Abstract
Purpose
To determine the prevalence and causes of decreased visual acuity (VA) in a population-based study of minority preschool children.
Design
Population-based cross-sectional study.
Participants
Children 30 to 72 months of age in 44 census tracts in Los Angeles County.
Methods
A population-based sample of children underwent comprehensive ophthalmic evaluation including monocular VA testing, cover testing, cycloplegic autorefraction, anterior segment and fundus evaluation, and VA retesting with refractive correction. The prevalence and etiology of decreased VA were determined, for both presenting and best-measured VA, and better eye and worse eye.
Main Outcome Measures
Prevalence of decreased presenting and best-measured VA with an identifiable ophthalmic etiology.
Results
Presenting VA was assessed in 3207 children and best-measured VA in 3364 children. While nearly a third of cases of worse-eye decreased presenting VA were without an identifiable ophthalmic etiology, this proportion decreased with increasing age. Decreased presenting VA that resolved with retesting and was associated with uncorrected refractive error was present in the worse eye of 4.3% of African-American children and 5.3% of Hispanic children, and in the better eye of 1.9% of African-American children and 1.7% of Hispanic children. Decreased best-measured VA that was not immediately correctable with spectacles and that was due to ocular disease, unilateral or bilateral amblyopia, or probable bilateral ametropic amblyopia, was seen in the worse eye of 1.5% of African-American children and 1.9% of Hispanic children, and in the better eye of 0.8% of African-American and 0.6% of Hispanic children. Amblyopia related to refractive error was the most common cause.
Conclusion
Over 5% of African-American and Hispanic preschool children in Los Angeles County have either correctable visual impairment from uncorrected refractive error, or visual impairment from amblyopia related to refractive error.
Introduction
Little has been published on the prevalence of visual impairment (VI) in children less than 6 years of age, partly because reliable visual acuity (VA) measurement is challenging in children. Registries and blind schools track severe bilateral childhood VI,1 2 but only population-based studies can accurately assess the prevalence of unilateral and moderate bilateral VI; these are less devastating than severe bilateral VI, but much more common. Unilateral amblyopia in childhood confers a significant lifetime risk of bilateral impairment from loss of vision in the better eye,3 4 while the impact of bilateral amblyopia on development and school readiness remains unknown. In addition, simple refractive error effectively causes VI in children so long as it remains uncorrected.5
Childhood VI prevalence estimates from countries practicing population-wide vision screening6 7 use varying amblyopia definitions that don’t always include amblyopia risk factors.8 9 10 11 12 13 14 Recently the standardized Refractive Error Study in Children (RESC) protocol15 has been used to assess refractive error and VI in 5- to 15-year-old children around the world, 15 16 17 18 19 20 21 22 23 24 25 and similar methodology was used to study 6- to 7- year-old Australian children.26 27 28 Data for the United States (US), however, are sorely lacking. The often-cited study of Preslan and Novak was designed only to assess the need for and efficacy of screening in a single school;29 until now, there has not been a population-based study of early childhood VI in the US for over four decades.30
The Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) and the Baltimore Pediatric Eye Disease Study (BPEDS),31 using identical methodology and shared protocols, examined the prevalence of early childhood VI in the US. This report from the MEPEDS details the prevalence and causes of unilateral and bilateral VI in a population-based sample of minority preschool children in Los Angeles County.
Methods
This study was supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health and was conducted in Los Angeles County, CA. The protocol and informed consent forms were reviewed and approved by the Institutional Review Board (IRB)/Ethics Committee of the Los Angeles County University of Southern California Medical Center, and complied with current Health Insurance Portability and Accountability Act regulations. A parent or guardian of each study participant gave written informed consent. Study oversight was provided by an independent data monitoring and oversight committee.
Study Cohort
The study population consisted of children, aged 30 to 72 months, living within 44 census tracts in and around the city of Inglewood in Los Angeles County, California who were participants in the MEPEDS. Details of the study design and sampling plan have been described previously.32 In brief, after a door-to-door census of all dwelling units within the targeted census tracts, parents of eligible children were given an explanation of the study and invited to participate. Written informed consent was obtained from prospective participants and an appointment was scheduled for a comprehensive eye examination.
