Abstract
Background & Aims
The commensal microbiota is believed to have an important role in regulating immune responsiveness and preventing intestinal inflammation. Intestinal microbes produce signals that regulate inflammation via Toll-like receptor (TLR) signaling, but the mechanisms of this process are poorly understood. We investigated the role of the anti-inflammatory cytokine, IL-10, in this signaling pathway using a mouse model of colitis.
Methods
Clinical, histopathological and functional parameters of intestinal inflammation were evaluated in TLR4-/-, IL-10-/- and TLR4 -/- x IL-10 -/- mice that were free of specific pathogens and in TLR4-/- x IL-10-/- mice following eradication and reintroduction of Helicobacter hepaticus. Regulatory T-cell (Treg) function was evaluated by crossing each of the lines with transgenic mice that express green fluorescent protein (GFP) under control of the endogenous regulatory elements of Foxp3. Apoptotic cells in the colonic lamina propria were detected by a TUNEL assay.
Results
TLR4-mediated signals have 2 interrelated roles in promoting inflammation in TLR4-/- x IL-10-/- mice. In the absence of TLR4-mediated signals, secretion of proinflammatory and immunoregulatory cytokines is dysregulated. Tregs (Foxp3+) that secrete IFN-© and IL-17 accumulate in the colonic lamina propria of TLR4-/- x IL-10-/- mice and do not prevent inflammation. Aberrant control of epithelial-cell turnover results in the persistence of antigen presenting cells that contain apoptotic epithelial fragments in the colonic lamina propria of Helicobacter-infected TLR4-/- mice.
Conclusions
In mice that lack both IL-10 and TLR4 mediated signals, aberrant regulatory T cell function and dysregulated control of epithelial homeostasis combine to exacerbate intestinal inflammation.
The incidence of the chronic inflammatory bowel diseases (IBD), Crohn's disease and ulcerative colitis, has increased during the last two decades1. Healthy individuals maintain a symbiotic relationship with the trillions of bacteria that populate their gastrointestinal tracts. When the complex mechanisms that maintain immunological homeostasis to this enormous antigenic load are perturbed, an intestinal inflammatory response is provoked in many types of experimentally induced mutant mice as well as in human patients with a growing list of genetic mutations 2. The prominent role of the microbiota in driving this aberrant response is highlighted by the finding that spontaneous colitis does not develop when mice are housed in a germ-free environment 3. Indeed, increasing evidence suggests that immunomodulatory molecules on commensal bacteria play an important role in regulating immune responsiveness and preventing intestinal inflammation 4 5. Previous work from our laboratory demonstrated aberrant Th2 biased hyperreactivity to food antigens in TLR4 mutant mice that are unable to receive TLR4 derived signals from commensal bacteria; when the microbiota was depleted by antibiotic treatment, TLR4 wild type mice became as susceptible to the induction of allergy as their TLR4 mutant counterparts 6. We now show that TLR4 signals from the commensal microbiota also influence the Th1/Th17 mediated inflammation associated with the development of colitis in IL-10-/- mice. The recent identification of a genetic locus that modifies TLR4 mediated signaling as a novel risk factor for IBD emphasizes the relevance of this model to our understanding of the pathogenesis of human disease 7.
MATERIALS AND METHODS
Mice
TLR4 -/- mice on an inbred C57Bl/6 background, originally provided by Shizuo Akira 8, were obtained from Dr. Evelyn Kurt-Jones (U. Mass. Medical School, Worcester, MA). C57Bl/6 IL-10-/- (B6.129P2-Il10tm1Cgn/J) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). TLR4-/- x IL-10-/- mice were created by crossing TLR4-/- mice with IL-10-/- mice and screening the tail DNA of the progeny by PCR. All three colonies were originally maintained in an SPF facility at Massachusetts General Hospital (MGH). The TLR4-/- x IL-10-/- line was rederived by delivery by Caesarean section and cross-fostering on a Helicobacter/Pasteurella pneumotropica (Hpp) free dam. In one experiment groups of Hpp free TLR4-/- x IL-10-/- mice were inoculated with H. hepaticus 3B1 (ATCC 51449) or sham inoculated as previously described 9 and monitored for eight weeks. Foxp3gfp.KI mice on a C57Bl/6 background 10 were generously provided by Vijay Kuchroo and Mohamed Oukka (Brigham and Women's Hospital, Boston, MA). The Foxp3gfp.KI mice were crossed with the TLR4-/-, IL-10-/- and TLR4-/- x IL-10-/- lines to create the homozygous mutant lines Foxp3gfp.TLR4-/-, Foxp3gfp.IL-10-/- and Foxp3gfp.TLR4-/- x IL-10 -/-. All animal experiments were conducted after approval and according to the regulations of the Subcommittee on Research Animal Care at MGH.
Confocal microscopy and image analysis
Living tissue specimens from the MLNs, isolated lymphoid follicle, cecum and colon from 3-5 Foxp3gfp, Foxp3gfp. TLR4-/-, Foxp3gfp.IL-10-/- and Foxp3gfp.TLR4-/- x IL-10 -/- mice were imaged with a Bio-Rad Radiance 2000 confocal microscope as previously described 11. Image acquisition was carried out with Laser Sharp Scanning Software and 3D reconstructions were completed with Volocity software (Improvision, Waltham, MA).
