Abstract
Embryonic insults during early gestation increase the risk of schizophrenia. Abnormal forebrain development during this period is often characterized by a shallow olfactory sulcus. The adjacent orbital sulcus does not develop until the third trimester and so is immune to early intrauterine insults. We measured olfactory and orbital sulcal depths in 36 patients and 28 control subjects. Patients had shallower olfactory sulci, but normal orbital sulci. Olfactory and orbital sulcal depths were correlated in controls, but not in patients. Olfactory sulcal depth may therefore be a biomarker denoting an early embryonic disruption in individuals at risk for schizophrenia.
Keywords: schizophrenia, olfactory sulcus, orbital sulcus, neurodevelopment, embryogenesis, vulnerability
1. Introduction
The period from late first through early second trimester of gestation is one of increased fetal risk for schizophrenia. The evidence that developmental disruptions during this period contribute to the etiology of schizophrenia is clear. Studies of maternal infection (Mednick et al., 1988), maternal stress (Malaspina et al., 2008) and famine (Susser and Lin, 1992) all suggest that gestational effects within a relatively narrow time window increase the risk of illness. Increased prevalence of midline developmental anomalies such as cleft palate (Christensen and Mortensen, 2002) and cavum septum pellucidum (Brown et al., 2009), which occur during this same period, provides additional evidence of an early embryonic disruption. Gross anomalies like cleft palate, however, are only apparent in a small percentage of schizophrenia patients. Typically there is neither a history nor obvious indicator of any gestational disturbance, although subtle anomalies may be present (O’Callaghan et al., 1991).
Olfactory structures are derived from the same embryonic processes that produce other midline structures, and development of the olfactory system coincides with this period of increased vulnerability (Farbman, 1991). The nasal placodes invaginate at week 6 and the nasal cavities are fully sculpted by week 11. Axons from peripheral receptor neurons reach the olfactory bulb by late first trimester and functional synapses appear by week 17. Consistent with this early development, the olfactory system is vulnerable to disruption, in utero, by physical and chemical teratogens. Multiple structural abnormalities of the olfactory sensory system have also been observed in schizophrenia (Moberg et al., 2004; Turetsky et al., 2000, 2003b). It is plausible, therefore, that embryonic disruptions that increase the risk for schizophrenia might also disturb the neural substrates associated with olfaction.
The depth of the olfactory sulcus is a measure that is directly linked to embryonic development of both the olfactory system and the cerebral cortex. This sulcus, which directly overlays the olfactory tracts, is among the earliest to develop and is identifiable in the fetal forebrain around gestational week 16 (Chi et al., 1977). It develops in concert with the projection of the olfactory tract from the olfactory bulb to the olfactory cortex and is dependent upon the integrity of this afferent fiber tract for its own development. Consistent with the right hemisphere preference for olfactory processing, the olfactory sulcus is usually deeper on the right side (Hummel et al., 2003). In developmental disorders such as Kallman syndrome or isolated congenital anosmia, that are characterized by dysgenesis of the olfactory bulb and tract, the olfactory sulcus tends to be either unusually shallow or absent (Abolmaali et al., 2002; Vogl et al., 1994). Olfactory sulcal depth may, therefore, be a pathognomonic marker for aberrant forebrain development during the first half of embryonic development.
This is in contrast to other forebrain sulci, such as the 'H-shaped' orbital sulcus. Although adjacent to the olfactory sulcus, it does not develop until week 28 of gestation. Due to the timing of its development, the orbital sulcus is more vulnerable to disturbances that occur late in gestation, including very pre-term birth (Giménez et al., 2006). However, it is relatively immune to the effects of intrauterine insults that occur earlier. To test the hypothesis that schizophrenia patients exhibit abnormalities associated with aberrant development of the basal forebrain early in gestation, we examined olfactory sulcal depth in schizophrenia patients and healthy participants and compared these to similar measures derived from the orbital sulcus.
2. Materials and Methods
This study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Thirty-six patients meeting DSM-IV criteria for schizophrenia (18 men, 18 women) were recruited by the University of Pennsylvania Schizophrenia Research Center. Twenty-eight healthy individuals (14 men, 14 women) with no family history of schizophrenia or affective illness were recruited from the community. Subjects were excluded for history of neurological disorder, head trauma, loss of consciousness, substance abuse, or any medical condition that might alter cerebral functioning.
