Abstract
The application of adenoviral gene therapy for cancer is limited by immune clearance of the virus as well as poor transduction efficiency, since the protein used for viral entry (CAR) serves physiological functions in adhesion and is frequently decreased among cancer cells. Cationic polymers have been used to enhance adenoviral gene delivery but novel polymers with low toxicity are needed to realize this approach. We recently identified polymers that were characterized by high transfection efficiency of plasmid DNA and a low toxicity profile. In this study we evaluated the novel cationic polymer EGDE-3,3′ for its potential to increase adenoviral transduction of the CAR-negative bladder cancer cell line TCCSUP. The amount of adenovirus required to transduce 50-60% of the cells was reduced 100-fold when Ad.GFP was pre-incubated with the EGDE-3,3′ polymer. Polyethyleneimine (pEI), a positively charged polymer currently used as a standard for enhancing adenoviral transduction, also increased infectivity, but transgene expression was consistently higher with EGDE-3,3′. In addition, EGDE-3,3′-supplemented transduction of an adenovirus expressing an apoptosis inducing transgene, Ad.GFP-TRAIL, significantly enhanced the amount of cell death. Thus, our results indicate that novel biocompatible polymers may be useful in improving the delivery of adenoviral gene therapy.
Keywords: Polymer, adenovirus, gene therapy, cancer, bladder cancer
Introduction
In the United States, bladder cancer is the second most common cancer of the urogenital tract with over 68,000 new cases and 14,000 deaths in 2008 1. While the majority of lesions are superficial at diagnosis, the risks of recurrence and progression are high and close long-term monitoring is required, which makes bladder cancer one of the costliest diseases 2. Despite therapy nearly half of all patients with superficial disease experience relapse or progression within 5 years. Patients who present with advanced disease have, even with multimodal therapy, only 20-40% survival at 5 years. Thus, more effective treatment strategies, aimed at reducing the recurrence and progression of superficial bladder cancer as well as improving therapeutic outcome in patients with advanced disease, are needed.
Gene therapy has garnered significant attention as a therapeutic approach for bladder cancer disease 3. Viral vectors that have been explored for bladder cancer gene therapy include herpes virus, retrovirus, adeno-associated virus (AAV), and adenovirus. Adenoviral delivery of therapeutic transgenes is particularly attractive since these vectors infect a wide range of cells with high efficiency but remain episomal thus minimizing the risk of insertional mutations. Despite the widespread use of adenoviral vectors for transgene expression, in vivo use of adenovirus faces significant hurdles. Neutralization of type 5 adenovirus by pre-existing or induced antibodies results in a significant decrease of target cell infection 4, 5. In addition, following intravenous delivery adenovirus is inactivated by interactions with cells of the innate immune system as well as platelets and erythrocytes 6, 7; these ‘non-specific’ interactions limit the efficacy of the delivered viral vectors and lead to toxicity 8. Since adenovirus can be delivered to malignant lesions in the bladder by intravesical instillation, complications associated with systemic delivery may be reduced but cannot be completely avoided. Additionally, challenges such as effective transduction of target cells remain. Adenovirus enters cells via a cell surface protein known as coxsackie and adenovirus receptor (CAR) that physiologically functions as an adhesion protein 9. In bladder cancer, decreased CAR expression has been associated with increased tumor invasiveness and poor prognosis 10. Thus the development of strategies to overcome obstacles associated with immune clearance and viral transduction of CAR negative cells are needed.
Cationic lipids and polymers have been employed to enhance adenovirus-mediated gene delivery 11-18. Cationic lipids and macromolecules can overcome the repulsion between the negatively charged adenoviral surfaces and anionic glycosaminoglycan coated epithelial cells, leading to enhanced adenoviral infection in cells that downregulate CAR19. For example, cationic polymers including poly-L-lysine11 and poly(ethylene imine) (pEI)16 have been previously used for enhancing adenoviral infection but the toxicity of these polymers is a significant limitation to their subsequent use. Thus, while enhancing adenoviral infectivity with cationic polymers remains a promising approach, new polymers that demonstrate high infectivity and low toxicities are needed in order to realize the promise of this strategy. We have recently generated and screened a library of cationic polymers for binding anionic plasmid DNA for cancer cell transfection 20. A number of candidate polymers demonstrated higher transfection efficacies and lower cytotoxicites compared to the commonly used polymer, pEI. In the current study, we investigated the ability of a candidate polymer from this library to enhance adenoviral transduction in CAR-negative bladder cancer cells. We observed that the candidate polymer, ethyleneglycol diglycidyl ether (EGDE)-3,3′-diamino-N-methyl dipropylamine (3,3′) or EGDE-3,3′, demonstrated higher efficacies for enhancing adenoviral transgene expression than pEI. In addition, complexing EGDE-3,3′ with an adenovirus expressing TRAIL enhanced bladder cancer cell death. Our results underscore the opportunities presented by novel biocompatible polymers for enhancing adenoviral infection and apoptosis of bladder cancer cells.