RESULTS
TLR4-/- x IL-10-/- mice develop rectal prolapse and colitis with earlier onset and higher incidence than IL-10-/- mice maintained in the same SPF colony
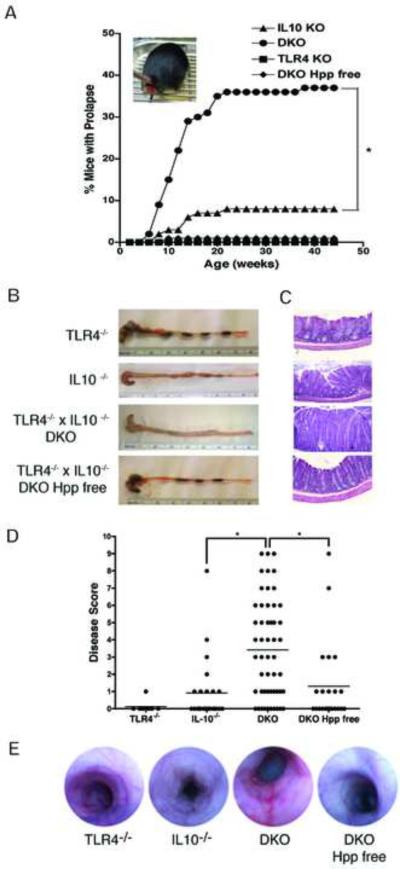
TLR4-/- x IL-10-/- (DKO) mice exhibited a high incidence of rectal prolapses first detectable at 6-8 weeks of age. Rectal prolapse is often associated with histological evidence of colitis in mice 12. Analysis of the prevalence of prolapse in the colony over the course of a year showed that, by 22 weeks, 37% of the TLR4-/- x IL10-/- mice developed rectal prolapse (Fig. 1A). This was significantly higher than the small percentage of IL-10-/- mice (8%) with rectal prolapse maintained in the same SPF room during the same time period. No rectal prolapse was detected in the TLR4-/- mice in this breeding colony. Enzootic infection with Helicobacter hepaticus is common in many SPF academic mouse colonies, including those at MGH, and has previously been reported to trigger both rectal prolapse and colitis in IL-10-/- mice 13. Rectal prolapse was virtually eliminated when TLR4-/- x IL10-/- mice were rederived Helicobacter/Pasteurella (Hpp) free (Fig. 1B). The incidence of rectal prolapse correlated with histopathologic evidence of colitis (Fig. 1 B-D). 82% of the SPF TLR4-/- x IL-10-/- mice exhibited histological evidence of disease (with a mean histological score of 3.5, Fig. 1D). This was significantly greater than the mean scores (of about 1) calculated for either SPF IL-10-/- mice or Hpp free TLR4 -/- x IL10-/- mice and nearly half of the SPF IL-10-/- and Hpp free TLR4-/- x IL10-/- mice showed no histological evidence of colitis. TLR4-/- mice maintained in the same room did not exhibit any sign of clinical disease although low-grade colonic inflammation was sometimes detectable histologically (Fig. 1 B-D). Colonoscopic imaging of mice from each line showed increased edema and loss of vascular markings, and increased erythema and the presence of linear erosions in colitic IL-10-/- and TLR4-/- x IL-10-/- mice but not in healthy TLR4-/- or Hpp free TLR4-/- x IL10-/- mice (Fig. 1E). When adult Hpp free TLR4-/- x IL-10-/- mice were inoculated with H. hepaticus 9 their weight gain was significantly reduced (Supplementary Fig. 1A). Although none of the deliberately infected mice developed severe colitis, the mean disease scores for the H. hepaticus infected mice (3) and the sham-infected mice (1, Supp. Fig. 1C) were the same as those derived from the colonies of SPF (3) and Hpp free (1, Fig. 1D) TLR4-/- x IL-10-/- mice. This indicates that H. hepaticus infection is sufficient to trigger colitis in TLR4-/- x IL-10-/- mice.
Figure 1. TLR4-/- x IL-10-/- mice develop rectal prolapse and colitis with earlier onset and higher incidence than IL-10-/- mice.
(A) Prevalence of rectal prolapse: TLR4-/- x IL-10-/- (DKO) mice (circle, n = 188), IL-10-/- mice (triangle, n = 123), TLR4-/- mice (square, n = 181) and TLR4-/- x IL-10-/- mice rederived Helicobacter /Pasteurella pneumotropica (Hpp) free (diamond, n = 85); * p < .05. (B) Representative tissue samples show the absence of formed stool and thickening of the intestinal wall in colons from IL-10-/- and TLR4-/- x IL-10-/- mice. (C) Histologic analysis of the same tissues, H&E stained, 20X (D) Disease score based on a combination of gross (0-3) and histologic (0-7) findings: TLR4-/- (n = 8), IL-10-/- (n = 24), SPF TLR4-/- x IL-10-/- (DKO, n = 51), Hpp free TLR4-/- x IL-10-/- (DKO Hpp free, n = 23); *, p < .05. (E) Representative colonoscopic images.
