Abstract
Large conductance Ca2+- activated K+ channels (BKCa) encoded by the Slo1 gene play a role in the physiological regulation of many cell types. Here, we show that the β1 subunit of Na+/K+-ATPase (NKβ1) interacts with the cytoplasmic COOH-terminal region of Slo1 proteins. Reduced expression of endogenous NKβ1 markedly inhibits evoked BKCa currents with no apparent effect on their gating. In addition, NKβ1 down-regulated cells show decreased density of Slo1 subunits on the cell surface.
Keywords: potassium channel, trafficking, ion transporter
Introduction
Large conductance Ca2+-activated K+ channels (BKCa channels) regulate physiology in a wide range of tissues [1]. BKCa channels can be preferentially targeted to different portions of polarized cells. For example, in hippocampal neurons, BKCa channels extensively localize to axons and presynaptic terminals [2-3] whereas in cerebellar Purkinje cells, they are observed primarily in the somato-dendritic region [3]. Similarly, in epithelial cells, BKCa channels can be preferentially expressed on either the apical or basolateral surface [4-5]. The pore-forming subunits of BKCa channels are encoded by the Slo1 gene, also known as KCNMA1. Alternative splicing and protein-protein interactions at the COOH-terminal tail region contribute significantly to regulation of BKCa channels [8-26].
The Na+/K+-ATPase is a ubiquitously expressed member of the P-type ATPase superfamily. The functional enzyme is a heterodimer comprised of a catalytic α-subunit as well as one of at least three highly conserved glycoprotein β-subunits [27-29]. Stoichiometrically unequal amounts of α- and β-Na+/K+-ATPase subunits are present in some tissues [30-32], and the two classes of subunits are subjected to different modes of degradation [33]. This raises the possibility that some Na+/K+-ATPase β-subunits may be free to interact with other proteins. More recent studies suggest that these subunits contribute to cell adhesion and formation of tight junctions [34-35].
We now report that Slo1 proteins biochemically interact with NKβ1 and that this interaction plays a role in regulating the steady-state expression of BKCa channels on the cell surface.
Methods
Plasmids and antibodies
An expression plasmid encoding NH2-terminal (ectofacial) Myc-tagged mouse Slo1 (VEDEC isoform) was provided by Dr. Min Li (Johns Hopkins University, Baltimore, MD). Other plasmids, including pGBKT7-Slo1G785-A985 and different pGEXKG-Slo1 constructs were created using PCR and confirmed by sequencing. Antibodies used in this work include: anti-Myc (9B11, Cell Signaling Technology, Inc); anti-Myc (06-549, Upstate Biotechnology, Inc); anti-NKβ1 (ab33144, Abcam Inc. Cambridge, MA), anti-α-Na+/K+-ATPase (anti-NKα1) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) and anti-Slo1 (APC-107, Alomone Laboratories, Jerusalem, Israel).
Yeast two-hybrid screen
Yeast two-hybrid screens of an embryonic day 9 (E9) chick ciliary ganglion (CG) cDNA library were carried out using the Matchmaker™ system (BD Biosciences, San Jose, CA) according to the manufacturer's instructions, as described in detail previously [10,24]. The bait construct was comprised of amino acids G785-A985 of mouse Slo1. NKβ1 emerged repeatedly in this screen.
Co-immunoprecipitation, cell surface biotinylation, and GST pull-down assays
CG were excised from E9 chick embryos and lysed in PBST (phosphate-buffered saline with 1% Triton) containing protease inhibitors (P2714, Sigma-Aldrich, Inc.). The extracts were centrifuged briefly, and the supernatants were incubated with anti-Slo1 or anti-NKβ1, and precipitates were isolated using Protein A/G PLUS-Agarose beads (sc-2003, Santa Cruz Biotechnology, Inc.). Beads were washed in PBST, SDS-sample buffer was added and samples were boiled for 5 min and then loaded onto gels. SDS-PAGE separation, immunoblot analysis, cell-surface biotinulation assays, and GST pull-down assays were performed as described previously [10,19,24].
