Abstract
While stress plays an important role in chronic widespread pain syndromes, such as fibromyalgia, the underlying mechanism has remained elusive. We have recently demonstrated, in a model of chronic widespread pain, that prolonged enhancement of immune mediator hyperalgesia, induced by unpredictable sound stress, requires a contribution of both the sympathoadrenal (epinephrine) and the hypothalamic-pituitary adrenal (corticosterone) neuroendocrine stress axes. Since this stress protocol produced sustained elevation of plasma epinephrine, in the current study we have tested the hypothesis that the sympathoadrenal axis also plays a role in maintenance of symptoms in this model of chronic widespread pain. After establishment, adrenal medullectomy abolished the enhancement of epinephrine-induced cutaneous and muscle hyperalgesia. Administration of stress levels of epinephrine to adrenal medullectomized rats reconstituted the pain phenotype. These observations suggest that the sympathoadrenal stress axis plays a major role in the induction as well as maintenance of stress-induced enhancement of mechanical hyperalgesia, mediated by prolonged elevation of circulating epinephrine.
Perspective
We present data showing mechanical hyperalgesia persisting for up to 28 days after exposure to sound stress, with evidence that the sympathoadrenal axis mediator, epinephrine, plays a major role. These findings could have clinical implications with regard to novel potential treatments for chronic widespread pain syndromes, such as fibromyalgia.
Keywords: Stress, epinephrine, adrenal medulla, muscle pain, hyperalgesia
INTRODUCTION
Stress markedly exacerbates pain in diseases such as fibromyalgia and rheumatoid arthritis 1-4, 8, and we have hypothesized that stress causes generalized hyperalgesia by enhancing pronociceptive effects of immune mediators (e.g. cytokines and prostaglandins). While the underlying mechanisms for the effect of stress on chronic pain have remained elusive, chronic or repeated exposure to stress induces both an immediate and a long-lasting increase in catecholamine synthesizing enzymes in the adrenal medulla 18-20, 26, the neuroendocrine organ that responds to stress by secreting catecholamines to cope with impending danger, perceived or real 19, 21. Based on our studies using a model of intermittent sound stress in rats, we have suggested that sound stress-induced enhancement of immune mediator hyperalgesia requires activity in both neuroendocrine stress axes: the sympathoadrenal (via release of epinephrine) and hypothalamic pituitary adrenal (HPA) (via release of corticosterone) 12. However, while plasma levels of corticosterone return to physiological levels, 24 hours post stress, epinephrine is still markedly elevated 21 days later 12. Stress-induced long-lasting changes in the catecholamine-synthesizing enzymes may contribute to this prolonged elevation of plasma epinephrine levels in sound stressed rats 12.
We have previously shown that removal of the adrenal medulla before exposure to sound stress prevented the stress-induced enhancement of epinephrine hyperalgesia. Given the long-term the molecular level, and the prolonged elevation of plasma epinephrine levels, we hypothesized that the adrenal medulla contributes to the maintenance as well as the onset of sound stress-induced enhancement of immune mediator hyperalgesia. We tested this hypothesis by assessing whether adrenal medullectomy could reverse stress-induced enhancement of epinephrine hyperalgesia after it is established by performing adrenal medullectomy, 14 days after the last exposure to sound stress, at which time, enhancement of mechanical hyperalgesia is fully established.
MATERIALS AND METHODS
Animals
Experiments were performed on adult male Sprague Dawley rats (300−400 g, approximately 9 − 12 weeks old; Charles River, Hollister, CA). Rats were pair housed in acrylic cages (30 × 75 × 20 cm) in the Laboratory Animal Resource Center of the University of California, San Francisco, under a 12-hour light/dark cycle. Animal care and use conformed to NIH guidelines. The University of California, San Francisco Institutional Animal Care and Use Committee approved experimental protocols. Concerted effort was made to reduce the suffering and number of animals used.
Epinephrine
Stock solutions (4 mg/ml) of L-epinephrine D-hydrogen tartrate (Sigma, St. Louis, MO) were prepared daily, in distilled water, with 4 mg/ml ascorbic acid (Sigma, St. Louis, MO) added to minimize oxidation. The stock solution was further diluted in 0.9% saline for use in experiments. Working solutions of epinephrine were kept on ice and in subdued light during experiments. Cumulative dosing was used as it markedly reduces the number of animals used. Intradermal injections were made at the site of nociceptive testing.
