Abstract
Fertility in mammals is dependant on females having an adequate primordial follicle pool to supply oocytes for fertilization. The formation of primordial follicles is called ovarian follicular assembly. In rats and mice progesterone and estradiol have been shown to inhibit follicle assembly with assembly occurring after birth when the pups are removed from the high-steroid maternal environment. In contrast, primordial follicle assembly in other species, such as cattle and humans, occurs during fetal development before birth. The objective of the current study is to determine if progesterone levels regulate primordial follicle assembly in fetal bovine ovaries. Ovaries and blood were collected from bovine fetuses. Interestingly, ovarian progesterone and estradiol concentrations were found to decrease with increasing fetal age and correlated to increased primordial follicle assembly. Microarray analysis of fetal ovary RNA suggests that progesterone membrane receptor and estrogen nuclear receptor are expressed. Treatment of fetal bovine ovary cultures with a higher progesterone concentration significantly decreased primordial follicle assembly. Observations indicate that progesterone affects ovarian primordial follicle assembly in cattle, as it does in rats and mice.
Keywords: Embryonic Development, Fetal Ovary, Ovarian Morphogenesis, Cow, Fetal Endocrinology
INTRODUCTION
Fertility in mammals is dependant on females having a supply of oocytes for fertilization. Oocytes are stored in mammalian ovaries in an arrested state in structures called primordial follicles. Each primordial follicle is composed of a single oocyte arrested in the diplotene stage of the first meiotic division and surrounded by a single layer of squamous pre-granulosa cells (Hirshfield 1991). The size (i.e. number) of the primordial follicle pool is one determinant of the reproductive lifespan of a female (Hirshfield 1994). The formation of primordial follicles is a process known as ovarian follicular assembly. During follicle assembly groups or ‘nests’ of oocytes, having completed mitotic proliferation and entered into meiosis, separate to form individual primordial follicles (Pepling M. E. 2006). Many of the oocytes undergo apoptotic cell death during nest breakdown (Pepling M. E. and Spradling 2001, Tilly 1996). Follicle assembly occurs after birth in rodents (Pepling M. E. 2006, Skinner 2005), and during fetal development in other mammalian species such as cattle (Sakai 1955, Tanaka et al. 2001), sheep (Mauleon 1974, Sawyer et al. 2002) and humans (Baker 1963). Cattle and humans have similar gestation lengths. The corresponding age during human fetal gestation at which primordial follicle assembly is observed varies in the literature (Abir et al. 2005, Hirshfield 1991, Sforza and Forabosco 1998, van Wagener and Simpson 1965), but appears to occur between 16-19 weeks (112-133 day) gestation. This is slightly later than the initiation of assembly observed in the current study of approximately 80 days in cattle, and likely reflects the fact that calves are born at a more developed physical stage than are human infants.
The factors that regulate follicle assembly are not well understood. Tumor necrosis factor alpha (TNFα) signaling is thought to be important in promoting the oocyte apoptosis that accompanies oocyte nest breakdown and follicle assembly (Marcinkiewicz et al. 2002, Morrison and Marcinkiewicz 2002). Administration of activin A, a member of the transforming growth factor beta (TGFβ superfamily of growth factors, during follicle assembly in mice results in an increased pool of primordial follicles (Bristol-Gould et al. 2006), indicating a role for this growth factor. Genetic studies with knockout mice suggest that several more growth factors may play a role in follicle assembly. Growth and differentiation factor 9 (GDF-9) (Yan et al. 2001), bone morphogenetic factor 15 (BMP-15) (Yan et al. 2001) and lunatic fringe (Lfng) (Hahn et al. 2005) knockout mouse adults have ovaries where some follicles have multiple oocytes. Multiple oocyte follicles are thought to be the result of faulty oocyte nest breakdown. Transgenic over-expression of inhibin alpha also results in multiple oocyte follicles (McMullen et al. 2001). In addition, nerve growth factor knockout mice have oocytes not enclosed in follicles at seven days of age, again suggesting faulty follicle assembly (Dissen et al. 2001).
Sex steroids have also been shown to play a role in follicle assembly. In rats progesterone, but not estrogen, was shown to inhibit follicle assembly both in vitro and in vivo (Kezele and Skinner 2003). Progesterone was also shown to decrease oocyte apoptosis during follicle assembly (Chen et al. 2009, Kezele and Skinner 2003). In mice estrogen as well as progesterone inhibit oocyte nest breakdown (Chen et al. 2007), suggesting that there may be species differences in the regulation of follicle assembly. In fetal sheep ovaries, steroidogenic cells have been identified and the speculation has been made that these steroids are important for formation of ovigerous cords (i.e. oocyte nests), and that a fall in steroid levels corresponds with entry of oogonia into meiosis (Juengel et al. 2002). In contrast, in some other species there are indications that steroid hormones, especially estrogen, promote follicle assembly. In baboons an experimental decrease in estradiol levels during pregnancy resulted in the fetal ovaries having fewer assembled follicles and an increase in un-assembled oocyte nests (Zachos et al. 2002). In hamsters the presence of estrogen promotes follicle assembly (Wang and Roy 2007, Wang et al. 2008), although this effect decreases at higher estrogen doses.
The fact that female sex steroids inhibit follicle assembly in rats and mice, and that rodent follicle assembly occurs in the first days after birth, has led to the ‘endocrine model’ of follicle assembly (Kezele and Skinner 2003). In this model, rodent embryos are exposed to high levels of maternal progesterone late in gestation, which inhibits follicle assembly. Right at birth the pups are removed from the high progesterone environment, levels of progesterone in the pups fall and follicle assembly commences. This endocrine model explains regulation of primordial follicle assembly in those species in which assembly occurs shortly after birth. However, as mentioned above there are species, including cattle and humans, in which follicle assembly occurs during fetal development before birth. The objective of the current study was to determine if sex steroid levels help regulate primordial follicle assembly in cattle prior to birth. The hypothesis tested was that progesterone inhibits primordial follicle assembly in bovine fetal ovaries in vitro and in vivo. The timing of normal bovine fetal ovarian follicle assembly was characterized, and levels of fetal progesterone and estrogen were determined. Fetal bovine ovaries were also cultured in the presence and absence of progesterone to test for an effect on follicle assembly. Understanding the mechanisms of follicle assembly in cattle could give insights into how follicle assembly is regulated in other species in which follicle assembly occurs prior to birth, including humans.
MATERIALS AND METHODS
Tissue collection and processing
Bovine fetal ovaries were collected from abattoirs by the Center for Reproductive Biology, Animal Reproduction Core Laboratory at Washington State University. The ovaries were collected from fetuses ranging in size from 6.5 to 50 cm of crown-rump length (CRL). This corresponds to fetuses estimated to be 60 to 170 days of gestation (Rexroad et al. 1974). From each fetus the ovaries were dissected free of surrounding bursal tissue, and one ovary was placed in Bouin's solution for 5-8 hours for fixation. The second ovary was placed in a sterile plastic bag with a small amount of phosphate buffered saline (PBS) and the bag filled with 50% oxygen/45% nitrogen/5% CO2 mix and placed on ice for transport (4-7 hours) to the laboratory. Some of these ovaries were used for steroid assays and others for organ culture experiments. Not all ovaries were assayed for both progesterone and estrogen. Fetal blood was also collected from each animal and transported on ice to the laboratory. Tissues were collected in accordance with Washington State University Institutional Animal Care and Use Committee approved protocols.
