Abstract
Background & Aims
c-Jun N-terminal kinase (JNK) plays a pivotal role in the development of the metabolic syndrome including NAFLD. However, the mechanism underlying the contribution of JNK to the progression from simple steatosis to steatohepatitis and liver fibrosis is unresolved.
Methods
Hepatic steatosis, inflammation, and fibrosis were examined in wild-type, jnk1−/−, or jnk2−/− mice fed a choline-deficient L-amino acid-defined (CDAA) diet for 20 weeks. The functional contribution of JNK isoforms in Kupffer cells was assessed in vitro and in vivo using chimeric mice in which the hematopoietic compartment including Kupffer cells was replaced by wild-type, jnk1−/−, or jnk2−/− cells.
Results
CDAA diet induced significantly less hepatic inflammation and less liver fibrosis despite similar level of hepatic steatosis in jnk1−/− mice as compared to wild-type or jnk2−/− mice. CDAA diet-induced hepatic inflammation was chronic and mediated by Kupffer cells. Pharmacological inhibition of JNK or gene deletion of jnk1 but not jnk2 repressed the expression of inflammatory and fibrogenic mediators in primary Kupffer cells. In vivo, CDAA diet induced less hepatic inflammation and liver fibrosis despite equivalent level of hepatic steatosis in chimeric mice with jnk1−/− hematopoietic cells as compared to chimeric mice with wild-type or jnk2−/− hematopoietic cells.
Conclusions
jnk1−/− mice are resistant to diet-induced steatohepatitis and liver fibrosis. JNK1 in hematopoietic cells, especially in Kupffer cells, contributes to the development of liver fibrosis by inducing chronic inflammation.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) and liver fibrosis,1–4 and is commonly associated with the clinical features of the metabolic syndrome such as obesity, type II diabetes, and dyslipidemia.2–5A “two-hit” model has been proposed for the development of NAFLD.6 The “first hit” is the initial hepatic lipid accumulation, but a “second hit” is required for liver injury, inflammation, and fibrosis. The major candidate for the “second hit” is hepatic oxidative stress that could cause cellular injury and trigger the recruitment of inflammatory cells.7–10 Kupffer cells have been shown to play a critical role in the development of hepatic inflammation in both patients with NASH and experimental animal models.11–13 Indeed, various pro-inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, and cyclooxygenase-2 are increased in the livers.14–16 Furthermore, recent studies have demonstrated that depletion of Kupffer cells or inhibition of these pro-inflammatory mediators substantially protect against the development of steatohepatitis and subsequent liver fibrosis, suggesting the pivotal role of chronic inflammation in the progression of NAFLD.12, 13, 17, 18
c-Jun N-terminal kinase (JNK) has been implicated to play a role in both of these “hits” of NAFLD. First, increased JNK activity can promote the insulin resistance that underlies the development of metabolic syndrome, including hepatic steatosis.19, 20 Second, hepatic oxidative stress can activate JNK to mediate inflammation through activator protein-1 (AP-1)-dependent transcription of pro-inflammatory cytokines. Indeed, the JNK isoforms, especially JNK1, contribute to the development of hepatic steatosis and steatohepatitis in mice induced by high fat diet (16 weeks) and methionine- and choline-deficient (MCD) diet (4 weeks), respectively.21, 22 Although deletion of JNK1 diminishes hepatic inflammation in these studies, it is unclear whether this is a direct effect of JNK1 deletion on inflammatory signaling or a secondary effect through less hepatic steatosis. Recently, Solinas et al. demonstrated that selective JNK1 deletion in hematopoietic cells by bone marrow transplantation prevents systemic and hepatic inflammation and subsequent development of insulin resistance induced by high fat diet without affecting the level of obesity or hepatic steatosis.23 Thus, not only JNK1 in insulin target cells such as hepatocytes but also in hematopoietic cells contributes to the development of NAFLD. Given the impact of these multiple roles of JNK in the pathogenesis of insulin resistance, obesity, hepatic steatosis, and inflammation, JNK might play a central role in the progression of NAFLD.24 However, the mechanisms underlying the contribution of JNK to the development of NASH-induced hepatic fibrosis, which leads to the most important clinical consequence of cirrhosis, liver failure, and hepatocelullar cancer, are still unresolved, partly because high fat diet fails to induce liver fibrosis, and MCD diet develops minimal fibrosis with substantial body weight loss.
To overcome these problems in studying the genetics of NASH-induced fibrosis, we used the choline-deficient L-amino acid-defined (CDAA) diet model (20 weeks) in mice, that has been previously demonstrated to mimic human NASH in both mice and rats by sequentially producing steatohepatitis, liver fibrosis, and liver cancer without any body weight loss.25–28 Our study shows that jnk1−/− mice were resistant to liver fibrosis as compared to wild-type or jnk2−/− mice. Moreover, utilizing a similar system of bone marrow transplantation as described by Solinas et al.,23 we found that JNK1 in hematopoietic cells, especially in Kupffer cells, promotes the progression from simple steatosis to steatohepatitis and subsequent liver fibrosis through mediating steatosis-induced chronic inflammation.
Materials and Methods
Animal Model
Male and female wild-type C57BL/6, jnk1−/−, and jnk2−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were bred and housed in a temperature- and light-controlled facility with unlimited access to food and water. For the CDAA diet-induced steatohepatitis/liver fibrosis animal model, male 10 to 12 week-old mice were fed CDAA diet or a corresponding control diet choline-supplemented L-amino acid-defined (CSAA) diet (Dyets Inc., Bethlehem, PA) for 4 or 20 weeks. Serum levels of alanine aminotransferase (ALT) were measured using a commercial kit (Biotron Diagnostic Inc., Hemet, CA). Serum endotoxin level was measured using a commercial kit (Lonza, Walkersville, MD). The studies were approved by the University of California San Diego Institutional Animal Care and Use Committee and followed the National Institutes of Health guidelines outlined in “Guide for the Care and Use of Laboratory Animals”.