Materials and Methods
Materials
The monomers, ethyleneglycol diglycidyl ether (EGDE) and 3,3′-diamino-N-methyl dipropylamine (3,3′), were purchased from Sigma-Aldrich. In addition, branched 25-kDa poly(ethylene imine) (Mn=10 kDa, Mw=25 kDa, catalog number: 408727; henceforth called pEI) was purchased from Sigma-Aldrich.
Cell lines and culture conditions
Human bladder cancer cells lines 5637, RT4, UM-UC3, TCCSUP, and SW780 were purchased from the American Type Culture Collection (Manassas, VA) and grown in RPMI containing 10% fetal calf serum, with the exception of RT4 cells which were grown in McCoy's medium containing 10% fetal calf serum. All media was supplemented with Gibco 15240 antibiotic-antimycotic mixture (Invitrogen, Grand Island, NY). TCCSUP were used between passages 5 to 15, by splitting nearly confluent cultures up to a 1:10 ratio every 3-4 days.
Adenovirus
Replication-deficient adenoviral vectors were generated by the Medical University of South Carolina (MUSC) vector core facility. The transgene cassette for Ad.GFP contains the green fluorescent protein (GFP) reporter under control of the cytomegalovirus (CMV) promoter. The GFP-TRAIL fusion protein was generated by polymerase chain reaction (PCR) amplification of human TNF-related apoptosis inducing ligand (TRAIL) cDNA and inserting it into the plasmid vector pEGFP-C1 (Clontech). The insert was verified by sequencing and subcloned into the RAd.T.mcs plasmid shuttle vector 21. The verified plasmid was then provided to the MUSC vector core for adenovirus generation as previously described for an adenovirus expressing Fas Ligand 21. Ad.GFP-TRAIL is comparable in potency to the Ad.TRAIL-IRES-GFP we previously generated22, indicating that the GFP fusion does not interfere with TRAIL function. One difference between these constructs is that transgene expression in Ad.TRAIL-IRES-GFP is under control of the Tet-On system, while Ad.GFP-TRAIL uses the Tet-Off system. A benefit of the GFP-TRAIL fusion protein is that subcellular localization of TRAIL can be studied by fluorescence microscopy to detect GFP.
Polymer Synthesis
Polymerizations were carried out as described previously20. Briefly, ethyleneglycol diglycidyl ether (EGDE; 2.3 mmol) was reacted with equimolar quantities of 3,3′-diamino-N-methyl dipropylamine (3,3′) resulting in the formation of the EGDE-3,3′ polymer. Neat, as-purchased solutions were employed in the polymerization reaction, which was carried out in 7 mL glass scintillation vials for 16 h. Following completion of the reaction, the resulting polymer was diluted to a concentration of 10 mg/mL in phosphate-buffered saline (PBS). The solution pH was adjusted to 7.4 using 30% hydrochloric acid in de-ionized (DI) water in order to compensate for the alkalinity of the cationic polymers. Polymer stock concentrations of 10 mg/mL were used in the experiments.
Kinetics of Polymerization
The polymerization reaction involves the reaction of epoxide groups on the diglycidyl ethers with primary and secondary amines of the polyamines. As a result, the disappearance of reactive (primary and secondary) amines with time was employed to follow the kinetics of the diglycidyl ether – polyamine reaction20. The ninhydrin assay23 results in a yellow-orange color in case of secondary amines and a dark blue / purple color in case of primary amines and was therefore used to determine the concentration of residual reactive amines using absorbance analyses at 570 nm at each time point as described previously20. The assay was carried out every 4 h and the concentration of reactive amines in the polymerization mixture at a given time point was normalized with the concentration of amines at the beginning of the reaction (t=0) in order to obtain percentage residual reactive amine values.
Polymer Characterization
Fourier Transform Infrared (FT-IR) Spectroscopy spectra of the polymer sample were analyzed at two different time points (t= 0 h and 16 h) using a Bruker IFS 66 v/S FT-IR spectrometer in order to ascertain the formation of the EGDE-3,3′ polymer. The polymer was loaded such that it covered a substantial area around the center of a germanium attenuated total reflectance (GATR) crystal. The sample chamber was maintained at 4 millibar pressure in order to minimize interference from atmospheric water and CO2. The absorption spectrum between 650 and 4000 cm-1 was measured and the background spectrum of the crystal was subtracted from the sample spectra. Polymer molecular weight and polydispersity were determined using gel permeation chromatography as described previously20.
