Abstract
Nonalcoholic fatty liver disease (NAFLD) has emerged as the leading cause of chronic liver disease in children and adolescents in the United States. A two- to three-fold rise in the rates of obesity and overweight in children over the last 2 decades is probably responsible for the epidemic of NAFLD. Emerging data suggest that children with NASH progress to cirrhosis which may ultimately increase liver-related mortality. More worrisome is the recognition that cardiovascular risk and morbidity in children and adolescents is associated with fatty liver. Pediatric fatty liver disease often displays a histologic pattern distinct from that found in adults. Liver biopsy remains the gold standard for diagnosis of NASH. Non-invasive biomarkers are needed to identify individuals with progressive liver injury. Targeted therapies to improve liver histology and metabolic abnormalities associated with fatty liver are needed. Currently, randomized-controlled trials are underway in the pediatric population to define pharmacologic therapy for NAFLD. Public health awareness and intervention are needed to promote healthy diet, exercise, and lifestyle modifications to prevent and reduce the burden of disease in the community.
Keywords: Nonalcoholic steatohepatitis, hepatic steatosis, systematic review, expert opinion, clinical trial, natural history of disease, pooled analysis, treatment end-points, fatty liver disease, fibrosis, serum aminotransferase levels, liver histology and histologic scoring systems
Introduction
Nonalcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease in children and adolescents in the United States (1). Over the last 2 decades, the rise in the prevalence rates of overweight and obesity likely explains the emergence of NAFLD as the leading cause of liver disease in pediatric populations worldwide (2, 3). Based upon the National Health and Nutrition Examination Survey (NHANES) Data, 17% of all children are overweight in the United States and among the age group of 2-19 years, adolescents have the highest rates of overweight (4).
Reflecting the extreme increase in obesity among adolescents, this age group is also the most vulnerable for developing NAFLD. Pediatric NAFLD has several distinct pathologic characteristics that are rarely seen in adult NAFLD (5). Whether these differences in liver histopathology between pediatric and adult NAFLD are due to differences in pathogenesis or represent two phenotypes in children that evolve into a typical adult pattern NAFLD over a period of time remains unclear. Better understanding of the pathogenesis and natural history of pediatric NAFLD should improve our understanding of genetic and environmental basis of NAFLD for all age groups. In addition, earlier recognition of NAFLD may help clinicians intervene at a younger age that may reduce future morbidity and mortality from NAFLD.
Despite several advances, there are limited data on the epidemiology, natural history, etiopathogenesis and treatment of pediatric NAFLD. This review article outlines the current understanding, recent advances and challenges surrounding pediatric NAFLD for both researchers and clinicians.
Definitions and Epidemiology
NAFLD is a clinico-pathologic entity defined as presence of hepatic steatosis in individuals who drink little or no alcohol and represents a spectrum of liver disease ranging from bland steatosis to nonalcoholic steatohepatitis (NASH), which is a progressive form of liver disease that may lead to advanced fibrosis, cirrhosis and hepatocellular carcinoma in a subset of affected individuals. NASH is characterized by macrovesicular steatosis (the fat globules vary in size from very small to nearly filling the hepatocyte), ballooning degeneration with or without Mallory bodies, with lobular or portal inflammation, with or without fibrosis (6). Diagnosis of NASH requires a liver biopsy and careful histologic examination by an expert pathologist, in an appropriate clinical context. In the section entitled “Histology”, key histologic features and available histologic scoring systems that may be helpful in diagnosing NASH will be detailed.
Prevalence
Pediatric NAFLD extends beyond North America according to centers in Europe Asia, South America and Australia (7-9). Although the prevalence of pediatric NAFLD is difficult to determine accurately, several epidemiologic studies used surrogate markers of NAFLD to estimate prevalence. Table 1 provides a summary of recent prevalence studies. Data derived from NHANES III (1988-1994) suggests that approximately 3% of adolescents have elevated serum ALT (ALT>30U/L) (10). Data derived from the Korean National Health and Nutrition Examination Survey conducted in 1998 showed that prevalence of elevated ALT was similar (3.2%) in their cohort (8). A study conducted in Japanese children, in 1989, using liver ultrasound as an alternative surrogate marker showed that prevalence of diffusely echogenic liver was 2.6% (11). Therefore, based upon above data it is reasonable to assume that prevalence of NAFLD in adolescents is at least 2.6-3.2 % because both elevated serum ALT and liver ultrasound underestimate the prevalence of NAFLD. The prevalence estimates vary based upon the type of test (and the cut-points used to define abnormal) used to assess fatty liver and also the age, sex, race and ethnicity of the population sampled (12, 13). Liver histology is the gold standard for assessing hepatic steatosis.
As it is not appropriate or feasible to perform liver biopsies for prevalence determination, alternative approaches have been utilized. We conducted an autopsy study by reviewing records and liver histology of 742 children between the ages of 2-19 y who died from unnatural causes in San Diego County between 1993 and 2003 (14). Fatty liver was defined as 5% or more of hepatocytes containing macrovesicular fat. The prevalence of fatty liver adjusted for age, gender, race, and ethnicity was estimated to be 9.6%, although the autopsy cohort was understandably biased relative to the general population due to over-representation with older teen Hispanic males. Multivariate analysis showed that older age, male gender, overweight and Hispanic ethnicity were independent predictors of fatty liver in this cohort (14).