Mechanical threshold in the skin
Nociceptive thresholds were quantified in lightly restrained rats using an Ugo Basile Algesymeter (Stoelting, IL), which applies linearly increasing mechanical pressure to the dorsum of the hind paw. To minimize variability of measurements, the rats were trained in the paw-withdrawal reflex test at 5-minute intervals for 1 hour each day for a period of 3 days 27. These training sessions also serve to familiarize the rats with the handling procedure and the restraining apparatus, a vented tubular acrylic holder with slanted openings on the side that allow easy access to the hind limb.
Intradermal injection of test agents
Mechanical nociceptive thresholds were determined both before (baseline threshold) and 10, 15, and 20 minutes after the intradermal injection (dorsal surface of hind paw) of epinephrine, in a volume of 2.5 μl. For each dose of epinephrine (1−1,000 ng cumulative dosing schedule, 30 min between doses), the mean of the nociceptive threshold at the 3 time points was determined and the percentage change in nociceptive threshold calculated as (post-injection nociceptive threshold)-(pre-injection nociceptive threshold) / (pre-injection nociceptive threshold) × 100.
Mechanical threshold in the gastrocnemius muscle
Since fibromyalgia syndrome also affects skeletal muscle, we tested whether there is increased sensitivity to hyperalgesic mediator, in skeletal muscle, in the sound stressed rats compared to sham stressed controls. Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a digital force transducer (Chatillon, Model DFI2, Amtek Inc., Largo, FL) 6, 7. Rats were restrained in a vented tubular acrylic holder with slanted openings on the side that allow easy access to the hind limb. A 6-mm diameter probe attached to a transducer was applied to the gastrocnemius muscle to deliver an increasing compression force, and the nociceptive threshold defined as the force, in Newtons (N), at which the rat withdrew its hind leg. Baseline withdrawal threshold was defined as the mean of 2 readings taken at 5-minute intervals before administration of test agent.
Intramuscular injection of test agents
Increasing doses of epinephrine (1−10,000 ng) were administered cumulatively, at 25-minute intervals, in a volume of 10 μl, into the belly of the gastrocnemius muscle. The skin over the injection site was marked, using an indelible pen, so that the same muscle site could be retested for mechanical nociceptive threshold. Mechanical nociceptive thresholds were measured before (baseline threshold), and again 15 and 20 minutes after injection of epinephrine. Percentage change in nociceptive threshold was calculated as for cutaneous threshold (vide supra).
Stress
Exposure to sound stress occurred over 4 days as described previously 12, 13, 25. Animals were placed 3 per cage and the cage placed 25 cm from a speaker that emitted 4 pure tones (5, 11, 15 and 19 kHz), whose amplitudes varied through time independently from 20−110 dB sound pressure level at random times each minute, lasting 5 or 10 s. Sham stressed animals were placed in the sound chamber for 30 minutes, but without exposure to the sound stimulus. Following sound or sham sound stress, rats were returned to their home cages in the animal care facility. Animals were exposed to the stressor on days 1, 3, and 4 12. Rats were used for nociceptive studies, 28 days after the last sound stress exposure.
Adrenal medullectomy
Rats were anesthetized with isoflurane (2.5% in 97.5% O2). The adrenal glands were located through bilateral incisions in the abdominal wall, the capsules incised and the medullae removed 17, 30. The fascia was closed with suture and the skin closed with wound clips. Rats were provided with 0.45% saline to drink for the first 7 days following surgery. Surgical removal of the adrenal medullae was performed 14 days after the last sound stress protocol, and nociceptive testing performed 14 days after the surgery (i.e., 28 days after the last sound stress).
Chronic administration of epinephrine
Chronic administration of stress levels of epinephrine was performed by implanting Alzet® mini-osmotic pumps (model 2004; Durect, Cupertino, CA) filled with epinephrine, subcutaneously in the interscapular region, to deliver epinephrine systemically at the rate of 5.4 μg/0.25 μl/h 12, 13. Epinephrine-filled pumps were implanted during the same surgery as for the removal of the adrenal medullae. We have previously shown that rats with these implants have epinephrine plasma levels of 720 ± 67 pg/mL 13. Nociceptive threshold was measured in adrenal medullectomized rats with epinephrine implants 14 days after surgery.
