Abstract
The primary objectives of this study were to determine if erythropoietin (EPO) is neuroprotective to the photoreceptors in the retinal degeneration slow (rds) mouse in the absence of an increase in hematocrit and to determine if deglycosylated EPO (DEPO) is less neuroprotective. We performed subretinal injections of 10U EPO, DEPO or hyperglycosylated EPO (HEPO) in postnatal day 7 rds mice. Whole eye EPO levels were quantified by ELISA at specified time points post-injection. TUNEL analysis, hematocrit, and immunohistochemistry were performed at postnatal day 20. Half of the amount of EPO measured immediately after injection was detected less than one hour later. Twenty four hours later, EPO levels were 1000 times lower than the amount originally detected. Uninjected rds mice contained 36±2 TUNEL-positive cells/mm retina and PBS injected mice contained 17±3 TUNEL-positive cells/mm retina. EPO, DEPO, and HEPO treated rds retinas contained 5±2, 9±2, and 3±1 TUNEL-positive cells/mm retina, respectively. The hematocrit was 43% in control and 41% in treated rds mice Previous studies have shown neuroprotection of the retina by treatment with as little as 24–39mU EPO/mg total protein in the eye. In this study, we detected 40mU/mg EPO in the eye 11 hours after injecti on of 10U EPO. Treatment with all forms of EPO tested was neuroprotective to the photoreceptors without a concomitant increase in hematocrit.
Keywords: Erythropoietin, photoreceptors, neuroprotection, rds mouse, dose-dependent, glycosylation
1. Introduction
Retinitis pigmentosa is a group of inherited retinal degenerations caused by mutations in any of over 190 genes (RetNet, 1996–2009). One of these causative genes is peripherin/rds, which encodes a structural protein located at the rims of the photoreceptor outer segment discs (Connell et al., 1991). The retinal degeneration slow (rds) mouse contains a 10kb insertion in the middle of the peripherin/rds gene (van Nie et al., 1978; Travis et al., 1989). These mice form shortened, abnormal outer segments, and they do not exhibit a recordable electroretinogram. The photoreceptors progressively die, with the peak of apoptotic cell death at postnatal day 20 (PD20) (Sanyal et al., 1980; Chang et al., 1993).
Due to the complex genetics of retinitis pigmentosa, a general neuroprotective strategy is attractive. Erythropoietin (EPO), a member of the type 1 cytokine superfamily, is produced and secreted primarily by the adult kidney and its main function is to increase the hematocrit under hypoxic conditions. EPO activates erythropoiesis by binding to the EPO receptor, inducing EPO receptor homodimerization and activation of a signal transduction cascade. More recently, EPO and its receptor were found in some non-hematopoietic tissues, including the eye (Grimm et al., 2002). The EPO receptor was localized to the photoreceptor inner segments and synaptic terminals and to the inner nuclear layer. Exogenous EPO blocks apoptosis in multiple models of neuronal cell death (for review see (Gassmann et al., 2003)). Direct delivery of EPO into the eye protects the retinal ganglion cells from death (Kretz et al., 2005; King et al., 2007; Zhong et al., 2007), protects both retinal vascular and neuronal cells in a model of diabetic retinopathy (Zhang et al., 2008), and protects photoreceptors from photo-oxidative stress induced cell death (Grimm et al., 2002; Grimm et al., 2004).
Previously, we showed that treatment with a recombinant adeno-associated virus carrying Epo (rAAV.Epo) prevents photoreceptor cell death in the rds mouse and the light-damaged rat (Rex et al., 2004). However, rescue was only attained with systemic administration, which also induced an increase in hematocrit. In this study, we treated the retina directly with EPO to determine if neuroprotection by intramuscular delivery of rAAV.Epo was due to EPO itself, or to the concomitant increase in hematocrit.
In our previous study, we transduced the retinal pigment epithelium and photoreceptors with rAAV.Epo, and detected 1257mU/ml EPO in the vitreous (Rex et al., 2004). However, there was no neuroprotection. Since the retina is a highly metabolically active tissue and EPO is a heavily glycosylated protein, we hypothesized that the EPO over-produced in the retina was under-glycosylated and therefore, was unable to block photoreceptor cell death. Mammary gland transduced with adenovirus.Epo produces high levels of EPO that is underglycosylated and unable to activate the EPO receptor homodimer to induce erythropoiesis (Toledo et al., 2006). Further, others have deglycosylated EPO and shown decreased secretion and biological activity as compared to equal amounts of EPO (Dordal et al., 1984; Dube et al., 1988; Delorme et al., 1992). To test whether glycosylation of EPO affects its ability to protect the photoreceptors, we treated rds mice with EPO, deglycosylated EPO (DEPO), and hyperglycosylated EPO (HEPO).
