Abstract
The tumor microenvironment provides multiple cues that may be exploited to improve the efficacy of established chemotherapeutics; furthermore, polypeptides are uniquely situated to capitalize on these signals. Peptides provide: 1) a rich repertoire of biologically specific interactions to draw upon; 2) environmentally-responsive phase behaviors, which may be tuned to respond to signatures of disease; 3) opportunities to direct self-assembly; 4) control over routes of biodegradation; 5) the option to seamlessly combine functionalities into a single polymer via a one-step biosynthesis. As development of cancer-targeted nanocarriers expands, peptides provide a unique source of functional units that may target disease. This review explores potential microenvironmental physiology indicative of tumors and peptides that have demonstrated an ability to target and deliver to these signals.
Keywords: EPR, Tumor, Hyperthermia, Peptides, Drug Delivery, Redox. Cancer, pH, Chemotherapy
1. Introduction
According to the American Cancer Society, 7.6 million people died from cancer in the world during 2007 [1] , and about 565,650 Americans were expected to die of cancer [1] . Cancer is the second most common cause of death in the US, exceeded only by heart disease [1] . The sustained prevalence of cancer continues to motivate the development of new therapies. Significant research efforts have been directed towards targeting cancer drugs to tumors using specialized drug carriers, and peptides have become an important component of these targeting approaches. The contents of this review address the current status of peptide-mediated targeting of drug carriers.
It is likely that drug carriers will play an increasing role in the treatment of cancer. Cancer treatment is multi-pronged, consisting of surgery, radiation, and drug-mediated chemotherapy, which varies depending on the nature of the tumor [1]. Among these three modalities, improvements to chemotherapy offer some of the most exciting opportunities to develop new approaches. Most traditional chemotherapeutic agents have a therapeutic index close to one, and they cause concentration dependent toxicity in non-cancerous tissues [2]. The mechanism of dose-limiting toxicity varies from drug to drug. For example, the administration of doxorubicin (DOX) is limited by cardiomyopathy that arises from oxidative stress [3]. In mice free DOX has a therapeutic index of 1.2; however, this has not prevented its widespread use over the past 4 decades [4]. In contrast, neuropathy and neutropenia are the dose-limiting toxicities for patients treated with paclitaxel, a microtubule stabilizing agent [5]. Presumably, the differences in mechanisms of action and toxicity between chemotherapeutic agents will significantly impact the optimization of the drug carrier strategy.
In addition, the concentration dependent cytotoxicity of these chemotherapeutics typically makes them unsuitable for administration routes that produce high local concentrations, such as oral, transdermal, or subcutaneous administration. To circumvent this localized toxicity near the site of administration, many of these agents are delivered via intravenous administration [6]. After systemic administration only a small percentage of the administered drug reaches the target site [7]; furthermore, encapsulation of chemotherapeutics inside drug carriers can increase this percentage. For example, liposomal DOX accumulation in tumors is 3 to 15 fold higher than for free drug [8]. Ultimately chemotherapeutics continue to produce dose-limiting toxicity by their interaction with healthy tissues, diminishing their clinical efficacy; furthermore, a major rationale for the development of nanoparticulate carriers has been to reduce drug exposure to normal tissues.
Without a mechanism for releasing drug, carrier encapsulation is typically insufficient to generate useful anti-tumor responses. To improve the therapeutic index, carriers must have an optimal rate and mechanism of in vivo drug release. For many carrier systems, the tumor drug concentration depends upon the mechanism of encapsulation/attachment, as well as the physicochemical properties of the drug. Most drugs differ in their rates of systemic and local clearance; therefore, each drug must be evaluated on a case by case basis [9]. With regards to the rate of drug release, these carriers may fall into three possible regimes: 1) release is too fast; 2) release is too slow; and 3) release is perfectly balanced [10]. If the release of a drug from a carrier is too rapid, then the drug may clear to the bloodstream prior to reaching the tumor. While negligible rates of release of drug into the blood can be tolerated, rapid and untargeted drug release is not a desirable property for a reliable delivery system. Under this scenario, the carrier is unable to prevent exposure at sites of toxicity, and may be unable to promote selective tumor accumulation beyond that of free drug. Under the second scenario, where the drug release rate is too slow, the local clearance of drug could be faster than release of free drug. The resulting concentration of drug available in the tumor may then be sub-therapeutic, even though the total concentration of encapsulated drug in the tumor may be high [9,10]. In the third scenario the rate of drug release from the carrier is perfectly balanced to yield both optimal tumor accumulation and localized release; however, this balance is difficult to achieve.
Various nanocarrier systems have been explored that approach optimal rates of tumor accumulation and availability [11]. Formulations including Doxil™ (lipid-mediated) and Abraxane™ (peptide-mediated) have partially overcome these barriers, and have been translated to the clinical setting [12–15]. Falling short of its promise, a substantial limitation of the liposomal formulation appears to be that it has a suboptimal rate of drug release in the tumor [16]. Here we describe a range of mechanisms for controlling localized accumulation and drug release; furthermore, we summarize peptides with potentially useful behaviors that may enhance delivery to the tumor microenvironment.
2. Mechanisms of peptide-mediated tumor targeting
Environmentally responsive delivery systems make use of tumor pathology to trigger release of therapeutic agents at the target site. The tumor microenvironment has been widely studied, generating a host of biomarkers suitable for targeted delivery [17–19]. The list of potential biomarkers is extensive; however, it is likely that only a subset of biomarkers can be engineered into suitable triggers for targeted drug delivery. The list of potential biomarkers provided by the tumor microenvironment can be broadly classified into physical or molecular triggers. Physical triggers are activated by the nature of the tumor microenvironment. For example, the tumor microenvironment contains regions of reduced pH [17] and increased oxidative potential [18,20], and increased vascular wall permeability. On the other hand molecular triggers include the target molecules that are upregulated in the tumor vasculature or within the tumor cells. These targets include vascular endothelial growth factor, integrins, matrix metalloproteases and tumor necrosis factors [19]. Antibodies against these biomarkers have been successful in tumor treatment studies [21], which have prompted more research into pathways associated with these markers. Another example of a biomarker in the tumor microenvironment is the upregulation of secreted phospholipase A2 [22]. Phospholipase A2 is involved in the production of prostagladins [22]. Phospholipase A2 mediates carcinogenesis by two pathways: release of arachidonic acid, which produces carcinogenic metabolites; and release of lysophospholipids, including lysophosphatidic acids (LPA) that induce cell growth [23]. Both physical and molecular biomarkers such as these are being explored to develop an array of new nanocarriers [24,25].
These microenvironmental biomarkers are being actively explored for the ability to produce environmentally responsive drug release in the tumor. One approach has been to develop environmentally sensitive polymers, including peptides that respond to tumor microenvironment with targeted delivery of drug [26,27]. Such approaches are intended to either increase the accumulation of drug carrier in the tumor or increase the release of active drugs from carriers that have already trafficked to the tumor. Continued study of the tumor microenvironment is expected to reveal new cues, which may be useful for controlled drug release. The remainder of this review focuses on targeting mechanisms employed in current research and plausible roles played by peptides/proteins.
2.1 Enhanced permeability and retention
Some of the most frequently applied mechanisms of tumor targeting utilize the properties of the tumor vasculature. The tumor neovasculature is ‘chaotic’ in nature, consisting of various loops, dead ends, and openings that lead directly into the perivascular space [28]. These openings provide a passive mechanism for targeting macromolecular or nanoparticulate entrapment within the tumor. Additionally, lack of lymphatic drainage prevents drug/carrier clearance from tumors. The combination of these two factors is commonly referred to as the enhanced permeability and retention (EPR) effect [29] (Fig. 1). To obtain nutrients and oxygen, solid tumors grow around existing blood vessels and produce new blood vessels to interconnect with the existing vasculature, through a process known as angiogenesis. These new vessels are characterized by high permeability and a haphazard arrangement [29,30]. Systemically administered carriers must pass through this dense arrangement of vessels to reach the cells of solid tumors. While tumors do recruit a blood supply, they fail to develop functional lymphatic drainage [28]. One of the primary functions of the lymphatic system is to provide a route for the clearance of extravascular proteins, particulates, and white blood cells. Without a lymphatic system, macromolecules and nanoparticulates that extravasate through the ‘leaky’ tumor vasculature accumulate to form a ‘depot’ in the perivascular space. The pore sizes of some typical tumor blood vessels have been estimated to have diameters around 400–600nm [31]. Experiments have shown that carriers with a diameter of less than around 100 nm [32,33] are ideal to target the tumor vasculature via EPR. Thus appropriately sized particles that pass through the tumor can accumulate locally, which sustains the total drug concentration compared to the parent small molecule. This approach is relatively specific for tumor tissue, in addition to other tissues with permeable endothelia, even though it does not require any specific molecular interactions to drive accumulation.
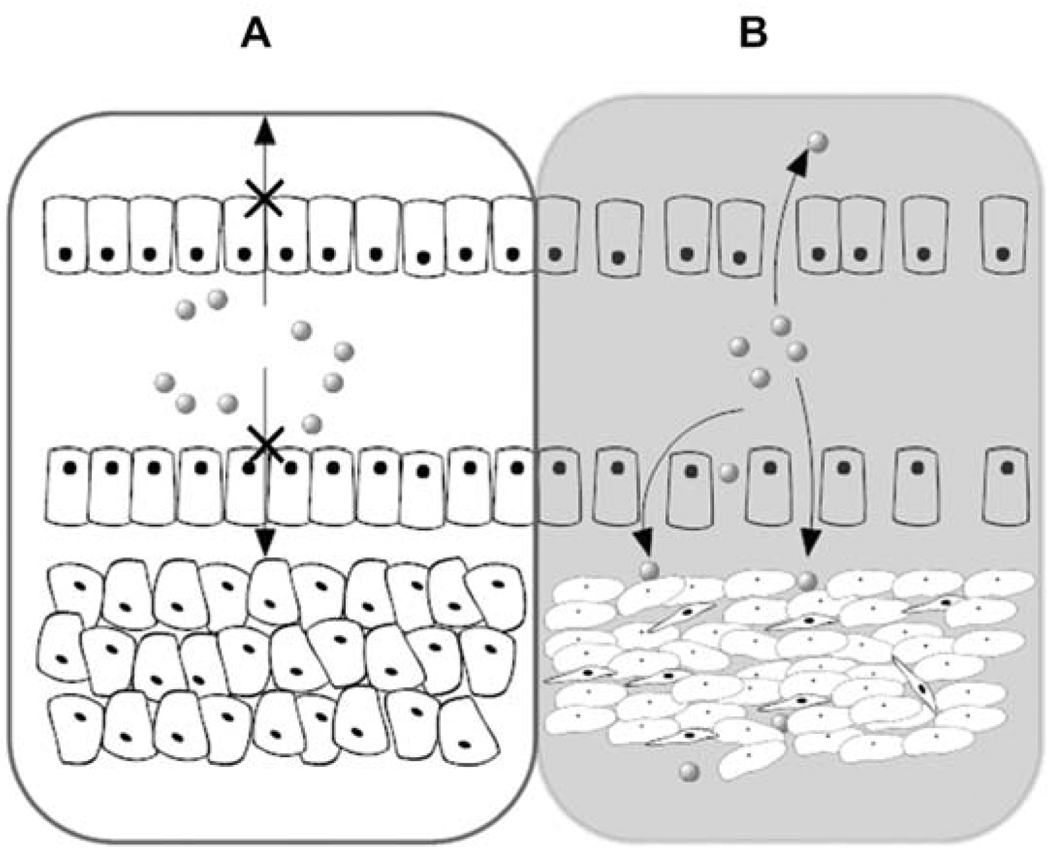
Figure 1.
