Abstract
Objective
To examine relationships of period of diagnosis of type 1 diabetes mellitus (T1DM) to the prevalence of visual impairment (VI).
Design
Population-based longitudinal study.
Participants
Nine hundred fifty-five persons (3,719 participant visits) 4–80 years of age at baseline who lived in an 11-county area in southern Wisconsin who were diagnosed with T1DM before age 30 years contributed to the prevalence of VI.
Methods
Five eye examination visits occurred in 1980–1982, 1984–1986, 1990–1992, 1994–1995, and 2005–2007. Age of diagnosis of T1DM was grouped as <1960, 1960–1969, 1970–1974, and 1975–1979. Best corrected visual acuity (VA) using a modification of the Early Treatment Diabetic Retinopathy protocol was measured.
Main Outcome Measures
VI defined as best corrected visual acuity in the better eye of 20/40 or worse.
Results
While controlling for duration of T1DM, there was a lower prevalence of VI for more recent periods of diagnosis of diabetes (odds ratio per category 0.91, 95% confidence interval, 0.88, 0.93; P <.0001). This remained while controlling for glycosylated hemoglobin, blood pressure, and other related factors.
Conclusions
More recently diagnosed T1DM was associated with lower prevalence of VI. This is likely due to the diminishing incidence of proliferative diabetic retinopathy (PDR) and clinically significant macular edema (CSME) due to better glycemic control and more timely interventions with photocoagulation for CSME and PDR in those with more recently diagnosed T1DM.
Persons with diabetes are at higher risk of visual impairment (VI) than nondiabetic persons.1 This is due to presence of proliferative diabetic retinopathy (PDR) and clinically significant macular edema (CSME) and to a lesser extent, a higher frequency of cataract and glaucoma in persons with diabetes compared to persons without diabetes.1–4 However, visual impairment may be decreasing as a result of implementation of findings from clinical trials that have showed that glycemic control reduces progression of diabetic retinopathy (DR) and that the treatment of vision-threatening diabetic retinopathy prevents vision loss.5–8 To address this possibility, we examined the relation of period of diagnosis of type 1 diabetes mellitus (T1DM) to the prevalence of VI over several time intervals when there has been significant change in the management of diabetes and its complications.
METHODS
Study Population
Case identification methods and descriptions of the population have appeared in previous reports.7,9–17 Briefly, the study area consisted of 11 counties in southern Wisconsin. From July 1, 1979 to June 30, 1980, 10,135 persons with diabetes were identified in the practices of 452 of 457 primary care physicians in the area. A two part sample of 2990 of these persons was invited to participate in the baseline examination from 1980 to 1982. The first part consisted of the entire population of persons taking insulin who were diagnosed as having diabetes before 30 years of age (n=1210) and the second part consisted of a probability sample of persons who were diagnosed as having diabetes at or after 30 years of age (n=1780). Based on C-peptide testing, the first group is referred to as T1DM and analyses are limited to this group. Surviving younger onset persons were invited to participate in follow up examinations in 1984–86, 1990–92, 1995–96, 2000–2002, and 2005–2007. The reasons for nonparticipation and comparisons between participants and nonparticipants at baseline and the 4-, 10-, 14-, and 25-year follow-ups have been presented elsewhere.7,9–17
Procedures
All examinations followed a similar protocol, which was approved by the institutional Human Subjects Committee of the University of Wisconsin, conformed to the tenets of the Declaration of Helsinki, and was Health Insurance Portability and Accountability Act compliant. The pertinent parts of the examination consisted of obtaining informed signed consent; measuring blood pressure; measuring refractive error and best corrected visual acuity for distance using a modified Early Treatment Diabetic Retinopathy Study (ETDRS) protocol in which the charts were reduced in size for a 2-meter distance18; dilating the pupils; administering a medical history questionnaire; performing an ophthalmoscopic examination; taking stereoscopic color fundus photographs of seven standard fields19; determining urine protein level; and determining blood glucose and glycosylated hemoglobin levels. Because the 2000–2002 examination was primarily focused on cardiovascular disease, measurements of refractive error, visual acuity, dilation of pupils, and fundus photography were not completed at this examination.
