Abstract
Wilson’s disease (WND) is an inherited disorder of copper homeostasis characterized by abnormal accumulation of copper in several tissues, particularly in the liver, brain, and kidney. The disease-associated gene encodes a copper-transporting P-type ATPase, the WND protein, the subcellular location of which could be regulated by copper. We demonstrate that the WND protein is present in cells in two forms, the 160-kDa and the 140-kDa products. The 160-kDa product was earlier shown to be targeted to trans-Golgi network. The 140-kDa product identified herein is located in mitochondria as evidenced by the immunofluorescent staining of HepG2 cells with specific mitochondria markers and polyclonal antibody directed against the C terminus of the WND molecule. The mitochondrial location for the 140-kDa WND product was confirmed by membrane fractionation and by analysis of purified human mitochondria. The antibody raised against a repetitive sequence in the N-terminal portion of the WND molecule detects an additional 16-kDa protein, suggesting that the 140-kDa product was formed after proteolytic cleavage of the full-length WND protein at the N terminus. Thus, the WND protein is a P-type ATPase with an unusual subcellular localization. The mitochondria targeting of the WND protein suggests its important role for copper-dependent processes taking place in this organelle.
Keywords: copper, subcellular localization
Copper is an essential trace element required for such diverse cell processes as oxidative metabolism, neurotransmitter synthesis, free radical detoxification, iron uptake, and maturation of connective tissue (1, 2). Disruption of copper transport across the cell membrane has devastating consequences for the organism, illustrated by Wilson’s disease (WND) and Menkes disease (MNK), genetic disorders of copper homeostasis. In WND, copper accumulates to a toxic level, particularly in the liver, causing a spectrum of hepatic and neurological abnormalities. MNK is characterized by copper deficiency in most tissues, leading to progressive neurological degeneration and connective tissue defects (3, 4).
Genes affected in WND and MNK have been recently identified and were shown to encode highly homologous proteins belonging to the family of cation-transporting P-type ATPases (5–8). Recent studies demonstrate that the MNK protein is glycosylated and is targeted to the membranes of the trans-Golgi network (9–11). An increase in copper concentration seems to induce the relocation of the MNK protein to the plasma membrane (9). The subcellular location of the WND protein is less clear. Recently, Hung et al. (12) found that the distribution of the WND protein within HepG2 cells was largely consistent with the trans-Golgi network targeting; however, the “Wilson-specific signal was also observed in punctate pattern” of unknown origin. Treatment of HepG2 cells with copper led to changes in the WND targeting pattern, which suggested the relocation of the WND protein to an unidentified intracellular organelle (12). The molecular mechanism underlying the copper-induced changes in the subcellular distribution of the WND and MNK proteins is unknown. It has been suggested that the N-terminal domains of the WND and MNK proteins, which specifically bind copper (13), may play an important role in copper-dependent targeting of the WND and MNK proteins to various cell membranes (9).
In this article, we demonstrate that in human hepatic cells the WND protein is modified, most likely cleaved, at the N-terminal portion of the molecule and that the 140-kDa WND product is localized to mitochondria. This unique subcellular location points to an unexpected role for the WND P- type ATPase in regulation of copper in the mitochondria.
EXPERIMENTAL PROCEDURES
Antibodies.
Antibodies were raised against synthetic peptides coupled to keyhole limpet hemocyanin (Sigma) as described (14) or against the purified recombinant protein corresponding to the central segment of the WND molecule (amino acids Lys-1,005 to Lys-1,321). The antibody aC-WND is directed against the peptide KWSLLLNGRD, corresponding to the C-terminal segment from Lys-1,450 to Asp-1,460 of the WND protein. The aN-WND was developed against the peptide GMTCASCVHNIE, which corresponds to the sixth metal-binding repeat Gly-572 to Glu-583 in the N-terminal domain of the WND protein. The antibody a-ABD was raised against the central hydrophillic domain of the WND molecule (residues Lys-1,005 to Lys-1,321) that was heterologously expressed as a His-Tag fusion in Escherichia coli. Control antibodies (αM5, αM6, and αM3M4, provided by J. H. Kaplan, Oregon Health Sciences University) were raised against synthetic decapeptides corresponding to the transmembrane segments of Na,K-ATPase. These peptides do not have structural homology to the WND protein and were coupled to keyhole limpet hemocyanin by the same experimental procedure used for the production of the aC-WND antigen. The mAb against mitochondria marker cytochome c oxidase (anti-COX) were from Molecular Probes. Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG and goat anti-mouse IgG-FITC antibodies were from GIBCO/BRL.
Tissue Culture.