Patient age ranged from 20 to 53 years (mean=31.1, SD=8.4); comparison subject age ranged from 18 to 56 years (mean= 29.0, SD=9.8). Group differences in sex (χ2=0.00, df=1, p=1.00) and age (t=0.94, df=62, p=0.35) were insignificant.
Magnetic resonance images were acquired on a GE Signa 1.5-T system with the following parameters: SPGR sequence; flip angle 35°; repetition time 35 ms; echo time 6 ms; field of view 24 cm; 1-mm slice thickness without gaps; transaxial images with 0.9375 × 0.9375-mm in-plane resolution. Sulcal measurements were quantified using BrainVISA 3.1 (http://brainvisa.info/). This software application constructs a 3-dimensional model of cortical folds and graphical representations of the various sulci. These are automatically classified through a neural network algorithm and summary measures, including maximum depth, are computed for each identified sulcus. Sulci identified by the program were visually inspected to ensure correct classification based on the Atlas of Cerebral Sulci (Ono et al., 1990). Figure 1 illustrates the results of this automatic sulcal detection algorithm. It is important to note that the automated algorithm was required only for measurement of the orbital sulcus which, due to its complex H-shape, is difficult to visualize on cross-sectional MRI slices. The olfactory sulcus can be readily visualized and manually measured using routine coronal MRI slices (Figure 2). Although not presented here, all automated measures for the olfactory sulcus were validated by manual sulcal depth measurements conducted by technicians who were blind to diagnosis, with inter-rater reliability (ICC) >0.90.
Figure 1.
Example of identification and 3-D reconstruction of olfactory and orbital sulci using BrainVISA software. A: inferior surface of the left frontal lobe, showing locations of the sulci in situ. B: 3-D reconstruction of olfactory sulcus (left) and 'H-shaped' orbital sulcus (right).
Figure 2.
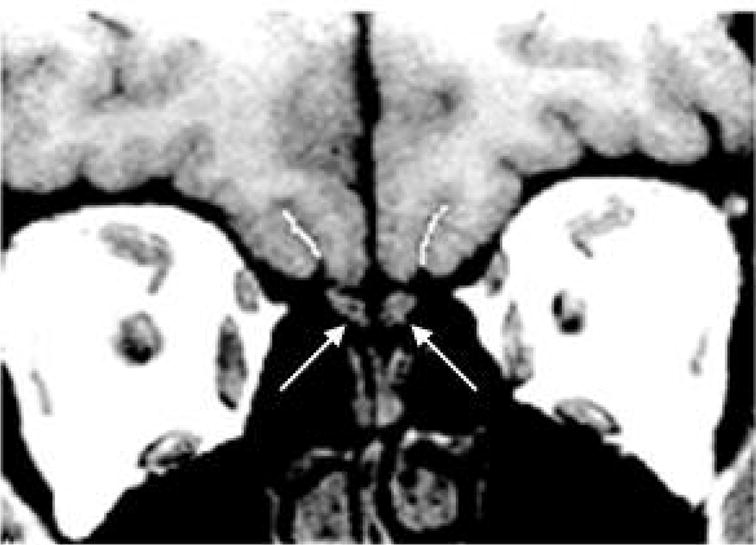
Coronal MRI scan through the forebrain. Left and right olfactory sulci are indicated by white lines. Arrows indicate the underlying olfactory bulbs.
Patient clinical symptomatology was assessed with the Brief Psychiatric Rating Scale (BPRS), the Scale for Assessment of Negative Symptoms (SANS) and the Scale for Assessment of Positive Symptoms (SAPS), by trained raters with inter-rater reliability>0.90. Additional clinical measures included age of onset, illness duration, deficit/non-deficit status and antipsychotic medication dose.
Separate analyses of variance were conducted for olfactory and orbital sulcal depth, with diagnosis as a categorical predictor, hemisphere as a repeated measures factor and age as a covariate. Relationships between MRI and clinical measures were examined in patients using Pearson correlation coefficients.
3. Results
Olfactory sulcal depth was significantly related to both diagnosis (F=4.17, df=1,62, p<0.05) and hemisphere (F=4.92, df=1,62, p<0.05). Sulcal depth was shallower in patients and deeper in the right hemisphere (Figure 2). Group comparisons within each hemisphere revealed a significant patient decrement on the right (F=5.38, df=1,62, p<0.05) but not on the left (F=1.38, df=1,62, p=0.24). Age was not significantly related to olfactory sulcal depth (F=2.87, df=1,61, p=0.10) and covarying for age did not alter the group difference in right hemisphere sulcal depth (F=4.88, df=1,61, p<0.05). No other clinical or demographic measures were related to the sulcal measures.