Demographic Predictors of NAFLD in Children
Age
Several studies demonstrate that prevalence of fatty liver is higher in adolescents than younger children. Factors potentially explaining the higher rate of NAFLD in adolescents include hormonal changes surrounding puberty or their increased control over unhealthy food choices and sedentary physical activity with age. Hormonal changes during puberty may potentiate accumulation of fat in the liver (15). Potau and colleagues have shown that puberty is associated with an increase in mean serum insulin levels. The rise in serum insulin levels coincides with Tanner stage 2, and these levels are sustained throughout adolescence (16). Moran and colleagues evaluated developmental changes in insulin resistance in 507 youth, 11- 19 years of age, and showed that transition from late childhood to adolescence is associated with an increase in insulin resistance in boys. This is associated with increased levels of serum triglycerides and a decrease in high density lipoproteins, despite a reduction in body fatness, whereas the reverse was found in girls (17).
Sex
NAFLD is more common in boys than girls (12). These sex differences implicate estrogens as potentially protective, or indicate that androgens may aggravate NASH. Estrogen has been shown to be anti-apoptotic and anti-fibrogenic in in vitro studies in various cancer cell lines (18) and in in vivo studies in rats and geese (19). Estrogen, especially 4-hydroxy estrone, reduces lipid peroxidation in lipoproteins or whole plasma by reducing the levels of both lipid (thiobarbituric acid-reactive substances) and cholesterol (oxysterols) oxidation products and thereby leads to reduced reactive oxygen species (20). Lipid peroxidation is one of the major pathways thought to increase the risk of progression from simple steatosis to NASH. It has also been suggested that estrogen may act as an anti-inflammatory agent by directly acting at the level of foam cells in macrophage cell lines, which reduces the risk of atherosclerosis (21).
The role of androgens in hepatic steatosis has also been studied. Hepatic steatosis is seen in male aromatase knockout mice (22). Estrogen treatment of these mice leads to reversal of hepatic steatosis (22). Women who are either deficient in estrogen or have higher androgen levels are at higher risk of NAFLD. It is not known if estrogen replacement in postmenopausal women reduces fatty liver.
Ethnicity
Ethnic disparity in the prevalence of NAFLD has also been consistently seen across studies derived from multi-ethnic patient populations. NAFLD is more common in Mexican Americans than Caucasian Americans (12). It is more common in young adults from Asian-Indian and Asian-American descent possibly due to higher rates of insulin resistance and visceral adiposity at equivalent BMI (23). As well, Asians and Mexican Americans may have higher rates of insulin resistance and visceral adiposity at equivalent BMI predisposing these groups to fatty liver disease (23). Ethnic differences also could relate to several factors including type of diet, exercise choice, socio-economic status and living location. The lowest rates of NAFLD uniformly are seen in African Americans despite the high rates of diabetes in this ethnic group. What guards African Americans from hepatic insulin resistance is an area of intense research. Genetic studies using a multiethnic cohort would be helpful in improving our understanding of factor(s) that protect African Americans.
Liver Pathology
Pediatric NASH can be a challenging diagnosis because ballooning degeneration, classic zone-3 fibrosis and parenchymal inflammation that are commonly seen in adult NASH are less common in children with definite NASH. To better define pediatric NASH, we conducted a study at our center by analyzing 100 consecutive liver biopsies of children with NASH (5). Most children (age range 2 -18 y) were either overweight or obese and 8% were diabetic. Simple steatosis was present in 16% of patients and the rest had NASH (5). Eight percent had advanced fibrosis and 3% had cirrhosis. An agglomerative cluster analysis revealed that two distinct pathologic sub-types were present in these children with NASH. This corroborated that histopathology of NASH in children often differed from that found in adults. Two-subtypes were proposed (Figure 1) (5). Type 1 (resembling an adult-type pattern) NASH was seen in only 17% of children and was characterized by steatosis with ballooning degeneration and lobular inflammation, with or without perisinusoidal fibrosis, and without portal inflammation. Type 2 NASH emerged as the predominant histologic injury pattern in 51% of children (5). Rest of the 32% of patients in this study had a pattern of “overlap” with features common to both the type 1 and 2 NASH pattern. Type 2 NASH was defined by macrovesicular hepatocellular steatosis with portal inflammation, with or without portal fibrosis, and no or minimal ballooning degeneration. Children with type 2 NASH are more likely to be male, younger, heavier and non-White. Kleiner and colleagues conducted a validation study to propose a histologic scoring system for NASH and confirmed the findings that a unique pattern distinct to children (termed borderline zone 1 pattern in that study) was seen in many children (6). They also found that isolated portal fibrosis is four times more likely in children than adults (6). Although these studies along with reports from other centers have strengthened these findings, it remains unclear whether these patterns differ in natural history, etiopathogenesis, prognosis or response to treatments. Some of the key features of type 1 and type 2 NASH are shown in Table 2. Prospective longitudinal cohort studies should address these issues.
Figure 1.