Human hepatoma cells (HepG2) were grown in MEM (GIBCO/BRL) supplemented with 10% fetal bovine serum (GIBCO/BRL), 1 mM sodium pyruvate (GIBCO/BRL), and 0.1 mM MEM nonessential amino acids (GIBCO/BRL) in a 37°C, 5% CO2/95% air incubator. Three days before fluorescence labeling, cells were split 1:20 onto Permanox chamber slides (Nunc) and grown as above. Cells [mouse embryonic liver (BNL), green monkey kidney (COS), Chinese hamster ovary (CHO), and human embryonic kidney (HEK)] were maintained according to protocols supplied by the American Type Culture Collection.
Western Blot Analysis of Cell Lysates.
Cells in 10-cm Petri dishes were grown to 70–85% confluency, rinsed with PBS (pH 7.4), and then were either solubilized with sample buffer [125 mM Tris⋅HCl, pH 6.8/8 M urea/10% SDS at 1:1:1 (vol/vol)] or lifted from plates by treatment with 0.05% trypsin, washed three times with 1 mM NaHCO3, containing 0.2 mM AEBSF [proteases inhibitor, 4-(2-aminoethyl)-benzenesulfonyl fluoride, ICN], and resuspended in homogenization buffer (HB buffer: 10 mM Hepes⋅NaOH/250 mM sucrose/0.2 mM AEBSF, pH 7.5). Protein concentration was measured by method of Lowry (15), and 25–35 μg of protein was taken for gel electrophoresis. Before electrophoresis 2-mercaptoethanol was added to a final concentration of 14.2 mM. Proteins were separated on a 7.5% or 15% Laemmli gel (16), transferred to poly(vinylidene difluoride) membrane as described by Matsudaria (17) and immunostained with antibodies (18). The following dilutions were used: aC-WND, 1:5,000–7,000; aN-WND, 1:6,500; a-ABD, 1:20,000.
In a series of experiments the effect of different denaturation procedures on the electrophoretic mobility of the aC-WND-positive protein has been tested. Cell lysates dissolved in Laemmli sample buffer were either heated at 100°C or incubated at room temperature for 3 min before loading on a gel. Also, in some experiments, urea was omitted from the sample buffer. The variations in the denaturation conditions did not cause changes in mobility of the 140-kDa protein.
Transfection of COS Cells with the Full-Length WND.
cDNA cloned into the pMT2 vector (kindly provided by K. Petrukhin, Columbia University) was done by using a standard DEAE-dextran protocol (19). Control cells were mock-transfected with either the pMT2 plasmid or buffer. After a 36-h incubation at 37°C, the growth medium was removed, cells were rinsed with PBS, scraped with rubber policeman, and resuspended in 1 mM NaHCO3 for a protein concentration of 15–20 mg/ml. Approximately 30–40 μg of protein was used for gel electrophoresis and Western blot analysis.
Fractionation of HepG2 Cell Membranes.
HepG2 cells from a 10-cm plate were resuspended in 1 ml of HB buffer and homogenized by 10–15 strokes in a Dounce homogenizer. The following membrane fractions were collected by centrifugation at 4°C: 1, 5 min at 500 × g; 2, 10 min at 3,000 × g; 3, 10 min at 6,000 × g; 4, 15 min at 20,000 × g; 5, 30 min at 250,000 × g. The pellets were resuspended in HB buffer and protein concentration in the fractions was determined. Protein (25–35 μg) from each fraction was separated by 7.5% Laemmli gel, transferred to poly(vinylidene difluoride) membrane, and immunostained with antibodies as described above. Staining with anti-COX, the antibody against mitochondria marker cytochrome c oxidase, was done according to the manufacturer’s suggestions. The mitochondria-enriched fraction was further separated from the plasma membrane and lysosomes with centrifugation through a stepwise gradient of metrizamide [steps of 10%, 19.78%, 24.35%, 26.34%, 32.84%, and 50% metrizamide in 10 mM Tris⋅HCl (pH 7.5)] as described (20). The WND protein was detected in the 32.84–50% metrizamide fraction, in agreement with the reported distribution of mitochondria proteins (20).
Reverse Transcription and PCRs.
These procedures were performed as suggested in the first-strand cDNA synthesis protocol (GIBCO/BRL) using SuperScript II RNase H− Reverse Transcriptase, 5 μg of TRIzol-purified total mRNA from HepG2 cells, and the antisense primer 5′-CTTGTGGTCGAAGTGATG-3′ (exon VI). The reverse transcription/cDNA mixture or the WND-pMT2 plasmid, as a control, were subjected to 32 amplification cycles. The sense primer used in the amplification was 5′-CCGAGAAACCAGGTCCAG-3′ (exon II). No difference between the PCR patterns produced with the control full-length WND cDNA and reverse transcription-synthesized cDNA has been observed.
Colocalization and Fluorescence Imaging.