Clinic Ocular Examination and Interview
The clinic visit included a structured interview administered by MEPEDS trained interviewers and a comprehensive eye examination performed by MEPEDS optometrists or ophthalmologists who were trained and certified using standardized protocols.32 Details of the interview are reported elsewhere.32 Ethnicity was determined by parental self-report. All age-appropriate clinical tests were attempted on all children, without exclusions for developmental delay or disability.
Ocular alignment was tested by unilateral cover testing (UCT) and alternate cover testing (ACT) of standardized duration, at 6 m fixation and 40 cm fixation, with and without correction (if worn). Hirschberg testing at near was used when UCT could not be performed.
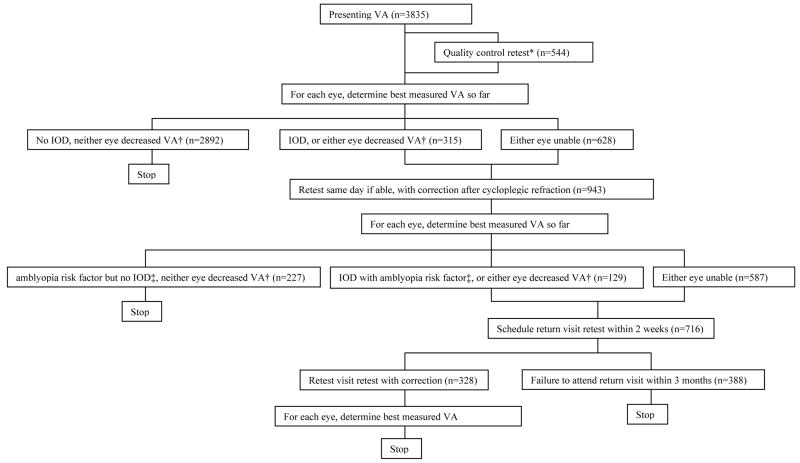
Monocular distance VA was tested in children aged ≥30 months with single-surround HOTV optotypes on the electronic VA tester33 with the Amblyopia Treatment Study protocol,34 with naming or matching of letters. The specific MEPEDS VA protocol has been described in detail.35 Presenting VA was tested with correction, if worn. On days when two examiners were available, a sample of children ≥36 months (including the first child for each examiner, and those with VA worse than 20/32) underwent quality control (QC) VA retesting by a different examiner (with presenting correction if worn). Presenting VA was defined as the first result. The better of presenting VA and QC VA (if performed) was evaluated for each eye: if VA was “decreased” in either eye (see below), or if there was a 2-line interocular difference (IOD) with VA 20/32 or worse in the worse eye, VA was retested after cycloplegic refraction, with full refractive correction. Taking the best of all test results for each eye, children who still had decreased VA in either eye, or who had a 2-line IOD with VA of 20/32 or worse in the worse eye and an amblyopia risk factor (see below), were scheduled for return-visit retesting. Here, VA was retested with correction without cycloplegia, to confirm that decreased VA was not an artifact of testing after dilation. Non-cycloplegic retesting employed full correction of myopia and astigmatism. Hyperopia was uncorrected if <1.50 diopters (D); if ≥1.50 D it was undercorrected by 1.50 D, allowing for incomplete relaxation of accommodation. Children untestable at the original visit were scheduled for a return visit and sent home with HOTV letters for practice. The final, “best-measured” VA for each eye was the best of all test results recorded for that eye. The protocol is summarized in Figure 1.
Figure 1. Visual acuity testing protocol.
VA: visual acuity. IOD: inter-ocular difference in visual acuity. *Quality control retest performed in children >=36 months under same conditions as presenting VA test (with child’s own glasses if worn), if a second examiner was available, for the first exam of the day for each examiner, and for children with VA worse than 20/32 in either eye. † IOD is defined as inter-ocular VA difference >=2 lines with 20/32 or worse in the worse eye. Decreased VA is defined as worse than 20/50 at any age, or worse than 20/40 if >=48 months of age. ‡ See Methods for definitions of amblyopia risk factors. Of the 388 children who did not attend the return visit retest, 326 (84%) had been unable to perform VA testing on the first visit.
