Abstract
Although sperm cryopreservation has been studied in at least 17 non-human primate species, systematic factor optimization for any single species is lacking. Gene banking of non-human primate sperm is still in its infancy. The objective of the present study was to initiate a systematic approach to optimize the process of sperm cryopreservation for rhesus macaques, specifically, factors related to pre-freezing conditions (eg. straw freezing position, sperm concentration, sperm washing, equilibration methods, and equilibration time periods). Straw position had no effect on post-thaw motility (P = 0.193). Sperm concentration was tested in a range from 5 × 106/mL to 5 × 108/mL; post-thaw motility of sperm samples frozen at 5 × 107 cell/mL (51.0 ± 10.6%; mean ± SD) and 5 × 108 cell/mL (48.1 ± 7.3%) were higher than samples frozen at 5 × 106 cells/mL (33.0 ± 12.0%, P = 0.003). Comparison of motility immediately after thawing between samples with (51.2 ± 6.2%) and without washing (53.9 ± 6.8%) revealed no differences (P > 0.05). However, washing improved sperm forward progression within 1 h after thawing, whereas unwashed sperm retained higher post-thaw motility and progression during extended incubation (4 h) after thawing (P < 0.05). Equilibration methods (with or without pre-cooling) made no difference on post-thaw motility (P > 0.05), and the most effective equilibration time was the duration required for samples to acclimate to 4 °C prior to freezing. Evaluation and optimization of these pre-freezing conditions will help to minimize sources of injury, maximize survival, and contribute to the development of an optimized cryopreservation protocol for rhesus macaque sperm.
Keywords: Cryopreservation, Sperm, Rhesus monkey, Macaca mulatta, Optimization
1. Introduction
The rhesus monkey has been one of the most widely used non-human primate models for various biomedical research fields, such as virology [1,2] and reproduction [3,4]. The captive population of rhesus macaques in the National Primate Research Centers (NPRCs) in the US was originally imported from India, but its genetic diversity has declined over time as animals are lost to disease, trauma or old age; these can not be easily replaced, as India has long banned further exportation of rhesus monkeys. Semen banking will help improve the diversity of the current rhesus monkey colonies at the NPRCs, by providing ways for centers to preserve the remaining founding genetic lines, promote sharing of genetics, and expand diversity by obtaining semen from rhesus monkeys in India. A reliable and optimized cryopreservation method is necessary to effectively bank semen. Currently, propagation in rhesus macaques using frozen-thawed sperm in standard AI has not been successful, and live births were only reported through either intrauterine insemination [5,6] or intracytoplasmic sperm injection (ICSI) [7]. The unique tortuous cervical canal of the rhesus macaque may act as the greatest barrier for the successful application of standard AI, where motility-compromised, frozen-thawed sperm are deposited into the female vagina or cervix. To achieve success with standard AI, there is a need for substantial improvement of current cryopreservation techniques for rhesus macaque sperm.
Sperm cryopreservation involves a series of sequential steps, and each of these steps may contribute to poor sperm quality after thawing [8]. Despite this, studies often tend to focus on a few major steps, such as the selection of cryoprotectant and cooling rate. A review of the non-human primate cryopreservation literature (approximately 50 reports) revealed that the main focus of these studies was on altering the extender, glycerol concentration, and freezing method. Other factors such as pre-freezing conditions, interactions among various factors, and post-thaw washing and incubation have rarely been examined. In addition, male/individual variation has been widely recognized in the literature (e.g., [9,10]); it is males with low semen quality post-thaw that pose the greatest challenge for current techniques of semen cryopreservation. Dong et al. [11] proposed a sperm tolerance model and hypothesized that individuals with a narrow tolerance to cryopreservation (poor freezers) could accommodate a more narrow range across protocols, and would benefit most from systematic procedure optimization. This was verified independently with recent studies on boar semen cryopreservation, in which optimization of the cryopreservation conditions improved the cryosurvival of spermatozoa, particularly in ejaculates with poor sperm freezing ability [12].
The goal of the present studies was to develop an optimized cryopreservation protocol for rhesus macaque, with the intention of establishing a rhesus semen cryobank, and concurrently developing a better protocol for males with poor sperm freezing ability. The present study was part of our systematic approach for the optimization process. In particular, factors related to pre-freezing conditions such as straw freezing position, sperm concentration, sperm washing, equilibration method, and equilibration time, were evaluated.