Colocalization and imaging were done with a Leica DM RB light microscope, a Fluor ×67 oil objective, and FITC and rhodamine flourescence filter sets. There was no detectable crossover signal of fluorophores between filter sets in control slides. Pictures were taken with a Leica DM RD camera system, using Kodak Royal Gold 1000 film.
Immunofluorescent Staining of HepG2 Cells.
Cells at 50–80% confluency on chamber slides were permeablized for 2 min in an ice-cold mixture of acetone and methanol (1:1, vol/vol). Cells were rinsed twice with PBS, blocked with 5% milk in PBS for 1 h at room temperature, and stained for 1.5 h at room temperature with the aC-WND or antibody to Na,K-ATPase (diluted 1:200 in PBS/1% milk/0.2% Tween 20), or anti-COX Ab (used as supplied, with milk added to 1% and Tween 20 to 0.2%). Excess antibodies were removed by washing the cells with Tris-buffered saline (pH 7.2) containing 0.2% Tween 20 (TBS-T). Cells were then stained with the secondary antibodies goat anti-rabbit IgG-FITC or goat anti-mouse IgG-FITC (diluted 1:25 in PBS/1% milk/0.2% Tween 20) for 1 h at room temperature. Excess antibody was removed, cells were washed with TBS-T and rinsed with PBS, and coverslips were mounted on the slides with 50% glycerol in water.
Fluorescent Labeling of HepG2 Cells for Colocalization Experiments.
Cells at 50–80% confluency on chamber slides were rinsed once with the growth medium prewarmed to 37°C and incubated for 5 min. Selective mitochondria marker MitoTracker (MitoTracker Red CMXROS, Molecular Probes) was added to growth medium to a final concentration of 50 nM, and the solution was added to the chamber slides. The cells were incubated in a 37°C, 5% CO2/95% air incubator for 1.5 h. The medium was discarded, the cells were rinsed twice with PBS and then fixed for 2 min in an ice-cold mixture of acetone and methanol. Fixed cells were rinsed with PBS and immunostained with aC-WND as described above.
RESULTS
To characterize the subcellular targeting of the WND protein, we developed an antibody against the C-terminal region 1450KWSLLLNGRD1460 of the WND protein. This segment has little similarity within the primary structure of the otherwise highly homologous MNK protein. In addition, the corresponding region of the mouse homologue of the WND protein has two amino acid substitutions (21) that provide an additional advantage for testing the antibody specificity. The antibody aC-WND was produced in two rabbits and the following results were identical for both sera.
The Antibody Raised Against the Synthetic C-Terminal Fragment of the WND Protein Specifically Recognizes the 140-kDa Protein in Human Hepatic Cells.
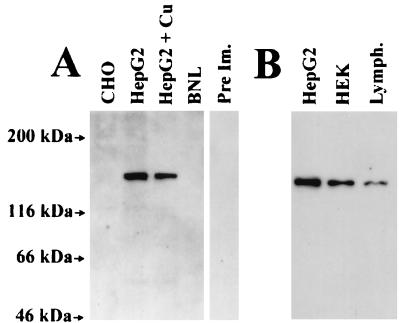
Liver cells contain the highest level of mRNA for the WND protein (3), consequently human hepatic cells (HepG2) were expected to produce an antibody-positive signal. Analysis of the HepG2 lysates revealed that in these cells aC-WND strongly recognizes a protein with a molecular mass of 140 kDa (Fig. 1A). This protein was not detected (i) with the preimmune serum, (ii) in the lysates of CHO-cells, which express a detectable amount of MNK protein (9), or (iii) in the lysates of mouse embryonic liver cells (BNL), in which the WND protein has two amino acid substitutions in the epitope region (Fig. 1A). The 140-kDa band was also not detected when lysates of HepG2 cells were stained with any of three antibodies raised against unrelated synthetic peptides coupled to keyhole limpet hemocyanin with the same protocol used for the production of the aC-WND antigen (data not shown). These results indicated that aC-WND recognizes the epitope specifically.
Figure 1.
(A) Immunostaining of the CHO, HepG2, and BNL cell lysates with aC-WND or preimmune serum (HepG2, PreIm). (B) The intensity of the 140-kDa product in cells with different level of WND mRNA. Thirty micrograms of protein was loaded to each lane, except the lymphoblasts sample, in which 60 μg of protein was used. aC-WND dilution is 1:5,000. The lysate of HepG2 cells grown in the presence of 20 μm CuCl2 is labeled HepG2+Cu.
The level of the WND mRNA is the highest in hepatic cells, kidney cells have less WND mRNA, and very little WND mRNA was detected in lymphoblasts (3). Fig. 1B illustrates that the amount of the 140-kDa protein detected by aC-WND in corresponding cells correlates well with the known levels of the WND mRNA, thus supporting our conclusion that the 140-kDa product is related to the WND protein.
