voglmayr, W.J. 3026 (WU 29169, culture C.P.K. 2493).
Notes: Stromata of H. thelephoricola look much like those of H. sinuosa or the American H. chlorospora, due to the bright yellow, projecting perithecia, particularly when young. Mature or old stromata resemble more those of H. strictipilosa. However, the specific association of H. thelephoricola with Steccherinum ochraceum permits easy identification. In addition, H. chlorospora has not been found in Europe, while a morphological distinction from the H. sinuosa teleomorph is possible due to slightly smaller ascospores and the peculiar orange reaction of the perithecial wall to KOH in H. thelephoricola, while the ostiolum remains colourless. The reaction to KOH and association with Steccherinum ochraceum was also verified in the holotype. The selection of the epithet thelephoricola is somewhat unfortunate, because the host genus is not a member of the Thelephoraceae, but was treated as an own family Steccherinaceae for a long time, and recently identified by gene phylogeny to belong to the Meruliaceae (Larsson 2007). The nearly gliocladium-like conidiation of T. thelephoricola is distinctive, but also the much slower growth and the absence of chlamydospores are further criteria for the distinction of T. thelephoricola from T. sinuosum and T. strictipile. Growth of T. thelephoricola is substantially faster on PDA and even faster on MEA (data not shown) than on CMD and SNA, indicating preference for rich media.
Acknowledgments
I want to express special thanks to Hermann Voglmayr for the collection of numerous specimens in separate and joint excursions and for friendship and support in many ways. Special thanks also to Christian P. Kubicek, Gary Samuels and Walter Gams for stimulating this work; C.P. Kubicek and his collaborators Irina Druzhinina, Monika Komon-żelazowska and Alexej Koptchinskiy for support at the TU Vienna; to Gary Samuels for technical instructions at the USDA-ARS, various materials and numerous discussions; and Walter Gams for specimens, excursion support in the Netherlands, and co-editorship of this manuscript. Thanks to Amy Rossman for editorship of the manuscript, and an anonymous person for a thorough review. Sincere thanks are due to Jan Holec, Ursula Peintner and Christian Scheuer for perfect organisation of joint excursions. Thanks to Alex Akulov, Hans-Otto Baral, Reinhard Geiter, Sven-Åke Hanson, Anton Hausknecht, Seppo Huhtinen, Peter Karasch, Wolfgang Klofac, Lothar Krieglsteiner, Thomas Læssøe, Kadri Põldmaa, Peter Roberts, Kerry Robinson, Klaus Siepe, Olga Sükösd, Alexander Urban, John Webster and Peter Wilberforce for specimens and/or support with excursions; Priscila Chaverri for discussions and papers; Irmgard Greilhuber for specimens and the long-term loan of Kornerup & Wanscher; Jean Mouchacca for search of Gliocladium type material in PC; Barrie Overton for discussions and gene sequences; Svengunnar Ryman and Roland Moberg (UPS) for excursion support in Sweden; Leif Ryvarden for help with issues in host identification; and Martina Vašutova for the translation of the description of H. cordyceps Vel. Sincere thanks are due to the curators Begoña Aguirre-Hudson and Brian Spooner (K), Anna-Lena Anderberg (S), Ellen Bloch (NY), János Gönczöl (BP), Antonio Graniti (ROM), Burghard Hein (B), Jan Holec and Jan Stepanek (PRC, PRM), Fernand Jacquemoud (G), Henning Knudsen (C), Genevieve Lewis-Gentry (FH), Rossella Marcucci (PAD), Uwe Passauer (W), Erin McCray (BPI), Walter Till (WU), Dagmar Triebel (M); the staff at the CBS, and Michael Brandstätter at Nikon Optoteam. Thanks to the editors of Mycologia for the permission to use data from earlier publications. The financial and other support by the Austrian Science Fund (FWF projects P16465-B03, P19143-B17) is gratefully acknowledged.