Cell culture and transfection
HEK293T cells were grown and transiently co-transfected with plasmids encoding Myc-Slo1 and si-NKβ1 (sc-36008, Santa Cruz Biotechnology, Inc.) using Lipofectamine 2000™ (Invitrogen) as described previously [10,19,24]. Control cells were co-transfected with Myc-Slo1 and non-specific siRNA (sc-37007, Santa Cruz Biotechnology, Inc.). Cells were used for physiology or biochemistry 48 hours after transfection. Neurons from E9 chick CG were dissociated and cultured as described previously [36-37].
Confocal microscopy
E9 CG neurons were maintained in culture for 3 hours before fixation in 4% paraformaldehyde for 10 min. Preparations were rinsed and incubated with rabbit anti-Slo1 (1:500 dilution) and mouse anti-NKβ1 (1:500 dilution) overnight at 4°C. This was followed by incubation with secondary antibodies and images were collected as described previously [10].
Electrophysiology and statistics
Inside-out patch recordings were made at room temperature (22° C) from HEK293T cells transiently co-expressing si-NKβ1 or non-specific siRNA, along with Myc-Slo1 and green fluorescent protein (GFP), which was used to allow identification of transfected cells during recording. Protocols for recording and analysis were described previously [10,24]. Student's unpaired t-test was used to compare experimental groups to a single control group with α = 0.05.
Results
We conducted a yeast two-hybrid screen of a chick CG cDNA library using as bait a conserved cytoplasmic domain (G785-A985) of mouse Slo1 (Fig. 1A). Based on the sequences of cDNAs that we isolated in these screens, the portion of NKβ1 that interacts with Slo1 channels includes residues between 228 to 299 in the extracellular COOH-terminal region of NKβ1 (Fig. 1B).
Fig. 1.
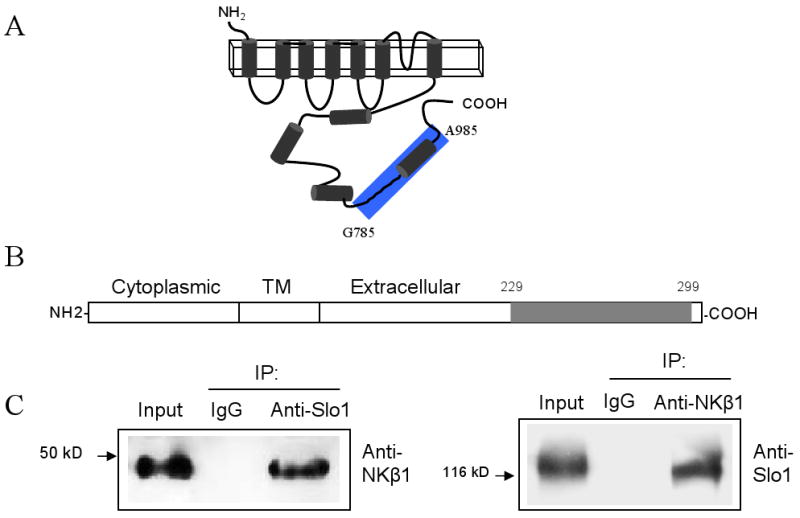
NKβ1 interacts with neuronal Slo1 channels. A, B Schematic representations of Slo1 and NKβ1 respectively. The bait fragment used in the yeast two-hybrid screen is highlighted in blue. C, Co-immunoprecipitation of NKβ1 and Slo1 from chick CG extracts. Initial immunoprecipitation was carried out using an antibody against Slo1 (left) or NKβ1 (right).
This interaction was also detected by co-immunoprecipitation from E9 chick CG extracts. NKβ1 could be detected in immunoprecipitates prepared using antibodies against Slo1 channels (Fig. 1C, left). Conversely, Slo1 could be detected when immunoprecipitation was carried out using anti-NKβ1 (Fig. 1C, right), whereas neither protein could be detected when immunoprecipitation was carried out with IgG. The Slo1- NKβ1 interaction was also seen using GST pull-down assays. Thus, GST-Slo1(G785-A985) could precipitate NKβ1 from CG extracts, whereas GST alone was ineffective (Fig. 2A). Additional GST fusion proteins, including GST-Slo1(L985-Q1108) were also able to precipitate NKβ1 from CG extracts (Fig. 2B). This construct comprises the most distal COOH-terminal portion of Slo1. It contains a caveolin-binding motif [15], and sorting motifs required for the apical expression of BKCa channels in certain epithelial cells [38]. In addition, we made four smaller GST fusion constructs of the bait region used in the initial screens. We detected NKβ1 interactions with GST-Slo1(T884-N936) and GST-Slo1(I937-A985). However, GST-Slo1(G785-L843) and GST-Slo1(Q844-I883) were unable to pull NKβ1 out of CG lysates (Fig. 2C). We carried out a similar set of analyses in HEK293T cells and obtained the same interaction pattern (data not shown).
