Abstract
Objective
To identify and characterize otolaryngologic markers for the early diagnosis of Turner Syndrome (TS).
Study Design
Prospective cohort survey.
Methods
Setting
Clinical Center of the National Institutes of Health (NIH).
Patients
Ninety-one females, 7 - 61 years old (average = 28.7 y), enrolled in a multidisciplinary study of karyotype-phenotype correlations in TS.
Main Outcome Measures
Age at diagnosis, X chromosome karyotype, history of chronic or recurrent otitis media (OM), sensorineural hearing loss (SNHL), palate dysmorphism, pinna deformity, pterygium colli, low posterior hairline, low-set ears, and micrognathia.
Results
Sixty-nine (76%) patients had a history of chronic or recurrent OM, 62 (68%) had a dysmorphic palate, 57 (63%) had SNHL, and 90 (99%) had one or more of these findings. 83 (91%; average age at diagnosis = 9.4 y) had one or more external craniofacial signs: pinna abnormalities, pterygium colli, low-set ears, micrognathia or a low posterior hairline. Eight patients (average age at diagnosis = 13.2 y) had no external craniofacial signs, although seven (88%) of these eight patients had a history of chronic or recurrent OM, dysmorphic palate or SNHL. The age at diagnosis was not significantly different between groups with or without external craniofacial signs (P = 0.126).
Conclusions
Patients with mild or incompletely penetrant TS phenotypes often present with otitis media, hearing loss, or both before the diagnosis of TS is established. Palatal dysmorphism, including ogival morphology, is another otolaryngologic marker for TS. Prompt recognition of these manifestations of TS could hasten its diagnosis and appropriate medical care.
INTRODUCTION
Turner Syndrome (TS) affects up to one in 2000 live female births and is characterized by the total or partial loss of one X chromosome1, 2. Frequently observed manifestations of TS include short stature, lymphedema, gonadal dysgenesis, cardiovascular anomalies, renal abnormalities, hypertension, hypothyroidism, glucose intolerance and hyperlipidemia1, 2. Some of the features frequently encountered in the otolaryngology clinic are sensorineural hearing loss (SNHL)3, recurrent otitis media (OM), pterygium colli and craniofacial dysmorphism1, 2. Commonly reported craniofacial findings include a low hairline, low-set ears, micrognathia, and oral palatal abnormalities1, 2.
TS patients are thought to have an increased incidence of cleft palate4-6. Less morbid palatal morphologies are more common and include a narrow, high-arched or ogival (steeple-shaped) palate, or a combination of these morphologies6-13. The ogival palate may be inaccurately or incompletely described as high-arched or narrow14, especially in the absence of cephalometric measurements. Similarly, prominent lateral ridges produce a different but distinctive palatal morphology that can also be mischaracterized as narrow or high-arched11, 15. Early diagnosis of TS is important for timely detection and surveillance of medically significant features such as congenital heart disease, and the prompt institution of therapies such as growth hormone, estrogen-progesterone and psychosocial counseling1, 2. Unfortunately, most girls with TS are not correctly diagnosed until age 10-12 when short stature and delayed pubertal development finally lead to cytogenetic testing16. However, many of these girls may present with distinctive otolaryngologic signs and symptoms prior to the diagnosis of TS. We sought to characterize the otolaryngologic manifestations of TS in order to facilitate its early detection in patients who may not have more obvious features.
SUBJECTS AND METHODS
Subjects
Ninety-one TS subjects with a wide spectrum of phenotypic severity were prospectively ascertained in a multidisciplinary study of genotype-phenotype correlations at the Clinical Center of the National Institute of Health (NIH). The average age at evaluation was 28.7 y (range = 7 - 61 y). The eligibility criteria comprised phenotypic characteristics of TS and karyotypic evidence of an X-chromosome abnormality with fewer than 30% normal cells on a 50-cell blood karyotype. Seventy-five subjects had monosomy X (45,X) and 16 had partial X monosomy comprising 46,X,delXp (n =2), 46,X,delXq (n = 2), 46,X,iXq (n = 5), and complex karyotypes including mosaics for 45,X and one of the above-mentioned X abnormalities (n = 7). Written informed consent was obtained from all subjects or parents of minor subjects. The study was approved by the Institutional Review Board of the National Institute for Child Health and Development, National Institutes of Health.