Intra tumoral vascular permeability permits entrapment of drug carriers. (A) Intact normal vasculature. (B) Semi porous tumor vasculature. Drug carriers selectively extravasate into tumor via pores [28]. This EPR targeting strategy provides a mechanism for extracellular drug carrier accumulation in the tumor, which may be further augmented via peptide-mediated targeting.
EPR targeting suffers from several limitations, in that it provides no mechanism for generating the active drug once the carrier localizes to the tumor and that vascular perfusion is not uniform either between or within tumors [34]. For some drug formulations, such as Doxil™ [13], the rate of nonspecific drug release is balanced enough to reduce tumor mass; however, the activity of novel EPR-targeted formulations should be specifically optimized to address the drug diffusion and permeability in the tumor and in the cell [30]. For carriers that target via the EPR effect, the tumor microenvironment must play a critical role in mediating release from the carrier. Typically mechanisms for achieving drug release are designed into the carrier, which include cleavable linkers, cell targeting ligands, or permeabilizing agents, many of which are derived from polymers and peptides. Peptides in particular provide a wealth of rational strategies for increasing the efficiency of drug delivery.
2.2 Ligand-mediated targeting
Peptides and proteins can be used to target delivery of anticancer agents via intracellular or extracellular release. These strategies typically benefit from passive EPR targeting prior to initiation of targeted interactions. Similar to free drug, extracellular release is influenced by cancer drug resistance mediated by: 1) activation of the P-gp proteins [35]; 2) activation of glutathione detoxification system [36]; and 3) by alterations in the genes and the proteins involved with apoptosis, such as p53 and Bcl-2 [37]. To circumvent this barrier, peptides can also mediate the intracellular delivery of drugs via targeting receptors at the cell surface (Table 1). For receptor targeting strategies, including transferrin [38–40], folate [41–48] and epidermal growth factor receptors [49–51], the EPR mediated accumulation of carrier is followed by improved internalization of the carriers into target cells, a mechanism known as receptor-mediated endocytosis (RME). Using RME strategies, tumors can be selectively targeted by increased localization of the carrier to the tumor and also by enhancement of internalization (Fig. 2) [25]. Conjugation of drugs to these carriers can in turn facilitate significant intracellular drug concentrations. Both imaging and chemotherapeutic agents have been targeted to tumors via RME [43–45,52].
Table 1.
Receptor & protein mediated targeting peptides.
| Peptides | *Properties | References |
|---|---|---|
| Transferrin and anti transferrin antibody | Iron binding proteins found in vertebrates. Enables 2-fold increase in tissue accumulation of non-specific antibody conjugated liposome. | [39,40,60–62] |
| Herceptin™/Trastuzumab | Antibody against HER2. Showed increased internalization of carrier and a 6-fold increase in internalization. | [50,64,65] |
| GE11 peptide | Ligand for human epidermal growth factor receptor. Contains the peptide sequence ‘YHWYGYTPQNVI.’ | [49,51] |
| Albumin | Human plasma protein soluble in water. Assists in transporting hydrophobic molecules. Used as a carrier for anticancer therapy. | [12,57,72,147] |
| HIV TAT peptide | The TAT protein enters cells when added exogenously. The transduction domain responsible for this property is RKKRRQRRR,’ which can be appended to other systems. | [148–150] |
| NGR peptide | This motif has 2 distinct binding sites 1) Aminopeptidase N (CD13) 2) αvβ3 Integrin. Plays a major role in cell adhesion. | [69,71] |
| RGD peptide | This motif is a recognized by integrins and is important for cell adhesion. | [66–68,151] |
| GFLG peptide | Acts as a cleavable peptide linker sensitive to proteolyses by lysosomal cathepsin B. | [73] |
| ALAL peptide | Acts as a cleavable peptide linker sensitive to proteolyses by lysosomal cathepsin B. | [72] |
| GPQGaAGQR peptide | Where a = Leucine, Isoleucine or Valine. This peptide sequence acts as a substrate for MMP-2,9 and can be used as a linker/trigger for drug release from polymer conjugates or drug carriers. | [76,79] |
A=Alanine, F=Phenylalanine, G=Glycine, H=Histidine, I=Isoleucine, K=Lysine, L=Leucine, N=Asparaginine, P=Proline, Q=Glutamine, R=Arginine, T=Theronine, V=Valine, W=Tryptophan Y=Tyrosine.
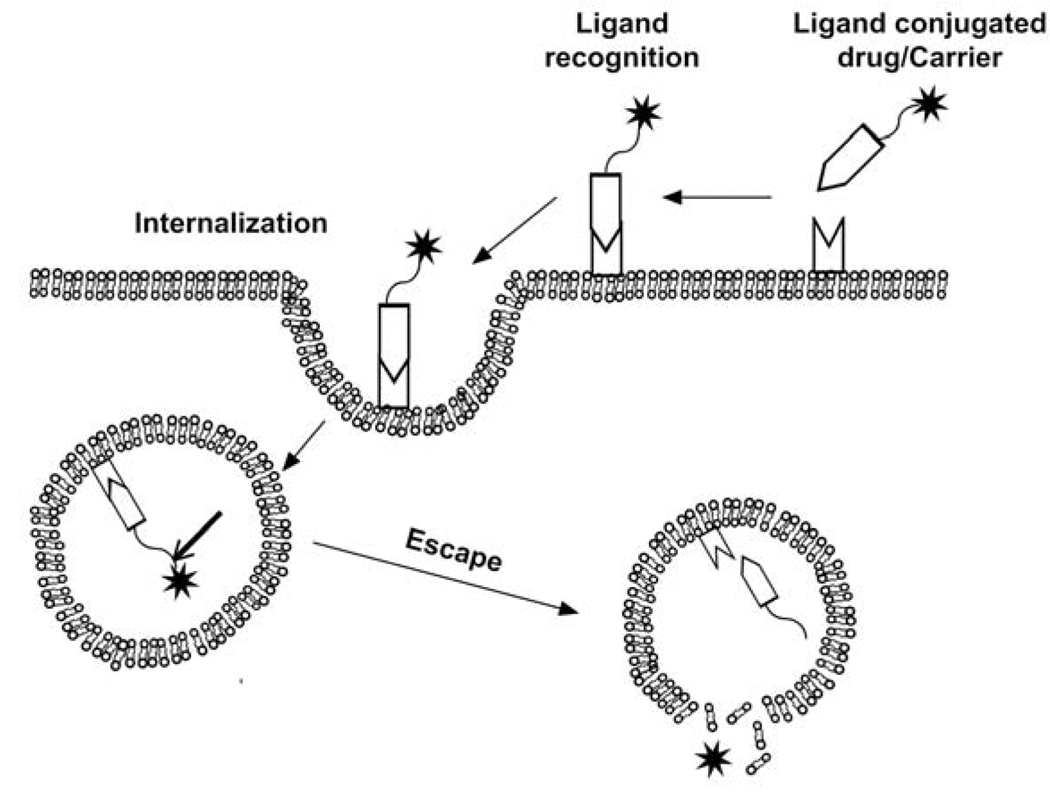
Figure 2.
Peptide-mediated targeting via ligand/cell-surface interactions. These strategies use ligands grafted onto the carrier/drug surface to increase the intracellular uptake of drug in target tissues (Table 2). A ligand is presented by the carrier, which binds to a moiety on the cellular surface, like specific receptors or nonspecific proteoglycans [141]. Surface binding may be followed by internalization through receptor-mediated endocytosis or other uptake mechanisms. Once internalized, the carrier or drug may be further processed via other cellular factors, such as pH or enzymatic activity.
Receptor-mediated peptide strategies have been extensively studied. Peptides have been used either to directly trigger drug release or to direct the carrier to increase specificity and internalization. This can be achieved by: 1) conjugating the ligand to carrier/drug; 2) conjugating an antibody against the receptor to the carrier/drug; and 3) using cell penetrating peptides [53] to promote nonspecific binding and internalization. For covalently attached drugs, it is important to incorporate a cleavable bond between the drug and carrier to facilitate drug-release. One recently successful linkage used in polymeric drug-delivery is the hydrazone bond, which demonstrates release and anti-tumor efficacy in a range of models [54]. One example of this hydrazone strategy is the antibody conjugated formulation Mylotarg™, which is a calicheamicin hydrazide derivative attached to the oxidized carbohydrates of the anti-CD33 antibody for the treatment of acute myeloid leukemia [54]. Ketal and disulfide linkages have also shown appreciable efficacy in intracellular drug delivery [55,56].
Albumin was one of the first proteins to be used as a drug carrier for a variety of drugs, including anticancer agents [57]. Albumin has a wide range of applications due to its versatility in binding to hydrophobic drugs. The first drug conjugate to be evaluated was methotrexate. Though the methotrexate-albumin conjugate was not successful clinically [58] it prompted further research in albumin bound therapy, which culminated in the success of albumin bound paclitaxel, Abraxane™. Although paclitaxel is not chemically conjugated to albumin, the hydrophobic binding affinity of the protein to drug is sufficient to be useful as a drug carrier. In the clinic, Abraxane™ was shown to have a higher anti-tumor activity than paclitaxel [59]. Abraxane™ targets the tumor by a combination of the EPR effect and albumin binding to Gp60 receptor [57] . This enhances the intratumoral concentration of drug and therefore increases the efficacy of the system. Another significant improvement of Abraxane™ over paclitaxel is the reduced incidence of hypersensitivity related to the use of harsh surfactant vehicles, such as Cremophor-EL™, which is unnecessary for the albumin-drug complexes [12].
Extensive work on transferrin-mediated targeting was done by Tanaka and coworkers [40]. Transferrin undergoes receptor-mediated endocytosis in a broad range of cells; furthermore, when Mitomycin C is conjugated to transferrin, the resulting conjugate demonstrates unaltered receptor binding properties. The mechanism of internalization is the same for normal and cancer cells; however, cancer cells achieved a higher concentration of drug in the tumors due to increased surface expression of the transferrin receptor [38]. Similarly, for liposomes decorated with transferrin, there was a higher concentration of drug observed in the tumor cells, which significantly improved efficacy in a rat tumor model [60]. Another avenue to utilize the transferrin trafficking pathways was demonstrated using anti-transferrin receptor antibodies [61,62]. The anti-transferrin receptor strategy boosted the accumulation of drug in the tumor two-fold compared to control liposomes. Fluorescence experiments suggested that these antibodies internalize into cells similar to transferrin via receptor-mediated endocytosis [62].
The folate receptor has also been a useful target in the development of anticancer peptide strategies. Conjugation of drug molecules to folic acid or other ligands specific to the folate receptor can increase the localization and internalization of the conjugate [42]. This effect has been demonstrated with cargo varying in size from small molecule drugs to liposomes. One of the first folate conjugated systems included a protein toxin called momordin [43]. The folate-momordin conjugate was shown to be specific to tumor cells [44]. Another early folate drug carrier system was developed using a folate-phosphatidyl ethanolamine lipid conjugate [47]. The lipid anchored the folate directly to a liposome surface, and these targeted vesicles had higher cellular uptake and higher cytotoxicity than the nontargeted drug, DOX. The folate targeting strategy has been extended to enzymes [48], DNA [46] and other non-lipid delivery systems.
