Abstract
N-methyl-D-aspartate receptors (NMDAR) play a pivotal role in excitatory neurotransmission, synaptic plasticity, and brain development. Clinical and experimental evidence suggests a dysregulation of NMDAR function and glutamatergic pathways in the pathophysiology of schizophrenia. We evaluated electrophysiological and behavioral properties of NMDAR deficiency utilizing mice that express of only 5-10% of the normal level of NMDAR NR1-subunit. Auditory and visual event related potentials yielded significantly increased amplitudes for the P20 and N40 components in NMDAR deficient (NR1neo-/-) mice suggesting decreased inhibitory tone. Compared to wildtypes, NR1neo-/- mice spent less time in social interactions and demonstrated reduced nest building. NR1neo-/- mice displayed a preference for open arms of a zero-maze and central zone of an open field, possibly reflecting decreased anxiety-related behavioral inhibition. However, locomotor activity did not differ between groups in either home cage environment or during behavioural testing. NR1neo-/- mice displayed hyperactivity only when placed in a large unfamiliar environment, suggesting that neither increased anxiety nor nonspecific motor activation accounts for differential behavioral patterns. Data suggest that NMDAR NR1 deficiency causes disinhibition in sensory processing, as well as reduced behavioral inhibition and impaired social interactions. The behavioral signature in NR1neo-/-mice supports the impact of impaired NMDAR-function in a mouse model with possible relevance to negative symptoms in schizophrenia.
Keywords: Models, Animal; Behavior; Deficiency; Evoked Potentials; Receptors, N-Methyl-D-Aspartate; Schizophrenia
Introduction
Schizophrenia is a highly disabling brain disorder. Recent advances in clinical and preclinical pharmacological, brain imaging and genetic studies suggest a central role for several neurotransmitters, in particular dopamine and glutamate (Coyle 1996; Javitt 2007; Javitt & Zukin 1991; Lewis & Lieberman 2000; Weinberger 1987). More specifically, N-methyl-D-aspartate receptors (NMDAR) have been implicated in the pathophysiology of schizophrenia. NMDAR are ionotropic glutamate receptors that play a pivotal role in excitatory neurotransmission, synaptic plasticity, and brain development (Dingledine et al. 1999; Goff & Coyle 2001). NMDAR antagonists such as phencyclidine (PCP) and ketamine provoke a syndrome in normal individuals that closely resembles the clinical presentation of schizophrenia (Javitt & Zukin 1991) and exacerbates symptoms in patients with schizophrenia (Lahti et al. 2001).
Studies using NMDAR antagonists in humans have been limited in that they only produce a temporary receptor hypofunction whereas the etiology of schizophrenia suggests a protracted developmental dysregulation. This has limited the generalizability of acute pharmacological studies and therefore, genetically altered mice have been a valuable tool for the study of developmental impairments in glutamatergic neurotransmission. Mohn et al have generated a mouse strain (NR1neo-/- mice) that only expresses 5-10% of normal NR1 NMDAR subunit protein (Mohn et al. 1999). Subsequent studies found decreased prepulse inhibition (PPI) (Bickel et al. 2008; Duncan et al. 2006a, b; Duncan et al. 2004; Moy et al. 2006), sociability (Duncan et al. 2004; Mohn et al. 1999; Moy et al. 2008), and mating behavior (Duncan et al. 2004; Mohn et al. 1999), as well as impaired olfaction and increased locomotor activity in NR1neo-/- mice . Based on these findings the NR1neo-/- strain has been described as a translational model for schizophrenia . Electrophysiological testing previously focused primarily on sensorimotor gating (Bickel et al. 2008; Duncan et al. 2006a; Duncan et al. 2004; Fradley et al. 2005; Moy et al. 2006). It remains unclear how sensory gating is affected by the knock-down of NMDA-NR1 subunits. In addition results regarding behavioral measures have been variable and it is uncertain whether the decreased socialbility of NR1neo-/- mice is a product of an anxiety or a genuine reduction in sociability (Duncan et al. 2004; Mohn et al. 1999; Moy et al. 2008). The latter suggests that NR1neo-/- mice can serve as a suitable model for negative symptoms of schizophrenia (Duncan et al. 2006b; Fradley et al. 2005; Miyamoto et al. 2004). The purpose of this study is to further elaborate following characteristics of mice with reduced NR1 NMDAR subunit expression:
Recording of auditory and visually ERPs is intended to clarify whether previously reported abnormal responses are limited to auditory processing or represent a general trait of sensory processing instead.
Sociability testing examines social interaction deficits in NR1neo-/- mice as a model of negative symptoms of schizophrenia.
Behavioral assessment of open field, elevated zero-maze and locomotor activity provide a more complete understanding of anxiety-like behaviors in NR1neo-/- mice as well as their relationship to social behavior.
Materials and methods
Mice
The NR1neo-/- mice (Mohn et al. 1999) were obtained from the laboratory of Dr. Beverly Koller (The University of North Carolina at Chapel Hill) and a breeding colony was established at AstraZeneca Pharmaceuticals LP (Wilmington DE 19850, USA). Their breeding involves three populations of mice: NR1neo+/- heterozygotes maintained on C57BL/6J background (Jackson Laboratory), NR1neo+/- heterozygotes maintained on 129S6/SvEvTac background (Taconic Farm), and an intercross between female C57BL/6J NR1neo+/- and male 129S6/SvEvTac NR1neo+/-. The progeny from the intercross are genetically identical F1 hybrids with the exception at the NR1 locus: 50% NR1neo+/-, 25% NR1neo-/- and 25% wild type (WT). A PCR protocol provided by B. Koller was used for genotyping these mice: NR1 (+) fwd primer (intron 20) 5'TGA GGG GAA GCT CTT CCT GT3'; NR1 (-) fwd primer (neo) 5'GCT TCC TCG TGC TTT ACG GTA T3'; NR1 common reverse primer (intron 20) 5'AAG CGA TTA GAC AAC TAA GGG T3'. For experiments described here, only male mice were used. Two cohorts of 16 NR1neo-/- and 16 wild type littermates were generated by AstraZeneca Neuroscience (Wilmington, DE 19850, USA) and arrived at the University of Pennsylvania when 8 weeks old. A third cohort of 8 NR1neo-/- and 8 wild type littermates was later generated and housed at Astra Zeneca Neuroscience to verify the results of locomotor activity testing collected from cohort 1 (Table 1). Animals were maintained on a 12-hour light/dark cycle (lights off at 19:00) in a temperature-controlled facility with food and water available ad libitum. Mice were housed four to five per cage during quarantine and during acclimation to the housing facility (1 week) prior to surgery. After electrode placement (before assessment of nest building for the 2nd cohort) and during all consecutive testing, each mouse was housed individually (Table 1). Surgeries, behavioral and electrophysiological testing occurred between 9-19 weeks of age. All tests were performed during the light phase between 14:00 and 17:00. Gonadectomized male A/J mice (8-30 weeks) used as stimulus mice for the social-choice paradigm were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Adequate measures were taken to minimize pain or discomfort. All protocols were approved by the AstraZeneca and University of Pennsylvania Institutional Animal Care and Use Committees and were conducted in accordance with National Institutes of Health guidelines.
Table 1.