Clinical evaluation
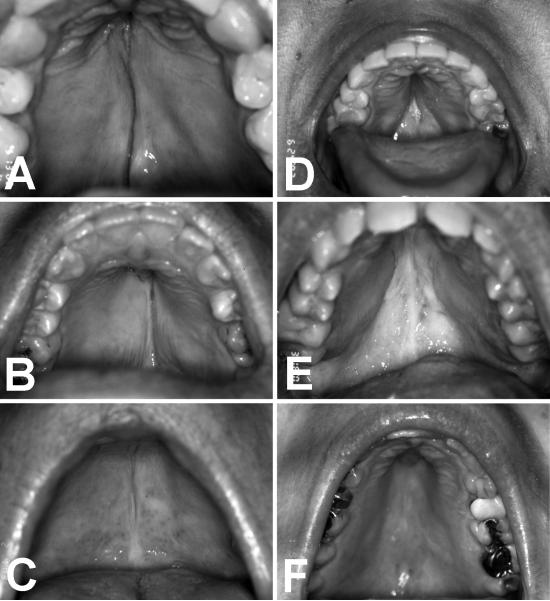
Subjects or their parents completed a questionnaire about their past and present otolaryngologic signs and symptoms of TS. Their responses were reviewed and discussed within the context of a medical history interview and physical examination by an otolaryngologist-head and neck surgeon. Past medical records were evaluated when available. Dysmorphic features such as pinna and palate malformations were photo-documented. Palates were considered dysmorphic if they were high-arched, ogival, or had prominent lateral ridges resulting in the appearance of a narrow or high arch (Figure 1). High-resolution temporal bone CT scans were obtained for the first 14 subjects and reviewed by a Neuroradiologist and an Otolaryngologist-Head and Neck Surgeon. Cochlear height and lateral semicircular canal bony island width was measured on coronal and axial sections, respectively. Hypoplasia and hyperplasia were defined as measurements outside normal ranges for cochlear height (4.4 - 5.9 mm) or lateral semicircular canal bony island width (2.6 - 4.8 mm)17.
Figure 1.
Palate dysmorphism in TS includes ogival (steeple-shaped) morphology (A,B,C) and prominent lateral palatal ridges (D,E,F).
The results of pure-tone and speech audiometry, tympanometry and stapedial reflex testing are described elsewhere3. A history of chronic or recurrent OM was defined by report of one or more episodes. SNHL was defined by a bone conduction threshold ≥ 25 dB HL at any test frequency with or without an air-bone gap, and thus included those with mixed hearing loss.
Statistical analysis
We tested 2 × 2 associations among clinical variables and karyotypes by Fisher’s Exact test. We compared average age at diagnosis by Student’s t-test and Mann-Whitney test.
RESULTS
The average age at diagnosis was 9.7 y (range = 0 - 38 y). The average age at diagnosis was 9.2 y for monosomic 45,X and 12.0 y for partial X monosomic patients. This difference was not significant (P = 0.197).
Sixty-nine (76%) subjects had a history of OM, 57 (63%) had SNHL, 62 (68%) had a dysmorphic palate (Figure 1) and two (2%) had a history of cleft palate. Among subjects with SNHL, an isolated mid-frequency hearing loss was observed in four (7%) subjects and a mid-frequency hearing loss in combination with high frequency hearing loss was observed in 38 (60%) subjects. Forty-eight subjects (53%) had pinna abnormalities, 35 (38%) had pterygium colli, 66 (73%) had a low posterior hair line, 34 (37%) had low-set ears, and 22 (24%) had micrognathia. Dysmorphic palate, low posterior hairline, low-set ears, and micrognathia each showed a significant association with monosomy 45,X (Table 1). The presence of any one or more of the five external craniofacial signs did not show a significant association with monosomy 45,X (P = 0.143).
Table 1. Associations of Clinical Features with Karyotype.
| No. of patients |
|||
|---|---|---|---|
| Clinical feature | Monosomy X (45,X) (n=75) |
Partial or mosaic monosomy Xa (n=16) |
P valueb |
| Otitis media (76%) | 57 | 12 | 0.579 |
| Low posterior hairline (73%) | 58 | 8 | 0.031* |
| Dysmorphic palate (68%) | 55 | 7 | 0.024* |
| Sensorineural hearing loss (63%) | 50 | 7 | 0.077 |
| Pinna deformity (53%) | 39 | 9 | 0.488 |
| Pterygium colli (38%) | 32 | 3 | 0.063 |
| Low-set ears (37%) | 32 | 2 | 0.019* |
| Micrognathia (24%) | 22 | 0 | 0.0073* |
| Any external signsc(91%) | 70 | 13 | 0.143 |
includes: 46,X,delXp; 46,X,delXq; 46,X,iXq; and complex X karyotypes including mosaics for 45,X or one of these abnormal karyotypes.
Fisher’s exact test, two-tailed.
pterygium colli, low posterior hairline, low-set ears, pinna deformity or micrognathia.
statistically significant.