Orbital sulcal depth did not differ between groups (F=1.78, df=1,62, p=0.19) or hemispheres (F=1.93, df=1,62, p=0.17). There was, however, an age effect, with orbital sulcal depth becoming shallower with increasing age (F=4.22, df=1,61, p<0.05). None of the other clinical or demographic measures were related to sulcal measures.
Olfactory and orbital sulcal depth measurements were significantly correlated in control subjects (r=0.57, p<0.001), but were independent in patients (r=0.23, p=0.17).
4. Discussion
These findings confirm that schizophrenia patients have abnormally shallow olfactory sulci. Although post-hoc testing indicated that this was confined to the right hemisphere, patients did not have a right-sided decrement compared to the left. Rather, they failed to exhibit the prominent augmentation of the right olfactory sulcus that is seen in healthy subjects (Hummel et al., 2003). They thus resembled individuals with known disruptions of early forebrain embryogenesis (Abolmaali et al., 2002; Vogl et al., 1994), in whom this sulcus is often underdeveloped. The fact that this anomaly was not seen in the adjacent orbital sulcus, which develops much later in gestation, supports the interpretation that this is an index of aberrant early neurodevelopment rather than a nonspecific correlate of cortical atrophy. The lack of an association with age, even though aging affected the orbital sulcus, also suggests that this abnormality is not due to more generalized atrophy. It is notable, in this regard, that olfactory and orbital sulcal depths were strongly correlated in control subjects but were dissociated in patients. This further indicates that the abnormality occurred during the first half of gestation, prior to formation of the orbital sulcus.
This developmental disturbance may represent either a genetic or an environmental “first hit” that leaves the individual vulnerable to subsequent pathology (Maynard et al., 2001). We do not yet know whether a similar abnormality is present in individuals who have an increased genetic risk of illness, and previous evidence for midline developmental abnormalities in unaffected 1st-degree relatives of schizophrenia patients has been inconsistent (Deutsch et al., 2000; Turetsky et al., 2003a, 2007). However, whether or not it is genetically mediated, this may be a simple vulnerability marker that implicates both a specific developmental process and a critical gestational time period in the etiology of schizophrenia. Since manual measurements of olfactory sulcal depth can be easily obtained from MRI scans of children and adolescents at risk for schizophrenia, its potential utility as a predictive biomarker in this population warrants further investigation.
Figure 3.
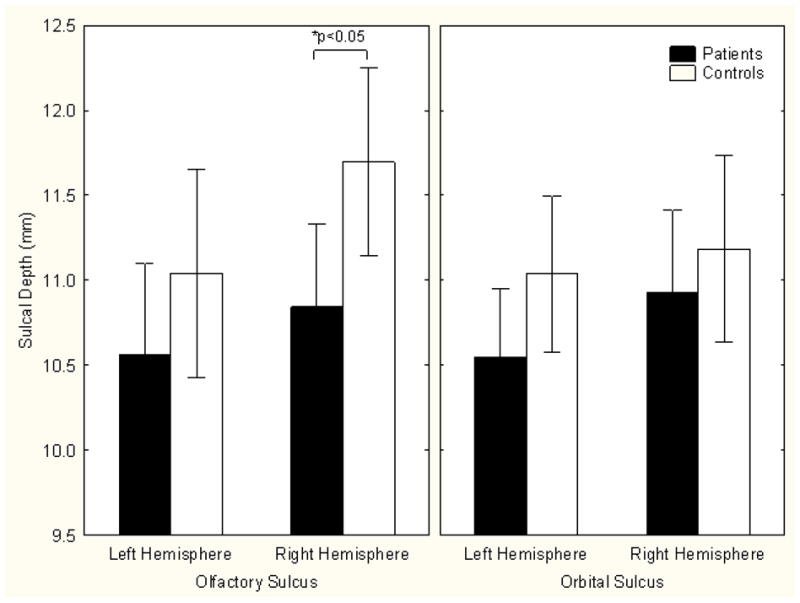
Mean olfactory and orbital sulcal depths by group and hemisphere (± 95% confidence interval). Patients have an abnormally shallow right olfactory sulcus.
Acknowledgments
Role of the Funding Source
Funding for this study was provided by NIMH Grants MH59852 and MH63381. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing or the report; and in the decision to submit the paper for publication.
