Abstract
Background and Aims
Receptor–stimulated Ca2+ influx is a critical component of the Ca2+ signal and mediates all cellular functions regulated by Ca2+. However, excessive Ca2+ influx is highly toxic resulting in cell death, which is the nodal point in all forms of pancreatitis. Ca2+ influx is mediated by store-operated channels (SOCs). The identity and function of the native SOCs in most cells is unknown.
Methods
Here, we determine the role of deletion of Trpc3 in mice on Ca2+ signaling, exocytosis, intracellular trypsin activation and pancreatitis.
Results
Deletion of TRPC3 reduced the receptor-stimulated and SOCs-mediated Ca2+ influx by about 50%, indicating that TRPC3 functions as SOC in vivo. The reduced Ca2+ influx in TRPC3−/− acini resulted in reduced frequency of the physiological Ca2+ oscillations and of the pathological sustained [Ca2+]i increase caused by supramaximal stimulation and by the toxins bile acids and palmitoleic acid ethyl ester. Consequently, deletion of TRPC3 shifted the dose response for receptor-stimulated exocytosis, and prevented the pathological inhibition of digestive enzyme secretion at supramaximal agonist concentrations. Accordingly, deletion of TRPC3 markedly reduced intracellular trypsin activation and excessive actin depolymerization in vitro and the severity of pancreatitis in vivo.
Conclusions
These findings establish the native TRPC3 as a SOC in vivo and a role for TRPC3-mediated Ca2+ influx in the pathogenesis of acute pancreatitis and suggest that TRPC3 should be considered a target for prevention of the pancreatic damage in acute pancreatitis.
Background and Aims
Acute pancreatitis is an inflammatory, multifactorial disease of the pancreas caused by generation of toxic mediators within the pancreas, resulting in mistargeting of digestive enzymes that eventually destroy the pancreatic parenchyma 1. The pancreatic acinar cells store the harmful digestive enzymes that destroy the pancreas. It is now well established that aberrant Ca2+ signaling perturbs many functions of acinar cells and is intimately associated with all forms and models of acute pancreatitis 2.
The physiological and pathological Ca2+ signal involves IP3-mediated Ca2+ release from the endoplasmic reticulum (ER) that causes the activation of Ca2+ influx channels at the plasma membrane, the so-called store-operated channels (SOCs). At physiological stimulus intensity, the SOCs sustain the receptor-stimulated Ca2+ oscillations and determine their frequency, reload the stores with Ca2+ at the termination of cell stimulation and provide the Ca2+ required for sustaining exocytosis, gene regulation and all long term functions regulated by Ca2+ 3–5. The pathological Ca2+ signal is caused by any stimulus that results in chronic depletion of the ER Ca2+ stores and consequently uncontrolled activation of the SOC channels that causes sustained and prolonged increase in cytosolic Ca2+ ([Ca2+]i) 2, 6.
The complement of the channels mediating the SOC activity in pancreatic acinar and other secretory cells is not known. Acinar cells express the TRPC1, TRPC3 and TRPC6 7 and the newly discovered Orai channels 8. The TRPC channels (TRPCs) function as Ca2+-permeable non-selective cation channels that mediate part of the receptor stimulated Ca2+ influx in many cells 9, 10. The Orai channels are highly selective Ca2+ channels and mediate the Ca2+-release activated Ca2+ (CRAC) current 8. The Orai 11, 12 and TRPC channels 13–15 are regulated by the ER Ca2+ sensor STIM1. STIM1 transmits the ER Ca2+ load to the plasma membrane SOCs to activate them 16, 17. Although STIM1 activates the two channel types by different mechanisms 15,18, the Orai and TRPC channels appear to interact 19 and affect the activity of each other 20, 21. Moreover, interaction of each channel type with STIM1 requires the presence of the other channel and SOC requires the pore function on both channels 22.
Because TRPC3 is prominently expressed in pancreatic and salivary gland acinar cells 7, and TRPC3 is regulated by STIM1 14, we asked whether TRPC3 contributes to the native SOCs in secretory cells and whether TRPC3-mediated Ca2+ influx contributes to aberrant Ca2+ influx responsible for pancreatitis. A definitive approach to these questions is deletion of Trpc3 in mice. Here we report that deletion of TRPC3 markedly reduced SOC activity in multiple secretory cell types, reduced the frequency of Ca2+ oscillations and consequently altered the dose response for agonist-stimulated exocytosis. Most notably, deletion of TRPC3 prevented the pathological inhibition of exocytosis observed at supramaximal receptor stimulation to reduce activation of trypsin within acinar cells and consequently the severity of acute pancreatitis in vivo. These findings establish the native TRPC3 as a SOC in vivo and a role for TRPC3-mediated Ca2+ influx in the pathogenesis of acute pancreatitis and suggest that TRPC3 should be considered a target for prevention of the pancreatic damage in acute pancreatitis.
Materials and methods
Reagents, preparation of acini, ducts and single acinar cells and general procedures
Trpc3−/− mice were generated as detailed in 23. Acini, ducts and single pancreatic acinar cells were prepared from the pancreas or submandibular gland of WT and Trpc3−/− mice as described previously 24. The acini and cells were maintained in solution A containing (mM) 140 NaCl, 5 KCl, 1 MgCl2, 10 HEPES (pH 7.4), 10 glucose and either 1 CaCl2 or 1 EGTA and adjusted to 310 mOsm. Amylase activity was measured with the Phadebas kit (Pharmacia &Upjohn) following the manufacturer instructions. Intracellular trypsin activity was measured using the synthetic substrate, rhodamine 110-(CBZ-Ile-Pro-Arg)2 25. Acini stimulated for 60 min at 37 °C, resuspended in a solution contaning (mM) 5 HEPES, 150 NaCl, 2 EDTA, pH 7.35, 10 µM substrate and incubated for additional 20 min to allow substrate hydrolysis. Bright-field and fluorescence images were captured and results were analyzed by counting the number of fluorescent cells. For western blots, lysates were prepared as before 24 and proteins (100 µg) were probed with a 1:500 dilution of phospho-PERK and PERK and 1:1000 dilutions of LC3, tubulin or actin.
