Abstract
Patients with diabetes are at increased risk for developing corneal disorders, termed diabetic keratopathy. Treatments for diabetic keratopathy are limited. Preclinical studies have demonstrated that topical administration of either naltrexone (NTX) or insulin (INS) accelerates corneal reepithelialization in Type I diabetic rats. This study determined whether the combination of NTX and INS would have additive effect(s) on the reepithelialization of corneal abrasions in diabetic male Sprague-Dawley rats beyond either agent alone. Type 1 diabetes (DB) (glucose levels >400 mg/dl) was induced with streptozotocin; glycemic levels were not controlled with INS. Eight weeks after induction of diabetes, a 5 mm diameter circular abrasion was created in the center of the cornea in one eye of each rat. Eye drops (0.05 ml) of INS [1U (~ 6 nM)] and NTX (10−5 M) in Vigamox were administered separately 4 times daily for 7 days (NTX/INS); DB control rats received drops of sterile vehicle (DB SV) 4 times daily. Two other groups of rats were given only NTX (DB NTX) or only INS (DB INS). Reepithelialization was monitored by fluorescein staining, and images were recorded with a CCD camera. Areal measurements were made using Optimas software, and the percentage of epithelial defect over a 40 hr period was calculated. Twenty-four hr after formation of an abrasion (~21.7±0.4 mm2 area), corneal wounds in DB rats treated with NTX, INS, or NTX/INS were significantly smaller (p<0.001) than those in DB SV rats, with reductions in the size of the defect ranging from 24–84%. DB rats treated with NTX or INS alone also were observed to have reductions in wound size of 22 and 29%, respectively, from subjects in the DB SV group at 16 h. At 16 h both the DB NTX and DB INS groups had defects that were 13 and 27%, respectively, smaller than those for the DB NTX/INS group, and at 40 h the DB INS animals had 78% smaller corneal wounds than in the DB NTX/INS group. Therefore, the DB NTX/INS group exhibited some slight delays in wound repair compared to the DB NTX and DB INS groups. Topical application of NTX and/or INS to the cornea had no effect on non-invasive measures that included ocular morphology, intraocular pressure, or corneal thickness. These data demonstrate that although NTX or INS accelerate wound healing, concomitant application of NTX and INS to corneal abrasions in diabetic animals does not have an additive effect on reepithelialization.
Keywords: Naltrexone, Insulin, Diabetes, Cornea, Wound healing, Opioid antagonist
1. Introduction
Diabetic keratopathy has been estimated to occur in 46–64% of diabetic patients during the course of their disease (Schultz et al., 1981). With an estimated 1 million individuals having type 1 diabetes (Diabetes Control and Complications Trial Research Group), 500,000 or more patients may experience diabetic keratopathy at some time in the course of their disease. Moreover, corneal transplantation, removal of the corneal epithelium during vitrectomy in the course of treatment for diabetic retinopathy, and procedures such as laser photocoagulation, cataract surgery, and refractive surgery are risk factors for abnormal corneal epithelial healing in diabetic patients (Kaji, 2005; Schultz et al., 1981; Sanchez-Thorin, 1998; Cisarik-Fredenburg, 2001). Some corneal disorders associated with diabetic keratopathy include nonhealing epithelial defects, infectious corneal ulcers, and secondary scarring, which may result in a decreased or even permanent loss of vision (Kaji, 2005; Schultz et al., 1981; Sanchez-Thorin, 1998; Cisarik-Fredenburg, 2001). Unfortunately, these diabetes-related epithelial defects may be resistant to conventional treatment regimens (e.g., bandage contact lens, lubricants and antibiotics) (Cavallerano, 1992; Kabosova et al., 2003). Moreover, none of these therapies address the fundamental biological processes in corneal healing that are perturbed secondary to the pathophysiology of diabetes (Kabosova et al., 2003). Recently, topical administration of autologous serum has been investigated as therapy for corneal epithelial abrasions in diabetic patients following vitrectomy (Schulze et al., 2006).
Naltrexone hydrochloride (NTX), an opioid antagonist, when applied topically in rabbits (Zagon et al., 1998a), systemically in normal and diabetic rats (Zagon et al., 1998b, 2002a), or included in organ cultures of human (Zagon et al., 2000) or rabbit (Zagon et al., 1998a) corneas, has been demonstrated to markedly accelerate epithelial DNA synthesis and corneal reepithelialization. The mechanism of action of NTX is by blockade of the opioid growth factor (OGF) interaction with the OGF receptor (OGFr) (Zagon et al., 2002a,b). The OGF-OGFr axis serves as a tonically repressive pathway that regulates cell proliferation through cyclin-dependent inhibitory kinases and nucleocytoplasmic transport (Cheng et al., 2009). The repercussions of NTX application in the homeostatic (i.e., normal, untreated, unwounded) cornea include a decreased epithelial transit time from the basal layer to the suprabasal layers, and increases in linear thickness of the epithelium, basal cell proliferation, and packing density of suprabasal cells secondary to a decrease in cell diameter (Zagon et al., 2006a).