Fig. 2.
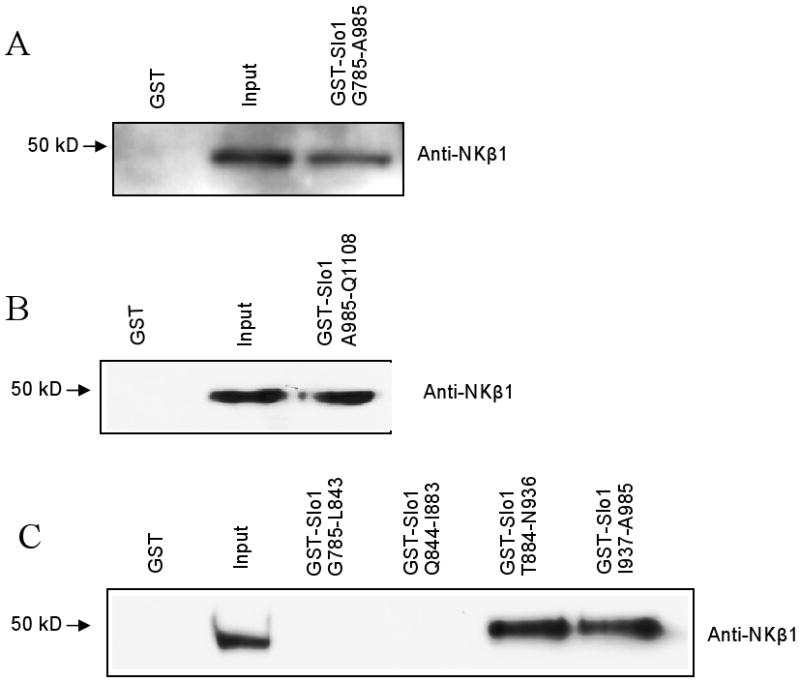
Interaction between NKβ1 and Slo1 occurs at multiple regions. A, GST pull-down assay carried out on chick CG lysates showing that a fusion protein containing the bait, GST-Slo1(G785-A985) binds to NKβ1. Additional fusion proteins from regions downstream and adjacent to the bait, GST-Slo1(L985-Q1108) also bind to NKβ1. B, Immunoblots of GST pull-down assays using smaller GST-fusion proteins from the bait region.
We also observed partial co-localization of NKβ1 and Slo1 by confocal microscopy in primary cultures of CG neurons (Fig. 3). Signals from anti-Slo1 and anti-NKβ1 show extensive overlap, especially in intracellular compartments of somatic areas. However, Slo1 signal in neurites does not appear to co-localize with NKβ1. Limits of resolution make it difficult to definitively observe co-localization at the cell surface.
Fig. 3.

Co-localization pattern of NKβ1 and Slo1. Top panel shows co-localization of NKβ1 (red) and Slo1 (green) in E9 chick CG neurons. The regions enclosed by white squares are magnified in the bottom panels to show neurites and cell bodies.