SPF TLR4-/- x IL-10-/- mice exhibit an exacerbated form of the Th17 mediated colitis previously reported in IL-10-/- mice
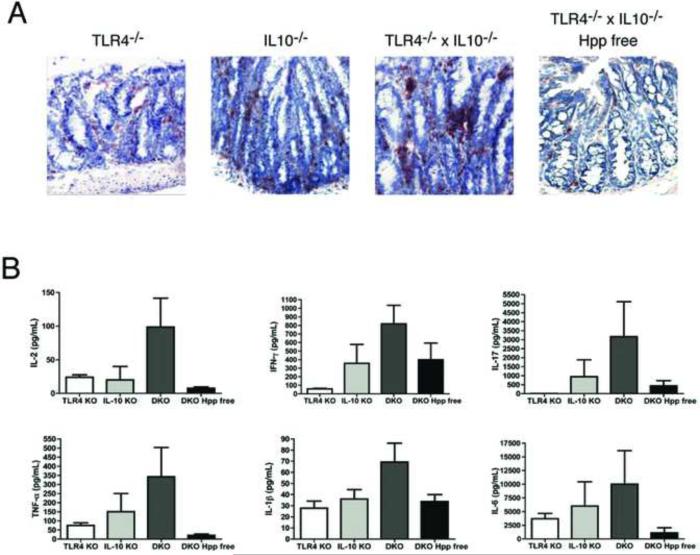
Spontaneous colitis in IL-10-/- mice is characterized by the expansion of inflammatory Th17 cells 14. When we examined the cytokines detectable in supernatants from cultured colonic tissue from 6-8 month old mice we found that the intestinal inflammatory response in SPF TLR4-/- x IL-10-/- mice is an exacerbated form of the Th17 mediated disease described in IL-10-/- mice (Fig. 2). More CD4+ T cells were present in the inflamed colons of SPF TLR4-/- x IL10-/- mice compared to the other three lines (Fig. 2A). Stimulation of colonic T cells from SPF TLR4-/- x IL10 -/- mice with anti-CD3/anti-CD28 Abs resulted in the production of the proinflammatory cytokines previously associated with Th17 mediated intestinal inflammatory responses (Fig. 2B)15. Culture supernatants from the colons of colitic SPF TLR4-/- x IL-10 -/- mice contained 2-3 fold higher levels of IFN-γ, IL-17, TNF-α, IL-1β and IL-6 than those from IL-10-/- mice. All of the inflammatory cytokines tested were present at lower levels in culture supernatants from the colons of TLR4-/- x IL-10-/- mice that have been rederived Hpp free. No IL-17 was detectable in the culture supernatants of non-inflamed colonic tissue from TLR4-/- mice (Fig. 2B).
Figure 2. SPF TLR4-/- x IL-10-/- mice exhibit an exacerbated form of the Th17 driven colitis previously reported in IL-10-/- mice.
(A) Immunohistochemical staining for CD4+ T cells in colonic tissue of TLR4-/-, IL-10-/-, SPF TLR4-/-x IL-10-/- and Hpp free TLR4-/- x IL-10-/- mice, 20X. (B). Cytokine secretion into 48 hr. anti-CD3/anti-CD28 stimulated supernatants was examined by either ELISA or Luminex assay. Bar graphs represent the mean +/- SEM of duplicate or triplicate samples from cultures of individual mice; TLR4-/- (white, n = 4), IL-10-/- (light gray, n = 5), SPF TLR4-/- x IL-10-/- (DKO, dark gray, n = 7), and Hpp free TLR4-/- x IL-10-/- (DKO, black, n = 5).
Activated CD4+Foxp3- Teff cells from the spleen and MLN of Foxp3gfp.IL-10-/- and Foxp3gfp.TLR4-/- x IL-10-/- mice secrete an inflammatory Th1/Th17 cytokine profile prior to the development of disease
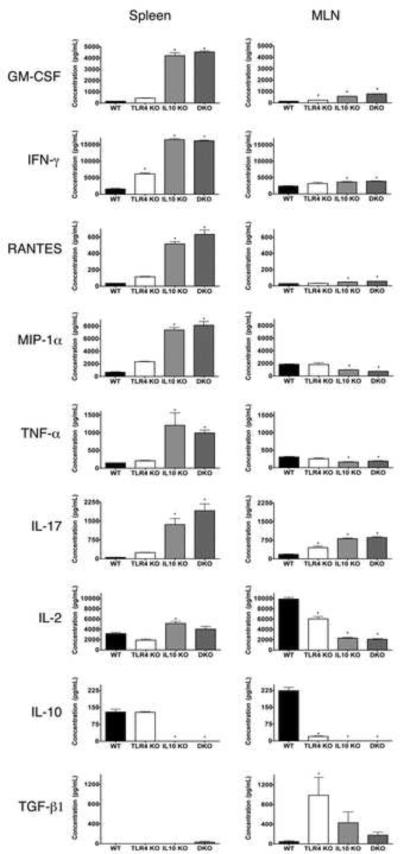
To explore TLR4 mediated control of effector and regulatory T cell function in the context of chronic intestinal inflammation we crossed mice in which GFP expression is knocked in under the control of the endogenous regulatory elements of the Foxp3 promoter (Foxp3gfp.KI 10) with the SPF TLR4-/-, IL-10-/- and TLR4-/- x IL-10-/- lines. In the resulting mutant reporter lines, GFP-expression faithfully reports the transcription of the Foxp3 gene. To examine Teff function in the mutant Foxp3 reporter lines, we sorted CD4+Foxp3- T cells (hereafter, Teff) from the spleen and MLN of each line and analyzed anti-CD3/anti-CD28 stimulated culture supernatants for the secretion of a panel of cytokines and chemokines by multiplex analysis. Teff from spleen of pre-colitic IL-10-/- and TLR4-/- x IL-10-/- mice Foxp3 reporter mice secreted significantly higher levels of the proinflammatory cytokines/chemokines GM-CSF, IFN-γ, RANTES, MIP-1α, TNF-α and IL-17 than were detectable in supernatants from WT controls (Figure 3). With the notable exception of IFN-γ, significantly elevated production of inflammatory cytokines /chemokines was not detectable in cultures of Teff isolated from the spleen of TLR4-/- Foxp3 reporter mice, which do not develop spontaneous intestinal inflammation.