Morphological evaluation of follicle assembly
Bouin's-fixed ovaries collected from bovine fetuses were paraffin embedded and sectioned at 5 μm thickness. Sections were made parallel to the longitudinal axis of the ovary and those sections with the largest cross-section (through the center of the ovary) were chosen for morphometric evaluation. Sections were stained with hematoxylin and eosin according to standard protocols. Oocytes or pre-meiotic germ cells were recognized by morphological characteristics, including having a large round nucleus surrounded by clear, non-basophilic cytoplasm. An oocyte that was separate from other oocytes and surrounded by granulosa cells was considered to have undergone follicle assembly and to be part of a primordial or developing follicle. Two regions in each ovary were evaluated, and each oocyte/germ cell in each region was classified as either un-assembled or assembled into a follicle. The regions evaluated for each ovary were, at minimum, strips within the plane of the chosen histologic section that were 0.1mm high by 1.0mm wide and that ran perpendicular to the long axis of the ovary from the ovarian surface to the center of the ovary. Two strips meeting the above criteria were located randomly within an ovary section. In smaller fetal ovaries 300-450 germ cells are present within two such strips. In larger ovaries from older animals oocyte density was lower, so larger portions of an ovary section (one quarter section, one half or entire ovary sections) were counted to ensure that at least 200 oocytes were counted and categorized per ovary.
Steroid assays
Fetal blood samples were evaluated for their progesterone content, and fetal ovaries were evaluated for their progesterone (P4) and estradiol (E2) content. Briefly, fetal whole blood was allowed to clot, then centrifuged and serum collected for assays. Fetal ovaries were weighed (wet weight), homogenized in phosphate buffered saline (PBS), and submitted for assay. Both serum samples and fetal ovary samples were subjected to an organic phase extraction procedure to concentrate the samples. The extracted samples were re-constituted into the same buffer as used with the assay standards. Both sample concentration procedures and steroid radioimmunoassays were performed at the Assay Core Laboratory, Center for Reproductive Biology, Washington State University. Steroid recovery was >95% and linearity was within acceptable criteria for the Assay Core Laboratory. Intra assay coefficient of variation (CV) for the progesterone assay (Kit DSL-3400, Diagnostic System Laboratories, Webster, TX) was 3.8%. For the estradiol assay (Kit DSL-39100, Diagnostic System Laboratories, Webster, TX) CV was 5.6%. Cross-reactivity data is available from the manufacturer.
Ovary culture
Ovaries from four bovine fetuses ranging from an estimated 73-117 days of gestation were shipped to the laboratory from abattoirs as described above and then each dissected so as to separate the outer ovarian cortex from the inner medulary tissue after which the cortex was cut into pieces approximately 1mm3. Ovary fragments were cultured as previously described (Wandji et al. 1996) on floating filters (0.4 μm Millicell-CM, Millipore, Bedford, MD, USA) in 0.5 ml Waymouth medium containing 10% calf serum (Hyclone; Logan, UT USA), 25μg/ml pyruvic acid, one-half suggested concentration ITS-X (0.335 μg/ml, sodium selenite, 0.5 mg/ml insulin, 0.275 mg/ml transferrin, 0.1 mg/ml ethanolamine) (Invitrogen Corp., Grand Island, NY, USA), and 50μg streptomycin sulfate and 75μg penicillin/ml (Invitrogen Corp., Grand Island, NY, USA). Alternatively, in one experiment ovaries were cultured in 50% Ham's F12 / 50% DMEM (Dulbecco's Modified Eagles’ Medium), (Gibco BRL, Gaithersburg, MD) containing 0.1% bovine serum albumin, 0.1% Albumax (Gibco BRL, Gaithersburg, MD) 27.5μg/ml transferrin, 200 ng/ml insulin (human recomginant, Sigma) and 50 mg/ml L-ascorbic acid. There was no obvious difference in response to culture for this experiment so results were combined. The culture experiments occurred on four different days. Ovaries were cultured in 0.5mL medium for two days at 37° in an incubator with 5% CO2. Treated ovaries received progesterone (P4) (Sigma) at 10–6 M (314 ng/ml). After culture, ovaries were fixed, sectioned at 3 μm, and stained with hematoxylin/eosin for use in morphological analysis.
RNA isolation and microarray analysis
Ovaries from three bovine fetuses (67, 72 and 144 days gestation estimated by crown rump length, CRL) were collected and stored at −20° until the time of RNA isolation. Total RNA was isolated after homogenizing ovaries in Trizol® reagent (Invitrogen, USA), using manufacturer's protocols. RNA quality was evaluated by examining a portion on an RNA gel. 18S and 28S bands were clear, and there was little smearing, indicating that quality was acceptable. However the 18S band was not as dark as expected, suggesting that some slight RNA degradation had occurred. RNA was used for microarray analysis with the Affymetrix (Affymetrix, Santa Clara, CA) bovine genome array chip. This chip contains probes for 23,000 genes. The Genomics Core in the Center for Reproductive Biology at Washington State University performed the analysis using standard Affymetrix protocols. The microarray was scanned on a Hewlett-Packard Gene Array Scanner (Hewlett-Packard Co., Palo Alto, CA). Microarray data were pre-processed using the Microarray Suite (MAS-5) statistical algorithm. All chips were scaled to target signal of 260 in order to keep scaling factor to 1. Using Gene Chip Operating System (Affymetrix, Santa Clara, CA) an absolute analysis was performed to assess the relative abundance of the transcripts based on signal and detection (present, absent, or marginal). MAS-5 pre-processed data were obtained from GCOS and imported into GeneSpring GX 7.3. Pre-processed data were used for analysis in GeneSpring. Quality measures included evaluation of 3’-end degradation, which indicated that all three samples had similar 3’-end degradation, and so are comparable to each other. The quantitative polymerase chain reaction (PCR) procedure used was a Realtime procedure previously described (Nilsson et al. 2006). Primers were designed to amplify products that cross intron – exon bounderies (except 3β HSD), and melting curve analyses were performed at the end of the real-time run. These melting curve analyses indicated there was minimal secondary product generated. The primers used for CYP11A1 were:
Set 1: Forward 5’AGA CGT GGC CCA TCT CTT CAA GTT-3’,
Reverse 5’TTA TTG CCT CTG GAG CCA TCA CCT-3’;
Set 2: Forward 5’-CGG AAA GTT TGT AGG GGA CA -3’,
Reverse 5’-ACG TTG AGC AGA GGG ACA CT -3’;
and for PGRMC1 were:
Forward 5’-CAGGACCCGCGTATACTCAT -3’,
Reverse 5’-AGGAGTGAGGTCGGAAAGGT -3’;
and for 3βHSD:
Set 1: Forward 5’-AGA CAT TCT GGA TGA GCA GTG CCT-3’,
Reverse 5’-ACA GCA GCT GGG TAC CTT TCA CAT-3’;
Set 2: Forward 5’-ACC AGA AGT TCG GGA GGA AT -3’,
Reverse 5’-TTC TAT GGT GCT GGT GTG GA-3’;
And for house keeping gene, ribosomal protein S2, rat:
Forward 5’- CTGCTCCTGTGCCCAAGAAG -3’,
Reverse 5’- AAGGTGGCCTTGGCAAAGTT -3’.