For each eye, the best corrected visual acuity was recorded as the number of letters read correctly from 0 ( 20/250) to 70 (20/10).12 For eyes with visual acuity worse than 20/250, one of six levels of visual acuity was recorded: 20/320, 20/400, 20/800, hand motions, light perception, and no light perception. The participants’ visual acuity is defined as the visual acuity in the better eye. In this study, we define severe VI as a visual acuity of 20/200 or less in the better eye. Any VI is defined as a visual acuity of 20/40 or less in the better eye.
To determine the severity of retinopathy in each eye, all fundus photographs were graded using a modification of the ETDRS classification scheme.10,19 Briefly, level 10 represents no retinopathy, levels 21 through 53 represent nonproliferative retinopathy of increasing severity, and levels 60 through 85 represent proliferative retinopathy of increasing severity. Macular edema was also determined from the fundus photographs as described previously.20 Macular edema was considered present if any area of the retina within 1 disc diameter from the center of the macula was thickened or if there was a prior history of macular edema with evidence of photocoagulation treatment consistent with it.
Current age was defined as the age at the time of the baseline examination. Duration of younger onset diabetes was the time interval between diagnosis of diabetes and the specific examination. Age at diagnosis was obtained from physician’s chart. Metabolic control was measured by glycosylated hemoglobin using a microcolumn technique.21,22 Hypertension was defined as a mean systolic blood pressure ≥ 160 mmHg and/or a mean diastolic blood pressure ≥ 95 mmHg or a history of antihypertensive medication at the time of examination in individuals ≥ 25 years of age or a mean systolic blood pressure of ≥ 140 mmHg and/or a mean diastolic blood pressure of ≥ 90 mmHg, and/or a history of antihypertensive medication at the time of examination in younger persons. Urine samples were collected and tested for gross proteinuria by means of a reagent strip (Labstix, Ames, Elkhart, IN). Urine protein was defined as absent (<0.30 g/l) or present (≥ 0.30/g/l). Questions about occupation, (“What is or was your occupation?” “What is or was the occupation of your spouse?”) and education (“What was the highest grade of school or year of college you completed?”) were asked. Information on occupation was coded according to the 1970 U.S. Census three-digit occupation coding system.23 A Duncan Socioeconomic Index score was assigned to each participant based upon the participant’s occupation.24 If a married participant had no occupation, we used the occupation of the spouse. When a single person had no occupation and lived with a parent, we used the parent’s occupation.
Statistics
SAS version 9 was used for analyzing the data (SAS Institute Inc., Cary, NC). Data were structured such that each participant contributed data for every examination visual acuity was measured whether or not they had previously obtained the VI outcome.
Effects of duration of diabetes and period of diagnosis of T1DM cohort on VI were assessed using logistic regression models with the generalized estimating equations approach to account for correlation between multiple assessments of the same person. We categorized duration of diabetes into 5-year bands and period of diagnosis of T1DM into 4 bands: 1922–59, 1960–69, 1970–74, and 1975–79. Multivariate models included the following variables that could potentially influence the relation between period of diagnosis and the prevalence of VI: age at the examination, gender, glycosylated hemoglobin A1, systolic and diastolic blood pressure, presence of proteinuria, and laser treatment. Additional models included the Duncan Socioeconomic Index. Interactions between period of diagnosis of T1DM cohort and each of these potential confounders were explored by including appropriate interaction terms in the model.