Bone Marrow Transplantation
Chimeric mice were generated by bone marrow transplantation as previously described.29 Briefly, liposomal clodronate was intravenously injected (200 μl/mouse) into male 8 week-old wild-type recipient mice to deplete endogenous Kupffer cells at one day before irradiation. Bone marrow cells were then harvested in a sterile fashion from the tibia and femur of donor mice and filtrated. After washing with Hank’s balanced salt solution, 1×107 bone marrow cells were injected into tail veins of lethally irradiated (12 Gy) recipient mice. Full reconstitution of hematopoietic cells including Kupffer cells by this system was evaluated by fluorescent-activated cell sorting (FACS) analysis of peripheral blood cells and immunofluorescent staining of liver sections in chimeric mice containing bone marrow cells from β-actin promoter-driven green fluorescent protein (GFP) transgenic mice (Jackson Laboratories, Bar Harbor, ME).29, 30 Eight weeks after transplanta tion, chimeric mice were fed CDAA diet or a corresponding control diet for 20 weeks.
Fractionation of liver cells
Liver cells of wild-type mice fed CDAA diet for four weeks were fractionated into four major cell populations i.e., hepatocytes, Kupffer cells, endothelial cells, and hepatic stellate cells as previously described.31 For details, please refer to Supplementary Materials and Methods.
Isolation, culture, and treatment of Primary Kupffer cells
Mouse Kupffer cells were isolated and cultured as previously described.29, 32 For details, please refer to Supplementary Materials and Methods.
Histological Analysis
Livers were removed, fixed with 10% buffered formalin, embedded in paraffin, and cut into 5-μm-thick sections. For frozen sections, liver tissues were fixed with 4% buffered formalin and embedded in OCT compound. All specimens were stained with hematoxylin & eosin (HE) and examined in a blinded fashion by a single pathologist. The degree of inflammation was graded by the presence or absence of inflammatory cells with 0=none, 1=mild inflammation, 2=moderate inflammation, and 3=marked inflammation. Routine Sirius red (saturated picric acid containing 0.1% Direct Red 80 and 0.1% Fast Green FCF) staining was performed to visualize collagen deposition. The sirius red positive area was calculated in five random low-power views on each slide using Image J Software (NIH). Immunohistochemical staining or immunofluorescent staining for Kupffer cells was done with antibody against F4/80 (eBioscience, San Diego, CA) on paraffin or frozen sections, respectively. Oil red O staining was performed with frozen sections for analysis of lipid accumulation in the liver.
Other Materials and Methods
Other materials and methods for protein analysis, quantitative real-time polymerase chain reaction (PCR), glucose tolerance test, insulin tolerance test, and hepatic triglyceride measurement are described in the Supplementary Materials and Methods.
Statistical Analysis
Results were expressed as mean ± standard error of mean from at least three independent experiments. Data between groups were analyzed by two-tailed t test. A P value of less than 0.05 was considered statistically significant.
Results
jnk1−/− Mice Are Resistant to CDAA diet-Induced Liver Fibrosis
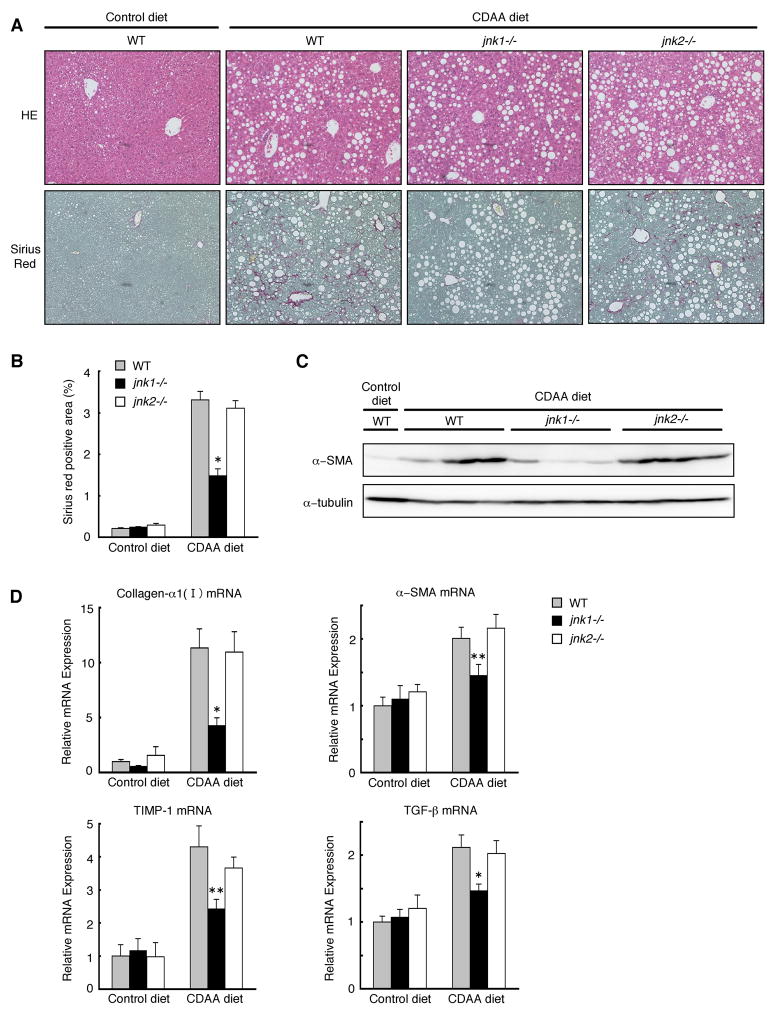
To analyze the distinct roles for JNK isoforms in the development of NASH-induced liver fibrosis, wild-type, jnk1−/−, and jnk2−/− mice were fed a control diet or a CDAA diet for 20 weeks. In contrast to previous reports with mice fed an MCD diet for shorter periods,7, 21 all three strains of mice fed an CDAA diet did not show any body weight loss, allowing us to observe these mice for 20 weeks without any dropouts (Table 1). CDAA diet for 20 weeks induced hepatic steatosis and liver fibrosis as previously reported (Figure 1A).25–28 Although wild-type mice fed a CDAA or a control (CSAA) diet showed impaired insulin sensitivity as compared to mice fed a standard chow (data not shown, manuscript in preparation), there was no difference in insulin sensitivity between mice on the CDAA diet and those on the control (CSAA) diet. (Supplementary Figure S1). Levels of liver fibrosis assessed by Sirius red staining were markedly reduced in jnk1−/− mice as compared to those of wild-type or jnk2−/− mice (Figure 1A, 1B). This result was confirmed by decreased protein and mRNA levels of fibrosis markers such as α-SMA, collagen-α1(I), TIMP-1, and TGF-β in the livers of jnk1−/− mice fed CDAA-diet (Figure 1C, 1D). Thus, jnk1−/− mice are resistant to CDAA diet-induced liver fibrosis.