Order of interventions and tests performed with each cohort: Please see the Materials and Methods section for a detailed description of each procedure. Mice were singly housed prior to testing, which was following surgery in the 1st cohort. Note that the number of mice decreases for the following reasons as follows. For Sociability Testing, 3 NR1neo-/- mice were excluded after having a seizure. For Elevated Zero Maze, 3 NR1neo-/- mice were removed. Two NR1neo-/- mice jumped off the platform and 1 NR1neo-/- had a seizure. These mice were excluded from statistical analysis.
| Group | n | Location | ⇒ Oder of Interventions/Testing | ||||
|---|---|---|---|---|---|---|---|
| 1st Cohort | 16 NR1neo-/- 16 WT | University of Pennsylvania | Surgery 16 NR1neo-/- 16 WT | Auditory Event Related Potentials 16 NR1neo-/- 15 WT | Sociability 7 NR1neo-/- 12 WT | Locomotor Activity 7 NR1neo-/- 10 WT | Visual Event Related Potentials 7 NR1neo-/- 8 WT |
| 2nd Cohort | 16 NR1neo-/- 16 WT | University of Pennsylvania | Nesting 16 NR1neo-/- 16 WT | Surgery 16 NR1neo-/- 16 WT | Elevated Zero Maze 8 NR1neo-/- 12 WT | Open Field 12 NR1neo-/- 12 WT | - |
| 3rd Cohort | 8 NR1neo-/- 8 WT | Astra Zeneca | Locomotor Activity 8 NR1neo-/- 8 WT | - | - | - | - |
Auditory Evoked Potentials
Surgery
Animals underwent stereotaxic implantation of tripolar electrode assemblies (PlasticsOne, Roanoke, VA, USA) for non-anesthetized recording of auditory ERPs. Animals were anesthetized with isoflurane. Three stainless steel electrodes, mounted in a single pedestal, were aligned along the sagittal axis of the skull at 1mm intervals with precut lengths of 3mm (positive) and 1mm (ground and negative). Positive electrodes were placed in the right CA3 hippocampal region, 1.8mm posterior, 2.65mm right lateral, and 2.75mm deep relative to bregma (junction of the sagittal and coronal sutures used as surgical point of reference). Negative electrodes were placed adjacent to positive and ground electrodes on the ipsilateral cortex at 0.2mm anterior, 2.75mm lateral and 0.75mm deep to relative to bregma. Ground electrodes were located between positive and negative electrodes on the ipsilateral cortex at 0.8mm posterior, 2.75lateral, and 0.75mm deep to bregma. The electrode pedestal was secured to the skull with ethyl cyanoacrylate (Loctite, Henkel, DÜsseldorf, Germany) and dental cement (Ortho Jet, Lang Dental, Wheeling IL, USA).
Electrophysiology
Recording of brain activity for auditory and visual ERPs was performed 2 weeks after electrode implantation. Each animal was placed in its home cage in a sound attenuated recording chamber inside a Faraday electrical isolation cage. Background white noise was at 63dB. Electrode pedestals were connected to a 30cm tripolar electrode cable that excited the chamber to connect to a high impedance differential AC amplifier (A-M Systems, Carlsborg, WA, USA).
Auditory Event Related Potentials
Auditory ERPs were recorded as previously described (Connolly et al. 2004; Maxwell et al. 2006). Stimuli were generated by Micro 1401 hardware with Spike 5 software (Cambridge Electronic Design, Cambridge, UK) and were delivered through speakers attached to the cage top. A series of 1250 paired stimuli (1500Hz, 85dB sound pressure, and 10ms duration) was presented 500ms apart, with a 9sec inter-pair interval. Spike 5 software on a Pentium V personal computer (PC) connected to a Micro 1401 II interface module (CED, Cambridge, UK) and high impedance differential AC amplifier (A-M Systems, Carlsborg, WA, USA) was used to record brain activity. Recording sessions were preceded by a 15min acclimation phase and subsequent stimulus presentation and data collection. For auditory ERPs, the P20 (maximal positive deflection 10-30ms) and N40 (maximal negative deflection 25-60ms) amplitudes were determined for 1st stimulus (S1) and 2nd stimulus (S2). P20 and N40 amplitudes were analyzed using repeated-measures-ANOVA. Genotype was designated as the independent variable with stimulus condition (1st vs. 2nd) as a repeated measure within each mouse. Significant main effects were followed by Fisher LSD post hoc comparisons. The level of significance was set at p<0.05 for all statistical comparisons reported subsequently.
Visual Event Related Potentials
Visual stimuli were delivered through a flash box (PS40/R Photic Stimulator, Grass Technologies, West Warwick, RI) 30cm above home cages with transparent cage tops. During the 15min acclimation period and subsequent stimulus presentation, mice were entirely in the dark. A 500ms prestimulus baseline and a 500ms poststimulus interval were recorded for each stimulus (sample rate 1000Hz). Electroencephalographic signal was digitally filtered between 100 and 450Hz. Average waves were created for the response to the first and second stimulus for each mouse separately. Recording sessions consisted of an acclimation phase (15min) and subsequent data collection. Time intervals for the 1st positive (P1) and 1st negative (N1) deflection were defined as two standar errors of the mean (SEM) around mean latency values (NR1neo-/- mice: P1=30-53ms, N1=49-77ms; WT mice: P1=19-31ms, N1=28-68ms). Analysis was performed as described above for auditory ERPs.
Behavioral Tests
Sociability
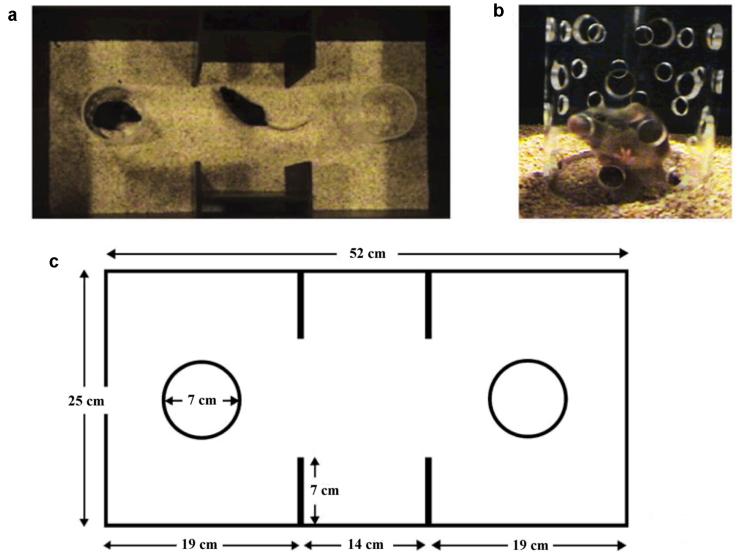
The sociability of 7 NR1neo-/- mice was compared to that of 12 WT mice. The degree of social approach for a test mouse (either a NR1neo-/- or WT mouse) towards a novel, unfamiliar stimulus mouse was measured in a social choice task as previously described (Brodkin 2007; Brodkin et al. 2004; Sankoorikal et al. 2006). The social choice test was carried out in a three-chambered apparatus with no top or bottom and that consisted of a center chamber and two end chambers (Figure 1). Behavioral testing was videotaped with a Sony digital video camera with NightShot® (infrared) feature for recording in low light. To minimize the general stress level of the mice, the testing room was dimly lit, with lighting in all chambers measured 1-2 lux during testing. Prior to the start of the test, one end chamber was designated as the “social chamber,” into which a stimulus mouse would be introduced, and the other end chamber was designated as the “nonsocial chamber.” The end chamber designated as the social chamber was varied in a counterbalanced sequence among tests. Before each test, the apparatus was placed on a clean mat and clean mouse bedding. Two identical clear, Plexiglas cylinders (7cm in diameter, 12cm tall) with removable black, Plexiglas lids were placed in the apparatus, one in each end chamber. The stimulus mouse could move around easily within the cylinder. The cylinders had multiple holes (1cm in diameter) to allow for air exchange between the inside and outside of the cylinder. Auditory, visual, and olfactory investigation between a mouse inside and a mouse outside the cylinder was thus possible. Between tests, the apparatus and cylinders were all washed with copious amounts of water and dried before testing the next mouse.
