Abstract
The T cell receptor (TCR) detects the presence of infectious pathogens and activates numerous intracellular signaling pathways. Protein tyrosine phosphorylation and ubiquitination serve as key regulatory mechanisms downstream of the TCR. Negative regulation of TCR signaling pathways is important in controlling the immune response, and the Suppressor of TCR Signaling proteins (Sts-1 and Sts-2) have been shown to function as critical negative regulators of TCR signaling. Although their mechanism of action has yet to be fully uncovered, it is known that the Sts proteins possess intrinsic phosphatase activity. Here, we demonstrate that Sts-1 and Sts-2 are instrumental in down-modulating proteins that are dually modified by both protein tyrosine phosphorylation and ubiquitination. Specifically, both naïve and activated T cells derived from genetically engineered mice that lack the Sts proteins display strikingly elevated levels of tyrosine phosphorylated, ubiquitinated proteins following TCR stimulation. The accumulation of the dually modified proteins is transient, and in activated T cells but not naïve T cells is significantly enhanced by co-receptor engagement. Our observations hint at a novel regulatory mechanism downstream of the T cell receptor.
Keywords: T cells, Signal transduction, T cell activation, T cell phosphatase, ubiquitination
1. Introduction
Sustained engagement of the T cell receptor (TCR) by antigen presenting cells activates T cells (Iwashimi, 2003). The intracellular signaling cascades downstream of the TCR turn on expression of multiple cytokine genes, promote entry into the cell cycle, and control execution of T cell effector functions (Huang and Wange, 2004). Numerous tyrosine kinases, among them members of the Src, Syk, Itk, and Abl/Arg families of kinases, are rapidly activated by triggering of the TCR and play critical roles in T cell signaling pathways (Wange, 2004). For example, phosphorylation of key residues within the cytoplasmic portions of TCR subunits by membrane-bound Lck and Fyn generate docking sites for the cytoplasmic kinase Zap-70 (Latour and Veilette, 2001). Recruitment of Zap-70 into the TCR complex leads to its activation by tyrosine phosphorylation, enabling it to phosphorylate numerous downstream substrates (Chu et al., 1998). Substrates include enzymes whose activity is regulated by tyrosine phosphorylation and many adaptor proteins that require tyrosine phosphorylation for assembly of higher order signaling complexes. Two adaptor proteins, LAT (Linker of Activated T cells) and Slp-76 (SH2 domain containing leukocyte protein of 76 kDa), are critical substrates of Zap-70 and are heavily phosphorylated following TCR stimulation (Samelson, 2002).
In order to inhibit proximal signaling pathways activated by tyrosine kinases, T cells possess a number of negative regulatory mechanisms (Acuto et al., 2008). For example, down-modulaton of cell surface TCR expression following ligand binding reduces receptor availability (Liu et al., 2000; Geisler, 2004). Numerous protein tyrosine phosphatases directly dephosphorylate tyrosine kinases and their phosphorylated substrates in order to switch off their activities (Pao et al., 2007). In addition, ubiquitin ligases promote the mono-, multi-, or poly-ubiquitination of target proteins to affect TCR-dependent tyrosine kinase signaling pathways (Liu, 2004). The covalent addition of one ubiquitin moiety (mono-) or multiple single ubiquitin moieties (multi-) to a protein can modify a protein’s function and/or activity or alter its subcellular localization (Hicke et al., 2005). The addition of poly-ubiquitin chains to a protein generally results in proteosome-dependent degradation, if the poly-ubiquitin chain is extended via ubiquitin lysine-48 linkages (Pickart and Fushman, 2004). Within T cells, activation-induced mono-ubiquitination of TCRζ subunits by the E3 ubiquitin protein ligase Cbl is thought to drive internalization of the TCR via the endosomal pathway and its subsequent degradation within the lysosome (Naramura et al., 2002; Geisler, 2004). Current evidence also suggests that Cbl-mediated ubiquitination plays a role in down-modulating the signaling molecules Lck, Zap-70, PI3 kinase, LAT, and Vav (Rao et al, 2002; Thien and Langdon, 2005; Fang and Liu, 2001; Balagopalan et al., 2007; Miura-Shimura et al., 2003). Other ubiquitin ligases, the two most well characterized being Grail and Itch, are thought to target other intracellular targets (Mueller, 2004). Thus, protein dephosphorylation and protein ubiquitination are two important mechanisms involved in attenuating TCR-induced signals in order to ensure an appropriate and balanced immune response. The importance of these mechanisms is highlighted by numerous mouse genetic models demonstrating that ablation of important regulatory components involved in protein dephosphorylation and protein ubiquitination often leads to severe autoimmunity (Mustelin et al., 2005; Lin and Mak, 2007).
Two proteins, Sts-1 and Sts-2, belonging to the Suppressor of TCR Signaling family have been identified as negative regulators of TCR signaling (Carpino et al., 2004). Family members are characterized by a unique tripartite structure comprised of an N-terminal UBA (Ubiquitin-association) domain that can bind ubiquitin, a central SH3 (Src homology 3) domain that can participate in protein-protein interactions, and a C-terminal PGM (phosphoglycerate mutase) — homology domain. The PGM motif is found in a group of structurally related enzymes that are known to act as phosphatases or phosphotransferases (Jedrzejas, 2000; Rigden, 2008). Recently, the PGM domain of Sts-1 was demonstrated to have robust tyrosine phosphatase activity that could target numerous proteins, including Zap-70, Syk, and the EGF receptor (Mikhailik et al., 2007; Raguz et al, 2007; Agrawal et al., 2008). Consistent with the notion that the Sts proteins function in part as phosphatases, naïve peripheral T cells isolated from mice engineered to lack both Sts-1 and Sts-2 (Sts-1/2-/-) display hyper-phosphorylation of key signaling molecules following TCR stimulation (Carpino et al., 2004). The identity of additional in vivo Sts substrates remains to be determined.