2. Materials and methods
2.1. Semen collection and processing
Ten adult male rhesus monkeys were individually caged at the California National Primate Research Center (CNPRC) with lights on from 06:00 to 18:00 h at 25–27 °C. Monkeys were fed a diet of Purina monkey chow and water ad libitum. The males were trained to chair restraint and semen were collected by direct penile stimulation with a Grass 6 stimulator equipped with EKG pad electrodes (30 to 50 V, 20 ms duration, 18 pulses/s) [13]. Semen was collected in the morning every Tuesday and Thursday, and one ejaculate was collected from each male every week. All samples were obtained between May and August 2007. For all experiments in this study, sperm suspensions from each ejaculate were used as replicates, and there were no mixture of ejaculates from different males. A total of 43 ejaculates were used in this study (2 to 6 ejaculates per male), and the mean volume of ejaculates ranged from 56 to 553 μL (Table 1). Samples were allowed to liquefy for 30 min before processing. A dilution of 1:20 (v/v) of semen to a modified Tyrode’s medium supplemented with bovine serum albumin (TL-BSA) [14] was used for sperm motility estimation of fresh semen, and a second dilution of 1:20 (v/v) of sperm to distilled water was used for hemacytometer counts (Hausser Scientific, Horsham, PA, USA). The mean sperm density ranged from 6.4 to 91.0 × 108/mL, and initial motility varied from 45 to 93% (Table 1). Sperm suspensions were adjusted to 1 × 108 cells/mL of total motile sperm (sperm density × initial motility) with TEST-yolk solution (43.25 g TES, 10.265 g Tris, 10 g glucose in 1 L distilled water, 20% egg yolk (v/v), pH 7.4, 350 mOsm/kg, modified from [5]). Suspended semen were then mixed with 6% glycerol to obtain a final sperm concentration of 5 × 107 cells/mL with 3% glycerol (except for the experiments addressing freezing concentration). Glycerol solutions were prepared within 1 wk of use, with TEST-yolk as the diluent, and were stored at 4 °C. All chemicals used for preparation of solutions were of reagent grade (Sigma Chemical Corporation, St. Louis, MO, USA).
Table 1.
Mean (± SD) basic characteristics of ejaculated semen of the 10 rhesus monkey males used.
| Male number | Age(yr) | Weight(kg) | No. ejaculates | Volume per ejaculate (μL) | Sperm density(× 108/mL) | Initial motility(%) |
|---|---|---|---|---|---|---|
| 1 | 18 | 13.6 | 3 | 123 ± 117 | 27.2 ± 11.6 | 53 ± 38 |
| 2 | 16 | 13.7 | 4 | 284 ± 97 | 11.0 ± 2.6 | 60 ± 28 |
| 3 | 15 | 13.8 | 6 | 248 ± 133 | 24.4 ± 11.4 | 93 ± 3 |
| 4 | 15 | 12.6 | 5 | 234 ± 103 | 20.6 ± 9.2 | 90 ± 6 |
| 5 | 15 | 12.6 | 2 | 200 ± 0 | 91.0 ± 0.0 | 60 ± 0 |
| 6 | 13 | 9.8 | 2 | 235 ± 21 | 6.4 ± 2.6 | 45 ± 21 |
| 7 | 12 | 11.1 | 5 | 56 ± 29 | 53.7 ± 10.4 | 93 ± 3 |
| 8 | 11 | 12.1 | 6 | 553 ± 97 | 10.2 ± 2.5 | 68 ± 23 |
| 9 | 10 | 12.2 | 5 | 403 ± 182 | 11.6 ± 4.8 | 86 ± 10 |
| 10 | 6 | 13.7 | 5 | 142 ± 130 | 26.0 ± 16.7 | 85 ± 15 |
2.2. Freezing and thawing procedure
Aliquots (50 μL) of sperm suspensions containing 3% glycerol-TEST-yolk were drawn into 0.25-mL French straws (IMV International, Minneapolis, MN, USA) manually with a 1 mL syringe, and heat-sealed. Except for the equilibration method and time duration experiments, straws were placed into a 600-mL glass beaker containing 500 mL of room temperature distilled water, and equilibrated at 4 °C in a refrigerator for 2 h before initiation of the freezing process. Freezing followed the methods described by [15]. In brief, a Styrofoam box (inside dimensions: 33×24×23 cm) was filled with a depth of 4 cm liquid nitrogen and a 1 cm thick Styrofoam ‘boat’ was floated on top of it for 10 min, then straws were placed on top of the ‘boat’, and equilibrated for 10 min before being plunged into liquid nitrogen. The average cooling rate from −10 to −70 °C was ~220 °C/min. After a minimum of 12 h, four straws per treatment were thawed in a 37 °C water bath for 30 s (ISOTEMP 102, Fisher Scientific, Pittsburg, PA, USA) to estimate post-thaw motility.
2.3. Motility estimation
A 10-μL drop of pre-freeze or post-thaw semen, covered with a 22 mm square coverglass, was visualized with 20 × positive-phase objective and a condenser setting of 100 (pseudo-dark field) on an Olympus BH-series phase-contrast microscope (Scientific Instrument Co, Sunnyvale, CA, USA). An air curtain incubator (Sage Instruments, Model 279, Orion Research Inc, Cambridge, MA, USA) maintained the microscope stage at 37 °C. Post-thaw motility was estimated without any dilution or washing immediately after thawing or incubation at room temperature or 37 °C for various time periods (detailed below). The percent forward progression was subjectively evaluated on a five point scale, with 0 indicative of no motility, 1 represented slight movement with > 75% of sperm showing vibration only, 2 represented moderate forward movement in about 50% of sperm, and 3 and 4 represented very active forward movement observed in about 70% and more than 90% of sperm, respectively [16]. To illustrate the swimming motion of post-thawed sperm, an adjusted motility index (AMI) was used to incorporate the scale value and the percent motility into a single number, with the formula as follows: AMI = (Scale value/4) × percent motility. Samples were presented in random order each time so that the operator did not know their identity.