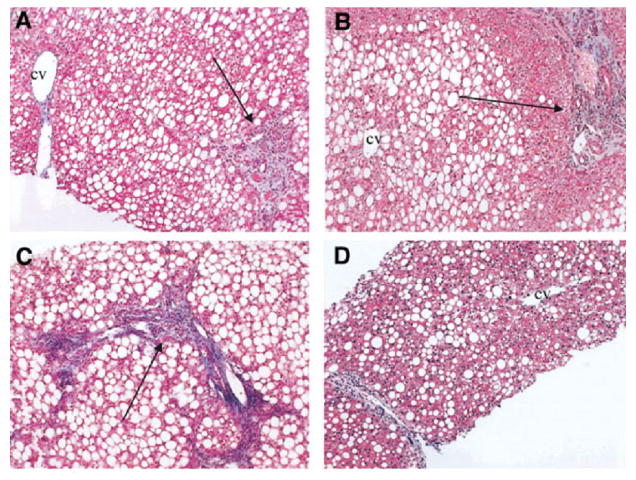
Panel A-D represent typical histologic pattern seen in children with type 2 NASH. Panel A and B shows absence of ballooning degeneration and peri-sinusoidal fibrosis in the presence of steatosis and portal inflammation. Panel C demonstrates portal fibrotic expansion and marked steatosis. Panel D shows peri-portal inflammation without steatosis in zone 1 (around portal tract) and mild portal fibrosis in the absence of any fibrosis in zone 3 (around central vein). Reproduced with permission from Hepatology (Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Hepatology. 2005 Sep;42(3):641-9)
Pediatric NAFLD interpretation has a lesser degree of inter-observer agreement than adult interpretations of NAFLD (6). Therefore, standard nomenclature and a uniform histologic scoring system for reading biopsy specimens of patients with suspected NASH is important.
Histologic scoring system
Several histologic scoring systems have been proposed in patients with NASH mainly based upon adult patients with NASH and NAFLD. Until further refinements in the pediatric NAFLD scoring system are developed, we suggest that investigators utilize the NAFLD scoring system designed and validated by the pathology committee of the NASH-Clinical Research Network (NASH-CRN) (6). This system addresses the entire spectrum of lesions of NAFLD in both adult and pediatric populations. The scoring system is composed of 14 histologic features; four of the key features are evaluated semi-quantitatively including steatosis (0-3), lobular inflammation (0-2), hepatocellular ballooning (0-2), and fibrosis (0-4). The other nine features, which include microvesicular steatosis, acidophil bodies, microgranulomas, lipogranulomas, portal inflammation, pigmented macrophages, megamitochondria, Mallory hyaline, and glycogenated nuclei, are qualitatively assessed as present or absent. This system also provides a NAFLD Activity Score (NAS) that can be utilized in clinical trials of NASH. NAS (range: 0-8) is a sum of steatosis, lobular inflammation, and ballooning degeneration scores. A NAS score of 5 or more correlates well with a diagnosis of NASH, and biopsies with scores of less than 3 are generally classified as “non-NASH”. NAS scores of 3 or 4 are considered borderline for a diagnosis of NASH and these cases may benefit from assessment of the entire biopsy specimen, utilizing other features of NASH histology. NAS is not a measure of rapidity of disease progression, and it is unclear if patients with NASH who have a higher NAS score have a worse prognosis. We would like to emphasize that NAS may not be used as a diagnostic tool for NASH but rather an objective scoring system for reporting and assessing treatment response in NASH studies in a standardized manner.
Pathogenesis
NAFLD is a complex metabolic disease that is strongly associated with visceral adiposity and insulin resistance. Day and colleagues proposed a “two-hit” hypothesis, which has been further refined by others (24). Most experts believe that fat accumulation in the liver is the first step but alone may not be enough to induce progressive liver damage. Additional co-factors or hits including oxidative stress (from deficiency of dietary antioxidants, glutathione depletion, mitochondrial dysfunction, hormonal imbalance, hypoxia from obstructive apnea), lipotoxicity, adipocytokines, alterations in mitochondrial permeability and stellate cell activation, among others, are potential mediators in inducing persistent liver injury leading to NASH. We refer the readers to previously published reviews on pathogenesis of NAFLD and NASH (25-27).
Fetuin-A
Fetuin, a protein secreted by liver, is increasingly thought to be an important mediator of hepatic insulin resistance. Reineher and Roth conducted a study in children with metabolic syndrome and fatty liver including 36 obese and 14 lean children (28). These children underwent an intervention for 1 year that included exercise and lifestyle modification. Obese children with NAFLD had significantly higher fetuin-A levels than obese children without NAFLD and the lean children. Fetuin-A levels were cross-sectionally associated with other biomarkers of insulin resistance at baseline and longitudinally decreased significantly in those who lost weight, suggesting that fetuin-A could be a potential biomarker for diagnosis and treatment response in NAFLD. The exact mechanism of fetuin-A in promoting fatty liver remains unknown.
Genetic studies
A recent genome-wide association study conducted in a multi-ethnic cohort including Hispanics, African-Americans and European Americans found that an allele in PNPLA3 (rs738409[G], encoding I148M) was strongly associated with increased hepatic fat levels and with hepatic inflammation (29). Little is known about the function of this gene or its product. The authors also found that sequence variation in PNPLA3 may also explain ethnic differences in the prevalence of NAFLD. Confirmation of these findings and elucidation of the effect of PNPLA3 gene on susceptibility towards NAFLD is needed.
Familial associations
NAFLD aggregates in families. A recent epidemiologic study derived from the Framingham Offspring Study showed that early-onset paternal obesity increased the risk of suspected NAFLD in offspring (30). A study conducted at UCSD using MRI to quantitate hepatic fat fraction evaluated the heritability of nonalcoholic fatty liver. Seventeen percent of siblings and 37% of parents of obese children without NAFLD had fatty liver by MRS compared to 59% of siblings and 78% of parents of children with biopsy-confirmed NAFLD. Although BMI was highly correlated with hepatic fat fraction, the correlation was significantly stronger for families of children with NAFLD than those without NAFLD (31). The heritability of fatty liver after adjustment for age, sex and BMI was 1.0. This establishes the significant genetic contribution to both fatty liver disease and degree of hepatic fat accumulation. Further studies from diverse ethnic and geographic cohorts will help pave the way for determining relative genetic and environmental contributions.