Adenoviral transduction
Cells were plated overnight at a density of 6×104/well in a 24-well plate. The next day, Ad.GFP was diluted in growth medium to the appropriate multiplicity of infection (MOI) and growth medium in the wells was replaced with medium containing adenovirus. For experiments with polymers, virus was diluted to the appropriate MOI and pre-incubated for 10 minutes at room temperature with 2 μl/well polymer (10 mg/ml stock) in a final volume of 67 μl/well growth medium. Following the 10-minute incubation, 100 μl/well growth medium was added to the tube and the growth medium in each well replaced with 169 μl polymer/virus mixture. After 4 hours, an additional 400 μl growth medium was added to each well to ensure cells had sufficient nutrients for the assay period. Cells were assayed for GFP expression and/or cell death 48 hours post-infection.
Western blot analysis
Protein was prepared and analyzed as described previously24. The antibody recognizing CAR (M-150) was purchased from SantaCruz Biotechnology (Santa Cruz, CA) and was used at a dilution of 1:1000.
Flow cytometry
All cells (non-adherent and adherent) were included in the analysis. Briefly, 48 hours post-infection, media were collected and adherent cells rinsed with PBS prior to adding trypsin. The PBS wash was added to the tubes containing the spent media. After cells had been detached with trypsin, they were pooled with the non-adherent cells. Cells were pelleted at 1500 rpm for 10 minutes and pellets resuspended in PBS. Viaprobe (20 μl) a cell viability detection reagent containing 7-amino-actinomycin D, (7-AAD) (BD Bioscience Pharmingen, San Jose, CA) was added where appropriate. Samples were analyzed in the MUSC core facility using a FACSCalibur (BD). A minimum of 10,000 events were evaluated and analyzed with the CellQuest software.
Results and Discussion
TCCSUP bladder cancer cells are highly resistant to adenoviral transduction due to lack of CAR expression
The first goal for testing whether cationic polymers from our newly developed library can enhance adenoviral transduction and transgene expression was to identify a cell line that is resistant to adenoviral infection. Adenovirus infects cells via receptor-mediated endocytosis following binding to the cell surface protein CAR (coxsackie and adenovirus receptor). Since CAR expression is frequently decreased in bladder cancer, we screened a panel of bladder cancer cell lines for infectivity and CAR expression. Cells were exposed to increasing concentrations of a replication-deficient adenoviral construct that expresses a CMV-driven GFP reporter gene (Ad.GFP). The percentage of GFP positive cells was assessed by flow cytometry at 48 hours post-infection. As shown in Figure 1, transduction rates of 5637, RT4, SW780, and UM-UC-3 cells were similar with 50% infectivity achieved at an MOI of 10. In TCCSUP cells, however, even a 10-fold higher concentration of adenovirus resulted in transduction of only 20%, suggesting these cells may have reduced CAR expression. Analysis of CAR expression by Western blot confirmed that the protein is absent or at least below detectable levels in TCCSUP cells (Fig. 1, insert). TCCSUP cells were isolated from a patient with transitional cell carcinoma that later metastasized to the bone 25. Lack of CAR expression, which decreases cellular adhesion and correlates with bladder cancer aggressiveness, may offer an explanation for this outcome 10. In addition to down-regulated CAR, the abundant expression of negatively charged glycosaminoglycans on bladder cancer epithelial cells results in repulsion of adenoviruses due to the anionic nature of their outer surfaces (e.g. hexons)18, 19. Excess cationic charge on the virus surface from the cationic polymer can facilitate electrostatic interactions between the polymer:virus complex (positively charged) and GAGs (negatively charged), facilitating cellular binding and uptake, which in turn results in increased infection. In addition, polymers can mediate viral aggregation towards the surface of targeted cells which also promotes increased transduction26.
Figure 1.

Adenoviral infectivity and CAR expression. Bladder cancer cells were plated overnight and exposed to the indicated amount of Ad.GFP for 4 hours. GFP expression was quantified by flow cytometry at 48 hours post-infection. Data represent the mean ± SD from at least 3 independent experiments, except RT4 cells which were tested only once. Insert: Total CAR expression was assessed by Western blot (U=UM-UC-3, T=TCCSUP). Actin is included as a loading control. Similar results were obtained in at least 2 independent experiments.
Characterization of Cationic Polymers for adenoviral transduction
The observation that TCCSUP cells lack CAR expression and are extremely difficult to transduce with adenovirus allowed us to evaluate the efficacy of polymers on viral uptake and gene expression. Cationic polymers neutralize the anionic charge on plasmid DNA and viral surfaces by means of electrostatic interactions 27, 28. As a result cationic polymers have been employed for the delivery of plasmid DNA and viral based gene expression systems to mammalian cells11, 14, 17, 26, 29-47. Recent results in our laboratory led to the identification of cationic polymers that demonstrated high DNA binding and gene transfection efficacies20. We therefore hypothesized that candidate cationic polymers from this library would be able to neutralize the anionic charge on adenoviral surfaces leading to enhanced transduction of TCCSUP cells that have undetectable CAR expression. The cationic polymer, EGDE-3,3′ was among the highest DNA-binding polymers identified in our previous screen20. Consequently, we tested the efficacy of both pEI and EGDE-3,3′ on adenoviral transduction.