Epidermal growth factor receptor (EGFR/HER2) expression is frequently upregulated in tumor cells [49]. A successful antibody against HER2 has been clinically approved, Herceptin™ [63], which has been used to increase specificity of gelatin/albumin nanocarriers [50]. For this strategy, avidin is directly conjugated to the drug carrier, which is subsequently modified by biotinylated HER2 [50]. The authors reportedly attached 370 antibodies onto the surface of each carrier [50]. The internalization of the carrier depends strongly on the concentration of HER2 receptors on the cell surface; furthermore, these HER2 receptor targeted carriers demonstrated enhanced binding and internalization compared to an untargeted control [50]. An alternative use for the anti-HER2 receptor antibodies has been the modification of DOX carrying liposomes [64]. Interestingly, these antibody-grafted liposomes showed higher internalization inside cells (6-fold) but did not substantially increase the amount in the tumor vasculature [65]. A simpler alternative to antibody targeting is to exploit short peptides with HER2 receptor binding, such as the GE11 peptide (Table 1) [51]. GE11 modified liposomes showed efficient transfection of cells, and increased cytotoxicity when compared to either free DOX or unmodified DOX liposomes [49].
Even short peptide motifs are capable of directing tumor targeting to specific membrane receptors. The RGD (Arg-Gly-Asp) peptide is one of the most widely studied motifs. RGD is recognized by integrins that promote endocytosis of the polymer or particulate presenting the peptide. The RGD motif has been used widely to target tumor cells with therapeutic drugs/proteins/liposomes/imaging agents [66]. For example, RGD conjugates have been used to target the synthetic polymer hydroxypropyl methacrylamide (HPMA) specifically to tumor epithelial cells [67]. An RGD-HPMA conjugate had 3.7 times higher tumor localization than compared to a control peptide conjugate [67]. Alternatively, liposomes designed with the RGD motif can decrease tumor growth; however, off-target interactions in the liver and spleen were observed [68]. Also incorporation of RGD lipopeptides in the liposomes formulation reduced the circulation time drastically [68]. More recently, other short motifs have been identified that promote receptor-mediated uptake including NGR (Asn-Gly-Arg) and GSL (Gly-Ser-Leu). These short motifs are tumor specific because their specific targets are upregulated in the tumor vasculature and not to the same extent in normal tissues [69]. The NGR, RGD, and GSL motifs can home in to cancers in vivo [70]. The NGR motif has two distinct homing mechanisms: binding to aminopeptidase N (CD13) which is a membrane bound matrix metalloproteinase; and binding to vascular integrins [71]. A major advantage of using these short peptide motifs is that they home in to the tumor vasculature, which is less dependent on the variability of receptors expressed directly on the tumor cell surface [69,70].
A related approach where peptides have contributed to drug targeting is in the use of enzymes that direct tissue or cell specific cleavage of active drug. For many drugs, the carrier-drug conjugate is inactive, and release is a prerequisite for activity. From a practical perspective drug cleavage should not occur during circulation in the plasma. One elegant solution is to use the proteolytic capacity confined within lysosomes. To gain access to these compartments, the nanocarriers must be taken into cells and internalized to lysosomes, such as through RME. Once in the lysosome, there are multiple enzymes that can mediate degradation; however, the most frequently discussed is cathepsin B [58,72,73]. Other interesting enzyme targets include matrix metalloproteases (MMP’s). 26 different kinds of MMP’s exist [74], and studies on MMP-9 specific release studies have shown encouraging results [75,76]. MMP-9 and MMP-2 are therapeutically relevant, both being upregulated in glioblastoma multiforme [77,78]. It is also interesting to note that MMP-2 and MMP-9 have common substrates specificities derived from collagen [79]. A consensus sequence has been observed, GPQGaAGQR where a = Leucine, Isoleucine, or Valine [76]. By conjugating this peptide to a lipid anchor, enzyme-dependent liposome rupture has been demonstrated [76]. Hence, by incorporating appropriate peptides into a linkage between carrier and drug, it is possible to develop rapid release in the presence of target enzymes without appreciably contributing to drug loss during circulation in the central blood compartment.
2.3 Temperature-mediated targeting
Thermo-responsive polymers respond to their surrounding temperature by altering their physicochemical properties; furthermore, as more advanced methods for achieving local-regional deposition of heat become available these polymers are becoming attractive targeting options to direct tumor-specific delivery. Peptides make excellent candidates for thermo-responsive polymer, and there have been exciting advances in the development of thermally responsive peptides that confer temperature dependence onto drug carriers [27,80]. This section deals with the application of hyperthermia as well as the use of thermally sensitive peptides as triggers in drug release (Fig 3).
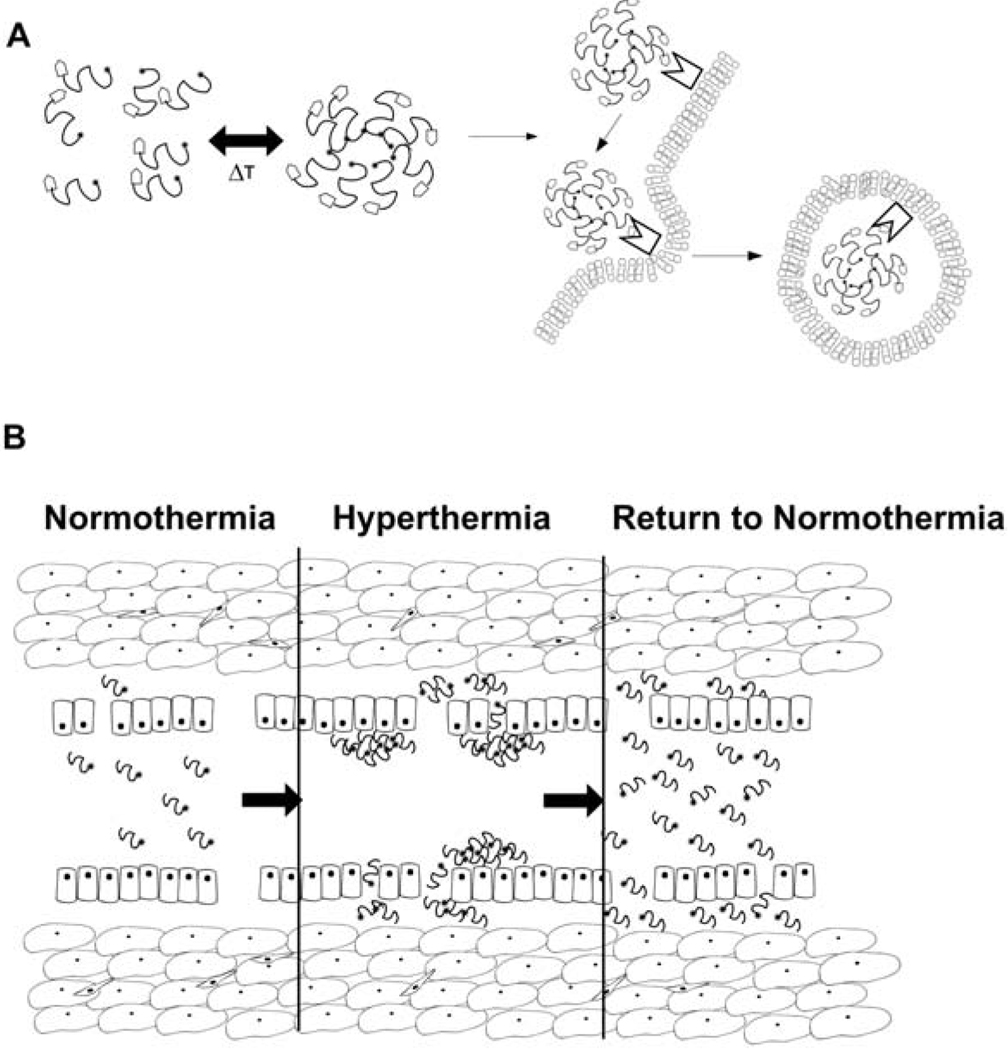
Figure 3.
Strategies for temperature-mediated peptide targeting. The application of local and regional hyperthermia provides an opportunity to change the carrier behavior in the tumor region. (A) Temperature-directed assembly from single peptides into multivalent nanoparticles. The multivalency may improve the avidity for cell surface targets that promote cellular internalization [26]. (B) For peptides that transition from soluble to aggregated states under hyperthermia, the sustained application of heat increases the accumulation of the carrier in the tumor vasculature. Upon return to normal temperatures, the dissolution of peptides produces a high local concentration that drives extravascular accumulation of carrier, a ‘thermal-pump’ [27].
2.3.1 Application of hyperthermia
To synergize with current hyperthermia research, thermo-responsive drug carriers should be able to respond to mild hyperthermia conditions between about 37 and 42 °C [81] . Sustained temperatures above this range induce protein denaturation and cell death without the need for chemotherapy; however, the application of high temperature is often limited by the need to spare critical anatomical structures. In contrast, mild hyperthermia produces effects that complement drug delivery. For example, tumor cells are more sensitive to hyperthermia-induced damage [82,83]. Also, hyperthermia increases vascular perfusion and permeability, which contributes to improved passive targeting and anti-tumor effect [81] . Tumors can be heated either by direct or indirect heating [81] . Under direct heating, energy is distributed via conduction and convection [81] . Due to the efficiency of thermal homeostasis, this approach is only suitable to a limited penetration depth [81] . To achieve deep tissue penetration, indirect heating is used to deposit energy via radiating waves from either ultrasonic or electromagnetic sources. Whole body hyperthermia can be induced by administration of pyrogens; however, pyrogenic hyperthermia is perhaps undesirable compared to the other routes of localized heating [81] . One promising heat application technology permits both localized heating and deep tissue penetration and is based upon high intensity focused ultrasound (HIFU) [84]. HIFU is a noninvasive procedure that can be used to induce mild hyperthermia or tumor ablation (70 °C). While HIFU can heat deep seated tumors, ultrasonic waves have two main drawbacks: 1) they are unable to safely penetrate gas phases, which leads to inefficient heating in the lung and bowel; and 2) they are strongly absorbed in bone, which can damage the skeletal tissue [84]. Despite these caveats, ultrasound heating can be used as an adjuvant to nanocarrier chemotherapy [80]. One alternative method to achieve indirect heating method is to use strong magnetic fields in order to heat iron oxide carriers [85]. Nanocarriers composed from both lipids [16,80] or peptides [27,86] have been observed to increase exposure of the tumor to the cytotoxic agent. For example, lipids that undergo sol-gel phase transitions including DPPC [87] as well as polymers that display Lower Critical Solution Temperature (LCST) are being developed for thermo-responsive delivery [88] and a few interesting examples have been summarized in the next section. Recent reviews [81,89] have summarized the application of hyperthermia in more detail and will make interesting reading.
2.3.2 Temperature mediated release
Hyperthermia-based strategies are under investigation that explore both polymer-drug conjugates or thermo-sensitive particulates that encapsulate drugs (Table 2). Some significant research into temperature-sensitive targeting focuses on peptides that undergo unique biophysical behavior, and in several cases these peptides can be reduced to repetitive amino acid sequences (Table 2). This section focuses exclusively on the properties of some temperature dependent peptides. The most widely studied thermally-responsive peptides are elastin-like polypeptides (ELPs), collagen, leucine zippers, and silk [89]. Each of these peptides display temperature dependent phase transitions, with some being reversible and others irreversible. In most cases, the thermal sensitivity of these peptides can be modulated by varying: 1) the MW of the peptide; 2) the identity of specific amino acid residues; and 3) the concentration [90]. Leucine zippers are heptameric repeats of [abcdefg]n that form α-helical structures, which can self-assemble (Table 2) [91,92]. Leucine zippers are stable at low temperatures, and dissociate under heating at a temperature that depends on their specific peptide sequence. These zippers could potentially be used in delivery strategies that drive assembly of particulate drug carriers that trap the drug in a carrier until release. Also, leucine zippers can be used as switchable hydrogels, which serve as matrices for controlled release under elevated temperatures [93,94].
