Abstract
The gel electrophoresis mobility shift assay (EMSA) is used to detect protein complexes with nucleic acids. It is the core technology underlying a wide range of qualitative and quantitative analyses for the characterization of interacting systems. In the classical assay, solutions of protein and nucleic acid are combined and the resulting mixtures are subjected to electrophoresis under native conditions through polyacrylamide or agarose gel. After electrophoresis, the distribution of species containing nucleic acid is determined, usually by autoradiography of 32P-labeled nucleic acid. In general, protein-nucleic acid complexes migrate more slowly than the corresponding free nucleic acid. In this article, we identify the most important factors that determine the stabilities and electrophoretic mobilities of complexes under assay conditions. A representative protocol is provided and commonly used variants are discussed. Expected outcomes are briefly described. References to extensions of the method and a troubleshooting guide are provided.
Keywords: Nucleic acid-protein interactions, Gel retardation, Electrophoretic mobility shift assay, Binding detection, Binding analysis
INTRODUCTION
The electrophoretic mobility shift assay (EMSA) is a rapid and sensitive method to detect protein-nucleic acid interactions1–6. It is based on the observation that the electrophoretic mobility of a protein-nucleic acid complex is typically less than that of the free nucleic acid (Fig. 1). The current, widely-used assay differs little from that originally described by Fried and Crothers7 and Garner and Revzin8, although precursors to the technique can be found in the earlier literature9–11. Mobility-shift assays are often used for qualitative purposes, although under appropriate conditions they can provide quantitative data for the determination of binding stoichiometries, affinities and kinetics3, 6, 12. Methods used in performing the assay differ for each purpose, and a large number of variants have been described in the literature (see Table 1).
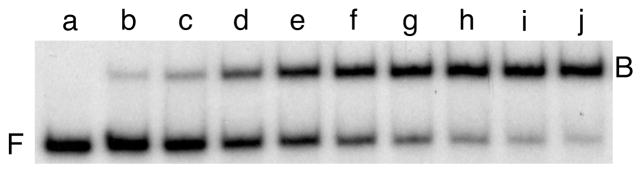
Figure 1. Titration of a 214 bp lac promoter DNA fragment with E. coli CAP protein.
Sample compositions are given in Table 4. The complex consists of a single molecule of CAP bound predominantly to the highest affinity CAP site in the lac promoter (CAP site 149, 66). Electrophoresis was carried out with a 10% w/v polyacrylamide (75:1 acrylamide:bisacrylamide) gel, cast and run in the Tris-acetate EDTA buffer described in the protocol shown in Table 5. Cyclic AMP (final concentration 20 μM) was added to the electrophoresis running buffer. Pre-electrophoresis ensured that the cAMP was distributed throughout the gel before the samples were loaded. Band designations: F, free DNA; B, bound DNA (i.e., protein-DNA complex).
Table 1.
EMSA Variants
| Method and Reference(s) | Purpose |
|---|---|
| 1-dimensional methods | |
| Time course7, 39 | Measurement of association and/or dissociation kinetics |
| Double-label assays31, 32 | Determination of binding stoichiometry |
| Continuous variation33, 91 | Determination of binding stoichiometry |
| Circular permutation92 | Detection of DNA bending |
| Phased bends analysis79 | Determination of direction of a protein-induced bend with respect to a standard bend locus |
| Binding partition analysis3, 12, 18, 93, 94 | Evaluation of binding cooperativity for nucleic acids with multiple protein binding sites; comparison of binding affinities of one protein for several target sequences, determination of the effect of one protein on binding affinity of a second. |
| Reverse EMSA 95 | Detection of nucleic acid binding, measurement of binding affinity. In this assay, the tracer species is the protein. In the classical EMSA the nucleic acid is the tracer species. |
| Topoisomer EMSA22 | Detection of complexes formed with supercoiled DNA |
| Cryogenic-EMSA96 | Detection of labile complexes |
| Antibody supershift84 | Identification of protein(s) carrying a specific epitope in mobility-shifted complex(es) |
| Nucleosome shift 97,98 | Detection of protein(s) that bind nucleosomes |
| Heterogeneous subunit assay 99 | Determination of whether binding activity is due to a protein monomer or a protein oligomer |
| Binding site selection 100 | Selection of oligonucleotides containing a specific protein binding site from an ensemble of partially randomized sequences, allowing subsequent amplification and sequencing |
| Protein-protein101 | Detection of protein interactions in the absence of nucleic acids |
| DNA-DNA and RNA-RNA 102,103,104 | Detection of nucleic acid interactions in the absence of proteins |
| Protein-oligosaccharide 105 | Detection of protein binding to charged oligosaccharides |
| 2-dimensional methods | |
| EMSA followed by high temperature electrophoresis106 | Identification of DNA fragments containing specific binding sites for a given protein |
| EMSA as an adjunct to footprinting37 | Reduction of signal from free nucleic acid in footprint pattern |
| EMSA followed by electron microscopy107 | Characterization of conformation |
| EMSA followed by SDS-polyacrylamide gel electrophoresis with Western blot detection 85,86 | Identification of unknown binding proteins |
| EMSA followed by MS87, 88 | Identification of unknown binding proteins |
Advantages and limitations of EMSA
The mobility shift assay has a number of strengths. The basic technique is simple to perform, yet it is robust enough to accommodate a wide range of binding conditions (see Table 2 for representative ranges). Using radioisotope-labeled nucleic acids, the assay is highly sensitive, allowing assays to be performed with small protein and nucleic acid concentrations (0.1 nM or less) and small (≤20μL) sample volumes. When such high sensitivity is not needed, variants or the assay using fluorescence, chemiluminescence and immunohistochemical detection are also available13–17. A wide range of nucleic acid sizes (lengths from short oligonucleotides to several thousand nt/bp18, 19) and structures (single-stranded, duplex, triplex 20 and quadruplex 21 nucleic acids as well as small circular DNAs22) are compatible with the assay. Under favorable conditions, the distribution of proteins between several nucleic acid molecules can be monitored within a single solution 18, 23, as can the presence of complexes differing in protein stoichiometry and/or binding site distribution 7, 24. Proteins ranging in size from small oligopeptides to transcription complexes with Mr ≥ 106 can give useful mobility shifts 25, 26 and the assay works well with both highly-purified proteins and crude cell extracts 27. These capabilities account in large part for the continuing popularity of the assay.
Table 2.
Representative Ranges of Conditions for EMSA Using Polyacrylamide Gels Unless indicated, conditions refer to sample equilibration prior to electrophoresis.
| Quantity | Range | Comment | Reference |
|---|---|---|---|
| Temperature (T) | 0°C ≤ T ≤ 60°C | Temperature range applies to electrophoresis as well as sample equilibration. The observed range was limited by the incubator used, not the assay. This range applies to electrophoresis using polyacrylamide gels only. As a result of changes in agarose gel properties with temperature our tests have not exceeded 37°C. | M. Fried, unpublished result |
| Pressure (P) | ambient ≤ P ≤ 500 bar | Sample and gel incubated in pressure chamber, electrophoresis performed under pressure. | 108 |
| pH | 4.0 ≤ pH ≤ 9.5 | Care was taken to match conductivity of sample and electrophoresis buffer. | M. Fried, unpublished result |
| Monovalent salt (M+X−) | 1 mM ≤ [M+X−] ≤ 300 mM | Care was taken to match conductivity of sample and electrophoresis buffer. High-salt experiments were run in cold room to limit heating of gel. Buffer was circulated between cathodic and anodic reservoirs during electrophoresis of samples containing ≤20 mM salt. CAUTION! Electrolysis of chloride salt solutions generates chlorine gas. Where the chance of chlorine generation exists, electrophoresis should be performed in a well-ventilated area and preferably a fume hood. |
7,109,110,49 |
| Oligovalent ions | [Calcium acetate] ≤ 20 mM [Magnesium acetate] ≤ 20 mM [Spermidine chloride] ≤ 10 mM [Potassium phosphate] ≤ 300 mM [Sodium borate] ≤ 200 mM [Potassium sulfate] ≤ 300 mM |
Approximate limits, obtained for each salt separately in studies of the CAP- and lac repressor-DNA interactions. Care was taken to match conductivity of sample and electrophoresis buffer. High-salt experiments were run in cold room to limit heating of gel. Limits reflect the stability of these protein-DNA complexes, not the resolution of the electrophoretic process. | 109 M. Fried, unpublished result. |
| Zwitterions (Z) (e.g., glycine, proline, betaine) | [Z] ≤ 2M | Solutes used in samples for osmotic stress experiments. | 65,66,111,112 |
| Neutral solutes (N) (e.g., glycerol, ethylene glycol, sucrose) | [N] ≤ 2 M | Sometimes added to samples to aid loading of gels. Used in samples for osmotic stress experiments. Use in gel buffer can stabilize some complexes during electrophoresis. | 65,66,113, 114,111,112 |
| Neutral polymers (e.g., Polyethylene glycol 8000, linear polyacrylamide) | [polymer] ≤ 10% w/v | Limited by difficulty in loading gels with viscous samples. Electrophoretic resolution was unaffected, even at the highest neutral polymer concentrations. | 29, 66 |
| Reducing agents | [dithiothreitol] ≤ 10 mM [2-mercaptoethanol] ≤ 10mM [thioglycolic acid] ≤ 5 mM |
Dithiothreitol and 2-mercaptoethanol were added to samples only. Due to its negative charge, thioglycolate can be included in the gel running buffer and will migrate into the gel to ensure the presence of a reducing environment within the gel matrix. | 33 M. Fried, unpublished result. |
On the other hand, the EMSA is not without limitations. One theme of this article is the identification of potential problems and the suggestion of strategies that avoid or mitigate the most severe. Table 3 contains a guide for troubleshooting the most common problems that we have encountered. Perhaps the most important limitation is that samples are not at chemical equilibrium during the electrophoresis step. Rapid dissociation during electrophoresis can prevent detection of complexes, while even slow dissociation can result in underestimation of binding density. On the other hand, many complexes are significantly more stable in the gel than they are in free solution 28–30; when this is the case, short electrophoresis times allow the resolution of patterns that closely approximate the distributions of species present in the samples at the start of electrophoresis. A second limitation is that the electrophoretic mobility of a protein-nucleic acid complex depends on many factors other than the size of the protein. Thus, an observed mobility shift does not provide a straightforward measure of the molecular weights or identities of proteins that are present in the complex 12. The electrophoretic “supershift” assay and assays that combine EMSA with western blotting or mass spectroscopy have been devised to allow identification of nucleic acid-associated proteins (summarized in Table 1), while a range of EMSA-based and non-EMSA methods can be used for evaluation of binding stoichiometries 31–34. A third limitation is that the electrophoretic mobility of a complex provides little direct information about the location of the nucleic acid sequences that are occupied by protein. This information is available from nuclease and chemical footprinting assays that can be performed independently of EMSA or in concert with it35–38. Finally, the time resolution of the current assay is defined by the interval required for manual solution handling. This limits kinetics studies to processes with relaxation times significantly larger than the ~1 min required to mix reaction components and for electrophoretic migration into the gel matrix 39. Strategies designed to improve the time resolution of the technique are in development (M. Fried, unpublished results).