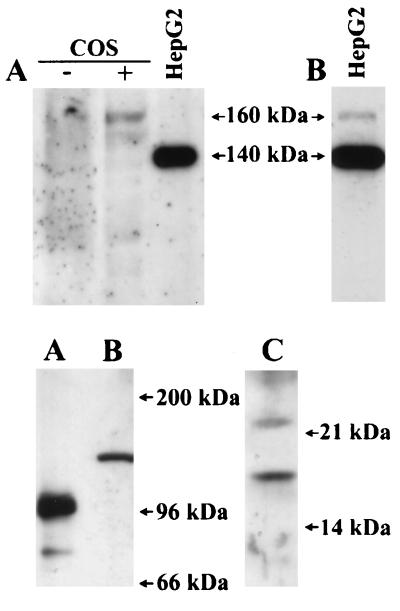
The calculated molecular mass of the full-length WND protein is 157 kDa, which is noticeably different from the observed molecular mass of 140 kDa. This result indicated that the WND protein by itself had an unusual electrophoretic mobility or was posttranslationally modified to produce a 140-kDa product or that the WND mRNA underwent alternative splicing. We did not find any evidence for alternative splicing for the WND mRNA in HepG2 cells by using reverse transcription-coupled PCR and primers corresponding to two different exons (II and VI) in the N-terminal portion of the molecule (data not shown). Also, the variations in protein denaturation conditions before gel electrophoresis did not lead to changes in the mobility of the 140-kDa band, arguing against the possibility that tight folding or copper binding to the WND protein alters its mobility on a gel. Thus, to determine whether the WND was posttranslationally modified, the full-length WND protein was expressed in COS cells (Fig. 2 Upper). The immunostaining of COS cell lysates revealed that the expressed full-length WND protein migrates as a 160-kDa band in agreement with its calculated molecular mass.
Figure 2.
(A Upper) The full-length WND protein is expressed in COS cells as a 160-kDa protein. COS cells were transfected with the WND cDNA. Twenty-five micrograms of mock-transfected (−) and WND cDNA-transfected (+) cell lysates were analyzed and compared with HepG2 lysate. (B Upper) The longer exposure reveals a 160-kDa band in addition to the 140 kDa product in HepG2 sample. (Lower) The antibody aN-WND detects the recombinant N-terminal domain of the WND protein (A) and specifically recognizes the 140-kDa protein (B) and 16-kDa protein (C) in HepG2 cell lysates. The recombinant domain was obtained as described (13).
These results confirmed the ability of the aC-WND to recognize the WND protein and suggested that in HepG2 cells the 160-kDa protein was posttranslationally modified or cleaved to yield the 140-kDa product. In fact, longer exposure of the immunoblots demonstrates the presence of small quantities of the 160-kDa band, which presumably represents the nonprocessed WND protein (Fig. 2B Upper). The low efficiency of the aC-WND in immunoprecipitation assays precluded the direct testing of this hypothesis in metabolically labeled cells. Thus, to determine whether the N-terminal region of the WND had been cleaved or modified, we used the antibody against the synthetic peptide corresponding to the repetitive sequence in the N-terminal region of the WND protein.
Antibody Against the Repetitive Sequence in the N-Terminal Portion Recognizes Two Protein Bands in Lysates of Human Hepatic Cells, the 140-kDa and 16-kDa Products.
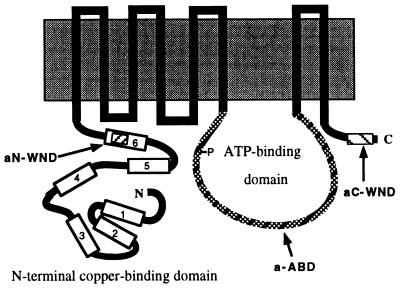
The N terminus of the WND protein contains six repetitive 70-amino acid sequences distributed evenly along the N-terminal region (Fig. 3). Each of these repeats includes a highly conserved sequence motif GMTCXXCXXXIE. The antibody aN-WND was raised against the synthetic peptide GMTCASCVHNIE, corresponding to the last (sixth) repeat (residues 572–583) of the WND molecule (see Fig. 3). Fig. 2 Lower illustrates that aN-WND recognizes the recombinant N-terminal domain of the WND protein and detects a 140-kDa band in lysates of HepG2 cells, confirming that the 140-kDa product is indeed the modified WND protein. Interestingly, when 15% Laemmli gel was used to better separate the low molecular weight proteins of the HepG2 cell lysate, an additional 16-kDa protein band was detected by aN-WND (Fig. 2 Lower). This band was not stained with the preimmune serum or with aC-WND (data not shown). Because sequence identity between the repeats in the N-terminal domain is very high (for example, repeat 4 has only 1 amino acid substitution in the epitope region), the 16-kDa protein is likely to represent a cleaved N-terminal fragment of the WND protein. This conclusion is particularly appealing because the molecular mass of the 16-kDa fragment corresponds closely to the difference between the full-size WND protein and the observed 140-kDa product.