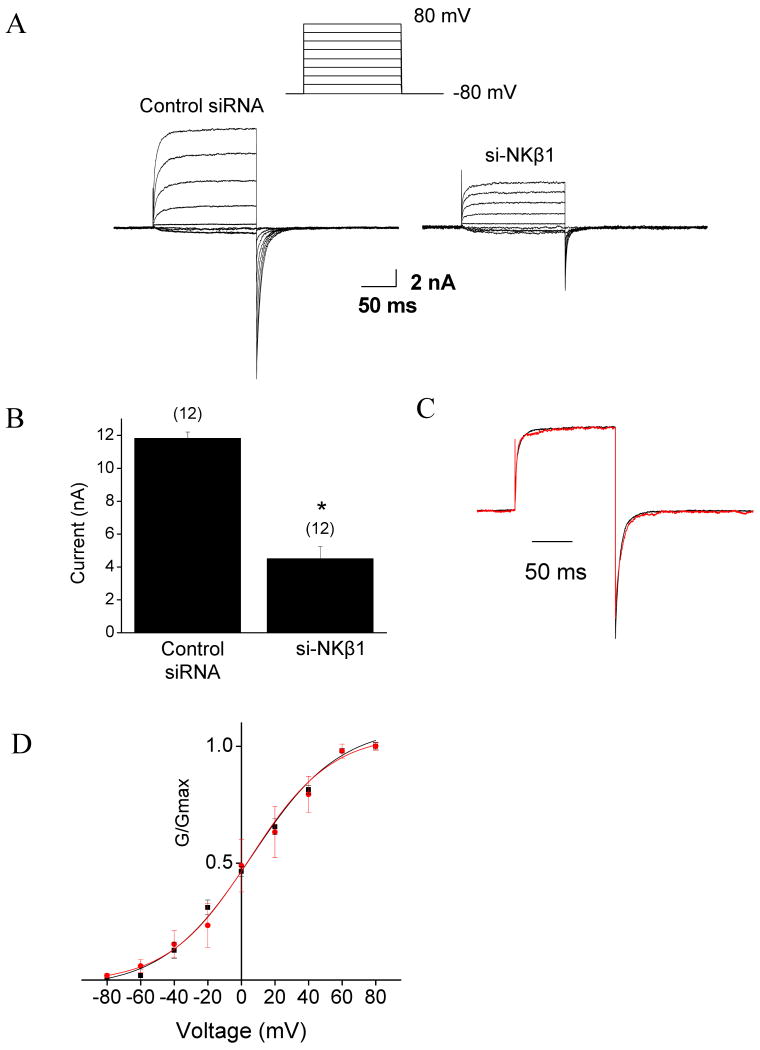
In order to examine the functional significance of the Slo1-NKβ1 interaction, we used an siRNA targeting NKβ1 transcripts (si-NKβ1) to reduce the endogenous expression of NKβ1 protein in HEK293T cells (Fig. 4). Treatment with si-NKβ1 had no effect on the expression of Na+/K+-ATPase α-subunits (NKα) compared to controls (Fig. 4A). To determine the functional implications of Slo1- NKβ1 interactions, inside-out patch recordings were made from HEK293T cells transiently co-transfected with si-NKβ1 or non-specific siRNA together with Myc-tagged Slo1. Large BKCa currents were observed in patches excised from Slo1-expressing cells treated with non-specific siRNA. However, we observed significantly reduced currents at all membrane potentials in cells treated with si-NKβ1 (Fig. 5A, B). There was no difference in the voltage-dependence of BKCa activation (Fig. 5D) or activation or deactivation kinetics due to NKβ1 siRNA treatment (Fig. 5C). We also examined the effects of si-NKβ1 or non-specific siRNAs on the distribution of Myc-Slo1 protein using a cell-surface biotinylation assay. These analyses revealed a reduced surface expression of Slo1 in si-NKβ1-transfected cells in comparison to control cells (Fig. 6A, top). There was no change in the protein expression levels of Slo1 as revealed by immunoblot analyses of the whole cell lysates (Fig. 6A, bottom). Quantitative analysis of the cell surface assays showed a significant reduction in the signal derived from the cell surface in NKβ1 down-regulated cells (Fig. 6B). Probing the same blots with anti-NKβ1 antibody showed a corresponding reduction in the cell surface expression of NKβ1.
Fig. 4.

Reduction of endogenous NKβ1 protein expression by si-RNA in HEK293T cells. A, Representative immunoblots showing expression of NKβ1 and NKα in HEK293T cells transfected with either non-specific si-RNA or si-NKβ1. B, Densitometric analysis of four repetitions of this experiment (mean ± S.E.M.).
Fig. 5.