Table 3.
Troubleshooting
| Problem | Possible Causes | Possible Remedies |
|---|---|---|
| No bands visible after electrophoresis | Low nucleic acid concentration | Verify nucleic acid concentration. Repeat experiment with appropriate concentration of nucleic acid. |
| Inefficient labeling | Check reaction components for concentration and enzyme activity | |
| Nucleic acid degradation | If sample is radioactive, verify that nucleic acid is intact DNA by running a sequencing gel. If nuclease activity is suspected, treat buffers with diethyl pyrocarbonate. Exclude divalent cations wherever possible. Commercially available RNase inhibitors are useful to protect RNA substrates. Commercially-available phosphatase inhibitors can prevent substrate dephosphorylation. | |
| All bands are smeared or streaked | Uneven gel polymerization | Use fresh gel components. Degas thoroughly before polymerization. If polymerization interfered with casting gel, reduce TEMED concentration. If gel required >1h to polymerize, increase ammonium persulfate concentration. |
| Excessive gel heating | Check concentrations of gel and running buffer. If they are correct, reduce voltage during electrophoresis. | |
| Sample conductivity too high. | Reduce salt concentration in nucleic acid or sample buffer. | |
| Free nucleic acid migrates normally. Nucleic acid mobility unchanged in presence of protein | Low protein concentration | Verify protein concentration. Use larger volumes of protein stock or more concentrated stock in preparing EMSA samples. |
| Protein is inactive | Run SDS-polyacrylamide gel electrophoresis to rule out degradation. If the binding protein is an enzyme, test for activity. Test higher concentrations of protein to detect residual binding activity. A new, more active preparation of protein may be necessary. | |
| Protein is negatively charged and co-migrates with nucleic acid | Check migration of protein alone under EMSA conditions. If protein co-migrates with nucleic acid, reduce pH of binding and electrophoresis buffers 64. | |
| Free nucleic acid migrates normally. No nucleic acid detectable in samples containing protein. | Nucleic acid degradation | Verify that nucleic acid is intact. If nuclease activity is suspected, treat glassware and buffers with diethyl pyrocarbonate. Exclude divalent cations wherever possible. Use commercial RNase and phosphatase inhibitors. |
| Binding occurred but complexes dissociated during electrophoresis. Liberated nucleic acid is too diffuse to detect. | Minimize gel running times; use more concentrated gel; include stabilizing solutes in gel buffer; reduce salt concentration in binding and electrophoresis buffers to increase electrostatic stabilization; lower electrophoresis temperature (run gel in cold room); reduce or eliminate competing nucleic acid (this can be added back with care once a useful binding signal is obtained). | |
| Free nucleic acid migrates normally. Complex bands smeared or streaky | Binding occurred but complexes dissociated in gel during electrophoresis. | Minimize gel running times; use more concentrated gel; include stabilizing solutes in gel buffer; reduce salt concentration in binding and electrophoresis buffers to increase electrostatic stabilization; lower electrophoresis temperature (run gel in cold room); reduce or eliminate competing nucleic acid. |
| Binding occurred but complexes dissociated in the well prior to electrophoresis. | Minimize interval that sample is in well before electrophoresis. Complexes may be destabilized by component(s) of running buffer. If so they may be more stable in gel and running buffers that more closely resemble the composition of binding buffer. | |
| Binding occurred but complexes dissociated in the well during electrophoresis. | To minimize gel “dead time”, use smallest sample volume possible; conduct electrophoresis at high voltage (~50 V/cm) until samples enter the gel, then reduce to ~10V/cm. | |
| Binding occurred but samples containing protein are too salty. | Reduce salt concentration in protein stock and/or in binding buffer. | |
| Free band is sharp, complex band(s) are broad and indistinct | Heterogeneous protein. | Multiple species may be due to post-translational modification or to partial degradation without loss of binding activity. |
| Complex and free bands are broad and indistinct | Sample zone is too large (measured from top of sample to bottom of well) at the start of electrophoresis | Reduce sample volume. Increase density of sample (e.g., increase glycerol concentration) to facilitate gel loading. Minimize time between loading and electrophoresis |
| Electrophoresis period too long | Reduce run-time | |
| Nucleic acid degradation | Verify that nucleic acid is intact. If nuclease activity is suspected, treat extracts and buffers with diethyl pyrocarbonate. Exclude divalent cations wherever possible. Use RNase and phosphatase inhibitors. | |
| Nucleic acid stuck in well, no free species visible | Protein/nucleic acid ratio is too high | Reduce the concentration of protein or increase the concentration of unlabeled nonspecific competitor |
| Protein is aggregated | Change binding conditions to improve protein solubility. Possible modifications: add solutes that stabilize folded (compact) forms of proteins (e.g., glycerol); keep protein stocks and binding reactions at ice temperature; avoid freeze-thaw cycles with protein stocks; include non-ionic detergents in protein storage buffer and/or binding buffer | |
| Free nucleic acid and complexes are too large for gel system | Try lower percentage polyacrylamide or reduce the acrylamide/bisacrylamide ratio. Test agarose gel as alternative to polyacrylamide. |
Alternatives to EMSA
Many techniques are available for the detection and characterization of protein-nucleic acid complexes and most have advantages and disadvantages that differ from those of the EMSA. The most widely used alternative assays are nitrocellulose filter-binding 40, 41 and footprinting 35, 36, 42.
Filter binding is simple to perform and the manipulations are rapid enough to allow kinetic studies as well as equilibrium measurements 43, 44. Under favorable conditions, the assay can be highly sensitive and with the exception of the scintillation counter, the required equipment is inexpensive. Like the EMSA, filter binding is a non-equilibrium technique. As a result, quantitative analyses require careful evaluation of filter-retention efficiency 44, 45. The assay is not limited by the salt concentration of the sample and it can accommodate very large nucleic acids (e.g., the phage λ genome (48,502 bp46)47. In contrast, the electrophoretic process limits the salt concentrations of EMSA samples to ≤300 mM (1:1 salt) and to DNAs of 5000 bp18, 48, 49 (note that these limits are approximate and may vary with sample and gel compositions). The presence of more than one binding protein complicates filter binding analysis, since retention of labeled nucleic acid is detected, not the identity of bound proteins or the proportion of binding activity attributable to each. In addition, conditions that give efficient filter-retention of 1:1 protein-nucleic acid complexes do not result in significantly increased retention of higher stoichiometry complexes 44, so the classical assay is poorly suited for distinguishing complexes containing one protein from those containing more than one. Finally, under some solution conditions, single-stranded nucleic acids are retained by nitrocellulose filters 50, resulting in a background of retained radioactivity that can mask the binding signal. Thus, for applications involving more than one nucleic acid-binding protein, the detection of more than one protein-nucleic acid complex in a mixture and/or binding to single stranded nucleic acids, mobility shift assays offer clear advantages.
Footprinting assays 35, 51 exploit the observation 42 that a protein bound to a specific nucleic acid sequence will interfere with the chemical or enzymatic modification of that sequence. A large number of chemical and enzymatic agents are available for this purpose. In the classical assay, a radioisotope label is located at one end of one strand of the nucleic acid target. Following modification and any steps needed to cleave the nucleic acid at modification sites, fragments are resolved on a denaturing polyacrylamide gel. The resulting ladder of bands is visualized by autoradiography and gaps in the array indicate sites of protection by the protein(s) in the test mixture. Comparison of the protection pattern with sequencing reaction products can allow identification of protected sequences with single-nucleotide resolution. In addition to protection, the appearance of sites that are hypersensitive to modification can provide evidence of conformational change in the target nucleic acid 42. Because most other methods provide little direct information about the identities of protein binding sites, this method remains the “gold standard” for the identification of nucleic acid sequences within or near binding sites. Since sequences of several hundred residues can be resolved on a typical sequencing gel, the technique is well suited for the analysis of the binding of several protein molecules to a single nucleic acid (a strength that footprinting shares with EMSA). In addition, the footprint signal can be obtained under conditions of binding equilibrium for the protein of interest. This is an important advantage over non-equilibrium assays such as EMSA. Variants of the footprinting assay optimized for quantitative detection of binding have been described 35, 52 and time-resolved methods have been developed that allow the analysis of binding kinetics as well as equilibria 53, 54.