Current measurement
The whole cell current was measured by dialyzing the cells with a pipette solution containing (mM) 140 Cs-aspartate, 6 MgCl2, 10 BAPTA and 10 HEPES to passively deplete t ER Ca2+. The cells were perfused with a bath solution of (mM) 140 NaC1, 5 CsCl, 1 MgCl2, 10 CaCl2, 10 Hepes, 10 Glucose. After 5 min the bath solution was changed to divalent-free medium (DFM) (mM: 140 NaC1, 5 KCl, 0.5 EGTA, 10 Hepes). Current was recorded by applying 400 ms RAMPs from −100 to +100 mV from a holding potential of 0 mV at 5 sec intervals. The current recorded at −100 mV was used to calculate current density in pA/pF. The current output was filtered at 20 Hz, stored online with a Digi-Data 1200 interface and analyzed offline with pclamp 9.2 software.
Assay of luminal actin
Acini in solution A were stimulated with 10 nM CCK8 for 20 min at 37°C, plated on poly-L-lysine coated cover glass, rinsed with PBS and fixed with 4% paraformaldehide for 10 min. The acini were permeabilized with 0.1% Triton X-100 for 5 min and the actin was stained with 0.15 µM FITC-Phalloidin for 20 min. Luminal fluorescence intensity of confocal images was analyzed with MetaMorph.
[Ca2+]i measurement
Acini and ducts loaded with Fura-2 were perfused (37°C) with solution A. [Ca2+]i was measured at the 340 and 380 nm excitation wavelengths and the emitted light was collected by a digital camera with a cutoff filter at 510 nm and analyzed with Metafluor. Results are presented as the 340/380 ratios.
Induction of acute pancreatitis in mice
Mice starved over night were injected hourly in the abdominal cavity over 4 hours with caerulein at 40 ng/g body weight. Four injections were used since eight injections induced severe pancreatitis in WT and Trpc3−/− mice. Two hours after the last injection, the mice were sacrificed to excise the pancreas and collect blood. The blood was spun down and the plasma was used to measure serum amylase. The pancreas was immediately embedded in OCT and fixed sections of 5 µm were stained with hematoxylin and eosin. Images were recorded while marinating constant exposure times and resolutions. Damaged area (edema) for each section was determined using MetaMorph. Each image was converted into a monochrome setup to set the same threshold. The damaged area was marked and converted into pixels. At least 5 randomly collected images showing tissue damage were analyzed from each pancreas and the damaged area was calculated as % of the damage in mice injected with saline.
Statistics
Results are expressed as mean±s.e.m of the indicated number of observations obtained from 3–5 independent experiments and mice. Statistical significance was determined by analysis of variance.
Results
Deletion of TRPC3 alters Ca2+ signaling
Stimulation of pancreatic acini with high agonist concentration (0.5 mM carbachol) generated a transient increase in Ca2+ with a plateau stabilizing at about 250 nM with a half time of about 65 sec (Fig. 1a). A similar Ca2+ signal was evoked by 10 nM CCK8. Termination of cell stimulation is followed by reloading of the ER with Ca2+. The rate of Ca2+ reloading can be reliably estimated from the recovery of the CCK signal following stimulation with carbachol and inhibition with atropine 26. In wild-type (WT) acini, about 85% of the response to CCK8 was recovered after 90 sec incubation with atropine. Stimulation of WT acini with physiological concentration of 0.5 µM carbachol (not shown) or 10 pM CCK8 (Fig. 1b) induced typical Ca2+ oscillations.
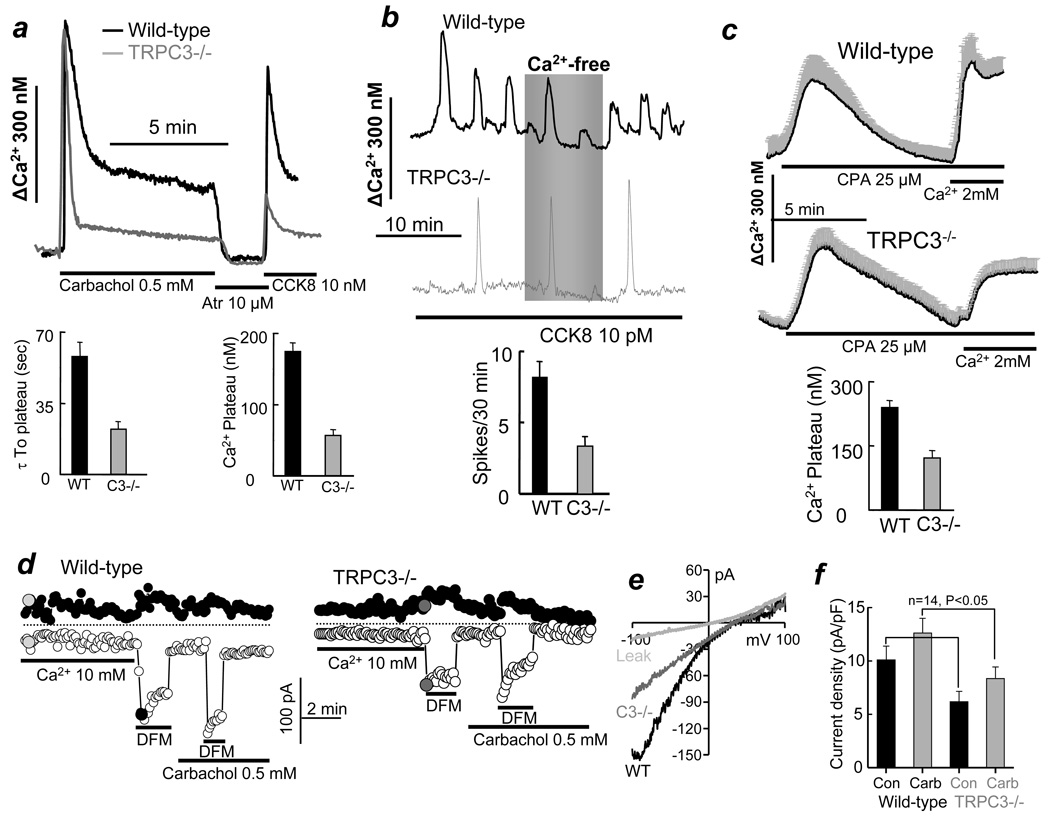
Fig. 1. Deletion of TRPC3 in mice alters Ca2+ signaling in pancreatic acini.
