Abstract
Objective
A burgeoning area of research has focused on motivational deficits in schizophrenia, producing hypotheses about the role that motivation plays in the well-known relationship between neurocognition and functional outcome. However, little work has examined the role of motivation in more complex models of outcome that include social cognition, despite our increased understanding of the critical role of social cognition in community functioning in schizophrenia, and despite new basic science findings on the association between social cognitive and reward processing in neural systems in humans. Using path analysis, we directly contrasted whether motivation 1) causally influences known social cognitive deficits in schizophrenia, leading to poor outcome or 2) mediates the relationship between social cognitive deficits and outcome in this illness.
Method
Ninety one patients with schizophrenia or schizoaffective disorder completed interview-based measures of motivation and functional outcome as well as standardized measures of neurocognition and social cognition in a cross-sectional design.
Results
In line with recent research, motivation appears to mediate the relationship between neurocognition, social cognition and functional outcome. A model with motivation as a causal factor resulted in poor fit indicating that motivation does not appear to precede neurocognition.
Conclusions
Findings in the present study indicate that motivation plays a significant and mediating role between neurocognition, social cognition, and functional outcome. Potential psychosocial treatment implications are discussed, especially those that emphasize social cognitive and motivational enhancement.
Keywords: schizophrenia, social cognition, neurocognition, motivation, functional outcome, path analysis
1. Introduction
It is now well-established that neurocognition and functional outcome are associated in schizophrenia (Gottesman, 1994; Green, 1996; Green et al., 2000) and compelling evidence indicates that deficits in social cognition mediate this relationship (Brekke et al., 2005; Couture et al., 2006; Yager and Ehmann, 2006). For example, using path analysis, Brekke and colleagues (2005) confirmed that social cognition and social support mediate the relationship between neurocognition and functional outcome, both cross-sectionally and prospectively. Although the model fit was excellent, the authors suggested further investigation of additional contributing factors unspecified by the model in order to identify other critical variables that play a role in community outcome.
One important factor that has received increasing attention in schizophrenia is motivation. As several researchers have noted, motivation likely plays an important role in the cognitive dysfunction of schizophrenia, with important associations to functioning (Barch, 2005; Barch et al., 2008; Docherty et al., 2001; Gold et al., 2008; Heerey and Gold, 2007). However, it is less clear what the causal relationships might be between motivation, neurocognition and social cognition, and it is also unclear how these variables interact to alter outcome. Historically, theorists and clinicians have considered emotion and motivation impairments to be a central feature of schizophrenia, and even one of the predisposing or causal factors in the development of the disorder. Bleuler (1911/1950) and Kraeplin (1919/1913) regarded “indifference” in patients as one of the fundamental symptoms of the illness. Later theorists (e.g., Meehl, 1962; Rado, 1953) expanded on this, positing that anhedonia is a congenital trait, primary among the factors in the development of schizophrenia. They argued that the result of this pleasure deficiency was two-fold: 1) pleasures resulting from goal attainment are not present and therefore do not reinforce positive or social behaviors, and 2) without the ability to experience pleasure, there is no ‘softening’ of negative emotional states, both of which lead to poor outcomes (e.g., Meehl, 1975). From this view, impaired motivation can be thought of as a causal factor influencing disease expression, cognitive performance, social interactions, and ultimately, outcome.
In contrast, recent work has framed motivation as a mediator between cognition and outcome. Drawing on behavioral neuroscience research which shows a distinction between in-the-moment pleasure and anticipated pleasure (Berridge and Robinson, 1998; Schultz, 2007; Wise, 2002), researchers have shown that schizophrenia patients appear to have a deficit in the more cognitively complex aspects of motivation – or anticipating that things will bring them pleasure (Gard et al., 2007). Others have linked anhedonia and other motivationally relevant deficits to problems with working memory (e.g., Burbridge and Barch, 2007). These findings suggest that the inability to maintain enduring representations of a pleasurable outcome, such as a positive social interaction, impedes patients from successfully engaging with goal-directed everyday activities, and thus ultimately leads patients to poorer functional status. Recently, structural equation modeling was used to test whether motivation acts as a mediator or a moderator between neurocognition and outcome (Nakagami et al., 2008). In other words, motivation was assessed on whether it accounted for the relationship between neurocognition and outcome (i.e., motivation as a mediator) or whether the relationship between neurocognition and outcome was explained by individuals who were particularly high or low in motivation (i.e., motivation as a moderator). In this analysis, motivation appeared to best fit as a mediator – explaining most of the relationship between neurocognition and functional outcome. However, in this study, there was no direct test of whether motivation would fit better as a factor preceding the relationship between neurocognition and outcome (e.g., using path analyses or other methods). In addition, social cognition was not included as a variable in the model.