Table 1.
Weight Gain, Final Body Weights, and Liver-to-Body Weight Ratios of Wild-Type, jnk1−/−, and jnk2−/− Mice Fed a Control or CDAA diet for 20 Weeks
| WT |
jnk1−/− |
jnk2−/− |
||||
|---|---|---|---|---|---|---|
| Cont. | CDAA | Cont. | CDAA | Cont. | CDAA | |
| Weight gain/20w (g) | 15.1±0.7 | 15.1±0.8 | 11.3±0.8 | 11.0±1.9 | 16.0±3.0 | 17.4±3.4 |
| Final body weight (g) | 41.0±1.1 | 40.2±1.2 | 33.6±0.7 | 32.6±1.1 | 43.0±2.0 | 45.4±1.5 |
| Liver/Body (%) | 4.6±0.2 | 5.6±0.3 | 4.4±0.2 | 5.3±0.2§ | 5.0±0.3 | 5.8±0.2 |
Note. Liver weight is expressed as a percentage of body weight. Data are expressed as the mean±standard error (n = 4–6/control diet, n = 6–8/CDAA diet).
Abbreviations: CDAA, choline-deficient L-amino acid-defined; Cont., control; WT, wild-type.
There is no statistically significant difference in this value from wild-type on CDAA diet.
Figure 1. jnk1−/− mice are resistant to CDAA diet-induced liver fibrosis.
Wild-type, jnk1−/−, or jnk2−/− mice were fed a CDAA diet (n = 6–8) or a control diet (n = 4–6) for 20 weeks. (A) HE staining of liver sections is shown (upper panel). Collagen deposition was evaluated by Sirius red staining (lower panel). (B) Sirius red positive area was quantified. (C) Protein expression of α-SMA and α-tubulin as loading control in whole liver extracts was analyzed by Western blot analysis. (D) Hepatic expression of collagen-α1(I), α-SMA, TIMP-1, and TGF-β mRNA was measured by quantitative real-time PCR and normalized to 18S mRNA expression. Values are mean±standard error. *P<0.01, **P<0.05 (jnk1−/− vs wild-type). WT, wild-type.
CDAA diet-Induced Hepatic Steatosis does not differ between Wild-type, jnk1−/−, and jnk2−/− Mice
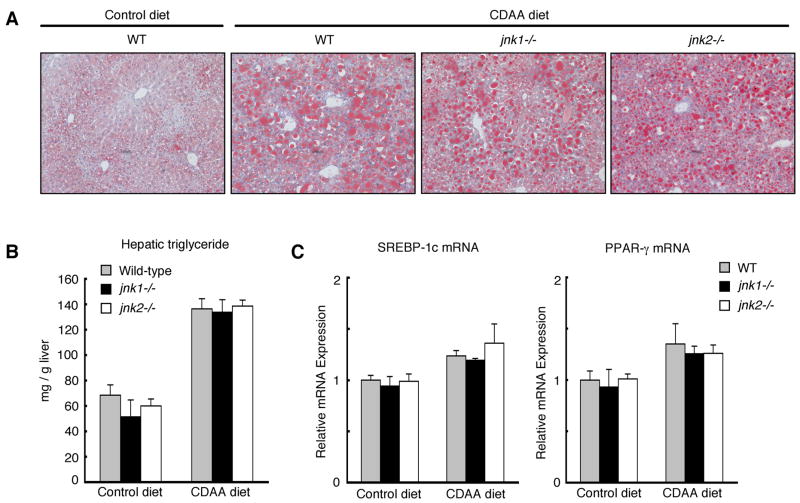
JNK1 plays a critical role in the development of hepatic steatosis by high fat diet or MCD diet in mice.21, 22 However, in our experimental model, levels of hepatic steatosis in wild-type, jnk1−/−, and jnk2−/− mice fed the CDAA diet for 4 weeks or 20 weeks were comparable as assessed by Oil red O staining (Figure 2A, Supplementary Figure S2A). Hepatic triglyceride content and mRNA expression of lipogenic genes such as SREBP-1c and PPAR-γ induced by CDAA diet were also equivalent in these three strains of mice (Figure 2B, 2C, Supplementary Figure S2B, S2C). Furthermore, the ratios of liver weight to body weight that reflect the hepatomegaly due to hepatic steatosis were similar in wild-type, jnk1−/−, and jnk2−/− mice fed CDAA diet (Table 1). From these results, at least at the end of the 4 weeks or 20 weeks period, CDAA diet-induced hepatic steatosis in wild-type, jnk1−/−, and jnk2−/− mice is similar and does not explain the differences observed in liver fibrosis.
Figure 2. CDAA diet-induced hepatic steatosis does not differ between wild-type, jnk1−/−, and jnk2−/− Mice.
Wild-type, jnk1−/−, or jnk2−/− mice were fed a CDAA diet (n = 6–8) or a control diet (n = 4–6) for 20 weeks. (A) Lipid accumulation was evaluated by Oil red O staining of liver sections. (B) Hepatic triglyceride content was measured. (C) Hepatic expression of SREBP-1c and PPAR-γ mRNA was measured by quantitative real-time PCR. Values are mean±standard error.