Measurement of epinephrine
One day prior to initiation of sound stress and 28 days following its completion, peripheral blood samples (0.5 ml) were collected from isoflurane-anesthetized rats and immediately placed on ice. Plasma was isolated by centrifugation and stored at −80°C until analysis. For analysis, catecholamines were extracted from plasma by alumina adsorption, and levels determined using high performance liquid chromatography with electrochemical detection (5200 electrochemical detector, ESA Inc, Chelmsford, MA) 14.
Statistical analysis
Data are presented as mean ± SEM and analyzed using 2-way repeated measures analysis of variance (ANOVA), or Student's t-test, as appropriate. Where the overall ANOVA showed significant differences between groups, Scheffe's post hoc test was used to determine the pairs of groups that were different. The accepted level of significance was P < 0.05. The P-values for main effects are from an ANOVA, and all subsequent P-values are from Scheffe's post hoc tests, unless otherwise stated.
RESULTS
While sound stress had no effect on nociceptive threshold (data not shown), consistent with our earlier finding that sound stress-induced enhancement of epinephrine hyperalgesia was still sustained 21 days after the last exposure to the 4-day stress protocol 12, we observed in the current study that even 28 days after the last exposure to stress, the hyperalgesia was still enhanced in the skin (Fig. 1A) and skeletal muscle (Fig. 1B). Nociceptive thresholds in repeat vehicle-injected controls were unchanged. In addition, 28 days after stress, plasma epinephrine levels were significantly higher compared to non-stressed control (470 ± 28 pg/ml (n = 12) vs. 242 ± 43 pg/ml (n = 17); P < 0.01, unpaired two-tailed Student's t-test).
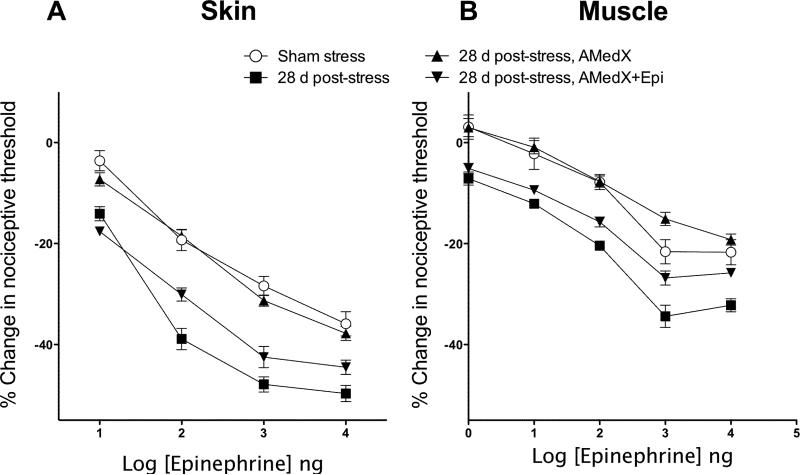
Figure 1.
Reversal of stress-induced enhancement of epinephrine hyperalgesia (28 d post stress, filled squares) by adrenal medullectomy (28 d post-stress, AMedX, filled triangles)) and reconstitution of the enhanced hyperalgesia (28 d post-stress, AMedX, + Epi, filled inverted triangles) by implants of epinephrine in A) the skin (dorsal aspect of the hind paw) and B) skeletal (gastrocnemius) muscle of the rat. Adrenal medullectomy, with or without epinephrine implants, was performed 14 days after the last sound stress exposure and rats were used for experiments 14 days after surgery.
Figure 1 shows that both in the skin (Fig. 1A) and muscle (Fig. 1B), the enhancement of epinephrine hyperalgesia was reversed 14 days after adrenal medullectomy in rats that had been exposed to sound stress 14 days prior. Overall ANOVA of the skin hyperalgesia data showed significant differences between the treatment groups (F3, 46 = 48.8, P < 0.001). Post hoc analysis showed that the stressed adrenal medullectomized group (n = 18) was significantly different from the non-medullectomized stressed group (n = 16; P < 0.001), but significantly different from sham stressed group (n = 6; P>0.05). In muscle, repeated measures ANOVA also demonstrated a significant difference between groups (F3,44 = 46.0; P < 0.001), with post-hoc analysis showing that the stressed adrenal medullectomized group (n = 24) was significantly different from the non-medullectomized stressed group (n = 6; P < 0.001), but was not significantly in naïve animals is not significantly different from the dose response curve after sham surgery {Khasar et al., 1999, #52001} and vehicle control for the epinephrine implant {Khasar et al., 2005, #64084}.