Our position is that, in order to more fully understand the unique role of motivation in functional outcome in schizophrenia, it is important to consider its relationship to social cognition as well as to general neurocognition. Accurate processing of socio-emotive stimuli- which are among the most highly salient stimuli for the human brain- is intimately integrated with neural systems related to reward, learning, and motivation (Grace et al., 2007; Murray et al., 2008a; 2008b; Waltz and Gold, 2007). Thus, it is possible that deficits in processing social stimuli have an adverse effect on motivational state in schizophrenia, and through this mechanism show a negative influence on functional status. This would be an important relationship to delineate, since it has potentially useful treatment implications, suggesting that focused treatment of social cognitive deficits could have a direct impact on motivational features of schizophrenia and thus on community functioning.
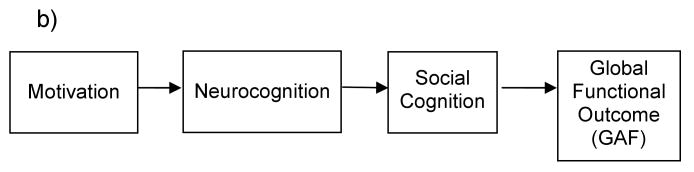
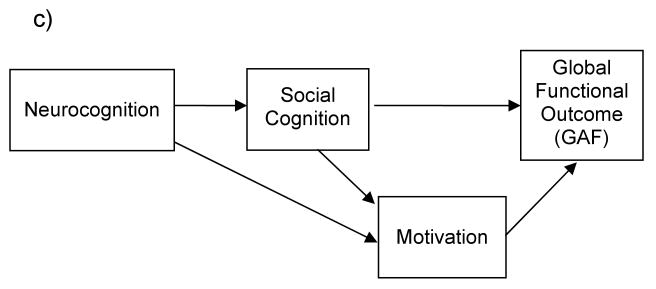
Therefore, in the present study we sought to: 1) replicate earlier findings that neurocognition is related to functional outcome and mediated by social cognition, 2) test motivation as a causal (or exogenous) factor (preceding neurocognition and social cognition) or 3) as a mediating variable (between neurocognition, social cognition and outcome) (see Figure 1a-c for examples of these models). Using path analysis, and model fit indices, the present study tested these alternative models of functional outcome in a cross-sectional design. Path analysis is a useful technique in testing the relationships among variables, and though it cannot directly confirm causality with correlated variables, model fit statistics can illuminate whether variables are better construed as exogenous or endogenous.
Figure 1.
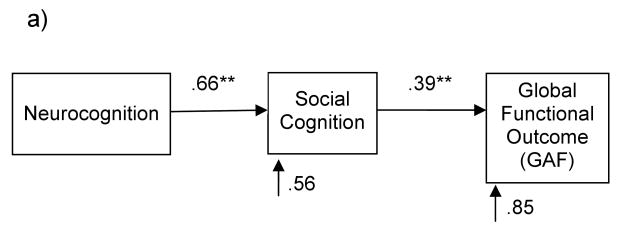
a) Green and colleagues' model of Global Functional Outcome is shown below (Brekke et al., 2005). Values represent standardized beta coefficients. An excellent fit to the data is provided.
b) A hypothesized model of the relationship between Neurocognition, Social Cognition, Motivation and Functional Outcome, with Motivation as a causal or exogenous factor.
c) A hypothesized model of the relationship between Neurocognition, Social Cognition, Motivation and Functional Outcome, with Motivation as a mediating factor.