CDAA diet-Induced Hepatic Inflammation Is Reduced in jnk1−/− Mice
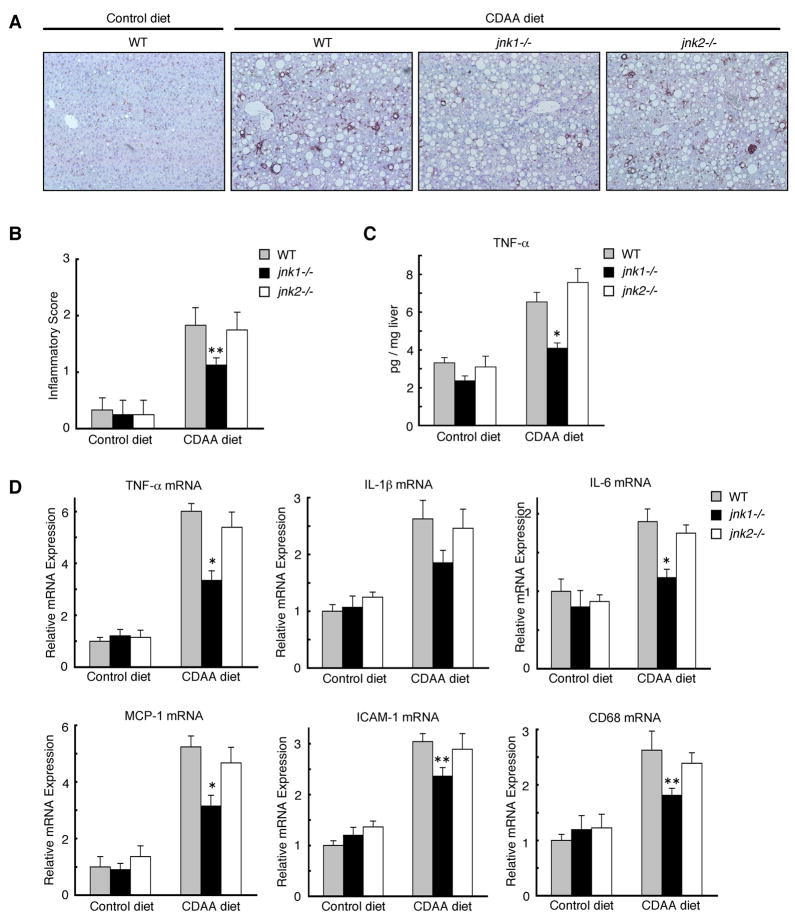
We next analyzed the levels of hepatic inflammation induced by CDAA diet in mice. Immunohistochemical staining of liver sections for F4/80, a marker for Kupffer cells, revealed that Kupffer cell accumulation was markedly greater in wild-type and jnk2−/− mice fed CDAA diet than that of jnk1−/− mice despite similar levels of hepatic steatosis (Figure 3A, Supplementary Figure 3A). The decreased inflammation in jnk1−/− mice was further documented by a lower inflammatory score, lower ALT level, and lower hepatic protein and mRNA expression of inflammatory markers such as TNF-α, IL-1β, IL-6, MCP-1, ICAM-1, and CD68 (Figure 3B, 3C, 3D, Supplementary Figure S3B). Since jnk1−/− mice had less liver fibrosis and less inflammation despite similar levels of hepatic steatosis as compared to wild-type or jnk2−/− mice, hepatic inflammation rather than steatosis correlates with liver fibrosis.
Figure 3. CDAA diet-induced hepatic inflammation is reduced in jnk1−/− mice.
Wild-type, jnk1−/−, or jnk2−/− mice were fed a CDAA diet (n = 6–8) or a control diet (n = 4–6) for 20 weeks. (A) Immunohistochemical staining for F4/80 on liver sections is shown. (B) The degree of hepatic inflammation was graded. (C) Hepatic TNF-α protein level was analyzed by ELISA. (D) Hepatic expression of TNF-α, IL-1β, IL-6, MCP-1, ICAM-1, and CD68 mRNA was measured by quantitative real-time PCR. Values are mean±standard error. *P<0.01, **P<0.05 (jnk1−/− vs wild-type).
Kupffer Cells Contribute to the Development of CDAA diet-Induced Hepatic Inflammation
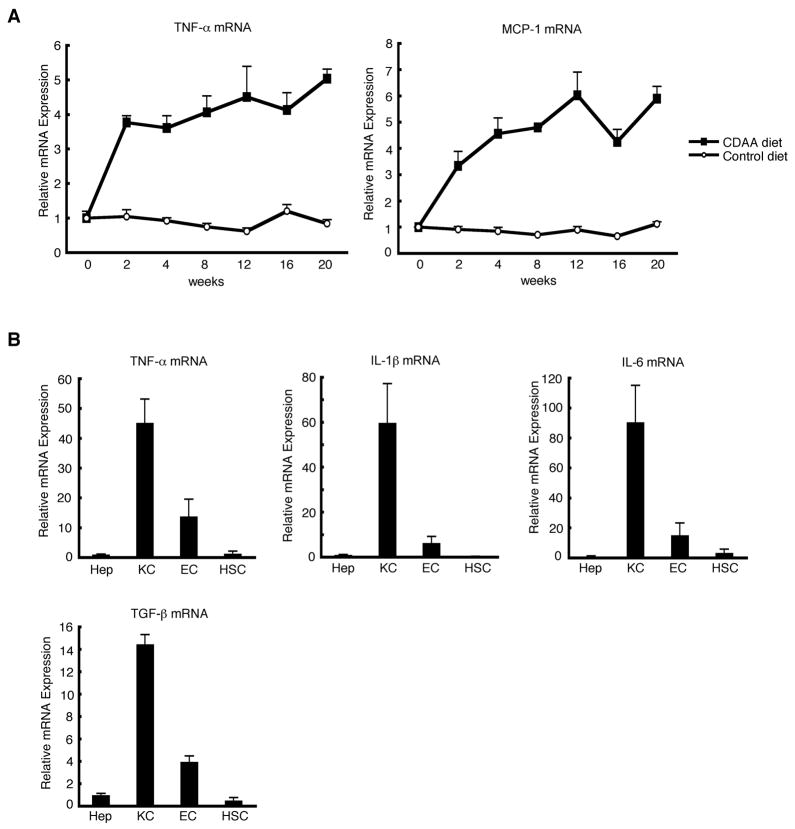
To characterize the hepatic inflammation in CDAA diet model, hepatic expression of inflammatory genes was analyzed at several time points after the CDAA or control diet was started. Hepatic mRNA levels of TNF-α and MCP-1 were already up-regulated in mice fed CDAA diet for 2 weeks, and maintained high levels of expression until the end of the 20 week period, suggestive of chronic inflammation in the liver (Figure 4A). To identify the cells responsible for this persistent inflammation, liver cells of mice fed CDAA diet for four weeks were fractionated into four major cell populations i.e., hepatocytes, Kupffer cells, endothelial cells, and hepatic stellate cells, and expression of proinflammatory genes was analyzed. TNF-α, IL-1β, and IL-6, were predominantly expressed in the Kupffer cell fraction (Figure 4B). In addition to these genes, TGF-β, a pro-fibrogenic gene, was also predominantly expressed in the Kupffer cell fraction, suggesting the crucial role of Kupffer cells in the development of both hepatic inflammation and liver fibrosis (Figure 4B).
Figure 4. CDAA diet-induced hepatic inflammation is mediated by Kupffer cells.