2.4. Effect of straw freezing position
To examine whether the straw position on the ‘boat’ would affect post-thaw sperm motility, ejaculates collected from four males were used. The capacity of the Styrofoam ‘boat’ was 40 straws (0.25-mL) without overlap; thus, 40 straws per male were used, and each straw was labeled with a position code from 1 (starting on the left) to 40. The 40 straws were fixed in position by taping the cotton ends with a strip of paper tape, and were frozen and thawed as described above. Motility was evaluated immediately after thawing.
2.5. Effect of sperm concentration
There were two trials in this experiment; for the first trial, ejaculates collected from nine males were used to evaluate the final sperm freezing concentrations of 5 × 108, 5 × 107, and 5 × 106 cells/mL. Percent motility and forward progression were estimated immediately after thawing. For the second trial, eight ejaculates collected from seven males were used to evaluate the final sperm freezing concentrations of 1 × 108, 5 × 107, 2.5 × 107, and 1.25 × 107 cells/mL. Motility was estimated immediately after thawing.
2.6. Effect of washing through centrifugation
Semen from eight males was used in this experiment. Each ejaculate was divided into two samples, with one washed twice with TL-BSA (290 mOsm/kg) at 300 × g for 10 min, and the other was not washed. Post-thaw motility and forward progression were estimated after incubation at room temperature for 0, 1, and 4 h.
2.7. Effect of equilibration methods
The term ‘equilibration’ has been loosely used to describe the interval between dilution and cryoprotectant addition, and the interval between cryoprotectant addition and the initiation of freezing, or the combination of both periods. In the present study, we restricted its use for after cryoprotectant addition and before freezing. The cooling period between dilution and cryoprotectant addition was referred to as ‘pre-cooling’. Ejaculates from eight males were used in this experiment. Each ejaculate was washed twice with TL-BSA (as described in the previous experiment), and the resulting sperm suspension was divided into two equal portions. One portion was subjected to the equilibration method as described above (equilibration without pre-cooling). The other portion (~ 150 μL) was transferred to a 1.5 mL tube, placed in a floater (maximum, four tubes per floater) in a 600-mL glass beaker containing 500 mL of room temperature distilled water, and equilibrated at 4 °C in a refrigerator for 2 h before the addition of cryoprotectant (equilibration with pre-cooling). Straws were then loaded on crushed ice (to minimize temperature shock) and quickly returned to 4 °C after sealing, and were equilibrated at 4 °C for an additional 30 min. Post-thaw motility and forward progression were estimated after incubation at room temperature for 0, 4, and 7 h.
2.8. Effect of equilibration time periods
Semen from six males was used in this experiment and each ejaculate was washed twice with TL-BSA as described above. The resulting sperm suspension was diluted with 3% glycerol-TEST-yolk, loaded into 24 straws with four straws for each of the following equilibration time periods: 30 min at room temperature, 30 min or1, 2, 3, and 4 h at 4°C in a glass beaker containing 500 mL of room temperature distilled water. Post-thaw motility and forward progression were estimated after incubation at 37 °C in 5% CO2 in air for 0 and 1 h.
2.9. Data analysis
Data were analyzed using one-way ANOVA or repeated measures ANOVA. When a significant difference (P = 0.05) was observed among treatments, Bonferroni correction was used for post-test comparisons. Percent motility was arcsine-square root transformed and means of four straws per treatment were used for analysis. Values presented are means ± SD.
3. Results
3.1. Effect of straw freezing position
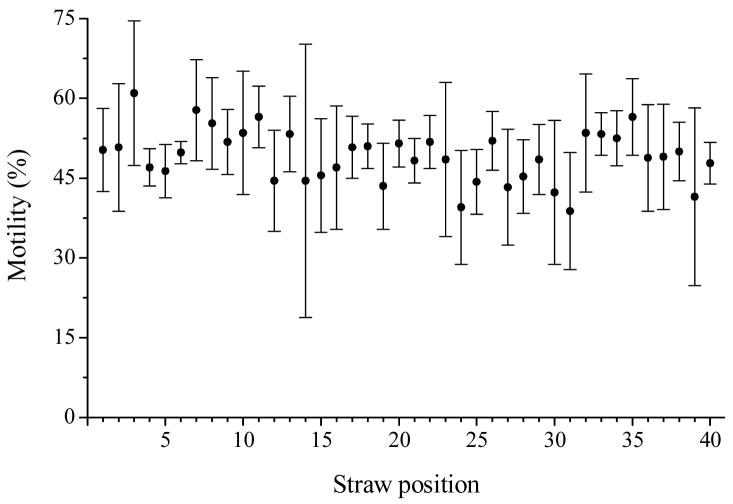
No difference in post-thaw motility was observed for sperm samples frozen in 0.25-mL French straws at various positions on the ‘boat’ (P = 0.193). Although post-thaw motility varied from 38.8 to 61.0%, straw positions with lower motility were not associated with a particular region (Fig. 1).
Fig. 1.
Post-thaw motility of rhesus monkey ejaculated sperm samples suspended with 3% glycerol-TEST-yolk, frozen on a 1 cm Styrofoam ‘boat’, at various positions ranging from 1 to 40 from one side of the ‘boat’ to the other side. Values presented were means (± SD) of four males.
