Abstract
To evaluate the potential use of recombinant murine cytomegalovirus (MCMV) as an antigen delivery vector, we examined the cytokine and CD80 and CD86 expression profiles of MCMV encoding either enhanced green fluorescent protein gene (MCMV-EGFP) or human immunodeficiency virus-1 glycoprotein gp120 gene (MCMV-gp120) infected monocyte-derived dendritic cells (Mo-DC) and investigated the role of nuclear factor kappa B (NF-κB) in Mo-DC activation. Results showed that MCMV triggered the induction of inflammatory cytokines and/or CD80 and CD86 up-regulation in Mo-DC. UV-inactivated MCMV exhibited a reduced production of inflammatory cytokines and a lowered expression of CD80 and CD86 compared with live MCMV infection. Treatment of cells with a NF-κB peptide inhibitor prior to MCMV infection reduced the induction of cytokines and CD80 and CD86 up-regulation. Overall, the results suggest that recombinant MCMV vectors activate human Mo-DC in a NF-κB dependent pathway. The abortive infection or de novo gene expression greatly enhances the activation of Mo-DC by MCMV vectors.
Recombinant murine cytomegalovirus (MCMV) has been explored as a potential antigen delivery vector due to its ability to target human dendritic cells (DC) without compromising the antigen-presenting ability of DC (Wang et al., 2003). Additionally, MCMV undergoes an abortive infection in human cells and is safe for use in humans (Jurak and Brune, 2006; Tang and Maul, 2006; Wang et al., 2003). To further understand the mechanistic basis of DC activation, we examined the cytokine profiles and CD80/CD86 expression of recombinant murine cytomegalovirus encoding an enhanced green fluorescence protein gene (MCMV-EGFP) or human immunodeficiency virus-1 glycoprotein gp120 gene (MCMV-gp120)-infected DC and investigated the role of nuclear factor kappa B (NF-κB) in MCMV-mediated DC activation.
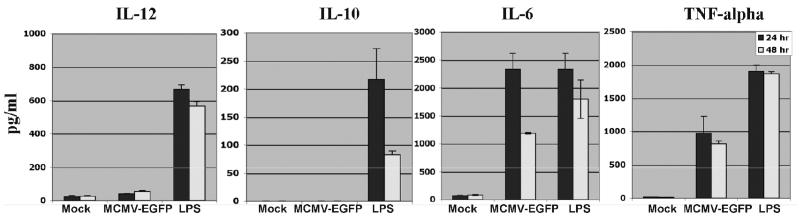
Previously, we have observed that MCMV-EGFP infected monocyte-derived dendritic cells (Mo-DC) exhibited a slightly increased expression of co-stimulatory molecules such as CD40 and CD86 (Wang et al., 2003). Here, we further characterized the cytokine profiles of MCMV-EGFP-infected DC. Mo-DC were prepared from peripheral blood mononuclear cells as described previously (Wang et al., 2003). We infected Mo-DC with 5 MOI (multiplicity of infection) of MCMV-EGFP and the production of inflammatory cytokines TNF-α, IL-6, IL-12, and anti-inflammatory cytokine IL-10 was measured using the quantitative ELISA kits from eBiociences (San Diego, CA). We observed that TNF-α, IL-6, IL-12, and/or IL-10 were induced by MCMV-EGFP at 24 and 48 h after virus infection (Fig.1A). The production of TNF-α induced by MCMV-EGFP ranged from 120 to 1,200 pg/ml in different donors (Fig.1B), suggesting a biological difference in responding to MCMV-EGFP.
Figure 1.