(A) TNF-α and MCP-1 mRNA expression in the liver of mice fed CDAA diet or control diet were analyzed by quantitative real-time PCR at indicated time points (n = 3/time point). Values are mean±standard error. (B) Livers from mice fed CDAA diet for four weeks were fractionated into four major cell populations as described in Materials and Methods section. Expression of TNF-α, IL-1β, IL-6, and TGF-β mRNA was measured by quantitative real-time PCR. Results are expressed relative to the expression in hepatocyte fraction. Values are mean±standard error. Hep, hepatocytes; KC, Kupffer cells; EC, endothelial cells; HSC, hepatic stellate cells.
JNK1 Is Required for Cytokine Production in Kupffer Cells
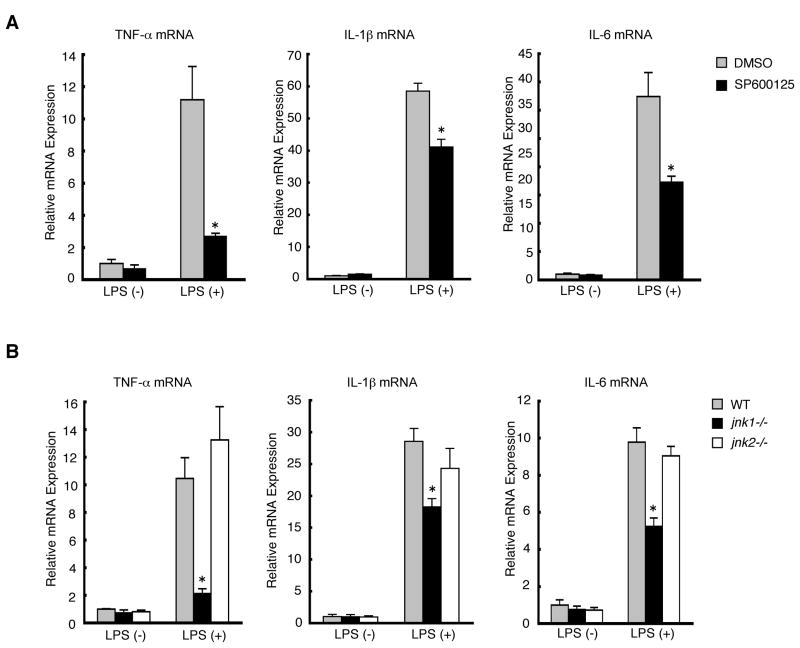
To assess the role of JNK isoforms in Kupffer cells, primary Kupffer cells were isolated from wild-type mice and treated with LPS, which is a potent activator of cytokine production in Kupffer cells and LPS plasma levels are elevated in both patients with NAFLD and experimental animal models, including our present study (Supplementary Figure S4).13, 33, 34 LPS treatment dramatically induced mRNA expression of TNF-α, IL-1β, and IL-6 in Kupffer cells as expected, whereas these gene inductions were significantly blocked by pretreatment with a JNK inhibitor, SP600125, suggesting the important role of JNK isoforms in Kupffer cells (Figure 5A). To further assess the distinct roles for JNK1 and JNK2, Kupffer cells were isolated from wild-type, jnk1−/−, and jnk2−/− mice, and treated with LPS. The results revealed that the mRNA expression of TNF-α, IL-1β, and IL-6 was significantly reduced in jnk1−/− Kupffer cells as compared to wild-type or jnk2−/− Kupffer cells (Figure 5B). These results demonstrate that JNK1 is required for the production of proinflammatory cytokines in Kupffer cells.
Figure 5. JNK1 is required for cytokine production in Kupffer cells.
Expression of TNF-α, IL-1β, and IL-6 mRNA was measured by quantitative real-time PCR in mouse primary Kupffer cells with or without LPS treatment (100 ng/ml) for 3 hours. (A) Primary Kupffer cells isolated from wild-type mice were pre-treated with DMSO or SP600125 followed by LPS treatment. Values are mean±standard error. *P<0.01 (SP600125 vs DMSO). (B) Primary Kupffer cells isolated from wild-type, jnk1−/−, and jnk2−/− mice were treated with LPS. Values are mean±standard error. *P<0.01 (jnk1−/− vs wild-type).
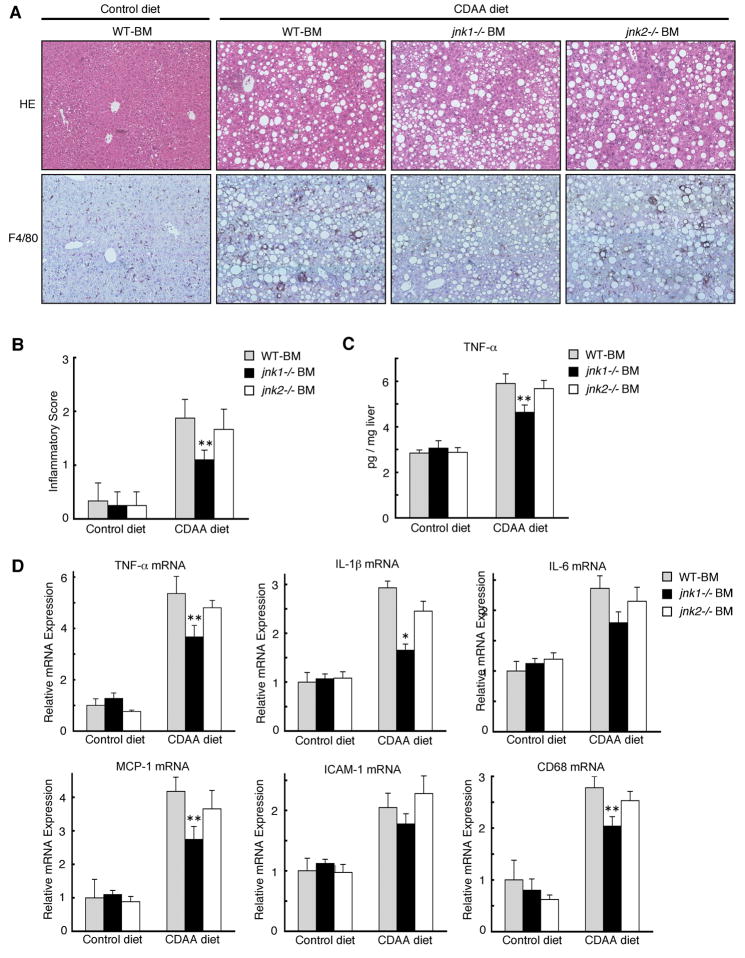
jnk1 Deletion in Hematopoietic Cells Decreases CDAA diet-Induced Hepatic Inflammation
To assess the role of JNK isoforms in Kupffer cells in the development of CDAA diet-induced hepatic inflammation in vivo, we generated chimeric mice in which wild-type, wild-type expressing transgenic GFP driven by the β-actin promoter, jnk1−/−, or jnk2−/− bone marrow cells were transplanted to lethally irradiated wild-type recipient mice in combination with liposomal clodronate to eliminate endogenous Kupffer cells. As previously reported,29 this method achieves full reconstitution of hematopoietic cells including Kupffer cells, confirmed by FACS analysis of peripheral blood and immunofluorescent staining of liver sections for F4/80 in chimeric mice containing bone marrow cells from transgenic mice expressing GFP driven by β-actin promoter (Supplementary Figure S5). Chimeric mice, in which resident non-hematopoietic cells were wild-type but hematopoietic cells were replaced by either wild-type, jnk1−/−, or jnk2−/− bone marrow cells, were then fed a CDAA diet or a control diet for 20 weeks. As expected, both body weights and liver-to-body weight ratios were similar (Supplementary Table S1), and also the levels of lipid deposition in the liver were similar between these chimeric mice fed CDAA diet (Figure 6A). On the other hand, even with the same levels of hepatic steatosis, Kupffer cell accumulation was markedly reduced in the chimeric mice with jnk1−/− bone marrow cells as compared to chimeric mice with wild-type or jnk2−/− bone marrow cells (Figure 6A, Supplementary Figure S6). The diminished inflammation in chimeric mice with jnk1−/− bone marrow cells was also confirmed by lower inflammatory score, and decreased protein and mRNA expression of TNF-α, IL-1β, IL-6, MCP-1, ICAM-1, and CD68 in the liver (Figure 6B, 6C, 6D). Thus, absence of jnk1 in hematopoietic cells including Kupffer cells decreases CDAA diet-induced hepatic inflammation.