Topically applied NTX for 7 days (4 times per day) has been shown to an efficacious and safe treatment for abraded corneas of animals that were normal (non-diabetic), diabetic and hyperglycemic, and diabetic but normoglycemic through the use of insulin (Zagon et al., 2006b, Klocek et al., 2007). These findings were determined using a variety of non-invasive and invasive measures, and the results suggest that NTX is safe for topical use across a wide range of biological conditions.
Topically applied insulin (1 U/0.05 ml) for 7 days (4 times a day) also has been shown to be efficacious in facilitating healing abraded corneas of hyperglycemic diabetic rats (Zagon et al., 2007). Topical insulin (INS) did not alter corneal thickness, intraocular pressure, or serum glucose. Diabetic rats had markedly decreased corneal sensitivity compared to non-diabetic rats, but rats treated with topical INS had sensitivity readings comparable to that of non-diabetic rats.
The present study was designed to examine the efficacy of concomitant administration of NTX and insulin in facilitating corneal wound healing when applied topically. Streptozotocin (STZ)-treated rats were used as a model system for type 1 diabetes. In a standard, reproducible manner, the ocular surface epithelium of the cornea was abraded from inner limbal margin to inner limbal margin, and rats were exposed to NTX, INS, NTX/INS, or vehicle 4 times per day for 7 days. The experiments assessed the size of the defect to examine whether NTX/INS can further accelerate reepithelialization. The outcome measures of the experiments included the size of the defect, and rate of repair as well as a wide range of non-invasive measures (intraocular pressure, corneal thickness, and corneal topography) and invasive parameters (histopathology, apoptosis/necrosis, and DNA synthesis in the peripheral cornea, limbus, and conjunctiva). Our results demonstrate that topical application of both NTX and INS does not significantly increase corneal reepithelialization beyond the efficacious properties of either NTX or INS alone.
2. Materials and methods
2.1 Animals and induction of diabetes
Six-week old male Sprague-Dawley rats weighing ~ 135 g were purchased from Charles River Laboratories (Wilmington, MA) and housed under standard laboratory conditions; water and food were continuously available. All investigations conformed to the regulations of the Association for Research in Vision and Ophthalmology, National Institutes of Health, and the Institutional Animal Care and Use guidelines of the Department of Comparative Medicine of The Pennsylvania State University.
Type 1 diabetes was induced by the method of Havel et al. (2000), which has been shown to avoid renal failure or loss of animals from hypoglycemia secondary to insulin release associated with β-cell destruction (Ahren et al., 1995). An intraperitoneal (i.p.) injection of 40 mg/kg STZ (Sigma, St. Louis, MO) in ice-cold 0.5 M citrate buffer (pH 4.5) was administered. A second dose of STZ (40 mg/kg) was injected 24 h later. This regimen produced insulin-deficient diabetes in 100% of the animals within 3 to 5 days; these animals were termed DB rats. Another group of animals received citrate buffer only, and were considered Normal.
Blood glucose levels were monitored from the tail vein using a True Track Smart System glucometer (Home Diagnostics, Inc., Ft. Lauderdale, FL) immediately prior to receiving STZ, and at 1 and 8 weeks after injection of STZ. Glucose levels of ≥ 400 mg/dl were considered to be the minimum blood glucose level compatible with a stable non-toxic diabetic state (Nakamura et al., 1997).
2.2. Corneal abrasions
The procedures for wounding and monitoring repair followed those reported earlier (Zagon et al., 2002a; Klocek et al., 2007). In brief, animals were anesthetized with a mixture of ketamine (70 mg/kg), xylazine (7 mg/kg), and acepromazine (10 mg/kg); Proparacaine Hydrochloride Ophthalmic Solution 0.5% (Baush & Lomb Inc., Tampa, FL) was administered topically to the eye immediately prior to surgery. Eyes were examined under a dissecting microscope (SZ-ET; Olympus, Tokyo, Japan), and a 5 mm diameter circle in the center of the cornea was marked with a disposable dermatological skin punch (Acuderm, Ft. Lauderdale, FL). The encircled corneal epithelium was removed with a No. 15 Bard-Parker scalpel blade. Care was taken not to injure the underlying corneal tissue. Wounds were created between 0730 and 0830 or 1630 and 1730. Any animal that experienced bleeding, inflammation, or infection was not included in the study. The right eye was abraded on the 9th week following injection of STZ.