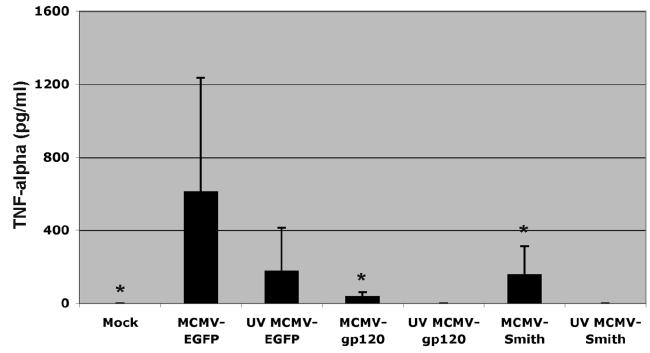
The efficient activation of DC by MCMV depends on the expression of immediate early and early genes as well as transgenes. A: IL-12, IL-10, IL-6, and TNF-α induction by MCMV-EGFP-infected and LPS (1μg/ml) treated DC at 24 and 48 h after infection. Representative results from one donor are shown. The experiment was repeated at least three times with cells derived from different donors and similar results were obtained. B: TNF-α induction by MCMV-EGFP, MCMV-gp120, MCMV-Smith and UV-inactivated MCMV-infected DC at 12 h after infection. Each column represents the data of four individual experiments from four donors (means + SD). An asterisk indicates a statistically significant difference (p-value < 0.05; ANOVA) compared to MCMV-EGFP group.
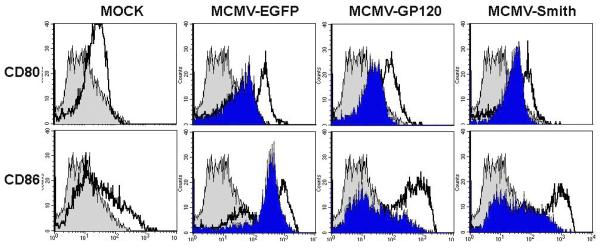
C: Up-regulated expression of CD80 and CD86 in MCMV infected DC at 48 h after infection. Gray filled histogram: PE labeled IgG1 isotype control antibody; Open histogram: Specific staining with either PE labeled CD80 or CD86 antibodies for live virus-infected cells. Blue filled histogram: Specific staining with either PE labeled CD80 or CD86 antibodies for UV-inactivated virus-infected cells. Dead cells were excluded from the flow cytometric analysis by using propidium iodide staining. The experiment was repeated twice using Mo-DC derived from two separate donors and similar results were obtained.
Previous studies have suggested that retroviral or adenoviral vectored GFP expression in human DC enhances the immunogenicity of DC (Re et al., 2004). Our previous study has shown that both immediate early 1 protein of MCMV and EGFP are abundantly expressed in MCMV-EGFP-infected human Mo-DC (Wang et al., 2003). To determine whether the cytokine induction by MCMV-EGFP is exclusively mediated by EGFP expression, we examined the cytokine induction by DC infected with either wild-type MCMV-Smith or recombinant MCMV-vectored HIV-1 gp120 gene. The construction of MCMV-EGFP and MCMV-gp120 has been described previously and both genes were inserted into the immediate early 2 region of MCMV backbone (Wang et al., 2003). We infected Mo-DC with 5 MOI of MCMV-gp120 or MCMV-Smith and measured the production of TNF-α. TNF-α was induced by MCMV-gp120 and MCMV-Smith infected Mo-DC, but much lower than MCMV-EGFP infected Mo-DC (ANOVA, p-value < 0.05, Fig.1B). A similar biological difference in TNF-α production (ranged from undetectable to 232 pg/ml for MCMV-Smith and from undetectable to 70 pg/ml for MCMV-gp120 in different donors) was observed for MCMV-gp120 and MCMV-Smith. This suggests that the induction of TNF-α by MCMV vectors is possibly modulated by both vector backbone itself and trangenes. Additionally, the result does support the reported role of EGFP in enhancing immunogenicity of human DC by promoting TNF-α production (Re et al., 2004).