Figure 6. jnk1 deletion in hematopoietic cells decreases CDAA diet-induced liver fibrosis.
Chimeric mice with wild-type, jnk1−/−, or jnk2−/− bone marrow cells were fed a CDAA diet (n = 8–10) or a control diet (n = 4–6) for 20 weeks. (A) HE staining of liver sections (upper panel) and immunohistochemical staining for F4/80 on liver sections (lower panel) are shown. (B) The degree of hepatic inflammation was graded. (C) Hepatic TNF-α protein level was analyzed by ELISA. (D) Hepatic expression of TNF-α, IL-1β, IL-6, MCP-1, ICAM-1, and CD68 mRNA was measured by quantitative real-time PCR. Values are mean ±standard error. *P<0.01, **P<0.05 (jnk1−/−BM vs WT-BM). WT-BM, chimeric mice with wild-type bone marrow cells; jnk1−/− BM, chimeric mice with jnk1−/− bone marrow cells; jnk2−/− BM, chimeric mice with jnk2−/− bone marrow cells.
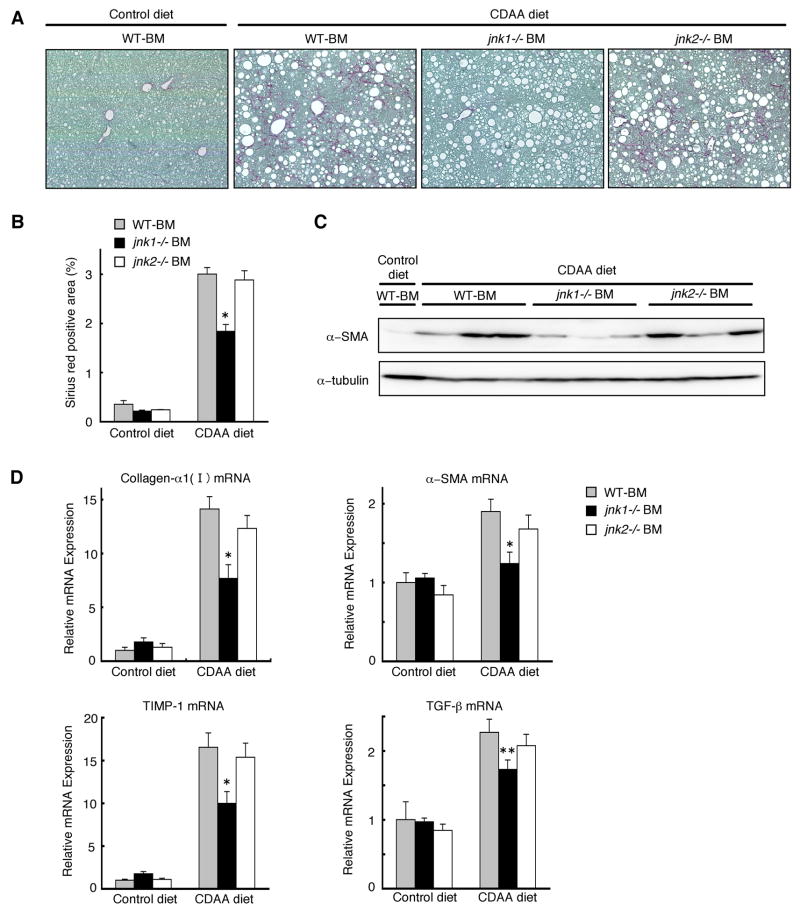
jnk1 Deletion in Hematopoietic Cells Decreases CDAA diet-Induced Liver Fibrosis
Finally, we assessed the levels of CDAA diet-induced liver fibrosis in these chimeric mice. Even with similar levels of hepatic steatosis, chimeric mice with jnk1−/− bone marrow cells had reduced liver fibrosis as compared to those chimeric mice with wild-type or jnk2−/− bone marrow cells as assessed by Sirius red staining (Figure 7A, 7B). The decreased liver fibrosis in chimeric mice with jnk1−/− bone marrow cells was further documented by less protein and mRNA levels of fibrosis markers such as α-SMA, collagen-α1(I), TIMP-1, and TGF-β in the liver (Figure 7C, 7D). Thus, deletion of jnk1 in hematopoietic cells including Kupffer cells decreases CDAA diet-induced hepatic fibrosis. These results suggested that the development of liver fibrosis is closely correlated with the level of hepatic inflammation that requires JNK1 in hematopoietic cells.
Figure 7. jnk1 deletion in hematopoietic cells decreases CDAA diet-Induced liver fibrosis.