2.3. Photography
For photography of corneal abrasions, animals were anesthetized in a Plexiglas chamber attached to an isoflurane vaporizer, and the residual epithelial defect was stained with topical fluorescein (Fluor-I-Strip; Ayerst Laboratories, Philadelphia, PA). Rat eyes were viewed using an Olympus dissecting microscope with a tungsten light source and a gelatin Wratten no. 47 filter, and photographed with a Sony CCD (charged couple device) camera at ×1.5 magnification. Photographs of rat eyes were taken immediately after abrasions (0 h) and 16, 24, 32, and 40 h later. No animal was photographed at intervals <8 h in order to prevent disruption of the epithelial healing process. The area of defect was determined using Optimas software and was calculated as the percentage of the original residual epithelial defect. Rates of healing (mm2/h) were calculated for only the first 24 h, because healing of the cornea does not occur in a linear manner (Crosson et al., 1986).
2.4. Topical administration of naltrexone and insulin
NTX (10−5 M, Sigma, Indianapolis, IN) and bovine insulin (1 Unit, Sigma-Aldrich) were prepared in Vigamox (moxifloxacin hydrochloride ophthalmic solution, Alcon, Inc. Ft. Worth, TX); these dosages were selected based on data from earlier reports (Zagon et al., 2007; Klocek et al., 2007). Compounds were given separately as single drop(s) (0.05 ml) to the central cornea of the injured eye, with the lower eyelid held away from the eye to avoid overflow. Eyedrops (Vigamox applicator) were delivered daily to unanesthetized animals at 0800, 1100, 1400, and 1800 h for 7 consecutive days. Diabetic rats were randomly assigned to receive NTX, INS, NTX/INS, or vehicle, thus establishing the following groups of Type 1 diabetic rats: DB NTX, DB INS, DB NTX/INS and DB-SV, respectively. Non-diabetic animals received sterile vehicle and comprised the Normal SV group.
2.5. Non-invasive measurements of the cornea
Three measures of ocular health were assessed on all rats for both eyes 3–4 days prior to drug administration and for the injured right eye 2 weeks post-wounding. These measures included general overall morphology and pathology with a hand-held slit lamp (Zeiss HSO 10 Hand Slit Lamp, Dublin, CA), corneal thickness with a pachymeter (DGH 550 Pachette 2, Pro Forma, Exton, PA), and intraocular pressure with a tonopen (Tono-Pen XL Tonometer, Medtronic, Jacksonville, FL) as described previously (Zagon et al., 2006b, 2007; Klocek et al., 2007). During intraocular pressure measurements, the cornea was locally anesthetized with Proparacaine Hydrochloride Ophthalmic Solution 0.5%. The slit lamp, and pachymeter were tested on rats anesthetized with a mixture of ketamine (70 mg/kg), xylazine (7 mg/kg), and acepromazine (10 mg/kg); measures with the slit lamp were conducted before and after dilation with Phenylephrine Hydrochloride Ophthalmic Solution 2.5% (Bausch & Lomb Inc., Tampa, FL) and Tropicamide Ophthalmic Solution (Falcon Pharmaceuticals Ltd., Forth Worth, TX). Intraocular pressure was obtained as 4 readings/eye, and corneal thickness was recorded as 20 readings/eye. All non-invasive measurements were conducted by observers masked as to treatment group
2.6. Histology and morphometric analysis
Animals were euthanized at either 48 h or 2 weeks post-wounding with an intraperitoneal injection of sodium pentobarbital (>100 mg/kg), decapitated, and the eyes proptosed and enucleated. Eyes were fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin, and sections (6 μm) of corneas were stained with hematoxylin and eosin and evaluated by light microscopy for cell death and structure of the basement membrane, epithelium, and stroma. Using at least 2 sections per rat cornea, and 3 animals per experimental group, assessment of the number of cell layers comprising the epithelium of the peripheral cornea, and the linear thickness of the corneal epithelium and stroma in the region of the peripheral cornea, were assessed in 4–6 independent fields per section.
2.7. DNA synthesis
Using histological preparations (see above), DNA synthesis in the corneal epithelium was assessed by BrdU labeling with anti-BrdU antibodies (Invitrogen, Eugene, OR), and counterstained with hematoxylin (Zagon et al., 2006b, 2007; Klocek et al., 2007). BrdU (100 mg/kg) was injected at 3 and 6 h prior to euthanasia. Basal and suprabasal cells of the peripheral corneal epithelium, limbus, and conjunctiva were evaluated from 2 sections per rat cornea, and 3 rats/treatment group 48 h post-treatment with an ocular grid (50 × 290 μm) at 400 magnification; 6 grids/section of the peripheral cornea and 4 grids of the limbus and the conjunctiva were utilized. The numbers of labeled cells were counted in basal and suprabasal layers of the cornea, limbus, and conjunctiva.
A labeling index was computed as the number of labeled cells divided by the total number of cells with nuclei × 100.