Inside-out patch recordings made from HEK293T cells expressing Slo1. A, Typical families of currents evoked by voltage steps (-80 mV to +80 mV) in inside-out patches from transfected HEK293T cells. The bath solution contained 20 μM Ca2+. No currents were detected in Ca2+-free bath solutions (data not shown). Note reduced current amplitudes in cell that received si-NKβ1 compared to cell treated with control siRNA. B, Quantitative analysis of peak currents obtained by a step to +80mV shows significant reduction in mean current amplitude (n = 8) in si-NKβ1 treated cells. C, Currents evoked by a step to +80mV in 20 μM Ca2+ normalized and superimposed to facilitate comparisons of activation and deactivation kinetics in a control cell (red trace) and a si-NKβ1 expressing cell (black trace). D, Normalized voltage activation curves of BKCa currents recorded at 20 μM Ca2+. Data points (mean ± S.E.M.) are shown with superimposed fitted Boltzmann curves. Eight cells were analyzed from each group. Treatment with siNKβ1 did not affect the voltage-dependence of BKCa activation.
Fig. 6.

NKβ1 down-regulation reduces the cell surface expression of Slo1 channels in HEK293T cells. A, Cell-surface biotinylation assay showing cell surface and total expression of Myc-Slo1 in HEK293T cell transiently cotransfected with si-NKβ1 or non-specific siRNA. Top panel shows that si-NKβ1 cause a a reduction in surface expression of Slo1 and and total NKβ1 but no effect on total Slo1 expression. B, The bar graph shows relative surface expression (mean ± S.E.M.) of Slo1 calculated from blots of three separate cell surface biotinylation assays.
Discussion
The functional expression of BKCa channels on the cell membrane is a highly regulated process in many tissues. Here we show that NKβ1 is an interaction partner of Slo1. The region of NKβ1 that interacts with Slo1 is highly conserved in all known Na+/K+-ATPase and H+/K+-ATPase β-subunits [27]. Down-regulation of NKβ1 expression caused a marked reduction in the surface expression of Slo1 in HEK293T cells. The effects of NKβ1 on the cell surface expression of BKCa channels may be related to other physiological effects recently ascribed to NKβ1. For example, NKβ1 plays a major role in the localization of tight junction-associated proteins in certain cell types and in the development of cell polarity [43-45]. In morphologically polarized cells such as neurons and epithelial cells, the interaction between Slo1 and NKβ1 may be responsible for targeting of BKCa channels to specific regions on the plasma membrane. There is evidence that formation of tight junctions in epithelia is sensitive to ionic conditions, including localized increases in intracellular Na+ concentration that lead to alterations in the subjacent cytoskeleton [44]. It is possible that co-localization of Slo1 channels with Na+/K+-ATPases contributes to local ionic homeostasis and maintenance of tight-junction integrity. Moreover, in cardiac myocytes and other tissues, NKβ1 regulates the concentration of Na+/K+-ATPases in caveolae [46] where these enzymes are pre-assembled with their partners [47]. Since NKβ1 is a ubiquitous protein, its ability to target proteins to caveolae may be a generalized mechanism that affects additional proteins, including BKCa channels [15-16].
Is NKβ1 an independent mediator of physiological regulation inside cells? Unequal amounts of α- and β-ATPase proteins are observed in many tissues [48]. In addition, mature β-subunits can be recovered independent of α-subunits, at least in the case of H+/K+-ATPases [29]. We have observed that Slo1 and NKα subunits interact with different regions on NKβ1 (see [27] for NKα binding sites), and it is possible that NKβ1 can interact with Slo1 and NKα simultaneously. We cannot exclude that the interaction between NKβ1 and BKCa channels may be facilitated by the presence of NKα subunits, or that the Slo1-NKβ1 interaction occurs within a larger complex formed between Na+/K+-ATPase, BKCa channels, and other proteins.
The possible existence of Slo1-NKβ1 interaction within a larger complex is intriguing in light of growing evidence that Na+/K+-ATPases can function as transducers for signaling pathways [49-50]. A number of studies point to the functional coupling between Na+/K+-ATPases and other ion channels [51-52] possibly including L-type Ca2+ channels [53]. Therefore, Na+/K+-ATPases may co-exist with a Cav-BKCa complex at the plasma membrane that plays a role in regulation intracellular Ca2+ levels. NKβ1 subunits may be important for formation or stabilization of such complexes.