Figure 3. Activated CD4+Foxp3- Teff from the spleen and MLN of Foxp3gfp.IL-10-/- and Foxp3gfp.TLR4-/- x IL-10-/- mice secrete an inflammatory Th1/Th17 cytokine profile prior to the development of disease.
Teff were sorted from the spleen and MLN of WT (n = 8), TLR4-/- (n = 6), IL-10-/- (n = 7) and DKO (TLR4-/- x IL-10-/-, n = 8) Foxp3 reporter mice. Cytokine/chemokine production by each of the sorted Teff populations was assayed 72 hrs. after stimulation with anti-CD3/anti-CD28 from 2-3 replicate cultures using multiplex analysis. * = p < .05.
Teff from the MLN of the IL-10-/- and TLR4-/- x IL-10-/- Foxp3 reporter mice also secreted significantly higher levels of the proinflammatory cytokines/chemokines GM-CSF, IFN-γ, RANTES and IL-17 than their wild type or TLR4-/- Foxp3 reporter counterparts (Figure 3). A population of TGF-β secreting Teff was highly enriched in the MLN (but not the spleen) of TLR4-/- reporter mice, (and to a lesser extent IL-10-/- mice) but reduced in both WT and TLR4-/- x IL-10-/- mice. Interestingly, Teff isolated from the MLN, but not the spleen, were impaired in their ability to make IL-10 in comparison to WT mice.
Activated CD4+Foxp3+ Tregs from the spleen and MLN of TLR4-/-, IL-10-/- and TLR4-/- x IL-10-/- mice secrete inflammatory cytokines but retain functional suppressive activity
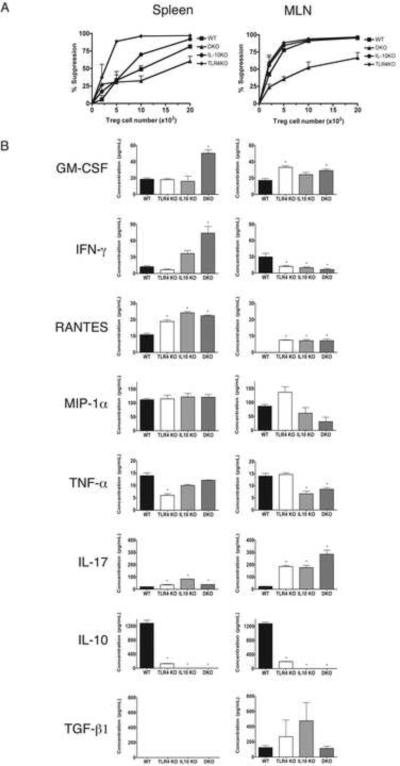
Cytokine secretion and functional suppressive activity were also examined by sorting CD4+Foxp3+ Tregs (hereafter Tregs) from the spleen and MLN of the mice analyzed in Figure 3. When we compared the ability of Tregs isolated from the spleen of WT, TLR4-/-, IL-10-/- and TLR4-/- x IL-10-/- mice to suppress the proliferation of Teff isolated from the same tissue we found that the suppressive capacity of all of the lines was similar to that seen in WT mice when analyzed using standard in vitro assays (Figure 4A). Tregs sorted from the MLN of TLR4-/- x IL-10-/- mice also suppressed the proliferation of Teff, albeit somewhat less effectively, than that noted for the other lines (Figure 4A). We also examined Treg functional activity in vivo. Tregs were sorted from the pooled spleen and MLN of both WT and TLR4-/- x IL-10-/- (DKO) reporter mice and each was co-transferred with DKO Teff to C57Bl/6 Rag-/- recipients. Transfer recipients that received proinflammatory DKO Teff alone died at 9-10 days post transfer. Tregs from WT and DKO mice were equally effective at preventing the mortality induced by the transfer of DKO Teff to immunodeficient Rag-/- recipients (data not shown). Multiplex analysis showed that activated Tregs from both the spleen and MLN of TLR4-/- mice were markedly deficient in their ability to secrete IL-10 (Fig. 4B). Treg IL-10 deficiency, due to mutation in either TLR4 or IL-10 itself, correlated with the secretion of significantly elevated levels of the inflammatory cytokines RANTES and IL-17 by Tregs isolated from both the spleen and MLN. However, only activated Tregs from the spleen of TLR4-/- x IL-10-/- mice secreted significantly higher levels of GM-CSF and IFN-γ. As was true for the Teff, TGF-β secretion was detectable exclusively in the MLN. TGF-β secretion by Tregs isolated from TLR4-/- x IL-10-/- mice was reduced in comparison to Tregs isolated from either TLR4-/- or IL-10-/- mice. Taken together, the data in Figs. 3 and 4 shows that T cell function in TLR4-/- x IL-10-/- mice is characterized by (1) highly elevated secretion of proinflammatory cytokines by activated Teffs (Fig. 3) (2) Treg secretion of inflammatory cytokines (Fig. 4), (3) the absence of IL-10 (4) impaired secretion of TGF-β by both Teffs (Fig. 3) and Tregs (Fig. 4) in the MLN and (5) retention of Treg functional suppressive activity when measured both in vitro and in vivo (Fig. 4).