Table 2.
Thermally-responsive peptides
| Peptide | *Motif trend | Properties | References |
|---|---|---|---|
| Elastin-like polypeptides | [aPGbG]n a = I , V b ≠ P n = 10 to 200 |
Reversible phase separation above adjustable transition temperature, which depends on concentration, length ‘n’, ‘a’ and ‘b’, ionic concentration. Accompanied by a change from a random coil to β-turn spiral conformation. | [89,90,95,98,152] |
| Leucine zippers | [abcdefg]n a-g ≠ P. a = hydrophobic d = L e,g = charged n = 5,6 |
Forms coiled-coil α-helical structures in solution that disassociate upon heating to form random coil structures. | [89,91,93] |
| Silk-like peptides | [GAGAGS]n n = 2 to 168 |
Irreversibly forms aggregates on exposure to high temperature. Accompanied by the formation of β-sheets. | [89,100,101] |
| Collagen-like peptides | [Gab]n a = often P b = often POH n = 100 to 500 |
Irreversibly dissociate on exposure to high temperature. Form triple helices in solution but dissociate to form fibrils on heating. | [89,92,153] |
| FEK16 peptide | [FEFEFKFK]2 | An indirect temperature sensitive system. Ca2+ dependent assembly of β-sheet structures when stimulated using temperature responsive liposomes. | [102,103] |
A=Alanine, E=Glutamic acid, F= Phenylalanine, G=Glycine, I=Isoleucine, K= Lysine, L=Leucine, P=Proline, POH=Hydroxyproline Q= Glutamine, S=Serine, V=valine.
Another class of thermally responsive peptides, the ELPs, are biologically inspired from human tropoelastin [95]. ELPs are pentameric repeats of [aPGbG]n that exhibit a first order phase transition from a water soluble phase to a two phase system. In vivo ELPs are sensitive to hyperthermia; furthermore, the ELP phase behavior is reversible. The phase transition temperature can be manipulated by modifying the guest residue in the a and b positions (Table 2). The trend is that substitution at the b guest residue with increasingly hydrophobic amino acids results in a lowering of the phase transition temperatures [89,95]. Functionally, ELPs have a wide range of applications: 1) they form temperature sensitive drug conjugates that promote tumor accumulation upon cycling the tumor temperature around the phase transition [27]; 2) ELP block copolymers can assemble nanoparticles that may encapsulate drugs (Fig. 3A) [96]; and 3) ELP tags can be used to purify fusion proteins [89]. Dreher and coworkers demonstrated that ELPs have a 2 fold increase in tumor concentration under local hyperthermia [27]. ELP-DOX conjugates also have demonstrated cellular uptake into low pH compartments, which is enhanced by the thermally triggered phase transition (Fig. 3B) [97]. The cytotoxicity of the ELP-DOX conjugate was also found to be nearly equivalent to free DOX. It was proposed by both Dreher [27] and Bidwell [98,99] that the mechanism of cytotoxicity differs from that of free DOX because the conjugate does not localize into the nucleus as rapidly as free DOX.
Another strategy has been developed that combines a ligand-mediated approach with a thermally responsive ELP [99]. This system employs a cell penetrating peptide derived from the HIV transacting transcriptional activator (TAT) to promote cellular uptake. TAT is an 86 amino acid peptide that enters cells when introduced to the surrounding media; furthermore, a short peptide from TAT is necessary and sufficient to impart this behavior to other proteins via a non-specific uptake mechanism (Table 1) [53]. An ELP linker is included between the DOX and TAT that confers thermal sensitivity to the conjugate. The optimized conjugate had a transition temperature of 40 °C. Incorporation of TAT increased the binding by 3-fold at 37 °C and by 6-fold at 42 °C, as compared to the same conjugate without TAT. Cytotoxicity followed a trend similar to that observed for uptake, suggesting that hyperthermia-responsive peptides may synergize with other peptide-ligand approaches.
Other examples of temperature sensitive peptides have been derived from silk and collagen repetitive motifs. Silk-like polypeptides (SLPs) are repeats of [GAGAGS]n [92], though [GA]n, and [AA]n are capable of similar behavior. SLPs form irreversible, aggregates on exposure to elevated temperatures, which can be used for designing depot systems for drug release (Table 2). The properties of these SLPs can be modulated by synthesizing block copolymers with other motifs, such as ELPs [89]. When mixed with gelatin SLP’s form a thermo-sensitive gel, which can be stabilized at 37 °C [100].
Collagen is a peptide polymer made from repetitive sequences that follow a trend of [GPPOH]n [89,92]. The second and third member of the collagen triplet can be nearly any amino acid; however, there is a strong bias for proline in the second position and hydroxyproline in the third position (Table 2). Collagen motifs form a unique triple helical structure that dissociates in response to heating. This process is irreversible, in that cooling of a melted collagen peptide produces a gelatinous hydrogel via random crosslinking between neighboring polypeptides [92]. Collagen-like peptides (CLPs) can be biosynthesized in bacteria; however, CLP stability depends on the degree of post-translational conversion to hydroxyproline in the peptide. Bacteria lack the required proline hydroxylase necessary to generate native collagens, but this deficiency can be remedied using genetic engineering [92]. Both collagen and denatured collagen are widely used in the pharmaceutical and food industries as gelling agents [92]. Having introduced, ELPs, CLPs, and SLPs, it is worth noting that a wide array of block copolymers have been evaluated that bring elements of two or more of these peptide families together. The most studied combination are the SLP-ELP hybrids, which have shown potential for use in local thermal delivery [101].
The development of hybrid polymers between peptides and synthetic polymers is also an active area for exploration. For example, dual-temperature sensitive peptide polymers have been synthesized by Stoica [102] that utilize N-isopropylacrylamide-co-acrylamide (NIPAAM) conjugated to an octapeptide ‘FEFEFKFK’. The ‘FEK16’ motif forms a hydrogel at room temperature (Table 2). This conjugate has a NIPAAM-mediated LCST at 30°C and a FEK16 induced gel melting temperature at 75 °C [103]. Due to their glutamic acid residues, both of the above polymers are pH sensitive, and their properties can be further modified to release the encapsulated contents in a pH dependent manner.
2.4 Redox-mediated targeting
Similar to pH mediated targeting, redox-mediated targeting can potentially be triggered extracellularly or intracellularly. The extracellular oxidative potential is maintained by redox-modulating proteins including NADPH oxidase, superoxide dismutase (SOD), and thioredoxin (TRX-SH2)/oxidized thioredoxin (TRX-SS), and free glutathione (GSH)/glutathione disulfide (GSSG) [104,105]. These enzymes are frequently used as markers to study redox stress in cancer. For example, TRX levels in blood are markers for oxidative stress in hepatocellular carcinoma [106]. In contrast, the ratio of reduced glutathione to oxidized glutathione disulfide (GSH:GSSG) is used to measure the intracellular redox state, where reduced glutathione is abundantly available in the cell [20]. Under normal homeostasis, reactive oxygen species are kept in check by glutathione (GSH) and superoxide dismutase (SOD) [18]. The intracellular levels of GSH in normal tissues ranges from 1–10 mM [107] compared to that of blood plasma which is 2µM [108]. This makes GSH a reasonably good target for intracellular delivery. In vitro the GSH levels in tumor cells were shown to have a 7–10 fold increase in GSH concentrations [18,109]. This combination of intracellular elevated GSH and the tumor-associated GSH make redox triggers interesting candidates for control over peptide structure and drug release [109,110]. Conversely SOD is frequently found at reduced concentrations in tumors compared to normal tissues [18]. Another issue observed with high levels of GSH is that it promotes cancer growth and resistance to chemotherapy [36]. Based on the elevated GSH levels intracellular release of small molecules is possible by using peptides stabilized by disulfide bonds [55]. The disulfide bond is broken down into two sulfhydryl moieties and the GSH is oxidized to GSSG, which can be achieved either by cell surface protein disulfide isomerase (PDI) or reductive cleavage in the cytoplasm (Fig. 4) [55]. Using non-peptide systems, multiple groups have demonstrated that the glutathione couple has potential anticancer application [111–116]. Also disulfide linkers can moderately increase carrier stability [117]. Work on redox mediated polymers dates back to the 1970’s using early polyplexes between DNA and poly(l-lysine) [118]. Polymers ranging from chitosan to poly (ethylene imine) (PEI) have been studied for redox-mediated targeting through disulfide linkages [118]; furthermore, redox targeting may be complementary with cystine peptide based targeting strategies. Despite the potential for stabilizing/destabilizing peptide secondary structures via redox targeting, relatively few studies have been reported that utilize redox sensitive peptides with chemotherapeutic drug delivery, and this may be an area of future opportunity.
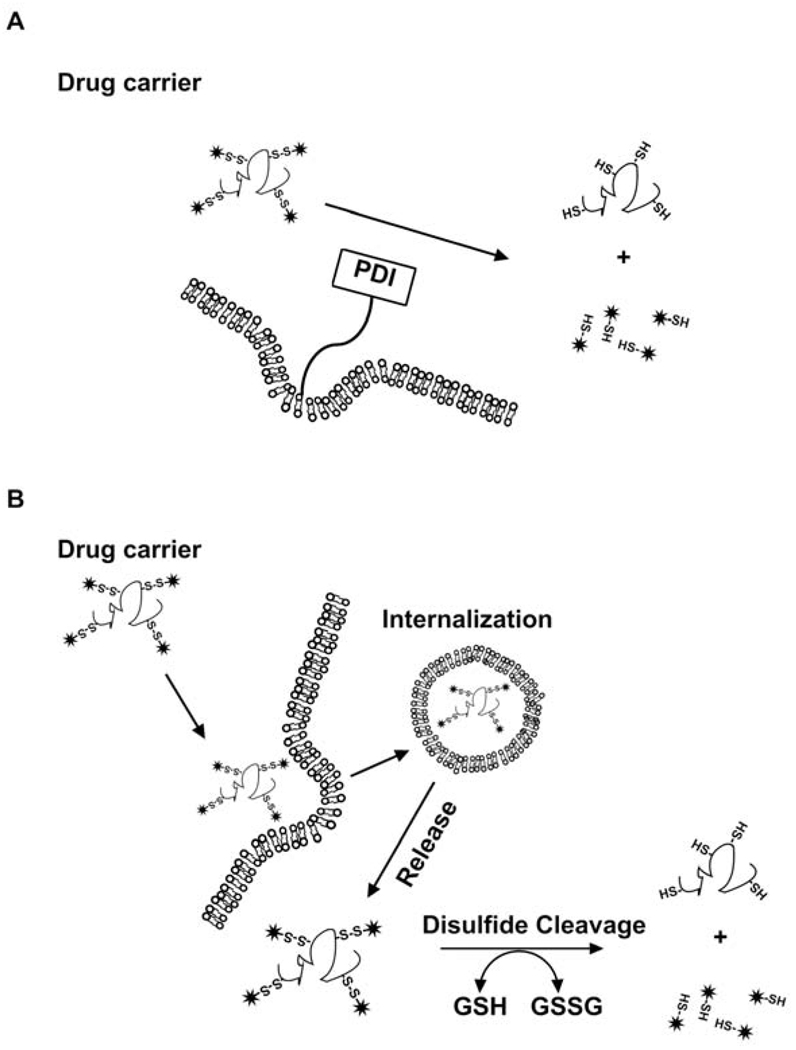
Figure 4.