In the future, we may find that numerous genes contribute to NAFLD susceptibility and these may differ based upon ethnicities and gene-environment interactions. Polymorphisms in pleiotropic gene regulators such as nuclear hormone receptors may be responsible for propensity to NAFLD in certain populations. The false-positive rates in SNP studies usually are high and overall contribution towards genetic susceptibility is low in polygenic complex disease such as NAFLD.
Clinical-pathologic correlation
Clinico-pathologic correlations associated with NAFLD include obstructive sleep apnea, hypothyroidism, and clinical signs associated with insulin resistance such as acanthosis nigricans (32). Norman and colleagues conducted a retrospective study in 109 adults with obstructive sleep apnea and reported that serum aminotransferase levels correlated (Pearson's r -0.38 to -0.31) with markers of oxygen desaturation (32). Studies are needed to define the mechanistic association between these disorders and susceptibility to develop hepatic steatosis independent of the confounding effect of obesity.
Investigators in our group conducted a study including 176 children (age range, 6-17 years) prospectively enrolled in the NASH-CRN to identify clinical-pathologic correlates of pediatric NAFLD (33). Increasing concentration of serum AST, GGT and higher titers of anti-smooth muscle antibody (ASMA) were independent predictors of severity of NASH, and increasing concentration of serum AST, higher white blood cell count and lower hemoglobin concentrations were independent predictors of advanced fibrosis. In this cohort, type 2 NASH was associated with age, prothrombin time and higher degrees of insulin resistance. Future studies may clarify whether ASMA positivity or elevated WBC count has a causal effect or a mere association with NAFLD.
NAFLD and cardiovascular disease risk factors: Elevated serum ALT (or suspected fatty liver disease) is associated with increased risk of cardiovascular and all-cause mortality (in addition to liver mortality) in adults (34). A case-control study from our group showed that children with biopsy proven NAFLD were more likely to have dyslipidemia, hypertension, insulin resistance, and more components of metabolic syndrome than age-sex-BMI matched controls without NAFLD (35). A study in Turkish children showed that carotid artery intima-medial thickness (IMT) is significantly higher in obese children with fatty liver than obese children without fatty liver or normal weight controls (36). Pacifico et al reported that carotid artery intima-medial thickness (IMT) was highest in obese children with echogenic liver and severity of liver fat based upon ultrasound was an independent predictor of IMT after adjustment for known cardio-vascular risk factors (37). These results suggest that hepatic steatosis may have more of an adverse health impact on cardiovascular outcomes than on liver disease itself.
Natural history of pediatric fatty liver disease
There are limited long-term data on mortality in pediatric NAFLD. However, recent data from adult studies suggests that NAFLD and elevated serum ALT may predict mortality in middle-aged adults. The most common cause of mortality in individuals with NAFLD is cardiovascular disease followed by cancer and liver disease. Feldstein et al reported a long-term study in 66 children from Mayo Clinic with NAFLD diagnosed based upon imaging (elevated serum ALT and/or liver biopsy proven NAFLD) (38). The primary outcome was transplant-free survival. The average age and BMI were 14 years and 31 kg/m2, respectively. At baseline visit, 40% had no fibrosis and 60% had some fibrosis. Two children had cirrhosis. These children with NAFLD had 13.6 higher odds of mortality or needing a liver transplant as compared to age-sex-matched controls from the general Minnesota population.
Non-invasive markers
Development of novel serum biomarkers to diagnose NASH is essential for population-based screening. Biomarkers may be divided into four categories:
Markers of Inflammation: Cytokine imbalance has been demonstrated in both adults and children with NASH. Patients with NASH have higher serum IL-6 and TNF-α compared to either patients with steatosis alone or controls without liver disease (39, 40). Serum adiponectin levels are lower in patients with NASH compared to age/sex/BMI-matched controls but no set point accurately predicts NASH versus steatosis alone with reasonable sensitivity and specificity.
Markers of oxidative stress: Oxidative stress related to excess free fatty acids is considered key in the progression of steatosis to steatohepatitis in humans and animal models of NASH. Chalasani and colleagues showed that oxidized low-density-cholesterol (ox-LDL) and thiobarbituric acid-reacting substance (TBARS) in serum were higher in biopsy-proven NASH patients compared to age, gender, BMI-matched controls without liver disease in un-adjusted analyses (41).
Biomarkers of apoptosis: Several groups report serum markers of hepatocyte apoptosis can discriminate NASH from benign steatosis. A major intermediate filament protein in the liver called CK-18 has been investigated in children with NASH (42). Caspase-generated CK-18 fragments are higher in the liver and in serum in those with NAFLD compared to age- and sex-matched controls (43). Serum CK-18 levels correlate with liver expression of CK-18 and both serum and liver CK-18 are independent predictors of NASH. A cut off value of 380 U/l provided a specificity of 94% and sensitivity of 91% for the diagnosis of NASH as compared to simple steatosis. The area under the curve was estimated to be 0.93 (95% CI: 0.83-1.00). Further studies are needed to confirm and validate these findings.