Fura2-loaded pancreatic acini from WT(black traces) or Trpc3−/− mice (gray traces) were used to measure [Ca2+]i. (a) Acini were stimulated with 0.5 mM carbachol and then inhibited with 10 µM atropine for 90 sec before re-stimulation with 10 nM CCK8. The columns on the left show the average time to reduction of Ca2+ from its peak to the stable plateau and the columns on the right show the [Ca2+]i levels at the plateau. (b) Ca2+ oscillations were induced by stimulation with 10 pM CCK8. The columns show the averaged oscillations frequency. (c) The internal stores were passively depleted by treating the acini with 25 µM CPA in Ca2+-free medium for 7.5–10 min and then measuring SOC activity by re-addition of 2 mM Ca2+ to the perfusate. The columns are the average SOC activity. All averages are given as mean±s.e.m of at least 12 acini and 32 cells from 4 or more mice of each strain. (d) CRAC-like current was measured in single acinar cells from WT and Trpc3−/− mice dialyzed with 10 mM BAPTA and perfused with bath solution containing 10 mM Ca2+ and than divalent-free medium containing 0.5 mM EGTA (DFM). Where indicated, the cells were stimulated with 0.5 mM carbachol that in almost all cell modestly increased the current. However, due to viable current density, when averaged from all cells, this effect of carbachol did not reach statistical difference. (e) shows example I/Vs recorded at the time indicated by the filed circles in (d). The columns in (f) show the current density in 14 cells obtained from two mice of each strain.
Deletion of TRPC3 has multiple effects on the Ca2+ signal. Although, the initial Ca2+ increase due to Ca2+ release from the ER was not affected by deletion of TRPC3, the subsequent reduction in [Ca2+]i was faster, with a half time of about 20 sec, and [Ca2+]i stabilization at a plateau of about 150 nM (Fig. 1a). Moreover, 90 sec after termination of cell stimulation only 25% of the response to CCK8 was recovered, indicating markedly impaired Ca2+ influx in the Trpc3−/− acini. Finally, deletion of TRPC3 reduced the frequency of the CCK8-evoked Ca2+ oscillation from 8.5 to 3.5 spikes/30 min. Similar results were obtained with carbachol-evoked Ca2+ oscillations.
Reduction in receptor-stimulated Ca2+ influx in the Trpc3−/− acini raised the question of whether TRPC3 contributes to SOC. SOC activity was measured by depleting the stores with 25 µM of the SERCA inhibitor cyclopiazonic acid (CPA) in Ca2+-free media for 7.5 min and then exposing the acini to media containing 2 mM Ca2+. Deletion of TRPC3 reduced SOC activity by about 50% (Fig. 1c), indicating that the native TRPC3 functions as a SOC in pancreatic acini.
To further analyze the role of TRPC3 in SOC we measured the whole cell current under conditions that isolate the Ca2+-release activated Ca2+ (CRAC) current 5. The cells were dialyzed with 10 mM BAPTA to passively deplete the stores and exposed to 10 mM external Ca2+. As with most non-hematopoietic cells, minimal or no current was measured under these conditions. Larger CRAC current can be observed when the cells are then exposed to a divalent-free medium (DFM) 8. Such a maneuver resulted in a modestly inward rectifying current (Fig. 1d,e) that was reduced by about 40% in Trpc3−/− cells (Fig. 1f). These findings further imply a role of TRPC3 in pancreatic acinar cells SOC.