Figure 4. Activated CD4+Foxp3+Tregs from the spleen and MLN of TLR4-/-, IL-10-/- and TLR4-/- x IL-10-/- mice secrete inflammatory cytokines but retain functional suppressive activity.
(A) In vitro suppression assay. CD4+Foxp3+ T cells sorted from the spleen and MLN of the mice analyzed in Figure 3 were co-cultured in triplicate with irradiated splenic APC, anti-CD3 and CD4+Foxp3- T cells sorted from the same tissue. (B) Cytokine/chemokine production by each of the sorted CD4+Foxp3+ Treg populations was assayed 72 hrs. after stimulation with anti-CD3/anti-CD28 from 2-3 replicate cultures using multiplex analysis. * = p < .05
IFN-γ secreting Foxp3+Tregs accumulate in the intestinal mucosa of IL-10-/- and TLR4-/- x IL-10-/- mice and fail to control disease
We next examined regulatory cell localization and function in the inflamed colonic tissues of each of the reporter lines. Confocal image analysis of freshly isolated, unfixed, tissue sections showed that in wild type Foxp3gfp.KI reporter mice, Tregs were readily detectable in the MLN and isolated lymphoid follicles (ILF) of the large intestine but completely absent from the diffuse lymphoid tissue of the lamina propria (LP) of the cecum and colon (Fig. 5A). The same was true of TLR4-/- reporter mice, which exhibit little or no intestinal inflammation (Fig. 5A). In contrast, Tregs were detectable in the inflamed LP of IL-10-/- reporter mice (Fig. 5A). In TLR4-/- x IL-10 -/- reporter mice, which have an exacerbated form of the colitis detectable in IL-10-/- mice, we noted a significant increase in the numbers of Tregs in the MLN and colonic LP (Fig. 5A, and graphically in 5B). Tregs sorted from the LP of colitic TLR4-/- x IL-10-/- reporter mice were effective at suppressing the proliferation of Teff isolated from the same tissue in an in vitro assay (Fig. 5C). As expected, activated Teff isolated from the inflamed colonic LP of TLR4-/- x IL-10-/- mice secreted very high levels of proinflammatory cytokines including GM-CSF, IFN-γ, TNF-α, IL-17 and IL-6 (Fig. 5D). Unexpectedly, however, activated Tregs isolated from the colonic LP secreted IFN-γ at a level similar to that secreted by the Teff (Fig. 5D). Tregs isolated from the colonic LP also secreted IL-17, at low levels similar to that secreted by Tregs isolated from the MLN of same mice (Fig. 4B).
Figure 5. Foxp3+ Tregs migrate into the cecum and colonic LP in association with inflammation but fail to control disease.
(A) Representative 3D reconstructions of confocal image analysis of Foxp3+ cells in the MLN, isolated lymphoid follicle (ILF), cecum and colon of 8-20 week old WT (Foxp3gfp.KI), TLR4-/- (Foxp3gfp.TLR4-/-), IL-10-/- (Foxp3gfp.IL-10-/-) and DKO (Foxp3gfp.TLR4-/- x IL-10-/-) mice. Multiple images of each tissue were collected from 3-5 mice of each reporter line (with and without rectal prolapse). White bars indicate 50 μm. (B) Graphic representation of GFP+ cells enumerated in multiple fields (0.1 mm2) of colonic sections from each Foxp3 reporter line; WT (n=6), TLR4-/- (n = 8), IL-10-/- (n = 4) and TLR4-/- x IL-10-/- (DKO, n = 17); *, p < .05. (C) In vitro suppression assay. Tregs sorted from the LP of colitic TLR4-/- x IL-10-/- Foxp3 reporter mice were co-cultured with irradiated splenic APC, anti-CD3 and Teff from the same mice. (D) Tregs and Teff were sorted from the LP of TLR4-/- x IL-10-/- reporter mice and cytokine/chemokine production was assayed 72 hrs. after anti-CD3/anti-CD28 stimulation using multiplex analysis.