In this report, we identify and characterize hyper-phosphorylated, ubiquitinated proteins in Sts-1/2-/- stimulated T cells. Our results illustrate a novel biochemical defect in cells lacking the Sts proteins and provide an important clue into the Sts mechanism of action by suggesting potential Sts targets.
2. Materials and Methods
2.1 Mice
The generation of mixed background (Bl/6 × Sv129) Sts-1/2-/- mice has been described elsewhere (Carpino et al., 2004). Wild-type and Sts-1/2-/- mice were housed and bred under specific pathogen-free conditions according to institutional guidelines. Animal work was conducted under guidelines established and approved by the Stony Brook Institutional Animal Care and Use Committee (IACUC).
2.2 Cell culture
T cells were obtained from spleens of wild-type and Sts-1/2-/- mice. To generate activated T cells, dissected spleens were crushed in PBS containing 2% FBS, red blood cells were lysed by addition of ACK lysis buffer (pH 7.2) containing 150 mM NH4Cl, 1 mM KHCO3, and 0.1 mM EDTA, and debris was removed by straining through a 70 uM filter (Becton Dickinson). Total splenocyte cultures were established, and T cells were activated and expanded for two days in T cell medium (RPMI media containing 10% FCS, 10 mM HEPES (pH 7.0), 2 mM glutamine, 1 mM sodium pyruvate, 50 uM b-mercaptoethanol, 0.1 mM nonessential amino acids and the appropriate concentration of penicillin/streptomycin) supplemented with 0.5 μg/ml anti-TCR antibody 145-2C11 (BD Biosciences) and 10 U/ml human IL-2 (Peprotech, Inc.). Then, T cells cultures were further expanded for two more days in fresh T cell media containing IL-2, collected by centrifugation, and utilized for biochemical or proliferation analyses.
2.3. Cell purification
To obtain naïve T cells (>90% pure), negative selection (utilizing a cocktail of PE-conjugated anti-B220, anti-Gr-1, anti-CD11b, and anti-Ter119) upon an AutoMACs (Miltenyi) was employed. Alternatively, T cells were isolated on a MACs separation column, utilizing CD90.2 microbeads (Miltenyi) to positively select desired cells.
2.4 Proliferation assays/T cell stimulation assays
For proliferation assays, anti-CD3 (145-2C11) diluted in PBS to the indicated concentrations was coated on the bottom of each well of a 96-well plate by incubation for 2 hr at 37°C. Alternatively, IL-2 at the indicated concentration was employed. 105 T cells of the indicated genotypes were placed in individual wells of a round bottom 96 well plate, and each stimulatory condition was conducted in triplicate. Cells were cultured for 24 hours, labeled with 1 μCi/well [3H]-thymidine for 12-14 hours, and harvested with a TOM-TECH Harvester 96 MachIII. Incorporated radioactivity was determined by scintillation counting. To stimulate T cells for immunoprecipitation or pulldown analyses, cells (2 × 107/point) were labeled on ice for 25 min with 20 μg/ml biotin-conjugated anti-CD3 (500A2, BD Biosciences), washed with 2% FBS in PBS, incubated for 20 on ice with 10 ug/ml streptavidin, placed in a 37°C waterbath either for two minutes or for the indicated times, and immediately lysed in cold lysis buffer (see below). Illustrated results are representative of multiple (≥3) assays. T cells were also incubated with Concanavalin A (Sigma) at the indicated concentrations for 10 minutes at 37°C, or with 1 mM pervanadate for 5 minutes at 37°C.
2.5. Antibodies and reagents
For T cell stimulation, anti-CD3e (purified 145-2C11 or biotinylated 500A2), biotinylated anti-CD4 (RM4-5), and biotinylated anti-CD8 (53-6.7) were employed. Anti-pTyr mAb 4G10 (Millipore), anti-ubiquitin mAb (Zymed), anti-Zap (mAb 1E7.2 and rabbit polyclonal LR, Santa Cruz Biotechnology), anti-Erk1/2 mAb (3A7) and anti-phospho-p42/p44 MAPK rabbit polyclonal (Cell Signaling Technologies), and anti-actin mAb (H11, Applied Biological Materials, Inc.) were also utilized. Concanavalin A was from Sigma and a 100X pervanadate stock was generated by mixing 100 mM H2O2 with 100 mM Na2VO4. For immunoblotting analysis, secondary antibodies were Alexa Fluor® 680-conjugated goat anti-mouse (Molecular Probes) and IRDye800-conjugated goat anti-rabbit secondary antibodies (LI-COR). IL-2 cytokine was purchased from R&D Systems or Peprotech, Inc.
2.6 Immunoprecipitations, pulldown analysis, and immunoblots
For immunoprecipitations, cells were lysed in ice-cold lysis buffer containing 50 mM Tris, pH 7.6, 150 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1% NP-40 substitute Igepal™ CA-630 (USB Corp.), 100 uM PMSF, and Complete™ protease inhibitors (Roche). Cell lysates were clarified by centrifugation, rotated at 4°C with specific antibody and 20 μl Protein A Sepharose 50% slurry (Sigma) for 1-2 hr. Beads were washed three times with cold wash buffer (20 mM Tris, pH7.6, 150 mM NaCl, 1 mM EDTA, 0.1% NP-40 substitute, 100 uM PMSF and 1 mM Na3VO4, and bound proteins were eluted with Laemmli sample buffer and separated by SDS-PAGE. Proteins were transferred to either nitrocellulose (Whatman) or PVDF (Millipore) using a semidry transfer apparatus (BioRad), blocked with 3% BSA in Tris-buffered saline (TBS, pH 8.0), incubated at 23°C for 1 hour or 4°C overnight with specific antibody, washed with TBS, incubated for 1 hr with the appropriate secondary antibody and developed with the ODYSSEY Infrared Imaging System (LI-COR). To conduct ubiquitin pulldown analysis, a ubiquitinated protein enrichment kit (Calbiochem) was utilized according to the manufacturer’s instructions.