Footprinting assays require simultaneous optimization of binding by the protein(s) of interest and the nucleic acid modification reaction(s) needed to produce the footprint signal. Thus, they are somewhat more difficult to perform than EMSA or filter binding assays. In addition, because the radioisotope label is distributed over many nucleic acid fragments, the detection of binding by footprinting is less sensitive than the detection of binding using EMSA. In addition, incomplete binding results in a footprint pattern that contains contributions from both free and bound nucleic acids. This makes the protected regions indistinct, because the pattern of free nucleic acid is visible within the protected footprint. A popular solution to this problem is to use EMSA to separate complexes from free nucleic acids after the footprinting modification reaction. Following this step with denaturing gel electrophoresis allows one to independently visualize the fragment patterns of free and bound nucleic acid populations 37, 38. Finally, some proteins that bind nucleic acids without sequence specificity may not produce distinct footprints. For such systems, EMSA and filter binding assays provide binding signals that are easier to interpret than those available from footprinting.
Strategic considerations that are relevant to EMSA
No single set of binding and electrophoresis conditions works well for all molecular systems. However, several variables can be optimized for the study of a particular interaction, including design of the nucleic acid target, binding reaction conditions and electrophoresis conditions, as discussed below.
Selection of nucleic acid target
Short nucleic acids are easily synthesized and inexpensive to purchase. The small number of non-specific protein binding sites in a small DNA or RNA can be advantageous when the binding protein has low sequence-specificity12. In addition, electrophoretic resolution of complexes from free nucleic acid is highest with small nucleic acids and this makes possible short electrophoresis times. On the other hand, all binding sites on a short nucleic acid are close to the molecular ends. This can result in aberrant binding due to structural and electrostatic end-effects55–57. Longer templates avoid these limitations but contain more non-specific binding sites, migrate more slowly (requiring longer electrophoresis times) and generally give a smaller mobility-shift on protein binding6.
In the classical EMSA assay, the electrophoretic mobility of the nucleic acid is monitored. Nucleic acids can be labeled with radioisotopes58, covalent or non-covalent fluorophores13, 59 or biotin 60, 61. These labels can be detected by autoradiography, fluorescence imaging, chemiluminescent imaging and/or chromophore deposition, respectively. Alternatively, unlabeled nucleic acid can be used in the binding and electrophoresis steps and detected by post-electrophoretic staining with chromophores or fluorophores that bind nucleic acids7, 8, 14. Labeling the nucleic acid at the 5′- or 3′-end with (32P)-phosphate is a widely used approach, as it is inexpensive, offers great sensitivity (≤10−18 mol of 5′ ends can be detected on a routine basis7) and does not introduce artificial structures that might influence binding. Thus, when radioisotope use is permissible, we consider 32P-labeling the method of choice for making nucleic acids detectable in the EMSA assay. When radioisotope use is not possible, covalent labeling of nucleic acid with a fluorophore allows both qualitative and quantitative EMSA applications 62, although with lower sensitivity than is possible with radioisotope-labeled nucleic acids.
Binding conditions
Protein-nucleic acid interactions are sensitive to mono- and divalent salt concentrations63 and pH64. Although a typical Tris-based sample buffer is used in the protocol detailed here, good results have been obtained with many different buffers, including HEPES, MOPS, Bis-Tris, Glycine and Phosphate. We favor buffers that approximate physiological salt concentrations and pH and provide any needed co-factors at appropriate concentrations. However, as long as the conductivity of the sample is not excessive, electrophoresis can be carried out with a wide range of sample compositions. This allows the concentration of binding buffer components to be adjusted to optimize complex formation.
Additives
Small neutral solutes such as glycerol or sucrose are often used to stabilize labile proteins. Such solutes can also enhance the stabilities of protein-nucleic acid interactions65, 66 and can be valuable additions to binding reaction mixtures. They are generally effective in the [solute] < 2M range, but less is known about their effects at higher concentrations, where high viscosity and surface tension complicate solution handling. Addition of modest concentrations (≤0.1 mg/mL) of a carrier protein (e.g., BSA) to the binding reaction can minimize non-specific losses of binding proteins during solution handling. Similarly, non-ionic detergents are sometimes helpful in maximizing protein solubility2. The useful concentrations of detergents vary with the identities of the detergent and the molecular system under study. Protease, nuclease and phosphatase inhibitors can be useful additives, especially when the protein sample is a partially-fractionated cell or nuclear extract27. Mixtures of inhibitors are commercially available and should be used according to the supplier’s instructions. Finally, some systems require specific co-factors for correct function. Examples include cAMP for the E. coli CAP protein67, ATP for recombinases such as E. coli RecA or human Rad5168, 69 and polyamines for some eukaryotic transcription factors70. Where necessary, small-molecule additives that stabilize complexes can be included in binding and gel buffers, to stabilize complexes during electrophoresis3,7.
Competing nucleic acid
Often a protein sample will contain more than one nucleic acid binding activity. When secondary binding activities obscure the one of interest, the addition of unlabeled competing nucleic acid to the reaction mixture can reduce the binding of secondary proteins to the labeled target27. This strategy works when the protein of interest binds the target nucleic acid with greater affinity than it binds the competitor and when the secondary binding activities do not discriminate between competitor and target sequences. Since competing nucleic acids also reduce the amount of specific binding, even under favorable conditions, it is best to test a range of competitor concentrations to optimize discrimination of specific and non-specific binding. Commonly used competitors include genomic DNAs, poly d(A–T) and poly d(I–C)5.
Electrophoresis conditions
The resolution of complexes depends on their stability during electrophoresis. Since many buffers are compatible with electrophoresis, the composition and concentration of gel and running buffer components can be adjusted to optimize the stability of complexes. The most popular buffers are variants of Tris-borate-EDTA71,72, Tris-acetate-EDTA73 or Tris-glycine27. As mentioned above, low molecular weight co-factors and/or non-specific stabilizers such as glycerol or ethylene glycol can be included in gel- and running buffers to enhance the stability of complexes. In some circumstances it may be feasible to perform electrophoresis in the buffer that was used in the binding reaction3, 6. This avoids subjecting the sample to a change of buffer conditions during gel loading and as reaction components migrate into the gel. This has the advantage of avoiding any perturbation to the molecular system that might be due to a change in buffer composition; it also eliminates the need to independently optimize binding and electrophoresis buffer conditions.
Although both polyacrylamide and agarose gels have been used for EMSA7,8,74, polyacrylamide gels offer better electrophoretic resolution for protein-DNA and protein-RNA complexes of Mr ≤ 500,0003. In addition, some complexes are significantly more stable in polyacrylamide matrices than in agarose gels or free solution7,29. While the mechanism of this effect is not completely understood, for a few molecular systems it has been shown that the dissociation rates of complexes decrease with increasing gel concentration28, 30. On the other hand, the resolution of large complexes and the duration of electrophoresis is much longer in high percentage gels. Thus, optimization of gel concentration can be a valuable precursor to the most critical EMSA applications. One effective optimization strategy is to start with a relatively low concentration gel (e.g., 5% w/v acrylamide) and to increase this concentration systematically until any improvement in complex stability is balanced by loss of electrophoretic resolution and/or the onset of impractically long electrophoresis times.
Given below is a protocol for a representative mobility shift assay. The protein is purified E. coli cyclic AMP receptor protein (CAP) 49, 67 and the nucleic acid is a 214 bp restriction fragment from the E. coli lac promoter-operator region 48. The binding buffer consists of 10 mM Tris (pH 7.5 at 20°C), 100 mM KCl, 1 mM EDTA, 0.1 mM DTT, 20 μM cyclic AMP, 5% v/v glycerol, 0.010 mg/mL BSA and the electrophoresis was carried out in a 10% w/v polyacrylamide (75:1 acrylamide:bisacrylamide) gel, cast in 40 mM Tris-acetate, 2.5 mM EDTA (pH 7.8 at 20°C) and run in 40 mM Tris-acetate, 2.5 mM EDTA (pH 7.8 at 20°C), supplemented with 20μM cyclic AMP. The results of this assay are shown in Fig. 1.
MATERIALS
Some materials and solutions described below are necessary only if the investigator intends to cast his/her own polyacrylamide gels. These are indicated with an asterisk (*). Pre-cast gels that are available commercially are a convenient and viable alternative to ones made in the laboratory although their use reduces the investigator’s ability to modify gel concentration, crosslinking or electrophoresis buffer compositions.
REAGENTS
TEMED* (N,N,N′,N′-Tetramethylethylenediamine) [Sigma, catalog number T9281]. Store tightly sealed at 4°C.
Ammonium persulfate* [Sigma, catalog number A3678]. Store in a desicator at 4°C.
Acrylamide monomer*, electrophoresis grade [Sigma, catalog number A3553]. Store refrigerated at 4°C. Protect from light.
N,N′-methylene bisacrylamide* [USB Corporation, catalog number 75821]. Store refrigerated at 4°C. Protect from light.
Other reagents should be “molecular biology grade” or better.
SOLUTIONS AND PREPARATIONS
-
Protein solution or cell extract containing nucleic acid binding activity. Purification methods vary according to the protein in question. Methods for preparing cell- and nuclear extracts that can be used for EMSA have been described elsewhere 75, 76,74.
▴ CRITICAL STEP: The preparation of protein solutions for assay can be time consuming. Since nucleic acid binding activities are often labile, samples should be prepared and stored under conditions that maximize the lifetime(s) of the active protein(s) and minimize the potential for undesirable modifications and proteolysis.
-
32P-labeled target nucleic acid. Methods are available for labeling DNA and RNA with 32P at 5′ ends, 3′ ends, or internally72,77.
! CAUTION Exposure to beta radiation and secondary X-radiation from 32P is hazardous. Most research institutions specify procedures for the safe handling of this isotope, which should be followed stringently.
10X Tris-acetate EDTA (TAE) electrophoresis buffer. 400 mM Tris, 25 mM EDTA, brought to pH 7.8 with acetic acid. As described in the Introduction, many variations of electrophoresis gel and running buffers can be used. Note: if a commercially prepared gel is used, it is best to use an electrophoresis buffer that matches the one with which the gel was cast. Consult the product literature for information on the selection and preparation of electrophoresis buffers.