Figure 3.
Schematic representation of transmembrane organization of the WND protein. Repetitive sequences in the N-terminal region are depicted by blocks and numbered; the arrows and corresponding letters indicate the position of the epitopes for the aC-WND, aN-WND, and a-ABD.
The 140-kDa WND Product Is Located in Mitochondria.
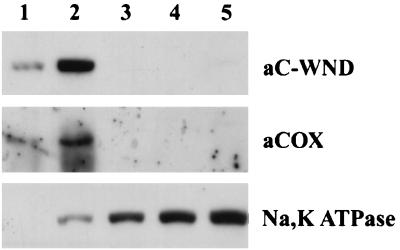
A combination of different techniques has been employed for subcellular localization of the 140-kDa WND product. The stepwise and gradient fractionation of HepG2 homogenates revealed that the 140-kDa protein is associated with the membrane fraction enriched in mitochondria (Fig. 4). The distribution of the WND proteins between different fractions was similar to the distribution of the mitochondria marker cytochome c oxidase and differed from the distribution of plasma membrane marker Na,K-ATPase (Fig. 4).
Figure 4.
Distribution of the 140-kDa WND product, cytochrome c oxidase, and Na,K-ATPase in HepG2 membrane fractions. HepG2 homogenate was centrifuged and membrane fractions were collected. Thirty micrograms of protein from each fraction was separated by 7.5% Laemmli gel and immunostained with anti-cytochrome c oxidase antibody (aCOX), aC-WND, or antibody directed against Na,K-ATPase.
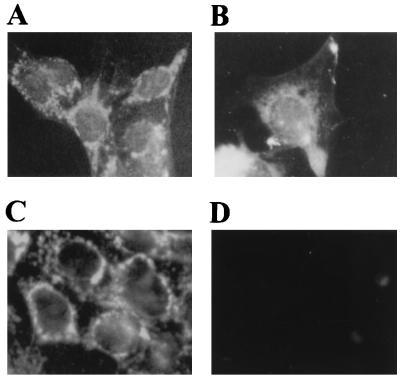
The immunocytochemistry experiments confirmed the results of membrane fractionation. Fig. 5A illustrates the pattern of fluorescent staining that was consistently obtained with aC-WND. This pattern demonstrates clearly that the WND protein has an intracellular location in HepG2 cells (compare with the plasma membrane pattern, Fig. 5B) that is very similar to the distribution of the mitochondria marker cytochrome c oxidase (see the anti-COX pattern, Fig. 5C). No fluorescence was seen with the preimmune serum (Fig. 5D).
Figure 5.
Fluorescent immunostaining of HepG2 cells obtained with aC-WND (A), antibody against plasma membrane marker Na,K-ATPase (B), antibody against mitochondria marker cytochrome c oxidase (C), and preimmune serum (D).
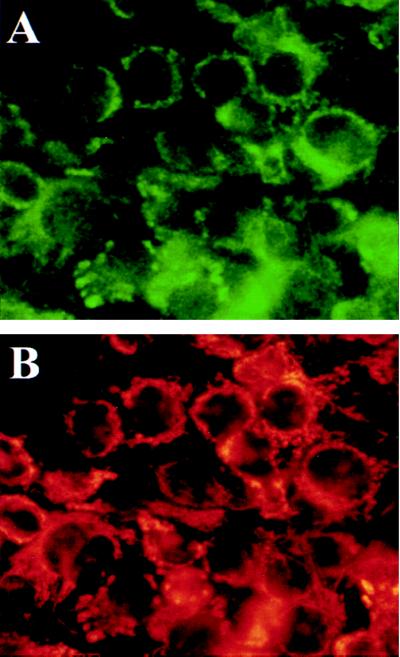
To confirm that the observed aC-WND staining pattern represents the mitochondria staining and to verify that this pattern is independent of the staining techniques the fluorescence colocalization experiments were carried out with aC-WND and the rhodamine-based chemical probe MitoTracker as a selective mitochondria marker. Live cells were stained first with the membrane-permeable MitoTracker and then cells were fixed and stained with the aC-WND and secondary FITC-coupled antibody. The comparison of the mitochondria-associated rhodamine fluorescence and FITC fluorescence patterns from aC-WND demonstrate that they are essentially identical (Fig. 6), confirming that the 140-kDa WND product is indeed targeted to mitochondria.
Figure 6.
WND protein is colocalized with mitochondria marker cytochrome c oxidase. Live HepG2 cells were incubated with selective mitochondria marker MitoTracker, and then cells were fixed and immunostained with aC-WND and secondary FITC-coupled antibody. Cells were photographed by using two filters to detect FITC fluorescence associated with the aC-WND staining (A) and the mitochondria-associated rodamine fluorescence of MitoTracker (B).