RESULTS
Nine hundred and ninety-five participants contributed 3,719 participant-visits for the analysis of the prevalence of VI. Characteristics of the remaining participants at the start of each examination are shown in Table 1. Persons participating at later examinations were older, had lower glycosylated hemoglobin A1 levels, higher systolic blood pressure, lower diastolic blood pressure, higher frequency of PDR and macular edema, and were more likely to have hypertension, to be college graduates, and have photocoagulation for PDR or macular edema than those seen at earlier examinations. There did not appear to be a difference in socioeconomic status, proteinuria or visual impairment status. Because the prevalence of severe VI was low (< 4%), further analyses are restricted to the prevalence of any VI.
Table 1.
Characteristics of Cohort at Beginning of Each Wisconsin Epidemiologic Study of Diabetic Retinopathy Examination.
| Factor | Baseline N=996 | 4-Year N=891 | 10-Year N=784 | 14-Year N=654 | 25-Year N=426 |
|---|---|---|---|---|---|
| Mean age, year | 29 | 32 | 37 | 41 | 50 |
| % Males | 49 | 49 | 49 | 50 | 48 |
| Mean glycosylated hemoglobin A1, % | 10.8 | 10.1 | 10.0 | 9.5 | 8.1 |
| Mean systolic blood pressure, mmHg | 124.6 | 123.2 | 126.1 | 126.8 | 132.8 |
| Mean diastolic blood pressure, mmHg | 78.7 | 77.2 | 76.2 | 74.5 | 73.2 |
| % Hypertension | 22 | 23 | 29 | 34 | 53 |
| % Proteinuria | 21 | 23 | 27 | 23 | 26 |
| % Education level, college graduate | 23 | 26 | 30 | 30 | 32 |
| Mean Duncan’s Socioeconomic Index | 61 | 61 | 61 | 62 | 62 |
| % Proliferative diabetic retinopathy | 23 | 28 | 41 | 43 | 48 |
| % Macular edema | 10 | 13 | 21 | 23 | 24 |
| % Photocoagulation* | 15 | 21 | 31 | 37 | 43 |
| % Visual impairment | 8 | 10 | 9 | 8 | 7 |
Photocoagulation= panretinal, macular grid and/or focal photocoagulation;
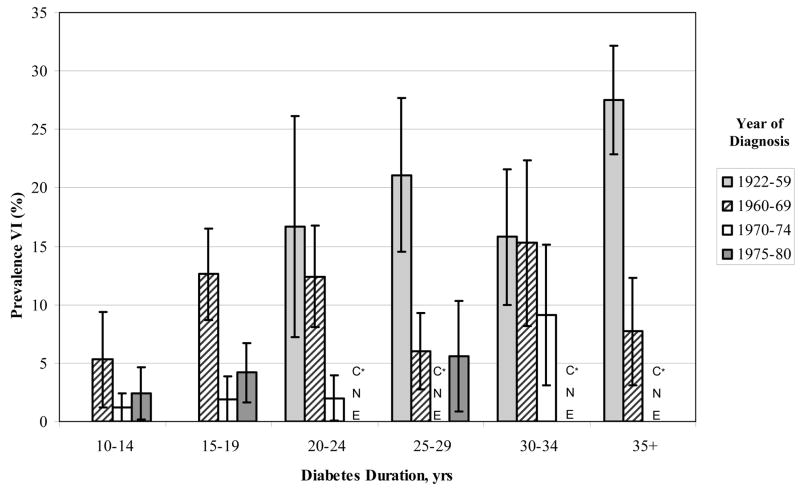
For most duration of diabetes groups, the prevalence of VI was lower in those persons diagnosed more recently than in those diagnosed earlier (Figure 1). For example, the prevalence of VI in people with 15–19 years of duration of T1DM at the time of examination was 13% among those diagnosed with T1DM in 1960–69, 2% among those diagnosed in 1970–74, and 4% among those diagnosed in 1975–79. The prevalence of VI in people with 30–34 years of duration of T1DM at the time of examination was 16% among those diagnosed with T1DM in 1922–59, 15% among those diagnosed in 1960–69, and 9% among those diagnosed in 1970–74.
Figure 1.