Figure 1. Testing apparatus used to assess social behavior of NR1neo-/- mice and wildtype littermates.
(a) The behavioral testing apparatus viewed from above. There is a clear Plexiglas cylinder in each of the two end chambers. Before the start of each test, one of the end chambers was arbitrarily designated the “social side” (the side into which the stimulus mouse would be introduced), and the other end chamber was designated the “nonsocial side.” A “test” mouse is shown in the center chamber of the apparatus, and a “stimulus” mouse is shown in the cylinder on the social side of the apparatus (the “social cylinder”). The cylinder on the nonsocial side of the apparatus (the “nonsocial cylinder”) is empty. For the purposes of the picture, no lids are shown on the cylinders. (b) Multiple holes are evenly spaced over the surface of the cylinders in each end chamber, and these holes are large enough for a mouse to poke its nose through for olfactory investigation. A stimulus mouse is shown in the cylinder. (c) Dimensions of the behavioral testing apparatus. (Figure with permission from (Sankoorikal et al. 2006))
The following modifications of the previously described procedure were made. Because we were interested in measuring affiliated social interactions of test mice, we tried to minimize aggressive and sexual motivations of the test mouse towards the stimulus mouse by using castrated A/J male stimulus mice paired with male test mice. Phase 1, during which time the test mouse was habituated to the apparatus, lasted 10min, which was split into two 5min intervals for data analysis (Phase 1A and Phase 1B). At the beginning of Phase 2, which lasted 5 minutes, a gonadectomized A/J stimulus mouse was placed in the “social cylinder” in the social side of the apparatus, and simultaneously, an inanimate object (black plastic block) was inserted into the nonsocial cylinder. During Phase 3, the cylinders were removed and test mouse and stimulus mouse were allowed to interact freely for another 5 minutes. Free interaction was terminated if there was more than 3 seconds of aggressive, attack behavior (biting, vigorous lunging). In the case of premature termination, data on time spent in affiliated social interaction in Phase 3 were not used for further analysis. The mean ± standard error (SEM) was calculated for the following variables for each group of mice defined by genotype: time in social and nonsocial chambers, time sniffing the social and the nonsocial cylinders, and numbers of transitions between chambers. These values were calculated for Phase 1A (stimulus mouse absent, 0-5 minutes) and Phase 1B (stimulus mouse absent, 5-10 minutes) as well as for Phase 2 (stimulus mouse present, 10-15 minutes). The mean ± SEM was calculated for the time that the test and stimulus mouse spent in direct contact during Phase 3. A chamber preference score was calculated for each mouse by subtracting the time spent in the nonsocial side from the time spent in the social side of the apparatus for Phase 1A (stimulus mouse absent, 0-5 minutes), Phase 1B (stimulus mouse absent, 5-10 minutes), as well as for Phase 2 (stimulus mouse present, 10-15 minutes). A positive score signified a predominance of approach towards the social side, whereas a negative score implied a predominance of approach towards the nonsocial side. Furthermore, a cylinder sniffing preference score was calculated by subtracting the time spent sniffing the nonsocial cylinder from the time spent sniffing the social cylinder for Phase 1A, Phase 1B, and Phase 2. A positive score indicated a predominance of social sniffing a behavior, whereas a negative score indicated a predominance of social avoidance. For statistical analysis, the mean of the two chamber preference scores for each mouse in Phase 1A and Phase 1B were calculated. This mean chamber preference score for Phase 1 was then subtracted from the chamber preference score for Phase 2 to yield a chamber preference change score. Similarly, the mean cylinder sniffing preference score for Phase 1 (mean of Phase 1A and Phase 1B) was subtracted from the cylinder sniffing preference score for Phase 2 to yield a cylinder sniffing preference change score. For each mouse, a positive value for the chamber preference change score or cylinder sniffing preference change score indicated a preference for the social chamber or social cylinder sniffing, respectively. NR1neo-/- vs. WT chamber preference change scores and cylinder sniffing preference change scores were compared using t-tests. The baseline locomotor activity (number of transitions in the 1st five minutes of Phase 1) of NR1neo-/- vs. WT was compared using a t-test. The percentage of NR1neo-/- vs. WT mice that showed aggressive attack behavior towards the stimulus mouse was compared using Fisher's exact test.
Elevated Zero-Maze
The zero-maze (Stoelting Co, Wood Dale, IL, USA) consisted of an elevated circular platform (grey-white plastic, 5.5cm wide track, 34cm diameter, 60cm elevation) with two open arms (1.2cm brims) and two closed arms (30.5cm walls). Each test started by placement of a mouse in the closed area. A Viewpoint tracking-system (Champagne au Mont d'Or, France) videorecorded and quantified the time spent in open/closed sectors, the number of entries into open/closed sectors and the total distance covered for the duration 300sec. We also determined the time until the mouse would first enter one of the open sectors, the number of head dips and the number of fecal boli dropped on the maze-maze during each test. Mice were acclimated to the room for 1 hour prior to testing. Testing was performed in dim lighting. Data from the elevated zero-maze testing was analyzed using Student's t-test comparison.
Open Field
The apparatus for the open-field included 4 rectangular translucent plastic boxes without lid (50cm × 35cm with 40cm sides). The floor was covered with a 2cm thick layer of clean bedding also used for the home cages. The boxes were illuminated by ambient fluorescent ceiling lights. Mice were placed individually in the periphery of the open field boxes and their movements were digitally recorded for a 5min with a cameras placed directly over each box. Using TopScan (Clever Systems Inc., Reston, VA, USA) a peripheral (11cm width) and a central zone (30cm × 11cm) were defined and the movements of each mouse were traced. Time of presence within the central zone, number of entries into the central zone and locomotor activity (covered distance during 5min) were determined. 12 NR1neo-/- and 12 WT mice were tested in 6 consecutive groups. After each run, the boxes were cleaned with ethanol and the bedding replaced. Data was analyzed using Student's t-test comparison.