Statistics
Steroid concentration data from ovaries and fetal blood were plotted against gestational age. Curve fitting suggested that an exponential decay formula provided a good fit of both progesterone and estrogen concentration data. In order to determine if there is a significant decrease in steroid concentrations over time, the data were linearized using the assumption that steroid concentrations followed an exponential decay curve, and the resulting linear regression was tested as to whether its slope was significantly non-zero. A slope different from zero indicates a change in steroid concentration over time.
For the cultured ovary experiments morphometric data was collected that determined the proportion of oocytes assembled into follicles versus those not assembled. Within each experiment the proportion of assembled follicles for each ovary fragment was divided by the proportion of assembled follicles in the control group. This normalized the data so that each control was equal to 1 and the proportions of assembled follicles in the progesterone-treated ovary fragments were expressed as ratios relative to their controls. Since all the control ovary values were now equal to 1, a one-sample t-test with relative mean equal to 1.0 was performed on cultured ovary follicle assembly data using GraphPad Prism version 4.0b for Macintosh, GraphPad Software, San Diego California USA, www.graphpad.com.
RESULTS
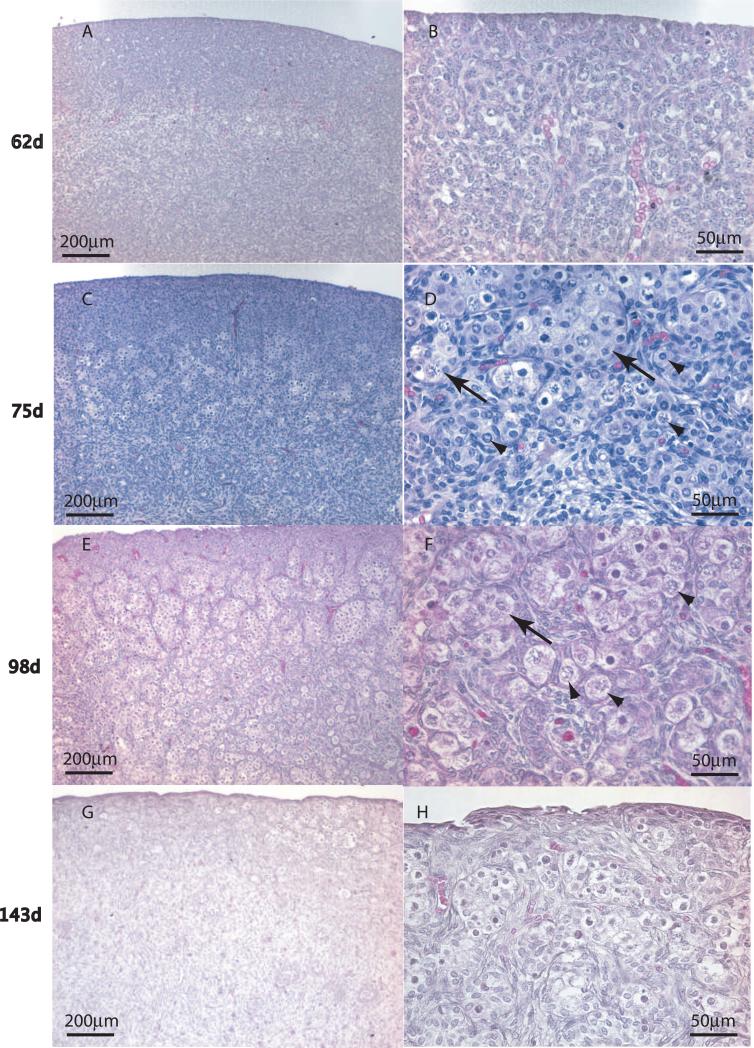
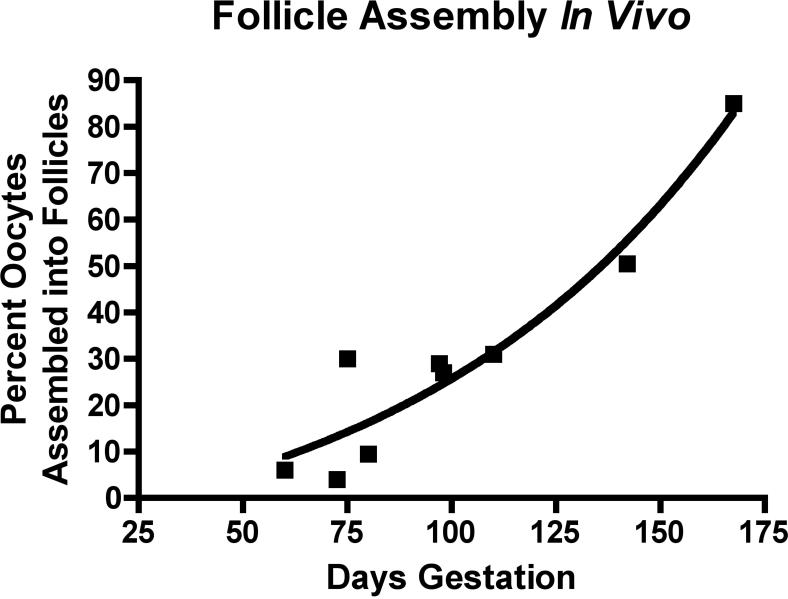
Ovaries were collected from 9 bovine fetuses measuring between seven and fifty cm crown rump length (CRL) in size, which corresponds to 62 to 170 days of gestation (Rexroad et al. 1974). Morphological evaluation of hematoxylin-eosin stained ovaries showed that germ cells are arranged in clearly identifiable clusters (i.e. ovigerous cords (Gondos 1973, Pepling M. E. 2006) by the time fetuses reached 75 days of age (12cm CRL). Some isolated oocytes were found assembled into primordial follicles by an estimated 75 days of development in the central portion of the fetal ovary (Figure 1). Both oocyte nests and primordial follicles were common at 98 days of development. Developing primary follicles, that had undergone primordial to primary follicle transition, were present in ovaries estimated as 143 days, along with many primordial follicles and some small oocyte nests (Figure 1). The proportion of oocytes in fetal bovine ovary cross-sections that were assembled into primordial follicles was determined morphometrically by microscopic examination of hematoxylin/eosin stained ovaries from bovine fetuses of different ages. Figure 2 shows the percent of assembled follicles as a proportion of total oocytes plotted against fetal gestational age. The proportion of assembled primordial follicles rises with increasing fetal age as expected, (Figure 2).
Figure 1.
Fetal bovine ovarian follicle assembly and development is shown at different estimated gestational ages, indicated left margin. Representative fetal ovaries were collected at different gestational ages, sectioned and hematoxylin/eosin stained. The right column of images (B,D,F,H) are a higher magnification (see scale bars) of the left column (A,C,E,G). Arrows = oocyte nests. Arrowheads = individual assembled follicles.