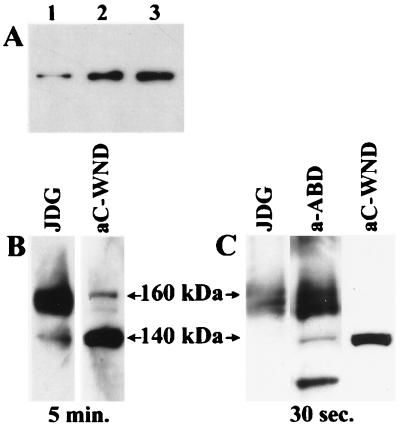
Finally, we tested whether the 140-kDa product is present in normal human tissues or only in cultured cells. Proteins from purified human mitochondria (kindly provided by N. G. Kennaway, Oregon Health Sciences University) were separated by gel electrophoresis and immunostained by using the Western blotting procedure. Fig. 7A illustrates the presence of a 140-kDa product in purified human mitochondria from skeletal muscle and from heart, this is identical to the WND product found in the HepG2 cells.
Figure 7.
(A) Identification of the 140-kDa WND product in human heart mitochondria (lane 1), skeletal muscle mitochondria (lane 2), and HepG2 (lane 3). Thirty micrograms of protein is in each lane. Identification of the 160-kDa and 140-kDa products in HepG2 lysates with JGD and aC-WND (5-min exposure) (B) or with JGD, a-ABD and aC-WND (30 sec exposure) (C). Twenty-five micrograms of protein is in each lane.
The Full-Length 160-kDa and the 140-kDa WND Products Have Different Subcellular Localization in HepG2 Cells.
Recently, Hung et al. (12) reported data that pointed to the trans-Golgi network as a major targeting site for the WND protein in HepG2 cells.
Hung et al. (12) used a polyclonal antibody directed against the 310-amino acid segment (residues 325–635) at the N-terminal portion of the WND molecule. This antibody detected a 165-kDa protein in both HepG2 cells and in yeast in which the WND protein was heterologously expressed (12). Interestingly, Hung et al. (12) found that in HepG2 cells the specific signal was also observed in punctate pattern that the authors ascribed to an unidentified intracellular organelle.
To resolve an apparent contradiction between our data and the results from Hung et al. (12), the Western blot patterns were compared for aC-WND, aN-WND, and JDG, the antibody used by Hung et al. (12), which was kindly provided by J. D. Gitlin (Washington University). The latter antibody detected the 140-kDa band at the position undistinguishable from the position of the aC-WND- or aN-WND-positive bands; however, the intensity of staining was much weaker than that of the 160-kDa protein (Fig. 7B). Staining of the 140-kDa protein with JGD was more evident when the mitochondria-enriched fraction was analyzed, in agreement with our results with the aC-WND (data not shown). Thus, difference in subcellular localization of the WND protein reported by Hung et al. (12) and obtained later by us reflects the opposite selectivity of JGD and aC-WND antibodies toward the 160-kDa and 140-kDa WND products. This opposite selectivity of the antibodies was puzzling and was investigated further.
The Ab directed against the recombinant protein corresponding to the central portion of the WND protein (from Lys-1,005 to Lys-1,321, Fig. 3) was produced. This antibody was expected to recognize the 160-kDa and the 140-kDa products with similar affinity, if both products were related to the WND protein. HepG2 lysates were separated by gel electrophoresis and immunostaining patterns for the aC-WND, JDG, and a-ABD were compared. Fig. 7C illustrates that antibody raised against the central portion of the WND protein detects both the diffuse 160-kDa protein and the 140-kDa product at dilution as low as 1:20,000, confirming that there are two distinct WND products in HepG2 cells. (The band of 90–100 kDa seen on Fig. 7C represents most likely the WND degradation product, because the intensity of this band increases significantly if cells or cell lysate are stored prior to analysis). The 160-kDa protein detected with JGD and a-ABD was found predominantly in microsomal fractions (10,000 × g and 100,000 × g, data not shown) in agreement with the results reported earlier by Hung et al. (12).
DISCUSSION
In this article, we provide evidence that the WND protein exists in a modified, most likely cleaved, form and that this 140-kDa WND product is located in mitochondria in both cultured hepatic cells and human tissues. In human hepatic cells, the 140-kDa WND product coexists with the full-length 160-kDa form, which was earlier identified by Hung et al. (12). The 160-kDa product was shown to colocalize with the trans-Golgi network markers; in addition, the WND-specific signal was seen in punctate pattern of unknown origin (12). Our data indicate that this punctate pattern is likely to reflect the staining of the 140-kDa product targeted to mitochondria. The diffuse character of the 160-kDa band revealed by JGD and a-ABD (Fig. 7) explains the poor recognition of this protein by aN-WND, which is a low-affinity antibody, and also suggests that the 160-kDa product could be modified or degraded at the C terminus and consequently be poorly recognized by the high-affinity anti-C-terminal antibody aC-WND.