Taxonomic novelties: Hypocrea aeruginea Jaklitsch, Trichoderma aerugineum Jaklitsch, T. dacrymycellum Jaklitsch, H. danica Jaklitsch, H./T. fomiticola Jaklitsch, H. longipilosa Jaklitsch, H./T. parepimyces Jaklitsch, H. parestonica Jaklitsch, T. parestonicum Jaklitsch.
References
- Bissett J (1984). A revision of the genus Trichoderma. I. Section Longibrachiatum sect. nov. Canadian Journal of Botany 62: 924–931. [Google Scholar]
- Bissett J (1991a). A revision of the genus Trichoderma. II. Infrageneric classification. Canadian Journal of Botany 69: 2357–2372. [Google Scholar]
- Bissett J (1991b). A revision of the genus Trichoderma. III. Section Pachybasium. Canadian Journal of Botany 69: 2373–2417. [Google Scholar]
- Brooks FT, Mathieson J (1950). A note on Chromocrea spinulosa (Fuckel) Petch n. comb. Transactions of the British Mycological Society 33: 350–351. [Google Scholar]
- Carey ST, Rogerson CT (1976). Taxonomy and morphology of a new species of Hypocrea on Marasmius. Brittonia 28: 381–389. [Google Scholar]
- Chamberlain HL, Rossman AY, Stewart EL, Ulvinen T, Samuels GJ (2004). The stipitate species of Hypocrea (Hypocreales, Hypocreaceae) including Podostroma. Karstenia 44: 1–24. [Google Scholar]
- Chaverri P, Candoussau F, Samuels GJ (2004). Hypocrea phyllostachydis and its Trichoderma anamorph, a new bambusicolous species from France. Mycological Progress 3: 29–36. [Google Scholar]
- Chaverri P, Castlebury LA, Overton BE, Samuels GJ (2003a). Hypocrea/Trichoderma: species with conidiophore elongations and green conidia. Mycologia 95: 1100–1140. [PubMed] [Google Scholar]
- Chaverri P, Castlebury LA, Samuels GJ, Geiser DM (2003b). Multilocus phylogenetic structure within the Trichoderma harzianum/Hypocrea lixii complex. Molecular Phylogenetics and Evolution 27: 302–313. [DOI] [PubMed] [Google Scholar]
- Chaverri P, Samuels GJ (2002). Hypocrea lixii, the teleomorph of Trichoderma harzianum. Mycological Progress 1: 283–286. [Google Scholar]
- Chaverri P, Samuels GJ (2003). Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): Species with green ascospores. Studies in Mycology 48: 1–116. [Google Scholar]
- Chaverri P, Samuels GJ, Stewart EL (2001a). Hypocrea virens sp. nov., the teleomorph of Trichoderma virens. Mycologia 93: 1113–1124. [Google Scholar]
- Chaverri P, Samuels GJ, Stewart EL, Umaña L (2001b). Hypocrea nigrovirens, a new species with a gliocladium-like anamorph. Mycologia 93: 758–763. [Google Scholar]
- Clemmensen LH, Hansen ME, Frisvad JC, Ersbøll BK (2007). A method for comparison of growth media in objective identification of Penicillium based on multi-spectral imaging. Journal of Microbiological Methods 69: 249–255. [DOI] [PubMed] [Google Scholar]
- Degenkolb T, Dieckmann R, Nielsen KF, Gräfenhan T, Theis C, Zafari D, Chaverri P, Ismaiel A, Brückner H, Döhren H von, Thrane U, Petrini O, Samuels GJ (2008a). The Trichoderma brevicompactum clade: a separate lineage with new species, new peptaibiotics, and mycotoxins. Mycological Progress 7: 177–219. [Google Scholar]
- Degenkolb T, Döhren H von, Nielsen KF, Samuels GJ, Brückner H (2008b). Recent Advances and Future Prospects in Peptaibiotics, Hydrophobin, and Mycotoxin Research, and Their Importance for Chemotaxonomy of Trichoderma and Hypocrea. Chemistry & Biodiversity 5: 671–680. [DOI] [PubMed] [Google Scholar]
- Degenkolb T, Gräfenhan T, Nirenberg HI, Gams W, Brückner H (2006a). Trichoderma brevicompactum Complex: Rich Source of Novel and Recurrent Plant-Protective Polypeptide Antibiotics (Peptaibiotics). Journal of Agricultural and Food Chemistry 54: 7047–7061. [DOI] [PubMed] [Google Scholar]
- Degenkolb T, Gräfenhan T, Berg A, Nirenberg HI, Gams W, Brückner H (2006b). Peptaibiomics: Screening for Polypeptide Antibiotics (Peptaibiotics) from Plant-Protective Trichoderma Species. Chemistry & Biodiversity 3: 593–610. [DOI] [PubMed] [Google Scholar]
- Dennis RWG (1981). British Ascomycetes. J. Cramer. Vaduz.