2.8. Apoptosis and necrosis
Using histological preparations, apoptosis was assessed using a DermaTACS In Situ Apoptosis Detect Kit (Trevigen, Gaithersburg, MD), whereas necrosis was determined in hematoxylin-eosin stained sections. Apoptotic cells were counted in the peripheral cornea as described above for DNA synthesis.
2.9. Data analysis
For body weights and glucose measurements, Student two-tailed t-tests were utilized. Data from apoptosis/necrosis, DNA synthesis, intraocular pressure, corneal thickness, and area of defect were analyzed with a one-way ANOVA and Newman-Keuls tests.
3. Results
3.1. Induction of diabetes
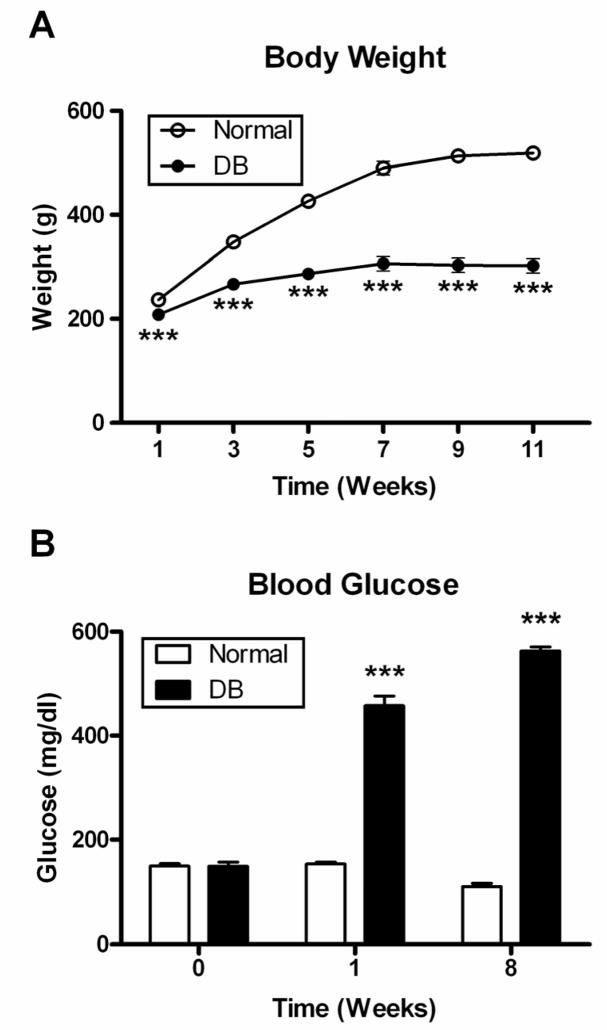
At least 12 rats were established for each treatment group. The animals weighed 164 ± 4 g at the time of STZ injections. Normal subjects (n = 12) gained ~ 350 g over the course of 11 weeks (Fig. 1A), and rats in the DB group (n = 50) weighed 11 to 41% less than Normal animals throughout the course of the study.
Fig. 1.
Body weights (A) and blood glucose levels (B) of rats in DB and Normal groups. Blood glucose levels were determined prior to STZ injection (0 week), as well as 1 and 8 weeks after administration of STZ. Values are means ± SEM for at least 12 animals/group at each time point. Significantly different from Normal rats at p<0.001 (***).
Baseline glucose readings were 148 ± 3 mg/dl for all rats, and these values were consistent in Normal animals for the entire study. Rats receiving STZ became hyperglycemic within 3–5 days, and had glucose levels >400 mg/dl (Fig. 1B). Serum glucose levels remained the same for each group throughout experimentation.