-
40% w/v acrylamide-bisacrylamide stock solution*. Acrylamide monomer, 39.5 g/100 mL; N,N′-methylene bisacrylamide 0.5 g/100 mL. Filter the resulting solution through a 0.45 micron filter. Store at 4°C in a brown glass bottle.
! CAUTION Acrylamide and bisacrylamide are neurotoxic. Weigh these reagents in a draft-free area. Wear a particle mask, gloves and eye protection when handling acrylamide powders. Never pipet solutions by mouth.
ALTERNATIVE Acrylamide-bisacrylamide stock solutions* can be purchased from commercial sources (for example, 40% w/v acrylamide-bisacrylamide solution for electrophoresis, 37.5:1 acrylamide:bisacrylamide [Sigma, catalog number A7168]). Although this product is convenient, the higher acrylamide:bisacrylamide ratio (approximately 75:1 w/w) of our formulation results in a gel with fewer crosslinks at a given acrylamide concentration than the 37.5:1 mixture. We believe that the 75:1 formulation gives better resolution of large complexes than do solutions containing greater proportions of bisacrylamide.
10X Dye + glycerol stock solution: 10mM Tris, 1mM EDTA, 50 % v/v glycerol, 0.001% w/v bromophenol blue, 0.001% w/v xylene cyanol FF.
10X Binding buffer: 100 mM Tris (pH 7.5 at 20°C), 10 mM EDTA, 1 M KCl, 1 mM DTT, 50% v/v glycerol, 0.10 mg/mL BSA. As described in the Introduction, many variations can be used.
REAGENT SETUP
Degassing gel polymerization solutions
The composition of the gel polymerization solution is given in Table 4. We have found that polymerization is more efficient and resulting gels more homogeneous if the solution is thoroughly degassed before the polymerization reaction is started. Our equipment for degassing gel solutions consists of a thick-walled side-arm flask with a volume at least 4× greater than the solution to be treated, a stopper for the flask, a magnetic stir bar and stir plate to agitate the solution and a vacuum hose connecting the side-arm flask to a mild vacuum source (“house” vacuum or a water aspirator are sufficient).
Table 4.
Composition of typical gel polymerization mixtures
| Components | Volume | ||
|---|---|---|---|
| Combine and perform steps in top-down order | |||
| 5% w/v gel | 10% w/v gel | 15% w/v gel | |
| 10X Tris acetate EDTA electrophoresis buffer. 400 mM Tris, 25 mM EDTA, brought to pH 7.8 with acetic acid | 2.0 mL | 2.0 mL | 2.0 mL |
| 40% w/v acrylamide-bisacrylamide stock solution | 2.5 mL | 5.0 mL | 7.5 mL |
| H2O | 15.5 mL | 13.0 mL | 10.5 mL |
| Ammonium persulfate | 0.05 g | 0.05 g | 0.05 g |
| Degas under mild vacuum 15 min with stirring. A stoppered side-arm flask connected to a water aspirator or “house vacuum” works well. | |||
| Start polymerization by adding TEMED (N,N,N′,N′-Tetramethylethylenediamine) | 12 μL | 12 μL | 12 μL |
! CAUTION Always interpose a trap between the side-arm flask and the vacuum source, to prevent contamination of the vacuum source with acrylamide.
! CAUTION Always interpose a trap between the side-arm flask and the vacuum source, to prevent contamination of the vacuum source with acrylamide.
The gel solution (minus the TEMED) is stirred at room temperature, under vacuum, until bubble formation stops. This typically requires 5–15 min. Polymerization should be started (by addition of TEMED) within 30 min of degassing treatment; if this is not possible, degas the solution (minus the TEMED) again before use.
! CAUTION Always wear eye protection, especially when working with glassware under vacuum.
EQUIPMENT
Electrophoresis power supply (250 V, 200 mA capacity recommended, 100 V, 25 mA capacity minimal). Most commercial power supplies sold for SDS-PAGE or DNA sequencing applications are suitable.
Vertical electrophoresis apparatus. Most commercially available vertical gel electrophoresis systems are suitable. This equipment includes the glass plates, spacers, well-forming combs, clamps and optionally, a gel-casting stand, needed to prepare and to run a polyacrylamide gel. While more elaborate equipment is available, the basic Studier “gel box”78 works well for most EMSA applications.
Side-arm flask & stopper, access to mild vacuum or water aspirator, to degas gel mixtures before polymerization.
Autoradiography film cassette and Kodak XAR-5 film or cassette and storage phosphor screen. Access to a darkroom equipped with film developer is needed for film autoradiography. Access to a phosphorimager instrument is needed for storage phosphor screen autoradiography.
Plastic food wrap. Types will relatively low “cling” such as Saran Wrap ® are easier to work with than others.
Accurately calibrated air-displacement pipettors (e.g., Gilson Pipetman). Instruments that deliver 1μl–10μL, 2μL–20μL and 20μL–200μL are needed to span the volume ranges given in the example protocol. Standard-taper tips are suitable for most solution handling, but narrow-taper gel-loading tips (e.g., Corning, catalog number 4853) are necessary for the gel-loading step.
PROCEDURE
Gel preparation (TIMING <3h)
If commercially available pre-cast gels are used, skip to step 4 (pre-electrophoresis).
Ensure that gel plates, spacers and comb are clean. Remove any fingerprints or other residue from glass plates with a swab saturated with methanol. Ensure that all components are dry before continuing.
-
Prepare the polymerization mixture. The amounts of reagents required for one gel with dimensions 0.08 × 12 × 18 cm (nominal volume 17.8 mL), containing 5%–15% w/v polyacrylamide, are shown in Table 4.
!CAUTION Acrylamide and bisacrylamide are neurotoxins. Wear gloves and use eye protection when handling the polymerization solution. Never pipet solutions by mouth.
▴CRITICAL STEP Polymerization of thoroughly degassed solutions can be rapid. Viscosity can become too great to allow the solution to be poured or transferred by pipet within ~5 min. Therefore, do not start the polymerization reaction until ready to cast the gel.
-
Pour the polymerizing mixture into the glass plate assembly, avoiding bubble formation. Insert the well-forming comb immediately and allow the gel to polymerize for at least 2h. Alternatively, precast native gels can be purchased from vendors, and the protocol can be started from step 4.
▴CRITICAL STEP Prevent bubble formation. Since bubbles do not conduct electrical current, they disrupt electrophoretic migration. Thus, it is important to minimize the number and size of bubbles trapped during polymerization. Pour the polymerizing mixture slowly to avoid bubble formation. Tilting the gel-plate assembly 45° from the vertical can minimize bubble formation during pouring. If bubbles are trapped in the solution, finish pouring, stand the assembly on end so that the plates are vertical and gently tap the assembly with a spatula to dislodge the bubbles. Once bubbles have floated to the top of the solution, they should be popped or removed before inserting the well-forming comb.
▪PAUSE POINT Polymerized gels can be stored at room temperature, with open edges sealed with plastic film, for several hours or up to 1 week at 4°C and 100% humidity. We use a plastic box in a refrigerator for this purpose. A paper wick attached to the side of the box and saturated with water is sufficient to maintain the humidity.
Pre-electrophoresis (TIMING 0.5–1.0h)
4. Remove comb and bottom spacer (if used) from gel and mount the gel in the vertical electrophoresis apparatus.
-
5. Fill the upper and lower buffer reservoirs with electrophoresis running buffer. For gels obtained with the polymerization protocol given above, the appropriate solution is 1X concentrated TAE buffer. Use a Pasteur pipette to rinse the submerged surfaces of the sample wells with buffer, to ensure that no debris is present. A Pasteur pipette with a 90° bend in the narrow end can be useful for displacing air bubbles trapped at the bottom of the gel.
▴CRITICAL STEP Charged reagents that are incompatible with the gel polymerization reaction can be moved into the gel by electrophoresis. An appropriate concentration of the reagent of interest is mixed with the running buffer prior to pre-electrophoresis. An example of this is the inclusion of cAMP in the electrophoresis buffer of the assay shown in Fig. 1.
-
6. Place ~5 μL of 1X dyes + glycerol solution in each well of the gel and conduct pre-electrophoresis at ~10 V/cm of gel length. Reduce this voltage if gel heating is evident.
▴CRITICAL STEP The dye migration pattern tests whether the gel density and conductivity are homogeneous and reveals any defect in the shape of the sample well. Discard any gel that does not give narrow, well-resolved dye bands at this stage.
Sample preparation and equilibration (TIMING 1–1.5h)
-
7. Prepare samples and equilibrate for 30 min at 20 ± 1 °C. This can be carried out, in part, while the gel is undergoing pre-electrophoresis. A titration protocol is normally used to determine the optimal concentration of protein required to interact with a constant amount of nucleic acid. Sample variation is minimized by preparing a premix of components that are present in constant concentrations and distributing a constant volume of this solution to individual sample tubes. All non-protein components should be mixed and brought to the reaction temperature prior to addition of the protein. This ensures that the protein encounters all solution components at close to their final concentrations. As an example, a typical titration protocol used for the study of the CAP-DNA interaction is given in Table 5. Note that cAMP is a special additive needed for sequence-specific DNA-binding activity in this molecular system.
▴CRITICAL STEP Equilibration time will depend on the identities of the interacting molecules and on many of the reaction conditions (examples include but are not limited to temperature, protein and nucleic acid concentrations and salt concentration). Failure to attain equilibrium may give irreproducible results. A minimal control for the attainment of equilibrium is to divide each sample in an assay into two aliquots and to incubate one subset for 30 min and the other subset for 60 min (or more). Analyze the mixtures using identical electrophoresis conditions. Equal mole-fractions of bound and free nucleic acids in corresponding samples are evidence of close approach to binding equilibrium.
Table 5.