Our results show that EPO protects the photoreceptors of the rds mouse without a change in hematocrit. Therefore, EPO itself is neuroprotective in this model of retinal degeneration. DEPO and HEPO also blocked photoreceptor cell death, indicating that the lack of neuroprotection seen in our previous intraocular gene therapy study was not due to underglycosylation of EPO.
2. Materials and methods
2.1 EPO preparation
EPO (a.k.a. Procrit; Ortho Biotech, Bridgewater, N.J.) and HEPO (a.k.a. Aranesp; Ortho Biotech, Bridgewater, N.J.) were concentrated 10-fold separately using 30kD MWCO/NMWL Microcon filters (Millipore Corp., Bedford, MA) to 10U/μL (based on a 1μg to 800U conversion for HEPO). DEPO was generated by the removal of sugar residues from EPO using the CarboRelease Kit (QA-Bio, Palm Desert, CA) according to manufacturer’s protocol. Successful deglycosylation of EPO was confirmed by Western blot analysis. Equal amounts of concentrated protein were loaded onto a 15% non-reducing Tris-glycine gel, transferred onto nitrocellulose, and probed with mouse anti-human EPO (1:1000; MAB2871, R&D Systems, Minneapolis, MN). The bands were detected by labeling with goat anti-mouse horseradish peroxidase (1:10,000; Invitrogen, Carlsbad, CA) and ECL Western blotting substrate (Pierce, Rockford, IL). The bands were imaged on a Kodak 4000MM imaging station (Carestream, New Haven, CT).
2.2 Injections
At PD7, rds mice were anesthetized by hypothermia. The eyelids were gently separated with fine forceps; the globe was punctured with a 30 gauge needle just posterior to the ora serrata; and a 10μl, 30 gauge Hamilton syringe was used to deliver 1μL (10U) of EPO, DEPO, or HEPO into the subretinal space. This dose was chosen because successful neuroprotection of retinal ganglion cells was demonstrated using a single administration of this dose (King et al., 2007). Additional rds mice were left uninjected, or were injected with PBS as negative controls. Dilute rAAV containing the transgene, enhanced green fluorescent protein, (rAAV.eGFP), was included in all injections to confirm a successful subretinal injection. Subretinal, but not intravitreal injections of rAAV.eGFP result in transduction of the retinal pigment epithelium and production of EGFP in these cells. At PD20, the mice were euthanized and the eyes were enucleated and processed for histological analysis. The hematocrit was determined by capillary centrifugation, and standard deviation was calculated. All experimental procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee at the University Tennessee Health Science Center.
2.3 EPO Enzyme-Linked Immuno-Sorbant Analysis (ELISA)
At 0, 15min, 30min, 1h, 3h, 6h, 15h, 24h, 30h, and 40h post-EPO injection, eyes were harvested, homogenized and sonicated in 200μl of sample diluent. Since EPO is a secreted protein, the entire eye was used for analysis to minimize loss of EPO during dissection. At PD 7 (age of injection) the lens is not completely developed, so homogenization and isolation of the supernatant is not a problem. The supernatants were assayed in duplicate using the Quantikine human erythropoietin ELISA according to manufacturer’s protocol (R&D Systems, Minneapolis, MN). The absorbance was detected on a BioTek plate reader (Winooski, VT). A minimum of four eyes were analyzed at each time point. Total eye protein content was determined using the BCA kit (Pierce; Rockford, IL).
2.4 Histological Analysis
Eyes were incubated overnight in 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4, cryopreserved in 30% sucrose in phosphate buffered saline (PBS) overnight at 4°C, and embedded in Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC). Eyes were serially sectioned on a LEICA CM1800 cryostat. Ten micron-thick sections were collected such that each slide contained approximately 20 sections representative of the entire eye.
2.5 Terminal transferase dUTP Nick-End Labeling (TUNEL)
The Apoptag in situ cell death detection kit (Millipore, Temecula, CA) was used according to manufacture’s protocol on representative sections of treated and control retinas. Mounting media containing 4′,6-diamidino-2-phenylindole (DAPI) was used on all slides in order to image the nuclei. TUNEL- positive cells were imaged using a Nikon Eclipse 80i fluorescence microscope and DXM1200C camera (Nikon, Japan). Only eyes in which the retinal pigment epithelium fluoresced green due to the presence of EGFP were analyzed. This assured that we only analyzed retinas that received a subretinal injection since all mice were co-injected with rAAV.eGFP and it only transduces the retinal pigment epithelium when injected into the subretinal space. The TUNEL-positive nuclei in the outer nuclear layer of each retina section analyzed were counted and divided by the length of the retina sections analyzed using NIS Elements software (Nikon, Japan). The status of each sample was unknown until after quantification was performed. The p value for each condition as compared to PBS-treated or EPO-treated was determined using one-way ANOVA and the Newman-Keuls multiple comparison test.