Biomarkers of fibrosis: Adult patients with NAFLD show that a combination of clinical, biochemical and/or extracellular matrix protein-associated serum markers may differentiate NASH from bland steatosis. ELF score, calculated from an algorithm incorporating a panel comprised of hyaluronic acid (HA), amino-terminal propeptide of type III collagen (PIIINP), and tissue inhibitor of metalloproteinase 1 (TIMP-1) has been shown to have reasonable predictive capability in adult NASH. Nobili et al assessed ELF score utility in predicting liver fibrosis in 112 children with NAFLD (44). Area under the receiver operating characteristics curve for diagnosing advanced fibrosis (stage 3 or more) was 0.99 at an ELF cut-point of 10.51. Larger and more diverse groups are needed to validate the diagnostic test characteristics of ELF in pediatric fatty liver disease.
Radiologic assessment
Although liver biopsy remains the gold standard for diagnosis of NASH, several radiologic techniques have been developed to estimate or measure hepatic fat content. Most studies testing radiologic modalities for evaluation of hepatic steatosis have been conducted in adults and there are limited data on the accuracy of imaging procedures in pediatric populations. Although the physics concepts underlying radiologic approaches for assessing liver fat apply similarly to both adults and children, the execution of imaging techniques may be more challenging in pediatric populations due to difficulties with breath holding, claustrophobia etc.
Three different modalities have been used for estimation of liver fat estimation including ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) or spectroscopy (MRS). Among these modalities, magnetic resonance shows the greatest promise. US is operator-dependent and its interpretation subjective. CT scan utilizes ionizing radiation, therefore it is not favored for evaluation of liver fat in the absence of another clinical indication. Unique among the three modalities, MR techniques can decompose the signal coming from the liver into its water and fat components, thereby permitting calculation of the fractional fat content of the liver as a measure of hepatic triglyceride concentration.
Several MR-based methods are possible. Of these, MRS is currently considered the most accurate for measurement of liver triglyceride concentration and it has been validated for this purpose in animal studies. However, MRS is not optimal as it is time consuming, restricted in spatial coverage, and requires off-scan analysis by an expert. Because of these limitations it is best suited for research studies at specialized centers and not appropriate for widespread utilization. As opposed to MRS, which provides exquisite biochemical information from small regions of interest in the liver, MRI can assess fat in the entire liver. MRI is easy to perform and interpret and, therefore, may be more suitable for widespread use. Pacifico and colleagues conducted a study in 50 children (mean age 10.5 years) who had hepatomegaly or elevated serum ALT. MRI fat fraction but not US determined steatosis severity was found to be an independent predictor of elevated serum ALT after adjustment for age, sex, BMI and pubertal status. Although US steatosis severity correlated with MRI fat fraction in this study (r = 0.69, p-value < 0.001) but was inaccurate in classifying participants into moderate versus severe steatosis, and no versus mild steatosis.
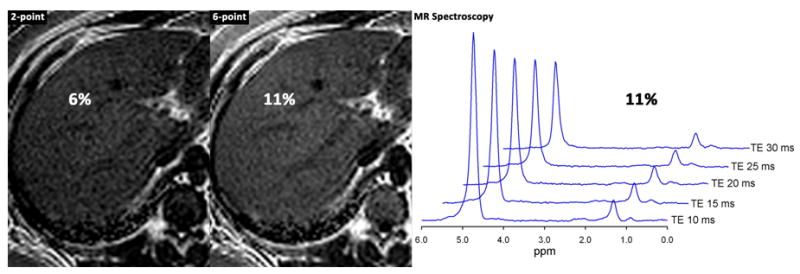
Investigators are now developing novel MR imaging techniques to generate better estimates of the hepatic fat content fraction. In initial clinical studies both in adults and children, these newer MR methods are more accurate for diagnosing fatty liver and quantifying the amount of fat than older methods, using spectroscopy as a reference standard (figure 2 a and b). These techniques are not yet commercially available but may become available in the course of few years for use in both adults and children.
Figure 2.
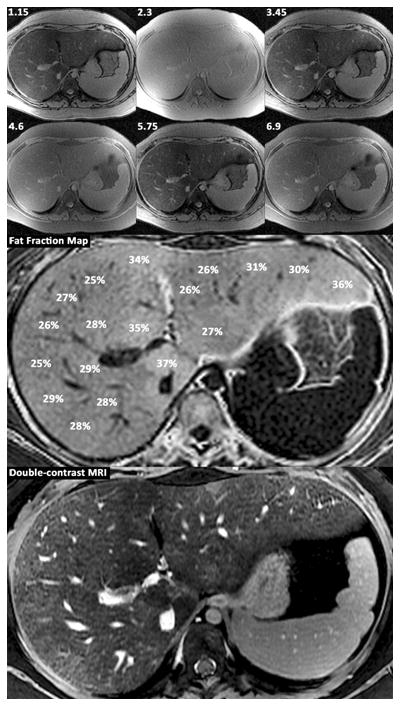
Panel A.
15 year old boy with cirrhosis due to NASH.
Shown are source images obtained at 3T with echo times of 1.15, 2.3, 3.45, 4.6, 5.75, and 6.9 msec; fat fraction map derived from the source images with T2* correction and spectrum modeling showing the percentage of fat in different portions of the liver; and double-contrast enhanced MR image depicting fibrotic reticulations throughout the liver, most pronounced in the periphery.
Panel B.
16 year old boy with NASH.
Shown are fat fraction maps from a 2-point Dixon technique (estimated fat fraction 6%) and from a T1-independent, T2*-corrected, spectrum-modeled 6-point technique (estimated fat fraction 11%). Also shown are spectra acquired using STEAM at multiple echo times from 10 to 30 msec. T2-corrected fat-fraction measured by spectroscopy = 11%.