- Dingley JM (1952). The Hypocreales of New Zealand III. The genus Hypocrea. Transactions of the Royal Society of New Zealand 79: 323–337. [Google Scholar]
- Dingley JM (1956). The Hypocreales of New Zealand, VII. A revision of records and species in the Hypocreaceae. Transactions of the Royal Society of New Zealand 83: 643–662. [Google Scholar]
- Dingley JM (1957). Life history studies in the genus Hypocrea Fr. Transactions of the Royal Society of New Zealand 84: 689–693. [Google Scholar]
- Dodd SL, Crowhurst RN, Rodrigo AG, Samuels GJ, Hill RA, Stewart A (2000). Examination of Trichoderma phylogenies derived from ribosomal DNA sequence data. Mycological Research 104: 23–34. [Google Scholar]
- Dodd SL, Lieckfeldt E, Chaverri P, Overton BE, Samuels GJ (2002). Taxonomy and phylogenetic relationships of two species of Hypocrea with Trichoderma anamorphs. Mycological Progress 1: 409–428. [Google Scholar]
- Dodd SL, Lieckfeldt E, Samuels GJ (2003). Hypocrea atroviridis sp. nov., the teleomorph of Trichoderma atroviride. Mycologia 95: 27–40. [DOI] [PubMed] [Google Scholar]
- Doi Y (1966). A revision of Hypocreales with cultural observation I. Some Japanese species of Hypocrea and Podostroma. Bulletin of the National Science Museum Tokyo 9: 345–357. [Google Scholar]
- Doi Y (1968). Revision of the Hypocreales with cultural observations II. Hypocrea dichromospora, sp. nov. and its Trichoderma state. Bulletin of the National Science Museum Tokyo 11: 185–189. [Google Scholar]
- Doi Y (1969). Revision of the Hypocreales with cultural observations IV. The genus Hypocrea and its allies in Japan. (1) General Part. Bulletin of the National Science Museum Tokyo, series B 12: 693–724. [Google Scholar]
- Doi Y (1971). Some species of the genus Hypocrea. Bulletin of the National Science Museum Tokyo 14: 387–400. [Google Scholar]
- Doi Y (1972). Revision of the Hypocreales with cultural observations IV. The genus Hypocrea and its allies in Japan. (2) Enumeration of the species. Bulletin of the National Science Museum Tokyo, series B 15: 649–751. [Google Scholar]
- Doi Y (1975). Revision of Hypocreales with cultural observations VII. The genus Hypocrea and its allied genera in South America (1). Bulletin of the National Science Museum Tokyo, series B 1: 1–33. [Google Scholar]
- Doi Y (1976). Revision of Hypocreales with cultural observations IX. The genus Hypocrea and its allied genera in South America (2). Bulletin of the National Science Museum Tokyo, series B 2: 119–131. [Google Scholar]
- Doi Y (1978). Revision of Hypocreales with cultural observations XI. Additional notes on Hypocrea and its allies in Japan (1). Bulletin of the National Science Museum Tokyo, series B 4: 19–26. [Google Scholar]
- Doi Y (1982). Type study on Hypocrea grandis Imai and Chromocrea nigricans Imai. Bulletin of the National Science Museum Tokyo, series B 8: 29–33. [Google Scholar]
- Doi Y (2001). A new species of Hypocrea (Ascomycota, Hypocreales) from Mikurajima Island, Japan. Memoirs of the National Science Museum Tokyo 37: 113–118. [Google Scholar]
- Doi Y (2006). Revision of the Hypocreales with cultural observations XIII. The Hypocreaceae of the Sagami Sea Maritime Forests, Japan. Memoirs of the National Science Museum Tokyo 42: 223–232. [Google Scholar]
- Doi Y, Liu P-G, Tamura M (2001). A new species of the Hypocreales (Ascomycota) from Mt. Changbaishan, Northeast China. Bulletin of the National Science Museum Tokyo, series B 27: 57–63. [Google Scholar]
- Druzhinina IS, Kopchinskiy AG, Komon M, Bissett J, Szakacs G, Kubicek CP (2005). An oligonucleotide barcode for species identification on Trichoderma and Hypocrea. Fungal Genetics and Biology 42: 813–828. [DOI] [PubMed] [Google Scholar]