The formation of the EGDE-3,3′ polymer was evaluated by the decrease in reactive amines over time using a ninhydrin assay based on absorbance spectroscopy. The amine concentration was determined based on a calibration curve generated using glycine standards. Approximately 70% primary reactive amines had disappeared 12 h after the reaction, after which the concentration of the reactive amines remained unchanged 20. This indicated that the polymer was formed rapidly and that the reaction rate remained largely invariant after 12 h. The molecular weights of the EGDE-3,3′ polymer determined by GPC were: Mn =14.52 (± 0.26) kDa, Mw = 72.06(± 0.72) kDa, resulting in a polydispersity of 4.96_20. The formation of the EGDE-3,3′ polymer was further analyzed using Fourier Transform Infrared (FT-IR) spectroscopy following the appearance and disappearance of bands at two different times (t=0 and 16 h). The reaction time of 16 h was chosen in order to allow the polymerization to reach completion based on the kinetics. At time t=0 h, peaks in the wave number range of 858-918 cm-1, attributed to the stretching and contraction of C-O groups, can be seen in the monomer mixture indicating the presence of epoxide groups (Figure 2B) 48. However, the reduction in transmittance in this range indicates the disappearance of epoxide groups, which is consistent with the chemistry of the polymerization reaction. The disappearance of the epoxide groups is accompanied by a concomitant increase in hydroxyl (-OH) groups. The appearance of broad bands in the wave number range 3382-3402 cm-1 is indicative of the hydroxyl groups generated as a result of the polymerization reaction 49. Characteristic spectral bands of primary amines were seen at 1100-1128 and 3358-3382 cm-1 due to the presence of C-N and N-H bonds, respectively. Taken together, FT-IR analysis further confirmed that the diglycidyl ether-polyamine reaction resulted in the formation of the EGDE-3,3′ polymer used in the current investigation and are along the lines of those observed previously for such polymerizations20.
Figure 2.
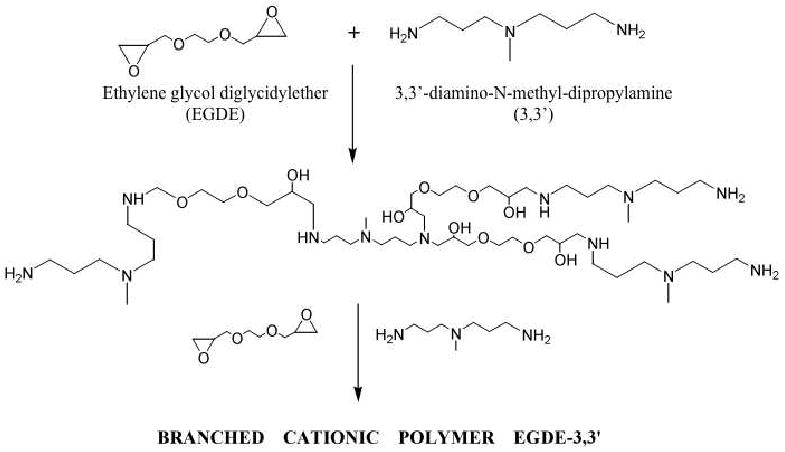

(A). Schematic of the polymerization reaction for generating the EGDE-3,3′ polymer based on ethyleneglycol diglycidyl ether (EGDE) and 3,3′-diamino-N-methyl dipropylamine (3,3′) monomers. (B). Fourier Transform Infrared (FT-IR). Spectroscopy of EGDE-3,3′ polymerization at t= 0 and 16 h. Polymerization reaction can be seen by the emergence of the hydroxyl peak from 3000-4000 cm-1 and the disappearance of the epoxide peak in the 860-950 cm-1 region of the FT-IR spectrum. These regions are marked with asterisks in the figure.