Measurement of Locomotor Activity
Prior to testing, mice (7 NR1neo-/- mice, 10 wild types) were allowed to habituate in their cages for 30min in the testing room. Home cages (31cm length, 19cm width, and 16cm height) were placed in automated locomotor activity frames (Med Associates Inc., St. Albans, VT, USA). The number of horizontal beam-breaks was counted and converted into cm as a measure of ambulation. Data was collected for 5min intervals over 30min and subsequently analyzed using a one-way ANOVA. Due to discrepancies with previously reported results, measurement of locomotor activity was repeated at another location (AstraZenca Neuroscience) with another cohort of 16 mice (8 NR1neo-/-, 8 wild type littermates). Two sets of conditions were tested, first using home cages (28cm length, 18cm width, 11cm height) as well as in large cages without bedding (41cm length, 41cm width, 20cm height) for 30 and 120 minutes. The distance travelled in cm was recorded and analyzed using a one-way ANOVA.
Nest Building Activity
Mice were housed individually and nesting was measured in home cages. Pressed white cotton square (5 × 5 cm, 2.5 g) were placed in each cage 1 hour before the dark phase (19:00) and left overnight. No other environmental enrichment items were present. Mice were left undisturbed over night and nests scored with a 5-point rating scale the next morning as previously described (Deacon 2006). Due to the non-normal distribution in nest building scores, a nonparametric Mann-Whitney U test was used for the analysis of this data.
Results
Electrophysiology
NR1neo-/- mice had significantly higher peak values for all auditory and visual ERPs components.
Auditory Event Related Potentials
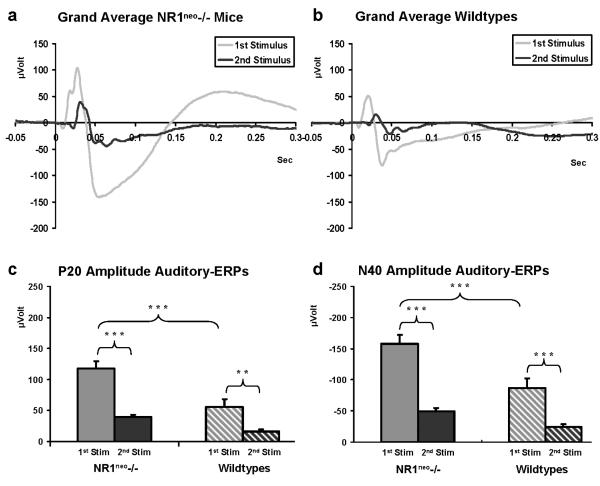
Figure 2 illustrates grand average traces for NR1neo-/- and WT mice for the 1st and 2nd stimulus responses. Analysis of P20 amplitudes revealed a stimulus by genotype interaction (F(1, 29)=6.2726, p=0.018) with higher amplitudes for 1st stimulus response in NR1neo-/- mice (N=16) when compared to WT (N=15) (Figure 2c). 1st stimulus amplitude was 117.65 ± 11.78μV for NR1neo-/- mice and 56.03 ± 12.17μV for WT mice. These results were statistically significant for the 1st stimulus response (Fisher LSD Post Hoc: Pooled MS=1188, df=54.711, p=0.0001). Across genotypes, 2nd stimulus responses were significantly lower than 1st stimulus responses (Fisher LSD Post Hoc: Pooled MS=1188, df=54.711, p 0.0001 for NR1neo-/- mice and p<0.001 for wildtype mice).
Figure 2. Increased auditory evoked brain activity in NR1neo-/- mice.
Following presentation of paired clicks, signal averaging was used to resolve the auditory event related potentials against the background of simultaneous brain activity. ERPs consist of different components with the P20 defined as the first major positive deflection (10-30ms) and the N40 defined as the following negative deflection (25-60ms). Traces in panel a and b show averaged first stimulus (S1, grey) and second stimulus responses (S2 black) for 16 NR1neo-/- and 15 WT mice (1 WT mouse lost the electrode pedestal during recording). The second response amplitude is decreased relative to the first at an interstimulus interval of 500ms, which allows for gating or habituation. This is also shown in panels c and d where S1 is significantly higher than S2 for NR1neo-/- and WT mice. Statistical analysis of amplitudes yields a significant increase for both P20 and N40 amplitude in NR1neo-/- mice compared to WT littermates (** p<0.01; *** p<0.001).
Analysis of N40 amplitudes (Figure 2d) yielded a stimulus by genotype interaction (F(1, 29)=7.3865, p=0.01) a similar pattern, with significantly larger S1 amplitude in NR1neo-/- than WT mice; -157.47 ± 14.59μV for NR1neo-/- mice and -86.73 ± 15.07μV for WT mice (Fisher Post Hoc: Pooled MS=1891.4, df=48.825, p<0.001). Both NR1neo-/- mice and WT littermates had significantly higher S1 than S2 responses (Fisher Post Hoc: Pooled MS=1891.4, df=48.825, NR1neo-/- mice: p<0.0001; WT: p<0.0001). There was no significant difference between S2 amplitude between genotypes for either the P20 or N40.
Visual Event Related Potentials
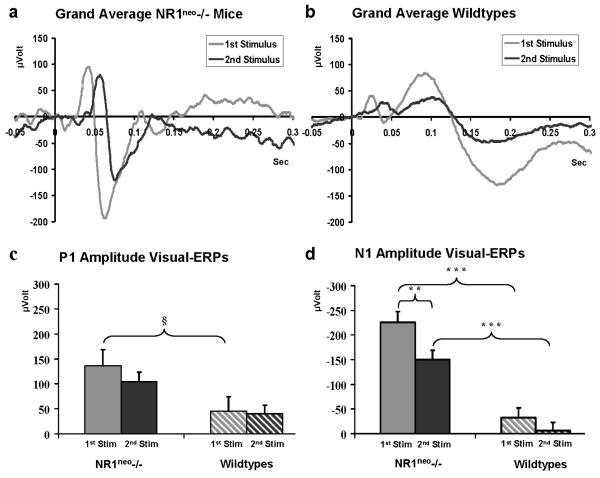
NR1neo-/- mice (7) had significantly larger N1 than WT (8) mice (F(1, 13)=53.46, p<0.001) across stimulus type. There was significant gating of S2 relative to S1 across genotypes (F(1, 13)=12.128, p<0.01). There was no interaction between genotype and stimulus condition, suggesting that NMDAR expression did not alter gating (Figure 3).
Figure 3. Increased visually evoked brain activity associated in NR1neo-/- mice.
Stimuli were presented as paired flashes; signal averaging was applied to resolve potentials against background brain activity. Visual ERPs consist of similar components as auditory-ERPs. Traces in panel a show grand average traces for S1 and S2 in NR1neo-/- mice, traces in panel b are grand averages for WT mice. Amplitudes for NR1neo-/- mice (7) were significantly larger than WT (8) for N1 (** p<0.01; *** p<0.001) while showing a trend towards significance for P1 (§ p=0.0597;). Mice which had seizures in previous tests (3 NR1neo-/-) or lost their electrode pedestal before or during recording of visual ERPs (6 NR1neo-/-, 8 WT mice) were excluded from statistical analysis.