3.2. Corneal reepithelialization
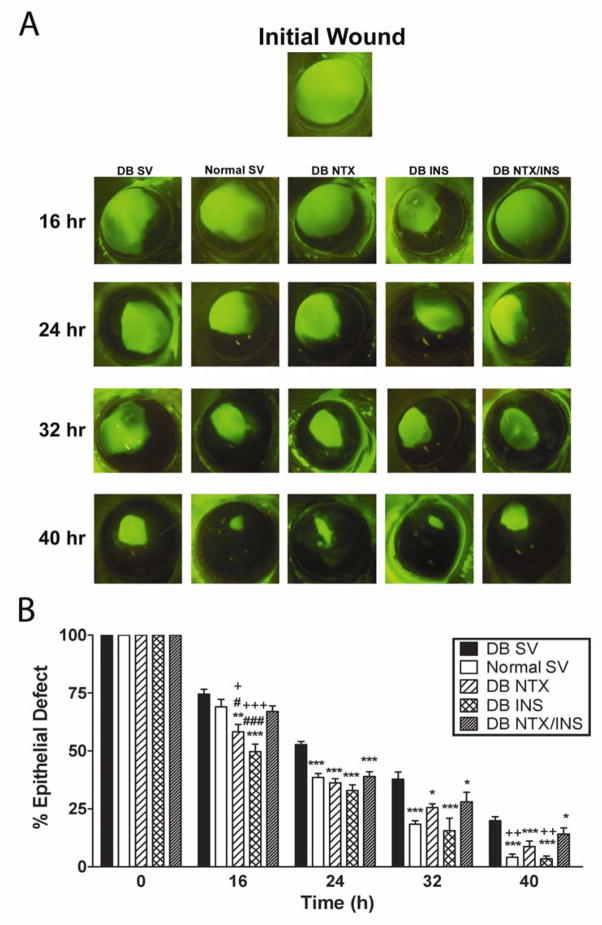
The 5-mm trephine demarcated the entire corneal region of the rat eye but did not encroach on the limbus or conjunctiva. The initial area of the abrasion ranged from 17.8–24.6 mm2, and corresponded to corneal injuries of 4.7 to 5.6-mm diameter. The size of initial abrasions was comparable between groups.
DB SV rats had corneal abrasions that reepithelialized significantly slower than Normal SV animals at 24, 32, and 40 h (Fig. 2), with DB SV rats exhibiting defects that were 27 to 79% greater than in Normal rats. DB NTX and DB INS rats had corneal abrasions that ranged from 22 to 84% smaller than DB SV animals at 16, 24, 32, and 40 h. The DB NTX/INS treated animals exhibited residual defects that were 24–27% smaller than those for the DB SV group at 24, 32, and 40 h. At 24 and 32 h no statistical differences in residual epithelial defects were noted between DB NTX, DB INS, or DB NTX/INS treatment groups. DB rats treated with NTX or INS alone also were observed to have reductions in wound size of 22 and 29%, respectively, from subjects in the DB SV group at 16 h. However, at 16 h both the DB NTX and DB INS groups had defects that were 13% and 27%, respectively, smaller than those for the DB NTX/INS group, and at 40 h the DB INS animals had 78% smaller corneal wounds than in the DB NTX/INS group.
Fig. 2.
A. Photographs of rat eyes stained with fluorescein immediately after initial wound or 16, 24, 32, and 40 hr later. DB rats received either sterile vehicle (SV), NTX, INS, or NTX/INS; photographs of eyes from Normal rats receiving SV are included for comparison. Images of the fluorescein stained corneas were captured with a Sony CCD camera (Olympus) and areas analyzed by Optimas software. B. Histograms of residual epithelial defect (%) were followed for 40 hr. Residual epithelial defects are presented as percentage of the original wound. Data are expressed as means ± SEM. Significantly different from DB SV rats at p<0.05 (*), p<0.01(**), and p<0.001(***). Significantly different from DB NTX/INS rats at p<0.05(+), p<0.01(++), and p<0.001 (+++). Significantly different from Normal SV rats at p<0.05 (#), p<0.001 (###).
Analysis of healing rates between 0 and 24 h revealed that the DB SV group had a mean rate of reepithelialization of 0.34 ± 0.02 mm2/h compared to the Normal SV animals (0.50 ± 0.02 mm2/h) (p<0.05). DB animals given NTX, INS, or NTX/INS had healing rates that were 32–37% greater (p<0.01) than the DB SV group, and were comparable to those for Normal SV animals. No differences in healing rates were recorded between the DB NTX or the DB INS groups in comparison to the DB NTX/INS cohort. Animals were followed one week beyond cessation of NTX, INS, NTX/INS or SV treatment, and no residual or recurrent corneal epithelial defects were identified.
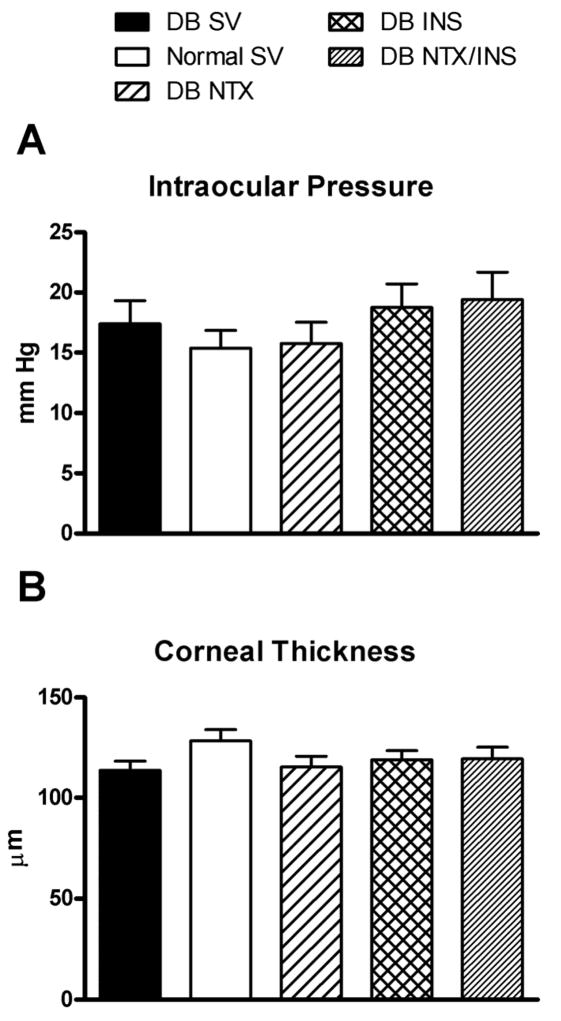
3.3. Non-invasive measurements of corneal integrity
Non-invasive measurements did not differ between right and left eyes in animals within each condition prior to wounding.