Cationic Polymers facilitate adenoviral transduction in TCCSUP cells
Having confirmed synthesis of EGDE-3,3′ polymer, we next determined the effect of adenovirus-polymer pre-incubation on viral transduction; pEI was included for comparison in our analysis. In the absence of polymer, even 3000 MOI of Ad.GFP resulted in only 50-60% infectivity. Although the extremely high concentration of adenovirus did not adversely affect TCCSUP cells, the amount is not physiologically relevant. When Ad.GFP was pre-incubated in the presence of polymers, adenoviral transduction was greatly enhanced and a rate of 50-60% infectivity was now achieved at 30 MOI (Figure 3A). In addition to infectivity, we also evaluated GFP intensity as a measure of transgene expression. Though both polymers enhanced infectivity to a similar extent with respect to the number of cells transduced, the EGDE-3,3′ polymer consistently resulted in higher transgene expression in the individual cells (Figure 3B, C). Brightfield analysis did not indicate any obvious adverse effects of the polymers at the concentrations used, but the long-term effect on viability will be further investigated in the future (Fig 3D). Fluorescent microscopy confirmed the increase in infectivity and reporter transgene expression (Figure 3D). These data indicate that pre-incubation with polymers decreases the amount of adenovirus required to transduce 50-60% of CAR-negative cells by 100-fold. Both pEI and EGDE-3,3′ polymers enhanced viral infectivity but the latter resulted in improved transgene expression. While the underlying reason for this difference will need to be further investigated it prompted us to examine EGDE-3,3′ in more detail.
Figure 3.
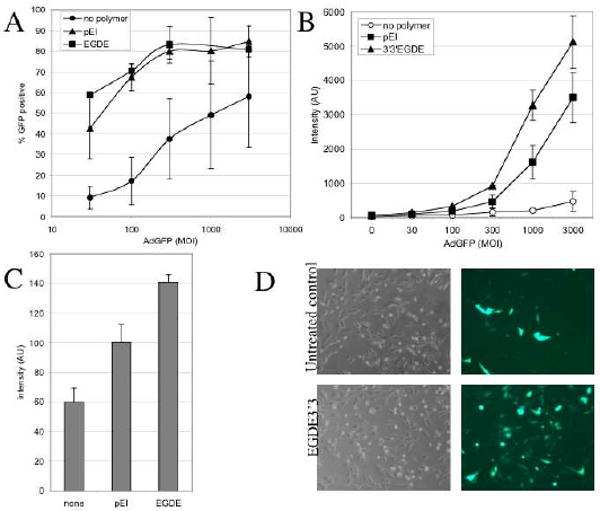
Effect of polymers on infectivity and transgene expression. TCCSUP cells were plated overnight and exposed to Ad.GFP either alone or complexed with polymers as described in the Materials and Methods. GFP expression was quantified by flow cytometry at 48 hours post-infection. (A) % infectivity based on the number of GFP positive cells, (B/C) GFP intensity as measure of transgene expression with increasing MOI (B) and at 30 MOI (C). Data shown are the mean ± SD from 3 independent experiments. (D) Representative images under brightfield and fluorescent microscopy of TCCSUP cells exposed to 30 MOI of Ad.GFP without (control) and with (EGDE-3,3′) polymer pre-incubation.
EGDE-3,3′ polymer enhances Ad.GFP-TRAIL-mediated cell death
To determine whether polymer-enhanced adenoviral delivery would translate into a biological effect, we quantified cell death achieved by adenoviral delivery of a GFP-TRAIL fusion protein (Ad.GFP-TRAIL). Fusion of the death ligand TRAIL to GFP has in our hands not altered the biological function of TRAIL (data not shown). In Figure 4A, we show that the percentage of GFP positive cells is enhanced when the Ad.GFP-TRAIL is pre-incubated with EGDE-3,3′ polymer, though the effect was less pronounced compared to Ad.GFP. Several possibilities can account for this discrepancy. One possibility is that cells infected with Ad.GFP-TRAIL are undergoing cell death, which may not allow accumulation of GFP fluorescence. The fusion protein also exhibits different subcellular distribution than GFP alone and may not be as brightly fluorescent when TRAIL is added to the GFP sequence. The discrepancy could also be related to the different promoters used in the viral vectors.
Figure 4.


Effect of polymers on efficacy of Ad.GFP-TRAIL-induced cell death. (A) Cells were infected with Ad.GFP-TRAIL at 30 MOI (as described for Ad.GFP) in the absence or presence of EGDE-3,3′ polymer. Infectivity was quantified by flow cytometry of GFP (B) Cells were infected with 30 MOI Ad.GFP or Ad.GFP-TRAIL either in the absence or presence of the EGDE-3,3′ polymer. Membrane integrity was quantified by flow cytometry following staining with 7-AAD. % specific 7-AAD uptake was calculated by subtracting background staining in Ad.GFP infected cells. All data shown are the mean ± SD from at least 3 independent experiments. Statistical significance between groups was calculated using the t-test (n.s. = not significant).
Quantification of cell death was performed by flow cytometry with 7-AAD, which intercalates into the DNA in cells with compromised membrane integrity. The effect of the EGDE-3,3′ polymer on cell death was approximately 5% and the effect of Ad.GFP-TRAIL alone was about 12%. The statistical difference did not reach significance (p>0.05). Preincubation of Ad.GFP-TRAIL resulted in a greater than additive effect reaching 32-40% of cell death. Statistical analysis indicates that cell death was significantly higher when Ad.GFP-TRAIL was pre-complexed with EGDE-3,3′ (Figure 4, compare AdGT to AdGT+E, p<0.0005). The data indicate that enhanced viral uptake following incubation of adenovirus with EGDE-3,3′ translates into a biological response.