The average age at diagnosis was 9.4 y for patients with one or more external craniofacial signs in comparison to 13.2 y for subjects without any external craniofacial signs. This difference was not significant (P = 0.687). Seven (88%) of eight subjects without external craniofacial signs had OM, SNHL, or an abnormal palate (Table 2). We detected no significant association of OM, SNHL, or palate dysmorphism with the presence or absence of external craniofacial signs (Table 2).
Table 2. Associations of Otolaryngologic Features with External Craniofacial Signs.
| No. of patients with or without external craniofacial signsa |
|||
|---|---|---|---|
| Otolaryngologic feature | With (n=83) | Without (n=8) | P valueb |
| Otitis media (OM), n=69 (76%) | 64 | 5 | 0.296 |
| Dysmorphic palate, n=62 (68%) | 59 | 3 | 0.064 |
| Sensorineural hearing loss (SNHL), n=57 (63%) | 52 | 5 | 0.635 |
| OM, SNHL or dysmorphic palate, n=90 (99%) | 83 | 7 | 0.087 |
pterygium colli, low hairline, low-set ears, pinna deformity or micrognathia.
Fisher’s exact test, two-tailed.
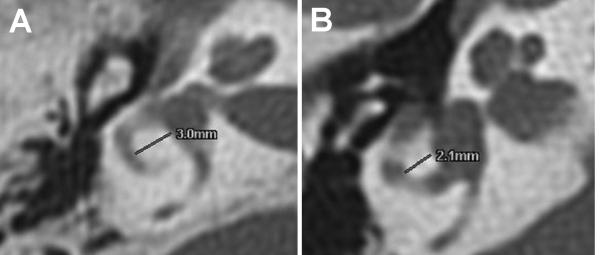
Fourteen patients had normal CT scans of the temporal bones. One patient had evidence of chronic OM and a previous right tympanoplasty-mastoidectomy (not shown). We observed hypoplastic lateral semicircular canals (1 bilateral, 1 left) in two other patients, all of whom had SNHL (Figure 2). One of these patients also had a posterior dehiscence of the right superior semicircular canal with the posterior fossa but she had no history of vertigo. There was no association between the presence of vestibular dysmorphism and karyotype (P > 0.5).
Figure 2.
CT scan images of normal (A) and hypoplastic (B) lateral semicircular canals. Lateral semicircular canal bony islands widths are indicated with lines and corresponding numerical measurements.
DISCUSSION
Our comprehensive and prospective study design with stringent karyotype documentation should provide a description of the otolaryngologic manifestations of TS that is less biased than previous retrospective or referral-based studies. Nevertheless, our observed prevalence of palatal abnormalities in TS patients is consistent with previous reports6-9, 11-13. The high-arched palate has been emphasized as a clinical marker for the timely diagnosis of TS12, although it can be a relatively common, nonspecific and subjective finding. Many palates that are qualitatively described as high-arched may actually be normal in height and more accurately described as narrow with prominent lateral ridges11, 15. An ogival or steeple-shaped palate may be more specific for TS and is less common or prone to subjective judgment than “high-arched”. There are reports of ogival palates noted in patients with Hutchinson-Gilford progeria18 and Cornelia de Lange syndromes19; however these extremely rare entities are not usually in the differential diagnosis.
Our observed frequencies of chronic or recurrent otitis media and SNHL in TS are also consistent with previous reports3, 20-22. Almost all of our TS subjects, even individuals with no external craniofacial signs, had recurrent or chronic OM, SNHL or a dysmorphic palate. Any of these findings in combination with one another or other manifestations of TS in a female patient should alert the clinician to this possible diagnosis. This would facilitate earlier detection and management of the significant medical and psychological manifestations of this disorder.
CONCLUSION
Patients with mild or incompletely penetrant TS phenotypes often present with otitis media, hearing loss, or both before the diagnosis of TS is established. Palatal dysmorphism, especially ogival morphology, is a key otolaryngologic marker for TS. Prompt recognition of these manifestations of TS could hasten its diagnosis and appropriate medical care.
Acknowledgements
We thank current and former NIDCD colleagues for clinical support and critical review of the manuscript. This study was supported by NIDCD and NICHD intramural research funds.
Footnotes
All authors do not have any financial disclosures for this manuscript.