Differences in prevalence of visual impairment by duration and period of diagnosis of type 1 diabetes mellitus in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. *CNE=cannot estimate (defined by < 50 persons at risk)
Models incorporating duration and period of T1DM cohort as categorical and, in addition, ordered factor variables are presented in Table 2. The prevalence of VI increased with longer duration of T1DM (Odds Ratio (OR) per duration group 1.15, 95% Confidence Interval (CI) 1.05, 1.27, P =.002). After controlling for age, sex, glycosylated hemoglobin A1, systolic and diastolic blood pressure, proteinuria, and photocoagulation status, the association was no longer evident (OR 0.93, 95% CI 0.79, 1.10, P =.38). There was a lower prevalence of VI with more recent diagnosis (OR per more recent period of diagnosis 0.91, 95% CI 0.88, 0.93, P <.0001). After controlling for, age, sex, glycosylated hemoglobin A1, systolic and diastolic blood pressure, proteinuria, and photocoagulation status, the association remained unchanged (OR 0.91, 95% CI 0.88, 0.94, P <.0001). By controlling further for Duncan’s Socioeconomic Index, the association remained unchanged (data not shown). There were no significant interactions between period of diagnosis of T1DM cohort and age, sex, glycosylated hemoglobin A1, and Duncan’s Socioeconomic Index and the prevalence of VI.
Table 2.
Diabetes Duration and Period of Diagnosis of Type 1 Diabetes Models for Visual Impairment.
| Models | Adjusted Models* | |||||
|---|---|---|---|---|---|---|
| Contrast | OR | (95% CI) | P-value | OR | (95% CI) | P-value |
| Duration of diabetes (years) | ||||||
| 10–14 vs 5–9 | 1.21 | (0.54–2.69) | .64 | 1.11 | (0.45–2.79) | .82 |
| 15–19 vs 10–14 | 2.35 | (1.33–4.16) | .003 | 1.10 | (0.58–2.10) | .76 |
| 20–24 vs 15–19 | 0.92 | (0.62–1.39) | .71 | 0.69 | (0.41–1.14) | .15 |
| 25–29 vs 20–24 | 0.82 | (0.56–1.19) | .29 | 0.76 | (0.47–1.22) | .25 |
| 30–34 vs 25–29 | 1.31 | (0.90–1.90) | .16 | 1.21 | (0.75–1.94) | .44 |
| 35+ vs 30–34 | 1.17 | (0.83–1.65) | .36 | 1.01 | (0.62–1.63) | .98 |
| Duration- ordered factor† | 1.15 | (1.05–1.26) | .003 | 0.93 | (0.78–1.10) | .40 |
| Period of diagnosis of diabetes | ||||||
| 1960–1969 vs 1922–1959 | 0.44 | (0.28–0.68) | <.001 | 0.42 | (0.24–0.71) | .001 |
| 1970–1974 vs 1960–1969 | 0.27 | (0.15–0.48) | <.001 | 0.29 | (0.15–0.57) | <.001 |
| 1975+ vs 1970–1974 | 1.46 | (0.72–2.98) | .30 | 1.85 | (0.81–4.22) | .15 |
| Period of diagnosis- ordered factor† | 0.91 | (0.88–0.93) | <.001 | 0.91 | (0.88–0.94) | <.001 |
Abbreviations: OR=odds ratio; CI=confidence interval.
Duration and diagnosis models further adjusted for: age at the examination, gender, glycosylated hemoglobin A1, systolic and diastolic blood pressure, presence of proteinuria, and laser treatment
Ordered factor effects were fit using the categorical variable as a linear effect in separate model
Changes in incidence of visual loss over the 25 year study period are presented in Table 3. To adjust for the variable lengths of the intervals between examinations, the rates are presented on an annual basis. For any VI, the annualized incidence rates decreased from 1.19% in the first follow-up interval to 0.30% in the fourth follow-up interval. Differences in annualized incidence rates based on intervals of greatly differing lengths may be exaggerated due to the greater cumulative impact of the competing risk of death in longer intervals. However, if we consider the interval between visits 2 and 4 as a single 11 year interval comparable in length to the last 10 year interval, the annualized incidence of VI for the earlier interval (0.52%) was still higher than that of the last interval (0.30%). This relationship persisted within strata based on duration of T1DM (data not shown).