In the absence of TLR4-mediated signals, apoptotic cells accumulate in the colonic LP
A role for TLR-mediated signals in the maintenance of intestinal homeostasis and barrier function in the gut has previously been established 16. Epithelial cell proliferation is decreased (and apoptosis is increased) in response to epithelial injury in TLR4-/- mice 17. Previous work has implicated impaired clearance of apoptotic cells from the colonic mucosa in the development of colitis 18, 19. We therefore examined whether increased numbers of apoptotic cells were present in the inflamed colonic LP of TLR4-/- x IL-10-/- mice. We found that apoptotic cells accumulated in the LP of both SPF IL-10-/- and TLR4-/- x IL-10-/- mice but not in TLR4-/- x IL-10-/- mice that had been rederived Hpp free (Figure 6A, B). Strikingly, apoptotic cells were also detectable in the LP of SPF (but not Hpp free) TLR4-/- mice, in the absence of overt inflammation (Figure 6B). Taken together these data suggest that the response of the intestinal epithelium to Helicobacter derived signals is impaired in TLR4-/- mice, resulting in the accumulation of apoptotic cells in the colonic LP. IL-10 appears to play a role in preventing the induction of an inflammatory response to these apoptotic remnants in TLR4-/- mice since, in the absence of TLR4 mediated protection against epithelial apoptosis, colitis is exacerbated in IL-10 -/- mice that also bear a mutation in TLR4.
Figure 6. Apoptotic nuclei accumulate in the colonic LP of SPF TLR4-/- mice.
Apoptotic cells in sections of colonic tissues were detected by TUNEL assay. (A) Representative images of sections prepared from WT C57Bl/6 mice and from the inflamed colon of an IL-10-/- mouse; apoptotic nuclei are indicated by black arrows, 20X (B). Representative images demonstrating the presence of apoptotic nuclei in sections from TLR4-/- and TLR4-/- x IL-10-/- (DKO, black arrows) mice maintained under SPF conditions and their reduction in mice that have been rederived Hpp free. (C) Graphic representation of TdT+ cells/1mm2 high power field (HPF) enumerated from sections of colonic tissue of each line. 30 fields were counted for each section prepared from C57Bl6/WT (n=4), Hpp free TLR4-/- (n=3), SPF TLR4-/- (n=3), Hpp free TLR4-/- x IL-10-/- (n=4), SPF TLR4-/- x IL-10-/- (n=5) and SPF IL-10-/- (n=6). ** p < 0.01.
DISCUSSION
Through clinical, histologic and functional analysis of the colonic inflammation in a large SPF colony of TLR4-/- x IL10-/- mice we have shown that this strain exhibits an exacerbated form of the Th1/Th17 driven colitis seen in IL-10-/- mice maintained in the same room. Tregs accumulate in the colonic LP of IL-10-/- and TLR4-/- x IL-10-/- mice but fail to control disease. Earlier work using the same Foxp3 reporter line documented the accumulation of Tregs in the inflamed central nervous system of mice with experimental autoimmune encephalomyelitis (EAE) 20. Other work has suggested that Tregs are ineffective at controlling Th17-mediated inflammatory diseases 21. CD4+Foxp3+ T cells with functional in vitro suppressive activity accumulate in the MLN and the inflamed colonic LP of patients with Crohn's disease 22. The intestines of patients with Crohn's disease also contain large proportions of T cells secreting IL-17 (Th17) and both IFN-γ and IL-17 (Th1/Th17) 23. We show here that elevated levels of IFN-γ, IL-17, TNF-α, IL-1 and IL-6 are associated with the colonic inflammation that spontaneously develops in TLR4-/- x IL-10-/- mice maintained in a Helicobacter hepaticus positive colony (Figure 2). Strikingly, Tregs directly isolated from the inflamed colonic LP of TLR4-/- x IL-10-/- mice secreted IFN-γ at levels comparable to those of Teff from the same site (as well as low levels of IL-17, Figure 5). Inflammatory cytokine secretion by Tregs was also dysregulated in the MLN and spleen of young, pre-colitic mice. Interestingly, Tregs isolated from both the spleen and MLN of TLR4-/- mice secreted strikingly less IL-10 than Tregs isolated from WT controls (Figure 4). IL-10 deficiency, due to mutation in either IL-10 itself, or in TLR4, correlated with the secretion of significantly elevated levels of the inflammatory mediators RANTES and IL-17 by activated Tregs from TLR4-/-, IL-10-/- and TLR4-/- x IL-10-/- mice when compared to Tregs from WT controls (Figure 4). A TGF-β secreting CD4+Foxp3- population was, however, enriched in the MLN of the same mice (Figs. 3 and 4). To our surprise, despite their abnormal secretion of inflammatory cytokines and their failure, ultimately, to control disease, Tregs sorted from the MLN and spleen of TLR4-/- x IL-10-/- mice retained functional suppressive activity in both in vitro and in vivo assays (Figure 4). Yet, Treg secretion of IL-10 is clearly important for the maintenance of homeostasis in the intestinal mucosa. In the normal colon, one third of the CD4+ T cells are Tregs and most of these are Foxp3+IL-10+. 24 Moreover, recent work has shown that colitis develops in fully immunocompetent mice with a Treg cell specific deletion of IL-10 25, emphasizing an essential role for Treg secretion of IL-10 in vivo in the prevention of intestinal inflammation. Taken together with our data these findings suggest that the mechanism of action of Tregs in vivo, particularly in inflamed tissue, may be substantially different from their mode of suppression in in vitro suppression assays or after transfer into lymphopenic mice.26 In our model, in the absence of IL-10, the dysregulated inflammatory cytokine microenvironment of the LP is likely to contribute to the impairment of Treg function in vivo. In particular IL-6, a cytokine commonly secreted by macrophages, has been shown in vitro to render naïve T cells resistant to Treg mediated suppression; T cell secretion of IL-6 has previously been associated with mucosal T cells in colitic mice27, 28. In agreement with the apparent ineffectiveness of Treg in situ, we found that only Teff isolated from the inflamed colonic LP of the TLR4-/- x IL-10-/- mice secreted IL-6; we did not detect secretion of IL-6 by Teff isolated from the MLN or spleen of any of the reporter lines. Alternatively, the effectiveness of the DKO Tregs in our functional suppression assays may suggest that they possess IL-10 independent suppressive activity that is eventually overwhelmed in the highly inflammatory microenvironment of the colitic LP.