Conflict of interest statement: None of the authors have relevant financial interests in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bondy CA. Care of girls and women with Turner syndrome: A guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 2.Frias JL, Davenport ML. Health supervision for children with Turner syndrome. Pediatrics. 2003;111:692–702. doi: 10.1542/peds.111.3.692. [DOI] [PubMed] [Google Scholar]
- 3.King KA, Makishima T, Zalewski CK, et al. Analysis of auditory phenotype and karyotype in 200 females with Turner syndrome. Ear Hear. 2007;28:831–841. doi: 10.1097/AUD.0b013e318157677f. [DOI] [PubMed] [Google Scholar]
- 4.Corona-Rivera JR, Corona-Rivera E, Bobadilla-Morales L, Garcia-Cobia TA, Corona-Rivera A. Cleft lip and/or palate in two cases of 46,X,i(Xq) Turner syndrome. Genet Couns. 2002;13:19–22. [PubMed] [Google Scholar]
- 5.Gorlin RJ, Cohen MM, Jr., Hennekam RCM. Syndromes of the Head and Neck. 4th ed. Oxford University Press; New York: 2001. [Google Scholar]
- 6.Lopez ME, Bazan C, Lorca IA, Chervonagura A. Oral and clinical characteristics of a group of patients with Turner syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:196–204. doi: 10.1067/moe.2002.121546. [DOI] [PubMed] [Google Scholar]
- 7.Johnson R, Baghdady VS. Maximum palatal height in patients with Turner’s syndrome. J Dent Res. 1969;48:473–476. doi: 10.1177/00220345690480032501. [DOI] [PubMed] [Google Scholar]
- 8.Laine T, Alvesalo L, Lammi S. Palatal dimensions in 45,X-females. J Craniofac Genet Dev Biol. 1985;5:239–246. [PubMed] [Google Scholar]
- 9.Lemli L, Smith DW. The Xo Syndrome. a Study of the Differentiated Phenotype in 25 Patients. J Pediatr. 1963;63:577–588. doi: 10.1016/s0022-3476(63)80368-6. [DOI] [PubMed] [Google Scholar]
- 10.Oberman JW. The prepuberal diagnosis of ovarian agenesis and its relationship to status Bonnevie-Ullrich. J Pediatr. 1955;47:48–59. doi: 10.1016/s0022-3476(55)80123-0. [DOI] [PubMed] [Google Scholar]
- 11.Perkiomaki MR, Alvesalo L. Palatine ridges and tongue position in Turner syndrome subjects. Eur J Orthod. 2008;30:163–168. doi: 10.1093/ejo/cjm118. [DOI] [PubMed] [Google Scholar]
- 12.Savendahl L, Davenport ML. Delayed diagnoses of Turner’s syndrome: proposed guidelines for change. J Pediatr. 2000;137:455–459. doi: 10.1067/mpd.2000.107390. [DOI] [PubMed] [Google Scholar]
- 13.Szilagyi A, Keszthelyi G, Nagy G, Madlena M. Oral manifestations of patients with Turner syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:577–584. doi: 10.1067/moe.2000.104475. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro BL, Gorlin RJ, Redman RS, Bruhl HH. The palate and Down’s syndrome. N Engl J Med. 1967;276:1460–1463. doi: 10.1056/NEJM196706292762603. [DOI] [PubMed] [Google Scholar]
- 15.Hanson JW, Smith DW, Cohen MM., Jr. Prominent lateral palatine ridges: developmental and clinical relevance. J Pediatr. 1976;89:54–58. doi: 10.1016/s0022-3476(76)80926-2. [DOI] [PubMed] [Google Scholar]
- 16.Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897–3902. doi: 10.1210/jc.2006-0558. [DOI] [PubMed] [Google Scholar]
- 17.Purcell DD, Fischbein NJ, Patel A, Johnson J, Lalwani AK. Two temporal bone computed tomography measurements increase recognition of malformations and predict sensorineural hearing loss. Laryngoscope. 2006;116:1439–1446. doi: 10.1097/01.mlg.0000229826.96593.13. [DOI] [PubMed] [Google Scholar]
- 18.Merideth MA, Gordon LB, Clauss S, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grau Carbo J, Lopez Jimenez J, Gimenez Prats MJ, Sanchez Molins M. Cornelia de Lange syndrome: a case report. Med Oral Patol Oral Cir Bucal. 2007;12:E445–448. [PubMed] [Google Scholar]
- 20.Barrenas M, Landin-Wilhelmsen K, Hanson C. Ear and hearing in relation to genotype and growth in Turner syndrome. Hear Res. 2000;144:21–28. doi: 10.1016/s0378-5955(00)00040-x. [DOI] [PubMed] [Google Scholar]
- 21.Dhooge IJ, De Vel E, Verhoye C, Lemmerling M, Vinck B. Otologic disease in turner syndrome. Otol Neurotol. 2005;26:145–150. doi: 10.1097/00129492-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto N, Tanaka T, Taiji H, et al. Hearing loss in Turner syndrome. J Pediatr. 2006;149:697–701. doi: 10.1016/j.jpeds.2006.06.071. [DOI] [PubMed] [Google Scholar]