Table 3.
Annualized Incidence of Visual Impairment in the Wisconsin Epidemiologic Study of Diabetic Retinopathy.
| Interval Number | Incident Visual Impairment | Annualized Incidence | |||||
|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Years between Visits | No. at Risk | N | % | % | (95% CI) |
| 1 | 2 | 4 | 832 | 39 | 4.69 | 1.19 | (0.82–1.57) |
| 2 | 3 | 6 | 693 | 34 | 4.91 | 0.83 | (0.56–1.12) |
| 3 | 4 | 5 | 566 | 21 | 3.71 | 0.75 | (0.43–1.08) |
| 4 | 6 | 10 | 368 | 11 | 2.99 | 0.30 | (0.13–0.48) |
Abbreviation: CI=confidence interval
DISCUSSION
Prevalence of VI is often examined by duration of diabetes strata. One advantage of the long-term study of population-based cohorts with multiple examinations is the opportunity to observe whether period of diagnosis of T1DM affects this relation. In the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), we found evidence of lower prevalence of VI in those diagnosed with T1DM more recently. This is consistent with our finding of decreasing incidence of visual impairment in persons examined in more recent periods.
To our knowledge, few other studies have examined a cohort effect on VI in persons with T1DM.8,25 Data from a clinic-based study in Denmark showed that the incidence of VI for a specific duration of T1DM declined for each subsequent five years at year of diagnosis from 1965–69 through 1979–80.8 This was associated with statistically significant trends of decreasing glycosylated hemoglobin A1, mean arterial blood pressure levels, earlier treatment of hypertension, and lower incidence of PDR and CSME in each subsequent period. In 2004, another study in Denmark found that the prevalence of VI was lower than anticipated from the existing literature.25
There are many possible reasons why persons diagnosed to have T1DM in more recent years have a lower prevalence of VI than persons with a similar duration of T1DM diagnosed in earlier years. One reason is that persons diagnosed in earlier years were less likely to receive retinal photocoagulation treatment for PDR or CSME because such treatment first became available in the early 1960s and its efficacy was demonstrated in randomized controlled clinical trials in the 1970s (Diabetic Retinopathy Study [DRS]) and 1980s (ETDRS).5,6,26 This is reflected in the WESDR where growing proportions of persons with PDR or CSME had been photocoagulated in subsequent examinations (Klein R, unpublished data). Implementation of results of the DRS and ETDRS into clinical practice in the late 1980s involved the development of guidelines, education, and screening programs for early detection of vision threatening retinopathy through dilated eye examinations.27,28
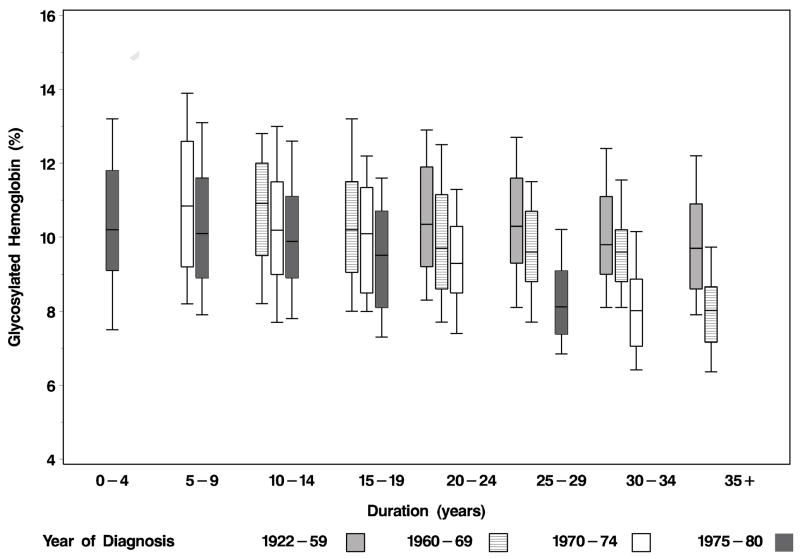
A second reason is earlier implementation of better glycemic control in those diagnosed to have T1DM more recently. These changes occurred as a result of the development of self-blood glucose monitoring and the more frequent use of intense glycemic control after the Diabetes Control and Complications Trial (DCCT) showed a statistically significant 60% and 34% reduction in progression of retinopathy in primary and secondary prevention arms, respectively, in those treated with intensive glycemic control compared to those treated with conventional control.7,29 Lower glycosylated hemoglobin A1 levels