We also identified a function for TLR4 mediated signals in the exacerbation of disease in the TLR4-/- x IL-10-/- mice. A role for MyD88 dependent TLR4-mediated signals in the homeostatic maintenance of the intestinal epithelial barrier has already been established 16. Epithelial injury results in decreased epithelial cell proliferation (and increased apoptosis) in TLR4-/- mice 17; this defect in epithelial repair has been linked to the failure of both intestinal epithelial cells and LP macrophages to upregulate the anti-inflammatory mediator Cox-2 in absence of TLR4 mediated signals 29. We show in Figure 6 that apoptotic cells apparently derived from the intestinal epithelium accumulate, in the absence of inflammation, in the colonic LP of Helicobacter positive TLR4-/- mice, as well as in the context of intestinal inflammation in both IL-10-/- and TLR4-/- x IL-10-/- mice. Apoptotic cells are not detectable in the colonic LP of either TLR4-/- or TLR4-/- x IL-10-/- mice that have been rederived Helicobacter free. Interestingly, an accumulation of apoptotic cells in multiple tissues (including the colon), in the absence of autoimmune inflammation, has also been reported in mice mutant for CD14, a co-receptor for TLR4 30. In WT mice, macrophage production of IL-10 during phagocytic clearance of apoptotic cells is critical to the restoration of homeostasis at inflammatory sites 31. Moreover, recent work has linked the colitis that develops in mice lacking αV integrins to the inability of gut associated macrophages and dendritic cells to efficiently clear apoptotic epithelial cells from the colonic mucosa and to induce functional Tregs 19.
Although Gram-negative bacteria typically signal the innate immune system via TLR4, TLR2 is the dominant receptor for gastrointestinal Helicobacter species and TLR2 mediated macrophage activation is enhanced in TLR4-/- mice 32. In vitro, both Helicobacter hepaticus, and its soluble LPS, inhibit TLR4 mediated activation of intestinal epithelial cells by E. coli LPS and prevent endotoxin tolerance, presumably by a receptor other than TLR4 33. Gnotobiotic IL-10-/-NF-κBEGFP reporter mice have also demonstrated a critical role for TLR-NF-κB signaling in the induction of commensal bacteria dependent T cell mediated colitis 34. However other work has shown that MyD88-/- x IL-10-/- mice are protected from the development of colitis 35, suggesting that MyD88 dependent NF-κB mediated signals can function to both enhance and limit intestinal inflammatory responses.
Taken together our data suggests a dual role for TLR4 in the regulation of intestinal inflammation in TLR4-/- x IL-10-/- mice. In the absence of TLR4 mediated signals, both proinflammatory and immunoregulatory cytokine secretion is dysregulated. IFN-γ/IL-17 secreting Foxp3+ Tregs accumulate in the colonic LP of TLR4-/- x IL-10-/- mice and fail to control disease. Dysregulated epithelial turnover leads to the accumulation of apoptotic epithelial cells in the colonic LP. In TLR4-/- mice, the macrophages/DCs that phagocytose the apoptotic epithelial remnants retain the ability to make IL-10 and this may allow them to avert an inflammatory response. We suggest that, in Helicobacter infected TLR4-/- x IL-10-/- mice, dysregulation of TLR4-mediated control of epithelial homeostasis accelerates the accumulation of apoptotic epithelial cells in the colonic LP and exacerbates intestinal inflammation in the absence of immunoregulatory IL-10.
Supplementary Material
ACKNOWLEDGMENTS
KSM would like to dedicate this manuscript to the memory of his mother Geeta Matharu. We thank Michelle Ahl and Kelly Farah, Center for Comparative Medicine, Mass General Hospital (MGH) for the Helicobacter free rederivation of the TLR4-/- x IL10-/- mice and Mohamed Oukka and Vijay Kuchroo (Brigham and Women's Hospital, Boston MA) for providing the Foxp3gfp.KI mice. We are grateful to Rodrigo Mora, Ramnik Xavier, Thorsten Mempel, Lynda Stuart, Adam Lacy-Hulbert and Daniel Podolsky (MGH) and Andrew Gewirtz (Emory, Atlanta, GA) for critical review of the manuscript and to Katrin Eurich and Satoko Toei-Shimizu (MGH) for technical assistance.