- Druzhinina IS, Kopchinskiy AG, Kubicek CP (2006). The first one hundred of Trichoderma species characterized by molecular data. Mycoscience 47: 55–64. [Google Scholar]
- Druzhinina IS, Komon-Zelazowska M, Kredics L, Hatvani L, Antal Z, Belayneh T, Kubicek CP (2008). Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology 154: 3447–3459. [DOI] [PubMed] [Google Scholar]
- Druzhinina IS, Kubicek CP, Komon-Zelazowska M, Belayneh T, Bissett J (2009). Trichoderma harzianum is a recent agamospecies isolated from numerous sibling relicts and several genetically close biological species. BMC Evolutionary Biology: in press. [DOI] [PMC free article] [PubMed]
- Engel H, Hanff B (1986). Pilzneufunde in Nordwestoberfranken 1985, I. Teil/B. Neue Ascomyceten–Funde 1985 (z. T. auch früher) in Nordwestoberfranken. Pilzflora Nordwest-Oberfrankens 10: 21–41. [Google Scholar]
- Fries E (1825). Systema Orbis Vegetabilis 1: i–vii, 369 pp. Sweden, Lund. [Google Scholar]
- Gams W, Bissett J (1998). Morphology and identification of Trichoderma. In: Kubicek CP, Harman GE, eds. Trichoderma & Gliocladium, Vol. 1, Basic Biology, Taxonomy, and Genetics. pp. 3–34. Taylor & Francis Ltd. London. [Google Scholar]
- Gherbawy Y, Druzhinina IS, Shaban GM, Wuczkowsky M, Yaser M, El-Naghy MA, Prillinger H-J, Kubicek CP (2004). Trichoderma populations from alkaline agricultural soil in the Nile valley, Egypt, consist of only two species. Mycological Progress 3: 211–218. [Google Scholar]
- Hanada RE, Jorge Souza T de, Pomella AWV, Prakash Hebbar K, Pereira JO, Ismaiel A, Samuels GJ (2008). Trichoderma martiale sp. nov., a new endophyte from sapwood of Theobroma cacao with a potential for biological control. Mycological Research 112: 1335–1343. [DOI] [PubMed] [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004). Trichoderma species — opportunistic, avirulent plant symbionts. Nature Reviews | Microbiology 2: 43–56. [DOI] [PubMed] [Google Scholar]
- Harman GE, Kubicek CP (1998). Trichoderma & Gliocladium. Vol. 2. Enzymes, biological control and commercial applications. Taylor & Francis Ltd. London. 393 pp. [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS (2005). Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 97: 1365–1378. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS (2006a). Hypocrea crystalligena sp. nov., a common European species with a white-spored Trichoderma anamorph. Mycologia 98: 499–513. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Samuels GJ, Dodd SL, Lu B-S, Druzhinina IS (2006b). Hypocrea rufa/Trichoderma viride: a reassessment, and description of five closely related species with and without warted conidia. Studies in Mycology 56: 135–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Kubicek CP, Druzhinina IS (2008a). Three European species of Hypocrea with reddish brown stromata and green ascospores. Mycologia 100: 796–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Gruber S, Voglmayr H (2008c). Hypocrea seppoi, a new stipitate species from Finland. Karstenia 48: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Põldmaa K, Samuels GJ (2008b). Reconsideration of Protocrea (Hypocreales, Hypocreaceae). Mycologia 100: 962–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindermann J, El-Ayouti Y, Samuels GJ, Kubicek CP (1998). Phylogeny of the genus Trichoderma based on sequence analysis of the internal transcribed spacer region 1 of the rDNA cluster. Fungal Genetics and Biology 24: 298–309. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008). Dictionary Of The Fungi, 10th Edition. CABI, UK.