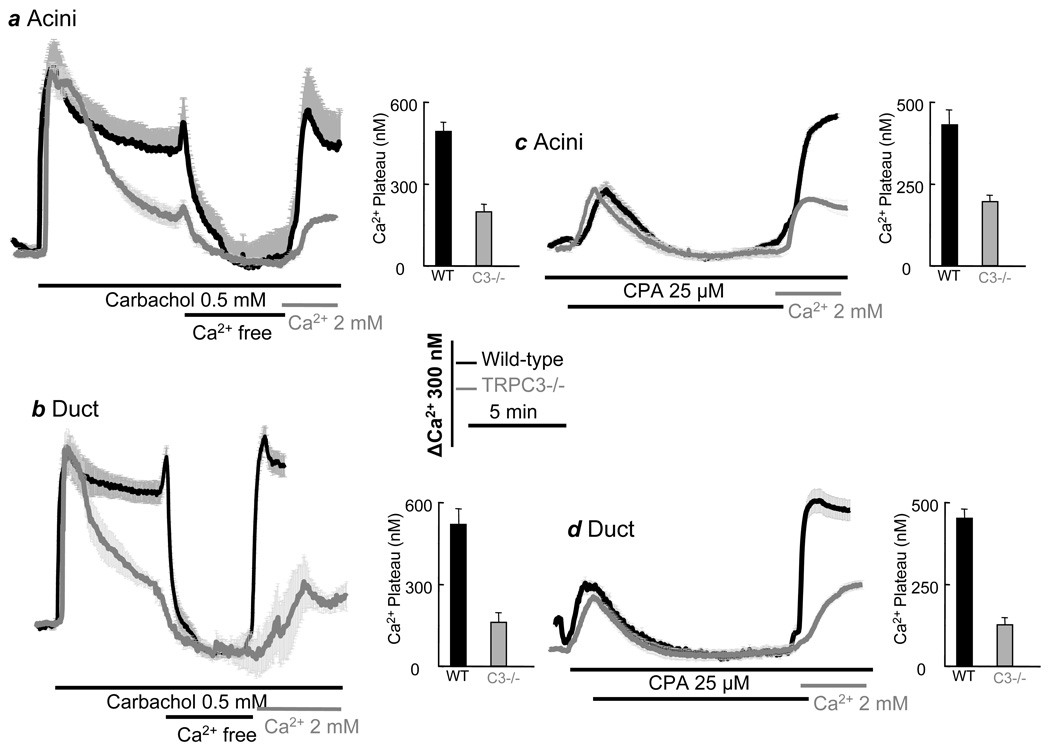
Next, we asked whether TRPC3 functions as SOC and contributes to receptor-stimulated Ca2+ influx in other cell types. We examined receptor- and CPA-induced Ca2+ influx in submandibular gland cells since they display particularly prominent SOC-mediated Ca2+ influx27. Figs. 2a,c (acini) and 2b,d (ducts) show that deletion of TRPC3 reduced agonist-stimulated and SOC-mediated Ca2+ influx Ca2+ in the two cell type. This was particularly prominent in the duct with both activities being reduced by about 70% in TRPC3−/− ducts.
Fig. 2. Deletion of TRPC3 in mice alters Ca2+ signaling in submandibular acini and ducts.
Fura2-loaded submandibular acini and ducts from WT(black traces) or Trpc3−/− mice (gray traces) were used to measure [Ca2+]i. (a, b) acini (a) and ducts (b) were stimulated with 0.5 mM carbachol and after stabilization of [Ca2+]i they were perfused with Ca2+-free medium and then Ca2+-containing medium to evaluate activation of Ca2+ influx. (c, d) acini (c) and ducts (d) were treated with 25 µM CPA in Ca2+-free medium for 7.5–10 min to measure SOC activity by readdition of 2 mM Ca2+ to the perfusate. The columns are the averages of the indicated conditions and are the mean±s.e.m of at least 8 acini and ducts from 3 mice of each strain.
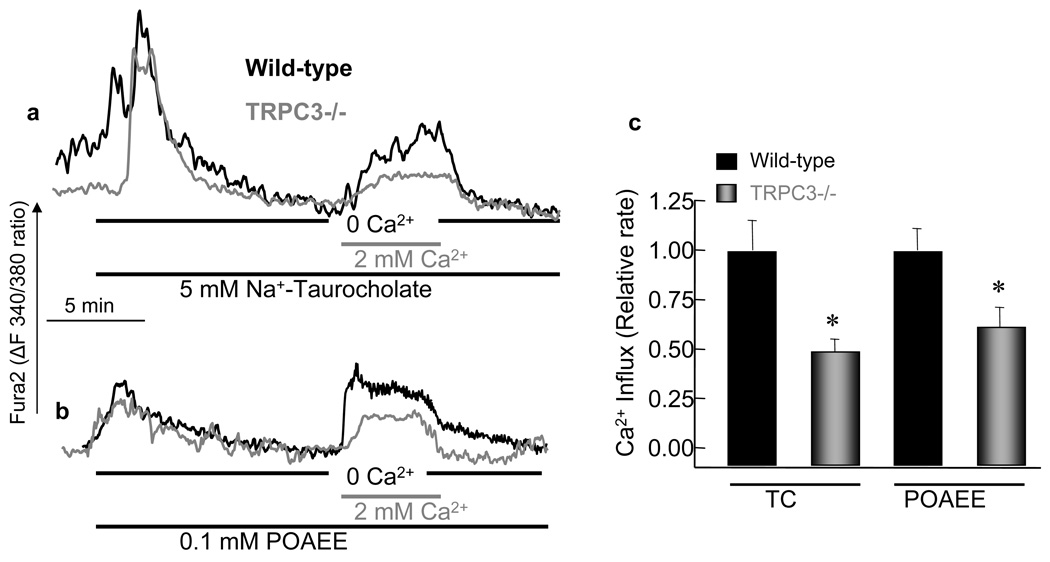
A pathological Ca2+ signal is induced by toxins, such as bile acids and the ethanol metabolites palmitoleic acid ethyl ester (POAEE), that act on pancreatic acinar cells. Reflex of bile acid into the pancreas 28 and ethanol consumption 1 are known causes of pancreatitis. Fig. 3 shows that deletion of TRPC3 has no effect of Ca2+ release by taurocholate (Fig. 3a) and POAEE (Fig. 3b). By contrast, deletion of TRPC3 reduced Ca2+ influx by about 50%. Since bile acids deplete the stores by inhibition of the SERCA pumps 28, these findings further indicate that the native TRPC3 functions as SOC.
Fig. 3. Deletion of TRPC3 in mice alters Ca2+ signaling evoked by cell stressors.
Fura2-loaded pancreatic acini from WT(black traces) or Trpc3−/− mice (gray traces) were treated with 5 mM Na+-taurocholate (a) or 100 µM POAEE (b) in Ca2+-free medium to measure Ca2+ release from internal stores and then in Ca2+-containing medium to measure activation of Ca2+ influx. The columns are the mean±s.e.m of at least 10 acini from 3 mice of each strain.