Abbreviations
- CG
Ciliary ganglion
- RCK
regulators of potassium conductance
- NKβ1
Na+/K+-ATPase β1 subunit
- NKα
Na+/K+-ATPase α-subunit
- BKCa
large-conductance Ca2+- activated K+ channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–31. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 2.Hu H, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–97. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravo-Zehnder M, Orio P, Norambuena A, Wallner M, Meera P, Toro L, Latorre R, Gonzalez A. Apical sorting of a voltage- and Ca2+-activated K+ channel alpha -subunit in Madin-Darby canine kidney cells is independent of N-glycosylation. Proc Natl Acad Sci U S A. 2000;97:13114–9. doi: 10.1073/pnas.240455697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, Toro L. A novel MaxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem. 2001;276:16232–9. doi: 10.1074/jbc.M008852200. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J. 1997;73:1355–63. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–4. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 8.Schmalhofer WA, et al. Role of the C-terminus of the high-conductance calcium-activated potassium channel in channel structure and function. Biochemistry. 2005;44:10135–44. doi: 10.1021/bi050527u. [DOI] [PubMed] [Google Scholar]
- 9.Zou S, Jha S, Kim EY, Dryer SE. A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane. Mol Pharmacol. 2008;73:359–68. doi: 10.1124/mol.107.039743. [DOI] [PubMed] [Google Scholar]
- 10.Kim EY, Ridgway LD, Dryer SE. Interactions with filamin A stimulate surface expression of large-conductance Ca2+-activated K+ channels in the absence of direct actin binding. Mol Pharmacol. 2007;72:622–30. doi: 10.1124/mol.107.038026. [DOI] [PubMed] [Google Scholar]
- 11.Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, Shipston MJ. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. Faseb J. 2006;20:2588–90. doi: 10.1096/fj.06-6152fje. [DOI] [PubMed] [Google Scholar]
- 12.Piao L, Ho WK, Earm YE. Actin filaments regulate the stretch sensitivity of large-conductance, Ca2+-activated K+ channels in coronary artery smooth muscle cells. Pflugers Arch. 2003;446:523–8. doi: 10.1007/s00424-003-1079-y. [DOI] [PubMed] [Google Scholar]
- 13.Ling S, Sheng JZ, Braun JE, Braun AP. Syntaxin 1A co-associates with native rat brain and cloned large conductance, calcium-activated potassium channels in situ. J Physiol. 2003;553:65–81. doi: 10.1113/jphysiol.2003.051631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cibulsky SM, Fei H, Levitan IB. Syntaxin-1A binds to and modulates the Slo calcium-activated potassium channel via an interaction that excludes syntaxin binding to calcium channels. J Neurophysiol. 2005;93:1393–405. doi: 10.1152/jn.00789.2004. [DOI] [PubMed] [Google Scholar]
- 15.Alioua A, Lu R, Kumar Y, Eghbali M, Kundu P, Toro L, Stefani E. Slo1 caveolin-binding motif, a mechanism of caveolin-1-Slo1 interaction regulating Slo1 surface expression. J Biol Chem. 2008;283:4808–17. doi: 10.1074/jbc.M709802200. [DOI] [PubMed] [Google Scholar]
- 16.Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee HC. Caveolae targeting and regulation of large conductance Ca2+-activated K+ channels in vascular endothelial cells. J Biol Chem. 2005;280:11656–64. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- 17.Lim HH, Park CS. Identification and functional characterization of ankyrin-repeat family protein ANKRA as a protein interacting with BKCa channel. Mol Biol Cell. 2005;16:1013–25. doi: 10.1091/mbc.E04-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridgway LD, Kim EY, Dryer SE. MAGI-1 interacts with Slo1 channel proteins and suppresses Slo1 expression on the cell surface. Am J Physiol Cell Physiol. 2009 doi: 10.1152/ajpcell.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim EY, Choi KJ, Dryer SE. Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface. Am J Physiol Renal Physiol. 2008;295:F235–46. doi: 10.1152/ajprenal.00140.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo S, Lee KH, Song S, Jung YK, Park CS. Identification and functional characterization of cereblon as a binding protein for large-conductance calcium-activated potassium channel in rat brain. J Neurochem. 2005;94:1212–24. doi: 10.1111/j.1471-4159.2005.03344.x. [DOI] [PubMed] [Google Scholar]