- Kiyuna T, An K-D, Kigawa R, Sano C, Miura S, Sugiyama J (2008). Mycobiota of the Takamatsuzuka and Kitora Tumuli in Japan, focusing on the molecular phylogenetic diversity of Fusarium and Trichoderma. Mycoscience 49: 298–311. [Google Scholar]
- Klein D, Eveleigh DE (1998). Ecology of Trichoderma. In: Kubicek CP, Harman GE, eds. Trichoderma & Gliocladium, Vol. 1, Basic Biology, Taxonomy, and Genetics. pp. 57–74. Taylor & Francis Ltd. London. [Google Scholar]
- Komon-Zelazowska M, Bissett J, Zafari D, Hatvani L, Manczinger L, Woo S, Lorito M, Kredics L, Kubicek CP, Druzhinina IS (2007). Genetically closely related but phenotypically divergent Trichoderma species cause green mold disease in oyster mushroom farms worldwide. Applied and Environmental Microbiology 73: 7415–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchinskiy AG, Komon M, Kubicek CP, Druzhinina IS (2005). TrichoBLAST: A multilocus database for Trichoderma and Hypocrea identifications. Mycological Research 109: 657–660. [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH (1981). Taschenlexikon der Farben. Zürich-Göttingen: Muster-Schmidt Verlag.
- Kredics L, Antal Z, Doczi I, Manczinger L, Kevei F, Nagy E (2003). Clinical importance of the genus Trichoderma. A review. Acta Microbiologica et Immunologica Hungarica 50: 105–117. [DOI] [PubMed] [Google Scholar]
- Kullnig CM, Krupica T, Woo SL, Mach RL, Rey M, Benítez T, Lorito M, Kubicek CP (2001). Confusion abounds over identities of Trichoderma biocontrol isolates. Mycological Research 105: 770–772. [Google Scholar]
- Kullnig-Gradinger CM, Szakács G, Kubicek CP (2002). Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycological Research 106: 757–767. [Google Scholar]
- Larsson K-H (2007). Re-thinking the classification of corticioid fungi. Mycological Research 111: 1040–1063. [DOI] [PubMed] [Google Scholar]
- Lieckfeldt E, Kuhls K, Muthumeenakshi S (1998). Molecular taxonomy of Trichoderma and Gliocladium and their teleomorphs. In: Kubicek CP, Harman GE, eds. Trichoderma & Gliocladium. Vol. 1. Basic biology, taxonomy and genetics. pp. 35–56. Taylor & Francis Ltd. London. [Google Scholar]
- Lieckfeldt E, Cavignac Y, Fekete C, Börner T (2000). Endochitinase gene-based phylogenetic analysis of Trichoderma. Microbiological Research 155: 1–9. [DOI] [PubMed] [Google Scholar]
- Link DH (1833). Handbuch zur Erkennung der nutzbarsten und am häufigsten vorkommenden Gewächse 3: 1–536. [Google Scholar]
- Liu YL, Whelen S, Hall BD (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lu BS, Samuels GJ (2003). Hypocrea stilbohypoxyli and its Trichoderma koningii-like anamorph: a new species from Puerto Rico on Stilbohypoxylon moelleri. Sydowia 55: 255–266. [Google Scholar]
- Lu B, Druzhinina IS, Fallah P, Chaverri P, Gradinger C, Kubicek CP, Samuels GJ (2004). Hypocrea/Trichoderma species with pachybasium-like conidiophores: teleomorphs for T. minutisporum and T. polysporum and their newly discovered relatives. Mycologia 96: 310–342. [PubMed] [Google Scholar]
- Mathieson J (1952). Ascospore dimorphism and mating type in Chromocrea spinulosa. Annals of Botany London, New Series 16: 449–466. [Google Scholar]
- McNeill J, Barrie F, Burdet HM, Demoulin V, Hawksworth DL, Marhold K, Nicolson DH, Prado J, Silva PC, Skog JE, Wiersema JH, Turland NJ (eds) (2006). International Code of Botanical Nomenclature (Vienna Code) adopted by the Seventeenth International Botanical Congress Vienna, Austria, July 2005. Gantner Verlag, Ruggell, Liechtenstein.