Although in early stages of development, US and MR elastography (MRE) has been used to measure stiffness of the liver as a surrogate marker of liver fibrosis. Nobili and colleagues conducted a study to assess the performance characteristics of US elastography in predicting fibrosis in pediatric NASH (45). They found that a value below 5 Kpa is associated with no fibrosis and a cut-point >9 Kpa is associated with advanced fibrosis. US elastography has limitations in evaluating obese individuals and in its current state of development may not be suitable for widespread utilization in obese children with NAFLD. MRE has the advantage of being less affected by body habitus and therefore, may be a more suitable modality in this population. But to date, there are no published studies on the use of MRE in children and this modality should be considered experimental. No imaging technique has been fully validated for estimating hepatic fibrosis and further research is needed in this area.
Treatment
There is no approved pharmacologic therapy for NAFLD or NASH. Therefore, current management paradigms are based upon the presence of associated risk factors in a particular patient with NAFLD. The overall goal is to improve an individual's quality of life and reduce long-term cardiovascular and liver morbidity and mortality. A summary of clinical trial characteristics in children with NAFLD is listed in Table 3.
Diet and exercise
As most NAFLD patients are overweight or obese, targeting pediatric obesity should help in reducing the burden of pediatric NAFLD. Studies in adults with NAFLD suggest that weight loss leads to significant improvement in serum ALT and liver histology (46). The relative efficacy of weight loss and degree of weight loss needed to induce histologic improvement in pediatric NAFLD is unknown. Based upon studies in adults, greater than 5% weight loss was associated with significant improvement in liver histology. A pediatric open label study showed that a mean weight loss of approximately 5 kg (baseline weight: 61 kg; age range: 5-19 years) resulted in improvement in serum ALT and AST in most children with NAFLD (47). However, randomized-controlled trials (RCT) are needed to assess the efficacy of weight loss in histologic improvement in pediatric NASH.
No information exists on recommending any type of diet. A low-carbohydrate diet has been shown to lead to reduction in serum ALT and hepatic steatosis (48). However, serum ALT alone is an unreliable marker for histologic severity. Low-glycemic index diet has been shown to be better than low fat diet in achieving weight loss in obese adolescents and adults (49) but have not been tested in patients with pediatric NASH. Further studies in pediatric NAFLD are needed to assess their efficacy.
Recommendations for overweight pediatric NAFLD patients should include consultation with a registered dietitian to assess quality of diet and measurement of caloric intake, adoption of American Heart Association dietary strategies (for children aged >2 years) and regular aerobic exercise progressing in difficulty as fitness allows (135)(50). Enlisting other willing family members to adopt diet and exercise goals may aid compliance. Future studies utilizing these interventions and behavioral modifications should be tested in pediatric obesity and NAFLD to develop more evidence-based recommendations (51).
Vitamin E
Vitamin E is an anti-oxidant potentially effective in reducing oxidative stress. As oxidative stress is considered a key component in NASH pathogenesis, vitamin E is under investigation as a treatment for pediatric NASH. An open label study of vitamin E (400 to 1200 units per day orally) given for 2-4 months led to improvement in serum ALT in obese children (52). Nobili and colleagues randomized 53 patients, aged between 5 and 19 years, to either lifestyle intervention with placebo or lifestyle intervention with anti-oxidants (alpha-tocopherol 600 IU/day plus ascorbic acid 500 mg/day) for 24 months (53). They reported that patient's in both arms lost weight (average of 5 kg approx) and weight loss was associated with improvements in liver histology. This study did not show any improved efficacy with addition of anti-oxidants to lifestyle intervention. Vitamin E is currently being evaluated for its efficacy in both children and adults in a histology-based, double-blind, randomized, placebo-controlled study in the NASH-CRN. The results from this trial will be available in 2010.
Ursodiol
The exact mechanistic role of ursodiol in the treatment of NASH is unknown. It may have a cytoprotective effect by possibly reducing bile-salt mediated mitochondrial injury within hepatocytes. However, it failed to show any benefit in RCT in adults with NASH and children with NAFLD. Vajro and colleagues conducted a RCT in obese children with elevated serum ALT and reported that ursodiol (10-12.5 mg/kg orally per day) was ineffective in improving serum ALT or steatosis by ultrasound (54). One RCT showed that ursodiol in combination with vitamin E may improve serum ALT and hepatic steatosis in NASH patients (55). Preponderance of evidence in literature is against ursodiol's efficacy in NASH.
Insulin sensitizers
Metformin is the only insulin-sensitizing agent evaluated for the treatment of NAFLD in children. Metformin improves insulin sensitivity via activation of LKB1, a tumor suppressor gene, which phosphorylates and activates AMP-activated protein kinase and thereby decreases gluconeogenesis in the liver. Studies in adults with NASH suggest that metformin improves NASH by inducing weight loss (56).