- 21.Rezzonico R, et al. Focal adhesion kinase pp125FAK interacts with the large conductance calcium-activated hSlo potassium channel in human osteoblasts: potential role in mechanotransduction. J Bone Miner Res. 2003;18:1863–71. doi: 10.1359/jbmr.2003.18.10.1863. [DOI] [PubMed] [Google Scholar]
- 22.Tian L, Coghill LS, MacDonald SH, Armstrong DL, Shipston MJ. Leucine zipper domain targets cAMP-dependent protein kinase to mammalian BK channels. J Biol Chem. 2003;278:8669–77. doi: 10.1074/jbc.M211661200. [DOI] [PubMed] [Google Scholar]
- 23.Grunnet M, Kaufmann WA. Coassembly of big conductance Ca2+-activated K+ channels and L-type voltage-gated Ca2+ channels in rat brain. J Biol Chem. 2004;279:36445–53. doi: 10.1074/jbc.M402254200. [DOI] [PubMed] [Google Scholar]
- 24.Zou S, Jha S, Kim EY, Dryer SE. The beta 1 subunit of L-type voltage-gated Ca2+ channels independently binds to and inhibits the gating of large-conductance Ca2+-activated K+ channels. Mol Pharmacol. 2008;73:369–78. doi: 10.1124/mol.107.040733. [DOI] [PubMed] [Google Scholar]
- 25.Kim EY, Alvarez-Baron CP, Dryer SE. Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes. Mol Pharmacol. 2009;75:466–77. doi: 10.1124/mol.108.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–8. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- 27.Geering K. The functional role of beta subunits in oligomeric P-type ATPases. J Bioenerg Biomembr. 2001;33:425–38. doi: 10.1023/a:1010623724749. [DOI] [PubMed] [Google Scholar]
- 28.Eakle KA, Kabalin MA, Wang SG, Farley RA. The influence of beta subunit structure on the stability of Na+/K(+)-ATPase complexes and interaction with K+ J Biol Chem. 1994;269:6550–7. [PubMed] [Google Scholar]
- 29.Jaunin P, Jaisser F, Beggah AT, Takeyasu K, Mangeat P, Rossier BC, Horisberger JD, Geering K. Role of the transmembrane and extracytoplasmic domain of beta subunits in subunit assembly, intracellular transport, and functional expression of Na,K-pumps. J Cell Biol. 1993;123:1751–9. doi: 10.1083/jcb.123.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laughery MD, Clifford RJ, Chi Y, Kaplan JH. Selective basolateral localization of overexpressed Na-K-ATPase beta1- and beta2-subunits is disrupted by butryate treatment of MDCK cells. Am J Physiol Renal Physiol. 2007;292:F1718–25. doi: 10.1152/ajprenal.00360.2006. [DOI] [PubMed] [Google Scholar]
- 31.Lavoie L, Levenson R, Martin-Vasallo P, Klip A. The molar ratios of alpha and beta subunits of the Na+-K+-ATPase differ in distinct subcellular membranes from rat skeletal muscle. Biochemistry. 1997;36:7726–32. doi: 10.1021/bi970109s. [DOI] [PubMed] [Google Scholar]
- 32.Mircheff AK, Bowen JW, Yiu SC, McDonough AA. Synthesis and translocation of Na+-K+-ATPase alpha- and beta-subunits to plasma membrane in MDCK cells. Am J Physiol. 1992;262:C470–83. doi: 10.1152/ajpcell.1992.262.2.C470. [DOI] [PubMed] [Google Scholar]
- 33.Gatto C, McLoud SM, Kaplan JH. Heterologous expression of Na+-K+-ATPase in insect cells: intracellular distribution of pump subunits. Am J Physiol Cell Physiol. 2001;281:C982–92. doi: 10.1152/ajpcell.2001.281.3.C982. [DOI] [PubMed] [Google Scholar]