Slit lamp
Ocular morphology in the Normal SV and DB groups was generally comparable at week 8 after STZ injection. However, 100% of the animals in the DB group developed cataracts. NTX, INS, or NTX/INS treatment had no effect on ocular surface morphology, or on the incidence of cataracts for DB and Normal rats.
Tonometry
Prior to treatment, mean intraocular pressures of Normal SV (15 ± 1 mm Hg) and DB (17 ± 2 mm of Hg) rats were similar (Fig. 3A). No changes in intraocular pressure measurements were found following treatment with NTX, INS, or NTX/INS.
Fig. 3.
Intraocular pressure (A) and corneal thickness (B) of rats rendered diabetic with STZ (DB), and untreated (Normal) rats receiving vehicle (SV) at 8 weeks following STZ injection. No differences between groups, with or without NTX, INS, or NTX/INS treatment were detected for intraocular pressure or corneal thickness. Values represent means ± SEM.
Pachymetry
Prior to drug treatment, data for corneal thickness of Normal SV (128 ± 5 μm) and DB (113 ± 4 μm) groups were comparable (Fig. 3B). No changes in pachymetry measurements were recorded following treatment with NTX, INS, or NTX/INS.
3.4. Histology and morphometric analysis
At 2 weeks after debridement, no differences in the morphology of the basal and suprabasal layers of the peripheral cornea, limbus, and conjunctiva, basement membrane, or stroma were observed in animals of the Normal SV and DB groups receiving vehicle, NTX, INS, or NTX/INS.
3.5. Apoptosis and necrosis
The number of apoptotic or necrotic cells in the corneas of rats in the Normal SV group (<1%) was comparable to those in the DB groups, with or without NTX, INS, or NTX/INS exposure.
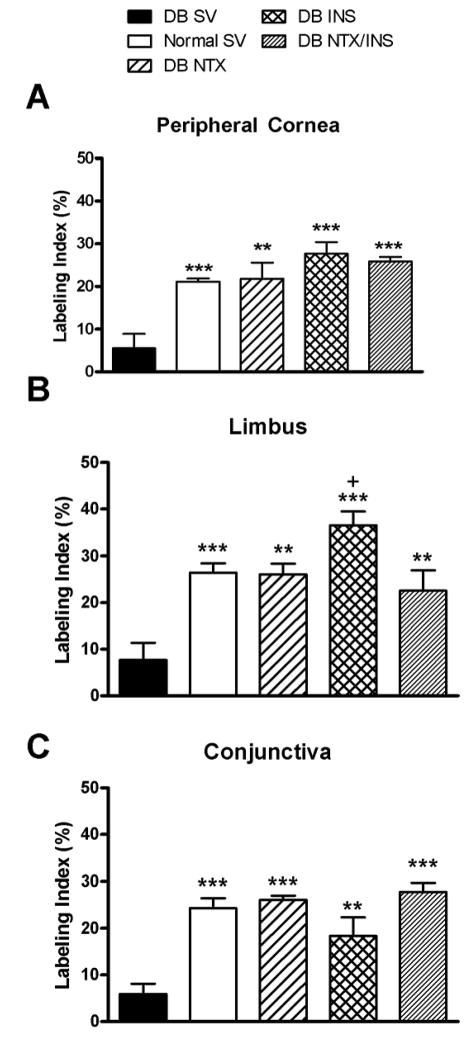
3.6. DNA synthesis
The numbers of BrdU-labeled cells located in the basal layers of the peripheral cornea, limbus, and conjunctiva of DB SV rats were decreased by 71–76% compared to Normal SV counterparts (Fig. 4). Treatment of DB animals with NTX, INS, or NTX/INS resulted in 3- to 5-fold increases in DNA synthesis in the basal layers of the peripheral cornea, limbus, and conjunctiva relative to those in DB SV rats. With the exception of DNA synthesis in the DB INS animals being 1.6-fold greater from the DB NTX/INS group, no marked differences in DNA synthesis in the peripheral cornea, limbus, or conjunctiva were noted between DB NTX, DB INS, DB NTX/INS, or Normal SV animals.