2.6 Immunohistochemistry
Sections were incubated in phosphate buffered saline (PBS), then blocked in 20% normal donkey serum in PBS containing 0.1% Triton-X-100 and 0.5% BSA (PBT) and for a minimum of two hours at room temperature. Polyclonal, anti-glial fibrillary acidic protein (GFAP, Dako, Carpinteria, CA) was diluted 1:400 in PBT. Sections were incubated in primary antibody at 4°C overnight in a humidified chamber, then rinsed with PBS and incubated in secondary antibody (Alexa 564, 1:200, Invitrogen, Carlsbad, CA) for two hours at room temperature. After a final rinse in PBS sections were mounted with Vectashield containing DAPI (Vector Labs, Burlingame, CA) and viewed on a Nikon Eclipse 80i fluorescence microscope and a DXM1200C camera (Nikon, Japan).
3. Results
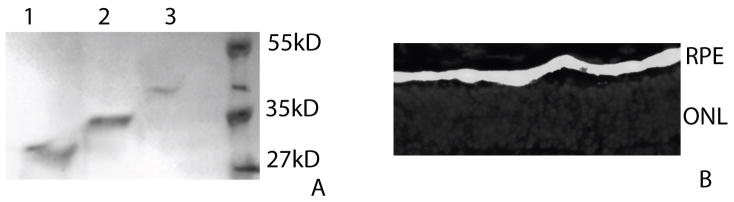
3.1 Confirmation of EPO deglycosylation, accurate subretinal injections, and no alteration in hematocrit levels
We confirmed successful removal of sugar groups from EPO by Western Blot analysis (Figure 1A). DEPO was detected at a lower molecular weight than EPO, and HEPO was detected at a higher molecular weight than EPO. Either proteins or buffer was injected, separately, into the subretinal space of rds mice. To confirm that the injections were subretinal and not intravitreal, rAAV.eGFP was included in each injection. rAAV.eGFP only transduces the retinal pigment epithelium when it is injected into the subretinal space, as seen by EGFP fluorescence (Figure 1B). Only eyes that exhibited EGFP fluorescence in the retinal pigment epithelium were used for TUNEL analysis. There was no difference in the hematocrit of treated and untreated rds mice. The hematocrit was 43% (± 7, n=12) in untreated rds mice, and 41% (± 2, n=4) in EPO treated rds mice.
Figure 1.
A. Western blot analysis confirms removal of EPO glycosylation to produce DEPO (lane 1) and confirmation of high levels of glycosylation on HEPO (lane 3) as compared to EPO (lane 2). B. Epifluorescence micrograph of an rds retina co-injected with EPO and rAAV.eGFP. A successful subretinal injection is demonstrated by visualization of EGFP fluorescence in the RPE. EGFP (green); DAPI (blue); RPE=retinal pigment epithelium; ONL= outer nuclear layer.
3.2 EPO is rapidly cleared from the eye
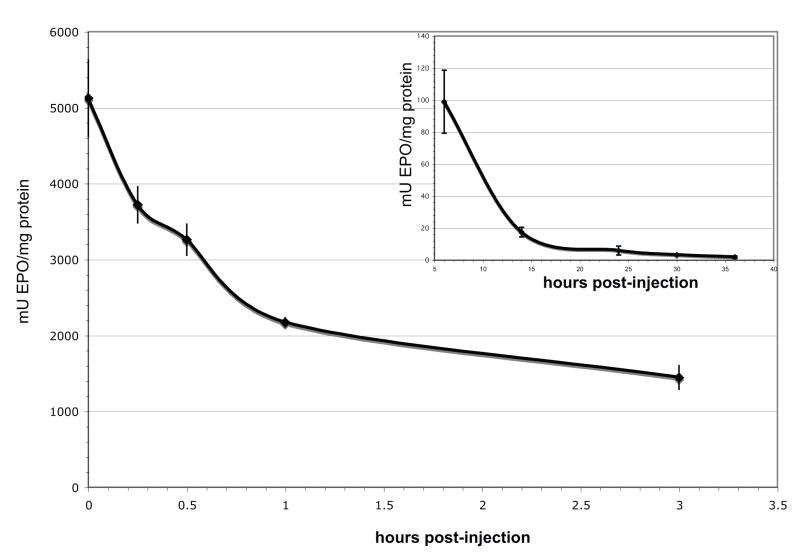
To determine the half-life of EPO in the eye, we measured EPO immediately after a single intraocular injection of 10U EPO into PD7 rds mice, and at specified time points post-injection (Figure 2A). At time zero, 5122±504mU EPO/mg total protein (n=4) was detected. EPO levels were 3720±238mU/mg (n=4) at 15 min, 3260±205mU/mg (n=4) at 30min, 2172±62mU/mg (n=4) at 1hr, and 1445±155 mU/mg (n=4) at 3hrs post–injection.