2. Method
2.1. Participants
Ninety one schizophrenia outpatients were recruited from psychiatric outpatient services and community mental health clinics in the San Francisco Bay area. A diagnosis of schizophrenia was confirmed with the Structured Clinical Interview for Diagnosis for the DSM-IV-Clinician Version (SCID: First et al., 1997), the Positive and Negative Syndrome Scale-Extended (PANSS: Kay and Sevy, 1990; Poole et al., 2000) and a medical chart review. Additional exclusion criteria included history of head trauma, substance dependence during the past 6 months, neurological disorders, and English as a second language. All subjects were clinically stable at the time of testing (no hospitalization in the last three months, and no change in medication or dosage in the last 30 days). Symptom severity varied broadly; with average PANSS-E ratings in the mild range (see Table 1).
Table 1.
Demographic characteristics and mean scores on composite measures for 91 subjects with schizophrenia.
| Mean | (SD) | |
|---|---|---|
| Age | 39.93 | (11.38) |
| Male/Female | 68/23 | NA |
| Education | 13.32 | (2.35) |
| WASI IQ | 96.38 | (11.56) |
| Age of First Symptoms Symptoms | 19.75 | (6.35) |
| PANSS Total1(Rating Average) | 2.89 | (0.52) |
| PANSS Positive1 Symptoms (Rating Average) | 3.33 | (1.08) |
| PANSS Negative1 Symptoms (Rating Average) | 3.02 | (0.83) |
| Motivation Composite | 2.80 | (1.19) |
| Global Cognition | -0.62 | (0.61) |
| Social Cognition | -0.48 | (0.83) |
| Global Assessment of Functioning (GAF) | 49.22 | (9.40) |
Positive and Negative Syndrome Scale
2.2. Procedures
The measures described below were administered within the context of a larger series of interviews and neuropsychological assessments over a 6 week period. All subjects provided written informed consent after experimental procedures were fully explained, and received nominal payment for their participation in this study.
2.3. Measures
2.3.1. Neurocognition
A composite score of neurocognition was calculated for each participant as the average age-adjusted z-score across the following measures: WAIS-R Digit Symbol, Trail Making Test Part A, Category Fluency, WAIS-R Digit Span, WMS-R Visual Memory Span, California Verbal Learning Test (immediate and delayed recall), Twelve-Point Visuospatial Memory Test (immediate and delayed recall) and Stroop Test (Interference Trial, total time and errors).
2.3.2. Social cognition
A social cognition composite score was calculated for each participant as the average z-score across five measures of social cognition (facial affect recognition, facial memory test immediate recognition, facial memory test delayed recognition, neutral prosody, and emotional prosody). The following measures were used: the Facial Affect Recognition Test (Poole et al., 2000); Immediate and delayed recognition of emotionally neutral faces from the Test of Memory and Learning facial memory test (Reynolds and Bigler, 1994); and Neutral and Emotional prosody subtests from the Florida Affect Battery (see Poole et al., 2000 for a full description of these measures). The social cognition composite was normally distributed, with acceptable internal-consistency reliability (Cronbach's alpha=0.72). In addition, the inter-item correlations of all social cognition were significant and ranged from .24 to .41.
2.3.3. Motivation
In line with previous research, a composite measure of motivation was computed for each participant as the average score on ratings of: Motivation (“ability to sustain goal-directed activities”), Curiosity (“degree to which one is interested in his/her surroundings”), and Sense of Purpose (“realistic integrated life goals”) items from the Quality of Life Scale (Heinrichs et al., 1984; Nakagami et al., 2008). This composite measure was designed with intrinsic motivation in mind or “interest in and enjoyment of an activity for its own sake” (Ryan and Deci, 1985). Although this composite may not be an ideal measure of motivation (as noted by Nakagami et al), there is not a current measure that best defines what specifically is impaired in schizophrenia. Indeed, it is not as yet clear what portion of motivation (extrinsic, intrinsic, etc.) is impaired in schizophrenia, and further research is needed (Barch et al., 2008; 2005).