in later examination periods of the WESDR may reflect this. This is reflected in the finding that in more recently diagnosed cohorts, glycosylated hemoglobin A1 levels were lower compared to earlier diagnosed cohorts (Figure 2). Thus, persons diagnosed to have T1DM in earlier periods are likely to have had differing exposures to factors (e.g., hyperglycemia, uncontrolled blood pressure) and different patterns of care for eye disease (e.g., timely detection and treatment of vision-threatening retinopathy, removal of cataract) that may affect the incidence of VI over time. However, when added to multivariable models, systolic and diastolic blood pressure and glycemic control resulted in minimal attenuation of the cohort effect. We cannot evaluate the effect of exposures and treatments prior to the beginning of the WESDR on the incidence of VI in different period of diagnosis of T1DM cohorts.
Figure 2.
Distribution (box for 25th to 75th and lines for 10th and 90th percentiles) of glycosylated hemoglobin A1 levels by duration and period of diagnosis of type 1 diabetes mellitus in the Wisconsin Epidemiologic Study of Diabetic Retinopathy.
Current forecasts of 25-year estimates of prevalent VI are based on the assumption that the earlier observed trends will be similar in future years.30 However, this assumption may not be correct if decreasing prevalence of VI continues with each new period of diagnosis of T1DM cohort as observed in the WESDR. Lower prevalence of VI might be expected in the future in persons with T1DM because current estimates are based on existing data and do not take into account possible drops in incidence as more recently diagnosed cohorts progress through their disease.
There are many strengths of the current study including the objective measurement of best corrected visual acuity measured at five examinations over a 25-year period in a large cohort. Nevertheless, the results from this study should be interpreted with caution. First, the current analysis was limited to participants who survived to enter the study in 1980. It is possible that risk factors (e.g., glycosylated hemoglobin A1, blood pressure, kidney disease) may have differentially affected the relationship between participation and mortality and prevalence of VI in different period of diagnosis of T1DM cohorts. However, when the joint outcome of VI and death was examined, we continued to see a reduction of VI or death in later period of diagnosis of T1DM cohorts making it less plausible that survival had a large influence on these results (Klein R, unpublished data). Second, it is likely that those from an earlier period of diagnosis of T1DM cohort were the healthiest, leading to an even more conservative estimate of the relationships reported. Third, we did not take into account changes in the management of other causes of VI (e.g., cataract, open angle glaucoma) that may have changed over the period of study.
In summary, we found that persons diagnosed to have T1DM in more recent years had a lower prevalence of VI than persons with similar durations of diabetes diagnosed to have T1DM in earlier periods. Observed differences in health care (e.g., better control of glycemia and blood pressure, and timely retinal photocoagulation) over the 25 years of the study may explain, in part, some of these findings.