Fig. 4.
Labeling indexes in basal epithelial cells of peripheral cornea (A), limbus (B), and conjunctiva (C), as measured by BrdU incorporation 48 hrs after abrasion. Comparison of DB rats that received either sterile vehicle (SV), NTX, INS, or NTX/INS, and Normal rats receiving SV. Values represent means ± SEM. Significantly different from DB SV at p<0.01(**) and p<0.001(***). Significantly different from DB NTX/INS at p<0.05(+).
4. Discussion
This study showed that although topical treatment of NTX and INS to the abraded cornea of an uncontrolled Type 1 diabetic rat accelerates corneal reepithelialization, these two agents in combination do not have an additive or synergistic effect on corneal wound healing. In fact, at some time points wound healing in the group with a combination of NTX and INS had larger residual defects relative to those animals receiving NTX or INS alone. Corneal reepithelialization in diabetic animals with uncontrolled hyperglycemia and treated with NTX or INS was restored to that of normal levels, validating previously published reports (Zagon et al., 2002a, 2006c, 2007, Klocek et al., 2007). We now show that the delay in corneal wound healing in diabetic rats with uncontrolled hyperglycemia can be restituted to that of normal values using a combined treatment with NTX and INS. However, neither the acceleration in the rate of closure over the initial 24 h nor the size of the defect was greater in the cohort of animals receiving both NTX and INS compared to those given either agent alone. The acceleration in wound closure by NTX or INS was not toxic, with corneal thickness, intraocular pressure, and overall morphology of the corneas of all animals unchanged due to either wounding or drug treatment, supporting earlier observations (Zagon et al., 2002a, 2006c, 2007, Klocek et al., 2007). Our study demonstrates that the combination of NTX and INS also did not alter these non-invasive measures, suggesting that co-administration of these drugs is not accompanied by unwanted adverse effects. Thus, based on efficacy data, although it would seem reasonable that combining NTX and INS would be a safe and non-toxic means to enhance corneal wound healing beyond that of either agent alone, there appears to be no benefit in such a regimen.
The mechanism of the effects of NTX and INS in accelerating wound healing of the cornea is related to modulating DNA synthesis of corneal epithelial cells rather than by increasing cell survival. NTX and INS each markedly elevated DNA synthesis in the peripheral cornea, limbus and conjunctiva 48 h after abrasion. The combination of NTX and INS also elevated DNA synthesis, and did so in the peripheral cornea, limbus, and conjunctiva. However, this increase in DNA synthesis of cells in the diabetic animals receiving either both NTX and INS was comparable to that with either NTX or INS. These data are consistent with our finding herein and previously (Zagon et al., 2006c, 2007; Klocek et al., 2007) demonstrating that the combination of NTX and INS can accelerate corneal reepithelialization in diabetic rats to the same magnitude as with combined therapy with NTX and INS. It is of interest to consider that if DNA synthesis is a common pathway of NTX and INS to accelerate corneal wound healing, there appears to be a limit to the magnitude this biological process can be driven in that the effects of NTX and of INS did not have an additive effect. Alternatively, they may share a common final pathway which can be maximized by either therapy.
Given the vital role of the corneal epithelium in maintaining vision, the frequency of corneal complications related to diabetes (diabetic keratopathy), and the problems occurring in diabetic individuals postoperatively (e.g., vitrectomy, cataract extraction) in which the ocular surface epithelium is disturbed, and that conventional therapies such as artificial tears and bandage contact lenses have failed (Pflugfelder, 2006), therapeutic modalities for healing postsurgical and persistent corneal epithelial defects are needed. Application of aldose-reductase inhibitor CT112 has been found to improve corneal epithelial barrier function, but did not change corneal sensitivity, in diabetic patients (Nakahara et al., 2005). Hourly exposure to autologous serum, but not hyaluronic acid (Vislube), accelerates closure of corneal epithelial abrasions by up to 40% (Schulze et al., 2006). Some of the advantages of topical application of either NTX or insulin for diabetic keratopathy are that these drugs are readily available and cost-effective, of known chemical consistency, easy to prepare, and require only 4 times daily dosing. Moreover, these treatments are not toxic to the cornea across a 10,000-fold range, exhibit no tolerance or adverse effects, have a fast rate of absorption and effectiveness, have minimal immunological reactions, and allow topical application. The present report reveals that although both agents are to be recommended for clinical trials, there is little to be gained when used in combination.