Figure 2.
The percentage of oocytes in an ovarian cross-section that are assembled into primordial follicles at different estimated gestational ages (days). Each point represents one ovary. Regression line is from a sigmoidal dose-response equation with variable slope (R2=0.91).
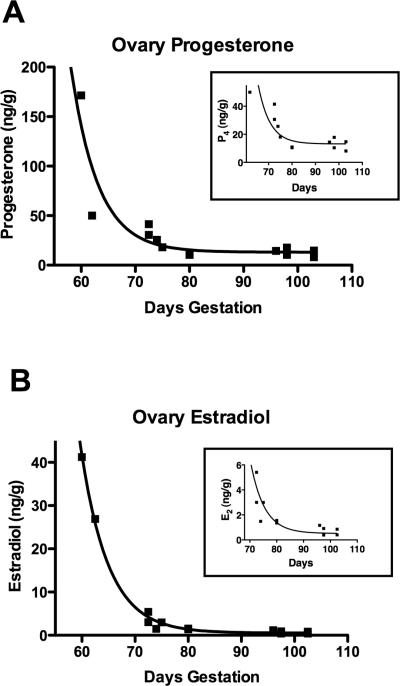
Experiments were performed to determine the levels of progesterone and estradiol present in fetal ovaries and progesterone in fetal blood serum. Steroid assays were performed on whole fetal ovaries from 60-103 days of gestation as described in the Methods. Ovarian progesterone concentration (ng per gram of wet tissue weight) was found to decrease with increasing fetal age (p=0.002, see Methods for statistical procedure) (Figure 3A). Ovarian estrogen levels also were found to decrease with increasing fetal age (Figure 3B) (p≥0.0005). Since the ovaries from the youngest bovine fetuses were very small, there was concern that inaccurate measurement of ovarian wet weight might give spurious results. Even with these earliest ovaries excluded there was a significant decrease in progesterone (p=0.006) and estrogen (p≥0.0005) concentrations (Fig. 3A and 3B, insets). Both progesterone and estrogen levels appeared to decrease until about 80 days gestation. Fetal blood serum progesterone levels were at the low limits of detection even after sample concentration (see Methods), so results must be viewed with caution. However, there was no indication that fetal serum progesterone levels changed with increasing fetal age (data not shown). Mean fetal serum progesterone concentration was 0.10 ± 0.015 ng/ml (mean ± standard deviation).
Figure 3.
The progesterone (A) and estradiol (estrogen) (B) concentration in fetal bovine ovaries (ng/g wet weight) taken from fetuses at different estimated gestational ages (days). Regression lines are fitted using exponential decay equations, R2=0.83 for progesterone regression, R2=0.99 for estrogen regression. The inset graphs are included to show more clearly steroid concentrations in older ovaries.
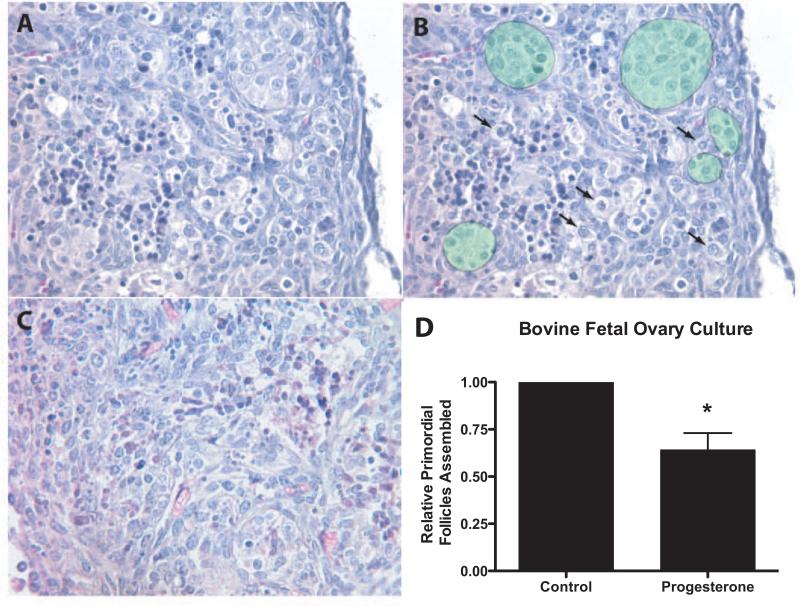
Bovine ovary organ culture experiments were performed to determine experimentally if high levels of progesterone can regulate the rate of primordial follicle assembly. Ovarian cortex pieces were dissected out of fetal ovaries from four bovine fetuses ranging from about 73-117 days of gestation, and placed into a floating filter culture system as described in the Methods. Ovarian cortex pieces from one of the ovaries from each fetus were cultured for two days in either the presence or absence of 10−6M progesterone. Histological examination determined the proportion of oocytes in each ovary sample that had undergone follicle assembly to form isolated primordial or developing follicles. This was accomplished by counting the number of oocytes assembled into follicles and those un-assembled in each of two serial sections, and averaging the counts (un-assembled vs. assembled) for each ovary. Since the ovaries came from bovine fetuses that varied in age, the starting percentage of primordial follicles prior to culture varied between ovaries. To account for this variable each ovary was assessed for the percentage of oocytes assembled into follicles after culture in the absence or presence of control and progesterone treatment. The percentage of oocytes assembled into follicles was normalized to the control culture percentage of assembled follicles by dividing by the percentage of assembled follicles in the control group of that experiment. A one-sample t-test of these data determined that this progesterone treatment significantly (p<0.05) reduced the proportion of assembled follicles (Figure 4D). The representative morphology of control and progesterone treated organ cultures is shown in Figure 4A-C. There is nuclear condensation of some cells visible after culture in both treated and control ovary fragments, which is likely a result of explant culture conditions. However, follicle assembly did occur. The decrease in primordial follicle assembly is reflected in the retention of oocyte nests and the decreased number of primordial follicles in progesterone-treated cultures, (Figure 4D).
Figure 4.
Representative ovarian cortex pieces after 2 days of ovary culture showing oocyte nests and assembled follicles (A) progesterone treated ovary, (B) duplicate of image (A) with the addition of green shaded areas showing oocyte nests, and arrows pointing to assembled primordial follicles. (C) Control un-treated cultured ovary. (D) The effect of progesterone on follicle assembly in cultured fetal bovine ovaries. Fetal ovary cortex pieces were cultured in the absence (control) or presence of progesterone (10−6M) for two days. After culture the pieces were sectioned, stained and the proportion of oocytes assembled into follicles determined. The percentage of assembled follicles was normalized to the control culture percentage of assembled follicles for each experimental replicate, for each fetal ovary (n=4). Data are presented as the mean ± S.E.M. as described in Methods. * indicates a statistical difference (p<0.05) between treatment and control by one-sample t-test.