Composition of binding reactions for the assay shown in Fig. 1.
| Component | Sample |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | |
| Volumes in μL | ||||||||||
| 10X Binding buffer: 100 mM Tris (pH 7.5 at 20°C), 10 mM EDTA, 1 M KCl, 1 mM DTT, 50% v/v glycerol, 0.10 mg/mL BSA | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 32P DNA: 214 bp lac promoter-operator fragment 48, 1.25 × 10−8M, ~4000 cpm/μL. | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| 200 μM cAMP | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| H2O | 22.5 | 21.5 | 20.5 | 18.5 | 16.5 | 14.5 | 12.5 | 7.5 | 20.5 | 18.5 |
| DNA-binding protein: | ||||||||||
| E. coli CAP, 1.4 × 10−8 M | 0 | 1 | 2 | 4 | 6 | 8 | 10 | 15 | ||
| E. coli CAP, 1.4 × 10−7 M | 2 | 4 | ||||||||
Electrophoresis (TIMING 30 min-5h depending on the electrophoretic resolution required)
-
8. At the end of the equilibration period, rinse out the wells of the gel and load the samples. In the experiment shown in Fig. 1 (reaction compositions given in Table 5), the entire sample (30 μL) was loaded. However, smaller sample volumes can be used (a practical lower limit is imposed by the need to cover the bottom surface of the well with a uniform layer of sample solution; for typical gels this can be as small as 5μL). Small sample volumes are advantageous because they result in narrow starting zones at the start of electrophoresis and, accordingly, sharper bands at the end of electrophoresis.
Gel loading is conveniently done using an air-displacement pipettor equipped with narrow-taper “gel-loading” tips (see equipment list, above). Many sample compositions are dense enough to layer cleanly at the bottom of the sample wells. For those that are not, inclusion of glycerol to a final concentration of 2–5% v/v will facilitate loading. (Many other dense solutes work equally well for this purpose.) Place the loaded tip within 1 mm of the gel surface and slowly expel the sample. Avoid expelling an air bubble after the sample, since the turbulence this causes will disrupt the narrow zone of the undisturbed sample.
9. Load tracking dye in flanking, unused wells as a marker, and perform electrophoresis at 10 V/cm. Reduce the voltage if the gel becomes warm during electrophoresis. To minimize complex dissociation, the electrophoresis interval should be as short as possible, consistent with resolution of the species of interest. Note that some molecular systems are not perturbed by the addition of tracking dyes to the samples. For these systems it is convenient to add dyes directly to the samples, prior to loading the gel.
Detection of electrophoretic bands (TIMING 30 min to several days, depending on the radioactivity of the sample)
-
10. At the end of electrophoresis, remove the gel and plate assembly from the electrophoresis device and dry it thoroughly with paper towels.
▴CRITICAL STEP The gel and plate assembly must be dry before separating the plates. Residual buffer trapped between the plates can promote vacuum formation as the plates are pried apart and this can distort the gel. In addition, transfer of free buffer to the gel surface can result in elution of radioactive nucleic acid from the gel during subsequent steps. This “free” radioisotope is a nuisance to autoradiography and a potential safety hazard.
11. Gently separate the plates, leaving the gel adherent to one of the plates. Wrap the gel and plate in a single layer of plastic food wrap. The wrapped surface of the gel should be free of air bubbles and wrinkles, since these will interfere with autoradiography. For short (≤ 24h) autoradiographic exposures, this preparation is sufficient. For longer exposures, drying the gel on a paper or membrane support or freezing it at −80°C (see below) will minimize band broadening due to diffusion.
12. Autoradiography. Working in a suitable darkroom (for film autoradiography) or in a laboratory with dim light (for phosphor screen autoradiography) place film or phosphor screen in an exposure cassette. Place the wrapped gel and plate assembly in the cassette, with gel-side toward film or screen. Close the cassette. Expose film or screen at 4°C for intervals up to 24h, and at −80°C for longer intervals. Note: Flexible cardboard or plastic film cassettes accommodate glass gel plates better than the traditional rigid metal cassette, but they do not absorb beta radiation emitted by the gel. If cassettes are stacked during the exposure interval, separating them with plexiglass sheets (5mm thick) will minimize cross-exposure fogging of adjacent films or phosphor screens. At the end of the exposure period, develop the latent image on phosphor screen or film, according to manufacturer’s instructions. Example gel pictures are shown in Figures 1–4.
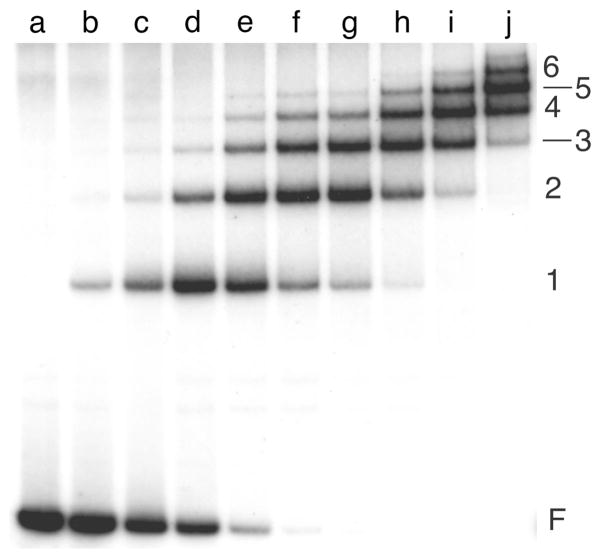
Figure 4. Titration of 1:1, 2:1 and 3:1 CAP-lac promoter complexes with lac repressor.
All samples contained a 214 bp E. coli lac promoter-operator DNA90 (3.7 × 10−10M). Samples b-h contained CAP protein (7.1 × 10−9M). Samples c-h contained lac repressor at 0.7, 1.5, 2.2, 2.9, 3.6 and 7.3 × 10−9M, respectively. The binding buffer was 10 mM Tris (pH 8.0 at 20°C), 1 mM EDTA, 50 mM KCl, 20 μM cAMP. Electrophoresis was carried out at room temperature in a 5% w/v polyacrylamide gel run in 45 mM Tris-borate (pH 8.0), 2 mM EDTA, 20 μM cAMP. Symbols: F, free DNA; C1, C2 and C3, complexes with 1, 2 and 3 CAP dimers bound per DNA molecule, respectively; C1R, C2R and C3R, complexes with one repressor tetramer and 1, 2 and 3 CAP dimers bound per DNA molecule, respectively.
TROUBLESHOOTING
The “pilot conditions” described above work well for many binding proteins. However, if binding is not detected the protocol may require modification. Outlined in Table 3 are some common problems, interpretations and solutions.
TIMING
Steps 1–3, Gel preparation: less than 3 hours
Steps 4–6, Pre-electrophoresis: 30 min. to 1 hour
Step 7, Sample preparation and equilibration: 1 to 1.5h. This step can be initiated during pre-electrophoresis (steps 4–6).
Step 8, Electrophoresis: 30min to 5 hours. The time required will vary depending on the experiment.
Steps 9–12, Detection of electrophoretic bands: typically 30 min. to several days. Autoradiographic exposure time will depend on concentration of nucleic acid and its specific radioactivity.
ANTICIPATED RESULTS
In a typical experiment, protein complexes migrate more slowly than free nucleic acid (Fig. 1). The mobility decrement that is observed depends on many factors including the sizes of protein and nucleic acid, the number of protein molecules bound, protein charge, and for rod-like DNAs, whether the DNA is bent on complex formation3, 79. Current versions of the EMSA provide little direct information about the location(s) of the binding site(s) that are occupied in protein-nucleic acid complexes. However, footprinting assays36, 42, 80 carried out in parallel with EMSA provide an efficient means of identifying occupied sequences.
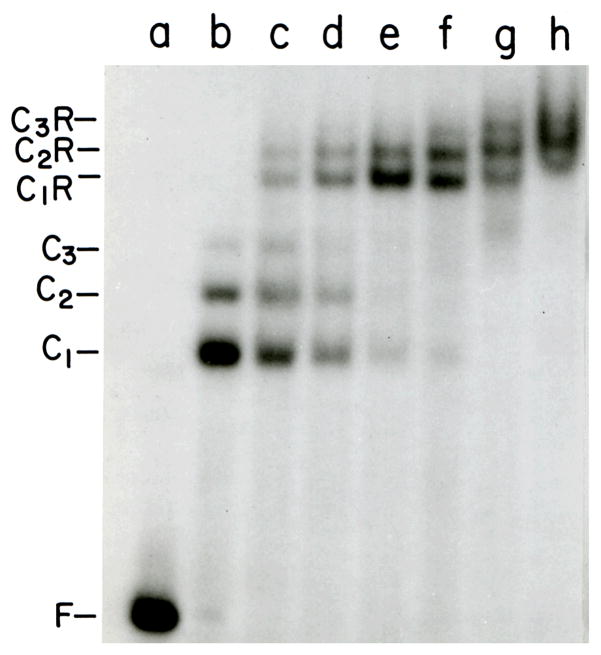
Although the CAP-DNA complex shown in Fig. 1 has a 1:1 stoichiometry, in other systems the first complex to form may have a different protein:nucleic acid ratio. For example, the first detectable complex to form in the titration of a 16 nucleotide (nt) single-stranded DNA with the human AGT DNA-repair protein has a 4:1 stoichiometry33 (Fig. 2). A range of EMSA methods are available for the determination of stoichiometries3, 6, 12 and investigators are encouraged to consider non-EMSA methods such as analytical ultracentrifugation81–83, as well.
Figure 2. Titration of a 16-residue single-stranded DNA with human AGT protein.
All samples contained 8.7 × 10−7 M DNA; samples b-l contained in addition 0.57, 1.14, 1.72, 2.29, 2.86, 3.44, 4.01, 4.58, 5.16, 5.73 and 6.60 × 10−6M AGT protein, respectively. The binding buffer contained 10 mM Tris (pH 7.6 at 20°C), 50 mM KCl, 1mM dithiothreitol, 10 μg/mL bovine serum albumin. Samples were resolved on a 10% w/v polyacrylamide gel cast and run in 40 mM Tris-Acetate, 2 mM EDTA, 50 mM KCl. Band designations: F, free DNA; B, protein-DNA complex. Continuous variation EMSA and analytical ultracentrifugation analyses support the conclusion that the complex has a 4:1 stoichiometry19, 33.