We wish to thank Julie Rosner, Dana Gatto and Dana Marchetto for their assistance in conducting the manual measurements to replicate and validate the automated methodology.
Footnotes
Contributors
Dr. Turetsky contributed to the study design and literature review, conducted the statistical analyses and wrote the manuscript. Mr. Crutchley implemented the automated computer algorithms, processed the MRI scans and conducted quality control analyses of the data. Mr. Walker contributed to the literature review and conducted preliminary image analyses. Dr. Gur contributed to the study design and provided the MRI scans. Dr. Moberg contributed to the study design, literature review and statistical analyses. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Drs. Turetsky and Gur receive unrelated research grant support from AstraZeneca and Pfizer Pharmaceuticals. All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abolmaali ND, Hietschold V, Vogl TJ, Hüttenbrink KB, Hummel T. MR evaluation in patients with isolated anosmia since birth or early childhood. AM J Neuroradiol. 2002;23:157–163. [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Penner JD, Kochetkova A, Kern D, Schaefer CA. Prenatal infection and cavum septum pellucidum in adult schizophrenia. Schizophr Res. 2009;108:285–287. doi: 10.1016/j.schres.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- Christensen K, Mortensen PB. Facial clefting and psychiatric diseases: a follow-up of the Danish 1936–1987 Facial Cleft cohort. Cleft Palate Craniofac J. 2002;39:392–396. doi: 10.1597/1545-1569_2002_039_0392_fcapda_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Deutsch CK, Hobbs K, Price SFR, Gordon-Vaughn K. Skewing of the brain midline in schizophrenia. NeuroReport. 2000;11:3985–3988. doi: 10.1097/00001756-200012180-00016. [DOI] [PubMed] [Google Scholar]
- Farbman A. Developmental Neurobiology of the Olfactory System. Raven Press; New York: 1991. [Google Scholar]
- Giménez M, Junqué C, Vendrell P, Narberhaus A, Bargalló N, Botet F, Mercader JM. Abnormal orbitofrontal development due to prematurity. Neurology. 2006;67:1818–1822. doi: 10.1212/01.wnl.0000244485.51898.93. [DOI] [PubMed] [Google Scholar]
- Hummel T, Damm M, Vent J, Schmidt M, Theissen P, Larsson M, Klussmann JP. Depth of olfactory sulcus and olfactory function. Brain Res. 2003;975:85–89. doi: 10.1016/s0006-8993(03)02589-7. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008;8:71. doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the "two-hit" hypothesis of schizophrenia. Schizophrenia Bull. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Roalf DR, Gur RE, Turetsky BI. Smaller nasal volumes as stigmata of aberrant neurodevelopment in schizophrenia. Am J Psychiatry. 2004;161:2314–2316. doi: 10.1176/appi.ajp.161.12.2314. [DOI] [PubMed] [Google Scholar]
- O'Callaghan E, Larkin C, Kinsella A, Waddington JL. Familial, obstetric, and other clinical correlates of minor physical anomalies in schizophrenia. Am J Psychiatry. 1991;148:479–483. doi: 10.1176/ajp.148.4.479. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of Cerebral Sulci. Thieme Medical Publishers; New York: 1990. [Google Scholar]
- Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Arch Gen Psych. 1992;49:983–988. doi: 10.1001/archpsyc.1992.01820120071010. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Yousem D, Arnold SE, Doty RL, Gur RE. Olfactory bulb volume is reduced in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. doi: 10.1176/appi.ajp.157.5.828. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Arnold SA, Doty RL, Gur RE. Low olfactory bulb volume in 1st-degree relatives of patients with schizophrenia. Am J Psychiatry. 2003a;160:703–708. doi: 10.1176/appi.ajp.160.4.703. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Roalf DR, Arnold SA, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003b;60:1193–1200. doi: 10.1001/archpsyc.60.12.1193. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Glass CA, Abbazia J, Kohler CG, Gur RE, Moberg PJ. Reduced posterior nasal cavity volume: A gender-specific neurodevelopmental abnormality in schizophrenia. Schizophr Res. 2007;93:237–244. doi: 10.1016/j.schres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl TJ, Stemmler J, Heye B, Schopohl J, Danek A, Bergman C, Balzer JO, Felix R. Kallman syndrome versus idiopathic hypogonadotropic hypogonadism at MR imaging. Radiology. 1994;191:53–57. doi: 10.1148/radiology.191.1.8134597. [DOI] [PubMed] [Google Scholar]