Chimeric mice with wild-type, jnk1−/−, or jnk2−/− bone marrow cells were fed a CDAA diet (n = 8–10) or a control diet (n = 4–6) for 20 weeks. (A) Collagen deposition was evaluated by Sirius red staining. (B) Sirius red positive area was quantified. (C) α-SMA and α-tubulin expression in whole liver extracts were analyzed by Western blot analysis with antibodies against α-SMA and α-tubulin. (D) Hepatic expression of collagen-α1(I), α-SMA, TIMP-1, and TGF-β mRNA was measured by quantitative real-time PCR. Values are mean±standard error. *P<0.01, **P<0.05 (jnk1−/−BM vs WT-BM). WT-BM, chimeric mice with wild-type bone marrow cells; jnk1−/− BM, chimeric mice with jnk1−/− bone marrow cells; jnk2−/− BM, chimeric mice with jnk2−/− bone marrow cells.
Discussion
JNK plays a central role in insulin resistance that underlies the development of the metabolic syndrome.19, 20, 23 In the liver, in addition to its effect on acute hepatocelluar injury,35–40 JNK activation is required for hepatic steatosis suggesting the important role of JNK in hepatocytes, an insulin target cell.21, 22 However, previous studies have not characterized the distinct contributions of JNK isoforms in the development of liver fibrosis, the final outcome of most chronic liver diseases including NAFLD. The present study shows that JNK1 in hematopoietic cells, non-insulin target cells, also plays a pivotal role in the formation of hepatic steatosis-induced chronic inflammation and liver fibrosis.
Although high fat diet and MCD diet are commonly used as models of steatohepatitis, the absence of collagen deposition and the loss of body weight are the major problems for long-term fibrogenesis experiments. Our present study uses the CDAA diet. The essential difference between the MCD and CDAA diets is the content of methionine. The CDAA diet is deficient in choline only, but contains methionine, allowing one to observe the sequential development of steatohepatitis, liver fibrosis, and cancer in a longer experimental period in both mice and rats without any body weight loss.25–28 This reproduces the natural course of human NAFLD. In our present study, no difference was found in body weights between mice fed a CDAA diet or a control diet, allowing us to observe these mice for 20 weeks without any dropouts. More importantly, we found a remarkable collagen deposition in wild-type and jnk2−/− mice but less in jnk1−/− mice, suggesting the important role of JNK1 in the development of liver fibrosis. On the other hand, unlike the high fat diet or other genetic models of metabolic syndrome such as ob/ob or A-ZIP/F1 mice, insulin sensitivity was not altered by CDAA diet in wild-type and jnk1−/− mice. Thus, insulin resistance (IR), another characteristic of NAFLD, was not reproduced by CDAA diet. However, this fact means that IR is not essential for the development of liver fibrosis, at least in this model. Given the role of JNK1 in fatty liver formation,21, 22 less liver fibrosis in jnk1−/− mice could be due to less hepatic steatosis. However, to our surprise, the levels of hepatic fat deposition were equivalent in wild-type, jnk1−/−, and jnk2−/− mice. This is in contrast to studies demonstrating less steatosis in jnk1−/− mice fed high fat diet or MCD diet. This is probably because the CDAA diet inhibits lipid secretion from liver that would induce hepatic steatosis independent from genetic background.41 On the other hand, the level of hepatic inflammation was less in jnk1−/− mice compared to those of wild-type and jnk2−/− mice. Thus, in our 20 weeks CDAA diet steatohepatitis/liver fibrosis model, jnk1−/− mice had less fibrosis with less inflammation despite similar levels of steatosis in the liver.
Similar to human NASH, the present study demonstrated chronic hepatic inflammation, which is mediated by Kupffer cells, in mice fed CDAA diet. Furthermore, consistent with previous reports,23 our in vitro experiments clearly showed that JNK1 but not JNK2 is required for the production of proinflammatory cytokines in primary Kupffer cells. Based on these results, we hypothesized that JNK1 in Kupffer cells plays a role in the development of CDAA diet-induced hepatic inflammation and subsequent liver fibrogenesis. JNK1 and JNK2 are ubiquitously expressed in most cells including hepatocytes, where have been implicated in hepatic steatosis and subsequent hepatocellular injury.21, 22, 38, 39 To separate the genetic analysis of Kupffer cells from other lineages of cells in vivo, we generated chimeric mice in which only the hematopoietic compartment including Kupffer cells is replaced by either wild-type, jnk1−/−, or jnk2−/− cells. Although these chimeric mice developed similar levels of hepatic steatosis on the CDAA diet, chimeric mice with jnk1−/− bone marrow cells had reduced hepatic inflammation as compared to those chimeric mice with wild-type or jnk2−/− bone marrow cells. Together with our in vitro data, these results suggested that JNK1 in hematopietic cells is required for hepatic steatosis-induced inflammation, at least in part, by Kupffer cell activation. jnk1 deletion in hematopoietic cells also resulted in less accumulation of Kupffer cells in the liver of mice fed CDAA diet. However, the number of Kupffer cells was equivalent between the three types of chimeric mice fed the control diet (Supplementary Figure S3, S6), jnk1−/− Kupffer cells had less induction of proinflammatory cytokines in vitro, and jnk1−/− macrophages display normal chemotactic activity,23 suggesting that less inflammation due to less Kupffer cell activation might cause less recruitment of peripheral monocytes. In accordance with this concept, hepatic expression of ICAM-1 was reduced in both jnk1−/− mice and chimeric mice with jnk1−/− bone marrow cells.
Finally, we evaluated the level of liver fibrosis in these chimeric mice. Chimeric mice with jnk1−/− bone marrow cells had reduced liver fibrosis as compared to chimeric mice with wild-type or jnk2−/− bone marrow cells. The observations that less hepatic inflammation and less liver fibrosis despite similar hepatic steatosis in chimeric mice with jnk1−/− bone marrow cells suggests that JNK1 in hematopoietec cells contributes to the development of liver fibrosis through mediating hepatic inflammation. Previous studies demonstrated that Kupffer cells can interact with hepatic stellate cells, accelerate their activation, and promote their fibrogenic responses in vitro.29, 42, 43 The present study demonstrated that Kupffer cells are the major producer of TGF-β, the most potent fibrogenic cytokine, in the livers of mice fed the CDAA diet. Taken together, activated Kupffer cells may promote the development liver fibrosis through directly activating hepatic stellate cells by producing TGF-β. Our present study does not exclude the possible contribution of JNK1 in non-hematopoietic cells such as hepatocytes, endothelial cells, or hepatic stellate cells to the development of liver fibrosis. Our ongoing experiments with bone marrow transplantation of wild-type bone marrow cells into jnk1−/− mice will quantitate any contribution of JNK1 in these cells.