3.2. Effect of sperm concentration
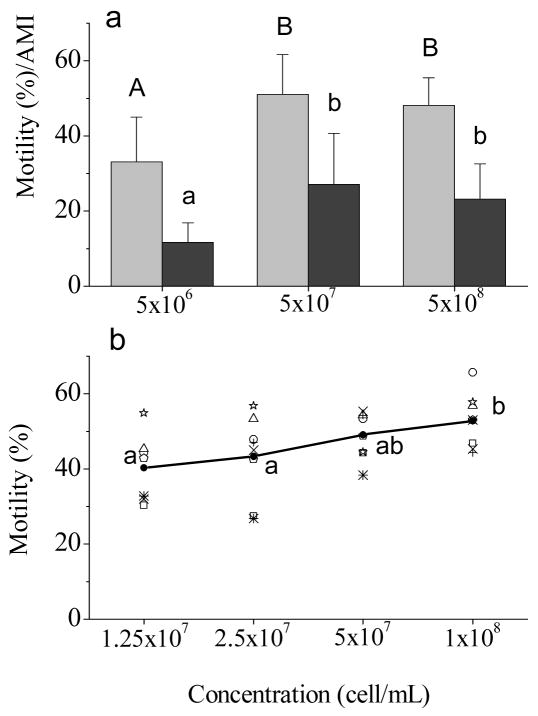
For the first trial (Fig. 2a), post-thaw motility of sperm samples frozen at 5 × 107 cell/mL (51.0 ± 10.6%) and 5 × 108 cell/mL (48.1 ± 7.3%) were higher than samples frozen at 5 × 106 cells/mL (33.0 ± 12.0%, P = 0.003). The adjusted motility index exhibited the same trend, with the highest value (27.1 ± 13.6) for samples frozen at 5 × 107 cell/mL. For the second trial (Fig. 2b), post-thaw motility of samples frozen at a sperm concentration of 1 × 108 cell/mL (52.8 ± 7.3%) was not different from that of samples frozen at 5 × 107 cell/mL (49.1 ± 6.2%, P > 0.05), but was higher than samples frozen at 2.5 × 107 (43.3 ± 11.0%) and 1.25 × 107 cell/mL (40.3 ± 9.0%, P = 0.005).
Fig. 2.
a) Post-thaw motility (grey bars) and adjusted motility index (AMI; dark bars) of rhesus monkey ejaculated sperm samples frozen at a final freezing sperm concentrations of 5 × 108, 5 × 107, and 5 × 106 cells/mL. Values presented were means (± SD) of nine males; b) Post-thaw motility of rhesus monkey ejaculated sperm samples frozen at final freezing sperm concentrations of 1 × 108, 5 × 107, 2.5 × 107, 1.25 × 107 cells/mL. Open symbols represent eight ejaculates from seven males; filled circles represent the means of all ejaculates. Bars or dots with a common superscript were not different (P>0.05).
3.3. Effect of washing through centrifugation
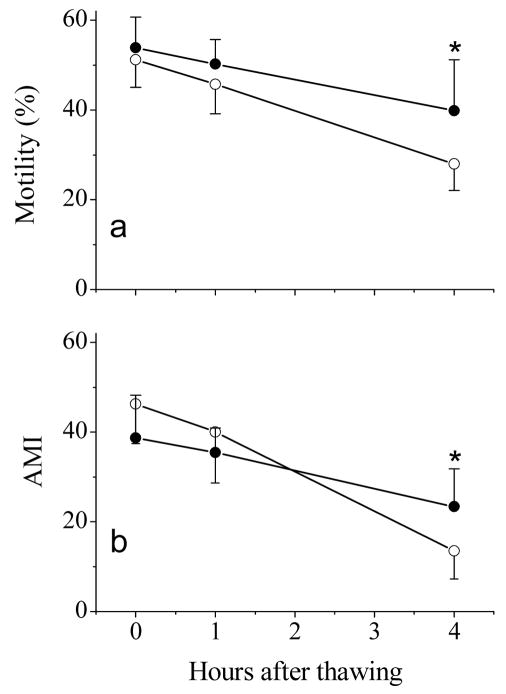
Semen samples frozen without washing retained higher (P = 0.038) post-thaw motility than samples washed with TL-BSA (Fig. 3a). However, the adjusted motility index was not different (P = 0.833, Fig. 3b), although higher values were observed at 0 and 1 h after thawing for washed samples. Both motility and adjusted motility index decreased (P < 0.001) over time for all thawed samples, with a more rapid decrease in washed samples.
Fig. 3.
Post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey ejaculated sperm samples after incubation at room temperature for 0, 1, and 4 h. Samples were used for freezing trials without wash (closed circles) or washed twice with TL-BSA (open circles). Values presented were means ± SD of eight males. Asterisks indicate significant difference between treatments.