3. Results
3.1 Hyper-responsiveness of activated Sts-1/2-/- T cells
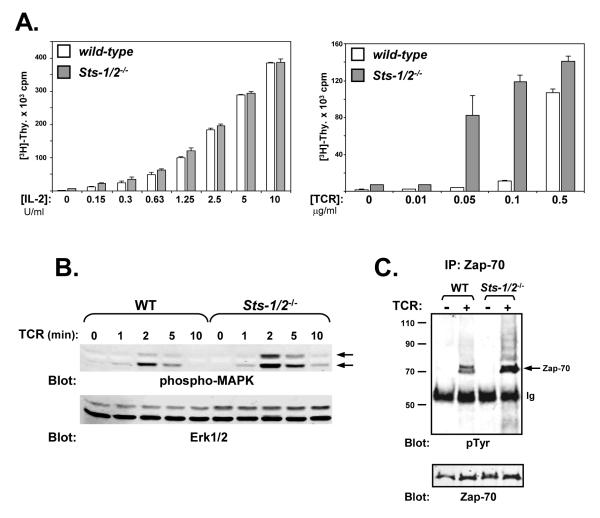
After naïve T cells are activated by antigen, they respond by proliferating, differentiating, and adopting various effector functions. Activated, effector T cells exhibit many different properties than naïve T cells (Farber et al., 1997; Iezzi et al., 1998; Krishnan et al., 2003; Chandok and Farber, 2003). Although the Sts proteins have been demonstrated to act as negative regulators of TCR signaling in naïve peripheral T cells, their functional role in activated T cells has not been evaluated. Therefore, to further characterize the role of the Sts proteins in regulating signaling pathways downstream of the TCR, we compared the response of activated wild-type and Sts-1/2-/- T cells. Splenic T cells were isolated, treated with a TCR stimulatory antibody and allowed to expand in the presence of IL-2 for several days. These culture conditions preferentially favor the expansion of activated CD8+ T cells (Moriggl et al., 1999). The out-growth of wild-type and Sts-1/2-/- CD8+ T cells under these culture conditions was indistinguishable, as was the cellular CD4+:CD8+ ratio following 4 days of expansion (data not shown). Indeed, when the proliferative response to IL-2 was examined, wild-type and Sts-1/2-/- activated T cells displayed identical responses (Figure 1A, left panel). In contrast, when these cells were stimulated with TCR antibody, cells lacking the Sts proteins demonstrated a marked hyper-sensitivity when compared with wild-type cells (Figure 1A, right panel). This increased responsiveness of activated Sts-1/2-/- T cells was most evident at low doses of TCR stimulation (0.01-0.1 ug/ml plate-bound anti-TCR), a result that mirrors the response of naïve Sts-1/2-/- T cells (Carpino et al., 2004). The observed hyper-proliferative response is accompanied by increased levels of MAPK activation. (Figure 1B).
Figure 1. Hyper-responsiveness of Sts-1/2-/- activated T cells to TCR engagement.
(A) The response of wild-type and Sts-1/2-/- activated T cells to either IL-2 or TCR stimulation was evaluated by [3H]-thymidine incorporation assay. Sts-1/2-/- cells display a hyper-proliferative phenotype in response to TCR stimulation. Each assay was conducted in triplicate, and data representative of over three separate experiments are displayed.
(B) Elevated MAPK activation in Sts-1/2-/- activated, cultured T cells. Stimulated T cells were lysed at the indicated time points. Whole-cell lysates were separated by SDS-PAGE and subjected to Western analysis with anti-phospho-MAPK antibodies (pMAPK indicated with arrows). The same blot was reprobed with anti-Erk1/2 antibodies, as a loading control (see lower blot).
(C) Hyper-phosphorylation of Zap-70 in Sts-1/2-/- activated T cells. Cells were stimulated for two minutes, lysed, and processed for IP/Western analysis. Half of the precipitated Zap-70 was assessed for levels of tyrosine phosphorylation and half was assessed for levels of Zap-70. Arrow indicates tyrosine phosphorylated Zap-70. This data is representative of over five experiments.
Zap-70 is a tyrosine kinase that plays a critical role in signaling pathways downstream of the TCR (Negishi et al., 1995). In naïve T cells that lack the Sts proteins, Zap-70 is hyper-activated following TCR stimulation, as evidenced by increased phospho-tyrosine content and increased in vitro kinase activity (Carpino et al., 2004). This suggests a role for Sts-1 and -2 in modulating Zap-70 activity downstream of the TCR within naïve T cells. To examine whether Zap-70 is regulated by the Sts proteins in activated T cells, its state of tyrosine phosphorylation following engagement of the TCR was examined in wild-type and Sts-1/2-/- activated T cells. An increase in Zap-70 stimulation-induced tyrosine phosphorylation was consistently observed in cells lacking the Sts proteins (Figure 1C). Increased tyrosine phosphorylation of additional higher molecular weight forms of Zap-70 that correspond to ubiquitinated Zap-70 was also observed in activated Sts-1/2-/- T cells, a phenotype that has been noted in naïve Sts-1/2-/- T cells (Carpino et al., 2004).