2.3.4. Functional outcome
Functional outcome was measured using the Global Assessment of Functioning (GAF) of the DSM-IV (in SCID, First et al., 1997). Studies of concurrent validity of the GAF with schizophrenia samples indicate that the GAF is a valid measure of global psychological, social, and occupational functioning (Schwartz, 2007), provided that patients are not suffering from acute psychosis (Startup et al., 2002). The present sample consisted of stable schizophrenia outpatients with average symptom ratings in the mild range on the Positive and Negative Syndrome Scale.
2.4. Data Analytic Plan
Three path analyses were conducted to test the three hypothesized models of functional outcome (see Figure 1a-c). The initial test was a confirmation of the results of Brekke et al. (2005) that social cognition is a mediator of the relationship between neurocognition and functional outcome. The final two analyses were completed to test whether motivation best fits in this model as a causal (or exogenous) factor preceding neurocognition and social cognition, or as a mediator between social cognition and functional outcome. Path analysis is used to test theory-driven causal relationships of constructs in a theoretical model. As with correlation analyses, the directionality (causation) among variables in the model cannot be determined, although path analysis is useful in assessing direct versus indirect effects, and in comparing the fit of multiple competing hypothesized models. A series of regression equations are calculated to determine the direct and indirect effects of variables in the model. Path coefficients describe numerically to what extent a change in a predictor variable affects a dependent variable, with all other variables in the model held constant. A number of prior studies in schizophrenia research have successfully used path analysis to test paths to relapse (Nuechterlein et al., 1992), the influence of expressed emotion on social adjustment (King and Dixon, 1996), and a model of the relationship between cognition, social support, social competence, and outcome (Brekke et al., 2005).
3. Results
Means and standard deviations for all variables are listed in Table 1 and all bivariate correlations between variables are listed in Table 2. There were no significant differences between men and women on any of the measured variables.
Table 2.
Correlation matrix.
| Motivation Composite | Global Cognition | Social Cognition | Global Outcome | |
|---|---|---|---|---|
| Motivation Composite | - | 0.27** | 0.31** | 0.35** |
| Global Cognition | 0.27** | - | 0.66*** | 0.22* |
| Social Cognition | 0.31** | 0.66** | - | 0.39*** |
| Global Outcome | 0.35** | 0.22* | 0.39*** | - |
p < .001
p < .01
p < .05
3.1. Model Fit
In line with previous research we used the following model fit indices for all models tested: χ2; the Normed Fit Index (NFI: values greater than .9 are considered strong); the Root Mean Square Residual (RMR – scores close to 0 indicate good model fit); Adjusted Goodness-of-Fit Index (AGFI – takes into consideration the number of degrees of freedom in the model and scores greater than .9 indicate good fit); the Expected Cross-Validation Index (ECVI – lower values indicate that the model will likely replicate with a different sample); and the Root Mean Square Error of Approximation (RMSEA - values of less than .08 are considered good);. See Table 3 for a summary of all model fit indices by models tested.
Table 3.
Model fit indices.
| Model | χ2 | NFIa | RMRb | AGFIc | ECVId | RMSEAe |
|---|---|---|---|---|---|---|
| Replication Model | 0.22; df = 1; p = .64 | .99 | 0.08 | 0.99 | .11; CI=(.12-.17) | .00; CI=(.00-.22) p(RMSEA<.05)=.68 |
| Motivation as causal/exogenous | 9.66; df = 3; p = .02 | .88 | 0.97 | 0.83 | .26; CI=(.20-.41) | .16; CI=(.05-.27) p(RMSEA<.05)=.05 |
| Motivation as a mediator | 1.39; df = 2; p = .50 | .98 | 0.06 | 0.96 | .19; CI=(.20-.27) | .00; CI=(.00-.19) p(RMSEA<.05)=.57 |
Normed Fit Index
Root Mean Square Residual
Adjusted Goodness-of-Fit Index
Expected Cross-Validation Index
Root Mean Square Error of Approximation
3.2. Test of previous model
We first tested whether we could generally replicate the findings of the final model presented in Brekke et al. (2005), as shown in Figure 1a – although noting that we did not include the measured variables of social competence and social support. In line with their model, we found that the significant direct relationship between neurocognition and outcome (β=.22, t=2.16, p<.05) was fully mediated by social cognition, resulting in a nonsignificant direct relationship between neurocognition and outcome (β=-.06, t=-.47, p=.64). Fifteen percent of the total variance in outcome was explained, slightly less than the 21% identified in the model of Brekke et al. (2005), but highly consistent with their data, given that we did not measure social competence and social support in the current study. See Figure 2a for a summary of the standardized beta weights and error terms. All model fit indices for Figure 2a were excellent (see Table 3).