3.4. Effect of equilibration methods
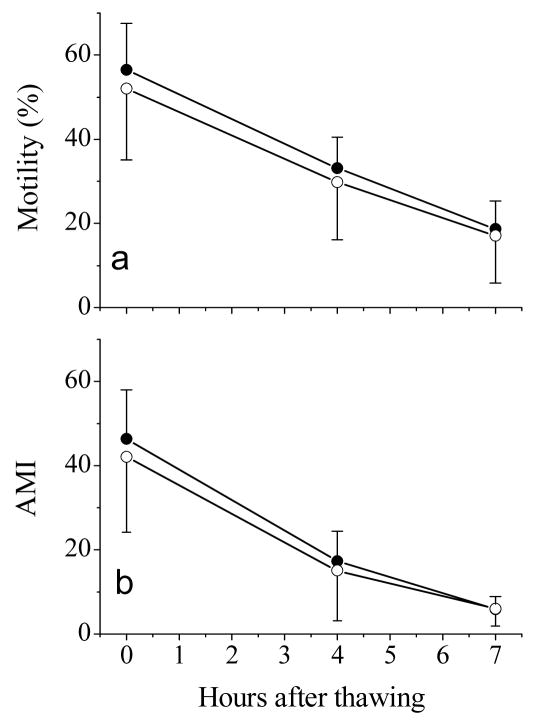
Equilibration of semen samples, either with or without pre-cooling, did not have any effect on post-thaw motility (P = 0.514, Fig. 4a) or adjusted motility index (P = 0.596, Fig. 4b), although equilibration without pre-cooling was slightly better. Similar to the above experiments, motility and adjusted motility index decreased with extended incubation time at room temperature. For example, motility declined from > 50% at 0 h to < 20% at 7 h after thawing, whereas the adjusted motility index declined from ~50 at 0 h to < 10 at 7 h after thawing. The decline rate over time was similar for the two equilibration methods.
Fig. 4.
Post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey ejaculated sperm samples after incubation at room temperature for 0, 4, and 7h. Samples were subjected to equilibration with (open circles) or without pre-cooling (closed circles). Values presented were means ± SD of eight males.
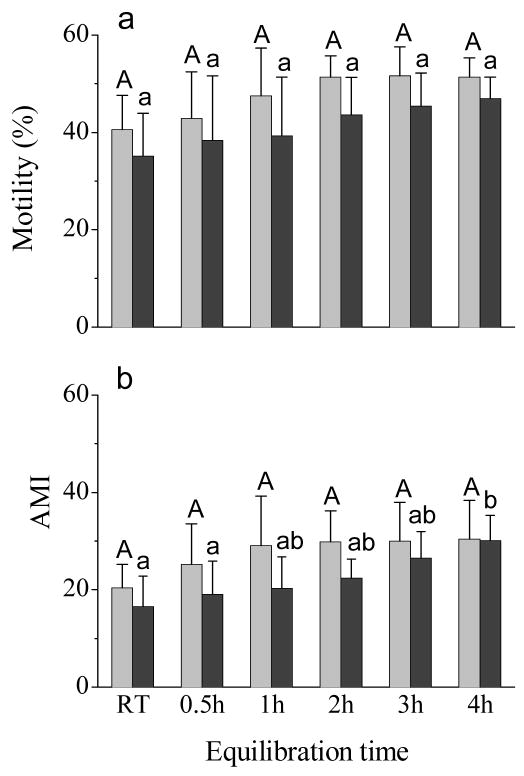
3.5. Effect of equilibration time periods
Post-thaw motility (Fig. 5a) and adjusted motility index (Fig. 5b) were lowest for samples equilibrated at room temperature for 30 min, and increased with the prolonged equilibration time for samples at 4 °C in a glass beaker containing 500 mL of room temperature distilled water. There were differences among various equilibration time periods for the adjusted motility index (P = 0.013), but not for the post-thaw motility (P = 0.071). Post-thaw motility and adjusted motility index declined (P < 0.001) after 1 h incubation at 37 °C.
Fig. 5.
Post-thaw motility (a) and adjusted motility index (AMI) (b) of rhesus monkey ejaculated sperm samples after incubation at 37 °C in 5% CO2 in air for 0 h (light gray bars) and 1 h (dark gray bars). Samples were equilibrated at room temperature for 30 min (RT) or at 4 °C in a glass beaker containing 500 mL of room temperature distilled water for 0.5, 1, 2, 3, and 4 h prior to freezing. Values presented were means ± SD of sixs males. Bars with a common superscript were not different (P>0.05).
4. Discussion
To date, sperm cryopreservation has been studied in at least 17 non-human primate species, with an emphasis on cynomolgus and rhesus macaques (based on our survey with the English literature). Despite that, few systematic studies have been performed to optimize the cryopreservation procedure for any single species. Successful gene banking of non-human primates relies on optimized cryopreservation protocols. Systematic optimization for a series of sequential cryopreservation steps will require a large number of treatment factors and levels to be evaluated in initial range-finding experiments. Therefore, a rapid assessment of post-thaw sperm quality is necessary to effectively perform these experiments. Motility is a simple and rapid estimator of sperm quality, and has been commonly used to assess thawed sperm, despite variable correlations with fertility in mammals [17,18]. However, in non-human primates, post-thaw motility was more sensitive than the sperm penetration assay in the lowland Gorilla [19], was positively correlated with membrane integrity in cynomolgus macaques [5,20], and was the most convincing parameter for sperm function analysis when compared with sperm acrosome integrity (evaluated with FITC-PNA) [21]. Thus, motility was used in the present study to estimate quality of post-thawed sperm.