RNA isolated from fetal bovine ovaries was used for a microarray analysis in order to determine whether selected genes important in steroid signaling and synthesis were expressed in these ovaries. Two ovaries (from fetuses estimated at 67 and 72 days gestation) were from the period of development when ovarian steroid concentrations are higher, and one (from a fetus of 144 days gestation) from when ovarian steroid concentrations are low. The results indicate which gene transcripts are expressed (i.e. present) or not (i.e. absent), Table 1. The presence of nuclear progesterone receptor is equivocal. The membrane progesterone receptor PGRMC1 is strongly present. Other membrane associated progesterone receptors including mPRα, mPRβ and mPRγ were not represented on the bovine microarray chip. Estrogen receptor ER1 (alpha) is not expressed, but ER2 (beta) is present. These data suggest that the components of steroid ligand-receptor signaling are present in fetal bovine ovaries. The presence of transcripts for certain steroidogenic enzymes was also investigated. CYP11A1 (i.e. P450scc), that converts cholesterol to pregnenolone, is present. 3βHSD, that converts pregnenolone to progesterone, is also present in the early stage ovaries, but not present in the later stage ovary. Transcripts for other steroidogenic enzymes leading to estradiol formation (i.e. aromatase) were also present.
Table 1.
Presence/Absence calls for selected genes from microarray analysis.
| Presence/Absence (Fetal ovary age) | ||||
|---|---|---|---|---|
| Common Name | Gene Symbol | 67day | 72day | 144day |
| Progesterone nuclear receptor (Genbank ID CK983148) | PGR | P | A | P |
| Progesterone receptor membrane component 1 | PGRMC1 | P | P | P |
| Estrogen receptor 1 (alpha) | ER1 | A | A | A |
| Estrogen receptor 2 (beta) | ER2 | P | P | P |
| Side-chain clevage enzyme | CYP11A1 (P450scc) | P | M | P |
| 3 beta-hydroxysteroid dehydrogenase | 3βHSD | P | P | A |
| 17 alpha-hydroxylase | CYP17 (P450c17) | P | A | A |
| Aromatase (Genbank ID NM_174305.1) | CYP19 (P450aro) | P | P | P |
| 17 beta-hydroxysteroid dehydrogenase | 17βHSD1 | A | A | A |
P=present, A=absent, M=marginal.
To confirm and extend the microarray analysis real-time PCR was performed on seven additional fetal ovary samples ranging from 70 to 150 days gestation for expression of 3βHSD, CYP11A1 and PGRMC1. Compared to the housekeeping gene ribosomal S2, expression of PGRMC1 was detectable and was at consistent levels across samples. CYP11A1 was also detectable, with expression levels quite variable between samples, and not correlated with fetal age. The detected expression level of 3βHSD was negligible throughout fetal ovary development (data not shown). Observations confirm that some components of steroid ligand receptor signaling are expressed in fetal bovine ovaries.
DISCUSSION
The objective of the current study is to determine if high progesterone levels regulate primordial follicle assembly in cattle prior to birth. To determine this, experiments were performed using fetal bovine ovaries collected from abattoirs. Abattoir ovary specimens were collected in central and western Washington State and came from a mixed population of beef and dairy cattle breeds. There was an increase in assembled follicles with increasing fetal size, as expected (Figure 2). There is some variation in the literature concerning at what stage of bovine fetal development particular stages of oocyte development occur (Erickson 1966, Kurilo et al. 1987, Marion and Gier 1971, Mbassa 1989, Russe 1983, Sakai 1955, Tanaka et al. 2001, Vigier et al. 1976). However, in the current study the CRL (crown-rump length) sizes and embryonic ages at which oocyte nests and primordial follicle assembly were first observed fall within the range expected from previous investigations. These observations indicate that the sample population used is appropriate for performing the current study.
Experiments were performed to determine the levels of progesterone and estrogen present in fetal ovaries and the levels of progesterone in fetal blood. Interestingly, the estrogen and progesterone concentrations in the fetal ovaries decline with increasing fetal age (Figure 3). This is consistent with the endocrine model of follicle assembly previously proposed (Kezele and Skinner 2003), that higher levels of progesterone inhibit assembly and that follicles assemble as progesterone levels decline (Figure 5). However, in cattle, humans and sheep, primordial follicle assembly is not synchronous after birth as it is in rodents (Mbassa 1989, Pepling M. E. 2006, Sakai 1955, Sawyer et al. 2002, Tanaka et al. 2001). In the bovine fetal ovary there is an extended period of time during which oocyte nests, primordial follicles and some developing follicles are all present. This suggests that progesterone is likely not the only regulatory factor involved in initiating follicle assembly. In fact, in progesterone receptor knock-out mice follicle assembly still occurs (Lydon et al. 1995). As mentioned, ovarian estrogen concentrations also decline, suggesting a role for estrogen in bovine follicle assembly. Cell-cell signaling via other locally produced growth factors (Pepling M. E. 2006) likely also plays a role in the assembly process.
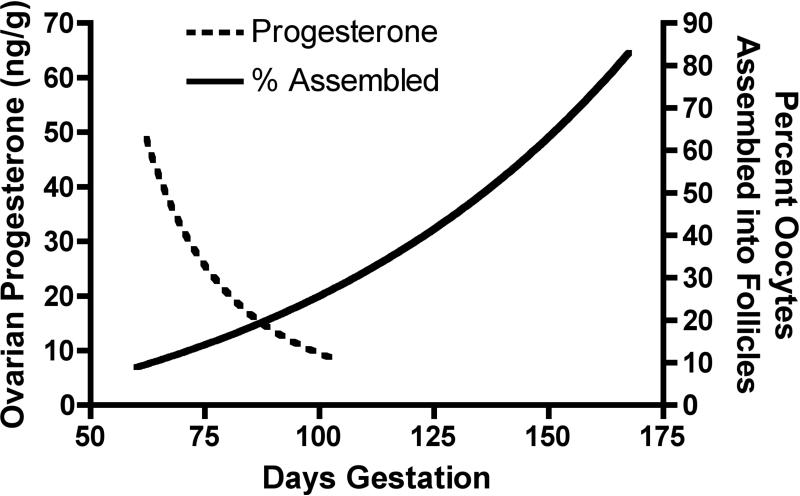
Figure 5.
Correlation of progesterone concentration in fetal bovine ovaries and the percentage (%) of oocytes assembled into primordial follicles as a function of estimated gestational age (days). Follicle assembly is initiated as ovarian progesterone concentration declines.
It is notable that fetal ovary progesterone levels decrease across a time period of gestation (estimated at 60-103 days) during which bovine maternal blood progesterone levels do not change (Echternkamp et al. 2006). In addition, maternal blood progesterone concentrations are at about 7 ng/ml between 50 and 100 days of gestation (Echternkamp et al. 2006), which is lower than the 20-150 ng/g progesterone concentration seen in fetal ovaries between 60 and 75 days of gestation. Previous literature has established that maternal and fetal progesterone levels are different from each other and may be regulated independently in macaques (Ducsay et al. 1985, Thau et al. 1976), humans (Donaldson et al. 1991), sheep (Nicol et al. 1997) and elephants (de Villiers et al. 1989). This suggests that ovarian fetal progesterone is regulated to control the initiation or progression of primordial follicle assembly, independent of maternal progesterone levels.