3.2 Tyrosine phosphorylated, ubiquitinated proteins evident in Sts-1/2-/- T cells
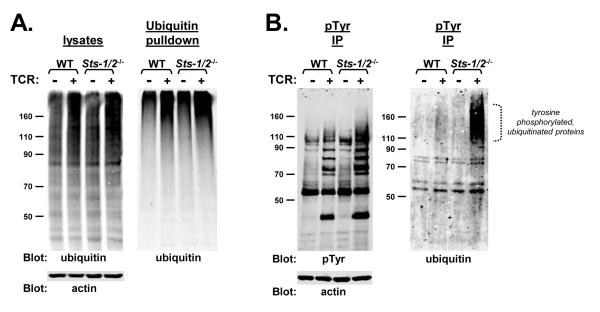
The results described above suggest a role for the Sts proteins regulating the intensity and/or duration of proximal TCR signaling in activated T cells. These latter signaling events are controlled in part by the post-translational modifications of tyrosine phosphorylation and ubiquitination. To investigate further the biochemical effects of Sts deficiency in T cells, wild-type and Sts-1/2-/- activated T cells were stimulated with anti-TCR antibodies and levels post-translational modifications were examined. Levels of intracellular poly-ubiquitination appeared similar, as assessed by anti-ubiquitin western analysis of whole-cell lysates (Figure 2A, left) and by examining levels of poly-ubiquitinated proteins captured by ubiquitin affinity chromatography (Figure 2A, right) (Chen and Madura, 2002). However, a striking increase in the level of ubiquitination on tyrosine phosphorylated proteins isolated from Sts-1/2-/- cells was observed (Figure 2B, right). The dually modified tyrosine phosphorylated, ubiquitinated protein(s) isolated from Sts-1/2-/- T cells migrated by SDS-PAGE in a broad range of molecular weights (see bracket, Figure 2B). This migration pattern may be indicative of additional post-translational modifications, such as glycosylation. Another feature of the dually modified proteins was that they appeared to migrate significantly faster by SDS-PAGE than the majority of poly-ubiquitinated species. Specifically, the broad migration pattern noted above centered somewhat near the 160 kDa molecular weight marker whereas the majority of poly-ubiquitinated proteins were retained near the top of the gel (compare Figure 2A with 2B). This suggests that the nature of the ubiquitin modification (mono, multi, or chains of poly-ubiquitin) on the tyrosine phosphorylated proteins might be different than that of the majority of poly-ubiquitinated proteins.
Figure 2. Stimulation — induced ubiquitination in wild-type and Sts-1/2-/- activated T cells.
(A) Wild-type and Sts-1/2-/- cultured T cells demonstrate similar levels of activation-induced ubiquitination. Resting (−) or TCR-stimulated (+) cells were lysed and levels of ubiquitination were assessed by western analysis. Total lysates (left panel) or proteins binding to a poly-ubiquitin affinity column (right panel) were separated by SDS-PAGE and ubiquitin levels were assessed by anti-ubiquitin western analysis.
(B) Tyrosine phosphorylated proteins in resting or TCR-stimulated activated T cells were isolated by immuno-precipitation. The pool of precipitated proteins was split in two, with half analyzed by anti-pTyr western analysis and half analyzed by anti-ubiquitin western analysis. An anti-actin immunoblot serves as a loading control. The dotted bracket indicates increased levels of proteins dually modified by tyrosine phosphorylation and ubiquitination that appear in stimulated Sts-1/2-/- activated T cells.
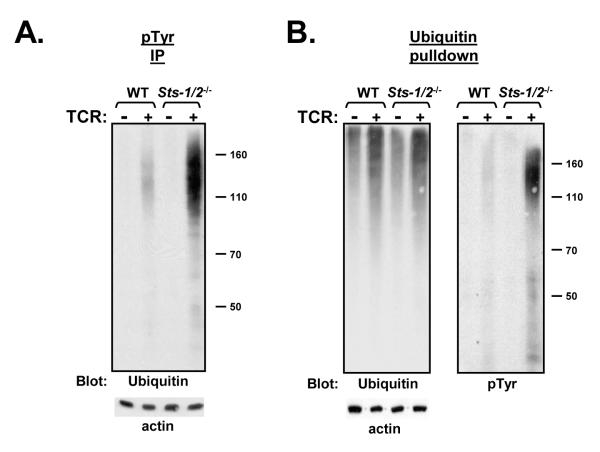
Freshly isolated naïve Sts-1/2-/- T cells were found to exhibit the same stimulation-induced accumulation of proteins that were dually modified by tyrosine phosphorylation and ubiquitination. Specifically, there was a significant increase in the presence of ubiquitinated proteins in phospho-tyrosine immunocomplexes isolated from lysates of stimulated naïve Sts-1/2-/- T cells (Figure 3A). To further explore the presence of the dually modified proteins in stimulated naïve Sts-1/2-/- T cells, ubiquitinated proteins were isolated by ubiquitin affinity pulldown (Chen and Madura, 2002) and subjected to immunoblot analysis. Steady state levels of precipitated ubiquitinated proteins in unstimulated cells were found to be comparable, as was the extent of stimulation-induced poly-ubiquitination. However, consistent with our previous observations, a subset of ubiquitinated proteins within stimulated naïve Sts-1/2-/- T cells was found to be significantly hyper-phosphorylated (Figure 3B). As noted previously, the hyper-phosphorylated ubiquitinated proteins migrated faster than the general population of ubiquitinated proteins by SDS-PAGE.. Overall, the results illustrated in Figures 2 and 3 confirm that the loss of the Sts proteins results in a similar phenotype in both naïve and activated T cells, namely a TCR-dependent increase in phosphorylation upon a subset of ubiquitinated proteins
Figure 3. Appearance of hyper-phosphorylated, ubiquitinated proteins in Sts-1/2-/- naïve T cells.
(A) Freshly isolated wild-type and Sts-1/2-/- splenic T cells were stimulated through the TCR, phosphorylated proteins were isolated by anti-pTyr immunoprecipitation and levels of ubiquitination were assessed by anti-ubiquitin western analysis. An anti-actin immunoblot demonstrates equivalent levels of protein in each lysates.
(B) Dually modified proteins in stimulated Sts-1/2-/- naïve T cells. Poly-ubiquitinated proteins from lysates of resting or stimulated naïve T cells were isolated by poly-ubiquitin affinity pull-down analysis. Levels of ubiquitinated proteins were assessed by anti-ubiquitin western analysis (left panel), following which the gel was stripped and reprobed with anti-pTyr antibodies (right panel). An anti-actin immunoblot was used as a loading control.