Cycloplegic refraction was performed with the Retinomax Autorefractor (Right Manufacturing, Virginia Beach, VA) at least 30 minutes after cycloplegia with two drops (five minutes apart) of 1% cyclopentolate. Cycloplegic retinoscopy was performed if Retinomax readings with confidence ratings of ≥8 were not obtained in both eyes after 3 attempts. If parents did not allow eyedrops, non-cycloplegic retinoscopy was performed. Anterior segment and fundus examinations followed refraction.
Definitions of Decreased Visual Acuity and Visual Impairment
Decreased VA was defined a priori by investigator consensus as worse than 20/50 in children aged 30–47 months, or worse than 20/40 for children ≥48 months. Retrospective analysis showed that ≥48 months, 99% of children without pathology or refractive error achieve 20/40 or better, compared to only 91% of normal children <48 months; 96% of these achieve 20/50 or better (Pan Y, Tarczy-Hornoch K, Cotter SA et al. Visual acuity norms in preschool children: the Multi-Ethnic Pediatric Eye Disease Study. Optom Vis Sci in press). Because some children had decreased VA without an identifiable etiology, visual impairment (VI) was defined as decreased VA with an identifiable ophthalmic etiology. Prevalence of decreased VA and of VI was analyzed for presenting and best-measured VA. Better and worse eyes were defined separately for presenting and best-measured VA. For equal VA, the right eye was defined as both the better and worse eye. VI severity was classified as mild (decreased VA, but better than 20/80), moderate (VA 20/80 or worse but better than 20/200), or severe (VA 20/200 or worse).
Decreased presenting VA had an identifiable ophthalmic etiology, and was classified as VI, if one of two conditions was met: (1) “non-resolving”: best-measured VA was also decreased, with an identifiable etiology (defined below); or (2) “resolving, with refractive error”: VA normalized with retesting, and visually significant uncorrected refractive error (defined below) was present in that eye at the time of presenting VA. Decreased presenting VA with no identifiable ophthalmic etiology was either: (1) “non-resolving”: best-measured VA was decreased without an identifiable etiology; or (2) “resolving”: VA normalized with retesting, but was not associated with visually significant uncorrected refractive error at presentation.
Visually significant uncorrected refractive error was defined a priori as spherical equivalent (SE) myopia of −0.5 D or more, SE hyperopia of 3.0 D or more, astigmatism (any axis) of 2.0 D or more if 30–36 months old, or 1.5 D or more for children >36 months of age. For children presenting with spectacles, decreased presenting VA that resolved was attributed to refractive error only if the residual uncorrected refractive error met the criteria above.
Children with decreased presenting VA and significant uncorrected refractive error sometimes normalized during QC retesting without refractive correction, and never qualified for retesting with correction. Because QC testing was done in only a subset of children, these cases are classified the same way as their peers who had no QC test and normalized during the retest with correction (“resolving, with refractive error.”) All children whose VA normalized during retesting could theoretically have normalized due to practice rather than refractive correction, so we performed a secondary analysis adjusting for the practice effect. Considering the subset of children in the “resolving, with refractive error” category who underwent QC retesting, the proportion whose VA normalized during the QC test was used to estimate the proportion of all children in that category whose presenting VA might be attributable to testing inexperience rather than refractive error.
Decreased best-measured VA was classified as VI if one or more of the following ophthalmic etiologies were identified on examination: (1) ocular disease (normal vision precluded by the presence of an anterior segment abnormality, fundus abnormality or nystagmus); (2) amblyopia (defined below); (3) probable amblyopia (defined below); (4) probable refractive error (visually significant uncorrected refractive error at presentation, unable to complete either the same-day or return-visit retest with correction). A pediatric ophthalmologist (KTH) reviewed every case of decreased VA to determine the appropriate classification.