Figure 2.
EPO levels drop rapidly immediately after intraocular injection, the half-life of EPO is 1 hour post-injection. The amount EPO detected reaches previously published neuroprotective levels 5–13hrs post-injection (Grimm et al., 2004; Kilic et al., 2005; Ranchon Cole et al., 2007). A. Whole eye EPO levels at 0, 15, 30, 60, and 180 min post-injection. B. EPO levels 6, 14, 24, 30, and 36 hours post-injection. Error bars represent S.E.M.
To determine when therapeutic levels of EPO were attained in the retina, we quantified EPO at later time points post-injection (Figure 2B). We detected 99±20 mU/mg EPO 6hrs post-injection (n=11 eyes), 18±3.0mU/mg EPO 14hrs post-injection (n=8), 6.0±2.8mU/mg EPO 24hrs post-injection (n=9), 3.3±0.5mU/mg EPO 30hrs post-injection (n=6), and 1.9±0.8mU/mg EPO 36hrs post-injection (n=6). The rate of decrease tapered off after the 6hr time point (Figure 2B). We did not measure EPO levels after 36hrs as we were nearing the detection limit of the EPO ELISA. The lower detection limit of the EPO ELISA is 2.5mU/ml, and at 36hrs we detected an average of 6mU/ml.
3.3 Apoptotic cell death and neuroprotection by EPO, DEPO, and HEPO
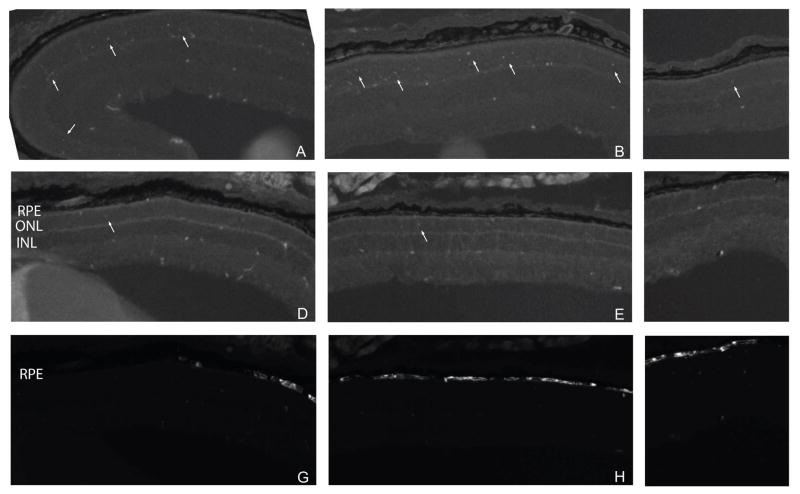
TUNEL-positive cells were located in all regions of the retina in untreated rds mice (Figure 3A–C). In treated rds mice, fewer TUNEL positive cells were detected throughout the retina (Figure 3D–F). There was no obvious difference in the number of TUNEL-positive cells in the injected area (i.e. EGFP-positive RPE) and uninjected areas (Figure 3D–I).
Figure 3.
Low magnification (10X) fluorescence micrographs of nasal (A, D, G), central (B, E, H), and temporal (C, F, I) regions of untreated (A–C) and EPO-treated (D–I) rds mouse retinas. Arrows indicate TUNEL-positive cells (A–F). Protection was not limited only to the area of injection as determined by the presence of EGFP in the RPE (G–I).
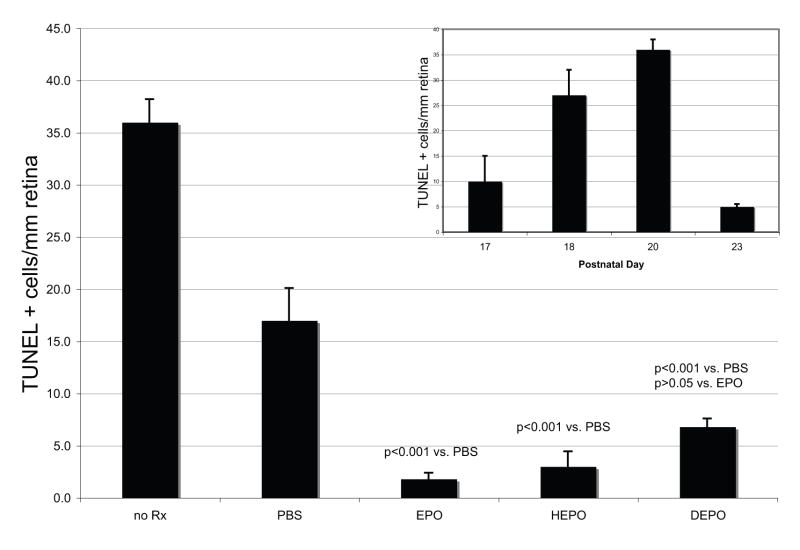
No TUNEL-positive photoreceptors were detected in age-matched (PD20) wild-type retina (Figure 4A). The number of TUNEL-positive cells in treated and untreated rds mice were quantified per mm retina in representative sections throughout the eye (Figure 5). TUNEL was performed on retina sections of rds mice at different ages to determine the peak of apoptotic cell death and the endpoint for the rest of the study. The number of TUNEL-positive cells/mm retina progressively increased until PD20 (Figure 5 inset). At PD23 only five cells per mm retina were TUNEL-positive.