Deletion of TRPC3 reduces cell stress associated with activation of SOCs
Induction of pancreatitis in vitro and in vivo is associated with cell stress, as evident from activation of the cell stress ER kinase PERK 29, which regulates the unfolding protein response. Accordingly, Fig. 4a shows that when the acini are treated with supramaximal CCK8 or with bile acid, PERK phosphorylation is increased. A recent notable finding is that induction of pancreatitis activates autophagy and inhibition of autophagy prevents acute pancreatitis 30. Autophagy can be reliably followed by lipidation of LC3-I to form LC3-II. Fig. 4b shows that treatment with CCK8 and bile acid increased the level of LC3-II. Similar results were obtained by treating the cells with 100 µM POAEE (not shown). Hence, all stressors that increase SOC activity and induce pancreatitis activate the ER stress response and induced autophagy in pancreatic acini.
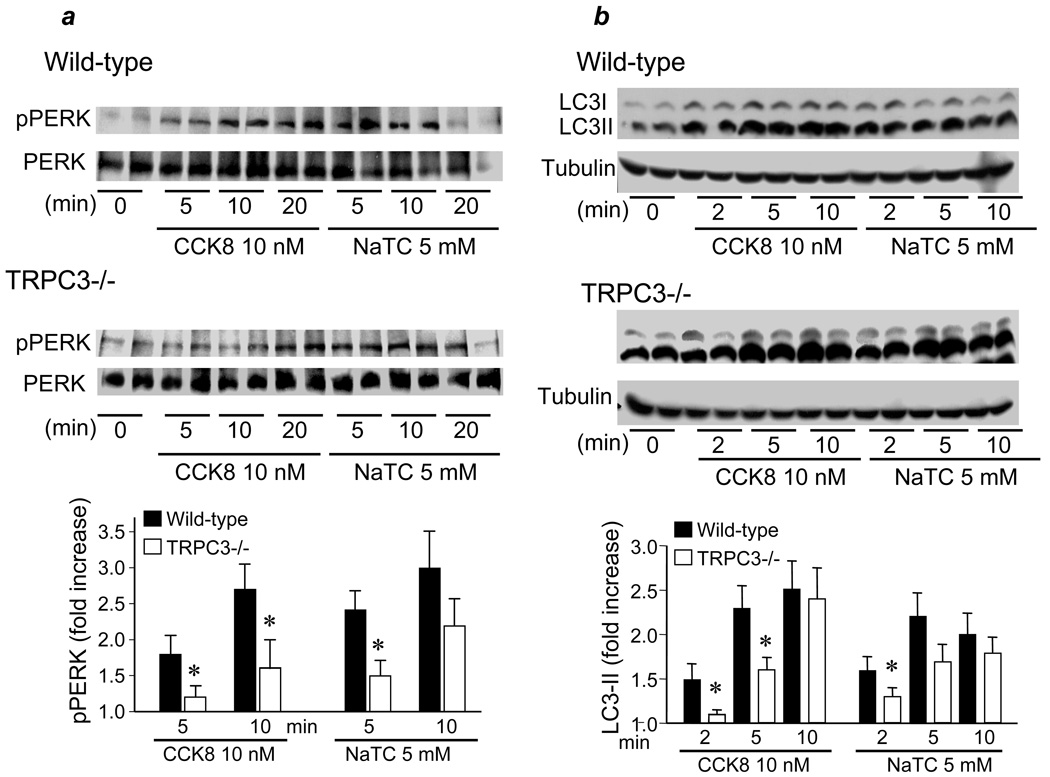
Fig. 4. Deletion of TRPC3 in mice reduces ER stress and activation of autophagy.
Pancreatic acini from WT(upper blots) of Trpc3−/− mice (lower blots) were stimulated with 10 nM CCK8 or treated with 5 mM Na+-taurocholate for the indicated times. The acini were rapidly collected and lysates prepared to determine phosphorylation of PERK (pPERK) (a) or generation of LC3II (b). The changes in pPERK were normalized relative to total PERK and were then calculated as an increase over unstimulated cells. Because of the damage to the acini treated with Na+-taurocholate, the 20 min time points are not reliable and thus results are presented only for the 5 and 10 min of treatment. The columns in (a) are the mean±s.e.m of 6 determinations from 3 mice. Note that, as shown by the manufacture for most cells, the anti-LC3 antibodies used detect better the mouse LC3-II than LC3-I in pancreatic acini. These antibodies were used since they detected better LC3 in pancreatic acini. The changes in LC3-II were normalized relative to tubulin and were then calculated as an increase over unstimulated cells. The columns in (a) are the mean±s.e.m of 6 determinations from 3 mice.
Significantly, deletion of TRPC3 reduced the rate of PERK phosphorylation (Fig. 4a) and the rate of activation of autophagy (Fig. 4b) in response to supramaximal CCK8 and to bile acids. Hence, the reduced Ca2+ influx in Trpc3−/− cells protected the cells by reducing ER stress and self-destruction by autophagy.