Unilateral amblyopia was defined a priori as a 2-line IOD in best-measured VA, and at least one of the following unilateral amblyopia risk factors: constant or intermittent strabismus on examination; strabismus surgery (from in-home interview); anisometropia consistent with the eye with worse VA (≥1.00 D SE anisohyperopia, ≥3.00 D SE anisomyopia, or ≥1.50 D anisoastigmatism); or evidence of past or present visual axis obstruction (e.g., cataract, pseudophakia, aphakia, corneal opacity, ptosis, or eyelid hemangioma). Bilateral amblyopia was defined a priori as bilateral decreased best-measured VA (see above) with either bilateral evidence of visual axis obstruction or bilateral ametropia (≥4.00 D SE hyperopia, ≥6.00 D SE myopia, or ≥2.50 D astigmatism). VI in children meeting amblyopia definitions but with co-existing fundus or anterior segment abnormalities precluding normal vision was attributed to the ocular abnormality rather than to amblyopia. Probable amblyopia was a combination of VA and refractive error just short of meeting bilateral ametropic amblyopia criteria. For the better eye, this meant bilaterally decreased VA, with, one eye meeting and the other within 0.25 D of meeting refraction criteria for bilateral ametropia. For the worse eye, probable amblyopia meant VI in the better eye was attributable to probable amblyopia, or both eyes were ametropic with the better eye one line short of decreased VA criteria. Neither amblyopia nor probable amblyopia was diagnosed as the cause of VI unless the child had at least one VA retest with correction.
Statistical Analysis
The analysis cohort for presenting VA was defined as all children able to complete presenting VA measurements in both eyes. For best-measured VA it was defined as all children able to complete VA measurements in both eyes by the end of the retesting protocol (see Figure 1). Prevalence was calculated as the ratio of the number of individuals with VI to the total number evaluated. Associations of VI with age group, ethnicity and gender were assessed using multivariate logistic regression (SAS software 9.1; SAS institute, Inc., Cary, NC). Fisher’s exact test was used for comparison of proportions between groups. Confidence intervals (CI) reported for proportions are exact binomial 95% confidence intervals. All analyses employed a 0.05 significance level.
Results
Seventy-seven percent of eligible children were examined. According to the in-home interview, the child’s vision was “very poor” or “blind” for 0.2% of non-participants and 0.3% of participants (p=0.78). Further comparison of participants and non-participants is published elsewhere.36 Of 6043 African-American and Hispanic children examined (51% male), 3835 (1917 Hispanic, 1918 African-American) were >=30 months of age. Of these, 1592 African-American children (83%) were able to perform VA testing for both eyes on the first attempt, as were 1615 Hispanic children (84%); these children constitute the analysis cohort for presenting VA. An additional 71 African-American children and 86 Hispanic children who were unable to perform VA testing at presentation were able to complete testing in both eyes by the end of the retesting protocol. Thus, best-measured VA was analyzed in 1663 African-American (87%) and 1701 Hispanic children (89%). Among these, cycloplegic eyedrops were refused by 68 African-American (4%) and 14 Hispanic participants (1%). Only 1.6% of children with presenting VA measurements wore refractive correction at the time of presentation.
Presenting visual acuity
Presenting VA was decreased in the worse eye of 8.6% of African-American children and 10.5% of Hispanic children, and in the better eye of 3.9% of African-American children and 3.5% of Hispanic children. For the worse eye, 69% of decreased presenting VA in African-American children and 67% in Hispanic children was attributable to an identifiable ophthalmic etiology and thus classified as VI. For the better eye, 71% of decreased presenting VA in both African-American and Hispanic children was attributable to an identifiable ophthalmic etiology and classified as VI. Decreased VA without an identifiable ophthalmic etiology is discussed further below.
VI at presentation was seen in the worse eye of 5.9% of African-American children and 7.1% of Hispanic children, and in the better eye of 2.8% of African-American children and 2.5% of Hispanic children (Table 1). Multivariate logistic regression adjusting for gender, ethnicity and age group found no association between VI in the worse eye and either gender (p=0.44) or ethnicity (p=0.18), but a significant association with age group (p=0.004). Children in the youngest age group were less likely to have VI in the worse eye than children in the oldest (reference) age group (OR 0.17; 95% CI 0.04 – 0.69), and 48–59 month-old children were more likely to do so than the oldest children (OR 1.48; 95% CI 1.06 – 2.08). For the better eye, there was no association of VI with gender (p=0.48), ethnicity (p=0.61), or age group (p=0.11).
Table 1.