Conclusions and Future Directions
Our results suggest that the EGDE-3,3′ polymer can enhance adenoviral transduction and transgene expression in cells that lack CAR expression. TCCSUP cells were extremely resistant to adenoviral transduction and the EGDE-3,3′ polymer reduced the amount of virus required to achieve 50-60% transduction by 100-fold, resulting in the ability to use adenovirus at a physiologically relevant concentration. While the use of polymer-adenovirus complexes restores infectivity of malignant bladder epithelial cells, there is a possibility that these complexes also infect non-malignant bladder cells. Studies that compare the relative infection efficacy of polymer-adenovirus complexes for normal and transformed bladder cancer cells will be carried out in the future in our laboratories. In addition, the use of targeting antibodies (e.g. anti-EGFR antibody19, 50, 51 since EGFR is overexpressed in bladder cancer disease) can further enhance selective targeting of bladder cancer cells over normal bladder epithelial cells. The presence of primary amines on our cationic polymers facilitates easy conjugation of targeting antibodies by means of simple, well-established chemistries (e.g. EDC-NHS coupling). These targeted complexes will also be explored in the future. Future directions will also include extension of our findings to other cells that transduce poorly with adenovirus and investigation of other biocompatible polymers from our library for enhancing adenoviral transduction. The use of adenoviral constructs in which therapeutic efficacy relies on local secretion of protein such as cytokines as well as translation into immunocompetent animal models will also be important.
Acknowledgments
We thank Drs. John Dong and Semyon Rubinchik of the MUSC viral vector core for generation of Ad.GFP.TRAIL and Mr. Rick Peppler of the MUSC flow cytometry core facility for technical expertise. This project was supported by grants from the National Cancer Institute (R01CA102218 to CVJ and R21CA133618 to KR), the National Science Foundation (CBET 0829128 to KR) ASU start-up funds (KR), and MUSC start-up funds (CVJ).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Stenzl A, Hennenlotter J, Schilling D. Can we still afford bladder cancer? Current opinion in urology. 2008;18(5):488–92. doi: 10.1097/MOU.0b013e32830b8925. [DOI] [PubMed] [Google Scholar]
- 3.Bochner BH. Gene therapy in bladder cancer. Current opinion in urology. 2008;18(5):519–23. doi: 10.1097/MOU.0b013e32830b86e3. [DOI] [PubMed] [Google Scholar]
- 4.Kreppel F, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Mol Ther. 2008;16(1):16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- 5.Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Human gene therapy. 2004;15(11):1034–44. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- 6.Seiradake E, Henaff D, Wodrich H, Billet O, Perreau M, Hippert C, Mennechet F, Schoehn G, Lortat-Jacob H, Dreja H, Ibanes S, Kalatzis V, Wang JP, Finberg RW, Cusack S, Kremer EJ. The cell adhesion molecule “CAR” and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog. 2009;5(1):e1000277. doi: 10.1371/journal.ppat.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker AL, Nicklin SA, Baker AH. Interactions of adenovirus vectors with blood: implications for intravascular gene therapy applications. Curr Opin Mol Ther. 2008;10(5):439–48. [PubMed] [Google Scholar]
- 8.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79(12):7478–91. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(26):15191–6. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto K, Shariat SF, Ayala GE, Rauen KA, Lerner SP. Loss of coxsackie and adenovirus receptor expression is associated with features of aggressive bladder cancer. Urology. 2005;66(2):441–6. doi: 10.1016/j.urology.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Fasbender A, Zabner J, Chillon M, Moninger TO, Puga AP, Davidson BL, Welsh MJ. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. The Journal of biological chemistry. 1997;272(10):6479–89. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 12.Meunier-Durmort C, Grimal H, Sachs LM, Demeneix BA, Forest C. Adenovirus enhancement of polyethylenimine-mediated transfer of regulated genes in differentiated cells. Gene therapy. 1997;4(8):808–14. doi: 10.1038/sj.gt.3300450. [DOI] [PubMed] [Google Scholar]
- 13.Meunier-Durmort C, Picart R, Ragot T, Perricaudet M, Hainque B, Forest C. Mechanism of adenovirus improvement of cationic liposome-mediated gene transfer. Biochim Biophys Acta. 1997;1330(1):8–16. doi: 10.1016/s0005-2736(97)00133-8. [DOI] [PubMed] [Google Scholar]