Strategy for redox-mediated peptide targeting. These approaches target the intratumoral ratio of GSH to GSSG. The disulphide bond confers redox sensitivity peptide carriers. (A) The extracellular concentration of GSH is very low but cleavage of disulfide bonds can be facilitated by the presence of membrane protein disulfide isomerase (PDI) which can lead to extracellular release of drug [142]. (B) The disulfide bond is cleaved by intracellular reductive activity after cellular uptake. A limitation of this approach is the stability of the disulfide linkage during circulation in the bloodstream [143].
2.5 pH-mediated targeting
pH mediated triggering may be achieved by sensitizing nanocarriers to the extracellular pH or the endosomal/lysosomal pH. The environment around the tumor is at a lower pH than compared to normal tissue [17]. Production of lactic acid and hydrolysis of ATP are the major contributors to the acidic environment around the tumor [119]. In certain extracellular regions, tumors have a lower pH (~ 6.5) than the blood (~ 7.4) [17]. Membrane ion transporters are responsible for maintaining intracellular pH [119]; therefore, the cytoplasmic pH of tumor cells is similar to that for normal cells. Also, similar to normal cells, the endosomal/lysosomal compartments within tumor cells also have a low pH that can be exploited to design pH sensitive delivery systems to release drugs (Fig. 5). Within the tumor, acidic drugs with pKa below the environmental pH or basic drugs with pKa above the environmental pH are ionized and do not penetrate lipid bilayers as efficiently as the uncharged species. To promote penetration across lipid bilayers, weakly acidic drugs with pKa’s in the range of 4.5–6.5 are optimal [120]. Similarly, acidic and basic moieties can be used to promote pH dependent accumulation or drug release from peptide conjugates and lipid nanocarriers (Fig. 5A–B). In order to achieve pH-mediated intracellular drug delivery, the carrier must first be taken up into cells, as described previously (Fig. 2) [121]. Cellular binding and uptake can be promoted using specific or nonspecific ligands, including peptides (Table 1). Following internalization, trafficking to low pH compartments can then be used to trigger drug release. The early endosome has a pH of 6 [122]; furthermore, the pH continues to decrease during trafficking as lysosomes fuse with the vesicles to activate proteolytic degradation [121].
Figure 5.
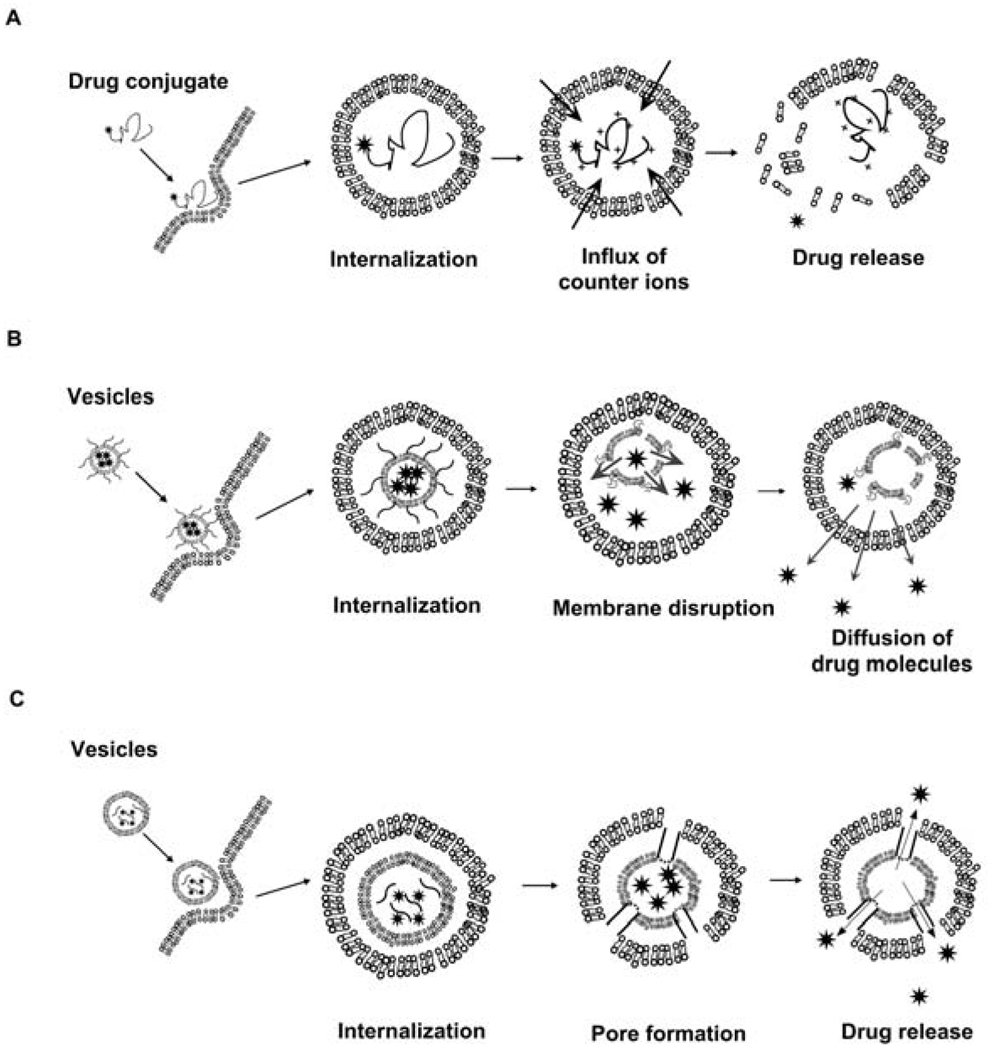
Strategies for pH-mediated peptide targeting. (A) Weakly basic peptides that protonate at endo-lysosomal pH may promote the lysosmotrophic release of contents into the cytoplasm [144]. (B) Lipid vesicles with surface-associated peptides may be ruptured via pH dependent change in peptide fold. Membrane disruption may release drug into endosomes, which can then traffic into the cytoplasm. (C) Lipid vesicles with encapsulated peptides may undergo a conformational change at endo-lysosomal pH, forming pores. These peptides may rupture the membranes of both the carrier and endosomes, promoting cytoplasmic drug release [145,146].
Although widely explored, extravascular acidity has been difficult to target in humans, partly due to the buffering capacity of the blood and the inhomogeneous nature of the extravascular pH gradient. The extravascular pH in tumors can range between 6.5 and 7.0; however, drugs and drug carriers must diffuse on the order of 50 µm away from capillaries to reach regions with this pH [123]. For a small molecule or drug, this penetration depth is easily achieved; however, for macromolecules and nanoparticulate drug carriers these distances may be unrealistic [124]. Alternatively, in regions where the extracellular pH remains normal (~7.4), pH dependent mechanisms that respond to cellular uptake have been successful. Cells within tumors continuously sample molecules and particles from their environment, which are often trafficked to low pH compartments for degradation [97], and the incorporation of pH-mediated strategies that detect cellular internalization reduces the need to target extracellular drug release. While this review focuses on peptides, many of the peptide-mediated delivery approaches are developed in combination with lipids or synthetic polymers; therefore, we will briefly summarize the pH responsive behavior of non-peptide systems.
Liposomes are one class of nanocarrier that have been extensively modified with peptides and proteins to improve the delivery of their contents. Liposomes share with many other nanocarriers an internalization pathway that results in endosomal/lysosomal entrapment and degradation [8]. To promote release of components into the cytoplasm, pH-sensitive liposomes have been optimized that change phases between pH 7.4 and 5.0 [125]. One way to achieve this is by preparing liposomes from phosphatidyl ethanolamine (PE) lipid. In the absence of a stabilizing amphiphile, PE lipids do not form a stable lamellar phase liposome at pH 7.4, but instead adopt a hexagonal aggregate structure [126,127]. Upon incorporation of amphiphiles, including peptides, polymers, or charged lipids, into the bilayer PE liposomes can be stabilized by either steric or electrostatic repulsion. Following cellular internalization and lysosomal trafficking, the reduction in pH induces loss of stabilization that drives a transition from lamellar to hexagonal phase and drug release [128]. The pH sensitivity of this behavior is a function of the amphiphile identity, MW, and concentration in the bilayer. Alternatively, Sudimack and coworkers described approaches to prepare pH sensitive liposomes that do not rely upon hexagonal phase lipids [129]. For example, polymer-lipid conjugates can fuse with membranes, such as the mixture of phosphatidylcholine (PC) with succinylated poly(glycidol) [130]. These formulations made from mixtures of cationic/anionic lipid combinations were found to be efficient vehicles for intracellular delivery. An alternative strategy to generate anionic pH-responsive liposomes is to prepare vesicles from a mixture of diolein/cholesterol hemisuccinate (6:4) [131]. These formulations are generally stable at physiological pH, but aggregate and release the encapsulated contents when the pH decreases to around 5.0. Similarly, peptides that provide steric or electrostatic stability to lipid vesicles may be useful for promoting drug release (Fig. 5B).
pH sensitive triggering is possible with many of the peptides that are temperature sensitive, such as ELPs, leucine zippers, and CLPs (Table 2). Similar to how temperature can drive these polypeptides to undergo conformational changes, the protonation of acidic and basic peptide residues can also shift the peptides from one conformation to another; therefore, many of these peptides can also be redesigned with pH sensitivity. For example, leucine zippers are stable coiled coil structures at low temperatures (Table 3) [89]. Substitution of an acidic group at the e and g position influences the stability of the secondary structure, and protonation of these residues is a dynamic way to control the stability of the complex. This property has been exploited by Stevens [132] and Ryadnov [133], and a similar principle also applies to ELPs [95].
Table 3.
pH-responsive peptides.
| Peptides | *Motif trend | Properties | References |
|---|---|---|---|
| Leucine zippers | [abcdefg]n a-g ≠ P. a = hydrophobic d = L e,g = charged n = 5,6 |
Acidic groups at ‘e’ and ‘g’ induce the formation of a rigid coiled coil structure at pH 4.5; but on increasing the pH (7–11) the structure disassembles. | [89,91,132,154] |
| Carboxymethyl poly(l-histidine) | [HOOCH2C-His]n | Cationic peptide polymer contains an imidazole ring and carboxymethyl group, which helps in conferring dual functionality to the polypeptide. | [137,138] |
| GALA peptide | WEAALAEALAE ALAEHLAEALAE ALEALAA |
Changes conformation from a random coil to an amphipathic α-helix when pH is lowered from 7 to 5. | [91,134,135] |
| KALA peptide | WEAKLAKALAK ALAKHLAKALAK ALKACEA |
Changes conformation from a random coil to an amphipathic α-helix when pH is increased from 5 to 7.5. | [136] |
A=Alanine, E=Glutamic acid, H=Histidine, K= Lysine, L=Leucine, P=Proline, W=Tryptophan.
The GALA peptide is another interesting pH-mediated system (Table 3). GALA is composed of tetrameric repeats of [EALA] [134], which under physiological pH adopts a random coil structure. When exposed to lower pHs (pH=5.7), the glutamic acid residues begin to neutralize, which enable the formation of α-helical structures. By incorporating GALA into a liposome, assembly of these helical structures results in pH dependent membrane disruption (Fig. 5C) [134]. Many variations on the GALA peptides have been developed, such as YALA and GALAdel3E [135]. Most of these have appreciable activity of a similar magnitude; however, they can be optimized to transition at different pHs [135]. One variation of GALA is the cationic peptide, KALA, which has lysine in place of glutamic acid [136]. The positive charge on the peptide interacts with oligonucleotides and also promotes membrane disruption and gene delivery [134].