Figure 2.
a) Similar results in the present study as Green and colleagues' model of Global Functional Outcome - social cognition fully mediates the relationship between neurocognition and functional outcome. Note that the present study did not include social competence or social support as measured variables.
b) The hypothesized model of the relationship between Neurocognition, Social Cognition, Motivation and Functional Outcome, with Motivation as a causal or exogenous factor, is not confirmed due to poor model fit (see Table 3).
c) A hypothesized model of the relationship between Neurocognition, Social Cognition, Motivation and Functional Outcome, with Motivation as a mediating factor is confirmed.
3.3. Motivation as causal/exogenous or a mediator
In line with theoretical and historical views of the role of motivation as a causal/exogenous contributor to neurocognition and social cognition, we first tested motivation as preceding neurocognition (a prerequisite to motivation being an exogenous variable) (Bailey, 1994; Cook and Campbell, 1979). However, with motivation preceding neurocognition, the model indicated very poor fit (see Table 3). In other words the data do not support motivation as a causal/exogenous factor as shown in Figure 2b.
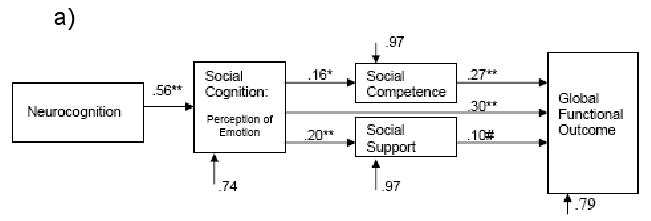
Motivation was subsequently tested as a mediator variable between social cognition and outcome – see Figure 2c.1 In this model, the relationship between neurocognition and motivation was mediated by social cognition (β=.27, t=2.72, p<.05 to β=.12, t=0.92, p=.36), thus the path between neurocognition and motivation was removed from the model. In this final model, motivation was significantly related to outcome (β=.25, t=3.80, p<.05), and the inclusion of motivation reduced the impact of social cognition on outcome from β=.39 to β=.31 (both significant at p<.01). Thus, motivation appears to partially mediate the relationship between social cognition and outcome.2 Furthermore, the total variance explained in outcome rose from 15% without motivation, to 21%. All fit indices for Figure 2c as shown in Table 3 were excellent.
3.3.1. Motivation as a partial mediator – tests of variables in miss-specified order
We tested the model in Figure 2c by randomly ordering the variables in a different configuration to test whether model fit would still hold. This test is important to investigate whether the order specified is important. In this miss-specified order results indicated that χ2 = 15.2 which was statistically significant (p<.001), NFI was .79, AGFI was .71 and RMR was .40. In other words the model in miss-specified order indicated very poor model fit reinforcing the order specified in the model in Figure 2c.
3.3.2. Motivation as a partial mediator – direct and indirect effects
Direct and indirect effects of all variables in Figure 2c are listed in Table 4. Direct effects are the standardized beta weights (direct lines) listed in Figure 2c and represent the independent effect of one variable on a subsequent one controlling for other effects on the endogenous variable. Indirect effects include subsequent effects that an exogenous variable can have on an endogenous variable through another variable. Total effects of an exogenous variable on an endogenous variable include the sum of direct and indirect effects.
Table 4.