Cooling of non-human primate sperm samples has often been conducted in liquid nitrogen vapor; the Styrofoam box was inexpensive, easy, reliable, and repeatable in studies with rhesus monkey sperm across three different laboratories [15]. In practice, when many straws were simultaneously loaded on top of a ‘boat’, it was common to have straws overlap or drop into liquid nitrogen. Therefore, we taped the cotton end of the straws together, thus aligning them into one single layer and avoiding the overlap and accidental dropping problems. The present study first tested whether the fixed position of straws (in reference to the distance from the sides of the ‘boat’) would affect the freezing outcome. There were some variations,but no significant differences, suggesting that the position will not affect treatment effect.
An early report of sperm cryopreservation from the squirrel monkey [22] indicated that the concentration of sperm in a sample affected post-thaw recovery of sperm motility, and their subsequent test suggested an optimal concentration range of 60 to 90 × 106/mL. Recent studies with aquatic organisms have also shown the importance of standardization of sperm concentration in freezing, which is especially relevant to the optimal concentration of cryoprotecant [23]. Studies reported using non-human primates revealed a wide variation of sperm freezing concentrations, ranging from 10 to 1000 × 106/mL, or reported as dilution ratios of 1:3 to 1:20, or simply not reported. The present study confirmed that sperm freezing concentration will affect the freezing outcome, and freezing at a higher concentration in an optimal range of 50 to 100 × 106/mL resulted in better post-thaw motility. This was in agreement with findings in horses [24] and dogs [25], where there was increased post-thaw survival for sperm frozen at high cell concentrations. Freezing of sperm at high concentrations also reduced the number of holding units required, but whether using high concentrations is practical for gene banking needs further evaluation. The use of semen dilution ratio when preparing sperm for freezing hindered or prevented the reproducibility of experimental conditions, especially when ejaculates vary in volume and sperm concentration from day to day or between different males, thus contributing to variations in treatment effect.
Sperm washing can help to remove dead sperm (thus improve motility) and meanwhile eliminate decapacitation activity of seminal plasma [14]. However, washing also reduced sperm number. In cases of experiments with multiple factors and treatment levels, unwashed samples may be desirable to ensure a sufficient number of sperm for a complete design. The present study compared the post-thaw motility of samples with and without washing; washing improved the forward progression scale immediately or within 1 h after thawing, whereas unwashed sperm retained higher post-thaw motility and progression scale for extended storage (4 h) after thawing. The presence of seminal plasma in unwashed samples may be beneficial for prolonged motility, as unbuffered semen retained motility longer than diluted samples in rhesus macaques [26]. The present study employed a simple dilution washing method, however, separation of motile and immotile spermatozoa can be performed through other methods such as Percoll gradients [27], the swim-up method [28], glass wool [29], and Sephadex filtration [30]. Future studies should explore whether these methods can help improve sperm motility for fresh and post-thawed samples, as well as for the sperm samples collected from epididymides where blood contamination is most likely.
Equilibration prior to freezing plays an important role in sperm survival during cryopreservation. The present study compared two commonly used equilibration methods (with versus without a pre-cooling period), with no significant difference between methods. Equilibration with pre-cooling requires a temperature controlled environment (e.g., 4 °C walk-in cooler) for straw loading and sealing (thus avoiding temperature shock), whereas equilibration without pre-cooling allowed straws to be loaded and sealed at room temperature, and thus was a more convenient method for practical use. Perhaps equilibration without pre-cooling only works well with low cryoprotectant concentration, as prolonged equilibration with high concentrations may be detrimental to sperm (see discussion below).
Equilibration was commonly carried out at 4 °C or with a gradual cooling process to 4 °C (e.g., the method used in this study). However, equilibration time used in sperm cryopreservation of non-human primates has varied from 1 min for ejaculated sperm of cynomolgus macaques [31] to 7 h for epididymal sperm in the gorilla [32], with 30 min to 2h used for the majority of studies. Previous studies with cynomolgus monkeys suggested a short equilibration of 30 min would improve sperm longevity after thawing [33]. However, in the present study, there was higher post-thaw motility (and progression scale) with longer equilibration time (2 to 4 h) when samples were evaluated at 0 and 1 h after thawing. This discrepancy may be due to the pre-cooling process employed in the study of [33]; an earlier study with cynomolgus monkey sperm demonstrated that motility decreased markedly when diluted semen was frozen without pre-cooling to 4 °C, regardless of the concentration of glycerol in the extender [16]. In the present study, samples equilibrated at room temperature without acclimation to 4 °C yielded the lowest post-thaw motility. Straws placed in a 600-mL glass beaker containing 500 mL of room temperature distilled water and equilibrated at 4 °C refrigerator were cooled at 0.12 °C/min and thus would take 2.5 h to reach 4 °C. The gradual increase of post-thaw motility along with the equilibration time from 0.5 to 2 h (or longer) in this study also highlighted the importance of temperature acclimation prior to freezing. Since it only takes seconds for glycerol to permeate the sperm cell [34], the protective function of equilibration time may rely on temperature acclimation, rather than cryoprotectant permeation for non-human primate sperm. In addition to the temperature acclimation protection that occurred at 2.5 h, higher post-thaw motility associated with longer equilibration time observed in this study could also have been due to the low glycerol concentration (3%) and rapid cooling rate (220 °C/min). Thus, it is necessary to evaluate multiple factors simultaneously in future studies.