3.3 Appearance of dually modified proteins is transient
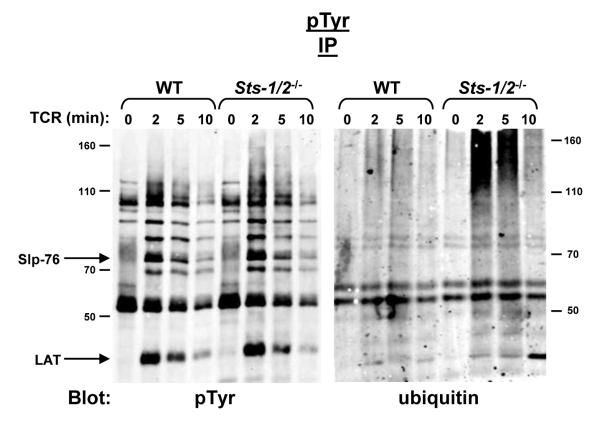
Having identified the hyper-phosphorylation of a subset of ubiquitinated proteins within Sts-1/2-/- T cells, we then assessed the kinetics of appearance and disappearance of the dually modified proteins. Wild-type and Sts-1/2-/- activated T cells were stimulated for varying lengths of time, tyrosine phosphorylated proteins were isolated by immunoprecipitation, and levels of ubiquitination were assessed by anti-ubiquitin immunoblot analysis. Maximal levels of tyrosine phosphorylated ubiquitinated proteins occurred shortly after TCR activation (ca. two minutes), within a similar timeframe as the appearance of peak levels of tyrosine phosphorylated proteins (see Figure 4). The level of tyrosine phosphorylated ubiquitinated species declined, with similar kinetics as the gradual disappearance of intracellular tyrosine phosphorylation. Thus, the appearance of aberrantly modified proteins in cells lacking the Sts proteins is a transient phenomenon with kinetics that correlate with the appearance and disappearance of tyrosine phosphorylated proteins. Whether their disappearance results from additional mechanisms that ensure their dephosphorylation, de-ubiquitination, or degradation is currently unclear.
Figure 4. Transient appearance of hyper-phosphorylated, ubiquitinated proteins in stimulated Sts-1/2-/- T cells.
IP/Western analysis with the indicated antibodies was used to detect the appearance and disappearance of hyper-phosphorylated, ubiquitinated proteins in Sts-1/2-/- cultured T cells along a time-course of TCR stimulation from 0 to 10 minutes.
3.4 Non-specific T cell activators suggest the aberrant modifications are TCR dependent
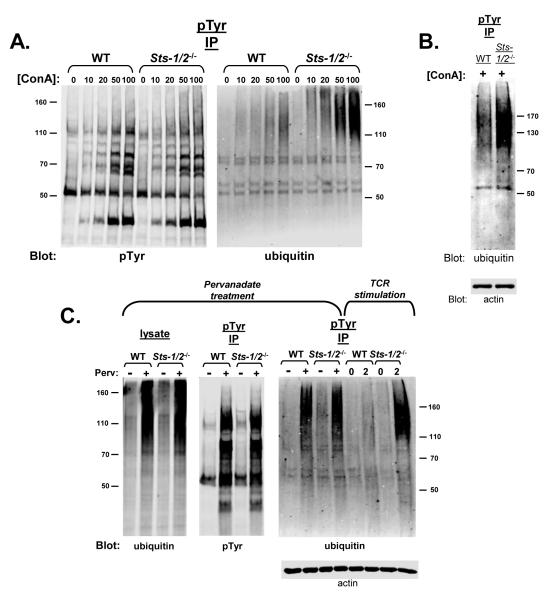
To further investigate the role of Sts-1 and -2 in regulating the balance of protein tyrosine phosphorylation and ubiquitination within T cell signaling pathways, wild-type and Sts-1/2-/- activated T cells were treated with different stimulatory agents. Concanavalin A (Con A) is a lectin mitogenic for T cells that binds cell surface glycoproteins and is thought to indirectly activate the T cell receptor (Chilson and Kelly-Chilson, 1989). Figure 5A, left illustrates the induction of intracellular tyrosine phosphorylation by T cells responding to increasing doses of Con A. Wild-type and Sts-1/2-/- activated T cells demonstrated similar levels of phospho-tyrosine containing proteins following Con A treatment. However, only Sts-1/2-/- cells displayed a clear dose-dependent accumulation of tyrosine phosphorylated, ubiquitinated protein species (Figure 5A, right). A similar accumulation of dually modified proteins was observed in Sts-1/2-/- naïve T cells stimulated with ConA. Thus, the Sts proteins appear to regulate signaling pathways within T cells that are activated by ConA stimulation in a similar fashion as they regulate signaling pathways downstream of the TCR.
Figure 5. Indirect stimulation of T cells suggests the Sts proteins regulate a TCR-dependent pathway.
(A) Wild-type and Sts-1/2-/- cultured T cells were incubated for five minutes with the indicated concentrations (μg/ml) of Concanavalin A, a lectin that binds surface glycoproteins and is thought to indirectly activate the TCR. The appearance of tyrosine phosphorylated, ubiquitinated proteins, as detected by IP/Western analysis with the indicated antibodies, was only evident in Sts-1/2-/- cells.
(B) Wild-type and Sts-1/2-/- purified splenic T cells were incubated for five minutes with 100 ug/ml ConA. IP/Western analysis was utilized to assess the appearance of dually modified proteins in Sts-1/2-/- naïve T cells.
(C) Wild-type and Sts-1/2-/- cultured T cells were treated for five minutes with pervanadate to inactivate intracellular tyrosine phosphatases. An equivalent level of overall treatment-induced protein tyrosine phosphorylation was evident in both samples (left panel). In addition, levels of phosphorylation-dependent ubiquitination were similar (middle panel), as assessed by anti-ubiquitin blotting of cell lysates. Both wild-type and Sts-1/2-/- pervanadate-treated cells displayed robust levels of tyrosine phosphorylated, ubiquitinated proteins (right panel). However, the SDS-PAGE migration pattern of the latter proteins differed significantly from the dually-modified proteins that appear in TCR-stimulated Sts-1/2-/- cells (see right panel), suggesting either the two modified protein populations are different or arise by different signaling mechanisms.