Behavioral Testing
Sociability
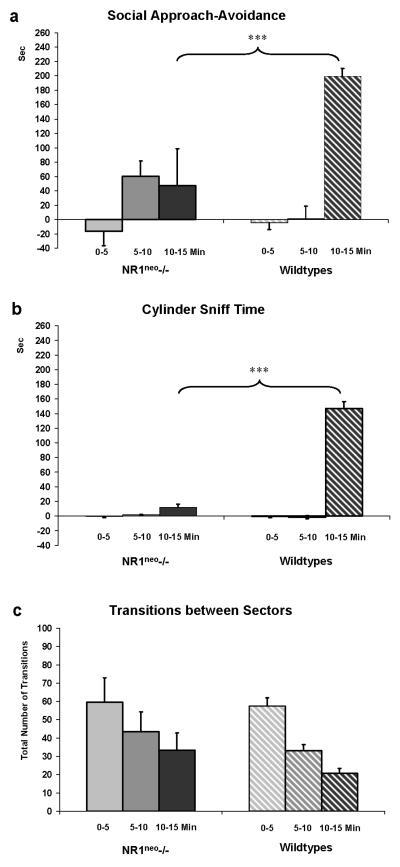
WT mice (N=12) exhibited a preference for the social chamber after a social stimulus mouse was presented (Figure 4a). The chamber preference score for WT mice shifted towards a positive number during Phase 2 (stimulus mouse present, between 10-15 minutes), indicating a preference for the social side (199±11sec). However, NR1neo-/- mice (N=7) showed significantly lower levels of approach toward the stimulus mouse (lower chamber preference changes scores) than WT mice during Phase 2 (t=-3.048, df=17, p<0.01). The cylinder sniffing preference score may be a more sensitive and direct measure of social investigation (Moy et al. 2007). This measure revealed no baseline preference for NR1neo-/- or WT mice in the absence of a stimulus mouse. However, when a stimulus mouse was inserted, WT mice demonstrated a preference for sniffing the social cylinder (147±9sec). This was in contrast to NR1neo-/- mice that showed a minimal sniffing at the social cylinder (12±5sec). The preference of NR1neo-/- mice for direct olfactory investigation of the stimulus mouse (cylinder sniffing preference change score) was significantly lower than that of WT (t=-6.154, df=17, p<0.001). Baseline locomotor activity, as measured by the number of transitions between chambers in the first 5min of Phase 1, did not differ in NR1neo-/- and WT mice. Six WT mice exhibited aggressive attack behavior toward the stimulus mouse in Phase 3, whereas no NR1neo-/- mice exhibited aggressive attack behavior, which was a significant genotype difference in aggressive behavior (p<0.05 by Fisher's exact test). The attack behavior in the 6 WT mice led to premature termination and exclusion from analysis of time spent in social contact during Phase 3 for these mice. For the remaining mice, the time sniffing a stimulus mouse in free interaction was qualitatively higher in WT than in NR1neo-/- mice, although this did not reach statistical significance (t=2.052, df=11, p=0.067) (data not shown).
Figure 4. Reduced sociability in NR1neo-/- mice.
Columns in panel a represent chamber preference scores, i.e. the amount of time mice spent in on the social side minus time spent on the non-social side of the 3-chambered social choice apparatus, in Phase 1A (stimulus mouse absent, 0-5min), Phase 1B (stimulus mouse absent, 5-10 min), and Phase 2 (stimulus mouse present, 10-15min). The social side refers to the side in which the stimulus mouse is placed. The non-social side refers to the side in which an inanimate object is placed. A negative value indicates a preference for the non-social side, whereas a positive value indicates a preference for the social side. NR1neo-/- mice (N=7) showed markedly reduced time spent in social interaction relative to WT mice (N=12). Panel b illustrates the cylinder sniffing preference score, i.e. the time mice spent sniffing the social cylinder minus time spent sniffing the non-social cylinder in Phase 1A (stimulus mouse absent, 0-5min), Phase 1B (stimulus mouse absent, 5-10min), and Phase 2 (stimulus mouse present, 10-15min). Again, a negative value indicates a preference for sniffing the cylinder on the non-social side, whereas a positive value indicates a preference for sniffing the cylinder on the social side. WT mice show a significantly higher value in social sniffing during Phase 2 (stimulus mouse present, 10-15min) than NR1neo-/- mice. The number of transitions between sectors (c) is comparable between genotypes, indicating that differences in locomotor activity do not account for differences in social choice measures shown in panels a and b (*** p<0.001). 3 NR1neo-/- mice had a seizure while tested in the sociability apparatus and were removed from subsequent testing.
Elevated Zero-Maze
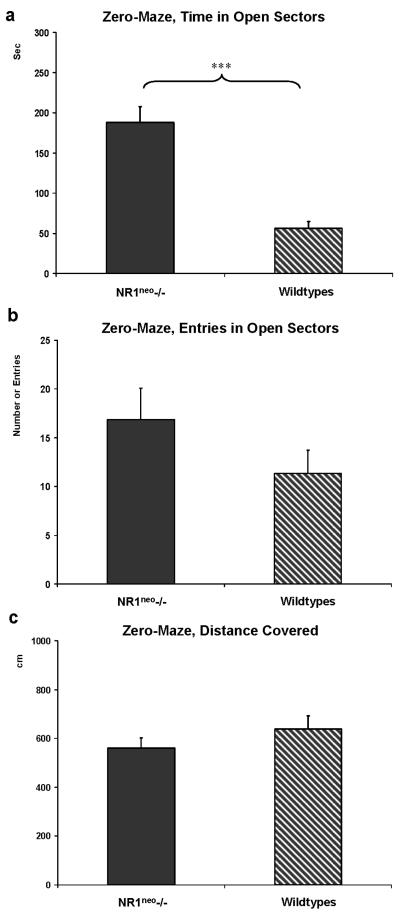
Zero-maze testing revealed a distinct difference in behavior between genotypes (Figure 5). NR1neo-/- mice (N=8) spent about 63% (190.6±20.6sec) of the total 300sec test time in the open arms of the zero-maze (Figure 5a). WT mice (N=12) spent less than 11% of the total test time in the open arms of the zero-maze (31.7±7.2sec). The difference between genotypes was significant (t=8.4468, df=18, p<0.001). The difference was not influenced by initial freezing/immobility. The time until the first entry into an open sectors was not significantly different between genotypes (14.1±5sec for NR1neo-/- vs. 10.8±3.1sec for wildtype mice; t=0.3218, p=0.75). The increased number of head dips in NR1neo-/- mice (45±7) was linked to the increased time spent in the open sectors (13±2; t=5.312, p<0.001). NR1neo-/- mice. The number of fecal boli was not different between genotypes. The number of entries into the open sectors was qualitatively higher for NR1neo-/- mice than wildtypes without reaching significance (t=-0.1687, df=18, p=0.868; Figure 5b). Locomotor activity in the zero-maze was not significantly different between genotypes (Figure 5c).
Figure 5. NR1neo-/- mice spend more time in open arms of an elevated zero-maze.
a) NR1neo-/- mice (N=8) spent about 60% of the total time in the open sectors whereas WT (N=12) littermates stayed in the enclosed sectors and spent less than ~10% of the time in the open sectors. b) The number of entries into the open sectors shows no significant difference between genotypes. c) This significant difference cannot be attributed to different locomotor activity patterns as the distance covered by NR1neo-/- and WT mice is comparable (***p<0.001). Three mice were excluded from analyses, two NR1neo-/- mice jumped off the platform and one NR1neo-/- mouse had a seizure during testing.