In summary, we concluded that the straw position had no effect on post-thaw motility; sperm concentration affected freezing outcomes, and freezing at higher cell concentrations yielded better post-thaw motility. There were no significant differences between washed and unwashed samples, but washed sperm had a higher progressive scale in a short incubation time of 1 h after thawing. Equilibration methods also had no effect on post-thaw motility, and the effective equilibration time was the duration required for samples to temperature acclimate to 4 °C prior to freezing.
Acknowledgments
This work was supported by NIH grants RR00169 and RR13439. We thank D. Hill and N. Sealey for assistance with cryopreservation, and L.M. Correa for critical review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haigwood NL. Predictive value of primate models for AIDS. AIDS Rev. 2004;6:187–198. [PubMed] [Google Scholar]
- 2.Hérodin F, Thullier P, Garin D, Drouet M. Nonhuman primates are relevant models for research in hematology, immunology and virology. Eur Cytokine Netw. 2005;16:104–116. [PubMed] [Google Scholar]
- 3.Abbott DH, Foong SC, Barnett DK, Dumesic DA. Nonhuman primates contribute unique understanding to anovulatory infertility in women. ILAR J. 2004;45:116–131. doi: 10.1093/ilar.45.2.116. [DOI] [PubMed] [Google Scholar]
- 4.Archer DF. Role of the nonhuman primate for research related to women’s health. ILAR J. 2004;45:212–219. doi: 10.1093/ilar.45.2.212. [DOI] [PubMed] [Google Scholar]
- 5.Tollner TL, VandeVoort CA, Overstreet JW, Drobnis EZ. Cryopreservation of spermatozoa from cynomolgus monkeys (Macaca fascicularis) J Reprod Fert. 1990;90:347–352. doi: 10.1530/jrf.0.0900347. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Partida LG, Maginnis G, Dominko T, Martinovich C, McVay B, Fanton J, Schatten G. Live rhesus offspring by artificial insemination using fresh sperm and cryopreserved sperm. Biol Reprod. 2000;63:1092–1097. doi: 10.1095/biolreprod63.4.1092. [DOI] [PubMed] [Google Scholar]
- 7.Yeoman RR, Mitalipov S, Gerami-Naini B, Nusser KD, Wolf DP. Low temperature storage of rhesus monkey spermatozoa and fertility evaluation by intracytoplasmic injection. Theriogenology. 2005;63:2356–2371. doi: 10.1016/j.theriogenology.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Leibo SP. Sources of variation in cryopreservation. In: Tiersch TR, Mazik PM, editors. Cryopreservation in aquatic species. World Aquaculture Society; Baton Rouge, Louisiana: 2000. pp. 75–83. [Google Scholar]
- 9.Leibo SP, Bradley L. Comparative cryobiology of mammalian spermatozoa. In: Gagnon C, editor. The male gamete. Cache River Press; 1999. pp. 501–516. [Google Scholar]
- 10.Holt WV. Fundamental aspects of sperm cryobiology: the importance of species and individual differences. Theriogenology. 2000;53:47–58. doi: 10.1016/s0093-691x(99)00239-3. [DOI] [PubMed] [Google Scholar]
- 11.Dong Q, Huang C, Eudeline B, Tiersch T. Systematic factor optimization for cryopreservation of shipped sperm samples of diploid Pacific Oysters, Crassostrea gigas. Cryobiology. 2005;51:176–197. doi: 10.1016/j.cryobiol.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez M, Roca J, Gil MA, Vazquez JM, Martinez EA. Adjustments on the cryopreservation conditions reduce the incidence of boar ejaculates with poor sperm freezability. Theriogenology. 2007;67:1436–1445. doi: 10.1016/j.theriogenology.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Sarason RL, Vandevoort CA, Mader DR, Overstreet JW. Electro-ejaculation by direct penile stimulation of restrained but unanesthetized macaques. J Med Primatol. 1991;20:122–125. [PubMed] [Google Scholar]
- 14.VandeVoort CA. High quality sperm for nonhuman primate ART: production and assessment. Reprod Biol Endocrinol. 2004;16:33. doi: 10.1186/1477-7827-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibo SP, Kubisch HM, Schramm RD, Harrison RM, VandeVoort CA. Male-to-male differences in post-thaw motility of rhesus spermatozoa after cryopreservation of replicate ejaculates. J Med Primatol. 2007;36:151–163. doi: 10.1111/j.1600-0684.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 16.Cho F, Honjo S. A simplified method for collecting and preserving cynomolgus macaque semen. Japan J Med Sci Biol. 1973;26:261–268. doi: 10.7883/yoken1952.26.261. [DOI] [PubMed] [Google Scholar]