- 14.Toyoda K, Ooboshi H, Chu Y, Fasbender A, Davidson BL, Welsh MJ, Heistad DD. Cationic polymer and lipids enhance adenovirus-mediated gene transfer to rabbit carotid artery. Stroke. 1998;29(10):2181–8. doi: 10.1161/01.str.29.10.2181. [DOI] [PubMed] [Google Scholar]
- 15.Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene therapy. 1998;5(7):995–1002. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- 16.Dodds E, Piper TA, Murphy SJ, Dickson G. Cationic lipids and polymers are able to enhance adenoviral infection of cultured mouse myotubes. J Neurochem. 1999;72(5):2105–12. doi: 10.1046/j.1471-4159.1999.0722105.x. [DOI] [PubMed] [Google Scholar]
- 17.Toyoda K, Nakane H, Heistad DD. Cationic polymer and lipids augment adenovirus-mediated gene transfer to cerebral arteries in vivo. J Cereb Blood Flow Metab. 2001;21(9):1125–31. doi: 10.1097/00004647-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Subr V, Kostka L, Selby-Milic T, Fisher K, Ulbrich K, Seymour LW, Carlisle RC. Coating of adenovirus type 5 with polymers containing quaternary amines prevents binding to blood components. J Control Release. 2009;135(2):152–8. doi: 10.1016/j.jconrel.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 19.van der Poel HG, Molenaar B, van Beusechem VW, Haisma HJ, Rodriguez R, Curiel DT, Gerritsen WR. Epidermal growth factor receptor targeting of replication competent adenovirus enhances cytotoxicity in bladder cancer. J Urol. 2002;168(1):266–72. doi: 10.1097/00005392-200207000-00089. [DOI] [PubMed] [Google Scholar]
- 20.Barua S, Joshi A, Banerjee A, Matthews D, Sharfstein ST, Cramer SM, Kane RS, Rege K. Parallel synthesis and screening of polymers for nonviral gene delivery. Mol Pharm. 2009;6(1):86–97. doi: 10.1021/mp800151j. [DOI] [PubMed] [Google Scholar]
- 21.Rubinchik S, Ding R, Qiu AJ, Zhang F, Dong J. Adenoviral vector which delivers FasL-GFP fusion protein regulated by the tet-inducible expression system. Gene therapy. 2000;7(10):875–85. doi: 10.1038/sj.gt.3301172. [DOI] [PubMed] [Google Scholar]
- 22.Voelkel-Johnson C, King DL, Norris JS. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Therapy. 2002;9(2):164–172. doi: 10.1038/sj.cgt.7700420. [DOI] [PubMed] [Google Scholar]
- 23.Friedman M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. Journal of Agricultural and Food Chemistry. 2004;52(3):385–406. doi: 10.1021/jf030490p. [DOI] [PubMed] [Google Scholar]
- 24.El-Zawahry A, Lu P, White SJ, Voelkel-Johnson C. In vitro efficacy of AdTRAIL gene therapy of bladder cancer is enhanced by trichostatin A-mediated restoration of CAR expression and downregulation of cFLIP and Bcl-XL. Cancer Gene Ther. 2006;13(3):281–9. doi: 10.1038/sj.cgt.7700905. [DOI] [PubMed] [Google Scholar]
- 25.Nayak SK, O'Toole C, Price ZH. A cell line from an anaplastic transitional cell carcinoma of human urinary bladder. British journal of cancer. 1977;35(2):142–51. doi: 10.1038/bjc.1977.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis HE, Rosinski M, Morgan JR, Yarmush ML. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophysical journal. 2004;86(2):1234–42. doi: 10.1016/S0006-3495(04)74197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. The Journal of general virology. 2000;81(Pt 11):2605–9. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 28.Mok H, Park JW, Park TG. Enhanced intracellular delivery of quantum dot and adenovirus nanoparticles triggered by acidic pH via surface charge reversal. Bioconjugate chemistry. 2008;19(4):797–801. doi: 10.1021/bc700464m. [DOI] [PubMed] [Google Scholar]
- 29.Akinc A, Anderson DG, Lynn DM, Langer R. Synthesis of poly(beta-amino ester)s optimized for highly effective gene delivery. Bioconjugate chemistry. 2003;14(5):979–88. doi: 10.1021/bc034067y. [DOI] [PubMed] [Google Scholar]
- 30.Anderson DG, Peng W, Akinc A, Hossain N, Kohn A, Padera R, Langer R, Sawicki JA. A polymer library approach to suicide gene therapy for cancer. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(45):16028–33. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen DJ, Majors BS, Zelikin A, Putnam D. Structure-function relationships of gene delivery vectors in a limited polycation library. J Control Release. 2005;103(1):273–83. doi: 10.1016/j.jconrel.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 32.Cohen H, Levy RJ, Gao J, Fishbein I, Kousaev V, Sosnowski S, Slomkowski S, Golomb G. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene therapy. 2000;7(22):1896–905. doi: 10.1038/sj.gt.3301318. [DOI] [PubMed] [Google Scholar]