Open Field
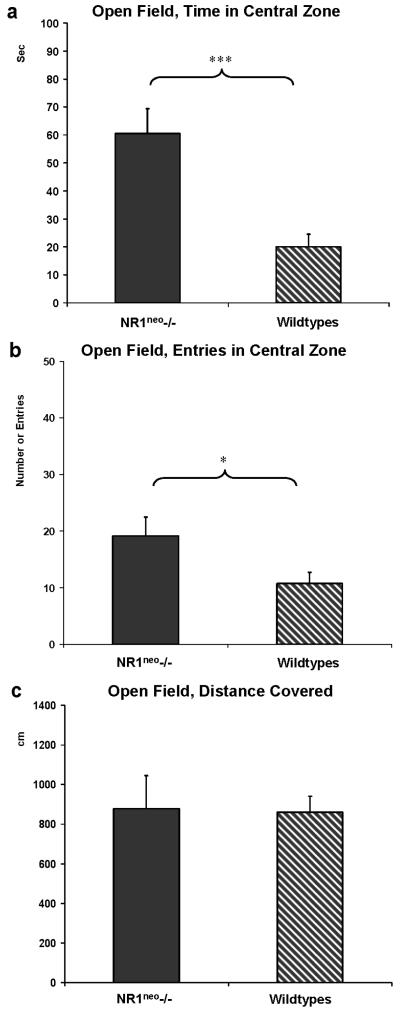
As a second well validated measurement of anxiety, open field testing showed that NR1neo-/-mice spent significantly more time in the central zone (60±9sec vs. 20±4sec, t=4.0367, df=22, p=0.0006). The number of entries into the central zone was higher for NR1neo-/- mice (19±3 entries vs. 11±2 entries, t=2.1809, df=22, p=0.04). The degree of ambulation as measured by the covered distance in both central and peripheral zone was not significantly different between knockdowns and wildtypes (Figure 6a, b, c).
Figure 6. NR1neo-/- mice spend more time in the central zone of the open field.
Panels a, b, and c display results of testing in the open field paradigm (* p<0.05; *** p<0.001). As shown in panel a NR1neo-/- mice (N=12) spent significantly more time in the central zone of the open field than WT mice (N=12). b) NR1neo-/- mice also entered the central zone significantly more often than WT mice. c) These differences cannot be attributed to different locomotor activity because the distance covered by NR1neo-/- and WT mice is comparable.
Locomotor Activity
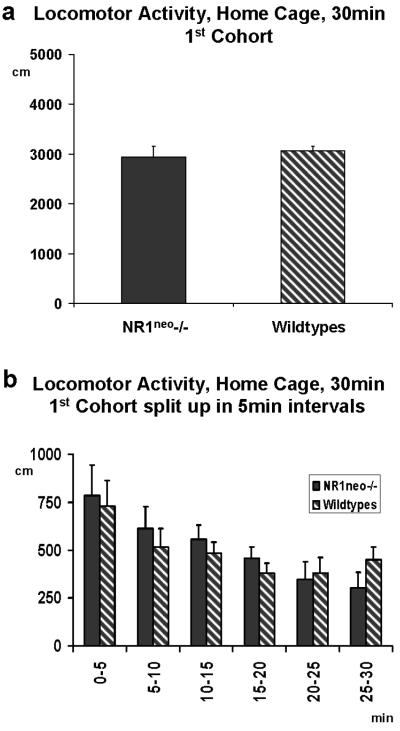
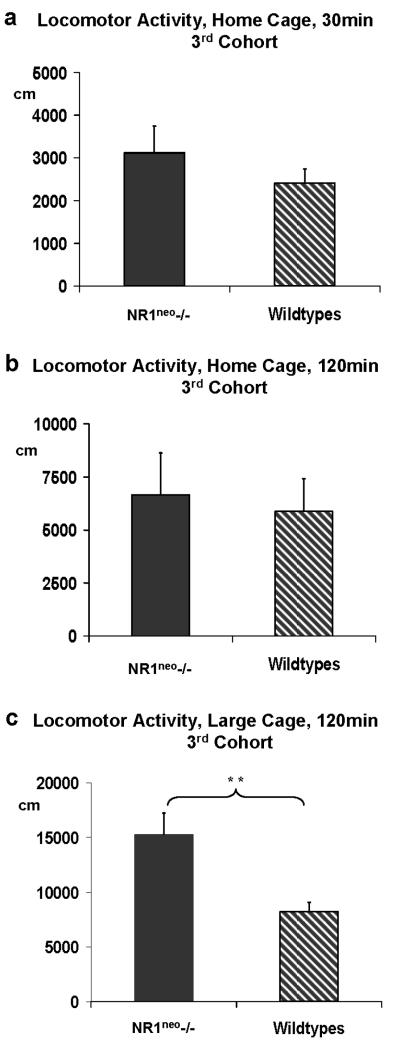
Figure 7a illustrates a 30 minutes comparison of locomotor activity in a home cage environment which revealed no significant effect of genotype on ambulation between NR1neo-/- (N=7) and WT (N=10) mice (F(1,15)=0.834, p=0.7767). A comparison of 5 minute time bins illustrated in Figure 7b shows a typical decrease of locomotor activity over time in NR1neo-/- mice and wildtypes alike. Because this finding was different from previous publications, we analyzed the locomotor activity data during zero-maze (Figure 5b), open field (Figure 6c) and social-choice (Figure 4c) to verify the consistency of this result. As described above, the total distance covered (zero-maze and open field) and the number of transitions between chambers (sociability) did not yield any significant difference in locomotor activity. In addition, measurement of locomotor activity was repeated with a second cohort of mice at AstraZeneca Neuroscience (Wilmington, DE, USA). Figure 8a illustrates the comparison of locomotor activity using a home cage environment for a cohort of 8 NR1neo-/- and 8 wild type littermates which replicated the initial results (F(1,14)=1.0195, p=0.3298). Next, we measured locomotor activity in an unfamiliar environment (large cages without bedding) to determine if these conditions would elicit an increase in locomotor activity. As shown in Figure 8c, NR1neo-/- mice traveled twice the distance compared to their wildtype littermates (F(1,14)=10.851, p=0.0053) when placed in large unfamiliar cages without bedding. The tested cohort had been unfamiliar with this environment before testing. These conditions and results were similar to those in the previous study by Mohn and colleagues (Mohn et al. 1999). This finding was obtained after an extension of the recording time to 120 minutes. A 30 minute comparison (Figure 7b) did not detect this difference (F(1,14)=0.1001, p=0.7564).
Figure 7. Locomotor activity in NR1neo-/- mice is comparable to the locomotor activity of wildtype littermates in a familiar environment.
Measurement of locomotor activity was performed with 2 cohorts of NR1neo-/- and WT mice. Panel 7a illustrates a 30 minute comparison of locomotor activity in the home cage environment for the 1st cohort. There was no significant effect of genotype on ambulation between NR1neo-/-(N=7) and WT (N=10) mice (F(1,15)=0.834, p=0.7767). Mice which had seizures during previous tests (3 NR1neo-/-) or lost the electrode pedestal (6 NR1neo-/-, 6 WT mice) were excluded from testing. 7b illustrates the comparison of individual 5 minute time bins. Both mutants and wildtypes show the typical decrease in locomotor activity over time.
Figure 8. NR1neo-/- mice display context dependent changes in locomotor activity.