Acknowledgments
This work was supported by National Institutes of Health Grant EY016666.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B, Stern JS, Gingerich RL, Curry DL, Havel PJ. Glucoagon secretory responses to hypoglycemia, adrenaline, and carbachol in streptozotocin diabetic rats. Acta Physiol Scand. 1995;155:215–221. doi: 10.1111/j.1748-1716.1995.tb09966.x. [DOI] [PubMed] [Google Scholar]
- Cavallerano J. Ocular manifestations of diabetes mellitus. Optometry Clin. 1992;2:93–116. [PubMed] [Google Scholar]
- Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. The OGF-OGFr axis utilizes the p16INK4a and p21Waf1/Cip1 pathways to restrict normal cell proliferation. Mol Biol Cell. 2009;20:319–327. doi: 10.1091/mbc.E08-07-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisarnik-Fredenburg P. Discoveries in research on diabetic keratopathy. Optometry. 2001;72:691–704. [PubMed] [Google Scholar]
- Crosson CE, Klyce SD, Beuerman RW. Epithelial wound closure in the rabbit. A biphasic process. Invest Ophthalmol Vis Sci. 1986;27:464–473. [PubMed] [Google Scholar]
- Diabetes Control Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Hahn TM, Sindelar DK, Baskin DG, Dallman MF, Weigle DS, Schwartz MW. Effects of Streptozotocin-induced diabetes and insulin treatment on the hypothalamic melanocortin system and muscle uncoupling protein 3 expression in rats. Diabetes. 2000;49:244–252. doi: 10.2337/diabetes.49.2.244. [DOI] [PubMed] [Google Scholar]
- Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res. 2003;77:211–217. doi: 10.1016/s0014-4835(03)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji Y. Prevention of diabetic keratopathy. Brit J Ophthalmol. 2005;89:254–255. doi: 10.1136/bjo.2004.055541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Topically applied naltrexone restores corneal reepithelialization in diabetic rats. J Ocul Pharmacol Ther. 2007;23:89–102. doi: 10.1089/jop.2006.0111. [DOI] [PubMed] [Google Scholar]
- Nakahara M, Miyata K, Otani S, Miyai T, Nejima R, Yamagami S, Amano S. A randomized, placebo-controlled clinical trial of the aldose reductase inhibitor CT-112 as management of corneal epithelial disorders in diabetic patients. Br J Opthalmol. 2005;89:226–268. doi: 10.1136/bjo.2004.049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sao N, Chikama TI, Hasegawa Y, Nishida T. Fibronectin facilitates corneal epithelial wound healing in diabetic rats. Exp Eye Res. 1997;64:335–359. doi: 10.1006/exer.1996.0216. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC. Is autologous serum a tonic for the ailing corneal epithelium? Am J Ophthalmol. 2006;142:316–317. doi: 10.1016/j.ajo.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Sanchez-Thorin JC. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998;38:19–36. [PubMed] [Google Scholar]
- Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WuH. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–189. [PMC free article] [PubMed] [Google Scholar]
- Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am J Ophthalmol. 2006;142:207–211. doi: 10.1016/j.ajo.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Jenkins JB, Sassani JW, Wylile JD, Ruth TB, Frey JL, Lang CM, McLaughlin PJ. Naltrexone, an opioid antagonist, facilitates reepithelialization of the cornea in diabetic rat. Diabetes. 2002a;51:3055–3062. doi: 10.2337/diabetes.51.10.3055. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Corneal safety of topically applied naltrexone. J Ocul Pharmacol Ther. 2006b;22:377–387. doi: 10.1089/jop.2006.22.377. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol. 2007;125:1082–1088. doi: 10.1001/archopht.125.8.1082. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, McLaughlin PJ. Re-epithelialization of the rabbit cornea is regulated by opioid growth factor. Brain Res. 1998a;803:61–68. doi: 10.1016/s0006-8993(98)00610-6. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, McLaughlin PJ. Re-epithelialization of the rat cornea is accelerated by blockade of opioid receptors. Brain Res. 1998b;798:254–260. doi: 10.1016/s0006-8993(98)00427-2. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, McLaughlin PJ. Re-epithelialization of the human cornea is regulated by endogenous opioids. Invest Ophthalmol Vis Sci. 2000;41:73–81. [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, McLaughlin PJ. Adaptations of homeostatic ocular surface epithelium to chronic treatment with the opioid antagonist naltrexone. Cornea. 2006a;25:821–820. doi: 10.1097/01.ico.0000224646.66472.aa. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, McLaughlin PJ. Insulin treatment ameliorates impaired corneal re-epithelialization in diabetic rats. Diabetes. 2006c;85:1141–1147. doi: 10.2337/diabetes.55.04.06.db05-1581. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr) Brain Res Rev. 2002b;38:351–376. doi: 10.1016/s0165-0173(01)00160-6. [DOI] [PubMed] [Google Scholar]