While fetal ovary progesterone levels were found to decrease during gestation, fetal blood progesterone levels were not correlated with fetal age. This observation suggests that fetal ovarian progesterone concentrations are being regulated independently from those of fetal blood. Previous literature has shown that ovarian tissue steroid concentrations change more rapidly than circulating blood concentrations (Peters and McNatty 1980). Certainly it is the case in the current study that fetal ovary progesterone concentrations (10-175 ng/g wet weight) are different from the fetal serum concentrations (<1ng/ml). Interestingly, past studies have also indicated that fetal and adult erythrocytes are able to metabolize progesterone (Chen et al. 1987, Chen et al. 1989, Nancarrow et al. 1984, Vahdat et al. 1984). This may be one mechanism by which fetal blood can maintain a different progesterone concentration than that of the fetal ovary and maternal blood.
Microarray analysis and real-time PCR of fetal ovary RNA indicates that the transcript for the surface membrane progesterone receptor PGRMC1 is present in the fetal ovary. This indicates that the actions of progesterone in regulating follicle assembly may be mediated, at least in part, through the surface membrane receptor. These data are consistent with findings in rat ovaries at the time of follicle assembly (Kezele et al. 2002, Nilsson et al. 2006). In previous work, the classic nuclear progesterone receptor was not detected in fetal sheep ovaries (Juengel et al. 2006). In the current study, the presence of mRNA for the nuclear progesterone receptor was equivocal. The transcript for estrogen receptor 2 (ER2) is present, similarly to what has previously been shown for fetal cattle and sheep ovaries (Gaverick et al. 2005, Juengel et al. 2006). In the current study ER1 mRNA was not detected. This is different from previous findings of ER1 in surface epithelial cells and some stromal cells of fetal sheep ovaries (Juengel et al. 2006). However, taken together, these data suggest that the components for steroid ligand-receptor signaling are present in bovine fetal ovaries.
Previous research suggests that the progesterone and estradiol detected in fetal bovine ovaries is, at least in part, produced locally in the ovaries. There have been several studies showing that mammalian gonads around the time of sexual differentiation synthesize steroids (George and Wilson 1994). In ovary culture experiments, fetal bovine ovaries have been shown to produce both progesterone and estradiol (Dominguez et al. 1988, Shemesh et al. 1978, Shore and Shemesh 1981, Tanaka et al. 2001). Both progesterone and estradiol have also been demonstrated to be produced by fetal sheep ovaries (Lun et al. 1998). In a recent study in which cultured fetal bovine ovaries were shown to produce estrogen and progesterone (Yang and Fortune 2008), it was shown that steroid production decreased with increasing fetal age, in concordance with the results of the current study in which ovarian steroid concentration decreased with age. Estradiol production by cultured ovaries was also seen to drop after about 70 days gestation in previous research (Dominguez et al. 1988, Shemesh et al. 1978). Tanaka et al. (2001) reported no change in total fetal ovary estradiol content from 60-100 days of gestation, during which time the ovarian mass increases considerably (Tanaka et al. 2001). Therefore, estradiol concentrations in these ovaries must decrease. In fetal sheep ovaries both P450scc (CYP11A1) and 3βHSD have been shown to be present at the time of morphological sexual differentiation (Quirke et al. 2001). In the current study, microarray analysis and real-time PCR show that CYP11A1, the enzyme that converts cholesterol to pregnenolone is present in fetal ovaries.
A limitation of the microarray analyses of the current study is that it was impractical to collect replicate RNA samples from ovaries of a particular developmental time point. This problem was inherent since the ovaries were from abbitoirs. This increases the chances of receiving false positive and false negative presence/absence determinations from the microarray analyses. To address this issue three genes were selected (i.e. 3βHSD, CYP11A1 and PGRMC1) to run real-time PCR on a larger number of ovaries and determine whether these genes were expressed. Results confirmed that PGRMC1 and CYP11A1 were expressed in fetal ovaries, although expression levels of CYP11A1 were variable and did not correlate well with fetal ovary age. The levels of 3βHSD detected were negligible. These results do not match exactly with the results of microarray analysis. This may reflect on the quality of the RNA samples, or reflect the robustness of microarray analysis, where each gene is evaluated with several probes, compared to real-time PCR, where each gene is evaluated with only one product. Although care must be taken in interpreting these results, as mentioned above previous research has demonstrated that fetal ruminant ovaries produce sex steroids (Dominguez et al. 1988, Lun et al. 1998, Shemesh et al. 1978, Shore and Shemesh 1981, Tanaka et al. 2001, Yang and Fortune 2008). This previous literature in combination with supporting data from the current experiment suggest local ovarian steroid production may be regulating primordial follicle assembly. Results indicate that progesterone receptor PGRMC1 is continuously expressed across the ovary development period of this study. A previous study confirmed the expression of PGRMC1 in primordial follicle assembly as well (Kezele and Skinner 2003). This raises the possibility that low progesterone levels may support continued follicle development, even while high levels are inhibitory to follicle assembly.
Fetal ovary estrogen concentrations also decreased with fetal age in the current study. Similar to progesterone, this occurs over a period of gestation during which both fetal and maternal blood serum estrogen concentrations have been shown to be either unchanging or gradually increasing (Challis et al. 1974, Eley et al. 1979, Robertson and King 1979). This observation suggests that bovine primordial follicle assembly may be regulated by progesterone and estrogen. It is also possible that the progesterone produced in fetal ovaries acts only as a precursor for estrogen synthesis. In contrast to the rat (Kezele and Skinner 2003), mouse follicle assembly appears to be regulated by both progesterone and estrogen (Chen et al. 2007), indicating that there may be species differences in control of follicle assembly. Previous research has indicated that the presence of estrogen, and perhaps progesterone, are required for follicle assembly to occur normally in some species (Anand Kumar 1968, Wang and Roy 2007, Wang et al. 2008, Zachos et al. 2002). These findings are not necessarily in conflict with the results of the current study. It is possible that high intro-ovarian steroid concentrations inhibit follicle assembly, while lower basal concentrations support the assembly process. Further research is needed to clarify this issue. Wang and Roy (2007) found in the hamster that higher estrogen doses did not promote follicle assembly as did lower doses. It is also possible that different species respond differently to estrogen signaling.
In order to definitively determine if a high level of progesterone can affect follicle assembly in fetal bovine ovaries, organ culture experiments were performed. It was found that the percentage of oocytes assembled into follicles was significantly decreased in progesterone-treated ovary cultures compared to untreated control cortex cultures from the same ovary (Figure 4). This supports the hypothesis that progesterone at this dose inhibits primordial follicle assembly in bovine fetal ovaries (Figure 5). The dose of progesterone used in these organ culture experiments was 314 ng/mL (10−bM). This is about twice the concentration seen in the youngest ovary measured in these experiments, but is less than the progesterone concentration found in follicular fluid. The effect of low doses of progesterone on bovine follicle assembly remains to be determined. The results of these studies also suggest that the ability to respond to progesterone may still exist in fetal ovaries after ovarian progesterone levels drop. Progesterone has also been found to inhibit follicle assembly in organ cultures of mouse and rat ovaries (Chen et al. 2007, Kezele and Skinner 2003). These observations suggest that progesterone inhibition may be a widely used mechanism of regulating follicle assembly across mammalian species.