- 34.Shoshani L, Contreras RG, Roldan ML, Moreno J, Lazaro A, Balda MS, Matter K, Cereijido M. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between beta-subunits located in neighboring cells. Mol Biol Cell. 2005;16:1071–81. doi: 10.1091/mbc.E04-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larre I, Ponce A, Fiorentino R, Shoshani L, Contreras RG, Cereijido M. Contacts and cooperation between cells depend on the hormone ouabain. Proc Natl Acad Sci U S A. 2006;103:10911–6. doi: 10.1073/pnas.0604496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron JS, Lhuillier L, Subramony P, Dryer SE. Developmental regulation of neuronal K+ channels by target-derived TGF beta in vivo and in vitro. Neuron. 1998;21:1045–53. doi: 10.1016/s0896-6273(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 37.Lhuillier L, Dryer SE. Developmental regulation of neuronal KCa channels by TGFbeta1: an essential role for PI3 kinase signaling and membrane insertion. J Neurophysiol. 2002;88:954–64. doi: 10.1152/jn.2002.88.2.954. [DOI] [PubMed] [Google Scholar]
- 38.Kwon SH, Guggino WB. Multiple sequences in the C terminus of MaxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci U S A. 2004;101:15237–42. doi: 10.1073/pnas.0404877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chae KS, Oh KS, Dryer SE. Akt activation is necessary for growth factor-induced trafficking of functional KCa channels in developing parasympathetic neurons. J Neurophysiol. 2005;94:1597–605. doi: 10.1152/jn.00796.2004. [DOI] [PubMed] [Google Scholar]
- 40.Wang SX, Ikeda M, Guggino WB. The cytoplasmic tail of large conductance, voltage- and Ca2+-activated K+ (MaxiK) channel is necessary for its cell surface expression. J Biol Chem. 2003;278:2713–22. doi: 10.1074/jbc.M208411200. [DOI] [PubMed] [Google Scholar]
- 41.Kim EY, Zou S, Ridgway LD, Dryer SE. Beta1-subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. J Neurophysiol. 2007;97:3508–16. doi: 10.1152/jn.00009.2007. [DOI] [PubMed] [Google Scholar]
- 42.Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett. 2007;581:1000–8. doi: 10.1016/j.febslet.2007.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madan P, Rose K, Watson AJ. Na/K-ATPase beta1 subunit expression is required for blastocyst formation and normal assembly of trophectoderm tight junction-associated proteins. J Biol Chem. 2007;282:12127–34. doi: 10.1074/jbc.M700696200. [DOI] [PubMed] [Google Scholar]
- 44.Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ, Jr, Bander NH, Peralta Soler A, Rajasekaran AK. Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell. 2001;12:279–95. doi: 10.1091/mbc.12.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krupinski T, Beitel GJ. Unexpected Roles of the Na-K-ATPase and Other Ion Transporters in Cell Junctions and Tubulogenesis. Physiology (Bethesda) 2009;24:192–201. doi: 10.1152/physiol.00008.2009. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Askari A. Beta-subunit of cardiac Na+-K+-ATPase dictates the concentration of the functional enzyme in caveolae. Am J Physiol Cell Physiol. 2006;291:C569–78. doi: 10.1152/ajpcell.00002.2006. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003;284:C1550–60. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- 48.Sznajder JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of Na+-K+-ATPase. J Appl Physiol. 2002;93:1860–6. doi: 10.1152/japplphysiol.00022.2002. [DOI] [PubMed] [Google Scholar]
- 49.Xie Z, Askari A. Na+/K+-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–9. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 50.Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–68. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 51.Mauerer UR, Boulpaep EL, Segal AS. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J Gen Physiol. 1998;111:161–80. doi: 10.1085/jgp.111.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Priebe L, Friedrich M, Benndorf K. Functional interaction between KATP channels and the Na+-K+ pump in metabolically inhibited heart cells of the guinea-pig. J Physiol. 1996;492:405–17. doi: 10.1113/jphysiol.1996.sp021317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marban E, Tsien RW. Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J Physiol. 1982;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]