Metformin has been shown to be safe and effective in the treatment of diabetes in children. A pilot study of metformin in pediatric NASH demonstrated that metformin (500 mg twice daily) treatment over six months resulted in improvement in serum ALT and reduction in hepatic steatosis as assessed with MRS (57). The NASH-CRN is conducting a 96-week double-blind, randomized controlled trial of metformin in pediatric NAFLD, for which the results will be available in 2010. Thiozolidinediones such as rosiglitazone and pioglitazone have been evaluated in RCT in adult patients with NASH and appear promising. Two RCTs showed that pioglitazone was effective in improving NASH histology but a recent rosiglitazone study did not show any benefit. There are serious concerns regarding the cardiovascular safety profile of rosiglitazone, therefore it is unlikely to be tested in children. A major side-effect of this group of medications is weight gain due to increase in adiposity that appears to persist even after cessation of medication, which may limit long term utility (58). Well-designed studies in pediatric NASH are necessary before considering pioglitazone in clinical practice.
Endpoints
NASH is a specific histology-based diagnosis and we recommend that this group of patients within the spectrum of pediatric NAFLD should be enrolled in pharmacologic intervention studies. Availability of histology allows for using the NASH scoring system developed by the NASH-CRN for inclusion and determination of treatment response. Uniform histologic assessment would allow for comparison and pooling of results through meta-analysis to guide clinical decision-making. We recently conducted a pooled analysis of placebo-treated patients in RCTs in NASH and found that neither steatosis nor serum ALT or AST alone can be used as an endpoint in a treatment study as they improve even in placebo-treated groups (59). Strict histologic improvement in NAS score by 2 or 3 points, as utilized in some previous NASH studies, is rarely seen in placebo-arms and may be a useful treatment endpoint to assess treatment efficacy.
Research priorities
NASH is a complex metabolic disease resulting from a wide variety of insults. Improved understanding of pathogenesis through genetic and familial studies is a priority. Liver biopsy is the gold standard and currently the only way to diagnose NASH. Therefore, we need non-invasive biomarkers for diagnosing patients with steatosis who are at highest risk of disease progression. Large population-based epidemiologic studies are needed to understand the true impact of NASH on long-term morbidity and mortality and its associations with cardio-metabolic-cancer risk factors. Lastly, we need effective and safe treatments for NASH that are practical, cost-effective and readily transferable to the community at large.
Summary
NAFLD has become the leading cause of pediatric liver disease in the United States and perhaps Western World. Trials are underway to establish pharmacologic treatment for patients with NASH but lifestyle interventions such as comprehensive approach to weight management, healthy diet, and exercise can reduce disease burden. Public health impact of NAFLD may require health and nutrition policy makers to mandate better food availability in the school cafeteria and home surroundings and develop better exercise habits in our youth.
Acknowledgments
Financial Support: This study was supported in part by U01DK61734 (JL), R01DK075128 (CS), and R24DK080506 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- NASH
nonalcoholic steatohepatitis
- NAFLD
nonalcoholic fatty liver disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- (NIDDK)
National Institute of Diabetes and Digestive and Kidney Diseases
- (NIH)
National Institutes of Health
Footnotes
Conflict of Interest: No conflict of interest exist
Role of sponsor: No conflict of interest exists.
References
- 1.Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: Hepatitis B, C and NAFLD: Summary of a single topic conference. Hepatology. 2006;44:1344–1354. doi: 10.1002/hep.21373. [DOI] [PubMed] [Google Scholar]
- 2.Matthiessen J, Velsing Groth M, Fagt S, Biltoft-Jensen A, Stockmarr A, Andersen JS, Trolle E. Prevalence and trends in overweight and obesity among children and adolescents in Denmark. Scand J Public Health. 2008;36:153–160. doi: 10.1177/1403494807085185. [DOI] [PubMed] [Google Scholar]
- 3.Ji CY. The prevalence of childhood overweight/obesity and the epidemic changes in 1985-2000 for Chinese school-age children and adolescents. Obes Rev. 2008;9 1:78–81. doi: 10.1111/j.1467-789X.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 6.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 7.Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Tani S, Goto M. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–149. doi: 10.2169/internalmedicine1962.27.142. [DOI] [PubMed] [Google Scholar]
- 8.Park HS, Han JH, Choi KM, Kim SM. Relation between elevated serum alanine aminotransferase and metabolic syndrome in Korean adolescents. Am J Clin Nutr. 2005;82:1046–1051. doi: 10.1093/ajcn/82.5.1046. [DOI] [PubMed] [Google Scholar]
- 9.Roberts EA. Non-alcoholic fatty liver disease (NAFLD) in children. Front Biosci. 2005;10:2306–2318. doi: 10.2741/1699. [DOI] [PubMed] [Google Scholar]
- 10.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–733. [PubMed] [Google Scholar]
- 11.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, Kusano Y. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–2009. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 12.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 13.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 14.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 15.Roberts EA. Pediatric nonalcoholic fatty liver disease (NAFLD): a “growing” problem? J Hepatol. 2007;46:1133–1142. doi: 10.1016/j.jhep.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Potau N, Ibanez L, Rique S, Carrascosa A. Pubertal changes in insulin secretion and peripheral insulin sensitivity. Horm Res. 1997;48:219–226. doi: 10.1159/000185519. [DOI] [PubMed] [Google Scholar]
- 17.Moran A, Jacobs DR, Jr, Steinberger J, Steffen LM, Pankow JS, Hong CP, Sinaiko AR. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008;117:2361–2368. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- 18.Lobanova YS, Scherbakov AM, Shatskaya VA, Evteev VA, Krasil'nikov MA. NF-kappaB suppression provokes the sensitization of hormone-resistant breast cancer cells to estrogen apoptosis. Mol Cell Biochem. 2008 doi: 10.1007/s11010-008-9985-0. [DOI] [PubMed] [Google Scholar]