Polyhistidine blocks are a simple example of pH responsive peptides (Table 3). The poly(l-histidine) polymers synthesized by Asayama and coworkers [137] are sensitive to the endosomal/lysosomal pH. The polymer is poorly soluble at physiological pH, but upon protonation in the endosomes the peptide becomes water-soluble (Fig. 5A). Modifying the poly(l-histidine) by carboxymethyl substitution on the polymer leads to the formation of anionic charge [138]. Coating of this polymer to the PEI/DNA complex leads to the formation of a ternary complex that enhances gene expression by 300 fold; furthermore, this peptide can deliver DNA to the nucleus, and can be used to inhibit cell division [138].
3. Discussion
The triggers mentioned above are being explored as parts of tumor-targeted delivery strategies. To complicate matters, most of these peptide-mediated delivery strategies are difficult to classify under a single strategy. Instead, these drug-carrier formulations are multifunctional, utilizing two or more targeting strategies. Each combination of strategies has its advantages and disadvantages. Some of the major limitations of the above mentioned strategies include: 1) decreased sensitivity of receptor to the ligand; 2) elimination of attached ligand during circulation in vivo; 3) off-target interactions; 4) immunological response to foreign antigens; and 5) limitation of drug-conjugate linker strategies. Using ligands to direct accumulation can lead to off target effects since other tissues may express the receptors that bind to the specific motif/ligand. When combined with cytotoxic chemotherapeutics, this could produce toxic effects and even decrease the therapeutic effect of the carrier. In addition, to overcome immunogenicity, it will become important to select peptide sequences carefully, to minimize the number of antigenic epitopes, and perhaps to use steric shielding to reduce the immuno-recognition where appropriate [139]. PEG shielding of protein therapeutics was shown to markedly decrease the immunogenicity of the formulation and increase circulation times [140]. One of the drawbacks of PEG modification is a loss of protein/peptide activity; therefore, the potential improvements provided by polymeric modification need to be balanced with decreases in activity [140]. One exciting possibility is that certain peptide sequences may have some of the properties of PEG; furthermore, peptides of this nature could be integrated seamlessly into genetically engineered constructs.
The tumor microenvironment provides multiple cues that may be exploited to improve the efficacy of established chemotherapeutics; furthermore, polypeptides are uniquely situated to capitalize on these signals. Peptides provide: 1) a rich repertoire of biologically specific interactions to draw upon; 2) multiple environmentally-responsive phase behaviors that can respond to disease signatures; 3) multiple opportunities to direct self-assembly; 4) extensive control over routes of biodegradation; 5) the ability to seamlessly combine functionalities into a single polymer or particle using biosynthesis. These opportunities are significant, and it is reasonable to expect that peptides will play a major role in the development of the next generation of environmentally responsive drug carriers.
4. Conclusions
Peptides provide a level of molecular specificity that is naturally suited to the development of environmentally responsive drug carriers. Most importantly, peptide secondary and tertiary structures enable a degree of control and functionality that surpasses what is easily achievable using lipids and nonbiological polymers. Here we have discussed some of the aspects of peptide-mediated drug delivery and present applications where peptides can be directed by temperature, pH, and specific biological interactions. As the development of cancer-targeted nanocarriers continues to expand, peptides are providing these formulations with critical functionalities necessary to target disease.
Acknowledgments
The authors would like to thank M Pastuszka, S Parakh, and V Valluripalli for their valuable suggestions in improving the manuscript. SM Janib is supported by a fellowship from the Malaysian Government. JA MacKay, SM Janib, and S Aluri are also supported by seed funding from the School of Pharmacy, by grant IRG-58-007-48 from the American Cancer Society, and by grant 5 P30 DK048522-15 from the NIH/NIDDK.
Abbreviations
- EPR
Enhanced Permeability & Retention
- HIFU
High intensity focused ultrasound
- LCST
Lower Critical Solution Temperature
- GSH/GSSG
Glutathione/Glutathione Disulfide
- PEG
Poly ethylene Glycol
- ELP’s
Elastin like polypeptides
- DOX
Doxorubicin
- HPMA
Hydroxypropyl Methacrylate
- NIPAAM
N-isopropylacrylamide-co-acrylamide
- PEI
Poly (Ethylene imine)
- RME
Receptor-mediated endocytosis
- TAT
Transacting transcriptional activator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures. 2008 [Google Scholar]
- 2.Chabner B, Longo DL. Cancer chemotherapy and biotherapy : principles and practice. Lippincott Williams & Wilkins. 2001 [Google Scholar]
- 3.Olson RD, Boerth RC, Gerber JG, Nies AS. Mechanism of adriamycin cardiotoxicity: evidence for oxidative stress. Life Sci. 1981;29:1393–1401. doi: 10.1016/0024-3205(81)90001-1. [DOI] [PubMed] [Google Scholar]
- 4.Di Marco A, Gaetani M, Scarpinato B. Adriamycin (NSC-123,127): a new antibiotic with antitumor activity. Cancer Chemother Rep. 1969;53:33–37. [PubMed] [Google Scholar]
- 5.Seidman AD, Hudis CA, Albanell J, Tong W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol. 1998;16:3353–3361. doi: 10.1200/JCO.1998.16.10.3353. [DOI] [PubMed] [Google Scholar]
- 6.Vokes EE, Golomb HM. Oncologic therapies. Springer. 2003 [Google Scholar]
- 7.Schilsky RL, Milano GRA, Ratain MJ. Principles of antineoplastic drug development and pharmacology. Dekker. 1996 [Google Scholar]
- 8.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing Liposomes for Delivery of Chemotherapeutic Agents to Solid Tumors. Pharmacol Rev. 1999;51:691–744. [PubMed] [Google Scholar]
- 9.Allen TM, Cullis PR. Drug Delivery Systems: Entering the Mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 10.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 11.Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59:491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Henderson IC, Bhatia V. Nab-paclitaxel for breast cancer: a new formulation with an improved safety profile and greater efficacy. Expert Rev Anticancer Ther. 2007;7:919–943. doi: 10.1586/14737140.7.7.919. [DOI] [PubMed] [Google Scholar]
- 13.Ranson MR, Cheeseman S, White S, Margison J. Caelyx (stealth liposomal doxorubicin) in the treatment of advanced breast cancer. Crit Rev Oncol Hematol. 2001;37:115–120. doi: 10.1016/s1040-8428(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 15.Gabizon A, Goren D, Cohen R, Barenholz Y. Development of liposomal anthracyclines: from basics to clinical applications. J Control Release. 1998;53:275–279. doi: 10.1016/s0168-3659(97)00261-7. [DOI] [PubMed] [Google Scholar]
- 16.Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res. 2000;60:6950–6957. [PubMed] [Google Scholar]
- 17.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 18.Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol. 2004;14:259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Mbeunkui F, Johann DJ., Jr Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol. 2009;63:571–582. doi: 10.1007/s00280-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 22.Laye JP, Gill JH. Phospholipase A2 expression in tumours: a target for therapeutic intervention? Drug Discov Today. 2003;8:710–716. doi: 10.1016/s1359-6446(03)02754-5. [DOI] [PubMed] [Google Scholar]
- 23.Cummings BS. Phospholipase A2 as targets for anti-cancer drugs. Biochem Pharmacol. 2007;74:949–959. doi: 10.1016/j.bcp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y, Tang H, Radosz M, Van Kirk E, Murdoch WJ. pH-responsive nanoparticles for cancer drug delivery. Methods Mol Biol. 2008;437:183–216. doi: 10.1007/978-1-59745-210-6_10. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Shiramoto S, Miyashita M, Fujishima Y, Kaneo Y. Tumor targeting based on the effect of enhanced permeability and retention (EPR) and the mechanism of receptor-mediated endocytosis (RME) Int J Pharm. 2004;277:39–61. doi: 10.1016/j.ijpharm.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 26.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc. 2008;130:687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67:4418–4424. doi: 10.1158/0008-5472.CAN-06-4444. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 29.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 30.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 31.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 32.Ahmed M, Lukyanov AN, Torchilin V, Tournier H, Schneider AN, Goldberg SN. Combined Radiofrequency Ablation and Adjuvant Liposomal Chemotherapy: Effect of Chemotherapeutic Agent, Nanoparticle Size, and Circulation Time. J Vasc Interv Radiol. 2005;16:1365–1371. doi: 10.1097/01.RVI.0000175324.63304.25. [DOI] [PubMed] [Google Scholar]
- 33.Kong G, Braun RD, Dewhirst MW. Hyperthermia Enables Tumor-specific Nanoparticle Delivery: Effect of Particle Size. Cancer Res. 2000;60:4440–4445. [PubMed] [Google Scholar]
- 34.Schroeder T, Yuan H, Viglianti BL, Peltz C, Asopa S, Vujaskovic Z, Dewhirst MW. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. Cancer Res. 2005;65:5163–5171. doi: 10.1158/0008-5472.CAN-04-3900. [DOI] [PubMed] [Google Scholar]
- 35.Saeki T, Tsuruo T, Sato W, Nishikawsa K. Drug resistance in chemotherapy for breast cancer. Cancer Chemother Pharmacol. 2005;56 Suppl 1:84–89. doi: 10.1007/s00280-005-0106-4. [DOI] [PubMed] [Google Scholar]
- 36.Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochemistry and Function. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 37.Stavrovskaya AA. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 2000;65:95–106. [PubMed] [Google Scholar]
- 38.Niitsu Y, Kohgo Y, Nishisato T, Kondo H, Kato J, Urushizaki Y, Urushizaki I. Transferrin Receptors in Human Cancerous Tissues. Tohoku Journal of Experimental Medicine. 1987;153:239–243. doi: 10.1620/tjem.153.239. [DOI] [PubMed] [Google Scholar]
- 39.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka T, Fujishima Y, Kaneo Y. Receptor mediated endocytosis and cytotoxicity of transferrin-mitomycin c conjugate in the HepG2 cell and primary cultured rat hepatocyte. Biological & Pharmaceutical Bulletin. 2001 24;:268–273. doi: 10.1248/bpb.24.268. [DOI] [PubMed] [Google Scholar]
- 41.Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J Pharm Sci. 2005;94:2135–2146. doi: 10.1002/jps.20457. [DOI] [PubMed] [Google Scholar]
- 42.Lu YJ, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54:675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 43.Leamon CP, Low PS. Cytotoxicity of Momordin-Folate Conjugates in Cultured Human-Cells. Journal of Biological Chemistry. 1992;267:24966–24971. [PubMed] [Google Scholar]