Deletion of TRPC3 alters agonist-stimulated exocytosis, intracellular trypsin activation and pathological actin depolymerization
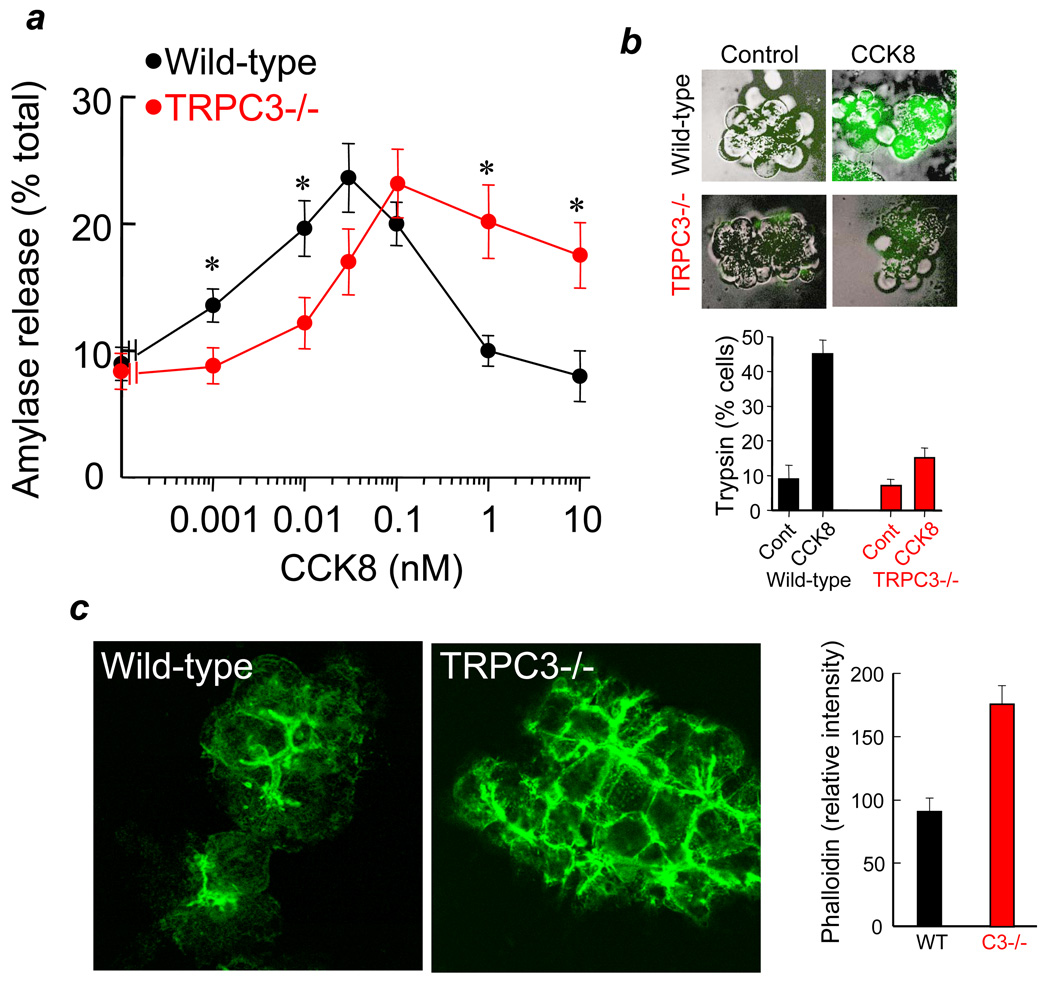
Exocytosis by pancreatic acinar cells is a Ca2+-triggered process. The initial phase of exocytosis is mediated by Ca2+ release from internal stores, whereas Ca2+ influx is essential to maintain exocytosis beyond the first 3–5 min 31. Since deletion of TRPC3 reduced Ca2+ influx and the frequency of Ca2+ oscillations (Fig. 1), we determined the effect of deletion of TRPC3 on exocytosis. Deletion of TRPC3 right shifted the dose response for CCK8-stimulated amylase release (Fig. 5a), as expected from the shift in the frequency of Ca2+ oscillations.
Fig. 5. Deletion of TRPC3 in mice alters receptor-stimulated exocytosis and intracellular trypsin activation.
Panel (a): Pancreatic acini from WT(black symbols) or Trpc3−/− mice (red symbols) were stimulated with the indicated concentrations of CCK8 and amylase released to the media was measured as a reporter of exocytosis. The results are the mean±s.e.m of 4 experiments with duplicate or triplicate determinations. * donates p<0.05. Note the shift in the dose response and the lack of inhibition of amylase release at supramaximal agonist concentrations. (b) Unstimulated pancreatic acini (control) and pancreatic acini stimulated with 10 nM CCK8 for 60 min from WT(black) of TRPC3−/− mice (red) were used to measure intracellular trypsin activity as detail in methods. Typical images at each condition are superimposed bright field and fluorescence images. A total of 60–140 cells in acini composed of 3–15 cells were counted under each condition and results are presented as the mean±s.e.m. of % cells showing intracellular trypsin activity. (c) Acini stimulated with 10 nM CCK8 were fixed, permeabilized and stained for actin. The columns show the meam±s.e.m of fluorescence intensity of more than 40 luminal spaces of multiple acini recorded in 8 randomly collected images from each WT and Trpc3−/− acini.
A particularly notable finding in Fig. 5a is the lack of inhibition of exocytosis at high concentrations of CCK8 in the Trpc3−/− acini. The key step in the pathogenesis of pancreatitis is mistargeting of digestive enzymes to the lysosomes via the autophagosomes. In in vitro models of pancreatitis this is manifested as inhibition of exocytosis at supramaximal agonist concentrations 1. Inhibition of exocytosis is due to excessive actin depolymerization at the terminal web that is observed at high, sustained increase in cytosolic Ca2+ 32. Indeed, direct estimation of actin depolymerization in WT and Trpc3−/− cells stimulated with the 10 nM CCK8 showed that deletion of TRPC3 reduced actin depolymerization by about 50% (Fig. 5c). Even more pronounced difference between WT and Trpc3−/− cells was observed on stimulated with 1 nM CCK8 (not shown). These findings suggest that deletion of TRPC3 reduces the severity of pancreatitis in vitro.
Mistargeting of digestive enzymes to the lysosomes results in their activation within the cells that can be followed by measuring intracellular trypsin activity 25. The lack of inhibition of exocytosis at supramaximal agonist concentration in Trpc3−/− cells predicts reduced intracellular trypsin activation in these cells. Fig. 5b shows strong intracellular trypsin activity in 45% of WT acini stimulated with 10 nM CCK8 for 1 hr. Deletion of TRPC3 reduced the number of cells with intracellular trypsin activity to 12% and most of these cells showed mild trypsin activity.