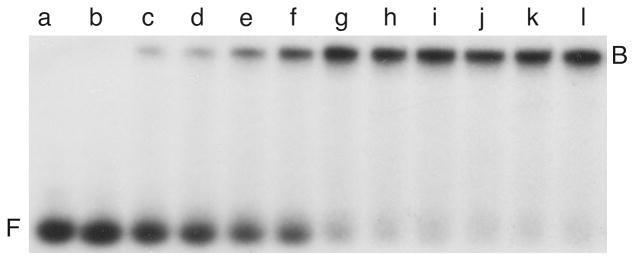
Occasionally, several complexes of differing stoichiometry will form in a single binding reaction. Under favorable conditions, these can be resolved, resulting in a “ladder” of bands in which each stoichiometric step is represented (Fig. 3). When more than one binding protein is present in the reaction mixture, complexes may form containing individual proteins or any possible combination of proteins. An example of such an assay with purified E. coli CAP and lac repressor proteins is shown in Fig. 4. Partition analysis methods7, 12, 18 are available for the characterization of multiple equilibria of the kinds shown in Figs. 3 and 4.
Figure 3. Titration of a 203 bp lac promoter DNA with E. coli lactose repressor.
All samples contained 1.91 × 10−9 M DNA; samples b-j contained 0.08, 0.16, 0.32, 0.48, 0.61, 0.83, 0.99, 1.32, 1.66 × 10−8 M repressor protein, respectively. The binding buffer contained 10 mM Tris (pH 8 at 20°C), 1 mM EDTA, 50 mM KCl, 100 μg/mL bovine serum albumin, 5% v/v glycerol. Samples were resolved on a 5% w/v polyacrylamide gel cast and run at room temperature (20 ± 2°C) in 45 mM Tris-borate (pH 7.8), 2.5 mM EDTA. Electrophoretic species are designated F, free DNA or numbered (1–6) to indicate the repressor:DNA ratio of the corresponding complex7. Under these conditions, repressor binds non-specific sites as well as its specific operator sequences.
When the source of binding activity is an unpurified or partially purified cell extract, the identification of the protein(s) present in a resolved complex is often the most important challenge. Because cell extracts contain large numbers of nucleic acid-binding proteins, it is risky to assume that the dominant binding activity associated with a nucleic acid is the one of interest to the investigator. The classical approach to this problem has been the electrophoretic supershift assay, in which an antibody against a candidate protein reduces the gel-mobility of the protein-nucleic acid complex, producing a secondary mobility shift84. More recently, 2-dimensional strategies in which EMSA is paired with SDS-PAGE with Western blot detection 85, 86, or paired with mass spectrometry 87, 88 have started to bring the power of proteomics to bear on this problem. A detailed protocol describing EMSA paired with SDS-PAGE and mass spectrometry for protein identification has recently been published 89.
Acknowledgments
This work was supported by NIH grant GM-070662. The experiment shown in Fig. 2 was performed by Dr. Joseph J. Rasimas. The experiment shown in Fig. 4 was performed by Dr. J. Michael Hudson. We thank Drs. J. A. Stead and K. J. McDowall for communication of their results prior to publication.
ABBREVIATIONS
- EMSA
electrophoretic mobility shift assay
- Tris
Tris(hydroxymethyl)aminomethane
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MOPS
3-(N-morpholino)propanesulfonic acid
- Bis-Tris
1,3-bis(tris(hydroxymethyl)methylamino)propane
- BSA
bovine serum albumin
- cAMP or cyclic AMP
3′-5′-cyclic adenosine monophosphate
- CAP
E. coli cAMP receptor protein
- ATP
adenosine triphosphate
- EDTA
ethylenediamine tetraacetic acid
- TEMED
N,N,N′,N′-tetramethylethylenediamine
- DTT
dithiothreitol
- SDS-PAGE
denaturing polyacrylamide gel electrophoresis carried out in the presence of sodium dodecylsulfate
Footnotes
COMPETING INTEREST STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Garner MM, Revzin A. The use of gel electrophoresis to detect and study nucleic acid-protein interactions. Trends Biol Sci. 1986;11:395–396. [Google Scholar]
- 2.Buratowski S, Chodosh LA. In: Current Protocols in Molecular Biology. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. John Wiley and Sons; New York: 1996. pp. 12.2.11–12.2.10. [Google Scholar]
- 3.Fried MG. Measurement of protein-DNA interaction parameters by electrophoresis mobility shift assay. Electrophoresis. 1989;10:366–376. doi: 10.1002/elps.1150100515. [DOI] [PubMed] [Google Scholar]
- 4.Carey J. Gel retardation. Methods Enzymol. 1991;208:103–117. doi: 10.1016/0076-6879(91)08010-f. [DOI] [PubMed] [Google Scholar]
- 5.Lane D, et al. Use of gel retardation to analyze protein-nucleic acid interactions. Microbiol Rev. 1992;56:509–528. doi: 10.1128/mr.56.4.509-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried MG, Garner MM. In: Molecular Biology Methods and Applications. Tietz D, editor. Elsevier; New York: 1998. pp. 239–271. [Google Scholar]
- 7.Fried MG, Crothers DM. Equilibria and Kinetics of Lac Repressor-Operator Interactions by Polyacrylamide Gel Electrophoresis. Nucl Acids Res. 1981;9:6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garner MM, Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon system. Nucl Acids Res. 1981;9:3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisinger J. Visible gel electrophoresis nd the determination of association constants. Biochem Biophys Res Comm. 1971;44:1135–1142. doi: 10.1016/s0006-291x(71)80204-8. [DOI] [PubMed] [Google Scholar]
- 10.Chelm BK, Geiduschek EP. Gel electrophoretic separation of transcription complexes: an assay for RNA polymerase selectivity and a method for promoter mapping. Nucl Acids Res. 1979;7:1851–1867. doi: 10.1093/nar/7.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varshavsky A, et al. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucl Acids Res. 1976;3:477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams C, Fried MG. In: Protein Interactions: Biophysical Approaches For The Study of Multicomponent Systems. Schuck P, editor. Academic Press; New York: 2007. pp. 417–446. [Google Scholar]
- 13.Rye HS, et al. Stable fluorescent dye-DNA complexes in high sensitivity detection of protein-DNA interactions. Application to heat shock transcription factor. J Biol Chem. 1993;268:25229–25238. [PubMed] [Google Scholar]
- 14.Jing D, et al. A sensitive two-color electrophoretic mobility shift assay for detecting both nucleic acids and protein in gels. Proteomics. 2003;3:1172–1180. doi: 10.1002/pmic.200300438. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay J, et al. Fluorescence resonance energy transfer (FRET) in analysis of transcription-complex structure and function. Methods Enzymol. 2003;371:144–159. doi: 10.1016/S0076-6879(03)71010-6. [DOI] [PubMed] [Google Scholar]
- 16.Berger R, et al. Nonradioactive gel mobility shift assay using chemiluminescent detection. Biotechniques. 1993;15:650–652. [PubMed] [Google Scholar]
- 17.Rodgers JT, et al. Use of biotin-labeled nucleic acids for protein purification and agarose-based chemiluminescent electromobility shift assays. Anal Biochem. 2000;277:254–259. doi: 10.1006/abio.1999.4394. [DOI] [PubMed] [Google Scholar]
- 18.Fried MG, Daugherty MA. Electrophoretic analysis of multiple protein-DNA interactions. Electrophoresis. 1998;19:1247–1253. doi: 10.1002/elps.1150190804. [DOI] [PubMed] [Google Scholar]
- 19.Rasimas JJ, et al. Interactions Of Human O6-Alkylguanine-DNA Alkyltransferase (AGT) With Short Single-Stranded DNAs. J Biol Chem. 2007;282:3357–3366. doi: 10.1074/jbc.M608876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musso M, et al. The yeast CDP1 gene encodes a triple-helical DNA-binding protein. Nucleic Acids Res. 2000;28:4090–4096. doi: 10.1093/nar/28.21.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolstonog GV, et al. Interaction in vitro of type III intermediate filament proteins with higher order structures of single-stranded DNA, particularly with G-quadruplex DNA. DNA Cell Biol. 2005;24:85–110. doi: 10.1089/dna.2005.24.85. [DOI] [PubMed] [Google Scholar]
- 22.Nordheim A, Meese K. Topoisomer gel retardation: detection of anti-Z-DNA antibodies bound to Z-DNA within supercoiled DNA minicircles. Nucleic Acids Res. 1988;16:21–37. doi: 10.1093/nar/16.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surgurladze N, et al. Interactions and reactions of ferritin with DNA. J Biol Chem. 2004;279:14694–14702. doi: 10.1074/jbc.M313348200. [DOI] [PubMed] [Google Scholar]
- 24.Hudson JM, Fried MG. Co-operative interactions between the catabolite gene activator protein and the lac repressor at the lactose promoter. J Mol Biol. 1990;214:381–396. doi: 10.1016/0022-2836(90)90188-R. [DOI] [PubMed] [Google Scholar]
- 25.Talanian RV, et al. Sequence-specific DNA binding by a short peptide dimer. Science. 1990;249:769–771. doi: 10.1126/science.2389142. [DOI] [PubMed] [Google Scholar]
- 26.Mita BC, et al. Interference of PR-bound RNA polymerase with open complex formation at PRM is relieved by a 10-base pair deletion between the two promoters. J Biol Chem. 1995;270:30428–30433. doi: 10.1074/jbc.270.51.30428. [DOI] [PubMed] [Google Scholar]
- 27.Varshavsky A. Electrophoretic assay for DNA-binding proteins. Methods Enzymol. 1987;151:551–565. doi: 10.1016/s0076-6879(87)51044-8. [DOI] [PubMed] [Google Scholar]
- 28.Fried MG, Liu G. Molecular sequestration stabilizes CAP-DNA complexes during polyacrylamide gel electrophoresis. Nucl Acids Res. 1994;22:5054–5059. doi: 10.1093/nar/22.23.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried MG, Bromberg JL. Factors that affect the stability of protein-DNA complexes during gel electrophoresis. Electrophoresis. 1997;18:6–11. doi: 10.1002/elps.1150180103. [DOI] [PubMed] [Google Scholar]