Direct and indirect effects of Figure 2c – Motivation as a partial mediator.
| Outcomes | ||
|---|---|---|
| Predictors | Motivation (t-value) | Global Functional Outcome (GAF) (t-value) |
| Neurocognition | ||
| Indirect effects | .20* (2.08) | .26** (2.63) |
| Total effects | .20* (2.08) | .26** (2.63) |
| Social Cognition | ||
| Direct effects | .31** (3.05) | .31** (3.13) |
| Indirect effects | - | .08 (0.71) |
| Total effects | .31** (3.05) | .39*** (3.93) |
p < .001
p < .01
p < .05
4. Discussion
Using path analysis, we examined the best fit for motivation in a model of the relationship between neurocognition, social cognition and functional outcome in schizophrenia. Our data confirm and extend previous findings indicating that social cognition mediates the relationship between neurocognition and functional outcome. Further, motivation appears to play an additional mediating role in the relationship between neurocognition, social cognition and functional outcome. Although previous theorists have hypothesized that motivation may be a causal factor in relation to neurocognition and social performance, the current data do not support this prediction. Instead, motivation appears to provide a similar relationship to functional outcome as social skills or social support (e.g., Brekke et al., 2005), playing an important role in the complex relationship between social cognitive abilities, and community functioning in schizophrenia.
A fuller understanding of this complex relationship is critical if our field is to create empirically-driven cognitive training approaches for schizophrenia that can optimize functional outcome. Our data indicate that, in addition to developing treatments that enhance general neurocognitive functioning, we must also pay close attention to the association between social cognitive abilities and motivation and to treatment approaches that can leverage this relationship. For example, the medial prefrontal cortex (mPFC) is an important neural correlate for the processing of both reward cues and social cognition tasks such as theory of mind (Brunet-Gouet and Decety, 2006; Harris et al., 2007). Indeed, the processing of social stimuli activates associated reward circuitry via MPFC and anterior cingulated cortex, as well as the ventral striatum (Grace et al., 2007; Murray et al., 2008a; Waltz and Gold, 2007). In schizophrenia, however, there are deficits in social information processing, as well as impairments in reward-related processes (Gold, et al. 2008). Interestingly, deficient neural activity in the ventral striatum in response to reward anticipation is related to the severity of negative symptoms (Kapur, 2003). Thus, compromised functioning of the ventral striatal system which uses reward-predicting cues (such as social stimuli) may contribute to the inability to use positive reinforcement to motivate behavior. This inability to activate motivation may then result in negative symptoms such as anhedonia, apathy, and loss of drive. Thus, cognitive treatments which directly target the improvement of social cognitive deficits may enhance motivation in schizophrenia. For example, exercises which improve the brain's ability to use reward-predicting social cues may improve negative symptoms and apathy. In addition, cognitive remediation approaches which emphasize the importance of intrinsic motivation by placing learning in a real-world context and by personalizing and allowing for some control of the learning environment, may be particularly effective in remediating deficits (Medalia et al., 2002).
The work of Burbridge and Barch (2007) suggests another angle by which our field might obtain some leverage into the motivational deficits of schizophrenia. These investigators found that individuals with schizophrenia report increased anhedonia on emotional questionnaires as compared to controls, despite having normal responses to stimuli with emotional content when immediately responding. Further, working memory accounted for a significant amount of the variance between subjects' immediate responses to emotional stimuli and their questionnaire responses. Specifically, intact working memory led to a stronger relationship between the questionnaire and the self-report of emotion experience to the images (Burbridge & Barch 2007). These data suggest that patients' have difficulty holding emotionally evocative stimuli in working memory, which could in turn could lead to decreased motivation, where patients are unable to maintain internal representations of normally rewarding social and emotional stimuli. Such findings indicate that enhancement of working memory functions might improve both social cognition and motivation. However, we have found no effect of successful cognitive training of verbal working memory on either social cognitive measures or measures related to motivation (Fisher, 2009). In contrast to this, in a small preliminary study of combined training of both auditory/verbal cognition and social cognition, we found that subjects showed significant gains in social cognitive measures and in symptom profiles (Aldebot et al., Under Review). In keeping with the results of the path analysis here, our findings suggest that, in order to optimize outcomes in schizophrenia, treatments should simultaneously and vigorously target the critical factors of general cognition, social cognition, and reward processing.