- 44.Leamon CP, Low PS. Selective Targeting of Malignant-Cells with Cytotoxin-Folate Conjugates. Journal of Drug Targeting. 1994;2:101–112. doi: 10.3109/10611869409015898. [DOI] [PubMed] [Google Scholar]
- 45.Leamon CP, Reddy JA. Folate-targeted chemotherapy. Adv Drug Deliv Rev. 2004;56:1127–1141. doi: 10.1016/j.addr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271:8481–8487. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 47.Lee RJ, Low PS. Folate-Mediated Tumor-Cell Targeting of Liposome-Entrapped Doxorubicin in-Vitro. Biochimica Et Biophysica Acta. 1995;1233:134–144. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- 48.Cho KC, Jeong JH, Chung HJ, Joe CO, Kim SW, Park TG. Folate receptor-mediated intracellular delivery of recombinant caspase-3 for inducing apoptosis. J Control Release. 2005;108:121–131. doi: 10.1016/j.jconrel.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Song S, Liu D, Peng J, Sun Y, Li Z, Gu JR, Xu Y. Peptide ligand-mediated liposome distribution and targeting to EGFR expressing tumor in vivo. Int J Pharm. 2008;363:155–161. doi: 10.1016/j.ijpharm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 50.Wartlick H, Michaelis K, Balthasar S, Strebhardt K, Kreuter J, Langer K. Highly specific HER2-mediated cellular uptake of antibody-modified nanoparticles in tumour cells. J Drug Target. 2004;12:461–471. doi: 10.1080/10611860400010697. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Zhao R, Wu X, Sun Y, Yao M, Li J, Xu Y, Gu J. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. Faseb J. 2005;19:1978–1985. doi: 10.1096/fj.05-4058com. [DOI] [PubMed] [Google Scholar]
- 52.Mathias CJ, Wang S, Lee RJ, Waters DJ, Low PS, Green MA. Tumor-selective radiopharmaceutical targeting via receptor-mediated endocytosis of gallium-67-deferoxamine-folate. J Nucl Med. 1996;37:1003–1008. [PubMed] [Google Scholar]
- 53.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, Hallett W, Tsou HR, Upeslacis J, Shochat D, Mountain A, Flowers DA, Bernstein I. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13:47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 55.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 56.Gillies ER, Goodwin AP, Frechet JMJ. Acetals as pH-Sensitive Linkages for Drug Delivery. Bioconjug Chem. 2004;15:1254–1263. doi: 10.1021/bc049853x. [DOI] [PubMed] [Google Scholar]
- 57.Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2005;108:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Kratz F, Abu Ajaj K, Warnecke A. Anticancer carrier-linked prodrugs in clinical trials. Expert Opin Investig Drugs. 2007;16:1037–1058. doi: 10.1517/13543784.16.7.1037. [DOI] [PubMed] [Google Scholar]
- 59.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R, Labao E, Hawkins M, Soon-Shiong P. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 60.Hisae Iinuma KM, Okinaga Kota, Sasaki Katsunori, Sekine Toshiyuki, Ishida Osamu, Ogiwara Naoko, Johkura Kohei, Yonemura Yutaka. Intracellular targeting therapy of cisplatin-encapsulated transferrin-polyethylene glycol liposome on peritoneal dissemination of gastric cancer. Int J Cancer. 2002;99:130–137. doi: 10.1002/ijc.10242. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki S, Inoue K, Hongoh A, Hashimoto Y, Yamazoe Y. Modulation of doxorubicin resistance in a doxorubicin-resistant human leukaemia cell by an immunoliposome targeting transferrin receptor. Br J Cancer. 1997;76:83–89. doi: 10.1038/bjc.1997.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huwyler J, Yang J, Pardridge WM. Receptor Mediated Delivery of Daunomycin Using Immunoliposomes: Pharmacokinetics and Tissue Distribution in the Rat. J Pharmacol Exp Ther. 1997;282:1541–1546. [PubMed] [Google Scholar]
- 63.Chang JC. HER2 Inhibition: From Discovery to Clinical Practice. Clin Cancer Res. 2007;13:1–3. doi: 10.1158/1078-0432.CCR-06-2405. [DOI] [PubMed] [Google Scholar]
- 64.Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, Marks JD, Papahadjopoulos D, Benz CC. Tumor targeting using anti-her2 immunoliposomes. J Control Release. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 65.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 66.Temming K, Schiffelers RM, Molema G, Kok RJ. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist Updat. 2005;8:381–402. doi: 10.1016/j.drup.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Mitra A, Mulholland J, Nan A, McNeill E, Ghandehari H, Line BR. Targeting tumor angiogenic vasculature using polymer-RGD conjugates. J Control Release. 2005;102:191–201. doi: 10.1016/j.jconrel.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 68.Holig P, Bach M, Volkel T, Nahde T, Hoffmann S, Muller R, Kontermann RE. Novel RGD lipopeptides for the targeting of liposomes to integrin-expressing endothelial and melanoma cells. Protein Eng Des Sel. 2004;17:433–441. doi: 10.1093/protein/gzh055. [DOI] [PubMed] [Google Scholar]
- 69.Ruoslahti E. Targeting tumor vasculature with homing peptides from phage display. Semin Cancer Biol. 2000;10:435–442. doi: 10.1006/scbi.2000.0334. [DOI] [PubMed] [Google Scholar]
- 70.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 71.Corti A, Curnis F, Arap W, Pasqualini R. The neovasculature homing motif NGR: more than meets the eye. Blood. 2008;112:2628–2635. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmid B, Chung DE, Warnecke A, Fichtner I, Kratz F. Albumin-binding prodrugs of camptothecin and doxorubicin with an Ala-Leu-Ala-Leu-linker that are cleaved by cathepsin B: synthesis and antitumor efficacy. Bioconjug Chem. 2007;18:702–716. doi: 10.1021/bc0602735. [DOI] [PubMed] [Google Scholar]
- 73.Rejmanova P, Kopecek J, Duncan R, Lloyd JB. Stability in rat plasma and serum of lysosomally degradable oligopeptide sequences in N-(2-hydroxypropyl) methacrylamide copolymers. Biomaterials. 1985;6:45–48. doi: 10.1016/0142-9612(85)90037-7. [DOI] [PubMed] [Google Scholar]
- 74.Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Tauro JR, Gemeinhart RA. Matrix metalloprotease triggered delivery of cancer chemotherapeutics from hydrogel matrixes. Bioconjug Chem. 2005;16:1133–1139. doi: 10.1021/bc0501303. [DOI] [PubMed] [Google Scholar]
- 76.Sarkar N, Banerjee J, Hanson AJ, Elegbede AI, Rosendahl T, Krueger AB, Banerjee AL, Tobwala S, Wang R, Lu X, Mallik S, Srivastava DK. Matrix metalloproteinase-assisted triggered release of liposomal contents. Bioconjug Chem. 2008;19:57–64. doi: 10.1021/bc070081p. [DOI] [PubMed] [Google Scholar]
- 77.Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG, Nicolson GL, Rao JS. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14:35–42. doi: 10.1007/BF00157684. [DOI] [PubMed] [Google Scholar]
- 78.Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79:1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagase H, Fields GB. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Peptide Science. 1996;40:399–416. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C399::AID-BIP5%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 80.Han HD, Choi MS, Hwang T, Song CK, Seong H, Kim TW, Choi HS, Shin BC. Hyperthermia-induced antitumor activity of thermosensitive polymer modified temperature-sensitive liposomes. J Pharm Sci. 2006;95:1909–1917. doi: 10.1002/jps.20646. [DOI] [PubMed] [Google Scholar]
- 81.Baronzio GF, Hager ED. Hyperthermia in cancer treatment a primer. Landes Bioscience. 2006 [Google Scholar]
- 82.Gerweck LE. Hyperthermia in Cancer Therapy: The Biological Basis and Unresolved Questions. Cancer Res. 1985;45:3408–3414. [PubMed] [Google Scholar]
- 83.Chang MSK, Song W, Rhee JuongG, Levitt SeymourH. Effect of hyperthermia on vascular function in normal and neoplastic tissues. Ann N Y Acad Sci. 1980;335:35–47. doi: 10.1111/j.1749-6632.1980.tb50735.x. [DOI] [PubMed] [Google Scholar]
- 84.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 85.Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine. 2007;2:23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- 86.Meyer DE, Kong GA, Dewhirst MW, Zalutsky MR, Chilkoti A. Targeting a genetically engineered elastin-like polypeptide to solid tumors by local hyperthermia. Cancer Res. 2001;61:1548–1554. [PubMed] [Google Scholar]
- 87.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 88.Kono K. Thermosensitive polymer-modified liposomes. Adv Drug Deliv Rev. 2001;53:307–319. doi: 10.1016/s0169-409x(01)00204-6. [DOI] [PubMed] [Google Scholar]
- 89.Mackay JA, Chilkoti A. Temperature sensitive peptides: engineering hyperthermia-directed therapeutics. Int J Hyperthermia. 2008;24:483–495. doi: 10.1080/02656730802149570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A. Drug targeting using thermally responsive polymers and local hyperthermia. J Control Release. 2001;74:213–224. doi: 10.1016/s0168-3659(01)00319-4. [DOI] [PubMed] [Google Scholar]
- 91.Mart RJ, Osborne RD, Stevens MM, Ulijn RV. Peptide-based stimuli-responsive biomaterials. Soft Matter. 2006;2:822–835. doi: 10.1039/b607706d. [DOI] [PubMed] [Google Scholar]
- 92.Hart DS, Gehrke SH. Thermally associating polypeptides designed for drug delivery produced by genetically engineered cells. J Pharm Sci. 2007;96:484–516. doi: 10.1002/jps.20755. [DOI] [PubMed] [Google Scholar]
- 93.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–392. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 94.Xu C, Breedveld V, Kopecek J. Reversible hydrogels from self-assembling genetically engineered protein block copolymers. Biomacromolecules. 2005;6:1739–1749. doi: 10.1021/bm050017f. [DOI] [PubMed] [Google Scholar]
- 95.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. Journal of Physical Chemistry B. 1997;101:11007–11028. [Google Scholar]
- 96.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc. 2008;130:687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dreher MR, Raucher D, Balu N, Michael Colvin O, Ludeman SM, Chilkoti A. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 98.Bidwell GL, 3rd, Davis AN, Fokt I, Priebe W, Raucher D. A thermally targeted elastin-like polypeptide-doxorubicin conjugate overcomes drug resistance. Invest New Drugs. 2007;25:313–326. doi: 10.1007/s10637-007-9053-8. [DOI] [PubMed] [Google Scholar]
- 99.Bidwell GL, 3rd, Fokt I, Priebe W, Raucher D. Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin. Biochem Pharmacol. 2007;73:620–631. doi: 10.1016/j.bcp.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 100.Gil ES, Frankowski DJ, Spontak RJ, Hudson SM. Swelling Behavior and Morphological Evolution of Mixed Gelatin/Silk Fibroin Hydrogels. Biomacromolecules. 2005;6:3079–3087. doi: 10.1021/bm050396c. [DOI] [PubMed] [Google Scholar]
- 101.Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv Drug Deliv Rev. 2002;54:1075–1091. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 102.Stoica F, Alexander C, Tirelli N, Miller AF, Saiani A. Selective synthesis of double temperature-sensitive polymer-peptide conjugates. Chem Commun (Camb) 2008:4433–4435. doi: 10.1039/b806782a. [DOI] [PubMed] [Google Scholar]
- 103.Collier JH, Hu BH, Ruberti JW, Zhang J, Shum P, Thompson DH, Messersmith PB. Thermally and photochemically triggered self-assembly of peptide hydrogels. J Am Chem Soc. 2001;123:9463–9464. doi: 10.1021/ja011535a. [DOI] [PubMed] [Google Scholar]