- 30.Vossen KM, Fried MG. Sequestration stabilizes lac repressor-DNA complexes during gel electrophoresis. Anal Biochem. 1997;245:85–92. doi: 10.1006/abio.1996.9944. [DOI] [PubMed] [Google Scholar]
- 31.Garner MM, Revzin A. Stoichiometry of catabolite activator protein/adenosine cyclic 3′,5′-monophosphate interactions at the lac promoter of Escherichia coli. Biochemistry. 1982;21:6032–6036. doi: 10.1021/bi00267a001. [DOI] [PubMed] [Google Scholar]
- 32.Fried MG, Crothers DM. CAP and RNA polymerase interactions with the lac promoter: binding stoichiometry and long range effects. Nucl Acids Res. 1983;11:141–158. doi: 10.1093/nar/11.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasimas JJ, et al. DNA-binding mechanism of O6-alkylguanine-DNA alkyltransferase. Effects of protein and DNA alkylation on complex stability. J Biol Chem. 2003;278:7973–7980. doi: 10.1074/jbc.M211854200. [DOI] [PubMed] [Google Scholar]
- 34.Ucci JW, Cole JL. Global analysis of non-specific protein-nucleic interactions by sedimentation equilibrium. Biophys Chem. 2004;108:127–140. doi: 10.1016/j.bpc.2003.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenowitz M, et al. Quantitative DNase footprint titration: a method for studying protein-DNA interactions. Methods Enzymol. 1986;130:132–181. doi: 10.1016/0076-6879(86)30011-9. [DOI] [PubMed] [Google Scholar]
- 36.Tullius TD, et al. Hydroxyl radical footprinting: a high resolution method for mapping protein-DNA contacts. Methods Enzymol. 1987;155:537–559. doi: 10.1016/0076-6879(87)55035-2. [DOI] [PubMed] [Google Scholar]
- 37.Hendrickson W, Schleif RF. A dimer of AraC protein contacts three adjacent major groove regions of the araI DNA site. Proc Nat’l Acad Sci U S A. 1985;82:3129–3133. doi: 10.1073/pnas.82.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revzin A. Gel electrophoresis assays for DNA-protein interactions. BioTechniques. 1989;7:346–355. [PubMed] [Google Scholar]
- 39.Gerstle JT, Fried MG. Measurement of binding kinetics using the gel electrophoresis mobility shift assay. Electrophoresis. 1993;14:725–731. doi: 10.1002/elps.11501401115. [DOI] [PubMed] [Google Scholar]
- 40.Papoulas O. Rapid separation of protein-bound DNA from free DNA using nitrocellulose filters. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & Sons; New York: 1989. pp. 12.18.11–12.18.19. [Google Scholar]
- 41.Hall K, Kranz J. Nitrocellulose filter binding for determination of dissociation constants. In: Haynes SR, editor. RNA Protein Interaction Protocols. Humana Press; Totowa, NJ: 1999. pp. 105–114. [DOI] [PubMed] [Google Scholar]
- 42.Galas DJ, Schmitz A. DNAase footprinting: a simple method of the detection of protein-DNA binding specificity. Nucl Acids Res. 1978;5:3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riggs AD, et al. The lac repressor-operator interaction. 3 Kinetic studies. J Mol Biol. 1970;53:401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- 44.Woodbury CP, Jr, von Hippel PH. On the determination of deoxyribonucleic acid-protein interactions parameters using the nitrocellulose filter-binding assay. Biochemistry. 1983;22:4730–4737. doi: 10.1021/bi00289a018. [DOI] [PubMed] [Google Scholar]
- 45.Oehler S, et al. Is nitrocellulose filter binding really a universal assay for protein DNA interactions? Analytical Biochemistry. 1999;268:330–336. doi: 10.1006/abio.1998.3056. [DOI] [PubMed] [Google Scholar]
- 46.Sanger F, et al. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982;162:729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- 47.Whitson PA, Matthews KS. Dissociation of the lactose repressor-operator DNA complex: effects of size and sequence context of operator-containing DNA. Biochemistry. 1986;25:3845–3852. doi: 10.1021/bi00361a016. [DOI] [PubMed] [Google Scholar]
- 48.Fried MG, Stickle DF. Ion exchange reactions of proteins during DNA binding. Eur J Biochem. 1993;218:469–475. doi: 10.1111/j.1432-1033.1993.tb18398.x. [DOI] [PubMed] [Google Scholar]
- 49.Stickle DF, Fried MG. Cation binding linked to a sequence-specific CAP-DNA interaction. Biophys Chem. 2007;126:106–116. doi: 10.1016/j.bpc.2006.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beattie KL, et al. Uptake of homologous single-stranded fragments by superhelical DNA. J Mol Biol. 1977;116:783–803. doi: 10.1016/0022-2836(77)90271-6. [DOI] [PubMed] [Google Scholar]
- 51.Tullius TD. Physical studies of protein-DNA complexes by footprinting. Annu Rev Biophys Biophys Chem. 1989;18:213–237. doi: 10.1146/annurev.bb.18.060189.001241. [DOI] [PubMed] [Google Scholar]
- 52.Brenowitz M, et al. DNase I footprint analysis of protein-DNA binding. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & sons; New York: 1987. pp. 12.14.11–12.14.16. [DOI] [PubMed] [Google Scholar]
- 53.Sclavi B, et al. Time-resolved synchrotron X-ray “footprinting”, a new approach to the study of nucleic acid structure and function: application to protein-DNA interactions and RNA folding. J Mol Biol. 1997;266:144–159. doi: 10.1006/jmbi.1996.0775. [DOI] [PubMed] [Google Scholar]
- 54.Shcherbakova I, et al. Fast Fenton footprinting: a laboratory-based method for the time-resolved analysis of DNA, RNA and proteins. Nucleic Acids Research. 2006;34:e48. doi: 10.1093/nar/gkl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naritsin DB, Lyubchenko YL. Melting of oligodeoxynucleotides with various structures. J Biomol Struct Dyn. 1991;8:813–825. doi: 10.1080/07391102.1991.10507847. [DOI] [PubMed] [Google Scholar]
- 56.Vallone PM, Benight AS. Thermodynamic, spectroscopic, and equilibrium binding studies of DNA sequence context effects in four 40 base pair deoxyoligonucleotides. Biochemistry. 2000;39:7835–7846. doi: 10.1021/bi000326k. [DOI] [PubMed] [Google Scholar]
- 57.Olmsted MC, et al. Monte Carlo description of oligoelectrolyte properties of DNA oligomers: range of the end effect and the approach of molecular and thermodynamic properties to the polyelectrolyte limits. Proc Natl Acad Sci USA. 1989;86:7766–7770. doi: 10.1073/pnas.86.20.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maxam A, Gilbert WS. A new method for sequencing DNA. Proc Natl Acad Sci USA. 1977;74:560–565. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forwood JK, Jans DA. Quantitative analysis of DNA-protein interactions using double-labeled native gel electrophoresis and fluorescence-based imaging. Electrophoresis. 2006;27:3166–3170. doi: 10.1002/elps.200500872. [DOI] [PubMed] [Google Scholar]
- 60.Kang J, Lee MS, Gorenstein DG. Quantitative analysis of chemiluminescence signals using a cooled charge-coupled device camera. Anal Biochem. 2005;345:66–71. doi: 10.1016/j.ab.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, et al. A modified quantitative EMSA and its application in the study of RNA-protein interactions. J Biochem Biophys Methods. 2004;60:85–96. doi: 10.1016/j.jbbm.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Man TK, Stormo GD. Non-independence of Mnt repressor-operator interaction determined by a new quantitative multiple fluorescence relative affinity (QuMFRA) assay. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.12.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Record MT, Mossing MC. Physical-chemical origins of stability, specificity, and control of protein-DNA interactions. In: Reznikoff WS, editor. RNA Polymerase and the Regulation of Transcription. Elsevier; New York, NY: 1987. pp. 61–83. [Google Scholar]
- 64.Carey J. Gel retardation at low pH resolves trp repressor-DNA complexes for quantitative study. Proc Natl Acad Sci USA. 1988;85:975–979. doi: 10.1073/pnas.85.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garner MM, Rau DC. Water release associated with specific binding of gal repressor. EMBO J. 1995;14:1257–1263. doi: 10.1002/j.1460-2075.1995.tb07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vossen KM, et al. The role of macromolecular hydration in the binding of the E. coli cyclic AMP receptor to DNA. Biochemistry. 1997;36:11640–11647. doi: 10.1021/bi971193e. [DOI] [PubMed] [Google Scholar]
- 67.Fried MG, Crothers DM. Equilibrium studies of the cyclic AMP receptor protein-DNA interaction. J Mol Biol. 1984;172:241–262. doi: 10.1016/s0022-2836(84)80025-x. [DOI] [PubMed] [Google Scholar]
- 68.Bell CE. Structure and mechanism of Escherichia coli RecA ATPase. Molecular Microbiology. 2005;58:358–366. doi: 10.1111/j.1365-2958.2005.04876.x. [DOI] [PubMed] [Google Scholar]
- 69.Chi P, et al. Roles of ATP binding and ATP hydrolysis in human Rad51 recombinase function. DNA Repair. 2006;5:381–391. doi: 10.1016/j.dnarep.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Panagiotidis CA, et al. Polyamines alter sequence-specific DNA-protein interactions. Nucleic Acids Res. 1995;3:1800–1809. doi: 10.1093/nar/23.10.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maniatis T, Efstratiadis A. Fractionation of low molecular weight DNA or RNA in polyacrylamide gels containing 98% formamide or 7M urea. Method Enzymol. 1980;65:299–305. doi: 10.1016/s0076-6879(80)65040-x. [DOI] [PubMed] [Google Scholar]