Figure 4.
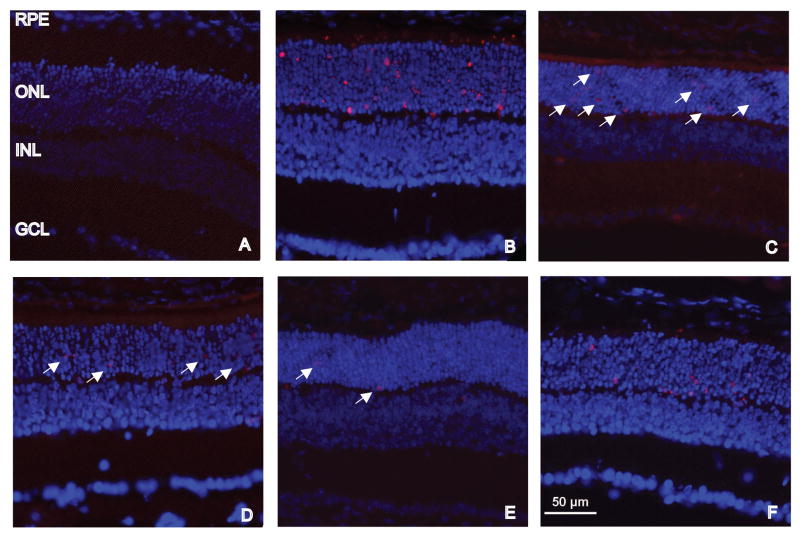
A single administration of EPO at PD7 blocks most photoreceptor cell death in the rds mouse. Fluorescence micrographs of PD20 retinas from normal (A) and rds (B–F) mice labeled with TUNEL (red) and DAPI (blue). No TUNEL-positive cells were detected in the normal retina (A). Many TUNEL-positive photoreceptor cells were detected in untreated (B) and PBS treated (C) retinas from rds mice. A few TUNEL-positive photoreceptor cells were detected in EPO (D), HEPO (E), and DEPO (F) treated retinas from rds mice. Arrows indicate TUNEL-positive cells. The scale bar in (E) is applicable to all images. INL= inner nuclear layer; GCL= ganglion cell layer
Figure 5.
Quantification of TUNEL-positive photoreceptor cells in untreated and treated rds eyes. The error bars represent the S.E.M.; p values compare treatment groups to the PBS-control group or to the EPO-treatment group, as indicated. There was no statistically significant difference between the treatment groups. Each of DEPO, EPO, and HEPO were more protective to the photoreceptors than PBS injection alone. Inset: Confirmation of the peak of TUNEL-positive cells in the rds mouse. Bar graph of TUNEL-positive cells per mm retina in PD 17, 18, 20, and 23 rds retinas. Error bars represent S.E.M.
Untreated retinas of PD20 rds mice had 36±2 (n=10) TUNEL-positive cells/mm retina (Figure 4B, Figure 5) and PBS treated retinas from PD20 rds mice had 17±3 (n=6) TUNEL-positive cells/mm retina (Figure 4C, Figure 5). In contrast, EPO treated retinas had 2±0.6 (n=8) TUNEL-positive cells/mm retina (Figure 4D, Figure 5). HEPO treated retinas had 3±1 (n=5) TUNEL-positive cells/mm retina (Figure 4E, Figure 5). DEPO treated retinas had 7±0.8 (n=9) TUNEL-positive cells/mm retina (Figure 4F, Figure 5). When treatment groups were compared to the PBS-injection control group, the p values were: p<0.001 for EPO, HEPO, and DEPO. There was no statistically significant difference between the EPO treatment group and either the HEPO or DEPO treatment groups (p> 0.05).
3.4 GFAP immunolabeling is diminished in EPO treated rds retinas
In the normal retina, GFAP immunolabeling was only detectable in the end feet of the Müller cells (Figure 5A). In the rds mouse retina, GFAP immunolabeling was increased (Figure 5B) as the Müller cells became reactive. GFAP labeling was detected in processes extending into the outer nuclear layer. In retinas treated with EPO, the amount of GFAP immunolabeling was decreased to near normal levels, most of the labeling being constrained to the endfeet of the Müller cells (Figure 5C).