For a number of viruses including adenovirus and human cytomegalovirus (HCMV), viral attachment and entry, not viral gene expression or viral DNA replication, is sufficient to activate the induction of inflammatory cytokines (Compton et al., 2003; Morelli et al., 2000). To examine if the same mechanism occurs in MCMV-infected Mo-DC, 5 MOI of UV-inactivated MCMV-EGFP, MCMV-gp120, and MCMV-Smith were used to infect Mo-DC and TNF-α induction was measured. We observed a reduced TNF-α induction in UV-inactivated MCMV-EGFP-infected Mo-DC compared to live MCMV-EGFP-infected cells (ANOVA, p-value > 0.05, Fig.1B). No TNF-α was detected in UV-inactivated MCMV-gp120 and UV-inactivated MCMV-Smith-infected cells (Fig.1B). This finding suggests that viral attachment and entry play some roles in TNF-α induction by MCMV-EGFP but abortive infection or active viral gene and transgene expression is critical to the efficient induction of TNF-α by MCMV vectors.
We next examined the expression level of co-stimulatory molecules such as CD80 and CD86 on MCMV-infected Mo-DC. Briefly, Mo-DC were infected with 5 MOI of MCMV-EGFP, MCMV-gp120, and MCMV-Smith. At 24 h and 48 h after infection, cells were harvested and analyzed for CD80/CD86 expression by flow cytometric analysis as described previously (Wang et al., 2003). A significant increase in the expression level of the two co-stimulatory molecules was observed in all three viruses-infected Mo-DC compared to mock-infected cells (Fig.1C). No obvious transgene specific difference in CD80/CD86 expression was observed in this case, suggesting the importance of viral vector backbone genes in triggering CD80/CD86 up-regulation. Interestingly, we did not detect an increased expression of CD80 by MCMV-EGFP in the previous study (Wang et al., 2003), which may be due to the fact that we used 1% autologous plasma instead of 10% fetal bovine serum in Mo-DC medium in that study.
To further determine whether the expression of viral immediate early and early genes as well as transgene is critical in up-regulating these co-stimulatory molecules, we assessed their expression levels after infection of Mo-DC with UV-inactivated MCMV. As shown in Fig.1C, a dramatic reduction in the expression of CD80 and CD86 was observed in UV-MCMV treated cells compared to live MCMV-treated cells, suggesting the role of gene expression or abortive infection in up-regulating CD80/CD86 expression. Nevertheless, virus attachment and entry alone appear sufficient to activate DC as evidenced by the up-regulated expression of co-stimulatory molecules (Fig.1C).
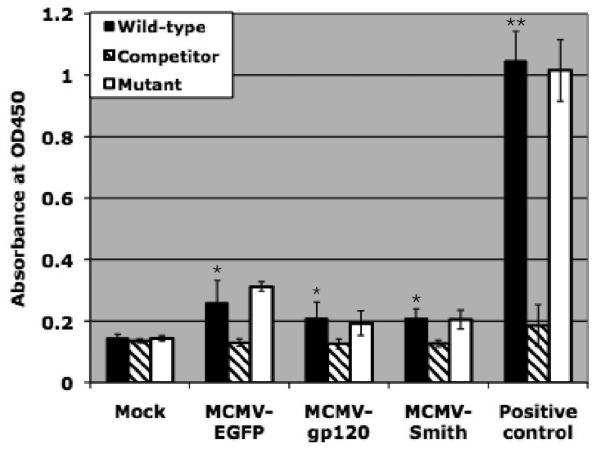
NF-κB is a ubiquitous DNA binding transcription factor that plays a key role in the activation of signal transduction pathways. NF-κB is a complex protein and consists of various homo- and heterodimeric associations of proteins including c-Rel, p65 (RelA), RelB, p50 (NF-κB1), and p52 (NF-κB2) (Karin and Delhase, 2000; May and Ghosh, 1998). To investigate the role of NF-κB in Mo-DC activation by MCMV, we examined the activation of NF-κB p65 profile at 12 h after infection using a no-shift transcription factor assay (Novagen, Madison, WI). Briefly, 1-2 × 106 Mo-DC were infected with 3 MOI of MCMV-EGFP, MCMV-gp120, or MCMV-Smith. At 12 h after infection, cells were harvested and nuclear extracts were prepared from the collected cells by following the manufacturer's instructions. The specific binding of the p65 in the nuclear extract to the NF-κB wild type, NF-κB mutant and NF-κB competitor DNA were measured in a 96-well plate using monoclonal antibody specific to human p65 followed by HRP-labeled secondary anti-mouse antibody and substrate for detection. We found that NF-κB was obviously activated by MCMV-EGFP, MCMV-gp120, and MCMV-Smith (Fig.2A). The relative low values obtained from MCMV-infected Mo-DC in comparison to the positive control (Hela cell nuclear extracts provided by the kit) may be due to the limited cell numbers (1-2 ×106) available for use in each group. Nevertheless, it is clear that all three MCMV activated the nuclear translocation of NF-κB in Mo-DC.