- Migheli Q, Balmas V, Komon-Zelazowska M, Scherm B, Fiori S, Kopchinskiy AG, Kubicek CP, Druzhinina IS (2008). Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environmental Microbiology (Online publication date: 1 October. 2008). [DOI] [PubMed]
- Moravec Z (1956a). Arachnocrea, un genre nouveau de la famille des Nectriaceae. Bulletin trimestriel de la Société Mycologique de France 72: 160–166. [Google Scholar]
- Moravec Z (1956b). Nové nebo méně známé tvrdohouby z řádu Hypocreales. Ceska Mykologie 10: 87–91. [Google Scholar]
- Müller E, Aebi B, Webster J (1972). Culture studies on Hypocrea and Trichoderma V. Hypocrea psychrophila sp. nov. Transactions of the British Mycological Society 58: 1–4. [Google Scholar]
- Nicholas KB, Nicholas Jr HB, Deerfield II DW (1997). Gene-Doc: analysis and visualization of genetic variation. EMBNET News 4: 1–4. [Google Scholar]
- Overton BE, Stewart EL, Geiser DM, Wenner NG, Jaklitsch W (2006a). Systematics of Hypocrea citrina and allies. Studies in Mycology 56: 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton BE, Stewart EL, Geiser DM (2006b). Taxonomy and phylogenetic relationships of nine species of Hypocrea with anamorphs assignable to Trichoderma section Hypocreanum. Studies in Mycology 56: 39–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MS, Bae KS, Yu SH (2006). Two new species of Trichoderma associated with green mold of oyster mushroom cultivation in Korea. Mycobiology 34: 11–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoon CH (1794). Dispositio methodica fungorum. Neues Magazin für die Botanik 1: 81–128. [Google Scholar]
- Petch T (1938). British Hypocreales. Transactions of the British Mycological Society 21: 243–305. [Google Scholar]
- Petrak F (1940). Mykologische Notizen XIII. Annales Mycologici 38: 181–267. [Google Scholar]
- Posada D, Crandall KA (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Rannala B, Yang Z (1996). Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. Journal of Molecular Evolution 43: 304–311. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ (1994). Taxonomy and phylogeny of Gliocladium analyzed by large subunit rDNA sequences. Mycological Research 98: 625–634. [Google Scholar]
- Rifai MA (1969). A revision of the genus Trichoderma. Mycological Papers 116: 1–56. [Google Scholar]
- Rifai MA, Webster J (1966). Culture studies on Hypocrea and Trichoderma II+III. Transactions of the British Mycological Society 49: 289–310. [Google Scholar]
- Rossman AY (1996). Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia 88: 1–19. [Google Scholar]
- Rossman AY (2000). Towards monophyletic genera in the holomorphic Hypocreales. Studies in Mycology 45: 27–34. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Samuels GJ (2006) Trichoderma: Systematics, the sexual state, and ecology. Phytopathology 96: 195–206. [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Doi Y, Rogerson CT (1990). Contributions toward a mycobiota of Indonesia: Hypocreales. Memoirs of the New York Botanical Garden 59: 6–108. [Google Scholar]
- Samuels GJ, Petrini O, Kuhls K, Lieckfeldt E, Kubicek CP (1998). The Hypocrea schweinitzii complex and Trichoderma sect. Longibrachiatum. Studies in Mycology 41: 1–54. [Google Scholar]
- Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O (2002). Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 94: 146–170. [PubMed] [Google Scholar]