Results of the 1st cohort led to a repetition with another cohort of mice (8 NR1neo-/- and 8 WT littermates) at AstraZeneca Neuroscience (Wilmington, DE, USA). Comparison of locomotor activity under comparable conditions replicated the initial results (F(1,14)=1.0195, p=0.3298, as shown in panel 8a. Figure 8c) Placement in an unfamiliar environment (large cage without bedding) elicited an increase in locomotor activity in NR1neo-/- mice relative to WT controls (** p<0.01). The recording time was extended to 120 minutes. Figure 8b) A shorter recording span did not detect this difference.
Nest building
Using a rating scale suggested by Deacon with a score of “1” being an almost untouched cotton nestlet and “5” an ideal nest (Deacon 2006), WT mice scored significantly higher during an assessment of their nest building activity than NR1neo-/- mice (U=1.5, Z=4.604, p<0.0001). The quality of their construction was better in that they had torn the pressed cotton squares almost entirely (median=5, 25-75% of scores between 4.5-5points, lowest/highest score=4/5points). WT mice build nests formed a crater with walls higher than the mouse and covering most of its circumference. NR1neo-/- mice either did not interact with the nest material or built less elaborated nests with minimal walls (median=1.5, 25-75% of scores between 1-2.5points, lowest/highest score=1/4points).
Discussion
Chronic deficiency of NMDAR expression in mice causes differences in electrophysiological and behavioral measures. Amplitudes for auditory and visual ERPs were increased for the P1 and N1 components, suggesting reduced inhibitory tone in NR1neo-/- mice. Similarly, the low anxiety-related behavior of the NMDAR deficient mice during zero-maze and open field testing may also indicate reduced behavioral inhibition as a result of chronic NMDAR deficiency. NMDAR deficient mice showed significantly decreased sociability which resembles reduced social interactions in schizophrenia without high levels of anxiety-related behavior. The decreased nest-building activities in NR1neo-/- mice are difficult to interpret, but may indicate a reduced ability to meet some basic needs for self care. Locomotor activity did not explain the behavioral differences. While behavioral results demonstrate the potential correlates for a use in translational schizophrenia research, electrophysiological results divert from previous findings in humans and rodents. However, the results demonstrate the importance of NMDAR function in the regulation of sensory processing and behavior. One of the major difficulties in advancing behavioral work has been the inconsistency across laboratories measuring apparently similar constructs. The current work addresses this important point by noting that social deficits appear to be a core feature of these animals that is stable across time, methods and settings.
Electrophysiological studies
Changes in prepulse inhibition of acoustic startle (PPI) and auditory ERPs are among the most widely investigated endophenotypes of schizophrenia. A majority of previous studies focused on the measurement of PPI reporting variable results for the effects of haloperidol and clozapine (Duncan et al. 2008; Duncan et al. 2006b; Fradley et al. 2005). The stimuli used in the present ERP paradigm are at sub-startling amplitude, making them comparable to the prepulse used in the PPI task. Thus, the electrophysiological response to the prepulse is the appropriate analogy. However, several studies have now demonstrated that PPI and gating of evoked potentials are not necessarily correlated in either mice or humans. Interestingly a study assessing sensory gating showed no significant difference for long interstimulus amplitudes (S1) but an increased amplitude at short intervals (S2) among NMDA NR1neo-/- mice, suggesting a disruption of auditory gating in NR1neo-/- mice (Bickel et al. 2008). However, in patients with schizophrenia impaired gating has been observed due to a decreased response to S1. Conversely, the current study did not find an increase in P20 and N40 2nd stimulus amplitudes but did find marked increase in 1st stimulus amplitudes in NR1neo-/- mice. One possible reason for the differential findings is the age of tested mice which was 5-6 months in the previous study in comparison to 3 months in our study. In addition, Bickel used male and female mice whereas this study was performed with male mice only. The previous study recorded from surface electrodes, which accounts for lower peak amplitudes in comparison with our approach where the recording electrode is placed in hippocampus. Type and characteristics of the electrodes used (impedance) may also influence the recording of electric brain activity. Lastly, Bickel and Umbricht anaesthetized their mice with ketamine (100 mg/kg). Our group has previously shown that a repetitive dose of 5 mg/kg ketamine causes long term adaptation of ERPs and hippocampal cell death (Majewski-Tiedeken et al. 2007; Maxwell et al. 2006).
Several previous studies have used NMDAR antagonists such as ketamine to examine the effects of reduced glutamatergic transmission on auditory gating. It is noteworthy that studies in mice and healthy human subjects do not find disruptive effects of ketamine on auditory gating, consistent with the current findings (Connolly et al. 2004; Oranje et al. 2002; van Berckel et al. 1998). In addition, we were also able to record a similarly increased response to visual stimuli thus confirming an abnormal sensory processing across modalities. To our knowledge there are no previous studies of visual ERPs in NMDA hypomorphic mice. In the current study, visual ERPs yielded the same pattern of abnormality seen in the auditory system with increased P1 and N1 amplitudes in NR1neo-/- mice, with a proportional increase in S2 amplitude. These data suggest that reductions in NMDAR lead to a pattern of disinhibition that is common to multiple sensory modalities.
Taken together results from sensorimotor (PPI) and sensory (ERPs) gating tests deliver a heterogeneous picture of the NR1neo-/- mouse strain and its suggested model character for schizophrenia.
Behavioral Studies
Previous studies reported an increase in locomotor activity among NMDA hypomorphic mice (Duncan et al. 2004; Mohn et al. 1999). Surprisingly, Miyamoto et al. were able to increase locomotor activity by injection of saline solution while a dose of 2mg/kg amphetamine had no effect and a dose of 4mg/kg slightly reduced locomotor activity in NR1neo-/- mice (Miyamoto et al. 2004). These findings may reflect a reaction to an unknown environment or novelty, rather than an endogenous difference in baseline ambulation. While Mohn et al. reported an increased locomotor activity for NR1neo-/- mice, the authors note that it only occurred during habituation to a novel environment. Motor activity of NR1neo-/- mice was reduced to that of wildtype mice after 4 hours (Mohn et al. 1999). Previous studies tested locomotor activity in an environment other than the home cage whereas mice in this study showed no effect of genotype on LMA when they remained in their home cage throughout testing. We found similar levels of ambulation between groups in a familiar environment and increased ambulation for NR1neo-/- mice in an unfamiliar environment, suggesting that NR1neo-/- mice react to a novel environment with a higher degree of exploratory behavior, resulting in a higher degree of ambulation. However, there were no differences in locomotor activity when mice remained in their home cage, where no habituation to a novel environment was required.