The mitochondria location of the 140-kDa WND protein suggests that the WND protein may play an important role in the copper-dependent processes taking place in this organelle. A number of key mitochondria enzymes require copper as a cofactor. Hepatic mitochondria isolated from copper-deficient animals were shown to be deficient in cytochrome oxidase activity and failed to synthesize heme from ferric iron (Fe III) and protoporphyrin at the normal rate (22). It was also reported that copper at low concentrations stimulates the activity of ferrochelatase, the key enzyme in heme synthesis, but that high concentrations of copper were inhibitory for biosynthesis of heme (23). The Cu2+-mediated regulation of cytochrome P450-dependent steroidogenic activity was observed in the adrenal mitochondria (24). Interestingly, the mitochondria of WND patients have characteristic morphological abnormalities (25), which are also seen in the mitochondria of Long–Evans Cinnamon rats [the WND animal model (26)] that could be associated with the malfunctioning of the WND protein in this organelle.
The WND protein is a P-type ATPase located in mitochondria. This unexpected finding illustrates that the function of heavy metal transporting P-type ATPases in the cell could be more diverse and complex than was anticipated. It was shown, for example, that PacS, the bacterial heavy metal transporting P type ATPase, is mainly located in the thylakoid membrane, in which the photosynthetic reactions take place (27). Recent studies on the fixGHIS operon, which includes the FixI gene for a heavy metal transporting P type ATPase homologous to the WND and MNK proteins, demonstrated that this operon is required for the formation of the high-affinity cbb3-type heme-copper oxidase that supports microaerobic respiration in Bradyrhizobium japonicum (28). These data illustrate that functional activity of some heavy metal transporting P type ATPases, including the WND protein, may be important for the energy-producing processes taking place in various cells.
Our data suggest that the 140-kDa WND product is formed as a result of proteolytic cleavage at the N-terminal portion of the full-length WND protein. It is likely, that the 16-kDa protein detected with the aN-WND represents a fragment cleaved from the WND molecule. Presently, we cannot, however, exclude the possibility that the 16-kDa protein is unrelated to the WND protein, because aN-WND was raised against the metal-binding motif found in a number of copper-binding proteins (29).
The cleavage of the WND protein presumably removes one or two metal-binding repeats (MRBs) from the N-terminal domain. Which of the N-terminal repeats are cleaved off and what could be a role for the removed fragment remains to be demonstrated. The very weak staining of the 140-kDa product with JDG provides some clues as to where the cleavage site could occur. This antibody was raised against the second half of the N-terminal domain and most likely recognizes the segment immediately preceding the MRB4, because it is different for the MNK and WND proteins (12). This sequence region and the MRB4 have several interesting features. (i) There is a number of Ser and Thr residues that can be phosphorylated and, consequently, alter the ability of the antibody to recognize this region efficiently. (ii) In the sequence of mouse and rat WND proteins this segment is substituted by the dissimilar sequence lacking the metal-binding motif (21, 30). In fact, the lack of the sequence MRB4 in the rat homologue of the WND protein led to the suggestion that not all repeats in the N-terminal region of the WND protein are important for copper binding (30). It is tempting to speculate that the observed 16-kDa product could be a proteolytically excised fragment of the WND protein that includes the MRB4. Excised segments (inteins) acting after cleavage as regulatory enzymes were found in a number of proteins, for example in vacuolar H+-ATPase, adenosyl cobalamin-dependent ribonucleotide reductase and other protozymes (31–33). Experiments are curently underway to determine precisely how the 140-kDa WND product is formed.
Acknowledgments
We are grateful to Dr. K. Petrukhin for his generous gift of the WND-pMT2 plasmid, to Dr. N. G. Kennaway for preparations of purified human mitochondria, and to Dr. J. D. Gitlin for the antibody against the WND protein. We thank Dr. J. H. Kaplan for stimulating discussions, Dr. D. Farrens for help with the WND expression in COS cells, and Dr. D. Kabat, Dr. E. Platt, and Dr. S. Kozak for advice and help with the immunofluorescence studies. This work was supported by start-up funds and Oregon MRF Grant 9727 (Lutsenko 898) for S.L.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: WND, Wilson’s disease; FITC, fluorescein isothiocyanate.