Figure 2.
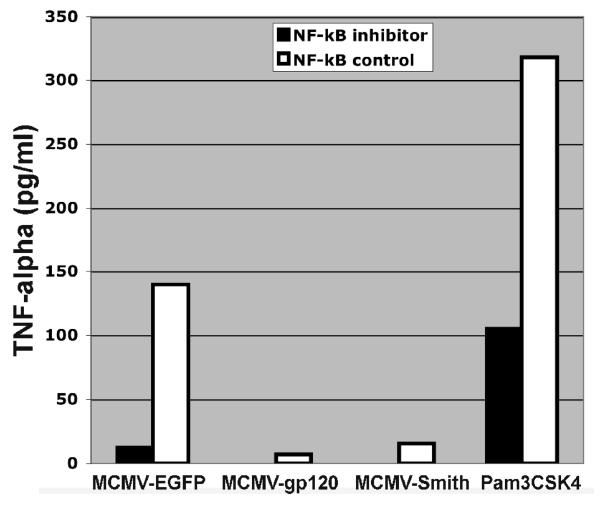
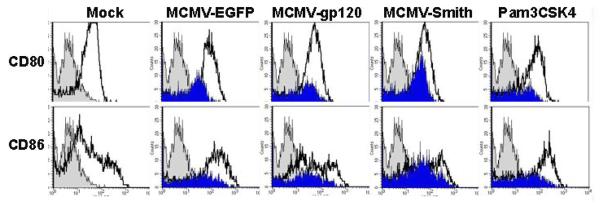
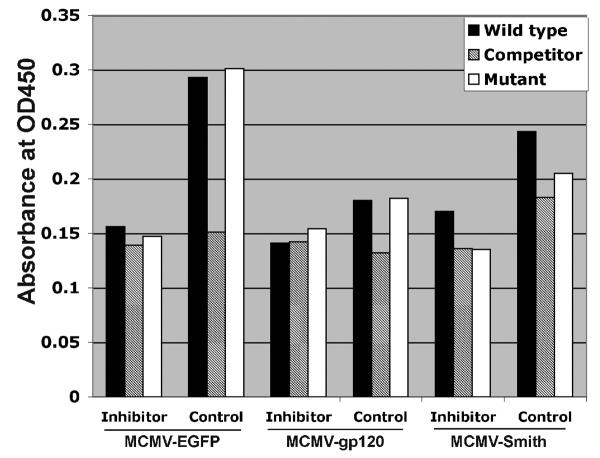
NF-κB activation is partly associated with DC activation by MCMV infection. A: NF-κB activation profile of MCMV-infected DC at 12 h after infection. Wild-type: NF-κB wild-type DNA. Competitor: NF-κB competitor DNA. Mutant: NF-κB mutant DNA. Positive control: HeLa Control Nuclear Extract. The experiment was repeated three times using Mo-DC prepared from three donors (Mean ± SD). * means p ≤ 0.05 compared to mock (t-test). ** means p < 0.001 compared to mock (t-test). B: TNF-α induction by MCMV-infected DC pre-treated with NF-κB inhibitor peptide or control peptide at 12 h after infection. Pam3CSK4 (1μg/ml), a known TLR2 ligand, serves as a positive control. The experiment was repeated twice and similar results were obtained. C: CD80 and CD86 expression by MCMV-infected DC pre-treated with NF-κB inhibitor peptide or control peptide at 48 h after infection. Gray filled histogram: PE labeled IgG1 isotype control antibody; Open histogram: Specific staining with either PE labeled CD80 or CD86 antibodies for control peptide treated cells. Blue filled histogram: Specific staining with either PE labeled CD80 or CD86 antibodies for NF-κB p65 inhibitor peptide treated cells. Pam3CSK4 serves as a positive control. Dead cells were excluded from the flow cytometric analysis by using propidium iodide staining. Representative results of two experiments are shown. D: NF-κB activation profile of MCMV-infected DC pre-treated with NF-κB inhibitor peptide or control peptide at 12 h after infection. Wild-type: NF-κB wild-type DNA. Competitor: NF-κB competitor DNA. Mutant: NF-κB mutant DNA. Representative results of two experiments are shown.