4. Discussion
In this study we have demonstrated histological rescue of photoreceptors in rds mice following direct administration of EPO into the subretinal space. This rescue was detected in the absence of an increase in the hematocrit. While we did not assess vascular alterations in the retina after intraocular delivery of EPO, Zhang et al., 2008 reported that there was no effect on the retinal vasculature after intraocular injection of EPO (Zhang et al., 2008). Therefore, the protection of photoreceptors is likely due to a direct effect of EPO on the retina. And, the protection that was detected in our previous study in the rds mouse and the light damaged rat by systemic gene delivery of rAAV.Epo (Rex et al., 2004) was also most likely due to a direct neuroprotective effect of EPO on the retina. This is further supported by studies that show: 1) EPO crosses the blood brain barrier at a similar rate as albumin (Banks et al., 2004; Xenocostas et al., 2005; Statler et al., 2007); 2) the blood brain barrier and the inner and outer blood retina barriers are very similar in terms of their barrier characteristics (Steuer et al., 2005); and 3) that the EPO receptor(Grimm et al., 2002) is located on the photoreceptor inner segments. Some protection was also detected after PBS injection, this was likely due to the release of endogenous factors including bFGF and CNTF (Cao et al., 1997). The protection by treatment with EPO, DEPO and HEPO was statistically significantly higher than the protection by PBS injection alone.
Glycosylation of EPO is necessary for erythropoiesis in vivo, but is not necessary for neuroprotection of the photoreceptors by subretinal injection. DEPO is less biologically active (i.e. erythropoietic) than EPO in vivo, some deglycosylated forms of EPO have no biological activity (Dordal et al., 1984; Dube et al., 1988; Delorme et al., 1992). However, DEPO has a higher affinity for the EPO receptor than does EPO due to lack of steric hinderance from the carboydrate groups (Elliott et al., 2004). The reasons for decreased in vivo biological activity are a lack of secretion from the EPO producing cells and/or more rapid clearance of DEPO from the body via the liver (Dordal et al., 1984; Dube et al., 1988; Delorme et al., 1992; Elliott et al., 2004). In contrast, we found that DEPO and EPO were equally neuroprotective to the photoreceptors after intraocular delivery. Since they were delivered exogenously, secretion was not an issue. The difference, then, could lie in the EPO clearance mechanisms in the eye versus the liver. It is possible that DEPO is not cleared from the eye more rapidly than EPO. Despite a rapid loss of EPO from the eye after injection, EPO was neuroprotective. If DEPO is not cleared much more rapidly than EPO, it should not be surprising that it was neuroprotective since it is able to bind and activate the EPO receptor with a higher affinity than EPO.
In our previous paper, intraocular delivery of rAAV.Epo in the rat produced up to 1257mU/ml EPO in the vitreous (Rex et al., 2004). Mouse vitreous is too small to sample, so the value in the transduced mouse eye is unknown. Since high levels of EPO were detected in the vitreous, there was no significant problem with secretion, as has been reported to occur with certain deglycosylated forms of EPO (Dordal et al., 1984; Dube et al., 1988; Delorme et al., 1992). Based on the results of the current study, this secreted EPO should have been neuroprotective whether or not it was underglycosylated. However, no neuroprotection was detected. This may be due to production of EPO at levels above the neuroprotective dose range of EPO. Neuroprotection by EPO has a bell-shaped dose-curve (Sakanaka et al., 1998; Weishaupt et al., 2004; Buhrer et al., 2007; Zhang et al., 2008). We detected photoreceptor rescue with a single injection of 10U of EPO thirteen days prior to analysis despite rapid clearance of the protein – only half the amount of EPO detected at time zero could be detected one hour later. Levels of EPO equivalent to previously published neuroprotective doses of 24–39mU/mg in the retina (Grimm et al., 2004; Kilic et al., 2005; Ranchon Cole et al., 2007) were reached about 11 hours post-injection. It is possible that a continuous dose of up to 1257mU/ml EPO in the eye was above the neuroprotective dose range of EPO.
In the same gene therapy study, systemic delivery of rAAV.Epo produced 24mU/mL EPO in the serum (Rex et al., 2004). EPO was not detected in the eye after systemic delivery of rAAV.Epo. However, the EPO ELISA is not sensitive to low levels of EPO that would still be in the neuroprotective range. Based on a calculated rate by which EPO crosses the blood brain barrier of 0.5–1% (Banks et al., 2004; Steuer et al., 2005; Xenocostas et al., 2005), this translates into 0.24mU/ml EPO in the eye. Using published mouse eye dimensions (Remtulla and Hallet, 1985) and an average protein content of 0.6mg in whole mouse eye, this translates to approximately 0.01mU/mg EPO in the mouse eye treated systemically with rAAV.Epo. The fact that the photoreceptors were protected after systemic gene delivery implies that continuous delivery of 0.01mU/mg EPO to the eye is within the therapeutic dose range for EPO. Future studies will further examine the dose-relationship of EPO neuroprotection in the eye, and on the development of non-erythropoietic, but neuroprotective forms of EPO that can be delivered systemically.