Prevalence of presenting visual impairment (decreased visual acuity with an ophthalmic etiology) stratified by age group, ethnicity and severity of visual impairment.
| Visual Acuity Level |
African-American | Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (months) | Age (months) | |||||||||
| 30–35 (n=101) |
36–47 (n=416) |
48–59 (n=537) |
60–72 (n=538) |
Total (n=1592) |
30–35 (n=98) |
36–47 (n=446) |
48–59 (n=529) |
60–72 (n=542) |
Total (n=1615) |
|
| Worse eye, stratified by severity: n (% of age group) (95% CI*) | ||||||||||
| >20/80 | 1 (1.0) | 6 (1.4) | 25 (4.7) | 23 (4.3) | 55 (3.5) | 0 | 14 (3.1) | 34 (6.4) | 23 (4.2) | 71 (4.4) |
| 20/80 to 20/160 | 1 (1.0) | 17 (4.1) | 13 (2.4) | 7 (1.3) | 38 (2.4) | 0 | 18 (4.0) | 13 (2.5) | 7 (1.3) | 38 (2.4) |
| <=20/200 | 0 | 0 | 0 | 1 (0.2) | 1 (0.1) | 0 | 1 (0.2) | 3 (0.6) | 1 (0.2) | 5 (0.3) |
| Total | 2 (2.0) (0.2–7.0) | 23 (5.5) (3.5–8.2) | 38 (7.1) (5.1–9.6) | 31 (5.8) (4.0–8.1) | 94 (5.9) (4.8–7.2) | 0 | 33 (7.4) (5.2–10.2) | 50 (9.5) (7.1–12.3) | 31 (5.7) (3.9–8.0) | 114 (7.1) (5.9–8.4) |
| Better eye, stratified by severity: n (% of age group) (95% CI) | ||||||||||
| >20/80 | 0 | 8 (1.9) | 12 (2.2) | 12 (2.2) | 32 (2.0) | 0 | 5 (1.1) | 18 (3.4) | 12 (2.2) | 35 (2.2) |
| 20/80 to 20/160 | 1 (1.0) | 5 (1.2) | 6 (1.1) | 0 | 12 (0.8) | 0 | 3 (0.7) | 1 (0.2) | 1 (0.2) | 5 (0.3) |
| <=20/200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 1 (1.0) (0–5.4) | 13 (3.1) (1.7–5.3) | 18 (3.4) (2.0–5.3) | 12 (2.2) (1.2–3.9) | 44 (2.8) (2.0–3.7) | 0 | 8 (1.8) (0.8–3.5) | 19 (3.6) (2.2–5.6) | 13 (2.4) (1.3–4.1) | 40 (2.5) (1.8–3.4) |
CI: 95% confidence interval; calculated for total visual impairment in each age category.
Pooling all children, 61% (126/208) of worse eye VI was classified as mild, and 80% (67/84) of better eye VI was mild. The proportion of VI cases that were mild increased with increasing age group (Table 1). In the worse eye, children under 48 months of age had mild VI in 36% of cases, compared to 70% in those ≥48 months of age (p=0.00001); in the better eye, children <48 months of age had mild VI in 59% of cases, compared to 87% in those ≥48 months of age (p=0.01).
Presenting visual acuity: visual impairment from uncorrected refractive error
Presenting VI was stratified according to whether or not VA normalized with retesting (Table 2). Most worse eye cases in both African-American and Hispanic children (73% and 75%, respectively) resolved and were associated with a pre-specified level of visually significant uncorrected refractive error (see Methods) (“Resolving, with refractive error”, Table 2). Similarly, most better-eye cases (68% in both ethnic groups) resolved and were associated with significant refractive error. The remaining cases did not resolve over the course of retesting, and were associated with a variety of other identifiable ophthalmic etiologies (“Not resolving”, Table 2). These etiologies are discussed below in the context of best-measured VA.
Table 2.