In order to confirm the role of adrenal medullary epinephrine in maintenance of sound stress-induced enhancement of epinephrine hyperalgesia, a group of adrenal medullectomized rats received continuous epinephrine via implanted mini osmotic pumps. In adrenal medullectomized rats with epinephrine implants, stress-induced enhancement of epinephrine hyperalgesia was present both in the skin (Fig. 1A) and in muscle (Fig. 1B). Post hoc analysis showed no significant differences in nociceptive threshold between the stressed medullectomized group with epinephrine implants (n = 4) compared to the sound stressed non-medullectomized group (P = 0.34). However, the sound stressed medullectomized group with epinephrine implants was significantly enhanced compared to the stressed medullectomized group without epinephrine implants (P < 0.001). Similarly, in muscle post hoc analysis showed no significant differences in nociceptive threshold between the stressed medullectomized group with epinephrine implants (n = 12) compared to the sound stressed non-medullectomized group (n = 6, P = 0.064) (Fig. 1B). Data in the figures is shown as a percentage change from baseline; Table 1 shows the raw data for cutaneous and muscle nociceptive thresholds.
Table 1.
Absolute values for mechanical nociceptive thresholds for cutaneous and muscle tissue
| Tissue |
Sham stress |
28d post-stress |
14d post-stress AMedx |
14d post-stress AMedx + Epi |
|---|---|---|---|---|
| Cutaneous (Grams) | 108.8±1.420 | 105.1±1.278 | 114.6±1.305 | 121.5±1.708 |
| Muscle (Newtons) | 3.18±0.05 | 3.47±0.02 | 3.22±0.04 | 3.51±0.03 |
DISCUSSION
In this study, we have demonstrated that the long-lasting sound stress-induced enhancement of mechanical hyperalgesia in both skin and muscle is adrenal medulla dependent. This is consistent with previous findings that chronic or repeated exposure to stress leads to long-lasting increased activity of catecholamine synthesizing enzymes in the adrenal medulla 18-20, 26. How elevated catecholamine levels are maintained after removal of the stressor remains to be established.
Epinephrine is an endogenous β2-adrenergic receptor ligand, mainly secreted by the adrenal medulla. Although the adrenal medulla secretes many other substances 15, 29, the observation that implants of epinephrine in adrenal medullectomized rats reconstituted the enhanced hyperalgesia of the intact phenotype, suggests that epinephrine plays a major role in the adrenal medulla-dependent maintenance of enhanced immune mediator hyperalgesia. The importance of the involvement of β2-adrenergic receptors in stress-induced enhancement of hyperalgesia is exemplified in a study showing the efficacy of pindolol, a β-adrenergic receptor antagonist as a potential therapeutic agent in fibromyalgia 33.
While corticosterone levels may be normal or lower in fibromyalgia patients 5, 16, 28, 31, 32, and in our model of fibromyalgia-like generalized hyperalgesia 12, HPA axis reflexes may be exaggerated 5, 28. Thus, our current findings do not rule out a contribution of the HPA axis in the maintenance of sound stress-induced enhancement of immune mediator hyperalgesia. As we previously demonstrated 12, activity in both sympathoadrenal and HPA axes is necessary for induction of the enhanced immune mediator-induced hyperalgesia. Moreover, glucocorticoids can increase the expression of β2-adrenergic receptors 9, an effect that outlasts the elevation of plasma glucocorticoids.
Of note, while we did not observe any change in nociceptive threshold following sound stress, others have reported long-lasting decreased (repeated swim stress) 10, 22, transient decrease (water avoidance stress) 23 or increased (repeated sound stress) 24 nociceptive thresholds. While there is not yet a definitive answer for the basis of these differences, there are several major differences between the studies worthy of consideration, including differences in the type of stress used, differences in the nociceptive testing used (i.e., thermal nociception vs. mechanical nociception), timing of nociceptive testing and predictability of stress — unlike some other studies, we used unpredictable stress. This latter point may be especially relevant since when we used continuous days of stress exposure, we do not see our effect of stress on inflammatory mediator-induced hyperalgesia (unpublished observations). Importantly, nociceptive threshold following exposure to stress correlates with rats’ behavioral response to the stress. 11 Given that there is a rather extensive literature on analgesic and hyperalgesic effects of stress, we suggest that such differences in stress protocols and nociceptive testing may be important. Of note, we have recently observed that visceromotor responses (as measured in an established colorectal distension model) to noxious mechanical stimulation was greater in rats exposed to our sound stress model (unpublished observation); these preliminary findings also consistent with our hypothesis that the sound stress paradigm we employ is a good model for chronic widespread pain.