- 104.Chaiswing L, Zhong W, Cullen JJ, Oberley LW, Oberley TD. Extracellular redox state regulates features associated with prostate cancer cell invasion. Cancer Res. 2008;68:5820–5826. doi: 10.1158/0008-5472.CAN-08-0162. [DOI] [PubMed] [Google Scholar]
- 105.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 106.Miyazaki K, Noda N, Okada S, Hagiwara Y, Miyata M, Sakurabayashi I, Yamaguchi N, Sugimura T, Terada M, Wakasugi H. Elevated serum level of thioredoxin in patients with hepatocellular carcinoma. Biotherapy. 1998;11:277–288. doi: 10.1023/a:1008032703468. [DOI] [PubMed] [Google Scholar]
- 107.Hassan SSM, Rechnitz GA. Determination of glutathione and glutathione reductase with a silver sulfide membrane electrode. Analytical Chemistry. 1982;54:1972–1976. [Google Scholar]
- 108.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 109.Russo A, DeGraff W, Friedman N, Mitchell JB. Selective Modulation of Glutathione Levels in Human Normal versus Tumor Cells and Subsequent Differential Response to Chemotherapy Drugs. Cancer Res. 1986;46:2845–2848. [PubMed] [Google Scholar]
- 110.Ilangovan G, Li HQ, Zweier JL, Kuppusamy P. In vivo measurement of tumor redox environment using EPR spectroscopy. Molecular and Cellular Biochemistry. 2002;234:393–398. [PubMed] [Google Scholar]
- 111.Neu M, Germershaus O, Mao S, Voigt KH, Behe M, Kissel T. Crosslinked nanocarriers based upon poly(ethylene imine) for systemic plasmid delivery: In vitro characterization and in vivo studies in mice. J Control Release. 2007;118:370–380. doi: 10.1016/j.jconrel.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 112.Kommareddy S, Amiji M. Poly(ethylene glycol)-modified thiolated gelatin nanoparticles for glutathione-responsive intracellular DNA delivery. Nanomedicine. 2007;3:32–42. doi: 10.1016/j.nano.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kommareddy S, Amiji M. Preparation and evaluation of thiol-modified gelatin nanoparticles for intracellular DNA delivery in response to glutathione. Bioconjug Chem. 2005;16:1423–1432. doi: 10.1021/bc050146t. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y, Chen P, Shen J. The development and characterization of a glutathione-sensitive cross-linked polyethylenimine gene vector. Biomaterials. 2006;27:5292–5298. doi: 10.1016/j.biomaterials.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 115.Carlisle RC, Etrych T, Briggs SS, Preece JA, Ulbrich K, Seymour LW. Polymer-coated polyethylenimine/DNA complexes designed for triggered activation by intracellular reduction. Journal of Gene Medicine. 2004;6:337–344. doi: 10.1002/jgm.525. [DOI] [PubMed] [Google Scholar]
- 116.Hong R, Han G, Fernandez JM, Kim B-j, Forbes NS, Rotello VM. Glutathione-Mediated Delivery and Release Using Monolayer Protected Nanoparticle Carriers. J Am Chem Soc. 2006;128:1078–1079. doi: 10.1021/ja056726i. [DOI] [PubMed] [Google Scholar]
- 117.Kakizawa Y, Harada A, Kataoka K. Glutathione-Sensitive Stabilization of Block Copolymer Micelles Composed of Antisense DNA and Thiolated Poly(ethylene glycol)-block-poly(l-lysine): A Potential Carrier for Systemic Delivery of Antisense DNA. Biomacromolecules. 2001;2:491–497. doi: 10.1021/bm000142l. [DOI] [PubMed] [Google Scholar]
- 118.Laemmli UK. Characterization of DNA Condensates Induced by Poly(Ethylene Oxide) and Polylysine. Proc Natl Acad Sci U S A. 1975;72:4288–4292. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tannock IF, Rotin D. Acid pH in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 120.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Molecular Cancer Therapeutics. 2006;5:1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 121.Har-el YE, Kato Y. Intracellular delivery of nanocarriers for cancer therapy. Current Nanoscience. 2007;3:329–338. [Google Scholar]
- 122.Murphy R, Powers S, Cantor C. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J. Cell Biol. 1984;98:1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO(2) gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nature Medicine. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 124.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 125.Straubinger RM, Duzgunes N, Papahadjopoulos D. Ph-Sensitive Liposomes Mediate Cytoplasmic Delivery of Encapsulated Macromolecules. Febs Letters. 1985;179:148–154. doi: 10.1016/0014-5793(85)80210-6. [DOI] [PubMed] [Google Scholar]
- 126.Gruner SM, Jain MK. X-Ray-Diffraction Demonstrates That Phosphatidyldiacylglycerol and Phosphatidylcholesterol Are Not Lamellar above the Main Transition-Temperature. Biochimica Et Biophysica Acta. 1985;818:352–355. doi: 10.1016/0005-2736(85)90009-4. [DOI] [PubMed] [Google Scholar]
- 127.Harper PE, Mannock DA, Lewis RNAH, McElhaney RN, Gruner SM. X-ray diffraction structures of some phosphatidylethanolamine lamellar and inverted hexagonal phases. Biophysical Journal. 2001;81:2693–2706. doi: 10.1016/S0006-3495(01)75912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Oliveira MC, Rosilio V, Lesieur P, Bourgaux C, Couvreur P, Ollivon M, Dubernet C. pH-sensitive liposomes as a carrier for oligonucleotides: a physico-chemical study of the interaction between DOPE and a 15-mer oligonucleotide in excess water. Biophysical Chemistry. 2000;87:127–137. doi: 10.1016/s0301-4622(00)00180-0. [DOI] [PubMed] [Google Scholar]
- 129.Sudimack JJ, Guo WJ, Tjarks W, Lee RJ. A novel pH-sensitive liposome formulation containing oleyl alcohol. Biochimica Et Biophysica Acta. 2002;1564:31–37. doi: 10.1016/s0005-2736(02)00399-1. [DOI] [PubMed] [Google Scholar]
- 130.Kono K, Zenitani K, Takagishi T. Novel Ph-Sensitive Liposomes - Liposomes Bearing a Poly(Ethylene Glycol) Derivative with Carboxyl Groups. Biochimica Et Biophysica Acta. 1994;1193:1–9. doi: 10.1016/0005-2736(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 131.Guo WJ, Gosselin MA, Lee RJ. Characterization of a novel diolein-based LPDII vector for gene delivery. J Control Release. 2002;83:31–37. doi: 10.1016/s0168-3659(02)00167-0. [DOI] [PubMed] [Google Scholar]
- 132.Stevens MM, Flynn NT, Wang C, Tirrell DA, Langer R. Coiled-Coil Peptide-Based Assembly of Gold Nanoparticles. Advanced Materials. 2004;16:915–918. [Google Scholar]
- 133.Ryadnov MG, Ceyhan B, Niemeyer CM, Woolfson DN. "Belt and Braces": A Peptide-Based Linker System of de Novo Design. J Am Chem Soc. 2003;125:9388–9394. doi: 10.1021/ja0352045. [DOI] [PubMed] [Google Scholar]
- 134.Li WJ, Nicol F, Szoka FC. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56:967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 135.Haas DH, Murphy RM. Design of a pH-sensitive pore-forming peptide with improved performance. J Pept Res. 2004;63:9–16. doi: 10.1046/j.1399-3011.2004.00098.x. [DOI] [PubMed] [Google Scholar]
- 136.Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC. Design, Synthesis, and Characterization of a Cationic Peptide That Binds to Nucleic Acids and Permeabilizes Bilayers. Biochemistry. 1997;36:3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 137.Asayama S, Kato H, Kawakami H, Nagaoka S. Carboxymethyl poly(L-histidine) as a new pH-sensitive polypeptide at endosomal/lysosomal pH. Polymers for Advanced Technologies. 2007;18:329–333. [Google Scholar]
- 138.Asayama S, Sudo M, Nagaoka S, Kawakami H. Carboxymethyl poly(L-histidine) as a new pH-sensitive polypeptide to enhance polyplex gene delivery. Molecular Pharmaceutics. 2008;5:898–901. doi: 10.1021/mp800094b. [DOI] [PubMed] [Google Scholar]
- 139.Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, Samokhin GP, Whiteman KR. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta. 2001;1511:397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- 140.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today. 2005;10:1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 141.Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 142.Jiang XM, Fitzgerald M, Grant CM, Hogg PJ. Redox control of exofacial protein thiols/disulfides by protein disulfide isomerase. J Biol Chem. 1999;274:2416–2423. doi: 10.1074/jbc.274.4.2416. [DOI] [PubMed] [Google Scholar]
- 143.Ishida T, Kirchmeier MJ, Moase EH, Zalipsky S, Allen TM. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim Biophys Acta. 2001;1515:144–158. doi: 10.1016/s0005-2736(01)00409-6. [DOI] [PubMed] [Google Scholar]
- 144.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 145.Goormaghtigh E, De Meutter J, Szoka F, Cabiaux V, Parente RA, Ruysschaert JM. Secondary structure and orientation of the amphipathic peptide GALA in lipid structures. An infrared-spectroscopic approach. Eur J Biochem. 1991;195:421–429. doi: 10.1111/j.1432-1033.1991.tb15721.x. [DOI] [PubMed] [Google Scholar]
- 146.Subbarao NK, Parente RA, Szoka FC, Jr, Nadasdi L, Pongracz K. pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry. 1987;26:2964–2972. doi: 10.1021/bi00385a002. [DOI] [PubMed] [Google Scholar]
- 147.Volk LD, Flister MJ, Bivens CM, Stutzman A, Desai N, Trieu V, Ran S. Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor A therapy. Neoplasia. 2008;10:613–623. doi: 10.1593/neo.08302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.MacKay JA, Li W, Huang Z, Dy EE, Huynh G, Tihan T, Collins R, Deen DF, Szoka FC. HIV TAT peptide modifies the distribution of DNA nanolipoparticles following convection-enhanced delivery. Molecular Therapy. 2008;16:893–900. doi: 10.1038/mt.2008.36. [DOI] [PubMed] [Google Scholar]
- 149.Gratton JP, Yu J, Griffith JW, Babbitt RW, Scotland RS, Hickey R, Giordano FJ, Sessa WC. Cell-permeable peptides improve cellular uptake and therapeutic gene delivery of replication-deficient viruses in cells and in vivo. Nat Med. 2003;9:357–362. doi: 10.1038/nm835. [DOI] [PubMed] [Google Scholar]
- 150.Eguchi A, Akuta T, Okuyama H, Senda T, Yokoi H, Inokuchi H, Fujita S, Hayakawa T, Takeda K, Hasegawa M, Nakanishi M. Protein Transduction Domain of HIV-1 Tat Protein Promotes Efficient Delivery of DNA into Mammalian Cells. J. Biol. Chem. 2001;276:26204–26210. doi: 10.1074/jbc.M010625200. [DOI] [PubMed] [Google Scholar]
- 151.Temming K, Lacombe M, van der Hoeven P, Prakash J, Gonzalo T, Dijkers EC, Orfi L, Keri G, Poelstra K, Molema G, Kok RJ. Delivery of the p38 MAPkinase inhibitor SB202190 to angiogenic endothelial cells: development of novel RGD-equipped and PEGylated drug-albumin conjugates using platinum(II)-based drug linker technology. Bioconjug Chem. 2006;17:1246–1255. doi: 10.1021/bc0600158. [DOI] [PubMed] [Google Scholar]
- 152.Karle IL, Urry DW. Crystal structure of cyclic (APGVGV)2, an analog of elastin, and a suggested mechanism for elongation/contraction of the molecule. Biopolymers. 2005;77:198–204. doi: 10.1002/bip.20214. [DOI] [PubMed] [Google Scholar]
- 153.Long CG, Braswell E, Zhu D, Apigo J, Baum J, Brodsky B. Characterization of collagen-like peptides containing interruptions in the repeating Gly-X-Y sequence. Biochemistry. 1993;32:11688–11695. doi: 10.1021/bi00094a027. [DOI] [PubMed] [Google Scholar]
- 154.Stevens MM, Allen S, Sakata JK, Davies MC, Roberts CJ, Tendler SJB, Tirrell DA, Williams PM. pH-Dependent Behavior of Surface-immobilized Artificial Leucine Zipper Proteins. Langmuir. 2004;20:7747–7752. doi: 10.1021/la030440e. [DOI] [PubMed] [Google Scholar]