The current study demonstrates that EPO is directly neuroprotective to the photoreceptors in the rds mouse model of retinal degeneration. Further, the protection was not limited to the area of injection, indicating that EPO is able to diffuse through and across the retina. This is not surprising considering rAAV.Epo transduction of the RPE resulted in high levels of EPO in the vitreous (Rex et al., 2004). We were surprised to discover that DEPO is as effective in protecting the photoreceptors from cell death as normally glycosylated EPO.
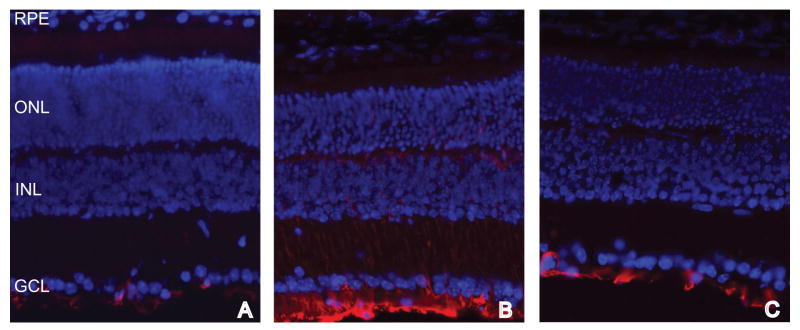
Treatment with EPO decreased GFAP reactivity in the Müller cells. This may be a secondary effect as preservation of the photoreceptors may prevent Müller cell hypertrophy. Alternatively, EPO could act directly on the Müller cells since they also appear to produce the EPOR (Grimm et al., 2002). Future studies will need to be performed to determine whether or not EPO can directly affect the Müller cells.
Figure 6.
Fluorescence micrographs of normal (A), untreated rds (B), and EPO treated rds (C) retinas labeled with anti-GFAP (red) and DAPI (blue). GFAP labeling is present only in the Müller endfeet in the normal and EPO-treated rds retinas. In contrast, GFAP labeling is present in the Müller cell endfeet and in processes extending through the retina in the untreated rds retina.
Acknowledgments
This project was funded by: Research to Prevent Blindness; NIH 5P30EY13080; Roche Foundation for Anemia Research; and Hope for Vision. The authors thank Dr. Albert Maguire, University of Pennsylvania for teaching Dr. Rex how to perform subretinal injections in mice. The authors thank Michael Herr for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Banks W, Jumbe N, Farrell C, Niehoff M, Heatherington A. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. European Journal of Pharmacology. 2004;505:93–101. doi: 10.1016/j.ejphar.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Buhrer C, Felderhoff-Mueser U, Wellmann S. Erythropoietin and ischemic conditioning - why two good things may be bad. Acta Paediatrica. 2007;96:787–789. doi: 10.1111/j.1651-2227.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Cao W, Wen R, Li F, LaVail M, Steinberg R. Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Experimental Eye Research. 1997;65:241–248. doi: 10.1006/exer.1997.0328. [DOI] [PubMed] [Google Scholar]
- Chang G, Hao Y, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- Connell G, Bascom R, Molday L, Reid D, McInnes R, Molday R. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proceedings of the National Academy of Sciences USA. 1991;88:723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme E, Lorenzini T, Giffin J, Martin F, Jacobsen F, Boone T, Elliott S. Role of glycosylation on the secretion and biological activity of erythropoietin. Biochemistry. 1992;31:9871–9876. doi: 10.1021/bi00156a003. [DOI] [PubMed] [Google Scholar]
- Dordal M, Wang F, Goldwasser E. The role of carbohydrate in erythropoietin action. Endocrinology. 1984;116:2293–2299. doi: 10.1210/endo-116-6-2293. [DOI] [PubMed] [Google Scholar]
- Dube S, Fisher J, Powell J. Glycosylation at specific sites of erythropoietin is essential for biosynthesis, secretion, and biological function. The Journal of Biological Chemistry. 1988;263:17516–17521. [PubMed] [Google Scholar]
- Elliott S, Egrie J, Browne J, Lorenzini T, Busse L, Rogers N, Ponting I. Control of rHuEPO biological activity: The role of carbohydrate. Experimental Hematology. 2004;32:1146–1155. doi: 10.1016/j.exphem.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Heinicke K, Soliz J, Ogunshola O. Non-erythroid functions of erythropoietin. Advances in Experimental Medicine and Biology. 2003;543:323–330. doi: 10.1007/978-1-4419-8997-0_22. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme C. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nature Medicine. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, Naash M, Gassmann M, Reme C. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. The Journal of Neuroscience. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazono Y, Sasaki K, Nitta H, Yazaki Y, Hirai H. Erythropoietin induces tyrosine phosphorylation of the beta chain of the GM-CSF receptor. Biochemical and Biophysical Research Communications. 1995;208:1060–1066. doi: 10.1006/bbrc.1995.1442. [DOI] [PubMed] [Google Scholar]