- 19.Xu JW, Gong J, Chang XM, Luo JY, Dong L, Jia A, Xu GP. Effects of estradiol on liver estrogen receptor-alpha and its mRNA expression in hepatic fibrosis in rats. World J Gastroenterol. 2004;10:250–254. doi: 10.3748/wjg.v10.i2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu I, Inoue H, Yano M, Shinomiya H, Wada S, Tsuji Y, Tsutsui A, et al. Estrogen receptor levels and lipid peroxidation in hepatocellular carcinoma with hepatitis C virus infection. Liver. 2001;21:342–349. doi: 10.1034/j.1600-0676.2001.210507.x. [DOI] [PubMed] [Google Scholar]
- 21.Abplanalp W, Ravi Subbiah MT. Uptake and protection against oxidative stress by estrogen esters in THP-1 human macrophage cell lines. Gynecol Obstet Invest. 2001;51:81–84. doi: 10.1159/000052898. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt KN, Pratis K, Jones ME, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145:1842–1848. doi: 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
- 23.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, Cobelli C, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 25.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:46–53. doi: 10.1038/ncpgasthep0084. [DOI] [PubMed] [Google Scholar]
- 27.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93:4479–4485. doi: 10.1210/jc.2008-1505. [DOI] [PubMed] [Google Scholar]
- 29.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loomba R, Hwang SJ, O'Donnell CJ, Ellison RC, Vasan RS, D'Agostino RB, Sr, Liang TJ, et al. Parental obesity and offspring serum alanine and aspartate aminotransferase levels: the Framingham heart study. Gastroenterology. 2008;134:953–959. doi: 10.1053/j.gastro.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norman D, Bardwell WA, Arosemena F, Nelesen R, Mills PJ, Loredo JS, Lavine JE, et al. Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. Sleep. 2008;31:121–126. doi: 10.1093/sleep/31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–1971. e1962. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TH, Kim WR, Benson JT, Therneau TM, Melton LJ., 3rd Serum aminotransferase activity and mortality risk in a United States community. Hepatology. 2008;47:880–887. doi: 10.1002/hep.22090. [DOI] [PubMed] [Google Scholar]
- 35.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–283. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demircioglu F, Kocyigit A, Arslan N, Cakmakci H, Hizli S, Sedat AT. Intima-media thickness of carotid artery and susceptibility to atherosclerosis in obese children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2008;47:68–75. doi: 10.1097/MPG.0b013e31816232c9. [DOI] [PubMed] [Google Scholar]
- 37.Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Anania C, Ferrara E, et al. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008;63:423–427. doi: 10.1203/PDR.0b013e318165b8e7. [DOI] [PubMed] [Google Scholar]
- 38.Feldstein AE, Charatcharoenwitthaya PST, Benson JT, Enders FB, Angulo P. The Natural History Of Nonalcoholic Fatty Liver Disease In Children: A Follow-Up Study For Up To 20 Years. Hepatology. 2008;48:64A. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954–960. doi: 10.1309/6VJ4DWGYDU0XYJ8Q. [DOI] [PubMed] [Google Scholar]
- 40.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 41.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 42.Vos MB, Barve S, Joshi-Barve S, Carew JD, Whitington PF, McClain CJ. Cytokeratin 18, a marker of cell death, is increased in children with suspected nonalcoholic Fatty liver disease. J Pediatr Gastroenterol Nutr. 2008;47:481–485. doi: 10.1097/MPG.0b013e31817e2bfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 44.Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160–167. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, Fruhwirth R, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442–448. doi: 10.1002/hep.22376. [DOI] [PubMed] [Google Scholar]
- 46.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nobili V, Manco M, Raponi M, Marcellini M. Case management in children affected by non-alcoholic fatty liver disease. J Paediatr Child Health. 2007;43:414. doi: 10.1111/j.1440-1754.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 48.Benjaminov O, Beglaibter N, Gindy L, Spivak H, Singer P, Wienberg M, Stark A, et al. The effect of a low-carbohydrate diet on the nonalcoholic fatty liver in morbidly obese patients before bariatric surgery. Surg Endosc. 2007;21:1423–1427. doi: 10.1007/s00464-006-9182-8. [DOI] [PubMed] [Google Scholar]
- 49.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 50.Barlow SE, Dietz WH. Management of child and adolescent obesity: summary and recommendations based on reports from pediatricians, pediatric nurse practitioners, and registered dietitians. Pediatrics. 2002;110:236–238. [PubMed] [Google Scholar]
- 51.Berg F, Buechner J, Parham E. Guidelines for childhood obesity prevention programs: promoting healthy weight in children. J Nutr Educ Behav. 2003;35:1–4. doi: 10.1016/s1499-4046(06)60318-7. [DOI] [PubMed] [Google Scholar]
- 52.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 53.Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, Piemonte F, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119–128. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 54.Vajro P, Franzese A, Valerio G, Iannucci MP, Aragione N. Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr. 2000;136:739–743. [PubMed] [Google Scholar]
- 55.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 56.Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, et al. Clinical trial: Pilot study of metformin for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2008 doi: 10.1111/j.1365-2036.2008.03869.x. (epub before publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–879. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 58.Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, Borg B, et al. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424–429. doi: 10.1002/hep.21661. [DOI] [PubMed] [Google Scholar]
- 59.Loomba R, Wesley R, Pucino F, Liang TJ, Kleiner DE, Lavine JE. Placebo in nonalcoholic steatohepatitis: insight into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol. 2008;6:1243–1248. doi: 10.1016/j.cgh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