- 17.Soderquist L, Rodriguez-Martinez H, Jansson L. Postthaw motility, ATP content and cytochrome C oxidase activity of A.I. bull spermatozoa in relation to fertility. J Vet Med. 1991;38:165–174. doi: 10.1111/j.1439-0442.1991.tb00998.x. [DOI] [PubMed] [Google Scholar]
- 18.Graham J. Assessment of sperm quality: a flow cytometric approach. Anim Reprod Sci. 2001;68:239–247. doi: 10.1016/s0378-4320(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 19.Pope CE, Dresser BL, Chin NW, Liu JH, Loskutoff NM, Behnke EJ, Brown C, McRae MA, Sinoway CE, Campbell MK, Cameron KN, Owens OM, Johnson CA, Evans RR, Cedars MI. Birth of a western lowland gorilla (Gorilla gorilla) following in vitro fertilization and embryo transfer. Amer J Primatol. 1997;41:247–260. doi: 10.1002/(SICI)1098-2345(1997)41:3<247::AID-AJP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Feradis AH, Pawitri D, Suatha IK, Amin MR, Yusuf TL, Sajuthi D, Budiarsa IN, Hayes ES. Cryopreservation of epididymal spermatozoa collected by needle biopsy from cynomolgus monkeys (Macaca fascicularis) J Med Primatol. 2001;30:100–106. doi: 10.1034/j.1600-0684.2001.300205.x. [DOI] [PubMed] [Google Scholar]
- 21.Li YH, Cai KJ, Kovacs A, Ji WZ. Effects of various extenders and permeating cryoprotectants on cryopreservation of cynomolgus monkey (Macaca fascicularis) spermatozoa. J Androl. 2005;26:387–395. doi: 10.2164/jandrol.04147. [DOI] [PubMed] [Google Scholar]
- 22.Denis LT, Poindexter AN, Ritter MB, Seager SWJ, Deter RL. Freeze preservation of squirrel monkey sperm for use in timed fertilization studies. Fert Steril. 1976;27:723–729. [PubMed] [Google Scholar]
- 23.Dong Q, Huang C, Tiersch T. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: evidence from sperm agglutination in oysters. Cryobiology. 2007;54:87–98. doi: 10.1016/j.cryobiol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Leipold SD, Graham JK, Squires PM, McCue PM, Brinsko SP, Venderwall DK. Effect of spermatozoal concentration and number on fertility of frozen equine semen. Theriogenology. 1998;49:1537–1543. doi: 10.1016/s0093-691x(98)00099-5. [DOI] [PubMed] [Google Scholar]
- 25.Pena AI, Johannisson A, Linde-Forsberg C. Validation of flow cytometry for assessment of viability and acrosomal integrity of dog spermatozoa and for evaluation of different methods of cryopreservation. J Reprod Fert Suppl. 2001;57:371–376. [PubMed] [Google Scholar]
- 26.Leverage WE, Valerio DA, Schultz AP, Kingsbury E, Dorey C. Comparative study on the freeze preservation of spermatozoa. Primate, bovine, and human Lab Anim Sci. 1972;22:882–889. [PubMed] [Google Scholar]
- 27.Ziebe S, Andersen CY. Separation of sperm and blood cells during isolation of motile human spermatozoa. J Assist Reprod Gen. 1993;10:175–176. doi: 10.1007/BF01207745. [DOI] [PubMed] [Google Scholar]
- 28.Overstreet JW, Yanagimachi R, Katz DF, Hayashi K, Hanson FW. Penetration of human spermatozoa into the human zona pellucida and the zona-free hamster egg: a study of fertile donors and infertile patients. Fert Steril. 1980;33:534–542. doi: 10.1016/s0015-0282(16)44720-5. [DOI] [PubMed] [Google Scholar]
- 29.Engel S, Weber H, Petzoldt R, Seidl B, Wiehe W, Sperl J. An improved method of sperm selection by glass wool filtration. Andrologia. 2001;33:223–230. doi: 10.1046/j.1439-0272.2001.00434.x. [DOI] [PubMed] [Google Scholar]
- 30.Januskauskas A, Lukoseviciute K, Nagy S, Johannisson A, Rodriguez-Martinez H. Assessment of the efficacy of Sephadex G-15 filtration of bovine spermatozoa for cryopreservation. Theriogenology. 2005;63:160–178. doi: 10.1016/j.theriogenology.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Mahone JP, Dukelow WR. Semen preservation in Macaca fascicularis. Lab Anim Sci. 1978;28:556–561. [PubMed] [Google Scholar]
- 32.Beehler BA, Piper A, Jacobs SC, Clowry LJ. Post mortem collection of sperm from a lowland gorilla. Ann Proc Am Assoc Zoo Vet. 1982:56–57. [Google Scholar]
- 33.Sankai T, Terao K, Yanagimachi R, Cho F, Yoshikawa Y. Cryopreservation of spermatozoa from cynomolgus monkeys (Macca fascicularis) J Reprod Fert. 1994;101:273–278. doi: 10.1530/jrf.0.1010273. [DOI] [PubMed] [Google Scholar]
- 34.Berndston WE, Foote RH. The freezability of spermatozoa after minimal pre-frezing exposure to glycerol or lactose. Cryobiology. 1972;9:57–60. doi: 10.1016/0011-2240(72)90010-7. [DOI] [PubMed] [Google Scholar]