- Jubinsky P, Krijanovski O, Nathan D, Tavernier J, Sieff C. The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood. 1997;90:1867–1873. [PubMed] [Google Scholar]
- Kilic U, Kilic E, Soliz J, Bassetti C, Gassmann M, Hermann D. Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating ERK-1/-2. The FASEB Journal. 2005;19:249–251. doi: 10.1096/fj.04-2493fje. [DOI] [PubMed] [Google Scholar]
- King C, Rodger J, Bartlett C, Esmaili T, Dunlop S, Beazley L. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Experimental Neurology. 2007;205:48–55. doi: 10.1016/j.expneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Kretz A, Happold C, Marticke J, Isenmann S. Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Molecular and Cellular Neuroscience. 2005;29:569–579. doi: 10.1016/j.mcn.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen A, Helboe L, Christensen S, Pedersen L, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie Q, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Ranchon Cole I, Bonhomme B, Doly M. Pre-treatment of adult rats with high doses of erythropoietin induces caspase-9 but prevents light-induced retinal injury. Experimental Eye Research. 2007;85:782–789. doi: 10.1016/j.exer.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Remtulla S, Hallet P. A schematic eye for the mouse, and comparisons with the rat. Vision Research. 1985;25:21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- RetNet. 1996–2009 from http://www.sph.uth.tmc.edu/retnet/
- Rex T, Allocca M, Domenici L, Surace E, Maguire A, Lyubarsky A, Cellerino A, Bennett J, Auricchio A. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Molecular Therapy. 2004;10:855–861. doi: 10.1016/j.ymthe.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proceedings of the National Academy of Sciences USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, De Ruiter A, Hawkins R. Development and degeneration of retina in rds mutant mice: light microscopy. Journal of Comparative Neurology. 1980;194:193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- Statler P, McPherson R, Bauer L, Kellert B, Juul S. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatric Research. 2007;61:671–675. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- Steuer H, Jaworski A, Elger B, Kaussmann M, Keldenich J, Schneider H, Stoll D, Schlosshauer B. Functional characterization and comparison of the outer blood-retina barrier and the blood-brain barrier. Investigative Ophthalmology and Visual Science. 2005;46:1047–1053. doi: 10.1167/iovs.04-0925. [DOI] [PubMed] [Google Scholar]
- Toledo J, Sánchez O, Seguí R, García G, Montañez M, Zamora P, Rodríguez M, Cremata J. High expression level of recombinant human erythropoietin in the milk of non-transgenic goats. Journal of Biotechnology. 2006;123:225–235. doi: 10.1016/j.jbiotec.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Travis G, Brennan M, Danielson P, Kozak C, Sutcliffe J. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds) Nature. 1989;338:70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- van Nie R, Ivanyi D, Demant P. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Anitgens. 1978;12:106–108. doi: 10.1111/j.1399-0039.1978.tb01305.x. [DOI] [PubMed] [Google Scholar]
- Weishaupt J, Rohde G, Polking E, Siren AL, Ehrenreich H, Bahr M. Effect of erythropoietin on axotomy-induced apoptosis in rat retinal ganglion cells. Investigative Ophthalmology and Visual Science. 2004;45:1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- Xenocostas A, Cheung W, Farrell F, Zakszewski C, Kelley M, Lutynski A, Crump M, Lipton J, Kiss T, Lau C, Messner H. The pharmacokinetics of erythropoietin in the cerebrospinal fluid after intravenous administration of recombinant human erythropoietin. European Journal of Pharmacology. 2005;61:189–195. doi: 10.1007/s00228-005-0896-7. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu Y, Jin Y, Ji F, Sinclair S, Luo Y, Xu G, Lu L, Dai W, Yanoff M, Li W, Xu G. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Investigative Ophthalmology and Visual Science. 2008;49:732–742. doi: 10.1167/iovs.07-0721. [DOI] [PubMed] [Google Scholar]
- Zhong L, Bradley J, Schubert W, Ahmed E, Adamis A, Shima D, Robinson G, Ng Y. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Investigative Ophthalmology and Visual Science. 2007;48:1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]