In most cases, the activation of NF-κB pathways will result in the subsequent induction of inflammatory cytokines such as TNF-α and the up-regulation of co-stimulatory molecules (Compton et al., 2003; Morelli et al., 2000). To verify that NF-κB p65 activation is indeed associated with the induction of TNF-α and the up-regulation of CD80 and CD86 by MCMV-EGFP, MCMV-gp120, and MCMV-Smith, we examined the TNF-α induction and CD80 and CD86 expression in cells treated with a NF-κB p65 phosphorylation inhibitory peptide or control peptide prior to MCMV infections as described previously (Takada et al., 2004). Treatment of cells with 150 μM of NF-κB p65 inhibitor peptide (Ser276, IMGENEX, San Diego, CA), but not control peptide, prior to MCMV infection reduced the induction of TNF-α (Fig.2B) and the expression of both CD80 and CD86 (Fig.2C). The successful inhibition of NF-κB activation by the peptide inhibitor was confirmed by the no-shift transcription factor assay (Fig.2D). Overall, the results suggest that MCMV triggers the induction of inflammatory cytokines and CD80 and CD86 up-regulation in human Mo-DC partly via activation of NF-κB. This is in agreement with the previous finding that the immediate early 1 protein of MCMV is a potent inducer of NF-κB pathway [6]. We have shown previously that the immediate early 1 protein is abundantly expressed in Mo-DC (Wang et al., 2003).
Taken together, our data suggest that MCMV activates Mo-DC to produce inflammatory cytokines and to increase the co-stimulatory molecules expression. Additionally, we have shown that MCMV activated Mo-DC partly depending on the NF-κB pathway and the abortive nature of replication that MCMV vector undergoes in human Mo-DC is critical to the efficient activation of human Mo-DC.
Acknowledgements
We thank Drs. Stephen Dewhurst and Martin Messerle for providing the recombinant MCMV for the study. We also thank Dr. Matthew Vukovich for his generous technical assistance. This work was supported by NIH grant R15 AI059216 to X.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–96. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurak I, Brune W. Induction of apoptosis limits cytomegalovirus cross-species infection. Embo J. 2006;25:2634–42. doi: 10.1038/sj.emboj.7601133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–8. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- Morelli AE, Larregina AT, Ganster RW, Zahorchak AF, Plowey JM, Takayama T, Logar AJ, Robbins PD, Falo LD, Thomson AW. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol. 2000;74:9617–28. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Srinivasan R, Igarashi T, Marincola F, Childs R. Green fluorescent protein expression in dendritic cells enhances their immunogenicity and elicits specific cytotoxic T-cell responses in humans. Exp Hematol. 2004;32:210–7. doi: 10.1016/j.exphem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Takada Y, Singh S, Aggarwal BB. Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappaB-mediated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279:15096–104. doi: 10.1074/jbc.M311192200. [DOI] [PubMed] [Google Scholar]
- Tang Q, Maul GG. Mouse cytomegalovirus crosses the species barrier with help from a few human cytomegalovirus proteins. J Virol. 2006;80:7510–21. doi: 10.1128/JVI.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Messerle M, Sapinoro R, Santos K, Hocknell PK, Jin X, Dewhurst S. Murine cytomegalovirus abortively infects human dendritic cells, leading to expression and presentation of virally vectored genes. J Virol. 2003;77:7182–92. doi: 10.1128/JVI.77.13.7182-7192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]