In conclusion, we have demonstrated that sound stress-induced enhancement immune mediator hyperalgesia as well as plasma epinephrine levels persist for up to 28 days after the last exposure to stress. Thus, our data suggest that a mediator of the sympathoadrenal axis, epinephrine, plays a major role in the maintenance of stress-induced enhancement of immune mediator hyperalgesia both in the skin and muscle.
Anowledgments
This research was supported by grants from NIAMS (AR054635, to JDL)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Adler GK, Manfredsdottir VF, Creskoff KW. Neuroendocrine abnormalities in fibromyalgia. Curr Pain Headache Rep. 2002;6:289–298. doi: 10.1007/s11916-002-0050-5. [DOI] [PubMed] [Google Scholar]
- 2.Amir M, Kaplan Z, Neumann L, Sharabani R, Shani N, Buskila D. Posttraumatic stress disorder, tenderness and fibromyalgia. J Psychosom Res. 1997;42:607–613. doi: 10.1016/s0022-3999(97)00009-3. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen H, Neumann L, Haiman Y, Matar MA, Press J, Buskila D. Prevalence of post-traumatic stress disorder in fibromyalgia patients: overlapping syndromes or post-traumatic fibromyalgia syndrome? Semin Arthritis Rheum. 2002;32:38–50. doi: 10.1053/sarh.2002.33719. [DOI] [PubMed] [Google Scholar]
- 5.Crofford LJ, Engleberg NC, Demitrack MA. Neurohormonal perturbations in fibromyalgia. Baillieres Clin Rheumatol. 1996;10:365–378. doi: 10.1016/s0950-3579(96)80022-7. [DOI] [PubMed] [Google Scholar]
- 6.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dina OA, Levine JD, Green PG. Muscle inflammation induces a protein kinase Cepsilon-dependent chronic-latent muscle pain. J Pain. 2008;9:457–462. doi: 10.1016/j.jpain.2008.01.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eijsbouts AM, Murphy EP. The role of the hypothalamic-pituitary-adrenal axis in rheumatoid arthritis. Baillieres Best Pract Res Clin Rheumatol. 1999;13:599–613. doi: 10.1053/berh.1999.0048. [DOI] [PubMed] [Google Scholar]
- 9.Fraser CM, Venter JC. The synthesis of beta-adrenergic receptors in cultured human lung cells: induction by glucocorticoids. Biochem Biophys Res Commun. 1980;94:390–397. doi: 10.1016/s0006-291x(80)80233-6. [DOI] [PubMed] [Google Scholar]
- 10.Hata T, Kita T, Itoh E, Kawabata A. The relationship of hyperalgesia in SART (repeated cold)-stressed animals to the autonomic nervous system. J Auton Pharmacol. 1988;8:45–52. doi: 10.1111/j.1474-8673.1988.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 11.Jorum E. Analgesia or hyperalgesia following stress correlates with emotional behavior in rats. Pain. 1988;32:341–348. doi: 10.1016/0304-3959(88)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 14.Khasar SG, Green PG, Miao FJ, Levine JD. Vagal modulation of nociception is mediated by adrenomedullary epinephrine in the rat. Eur J Neurosci. 2003;17:909–915. doi: 10.1046/j.1460-9568.2003.02503.x. [DOI] [PubMed] [Google Scholar]
- 15.Livett BG, Dean DM, Whelan LG, Udenfriend S, Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981;289:317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- 16.McLean SA, Williams DA, Harris RE, Kop WJ, Groner KH, Ambrose K, Lyden AK, Gracely RH, Crofford LJ, Geisser ME, Sen A, Biswas P, Clauw DJ. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis Rheum. 2005;52:3660–3669. doi: 10.1002/art.21372. [DOI] [PubMed] [Google Scholar]
- 17.Miao FJ, Benowitz NL, Basbaum AI, Levine JD. Sympathoadrenal contribution to nicotinic and muscarinic modulation of bradykinin-induced plasma extravasation in the knee joint of the rat. J Pharmacol Exp Ther. 1992;262:889–895. [PubMed] [Google Scholar]