Pervanadate is known to inactivate numerous intracellular protein tyrosine phosphatases, thereby shifting the equilibrium balance of protein tyrosine phosphorylation/dephosphorylation toward tyrosine phosphorylation (Gordon, 1991). Indeed, treatment of T cells with pervanadate has been shown to activate TCR signaling pathways in a manner which bypasses engagement of the TCR (Secrist et al., 1993). Pervanadate treatment of both wild-type and Sts-1/2-/- activated T cells leads to the rapid and equivalent accumulation of proteins altered by either tyrosine phosphorylation or protein ubiquitination (Figure 5B, left and middle panels). However, unlike stimulation of cells with TCR antibodies or with Con A (see above), pervanadate stimulation of both wild type and Sts-1/2-/- mutant cells also induces an equivalent level of intracellular proteins that are dually modified by tyrosine phosphorylation and ubiquitination (Figure 5B, right). Nonetheless, our results indicate that these dually modified proteins differ from those that are inducibly tyrosine phosphorylated and ubiquitinated by direct TCR stimulation in that they migrate slower than the latter during SDS-PAGE analysis. These results suggest that the Sts proteins modulate a signaling pathway(s) uniquely associated with the T cell receptor, and they do so by regulating the post-translational modification state of a specific set of unknown proteins.
3.5 Co-receptor stimulation enhances the appearance of tyrosine phosphorylated, ubiquitinated proteins
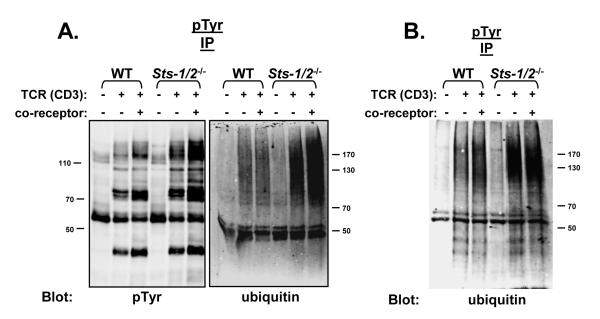
Although not strictly required for production of an antigen-driven immune response, the CD4 and CD8 T cell surface receptors enhance considerably TCR-mediated signaling effects, and are thus known as ‘co-receptors’ (Miceli and Parnes, 1993; Zamoyska, 1998). Src-family kinases are physically associated with the cytoplasmic tail of each co-receptor, and following combined TCR and co-receptor engagement the associated kinases strengthen downstream signals by augmenting intracellular tyrosine phosphorylation (Peri and Veilette, 1994). To investigate the effects of co-receptor stimulation on the appearance of hyper-phosphorylated ubiquitinated proteins, activated T cells were stimulated with a combination of anti-TCR and anti-CD8 antibodies. In both wild-type and Sts-1/2-/- T cells, simultaneous TCR and co-receptor engagement led to enhanced levels of tyrosine phosphorylation compared to that produced by TCR engagement alone (Figure 6A, left). Simultaneous TCR and co-receptor engagement of activated Sts-1/2-/- T cells also led to significantly enhanced levels of dually modified proteins (Figure 6A, right). This was not observed in wild-type cells, despite the co-receptor-induced increase in intracellular tyrosine phosphorylation. In contrast to activated Sts-1/2-/- T cells, naïve Sts-1/2-/- T cells lacking the Sts proteins did not display a significant degree of co-receptor — induced enhancement of the level of dually modified proteins compared to that observed following TCR stimulation alone (Figure 6B). This suggests differential regulation of a co-receptor - dependent signaling pathway in naïve vs. activated T cells.
Figure 6. Regulation of a co-receptor — dependent pathway by the Sts proteins.
(A) Co-receptor engagement stimulates increased levels of dually modified proteins in Sts-1/2-/- activated T cells. Wild-type and Sts-1/2-/- cultured T cells were stimulated with anti-TCR antibody or anti-TCR antibody plus antibodies to the CD8 co-receptor, as indicated. The level of intracellular tyrosine phosphorylation proteins and the level of dually modified proteins were assessed by IP/western analysis with the indicated antibodies.
(B) Co-receptor engagement has little effect on the level of dually modified protein in naïve cells. Wild-type and Sts-1/2-/- purified splenic T cells were stimulated with anti-TCR antibody or anti-TCR antibody plus antibodies to the CD4 and CD8 co-receptors. Levels of tyrosine phosphorylated, ubiquitinated proteins were assessed by IP/western analysis with the indicated antibodies.
4. Discussion
Our current results identify a population of TCR-induced phosphorylated, ubiquitinated proteins that might be downstream targets of Sts function. The transient accumulation in Sts-1/2-/- T cells of protein species that are tyrosine phosphorylated and ubiquitinated stands in glaring contrast to wild-type T cells, in which few, if any, dually modified proteins are observed. These results are consistent with earlier observations implicating the Sts proteins in the regulation of tyrosine phosphorylation levels within T cells. Previously, it was demonstrated that the Zap-70 tyrosine kinase was hyper-activated in Sts-1/2-/- T cells and that Sts-1 was a likely candidate phosphatase involved in Zap-70 dephosphorylation (Carpino et al., 2004; Mikhailik et al., 2007). Interestingly, the Zap-70 hyper-phosphorylation defect in Sts-1/2-/- T cells includes both unmodified Zap-70 as well as its ubiquitinated forms, a phenotype that is not seen with other putative negative regulators of Zap-70 activity such as Shp1 (Plas et al., 1996).