While reduced social interactions have been previously reported for NR1neo-/- mice, it remained unclear whether high levels of anxiety contribute to this characteristic (Duncan et al. 2004; Mohn et al. 1999{Moy, 2008 #80)}. Zero-maze and open field are well validated tests of anxiety-like behaviour in rodents. Both determine the response to a potentially dangerous environment and rely on the animal's conflict between the tendency to explore a novel environment and the aversive properties of a well lit, elevated open area (Shepherd et al. 1994). Surprisingly, NR1neo-/- mice exhibit a higher tendency to explore the open environment in both tests. While, a previous study with rats showed an increased level of anxiety-related behaviors after intermittent treatment with an NMDA antagonist at 8 weeks of age (Wedzony et al. 2008) another study with mice showed decreased anxiety after continuous treatment with an NMDA antagonist for 7 weeks (Minkeviciene et al. 2008). However, our results integrate into previous findings and elucidate that neither high anxiety nor increased LMA contributed to the lack of social interaction. Mice depend largely on olfactory sensory input and 2 studies indicate slight impairments in olfaction in NR1neo-/- mice (Duncan et al. 2004; Moy et al. 2008). However, with magnitude of social deficits a presumable olfactory deficit does not account for that. By giving wildtype and mutant mice a choice between novel social stimulus and novel unanimated object, we could elaborate that NR1neo-/- mice show significantly reduced sociability. Including the other behavioral results, it is not fear or hyperlocomotion that prevents social interaction but a genuine deficit in social interaction.
Deficits in social interactions and social withdrawal are frequent in schizophrenia. We sought to characterize the impact of NMDAR deficiency on behavior towards a social stimulus. NR1neo-/-mice had markedly reduced sociability, relative to WT mice. In contrast, WT mice show a marked preference for social interaction. These results are consistent with a previous report of reduced sociability of NMDAR1 deficient mice, as reflected by reduced huddling of NR1neo-/-mice in home cages with cagemates during sleep, reduced social investigation of a male NR1neo-/- resident mouse towards a male intruder mouse, and reduced mating behavior of male NR1neo-/-mice toward receptive female mice (Mohn et al 1999). They are also consistent with a previous report of reduced sociability of NMDAR1 deficient mice as measured in a social choice paradigm, as well as reduced inter-male aggressive behaviors in NMDAR1 deficient mice (Duncan et al 2004). The reduced sociability phenotype of the NR1neo-/- does not appear to be secondary to heightened anxiety, since the NR1neo-/- mice showed reduced anxiety-like behaviors in the zero maze and open field paradigm. It is noteworthy that NR1neo-/- mice also showed significantly less aggressive behaviors than WT mice toward the stimulus mice. Aggressive behaviors in WT mice may have been heightened by a prolonged period of individual housing (Miczek et al. 2002).
For small rodents, construction of a nest is important for heat conservation, reproduction and shelter (Van de Weerd et al. 1997). Genetic modifications as well as hippocampal lesions may alter nest building activities (Lijam et al. 1997). Interestingly, mice with a NMDA hypofunction due to alterations of the glycine binding site on the NR1 subunit exhibit reduced nest building behavior (Ballard et al. 2002). Assessment of nest building has not been assessed in NR1neo-/-mice before. Whether the observed decrease in nest building activities could be interpreted as potentially relevant to the self-neglect observed in schizophrenic patients where the individual neglects to attend to their basic needs, such as personal hygiene, appropriate clothing, feeding, or tending appropriately to any medical conditions has yet to be clarified.
In summary, genetic disruption of NMDA receptor function produces electrophysiological and behavioral alterations in a rodent model with similarity to a subset of sensory deficits and negative symptoms of schizophrenia. While behavioral data supports the hypothesis that a deficiency in NMDAR signaling is a contributing factor in the pathogenesis of schizophrenia, electrophysiological results are variable and await future clarification. The development of models with localized (e.g. prefrontal cortex) developmental impairments of glutamatergic neurotransmission would allow examination of regional differences in brain function as shown by functional imaging studies and add to the translational value of this rodent model for schizophrenia.
Acknowledgments
This study was supported by NIDA 5R01DA023210-02 (SJ Siegel), an NIMH P50 MH064045 SJ Siegel Project 5 PI, RE Gur Center PI)), the Deutsche Forschungsgemeinschaft DFG, IRTG 1328 (Training Fellowship, TB Halene), AstraZeneca Pharmaceuticals (SJ Siegel PI, EP Christian and GJ Jonak, Employees of AZ), P50-CA-084718 (SJ Siegel Project PI, C Lerman Center PI), KO8-MH068586 (ES Brodkin), R01MH080718 (ES Brodkin), and a Burroughs Wellcome Fund Career Award in the Biomedical Sciences (ES Brodkin). We thank Sybil O'Connor and Tanya West for an excellent animal care and Amanda Williams for the genotyping.
References
- Ballard TM, Pauly-Evers M, Higgins GA, Ouagazzal AM, Mutel V, Borroni E, Kemp JA, Bluethmann H, Kew JN. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J Neurosci. 2002;22:6713–6723. doi: 10.1523/JNEUROSCI.22-15-06713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S, Lipp HP, Umbricht D. Early Auditory Sensory Processing Deficits in Mouse Mutants with Reduced NMDA Receptor Function. Neuropsychopharmacology. 2008;33:1680–1689. doi: 10.1038/sj.npp.1301536. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behavioural brain research. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain research. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–1188. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Duncan GE, Inada K, Farrington JS, Koller BH. Seizure responses and induction of Fos by the NMDA agonist (tetrazol-5-yl)glycine in a genetic model of NMDA receptor hypofunction. Brain research. 2008;1221:41–48. doi: 10.1016/j.brainres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology. 2006a;184:190–200. doi: 10.1007/s00213-005-0214-1. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav. 2006b;85:481–491. doi: 10.1016/j.pbb.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behavioural brain research. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Fradley RL, O'Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behavioural brain research. 2005;163:257–264. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and Schizophrenia: Phencyclidine, N-Methyl-d-Aspartate Receptors, and Dopamine-Glutamate Interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, Wynshaw-Boris A. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Majewski-Tiedeken CR, Rabin CR, Siegel SJ. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2 and FVB inbred mouse strains. Drug Alcohol Depend. 2007 doi: 10.1016/j.drugalcdep.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology. 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Banerjee P, Tanila H. Cognition-enhancing and anxiolytic effects of memantine. Neuropharmacology. 2008;54:1079–1085. doi: 10.1016/j.neuropharm.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Snouwaert JN, Koller BH, Moy SS, Lieberman JA, Duncan GE. Amphetamine-induced Fos is reduced in limbic cortical regions but not in the caudate or accumbens in a genetic model of NMDA receptor hypofunction. Neuropsychopharmacology. 2004;29:2180–2188. doi: 10.1038/sj.npp.1300548. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behavioural brain research. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behavioural brain research. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain research. 2006;1089:186–194. doi: 10.1016/j.brainres.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Oranje B, Gispen-de Wied CC, Verbaten MN, Kahn RS. Modulating sensory gating in healthy volunteers: the effects of ketamine and haloperidol. Biol Psychiatry. 2002;52:887–895. doi: 10.1016/s0006-3223(02)01377-x. [DOI] [PubMed] [Google Scholar]
- Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Oranje B, van Ree JM, Verbaten MN, Kahn RS. The effects of low dose ketamine on sensory gating, neuroendocrine secretion and behavior in healthy human subjects. Psychopharmacology. 1998;137:271–281. doi: 10.1007/s002130050620. [DOI] [PubMed] [Google Scholar]
- Van de Weerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V. Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim. 1997;31:133–143. doi: 10.1258/002367797780600152. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Fijal K, Mackowiak M, Chocyk A, Zajaczkowski W. Impact of postnatal blockade of N-methyl-d-aspartate receptors on rat behavior: A search for a new developmental model of schizophrenia. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]