- 18.Nankova B, Kvetnansky R, McMahon A, Viskupic E, Hiremagalur B, Frankle G, Fukuhara K, Kopin IJ, Sabban EL. Induction of tyrosine hydroxylase gene expression by a nonneuronal nonpituitary-mediated mechanism in immobilization stress. Proc Natl Acad Sci U S A. 1994;91:5937–5941. doi: 10.1073/pnas.91.13.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nankova BB, Sabban EL. Multiple signalling pathways exist in the stress-triggered regulation of gene expression for catecholamine biosynthetic enzymes and several neuropeptides in the rat adrenal medulla. Acta Physiol Scand. 1999;167:1–9. doi: 10.1046/j.1365-201x.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- 20.Nankova BB, Tank AW, Sabban EL. Transient or sustained transcriptional activation of the genes encoding rat adrenomedullary catecholamine biosynthetic enzymes by different durations of immobilization stress. Neuroscience. 1999;94:803–808. doi: 10.1016/s0306-4522(99)00290-0. [DOI] [PubMed] [Google Scholar]
- 21.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 22.Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav. 2000;67:449–458. doi: 10.1016/s0091-3057(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 23.Schwetz I, McRoberts JA, Bradesi S, Miller JC, Zhou H, Mayer EA. NK1 receptors are involved in delayed stress-induced visceral hyperalgesia in Wistar rats. Soc Neurosci Abstr. 2003;451:12. [Google Scholar]
- 24.Shankar N, Awasthy N, Mago H, Tandon OP. Analgesic effect of environmental noise: a possible stress response in rats. Indian J Physiol Pharmacol. 1999;43:337–346. [PubMed] [Google Scholar]
- 25.Strausbaugh HJ, Green PG, Dallman MF, Levine JD. Repeated, non-habituating stress suppresses inflammatory plasma extravasation by a novel, sympathoadrenal dependent mechanism. Eur J Neurosci. 2003;17:805–812. doi: 10.1046/j.1460-9568.2003.02493.x. [DOI] [PubMed] [Google Scholar]
- 26.Tai TC, Claycomb R, Siddall BJ, Bell RA, Kvetnansky R, Wong DL. Stress-induced changes in epinephrine expression in the adrenal medulla in vivo. J Neurochem. 2007;101:1108–1118. doi: 10.1111/j.1471-4159.2007.04484.x. [DOI] [PubMed] [Google Scholar]
- 27.Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res. 1989;487:148–151. doi: 10.1016/0006-8993(89)90950-5. [DOI] [PubMed] [Google Scholar]
- 28.Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: a pilot study in fibromyalgia. Arthritis Rheum. 2000;43:872–880. doi: 10.1002/1529-0131(200004)43:4<872::AID-ANR19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 29.Unsicker K. The trophic cocktail made by adrenal chromaffin cells. Exp Neurol. 1993;123:167–173. doi: 10.1006/exnr.1993.1149. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson CW, Shinsako J, Dallman MF. Return of pituitary-adrenal function after adrenal enucleation or transplantation: diurnal rhythms and responses to ether. Endocrinology. 1981;109:162–169. doi: 10.1210/endo-109-1-162. [DOI] [PubMed] [Google Scholar]
- 31.Wingenfeld K, Heim C, Schmidt I, Wagner D, Meinlschmidt G, Hellhammer DH. HPA axis reactivity and lymphocyte glucocorticoid sensitivity in fibromyalgia syndrome and chronic pelvic pain. Psychosom Med. 2008;70:65–72. doi: 10.1097/PSY.0b013e31815ff3ce. [DOI] [PubMed] [Google Scholar]
- 32.Wingenfeld K, Wagner D, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim C. The low-dose dexamethasone suppression test in fibromyalgia. J Psychosom Res. 2007;62:85–91. doi: 10.1016/j.jpsychores.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Wood PB, Kablinger AS, Caldito GS. Open trial of pindolol in the treatment of fibromyalgia. Ann Pharmacother. 2005;39:1812–1816. doi: 10.1345/aph.1G014. [DOI] [PubMed] [Google Scholar]