Acknowledgments
Financial Support: This research is supported by National Institutes of Health grant EY03083 and EY016379 (Ronald Klein, MD, MPH, Barbara E.K. Klein, MD, MPH) and, in part, by the Research to Prevent Blindness (R. Klein and BEK Klein, Senior Scientific Investigator Awards), New York, NY. The National Eye Institute provided funding for entire study including collection and analyses of data; RPB provided further additional support for data analyses.
Footnotes
Financial disclosure: Authors with financial interest or relationships to disclose are listed after the references.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–60. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 2.Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes: the Beaver Dam Eye Study. Ophthalmology. 1994;101:1173–7. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- 3.Pasquale LR, Kang JH, Manson JE, et al. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology. 2006;113:1081–6. doi: 10.1016/j.ophtha.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities: the Beaver Dam Eye Study. Ophthalmic Epidemiol. 1995;2:49–55. doi: 10.3109/09286589509071451. [DOI] [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings. DRS report number 8 Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 6.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–64. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–6. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217–28. doi: 10.1001/archopht.1994.01090210105023. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–15. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE. Visual impairment in diabetes. Ophthalmology. 1984;91:1–9. [PubMed] [Google Scholar]
- 13.Moss SE, Klein R, Klein BE. The incidence of vision loss in a diabetic population. Ophthalmology. 1988;95:1340–8. doi: 10.1016/s0161-6420(88)32991-x. [DOI] [PubMed] [Google Scholar]
- 14.Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994;101:1061–70. doi: 10.1016/s0161-6420(94)31217-6. [DOI] [PubMed] [Google Scholar]
- 15.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 16.Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med. 2004;164:1917–24. doi: 10.1001/archinte.164.17.1917. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII. The twenty-five-year progression of retinopathy with persons with type 1 diabetes. Ophthalmology. 2008;115:1859–68. doi: 10.1016/j.ophtha.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Early Treatment Diabetic Retinopathy Study (ETDRS) Manual of Operations. Bethesda, MD: National Eye Inst.; 1985. pp. 101–19. NTIS PB85–223006. [Google Scholar]
- 19.Diabetic Retinopathy Study Research Group. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21:210–26. [PubMed] [Google Scholar]
- 20.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: IV. Diabetic macular edema. Ophthalmology. 1984;91:1464–74. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 21.Moss SE, Klein R, Klein BE, et al. Methodologic considerations in measuring glycosylated hemoglobin in epidemiologic studies. J Clin Epidemiol. 1988;41:645–9. doi: 10.1016/0895-4356(88)90116-3. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Moss S. A comparison of the study populations in the Diabetes Control and Complications Trial and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med. 1995;155:745–54. [PubMed] [Google Scholar]
- 23.U.S. Bureau of the Census. 1970 Census of population. Alphabetical index of industries and occupations. Washington, D.C.: U.S. Bureau of the Census, Population Div.; 1971. pp. 1–201. [Google Scholar]
- 24.Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Soc Sci Res. 1981;10:364–95. [Google Scholar]
- 25.Hove MN, Kristensen JK, Lauritzen T, Bek T. The prevalence of retinopathy in an unselected population of type 2 diabetes patients from Arhus County, Denmark. Acta Ophthalmol Scand. 2004;82:443–8. doi: 10.1111/j.1600-0420.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 26.Wetzig PC, Worlton JT. Treatment of diabetic retinopathy by light-coagulation: a preliminary study. Br J Ophthalmol. 1963;47:539–41. doi: 10.1136/bjo.47.9.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Eye Health Education Program. From Vision Research to Eye Health Education: Planning the Partnership. Bethesda, MD: National Eye Inst.; Mar, 1990. [Google Scholar]
- 28.American Diabetes Association. Eye care guidelines for patients with diabetes mellitus. Diabetes Care. 1988;11:745–6. doi: 10.2337/diacare.11.9.745. [DOI] [PubMed] [Google Scholar]
- 29.Jovanovic L, Peterson CM. Home blood glucose monitoring. Compr Ther. 1982;8:10–20. [PubMed] [Google Scholar]
- 30.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]