We note with great sadness the passing of our dear friend and collaborator David Schauer. David's insight was instrumental to the completion of this work. David was also an integral component of the research community of the Massachusetts General Hospital Center for the Study of Inflammatory Bowel Disease. Among his many contributions to our understanding of the pathogenesis of intestinal inflammation, David identified Helicobacter hepaticus as a common inciting agent for disease in many immunodeficient mouse strains and developed and characterized the use of Citrobacter rodentium, a murine attaching/effacing pathogen, as a model of infectious colitis. David's warm friendship and often inspiring advice in many generous collaborations will be truly missed by all who had the good fortune to know and work with him.
Grant support: This work was supported by the MGH CSIBD and NIH grants DK 55678 (CRN), DK 06818 (HCR), DK 64351 (AM), DK 64289, DK 74454 (EM), DK 052413 (DS), the Eli and Edythe L. Broad Medical foundation (EM, AM) and training fellowships T32 EY07156 (BM), F31AI054229 (OII) and T32DK007191 (DN). C.A.C was supported by the AGA Jon Isenberg International Scholar Award).
Glossary
Abbreviations
- TLR4
Toll-like receptor 4
- LPS
lipopolysaccharide
- MyD88
myeloid differentiation factor 88
- SPF
specific pathogen free
- RAG
recombinase activating gene
- GFP
green fluorescent protein
- MLN
mesenteric lymph node
- ILF
isolated lymphoid follicle
- Hpp free
Helicobacter /Pasteurella pneumotropica free
- LP
lamina propria, Teffector, Teff
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: None
Writing Assistance: None
Please see detailed supplemental information online for:
Clinical, histologic and immunohistochemical scoring of colitis Immunohistochemical analysis of cryosections Analysis of cytokine production Confocal microscopy and image analysis Analysis of Treg suppressive capacity in vitro Analysis of Treg suppressive capacity in vivo
REFERENCES
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Nagler-Anderson C. Man the barrier! Strategic defenses in the intestinal mucosa. Nature Reviews Immunology. 2001;1:59–67. doi: 10.1038/35095573. [DOI] [PubMed] [Google Scholar]
- 3.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 4.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 6.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–87. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 7.Labbe C, Goyette P, Lefebvre C, Stevens C, Green T, Tello-Ruiz MK, Cao Z, Landry AL, Stempak J, Annese V, Latiano A, Brant SR, Duerr RH, Taylor KD, Cho JH, Steinhart AH, Daly MJ, Silverberg MS, Xavier RJ, Rioux JD. MAST3: a novel IBD risk factor that modulates TLR4 signaling. Genes Immun. 2008;7:602–612. doi: 10.1038/gene.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR-4) deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 9.McBee ME, Zheng PZ, Rogers AB, Fox JG, Schauer DB. Modulation of actue diarrheal illness by persistent bacterial infection. Infect Immun. 2008;76:4851–4858. doi: 10.1128/IAI.00745-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 11.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 12.Fox JG, Dangler CA, Schauer DB. Inflammatory Bowel Disease in Mouse Models: Role of Gastrointestinal Microbiota as Proinflammatory Mediators. In: Ward J, Mahler J, Maronpot R, Sundberg J, editors. Pathology of Genetically Engineered Mice. Iowa State University Press; 2000. [Google Scholar]
- 13.Foltz CJ, Fox JG, Cahill R, Murphy JC, Yan L, Shames B, Schauer DB. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter. 1998;3:69–78. doi: 10.1046/j.1523-5378.1998.08006.x. [DOI] [PubMed] [Google Scholar]
- 14.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–65. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 18.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor a mutant mice. J. Exp. Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–8. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J. Exp. Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3(+) and Foxp3(-) precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–41. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 25.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–58. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 28.Mudter J, Amoussina L, Schenk M, Yu J, Brustle A, Weigmann B, Atreya R, Wirtz S, Becker C, Hoffman A, Atreya I, Biesterfeld S, Galle PR, Lehr HA, Rose-John S, Mueller C, Lohoff M, Neurath MF. The transcription factor IFN regulatory factor-4 controls experimental colitis in mice via T cell-derived IL-6. J Clin Invest. 2008;118:2415–2426. doi: 10.1172/JCI33227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–77. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devitt A, Parker KG, Ogden CA, Oldreive C, Clay MF, Melville LA, Bellamy CO, Lacy-Hulbert A, Gangloff SC, Goyert SM, Gregory CD. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14-/- mice. J Cell Biol. 2004;167:1161–70. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, Wang TC, Kurt-Jones EA. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;72:6446–54. doi: 10.1128/IAI.72.11.6446-6454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterzenbach T, Lee SK, Brenneke B, von Goetz F, Schauer DB, Fox JG, Suerbaum S, Josenhans C. Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells. Infect Immun. 2007;75:2717–28. doi: 10.1128/IAI.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10-/-;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–32. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 35.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–29. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.