These results draw attention to the complex series of post-translational modifications that occur among signaling proteins downstream of the TCR. Rapid TCR-induced tyrosine phosphorylation is known to be an important mechanism underlying the control of T cell activation (Singer and Koretzky, 2002). Tyrosine phosphorylation directly activates T cell kinases such as Zap-70 and other key signaling enzymes, and also serves an important role in promoting the formation of multi-molecular complexes necessary for signal propagation. In contrast to tyrosine phosphorylation, protein ubiquitination triggers a different set of intracellular responses. Among other functions, ubiquitination is known to ensure degradation of key signaling molecules and to direct other cellular processes such as internalization, down-modulation, and trafficking (Hicke et al., 2005). In recent years, it has become clear that ubiquitination is an important regulatory mechanism involved in many aspects of TCR signaling (Liu, 2004; Mueller, 2004). For example, the immunological synapse has been identified as a site of substantial enrichment for both tyrosine phosphorylation and ubiquitin (Wiedemann et al., 2005). This finding is consistent with the identification of the T cell receptor as a substrate for ubiquitin ligases (Cienciarelli et al., 1992; Hou et al., 1994; Wang et al., 2001). Not unexpectedly, there are instances when tyrosine phosphorylation and ubiquitination are utilized in a cooperative fashion to achieve the appropriate signaling outcome. For example, in addition to the various activating phosphorylation sites on Zap-70, phospho-Tyr-292 has been demonstrated to be a negative regulatory signal that recruits the ubiquitin ligase Cbl into the activated TCR complex (Kong et al., 1996). Cbl is thought to promote the ubiquitination of Zap-70 and other components of the proximal TCR signalosome, thereby contributing to the down-regulation of TCR signaling (Jang and Gu, 2003). Thus, protein tyrosine phosphorylation and protein ubiquitination are key regulatory mechanisms involved in modulating the various components of T cell signaling pathways.
An intriguing question yet to be resolved is whether the observed biochemical defect is not only correlated with the hyper-sensitive phenotype of Sts-1/2-/- T cells, but actually plays a causative role in their hyper-responsiveness. If this were the case, it would suggest that simultaneous modification by tyrosine phosphorylation and ubiquitination might have specific functional consequences. For example, prolonged phosphorylation of a tyrosine residue adjacent to a site of mono-ubiquitination that signals internalization and lysosomal-dependent degradation might inhibit the recruitment of ubiquitin-interacting factors that initiate the trafficking process. In such a scenario, ubiquitin-driven down-modulation in cells lacking the Sts proteins would be inhibited, thereby resulting in enhanced signaling. Conversely, a ubiquitin modification occurring adjacent to an activating tyrosine phosphorylation site might restrict accessibility to the pTyr site by negative regulatory proteins phosphatases. In this latter scenario, down-regulation would also be inhibited. Addressing these mechanistic possibilities, as well as the identification and subsequent analysis of the dually modified protein substrates evident in Sts-1/2-/- T cells, is a focus of on-going research.
5. Conclusion
T cells that lack the Sts proteins display an aberrant accumulation of tyrosine phosphorylated, ubiquitinated proteins following engagement of the TCR. Co-receptor engagement significantly enhances the phenotype in mutant T cells. These results suggest that the Sts proteins either directly or indirectly target tyrosine phosphorylated, ubiquitinated proteins within signaling pathways downstream of the TCR.
6. Acknowledgments
This work was supported by Stony Brook University, grants to NC from the Arthritis Foundation (LI07), NIH-NIAID (R21AI075176), and The National Multiple Sclerosis Society through a Collaborative MS Research Center Award (CA1044A1); and grants to NN from NIH (CA-115611) and DOD (NF060060). The authors would like to thank Laurie Levine for assistance with animal care. We also thank Jorge Benach for unfailing support, Jim Ihle for support during the very early stages of this project, Ando van der Velden and Joanna Luke for assistance with naïve T cell purification strategies, and N. Reich, and M. Hayman for helpful discussions and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Acuto O, Bartolo VD, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat. Rev. Immunol. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- Agrawal R, Carpino N, Tsygankov A. TULA proteins regulate activity of the protein tyrosine kinase Syk. J. Cell. Biochem. 2008;104:953–964. doi: 10.1002/jcb.21678. [DOI] [PubMed] [Google Scholar]
- Balagopalan L, Barr VA, Sommers CL, Barda-Saad M, Goyal A, Isakowitz MS, Samelson LE. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol. Cell. Biol. 2007;27:8622–8636. doi: 10.1128/MCB.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpino N, Turner S, Mekala D, Takahashi Y, Zang H, Geiger TL, Doherty P, Ihle JN. Regulation of Zap-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20:37–46. doi: 10.1016/s1074-7613(03)00351-0. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, Hou D, Hsu KC, Rellahan BL, Wiest DL, Smith HT, Fried VA, Weissman AM. Activation-induced ubiquitination of the T cell antigen receptor. Science. 1992;257:795–797. doi: 10.1126/science.1323144. [DOI] [PubMed] [Google Scholar]
- Chandok MR, Farber DL. Signaling control of memory T cell generation and function. Sem. in Immunol. 2003;16:285–293. doi: 10.1016/j.smim.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Chen L, Madura K. Rad23 promotes the targeting of proteolytic substrates to the proteosome. Mol. Cell. Biol. 2002;22:4902–4913. doi: 10.1128/MCB.22.13.4902-4913.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilson OP, Kelly-Chilson AE. Mitogenic lectins bind to the antigen receptor on human lymphocytes. Eur. J. Immunol. 1989;19:389–396. doi: 10.1002/eji.1830190225. [DOI] [PubMed] [Google Scholar]
- Chu DH, Morita CT, Weiss A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol. Rev. 1998;165:167–180. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- Fang D, Liu Y-C. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat. Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- Farber DL, Acuto O, Bottomly K. Differential T cell receptor-mediated signaling in naïve and memory CD4 T cells. Eur. J. Immunol. 1997;27:2094–2101. doi: 10.1002/eji.1830270838. [DOI] [PubMed] [Google Scholar]