Deletion of TRPC3 reduces the severity of acute pancreatitis
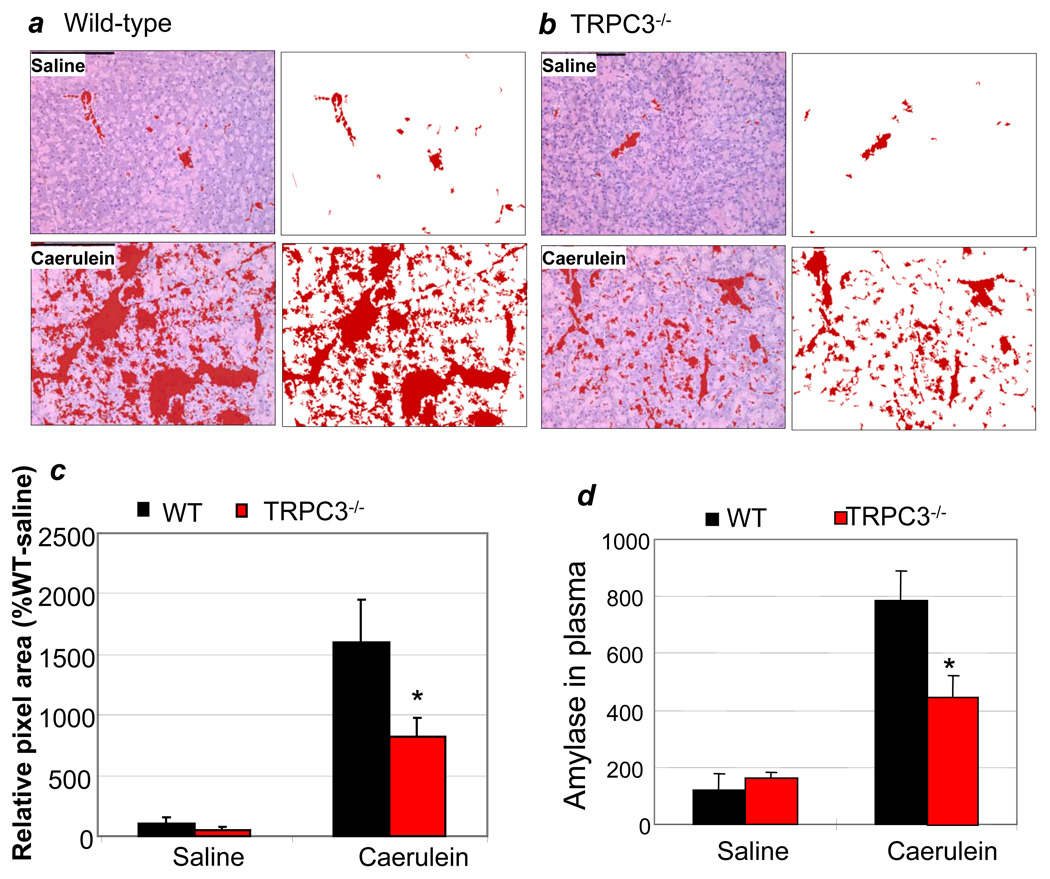
Direct evidence for reduced severity of pancreatitis in the Trpc3−/− mice was obtained with the caerulein model of pancreatitis. The severity of pancreatitis is evaluated by the extent of pancreatic edema and by mistargeting of amylase to the circulation. Figs. 6a and 6b and the summary in Fig. 6c show that deletion of TRPC3 reduced the edema by about 50%. Similarly, serum amylase reported about 50% reduction in the severity of pancreatitis in TRPC3−/− animals (Fig. 6d). Importantly, the reduction in serum amylase and the severity of pancreatitis was not due to reduced level of amylase in the Trpc3−/− pancreas. Total amylase content in WT and Trpc3−/− pancreas was 3.07±0.23 and 2.98± 0.15 mU/µl, respectively.
Fig. 6. Deletion of TRPC3 in mice reduces the severity of acute pancreatitis in vivo.
Wild-type (a) or Trpc3−/− mice (b) were injected with caerulein to induce acute pancreatitis and pancreatic slices were used to evaluate edema, as detailed in methods. The multiple sections obtained from each mice stain were used to determine the edematous area and the mean±s.e.m are shown in (c). Panel (d) shows the mean±s.e.m of the levels of serum amylase measured in the same mice. Each group of WT and Trpc3−/− mice injected with saline (control) or caerulein had 3 mice. Similar results were obtained with two additional experiments.
Discussion
The present study reports that TRPC3 functions as SOC in vivo to mediate significant portion of the receptor-stimulated Ca2+ influx in exocrine secretory cells; that the TRPC3-mediated Ca2+ influx affects the frequency of Ca2+ oscillations; and that excessive Ca2+ influx by TRPC3 during supramaximal receptor stimulation is toxic to acinar cells and is responsible in part to the cell stress and damage that occur in pancreatitis. Therefore, inhibition of acinar cell TRPC3 and other Ca2+ influx channels may be considered as a modality to control and reduce the severity of pancreatitis.