- 33.Coll JL, Chollet P, Brambilla E, Desplanques D, Behr JP, Favrot M. In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Human gene therapy. 1999;10(10):1659–66. doi: 10.1089/10430349950017662. [DOI] [PubMed] [Google Scholar]
- 34.Kaneda Y, Tabata Y. Non-viral vectors for cancer therapy. Cancer science. 2006;97(5):348–54. doi: 10.1111/j.1349-7006.2006.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little SR, Lynn DM, Ge Q, Anderson DG, Puram SV, Chen J, Eisen HN, Langer R. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(26):9534–9. doi: 10.1073/pnas.0403549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Reineke TM. Poly(glycoamidoamine)s for gene delivery. structural effects on cellular internalization, buffering capacity, and gene expression. Bioconjugate chemistry. 2007;18(1):19–30. doi: 10.1021/bc060029d. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Wenning L, Lynch M, Reineke TM. New poly(d-glucaramidoamine)s induce DNA nanoparticle formation and efficient gene delivery into mammalian cells. Journal of the American Chemical Society. 2004;126(24):7422–3. doi: 10.1021/ja049831l. [DOI] [PubMed] [Google Scholar]
- 38.Maheshwari A, Mahato RI, McGregor J, Han S, Samlowski WE, Park JS, Kim SW. Soluble biodegradable polymer-based cytokine gene delivery for cancer treatment. Mol Ther. 2000;2(2):121–30. doi: 10.1006/mthe.2000.0105. [DOI] [PubMed] [Google Scholar]
- 39.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–93. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 40.Pun SH, Davis ME. Development of a nonviral gene delivery vehicle for systemic application. Bioconjugate chemistry. 2002;13(3):630–9. doi: 10.1021/bc0155768. [DOI] [PubMed] [Google Scholar]
- 41.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 2. Charge center type. Bioconjugate chemistry. 2003;14(1):255–61. doi: 10.1021/bc025593c. [DOI] [PubMed] [Google Scholar]
- 42.Reineke TM, Davis ME. Structural effects of carbohydrate-containing polycations on gene delivery. 1. Carbohydrate size and its distance from charge centers. Bioconjugate chemistry. 2003;14(1):247–54. doi: 10.1021/bc025592k. [DOI] [PubMed] [Google Scholar]
- 43.Bielinska A, Kukowska-Latallo JF, Johnson J, Tomalia DA, Baker JR., Jr Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic acids research. 1996;24(11):2176–82. doi: 10.1093/nar/24.11.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boeckle S, Wagner E. Optimizing targeted gene delivery: chemical modification of viral vectors and synthesis of artificial virus vector systems. The AAPS journal. 2006;8(4):E731–42. doi: 10.1208/aapsj080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis HE, Morgan JR, Yarmush ML. Polybrene increases retrovirus gene transfer efficiency by enhancing receptor-independent virus adsorption on target cell membranes. Biophysical chemistry. 2002;97(23):159–72. doi: 10.1016/s0301-4622(02)00057-1. [DOI] [PubMed] [Google Scholar]
- 46.Landazuri N, Le Doux JM. Complexation of retroviruses with charged polymers enhances gene transfer by increasing the rate that viruses are delivered to cells. The journal of gene medicine. 2004;6(12):1304–19. doi: 10.1002/jgm.618. [DOI] [PubMed] [Google Scholar]
- 47.Lou YL, Peng YS, Chen BH, Wang LF, Leong KW. Poly(ethylene imine)-g-chitosan using EX-810 as a spacer for nonviral gene delivery vectors. J Biomed Mater Res A. 2009;88(4):1058–68. doi: 10.1002/jbm.a.31961. [DOI] [PubMed] [Google Scholar]
- 48.Loos MR, Coelho LAF, Pezzin SH, Amico SC. The effect of acetone addition on the properties of epoxy. Polimeros Ciencia e Tecnologia. 2008;18(1):76–80. [Google Scholar]
- 49.Lee JY, Shim MJ, Lee HK, Kim SW. Reaction kinetics of liquid crystalline epoxy cured with aromatic diamine: Its synthesis and mechanical and electrical characteristics. Journal of Applied Polymer Science. 2001;82(10):2372–2380. [Google Scholar]
- 50.Jung Y, Park HJ, Kim PH, Lee J, Hyung W, Yang J, Ko H, Sohn JH, Kim JH, Huh YM, Yun CO, Haam S. Retargeting of adenoviral gene delivery via Herceptin-PEG-adenovirus conjugates to breast cancer cells. J Control Release. 2007;123(2):164–71. doi: 10.1016/j.jconrel.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Miller CR, Buchsbaum DJ, Reynolds PN, Douglas JT, Gillespie GY, Mayo MS, Raben D, Curiel DT. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer research. 1998;58(24):5738–48. [PubMed] [Google Scholar]