- Geisler C. TCR trafficking in resting and stimulated T cells. Crit. Rev. Immunol. 2004;24:67–86. doi: 10.1615/critrevimmunol.v24.i1.30. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat. Rev Mol. Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- Hou D, Cenciarelli C, Jensen JP, Nguygen HB, Weissman AM. Activation-dependent ubiquitination of a T cell antigen receptor subunit on multiple intracellular lysines. J. Biol. Chem. 1994;269:14244–14247. [PubMed] [Google Scholar]
- Huang Y, Wange RL. T cell receptor signaling: beyond complex complexes. J Biol. Chem. 2004;279:28827–28830. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naïve and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Iwashima M. Kinetic perspectives of T cell antigen receptor signaling. A two-tier model for T cell full activation. Immunological Rev. 2003;191:196–210. doi: 10.1034/j.1600-065x.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- Jang I-K, Gu H. Negative regulation of TCR signaling and T-cell activation by selective protein degradation. Curr. Opin. in Immunol. 2003;15:315–320. doi: 10.1016/s0952-7915(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Jedrzejas MJ. Structure, function, and evolution of phosphoglycerate mutases: comparison with fructose-2,6-bisphosphatase, acid phosphatase, and alkaline phosphatase. Prog. in Biophy. and Mol. Biol. 2000;73:263–287. doi: 10.1016/s0079-6107(00)00007-9. [DOI] [PubMed] [Google Scholar]
- Kong G, Dalton M, Wardenburg JB, Straus D, Kurosaki T, Chan AC. Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol. Cell. Biol. 1996;16:5026–5035. doi: 10.1128/mcb.16.9.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Farber DL, Tsokos GC. T cell rewiring in differentiation and disease. J. Immunol. 2003;171:3325–3331. doi: 10.4049/jimmunol.171.7.3325. [DOI] [PubMed] [Google Scholar]
- Latour S, Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr. Opin. Immunol. 2001;13:299–306. doi: 10.1016/s0952-7915(00)00219-3. [DOI] [PubMed] [Google Scholar]
- Lin AE, Mak TW. The role of E3 ligases in autoimmunity and the regulation of autoreactive T cells. Curr. Opin. in Immunol. 2007;19:665–673. doi: 10.1016/j.coi.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Liu Y-C. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 2004;22:81–127. doi: 10.1146/annurev.immunol.22.012703.104813. [DOI] [PubMed] [Google Scholar]
- Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- Miceli MC, Parnes JR. Role of CD4 and CD8 in T cell activation and differentiation. Adv. Immunol. 1993;53:59–122. doi: 10.1016/s0065-2776(08)60498-8. [DOI] [PubMed] [Google Scholar]
- Mikhailik A, Ford B, Keller J, Chen Y, Nassar N, Carpino N. A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling. Mol. Cell. 2007;27:486–497. doi: 10.1016/j.molcel.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura-Shimura Y, Duan L, Rao NL, Reddi AL, Shimura H, Rotapel B, Druker BJ, Tsygankov A, Band V, Band H. Cbl-mediated ubiquitinylation and negative regulation of Vav. J. Biol. Chem. 2003;278:38495–38504. doi: 10.1074/jbc.M305656200. [DOI] [PubMed] [Google Scholar]
- Moriggl R, Topham DJ, Teglund S, Sexl C, McKay D, Wang D, Hoffmeyer A, van Deursen J, Sangster MY, Bunting KD, Grosveld GC, Ihle JN. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nature Immunol. 2004;9:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nature Rev. Immun. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- Naramura M, Jang I-K, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nature Imm. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- Negishi I, Motoyama N, Nakayama K, Nakayama K, Senju S, Hatkeyama S, Zhang Q, Chan AC, Loh DY. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu. Rev. Immunol. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- Peri KG, Veillette A. Tyrosine protein kinases in T lymphocytes. Chem. Immunol. 1994;59:19–39. [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, Thomas ML. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- Raguz J, Wagner S, Dikic I, Hoeller D. Suppressor of T-cell receptor signaling 1 and 2 differentially regulate endocytosis and signaling of receptor tyrosine kinases. FEBS Letters. 2007;581:4767–4772. doi: 10.1016/j.febslet.2007.08.077. [DOI] [PubMed] [Google Scholar]
- Rao N, Miyake S, Reddi AL, Bouillard P, Ghosh AK, Dodge IL, Zhou P, Fernandes ND, Band H. Negative regulation of Lck by Cbl ubiquitin ligase. Proc. Natl. Acad. Sci. USA. 2002;99:3794–3799. doi: 10.1073/pnas.062055999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigden DJ. The histidine phosphatase superfamily: structure and function. Biochem. J. 2008;409:333–348. doi: 10.1042/BJ20071097. [DOI] [PubMed] [Google Scholar]
- Samelson LE. Signal transduction mediated by the T cell receptor: the role of adapter proteins. Ann. Rev. Immunol. 2002;20:371–395. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- Secrist JP, Burns LA, Karnitz L, Koretzky GA, Abraham RT. Stimulatory effects of protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J. Biol. Chem. 1993;268:5886–5893. [PubMed] [Google Scholar]
- Singer AL, Koretzky GA. Control of T cell function by positive and negative regulators. Science. 2002;296:1639–1640. doi: 10.1126/science.1071551. [DOI] [PubMed] [Google Scholar]
- Thien CB, Langdon WY. Negative regulation of PTK signaling by Cbl proteins. Growth Factors. 2005;23:161–167. doi: 10.1080/08977190500153763. [DOI] [PubMed] [Google Scholar]
- Wang H-Y, Altman Y, Fang D, Elly C, Dai Y, Shao Y, Liu Y-C. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J. Biol. Chem. 2001;276:26004–26011. doi: 10.1074/jbc.M010738200. [DOI] [PubMed] [Google Scholar]
- Wange RL. TCR signaling: another Abl-bodied kinase joins the cascade. Current Biology. 2004;14:R562–R564. doi: 10.1016/j.cub.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Wiedemann A, Muller S, Favier B, Penna D, Martine G, Delmas C, Champagne E, Valitutti S. T-cell activation is accompanied by an ubiquitination process occurring at the immunological synapse. Immunol. Lett. 2005;98:57–61. doi: 10.1016/j.imlet.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Zamoyska R. CD4 and CD8: modulators of T-cell receptor recognition of antigen and of immune responses? Curr. Opin. Immunol. 1998;10:82–87. doi: 10.1016/s0952-7915(98)80036-8. [DOI] [PubMed] [Google Scholar]