References
- 1.Danks D M. In: Metabolic Basis of Inherited Disease. Scriever C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1989. pp. 1411–1432. [Google Scholar]
- 2.Vulpe C D, Packman S. Annu Rev Nutr. 1995;15:293–392. doi: 10.1146/annurev.nu.15.070195.001453. [DOI] [PubMed] [Google Scholar]
- 3.Tanzi R E, Petrukhin K, Chernov I, Pellequer J L, Wasco W, Ross B, Romano D M, Parano E, Pavone L, Brzustowicz L M, et al. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 4.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Nat Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 5.Bull P C, Thomas G R, Rommens J M, Forbes J R, Cox D W. Nat Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi Y, Heiny M E, Gitlin J D. Biochem Biophys Res Commun. 1993;197:271–277. doi: 10.1006/bbrc.1993.2471. [DOI] [PubMed] [Google Scholar]
- 7.Kaler S G, Gallo L K, Proud V K, Percy A K, Mark Y, Segal N A, Goldstein D A, Holmes C S, Gahl W A. Nat Genet. 1994;8:195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- 8.Petrukhin K, Lutsenko S, Chernov I, Ross B M, Kaplan J H, Gilliam T C. Hum Mol Genetics. 1994;3:1647–1656. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- 9.Petris M J, Mercer J F B, Culvenor J G, Lockhart P, Gleeson P A, Camakaris J. EMBO J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi Y, Heiny M E, Suzuki M, Gitlin J. Proc Natl Acad Sci USA. 1996;93:14030–14035. doi: 10.1073/pnas.93.24.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dierick H A, Adam A N, Escara-Wilke J F, Glover T W. Hum Mol Genet. 1997;6:409–416. doi: 10.1093/hmg/6.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung I H, Suzuki S, Yamaguchi Yu, Yuan D S, Klausner R D, Gitlin J D. J Biol Chem. 1997;27:21461–21466. doi: 10.1074/jbc.272.34.21461. [DOI] [PubMed] [Google Scholar]
- 13.Lutsenko S, Petrukhin K, Cooper M J, Gilliam T C, Kaplan J H. J Biol Chem. 1997;272:18939–18944. doi: 10.1074/jbc.272.30.18939. [DOI] [PubMed] [Google Scholar]
- 14.Pfaff E, Salfeld J, Gmelin K, Schaller H, Theilmann L. Virology. 1987;158:456–460. doi: 10.1016/0042-6822(87)90221-2. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Matsudaria P. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 18.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. , Chapter 16. [Google Scholar]
- 20.Khan M N, Posner B I, Verma A K, Khan R J, Bergeron J M. Proc Natl Acad Sci USA. 1981;78:4980–4984. doi: 10.1073/pnas.78.8.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theophilos M B, Cox D W, Mercer J F. Hum Mol Genet. 1996;5:1619–1624. doi: 10.1093/hmg/5.10.1619. [DOI] [PubMed] [Google Scholar]
- 22.Williams D M, Loukopoulos D, Lee G R, Cartwright G E. Blood. 1976;48:77–85. [PubMed] [Google Scholar]
- 23.Wagner G S, Tephly T R. Adv Exp Med Biol. 1975;58:343–354. doi: 10.1007/978-1-4615-9026-2_24. [DOI] [PubMed] [Google Scholar]
- 24.Veltman J C, Maines M D. Biochem Pharmacol. 1986;35:2903–2909. doi: 10.1016/0006-2952(86)90484-3. [DOI] [PubMed] [Google Scholar]
- 25.Sternlieb I. Hepatology. 1992;16:728–732. doi: 10.1002/hep.1840160319. [DOI] [PubMed] [Google Scholar]
- 26.Sternlieb I, Quintana N, Volenberg I, Schilsky M L. Hepatology. 1995;22:1782–1787. [PubMed] [Google Scholar]
- 27.Kanamaru K, Kashiwagi S, Mizuno T. Mol Microbiol. 1994;13:369–377. doi: 10.1111/j.1365-2958.1994.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 28.Preisig O, Zufferey R, Hennecke H. Arch Microbiol. 1996;165:297–305. doi: 10.1007/s002030050330. [DOI] [PubMed] [Google Scholar]
- 29.Klomp L W, Lin S J, Yuan D S, Klausner R D, Culotta V C, Gitlin J D. J Biol Chem. 1997;272:9221–9226. doi: 10.1074/jbc.272.14.9221. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Forbes J R, Chen H S, Cox D W. Nat Genet. 1994;7:541–545. doi: 10.1038/ng0894-541. [DOI] [PubMed] [Google Scholar]
- 31.Chong S, Shao Y, Paulus H, Benner J, Perler F B, Xu M Q. J Biol Chem. 1996;271:22159–22168. doi: 10.1074/jbc.271.36.22159. [DOI] [PubMed] [Google Scholar]
- 32.Riera J, Robb F T, Weiss R, Fontecave M. Proc Natl Acad Sci USA. 1997;94:475–478. doi: 10.1073/pnas.94.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper A A, Stevens T H. Bioessays. 1993;15:667–674. doi: 10.1002/bies.950151006. [DOI] [PubMed] [Google Scholar]