- 72.Maxam AM, Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65:449–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 73.Ogden RC, Adams DA. Electrophoresis in agarose and acrylamide gels. Methods Enzymol. 1987;152:55–61. doi: 10.1016/0076-6879(87)52011-0. [DOI] [PubMed] [Google Scholar]
- 74.Berman J, et al. An agarose gel electrophoresis assay for the detection of DNA binding activities in yeast cell extracts. Methods Enzymol. 1987;155:528–537. doi: 10.1016/0076-6879(87)55034-0. [DOI] [PubMed] [Google Scholar]
- 75.Dignam GD, et al. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1495–1487. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manley JL, et al. In vitro transcription: whole-cell extract. Methods Enzymol. 1983;101:568–582. doi: 10.1016/0076-6879(83)01038-1. [DOI] [PubMed] [Google Scholar]
- 77.Farrell RE. RNA Methodologies: A Laboratory Guide For Isolation And Characterization. Academic Press; San Diego: 1998. pp. 221–244. [Google Scholar]
- 78.Studier FW. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973;79:237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- 79.Crothers DM, Drak J. Global features of DNA structure by comparative gel electrophoresis. Methods in Enzymology. 1992;212:46–71. doi: 10.1016/0076-6879(92)12005-b. [DOI] [PubMed] [Google Scholar]
- 80.Pearson L, et al. Footprinting RNA-protein complexes following gel retardation assays: application to the R-17-procoat-RNA and tat-TAR interactions. Nucl Acids Res. 1994;22:2255–2263. doi: 10.1093/nar/22.12.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laue TM, Stafford WF., III Modern applications of analytical ultracentrifugation. Annu Rev Biophys Biomol Struct. 1999;28:75–100. doi: 10.1146/annurev.biophys.28.1.75. [DOI] [PubMed] [Google Scholar]
- 82.Lebowitz J, et al. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci. 2002;11:2067–2079. doi: 10.1110/ps.0207702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Daugherty MA, Fried MG. Analysis of transcription factor interactions at sedimentation equilibrium. Methods Enzymol. 2003;370:349–369. doi: 10.1016/S0076-6879(03)70031-7. [DOI] [PubMed] [Google Scholar]
- 84.Kristie TM, Roizman B. Alpha 4, the major regulatory protein of herpes simplex virus type 1, is stably and specifically associated with promoter-regulatory domains of alpha genes and of selected other viral genes. Proc Natl Acad Sci U S A. 1986;83:3218–3222. doi: 10.1073/pnas.83.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Granger-Schnarr M, et al. Specific protein-DNA complexes: immunodetection of the protein component after gel electrophoresis and Western blotting. Anal Biochem. 1988;174:235–238. doi: 10.1016/0003-2697(88)90540-4. [DOI] [PubMed] [Google Scholar]
- 86.Chen H, Chang GD. Simultaneous immunoblotting analysis with activity gel electrophoresis in a single polyacrylamide gel. Electrophoresis. 2001;22:1894–1899. doi: 10.1002/1522-2683(200106)22:10<1894::AID-ELPS1894>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 87.Woo AJ, et al. A proteomics approach for the identification of DNA-binding activities observed in the electrophoretic mobility shift assay. Mol Cellular Proteomics. 2002;1:472–478. doi: 10.1074/mcp.t200003-mcp200. [DOI] [PubMed] [Google Scholar]
- 88.Stead JA, et al. The identification of nucleic acid-interacting proteins using a simple proteomics-based approach that directly incorporates the electrophoretic mobility shift assay. Mol Cell Proteomics. 2006;5:1697–1702. doi: 10.1074/mcp.T600027-MCP200. [DOI] [PubMed] [Google Scholar]
- 89.Stead JA, McDowell KJ. Nature Protocols. 2007. Two-dimensional gel electrophoresis for identifying proteins that bind DNA or RNA. Submitted. [DOI] [PubMed] [Google Scholar]
- 90.Vossen KM, et al. The mechanism of CAP-lac repressor binding cooperativity at the E. coli lactose promoter. J Mol Biol. 1996;255:44–54. doi: 10.1006/jmbi.1996.0005. [DOI] [PubMed] [Google Scholar]
- 91.Huang CY. Determination of binding stoichiometry by the continuous variation method: the Job plot. Methods in Enzymology. 1982;87:509–525. doi: 10.1016/s0076-6879(82)87029-8. [DOI] [PubMed] [Google Scholar]
- 92.Crothers DM, et al. DNA bending in protein-DNA complexes. Methods Enzymol. 1991;208:118–146. doi: 10.1016/0076-6879(91)08011-6. [DOI] [PubMed] [Google Scholar]
- 93.Senear DF, Brenowitz M. Determination of binding constants for cooperative site-specific protein-DNA interactions using the gel mobility-shift assay. J Biol Chem. 1991;266:13661–13671. [PubMed] [Google Scholar]
- 94.Senear DF, et al. Effects of anomalous migration and DNA to protein ratios on resolution of equilibrium constants from gel mobility-shift assays. Electrophoresis. 1993;14:704–712. doi: 10.1002/elps.11501401112. [DOI] [PubMed] [Google Scholar]
- 95.Hope IA, Struhl K. GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: implications for general control of amino acid biosynthetic genes in yeast. Cell. 1985;43:177–188. doi: 10.1016/0092-8674(85)90022-4. [DOI] [PubMed] [Google Scholar]
- 96.Bain DL, Ackers GK. A quantitative cryogenic gel-shift technique for analysis of protein-DNA binding. Anal Biochem. 1998;258:240–245. doi: 10.1006/abio.1998.2626. [DOI] [PubMed] [Google Scholar]
- 97.Taylor IC, et al. Facilitated binding of GAL4 and heat shock factor to nucleosomal templates: differential function of DNA-binding domains. Genes Dev. 1991;5:1285–1298. doi: 10.1101/gad.5.7.1285. [DOI] [PubMed] [Google Scholar]
- 98.Li Q, Wrange O. Assays for transcription factor access to nucleosomal DNA. Methods. 1997;12:96–104. doi: 10.1006/meth.1997.0451. [DOI] [PubMed] [Google Scholar]
- 99.Hope IA, Struhl K. GCN4, a eukaryotic transcriptional activator protein, binds as a dimer to target DNA. EMBO J. 1987;9:2781–2784. doi: 10.1002/j.1460-2075.1987.tb02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blackwell TK, Wientraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990;250:1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- 101.Park SH, Raines RT. Green fluorescent protein as a signal for protein-protein interactions. Protein Sci. 1997;6:2344–2349. doi: 10.1002/pro.5560061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cooney M, et al. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988;241:456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- 103.Ferber MJ, Maher LJ. Quantitating oligonucleotide affinities for duplex DNA: footprinting vs electrophoretic mobility shift assays. Anal Biochem. 1997;244:312–320. doi: 10.1006/abio.1996.9901. [DOI] [PubMed] [Google Scholar]
- 104.Konarska MM, Sharp PA. Electrophoretic separation of complexes involved in the splicing of precursors to mRNAs. Cell. 1986;46:845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- 105.Kang J, et al. Chemiluminescence-based electrophoretic mobility shift assay of heparin-protein interactions. Anal Biochem. 2006;349:156–158. doi: 10.1016/j.ab.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 106.Boffini A, Prentki P. Identification of protein binding sites in genomic DNA by two-dimensional gel electrophoresis. Nucleic Acids Res. 1991;19:1369–1374. doi: 10.1093/nar/19.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jett SD, Bear DG. Snapshot blotting: transfer of nucleic acids and nucleoprotein complexes from electrophoresis gels to grids for electron microscopy. Proc Nat’l Acad Sci USA. 1994;91:6870–6874. doi: 10.1073/pnas.91.15.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lynch TW, et al. Dissecting the molecular origins of specific protein-nucleic acid recognition: hydrostatic pressure and molecular dynamics. Biophys J. 2002;82:93–98. doi: 10.1016/S0006-3495(02)75376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hudson JM, et al. Effects of anions on the binding of the cyclic AMP receptor protein to the lactose promoter. Eur J Biochem. 1993;212:539–548. doi: 10.1111/j.1432-1033.1993.tb17691.x. [DOI] [PubMed] [Google Scholar]
- 110.Stickle DF, et al. Analysis of the thermodynamic linkage of DNA binding and ion binding for dimeric and tetrameric forms of the lac repressor. Eur J Biochem. 1994;226:869–876. doi: 10.1111/j.1432-1033.1994.00869.x. [DOI] [PubMed] [Google Scholar]
- 111.Fried MG, et al. Role of Macromolecular Hydration in Lac Repressor-DNA Interactions. J Biol Chem. 2002;277:50676–50682. doi: 10.1074/jbc.M208540200. [DOI] [PubMed] [Google Scholar]
- 112.Sidorova NY, Rau DC. Differences between EcoRI nonspecific and “star” sequence complexes revealed by osmotic stress. Biophys J. 2004;87:2564–2576. doi: 10.1529/biophysj.104.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Robinson CR, Sligar SG. Changes in solvation during DNA binding and cleavage are critical to altered specificity of the Eco RI endonuclease. Proc Nat Acad Sci USA. 1998;95:2186–2191. doi: 10.1073/pnas.95.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lynch TW, Sligar SG. Macromolecular hydration changes associated with BamHI binding and catalysis. J Biol Chem. 2000;275:30561–30565. doi: 10.1074/jbc.M000266200. [DOI] [PubMed] [Google Scholar]