- Samuels GJ, Dodd S, Lu B-S, Petrini O, Schroers H-J, Druzhinina IS (2006a). The Trichoderma koningii aggregate species. Studies in Mycology 56: 67–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels GJ, Suarez C, Solis K, Holmes KA, Thomas SE, Ismaiel A, Evans HC (2006b). Trichoderma theobromicola and T. paucisporum: two new species isolated from cacao in South America. Mycological Research 110: 381–392. [DOI] [PubMed] [Google Scholar]
- Samuels GJ, Ismaiel A (2009). Trichoderma evansii and T. lieckfeldtiae: two new T. hamatum-like species. Mycologia 101: 142–156. [DOI] [PubMed] [Google Scholar]
- Seaver FJ (1910). The Hypocreales of North America - III. Mycologia 2: 48–92. [Google Scholar]
- Seifert KA, Samuels GJ (1997). Two new hypocrealean fungi with synnematous anamorphs. Mycologia 89: 512–520. [Google Scholar]
- Sivasithamparam K, Ghisalberti EL (1998). Secondary metabolism in Trichoderma and Gliocladium. In: Kubicek CP, Harman GE, eds. Trichoderma & Gliocladium, Vol. 1, Basic Biology, Taxonomy, and Genetics. pp. 139–191. Taylor & Francis Ltd. London. [Google Scholar]
- Swofford DL (2002). PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods), v. 4.0b10. Sinauer Associates, Sunderland, MA.
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000). Phylogenetic species recognition and species concepts in Fungi. Fungal Genetics and Biology 31: 21–31. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulasne L-R, Tulasne C (1865). Selecta Fungorum Carpologia. Vol. 3. Paris.
- Webster J (1964). Culture studies on Hypocrea and Trichoderma I. Comparison of perfect and imperfect states of H. gelatinosa, H. rufa and Hypocrea sp. 1. Transactions of the British Mycological Society 47: 75–96. [Google Scholar]
- Webster J, Rifai MA (1968). Culture studies on Hypocrea and Trichoderma IV. Hypocrea pilulifera sp. nov. Transactions of the British Mycological Society 51: 511–514. [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W (1994). Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research 22: 4354–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. eds. PCR Protocols: A Guide to Methods and Applications. pp. 315–322. San Diego, California, USA: Academic Press.
- Wuczkowski M, Druzhinina I, Gherbawy Y, Klug B, Prillinger H, Kubicek CP (2003). Species pattern and genetic diversity of Trichoderma in a mid-European, primeval floodplain-forest. Microbiological Research 158: 125–133. [DOI] [PubMed] [Google Scholar]
- Yang Z, Rannala B (1997). Bayesian phylogenetic inference using DNA sequences: a Markov chain Monte Carlo method. Molecular Biology and Evolution 14: 717–724. [DOI] [PubMed] [Google Scholar]
- Zachow C, Berg C, Müller H, Meincke R, Komon-Zelazowska M, Druzhinina IS, Kubicek CP, Berg G (2008). Fungal diversity in the rhizosphere of endemic plant species of Tenerife (Canary Islands): relationship to vegetation zones and environmental factors. ISME Journal 3: 79–92. [DOI] [PubMed] [Google Scholar]
- Zhang C-l, Druzhinina IS, Kubicek CP, Xu T (2005). Trichoderma biodiversity in China: Evidence for a North to South distribution of species in East Asia. FEMS Microbiology Letters 251: 251–257. [DOI] [PubMed] [Google Scholar]
- Zhang C-l, Liu S-p, Lin F-c, Kubicek CP, Druzhinina IS (2007). Trichoderma taxi sp. nov., an endophytic fungus from Chinese yew Taxus mairei. FEMS Microbiology Letters 270: 90–96. [DOI] [PubMed] [Google Scholar]