The premature assembly and development of primordial follicles during gestation could cause an abnormal endocrine state and/or the entry of oocytes into meiosis. Therefore, a mechanism to inhibit premature primordial follicle development appears to have evolved. Since progesterone is essential for pregnancy and fetal development, high levels of progesterone provide an ideal inhibitory factor for species where the completion of follicle assembly occurs after birth. The authors speculate that this endocrine model of follicle assembly is evolutionarily conserved. For those species that evolved to complete follicle assembly before birth (i.e. cattle and humans), there also evolved an independent local regulation of fetal ovarian steroids. As fetal and ovarian development proceeds, primordial follicle assembly is initiated and linked to the decline in steroids in the ovary itself. This occurs in larger mammals (i.e. cattle and humans) during mid-gestation.
In summary, fetal ovarian progesterone and estrogen concentrations decreased during mid-gestation, at the same time as follicle assembly was initiated in the bovine ovary. This occurred at a time during which maternal blood progesterone and estrogen levels were stable. The mRNA transcripts for certain progesterone and estrogen receptors are present in fetal ovaries, as are the transcripts for enzymes to synthesize progesterone. In cultured fetal bovine ovaries, treatment with high levels of progesterone significantly decreased follicle assembly. These data indicate that progesterone and possibly estrogen are important regulators of ovarian follicle assembly. Understanding these mechanisms in cattle could provide insight into how these processes are regulated in other species in which follicle assembly occurs prior to birth, including humans.
ACKNOWLEDGEMENTS
We acknowledge the expert technical assistance of Dr. Marina Savenkova in the Bioinformatics Core Laboratory and Ms. Nada Cummings in the Animal Reproduction Core Laboratory, Center for Reproductive Biology, Washington State University. We also thank Ms. Heather Johnson for assistance in preparing the manuscript. This research was supported by a grant from the National Institutes of Health, NICHD to Michael K. Skinner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abir R, Fisch B, Jin S, Barnnet M, Ben-Haroush A, Felz C, Kessler-Icekson G, Feldberg D, Nitke S, Ao A. Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol Hum Reprod. 2005;11:229–236. doi: 10.1093/molehr/gah164. [DOI] [PubMed] [Google Scholar]
- Anand Kumar T. The effects of gonadal hormones on primordial germ cell population in the slender loris. J. Reprod. Fertil. 1968;16:322–323. [Google Scholar]
- Baker TG. A Quantitative and Cytological Study of Germ Cells in Human Ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298:132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Challis JR, Kim CK, Naftolin F, Judd HL, Yen SS, Benirschke K. The concentrations of androgens, oestrogens, progesterone and luteinizing hormone in the serum of foetal calves throughout the course of gestations. J Endocrinol. 1974;60:107–115. doi: 10.1677/joe.0.0600107. [DOI] [PubMed] [Google Scholar]
- Chen QX, Nancarrow CD, Sweet F. Isolation of 3 beta,20 alpha-hydroxysteroid oxidoreductase from sheep fetal blood. Steroids. 1987;49:477–496. doi: 10.1016/0039-128x(87)90089-4. [DOI] [PubMed] [Google Scholar]
- Chen QX, Rosik LO, Nancarrow CD, Sweet F. Fetal lamb 3 beta, 20 alpha-hydroxysteroid oxidoreductase: dual activity at the same active site examined by affinity labeling with 16 alpha-(bromo[2'-14C]acetoxy)progesterone. Biochemistry. 1989;28:8856–8863. doi: 10.1021/bi00448a026. [DOI] [PubMed] [Google Scholar]
- Chen Y, Breen K, Pepling M. Estrogen Can Signal through Multiple Pathways to Regulate Oocyte Cyst Breakdown and Primordial Follicle Assembly in the Neonatal Mouse Ovary. J Endocrinol. 2009 doi: 10.1677/JOE-09-0109. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- de Villiers DJ, Skinner JD, Hall-Martin AJ. Circulating progesterone concentrations and ovarian functional anatomy in the African elephant (Loxodonta africana). J Reprod Fertil. 1989;86:195–201. doi: 10.1530/jrf.0.0860195. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Hirshfield AN, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology. 2001;142:2078–2086. doi: 10.1210/endo.142.5.8126. [DOI] [PubMed] [Google Scholar]
- Dominguez MM, Liptrap RM, Basrur PK. Steroidogenesis in fetal bovine gonads. Can J Vet Res. 1988;52:401–406. [PMC free article] [PubMed] [Google Scholar]
- Donaldson A, Nicolini U, Symes EK, Rodeck CH, Tannirandorn Y. Changes in concentrations of cortisol, dehydroepiandrosterone sulphate and progesterone in fetal and maternal serum during pregnancy. Clin Endocrinol (Oxf) 1991;35:447–451. doi: 10.1111/j.1365-2265.1991.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Ducsay CA, Stanczyk FZ, Novy MJ. Maternal and fetal production rates of progesterone in rhesus macaques: placental transfer and conversion to cortisol. Endocrinology. 1985;117:1253–1258. doi: 10.1210/endo-117-3-1253. [DOI] [PubMed] [Google Scholar]
- Echternkamp SE, Vonnahme KA, Green JA, Ford SP. Increased vascular endothelial growth factor and pregnancy-associated glycoproteins, but not insulin-like growth factor-I, in maternal blood of cows gestating twin fetuses. J Anim Sci. 2006;84:2057–2064. doi: 10.2527/jas.2005-714. [DOI] [PubMed] [Google Scholar]
- Eley RM, Thatcher WW, Bazer FW. Hormonal and physical changes associated with bovine conceptus development. J Reprod Fertil. 1979;55:181–190. doi: 10.1530/jrf.0.0550181. [DOI] [PubMed] [Google Scholar]
- Erickson BH. Development and radio-response of the prenatal bovine ovary. Reprod. Fert. 1966;10:97–105. [Google Scholar]
- Gaverick A, Juengel J, Smith P, D H, N B, J S, G P, M S, McNatty K. mRNA and protein expression of P450 aromatase (AROM) and estrogen receptors (ER) α and β during early development of bovine fetal ovaries. Biology of Reproduction. 2005:184. [Google Scholar]
- George F, Wilson J. The Physiology of Reproduction. 2nd edn. Raven Press; New York: 1994. Sex Determination and Differentiation in Neill EKJ, ed. [Google Scholar]
- Gondos B. Germ cell degeneration and intercellular bridges in the human fetal ovary. Z Zellforsch Mikrosk Anat. 1973;138:23–30. doi: 10.1007/BF00307075. [DOI] [PubMed] [Google Scholar]
- Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132:817–828. doi: 10.1242/dev.01601. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Relationship between the supply of primordial follicles and the onset of follicular growth in rats. Biol Reprod. 1994;50:421–428. doi: 10.1095/biolreprod50.2.421. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Heath DA, Quirke LD, McNatty KP. Oestrogen receptor alpha and beta, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep. Reproduction. 2006;131:81–92. doi: 10.1530/rep.1.00704. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Sawyer HR, Smith PR, Quirke LD, Heath DA, Lun S, Wakefield SJ, McNatty KP. Origins of follicular cells and ontogeny of steroidogenesis in ovine fetal ovaries. Mol Cell Endocrinol. 2002;191:1–10. doi: 10.1016/s0303-7207(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Kezele P, Nilsson E, Skinner MK. Cell-cell interactions in primordial follicle assembly and development. Front Biosci. 2002;7:d1990–1996. doi: 10.2741/kezele. [DOI] [PubMed] [Google Scholar]
- Kurilo LF, Tepliakova NP, Lavrikova GV. [Development of ovaries in bovine fetuses]. Ontogenez. 1987;18:500–506. [PubMed] [Google Scholar]
- Lun S, Smith P, Lundy T, O'Connell A, Hudson N, McNatty KP. Steroid contents of and steroidogenesis in vitro by the developing gonad and mesonephros around sexual differentiation in fetal sheep. J Reprod Fertil. 1998;114:131–139. doi: 10.1530/jrf.0.1140131. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr., Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz JL, Balchak SK, Morrison LJ. The involvement of tumor necrosis factor-alpha (TNF) as an intraovarian regulator of oocyte apoptosis in the neonatal rat. Front Biosci. 2002;7:d1997–2005. doi: 10.2741/A894. [DOI] [PubMed] [Google Scholar]
- Marion GB, Gier HT. Ovarian and uterine embryogenesis and morphology of the non-pregnant female mammal. J Anim Sci. 1971;32(Suppl 1):24–47. [PubMed] [Google Scholar]
- Mauleon P. The establishment of the primordial follicle reserve in the sheep embryo studied by labelling of oocytes with tritiated thymidine. Eur J Obstet Gynecol Reprod Biol. 1974;4:S133–139. [PubMed] [Google Scholar]
- Mbassa GK. Studies on the ovarian development in zebu cattle (Bos indicus). Anat Histol Embryol. 1989;18:143–149. doi: 10.1111/j.1439-0264.1989.tb00590.x. [DOI] [PubMed] [Google Scholar]
- McMullen ML, Cho BN, Yates CJ, Mayo KE. Gonadal pathologies in transgenic mice expressing the rat inhibin alpha-subunit. Endocrinology. 2001;142:5005–5014. doi: 10.1210/endo.142.11.8472. [DOI] [PubMed] [Google Scholar]
- Morrison LJ, Marcinkiewicz JL. Tumor necrosis factor alpha enhances oocyte/follicle apoptosis in the neonatal rat ovary. Biol Reprod. 2002;66:450–457. doi: 10.1095/biolreprod66.2.450. [DOI] [PubMed] [Google Scholar]
- Nancarrow CD, Connell PJ, Stevens D. Relationship between glucose and 20 alpha-hydroxysteroid dehydrogenase in fetal sheep erythrocytes. J Endocrinol. 1984;101:131–139. doi: 10.1677/joe.0.1010131. [DOI] [PubMed] [Google Scholar]
- Nicol MB, Hirst JJ, Walker D, Thorburn GD. Effect of alteration of maternal plasma progesterone concentrations on fetal behavioural state during late gestation. J Endocrinol. 1997;152:379–386. doi: 10.1677/joe.0.1520379. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006;131:1007–1015. doi: 10.1530/rep.1.00978. [DOI] [PubMed] [Google Scholar]
- Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–632. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Peters H, McNatty KP. A Correlation of Structure and Function in Mammals. University of California Press; Berkeley and Los Angeles: 1980. The Ovary. [Google Scholar]
- Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, McNatty KP. Ontogeny of steroidogenesis in the fetal sheep gonad. Biol Reprod. 2001;65:216–228. doi: 10.1095/biolreprod65.1.216. [DOI] [PubMed] [Google Scholar]
- Rexroad CE, Cassida LE, Tyler WJ. Crown-Rump Length of Fetuses in Purebred Holstein-Friesian Cows. J Dairy Sci. 1974;57:346–347. [Google Scholar]
- Robertson HA, King GJ. Conjugated and unconjugated oestrogens in fetal and maternal fluids of the cow throughout pregnancy. J Reprod Fertil. 1979;55:463–470. doi: 10.1530/jrf.0.0550463. [DOI] [PubMed] [Google Scholar]
- Russe I. Oogenesis in cattle and sheep. Bibl Anat. 1983;24:77–92. [PubMed] [Google Scholar]
- Sakai T. Studies on the development of the embryonic ovary in swine, cattle and horse. Jap. J. Vet. Res. 1955;3:183–194. [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Sforza C, Forabosco A. A morphometric approach to the study of human ovarian organogenesis. Ital J Anat Embryol. 1998;103:51–62. [PubMed] [Google Scholar]
- Shemesh M, Allenberg M, Milaguir F, Ayalon N, Hansel W. Hormone secretion by cultured bovine pre- and postimplantation gonads. Biol. Reprod. 1978;19:761–767. doi: 10.1095/biolreprod19.4.761. [DOI] [PubMed] [Google Scholar]
- Shore L, Shemesh M. Altered steroidogenesis by the fetal bovine freemartin ovary. J Reprod Fertil. 1981;63:309–314. doi: 10.1530/jrf.0.0630309. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakada K, Moriyoshi M, Sawamukai Y. Appearance and number of follicles and change in the concentration of serum FSH in female bovine fetuses. Reproduction. 2001;121:777–782. [PubMed] [Google Scholar]
- Thau R, Lanman JT, Brinson A. Declining plasma progesterone concentration with advancing gestation in blood from umbilical and uterine veins and fetal heart in monkeys. Biol Reprod. 1976;14:507–509. doi: 10.1095/biolreprod14.4.507. [DOI] [PubMed] [Google Scholar]
- Tilly JL. Apoptosis and ovarian function. Rev Reprod. 1996;1:162–172. doi: 10.1530/ror.0.0010162. [DOI] [PubMed] [Google Scholar]
- Vahdat F, Seguin BE, Whitmore HL, Johnston SD. Role of blood cells in degradation of progesterone in bovine blood. Am J Vet Res. 1984;45:240–243. [PubMed] [Google Scholar]
- van Wagener G, Simpson M. Embryology of the Ovary and Testis HomoSapiens and Macaca mulatta. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Vigier B, Prepin J, Jost A. [Chronology of development of the genital tract of the calf fetus]. Arch Anat Microsc Morphol Exp. 1976;65:77–101. [PubMed] [Google Scholar]
- Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biol Reprod. 1996;55:942–948. doi: 10.1095/biolreprod55.5.942. [DOI] [PubMed] [Google Scholar]
- Wang C, Roy SK. Development of primordial follicles in the hamster: role of estradiol-17beta. Endocrinology. 2007;148:1707–1716. doi: 10.1210/en.2006-1193. [DOI] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, Roy SK. G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology. 2008;149:4452–4461. doi: 10.1210/en.2008-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during mid-gestation and is associated with meiotic arrest of oocytes. Biol Reprod. 2008;78:1153–1161. doi: 10.1095/biolreprod.107.066688. [DOI] [PubMed] [Google Scholar]
- Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Developmental regulation of baboon fetal ovarian maturation by estrogen. Biol Reprod. 2002;67:1148–1156. doi: 10.1095/biolreprod67.4.1148. [DOI] [PubMed] [Google Scholar]