In summary, our results demonstrate that JNK1 in hematopoietic cells including Kupffer cells contributes to the development of diet-induced liver fibrosis through mediating the hepatic steatosis-induced chronic inflammation. Given the multiple roles of JNK signaling in hepatocyte survival or liver regeneration,44, 45 JNK1 in hematopoietic cells would be an attractive target for anti-inflammatory therapy against steatohepatitis and liver fibrosis.
Supplementary Material
Acknowledgments
This study is supported by NIH grants R01 DK072237 and R01 GM041804.
We thank Dr. Katsumi Miyai (UCSD), Dr. WuQiang Fan (UCSD), and Dr. Hidetaka Morinaga (UCSD) for their helpful discussions and technical assistance.
Abbreviations
- CDAA
choline-deficient L-amino acid-defined
- FACS
fluorescent-activated cell sorting
- GFP
green fluorescent protein
- HE
hematoxylin-eosin
- ICAM-1
intracellular adhesion molecule-1
- IL
interleukin
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MCD
methionine- and choline-deficient
- MCP-1
monocyte chemoattractant protein-1
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PCR
polymerase chain reaction
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- α-SMA
α-smooth muscle actin
- SREBP-1c
sterol regulatory element binding protein-1c
- TGF-β
transforming growth factor-β
- TIMP-1
tissue inhibitor of metalloproteinase-1
- TNF-α
tumor necrosis factor-α
- WT
wild-type
Footnotes
Conflicts of interest: There is no conflict of interest to disclose.
Contribution of authors: each author was involved with this study.
1. Yuzo Kodama: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript
2. Tatiana Kisseleva: technical support, material support
3. Keiko Iwaisako: acquisition of data, technical support
4. Kouichi Miura: acquisition of data, analysis and interpretation of data
5. Kojiro Taura: analysis and interpretation of data
6. Samuele Deminicis: technical support
7. Christoph H. Österreicher: acquisition of data, statistical analysis
8. Bernd Schnabl: critical revision of the manuscript for important intellectual content
9. Ekihiro Seki: technical support, interpretation of data
10. David A. Brenner: study concept and design, critical revision of the manuscript for important intellectual content, obtained funding, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–57. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 3.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 4.Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 5.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 7.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–75. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–50. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 9.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 10.Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670–8. doi: 10.1002/hep.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malaguarnera L, Di Rosa M, Zambito AM, dell’Ombra N, Nicoletti F, Malaguarnera M. Chitotriosidase gene expression in Kupffer cells from patients with non-alcoholic fatty liver disease. Gut. 2006;55:1313–20. doi: 10.1136/gut.2005.075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, Han JY, Kato S, Shimoda M, Oike Y, Tomizawa M, Makino S, Ohkura T, Saito H, Kumagai N, Nagata H, Ishii H, Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–24. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–9. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–96. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 15.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–14. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 16.Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–6. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dela Pena A, Leclercq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663–74. doi: 10.1053/j.gastro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Ip E, Dela Pena A, Hou JY, Sesha J, Pera N, Hall P, Kirsch R, Leclercq I, Farrell GC. COX-2 induction in mice with experimental nutritional steatohepatitis: Role as pro-inflammatory mediator. Hepatology. 2006;43:826–36. doi: 10.1002/hep.21108. [DOI] [PubMed] [Google Scholar]
- 19.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 20.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–53. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 21.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–72. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 22.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–6. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–97. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Kodama Y, Brenner DA. c-Jun N-terminal kinase signaling in the pathogenesis of nonalcoholic fatty liver disease: Multiple roles in multiple steps. Hepatology. 2009;49:6–8. doi: 10.1002/hep.22710. [DOI] [PubMed] [Google Scholar]
- 25.Denda A, Kitayama W, Kishida H, Murata N, Tsutsumi M, Tsujiuchi T, Nakae D, Konishi Y. Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn J Cancer Res. 2002;93:125–32. doi: 10.1111/j.1349-7006.2002.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takami T, Terai S, Yokoyama Y, Tanimoto H, Tajima K, Uchida K, Yamasaki T, Sakaida I, Nishina H, Thorgeirsson SS, Okita K. Human homologue of maid is a useful marker protein in hepatocarcinogenesis. Gastroenterology. 2005;128:1369–80. doi: 10.1053/j.gastro.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Denda A, Kitayama W, Kishida H, Murata N, Tamura K, Kusuoka O, Tsutsumi M, Nishikawa F, Kita E, Nakae D, Konishi Y, Kuniyasu H. Expression of inducible nitric oxide (NO) synthase but not prevention by its gene ablation of hepatocarcinogenesis with fibrosis caused by a choline-deficient, L-amino acid-defined diet in rats and mice. Nitric Oxide. 2007;16:164–76. doi: 10.1016/j.niox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Fujita K, Nozaki Y, Yoneda M, Wada K, Endo H, Takahashi H, Iwasaki T, Inamori M, Abe Y, Kirikoshi H, Kobayashi N, Kubota K, Saito S, Nagashima Y, Nakajima A. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut. 2008 doi: 10.1136/gut.2007.144550. [DOI] [PubMed] [Google Scholar]
- 29.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 30.Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest. 2006;116:2955–63. doi: 10.1172/JCI27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S, Brenner DA. Hepatic Stellate Cells Secrete Angiopoietin 1 That Induces Angiogenesis in Liver Fibrosis. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 32.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, Moh A, Fu XY, Pacher P, Kunos G, Gao B. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–58. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–25. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 34.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452–5. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 35.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–13. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–67. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–78. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 39.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–31. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kodama Y, Taura K, Miura K, Schnabl B, Osawa Y, Brenner DA. Antiapoptotic effect of c-Jun N-terminal Kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136:1423–34. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 41.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology. 2006;44:1487–501. doi: 10.1002/hep.21427. [DOI] [PubMed] [Google Scholar]
- 43.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–46. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 44.Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–32. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 45.Hasselblatt P, Rath M, Komnenovic V, Zatloukal K, Wagner EF. Hepatocyte survival in acute hepatitis is due to c-Jun/AP-1-dependent expression of inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:17105–10. doi: 10.1073/pnas.0706272104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.