It is still a matter of debate of whether and which of the TRPCs function as SOCs. Gating of TRPCs by STIM1 13–15 and interaction of TRPCs with Orai1 19–22, 33, 34 provide compelling evidence for the function of TRPCs as SOCs. Moreover, our recent work indicate that the function of both Orai1 and TRPCs is required for the native SOC 22 and the Orai1-TRPC complex mediate both SOC and receptor-stimulated Ca2+ influx 35. Nevertheless, in spite of all these findings, a recent report claims that TRPCs, including TRPC3, do not function as SOCs 36. The current findings add significantly to this topic by showing that the native TRPC3 functions as SOC in vivo. This is particularly important, since the behavior of TRPC3 is affected by its expression level 14, 37. Thus, the native TRPC3 functions as SOC, and as such significantly contributes to receptor-stimulated Ca2+ influx in secretory glands acinar and duct cells. Interestingly, the native TRPC1 functions as SOC in salivary glands acinar and duct cells 38. In model systems TRPC1 and TRPC3 are assembled into a heteromultimer by STIM1 and the function of TRPC3 as SOC requires TRPC1 14. Together, these results suggest that native TRPC1 and TRPC3 may require the activity of each other to function as Ca2+ influx channels.
The contribution of TRPC3 to receptor-stimulated Ca2+ influx is also evident from the reduced frequency of Ca2+ oscillations in Trpc3−/− cells, providing independent evidence that sustained Ca2+ oscillations requires refilling of Ca2+ stores by Ca2+ influx on a spike per spike basis. Through control of the frequency of Ca2+ oscillations TRPC3 determines the exocytotic response of acinar cells. The oscillatory Ca2+ signal that controls exocytosis is initiated by Ca2+ release from the ER at the apical pole 39, 40. The apical pole of pancreatic acini is enriched with all components of the Ca2+ signaling complex 4, including TRPC3 7. Localization of TRPC3 at the apical pole can explain its profound effect on the frequency of the Ca2+ oscillations and exocytosis.
It is now well established that sustained [Ca2+]i increase is responsible for the cell damage occurring in various modes of pancreatitis 6. However, there is no information on the molecular nature of the pathway mediating the sustained [Ca2+]i increase. The present work shows that TRPC3 mediates a significant fraction of the sustained [Ca2+]i increase evoked by the toxins supramaximal stimulation, bile acids and POAEE. In addition, deletion of TRPC3 prevented the pathological inhibition of exocytosis and intracellular trypsin activation in the in vitro model of pancreatitis and reduced the severity of pancreatitis in mice. Hence, it is clear that overactivation of TRPC3 by pathological depletion of ER Ca2+ mediates significant portion of the pathological Ca2+ influx associated with pancreatitis. Complete characterization of all the Ca2+ influx pathways in acinar cells should further clarify the role of Ca2+ influx channels in pancreatitis and other diseases of secretory cells and may provide viable molecular targets for developing drugs to reduce the severity of these diseases.
Acknowledgments
Grant Support: This work was supported by NIH grants DE12309 and DK38938 and the Ruth S. Harrell Professorship in Medical Research to S.M. and by the Intramural Research Program of the NIH (Z01-ES-101684) to L.B.
Abbreviations
- SOC
store-operated Ca2+ influx channels
- TRPC3
transient receptor potential (canonical) isoform 3 channel
- [Ca2+]i
free cytoplasmic Ca2+
- ER
endoplasmic reticulum
- POAEE
Palmitoleic acid ethyl ester
- CPA
cyclopiazonic acid (SERCA inhibitor)
- SERCA
sarcoplasmic/endoplasmic Ca2+ ATPase pump
- PERK
PKR-like ER kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- 2.Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27:113–120. doi: 10.1016/j.tips.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 4.Kiselyov K, Wang X, Shin DM, Zang W, Muallem S. Calcium signaling complexes in microdomains of polarized secretory cells. Cell Calcium. 2006;40:451–459. doi: 10.1016/j.ceca.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 6.Sutton R, Petersen OH, Pandol SJ. Pancreatitis and calcium signalling: report of an international workshop. Pancreas. 2008;36:e1–e14. doi: 10.1097/MPA.0b013e3181675010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006;281:32540–32549. doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- 8.Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 11.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 14.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci U S A. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MS, Zeng W, Yuan J, Shin DM, Worley P, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem. 2009 doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worely PF, Muallem S. Homer 1 mediates store- and IP3Rs- dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem. 2006 doi: 10.1074/jbc.M602496200. [DOI] [PubMed] [Google Scholar]
- 25.Kruger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muallem S, Khademazad M, Sachs G. The route of Ca2+ entry during reloading of the intracellular Ca2+ pool in pancreatic acini. J Biol Chem. 1990;265:2011–2016. [PubMed] [Google Scholar]
- 27.Won JH, Yule DI. Measurement of Ca2+ signaling dynamics in exocrine cells with total internal reflection microscopy. Am J Physiol Gastrointest Liver Physiol. 2006;291:G146–G155. doi: 10.1152/ajpgi.00003.2006. [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Kim KH, Lee JA, Namkung W, Sun AQ, Ananthanarayanan M, Suchy FJ, Shin DM, Muallem S, Lee MG. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology. 2002;122:1941–1953. doi: 10.1053/gast.2002.33617. [DOI] [PubMed] [Google Scholar]
- 29.Kubisch CH, Logsdon CD. Secretagogues differentially activate endoplasmic reticulum stress responses in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1804–G1812. doi: 10.1152/ajpgi.00078.2007. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto D, Ohmuraya M, Hirota M, Yamamoto A, Suyama K, Ida S, Okumura Y, Takahashi E, Kido H, Araki K, Baba H, Mizushima N, Yamamura K. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandol SJ, Schoeffield MS, Sachs G, Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem. 1985;260:10081–10086. [PubMed] [Google Scholar]
- 32.Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alicia S, Angelica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: Moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008 doi: 10.1016/j.ceca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci U S A. 2009;106:3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dehaven W, Jones B, Petranka J, Smyth J, Tomita T, Bird G, Putney J. TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009 doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez G, Lievremont JP, St JBG, Putney JW., Jr Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proc Natl Acad Sci U S A. 2001;98:11777–11782. doi: 10.1073/pnas.201238198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci U S A. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993;74:669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- 40.Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993;74:661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]