Several limitations of this study should be noted. First, path analysis cannot prove causality but only can test theoretically driven relationships between variables. Though the final model appears to best represent the data, these data are ultimately correlational and therefore cannot conclusively indicate that motivation is not a causal factor. In order to fully explicate the directionality of these variables a longitudinal design must be employed. Second, the measures of motivation and functional outcome we used were interview-based, which may provide a somewhat indirect measure of these constructs as opposed to behavioral or direct observations. For example, some researchers have used the cued reinforcement reaction time measure as a proxy measure of motivation (e.g., Cools et al., 2005; Murray et al., 2008c). In terms of functional outcome, some research has indicated that the GAF is predicted most heavily by clinical symptoms (e.g., Gaite et al., 2005). Thus the use of more direct measures of functioning as well as motivation might allow for a more clear measure of these constructs.
The results of our path analysis indicate that cognitive treatment development in schizophrenia must explicitly and aggressively focus not only on general cognition, but also on social cognition and reward-related processing (motivation). We must fully understand and systematically harness these factors if we are to maximize functional gains for our patients and if we are to assist individuals with schizophrenia to achieve an optimal, enduring, and meaningful reintegration into the community.
Footnotes
We also tested the role of motivation as a mediator without including social cognition in the model. Our findings indicated that motivation mediated the relationship between neurocognition and outcome.
One additional possible ordering of motivation as mediator would be to switch the placement of motivation and social cognition. In this model the hypothesized direction would suppose that faulty neurocognition leads to impairment with motivation, which subsequently causes difficulty in social cognition, resulting in poor outcome. We did not include this model because of the lack of previous data to support this ordering. Nonetheless, similar to the causal/exogenous model (Figure 2b) this model resulted in poor fit.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldebot S, Fisher M, Alexander P, Holland C, Genevsky A, Rose D, Hooker C, Vinogradov S. Combining Computerized Social Cognitive Training with Neuroplasticity-Based Auditory Training in Schizophrenia. doi: 10.3371/CSRP.SAFI.012513. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KD. Methods of Social Research. New York: The Free Press; 1994. [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31(4):875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Barch DM, Yodkovik N, Sypher-Locke H, Hanewinkel M. Intrinsic motivation in schizophrenia: Relationships to cognitive function, depression, anxiety, and personality. Journal of Abnormal Psychology. 2008;117(4):776–787. doi: 10.1037/a0013944. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox or the group of schizophrenias. Oxford, England: International Universities Press; 19111950. [Google Scholar]
- Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophrenia Research. 2005;80(23):213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148(23):75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. Journal of Abnormal Psychology. 2007;116(1):30–42. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- Cook TD, Campbell DT. Quasi Experimentation: Design and Analysis Issues for Field Settings. Hopewell, N.J.: Houghton Mifflin Co.; 1979. [Google Scholar]
- Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. Tryptophan depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005;30(7):1362–1373. doi: 10.1038/sj.npp.1300704. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The Functional Significance of Social Cognition in Schizophrenia: A Review. Schizophr Bull. 2006;32(Suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty NM, Rhinewine JP, Nienow TM, Cohen AS. Affective reactivity of language symptoms, startle responding, and inhibition in schizophrenia. Journal of Abnormal Psychology. 2001;110(1):194–198. doi: 10.1037//0021-843x.110.1.194. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-Based Cognitive Training in Schizophrenia: An Interim Report on the Effects 6 Months Later. Schizophrenia Bulletin. 2009 doi: 10.1093/schbul/sbn170. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaite L, Vazquez-Barquero JL, Herran A, Thornicroft G, Becker T, Sierra-Biddle D, Ruggeri M, Schene A, Knapp M, Vazquez-Bourgon J. Main determinants of Global Assessment of Functioning score in schizophrenia: a European multicenter study. Compr Psychiatry. 2005;46(6):440–446. doi: 10.1016/j.comppsych.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(13):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II. Schizophrenia epigenesis: Past, present, and future. Acta Psychiatrica Scandinavica Supplementum. 1994;90(384 Suppl):26–33. doi: 10.1111/j.1600-0447.1994.tb05887.x. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the ‘right stuff’? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Harris LT, McClure SM, van den Bos W, Cohen JD, Fiske ST. Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cogn Affect Behav Neurosci. 2007;7(4):309–316. doi: 10.3758/cabn.7.4.309. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology. 2007;116(2):268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: An instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a State of Aberrant Salience: A Framework Linking Biology, Phenomenology, and Pharmacology in Schizophrenia. American Journal of Psychiatry. 2003;130:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kay SR, Sevy S. Pyramidical Model of Schizophrenia. Schizophr Bull. 1990;16(3):537–545. doi: 10.1093/schbul/16.3.537. [DOI] [PubMed] [Google Scholar]
- King S, Dixon MJ. The influence of expressed emotion, family dynamics, and symptom type on the social adjustment of schizophrenic young adults. Arch Gen Psychiatry. 1996;53(12):1098–1104. doi: 10.1001/archpsyc.1996.01830120028007. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. In: Dementia praecox and paraphrenia. Barclay RM, translator. Edinburgh: E & S Livingstone; 19191913. [Google Scholar]
- Medalia A, Revheim N, Casey M. Remediation of problem-solving skills in schizophrenia: evidence of a persistent effect. Schizophr Res. 2002;57(23):165–171. doi: 10.1016/s0920-9964(01)00293-6. [DOI] [PubMed] [Google Scholar]
- Meehl PF. Schizotaxia, schizotypy, and schizophrenia. American Psychologist. 1962;17:827–838. [Google Scholar]
- Meehl PF. Hedonic capacity: some conjectures. Bulletin of the Menninger Clinic. 1975;39(4):295–307. [PubMed] [Google Scholar]
- Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, Robbins TW, Bullmore ET, Jones PB. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008a;34(5):848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Clark L, Corlett PR, Blackwell AD, Cools R, Jones PB, Robbins TW, Poustka L. Incentive motivation in first-episode psychosis: a behavioural study. BMC Psychiatry. 2008b;8:34. doi: 10.1186/1471-244X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, Jones PB, Bullmore ET, Robbins TW, Fletcher PC. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008c;13(3):239, 267–276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami E, Xie B, Hoe M, Brekke JS. Intrinsic motivation, neurocognition and psychosocial functioning in schizophrenia: Testing mediator and moderator effects. Schizophrenia Research. 2008;105(13):95–104. doi: 10.1016/j.schres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Snyder KS, Mintz J. Paths to relapse: possible transactional processes connecting patient illness onset, expressed emotion, and psychotic relapse. Br J Psychiatry Suppl. 1992;(18):88–96. [PubMed] [Google Scholar]
- Poole JH, Tobias FC, Vinogradov S. The functional relevance of affect recognition errors in schizophrenia. J Int Neuropsychol Soc. 2000;6(6):649–658. doi: 10.1017/s135561770066602x. [DOI] [PubMed] [Google Scholar]
- Rado S. Dynamics and classification of disordered behavior. American Journal of Psychiatry. 1953;110:406–426. doi: 10.1176/ajp.110.6.406. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Bigler ED. Test of Memory and Learning Examiner's Manual. Austin, Texas: 1994. [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schwartz RC. Concurrent validity of the Global Assessment of Functioning Scale for clients with schizophrenia. Psychol Rep. 2007;100(2):571–574. doi: 10.2466/pr0.100.2.571-574. [DOI] [PubMed] [Google Scholar]
- Startup M, Jackson MC, Bendix S. The concurrent validity of the Global Assessment of Functioning (GAF) Br J Clin Psychol. 2002;41(Pt 4):417–422. doi: 10.1348/014466502760387533. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93(13):296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Yager JA, Ehmann TS. Untangling Social Function and Social Cognition: A Review of Concepts and Measurement. Psychiatry: Interpersonal and Biological Processes. 2006;69(1):47–68. doi: 10.1521/psyc.2006.69.1.47. [DOI] [PubMed] [Google Scholar]