Prevalence of presenting visual impairment (decreased visual acuity with an ophthalmic etiology) stratified by age group, ethnicity and type of visual impairment.
| Type of Visual impairment |
African-American | Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (months) | Age (months) | |||||||||
| 30–35 (n=101) |
36–47 (n=416) |
48–59 (n=537) |
60–72 (n=538) |
Total (n=1592) |
30–35 (n=98) |
36–47 (n=446) |
48–59 (n=529) |
60–72 (n=542) |
Total (n=1615) |
|
| Worse eye, stratified by resolution with retesting protocol:* n (% of age group) (95% CI**) | ||||||||||
| Non-resolving | 0 | 5 (1.2) | 14 (2.6) | 6 (1.1) | 25 (1.6) | 0 | 6 (1.3) | 14 (2.6) | 9 (1.7) | 29(1.8) |
| Resolving, with refractive error | 2 (2.0) | 18 (4.3) | 24 (4.5) | 25 (4.6) | 69 (4.3) | 0 | 27 (6.1) | 36 (6.8) | 22 (4.1) | 85 (5.3) |
| Total | 2 (2.0) (0.2–7.0) | 23 (5.5) (3.5–8.2) | 38 (7.1) (5.1–9.6) | 31 (5.8) (4.0–8.1) | 94 (5.9) (4.8–7.2) | 0 | 33 (7.4) (5.2–10.2) | 50 (9.5) (7.1–12.3) | 31 (5.7) (3.9–8.0) | 114 (7.1) (5.9–8.4) |
| Better eye, stratified by resolution with retesting protocol: n (% of age group) (95% CI) | ||||||||||
| Non-resolving | 0 | 2 (0.5) | 9 (1.7) | 3 (0.6) | 14 (0.9) | 0 | 1 (0.2) | 8 (1.5) | 4 (0.7) | 13 (0.8) |
| Resolving, with refractive error | 1 (1.0) | 11 (2.6) | 9 (1.7) | 9 (1.7) | 30 (1.9) | 0 | 7 (1.6) | 11 (2.1) | 9 (1.7) | 27 (1.7) |
| Total | 1 (1.0) (0–5.4) | 13 (3.1) (1.7–5.3) | 18 (3.4) (2.0–5.3) | 12 (2.2) (1.2–3.9) | 44 (2.8) (2.0–3.7) | 0 | 8 (1.8) (0.8–3.5) | 19 (3.6) (2.2–5.6) | 13 (2.4) (1.3–4.1) | 40 (2.5) (1.8–3.4) |
Non-resolving: visual impairment by best measured as well as presenting visual acuity. Resolving, with refractive error: resolved during retesting protocol (Figure 1), with significant uncorrected refractive error at presentation: spherical equivalent (SE) hyperopia 3.0 diopter (D) or more, SE myopia 0.5 D or more, astigmatism 2.0 D or more at any age, or astigmatism 1.5 D or more for children >36 months of age.
CI: 95% confidence interval; calculated for total visual impairment in each age category.
The prevalence of worse eye VI at presentation attributed to refractive error was 4.3% in African-American children (95% C.I. 3.4, 5.5), and 5.3% in Hispanic children (95% C.I. 4.2, 6.5); this difference was not significant (p=0.25). For the better eye, VI at presentation was attributed to refractive error in 1.9% of African-American children (95% C.I. 1.3, 2.7), and 1.7% of Hispanic children (95% C.I. 1.1, 2.4) (p=0.69). The type of refractive error associated with cases of resolving VI differed according to ethnicity (Table 3). Among African-American children, 45% of the refractive errors associated with resolving worse eye VI had an astigmatic component, compared to 72% for Hispanic children (p=0.0009). SE myopia was present in 45% of African-American children with resolving refractive-error associated worse eye VI, and only 18% of Hispanic children (p=0.0003). Hyperopia was present in a similar proportion of refractive error-associated VI in both ethnicities (26% and 28% for African-American and Hispanic cases, respectively) (p=0.86).
Table 3.
Type and magnitude of refractive errors associated with presenting visual impairment in the worse eye stratified by ethnicity (resolving, with refractive error).
| Type of refractive error* | African-American (n=69) | Hispanic (n=85) | P value†† | ||||
|---|---|---|---|---|---|---|---|
| Frequency and proportion of cases** N (%) | Mean refractive error in diopters (SD†) | Refractive error range in diopters | Frequency and proportion of cases** N (%) | Mean refractive error in diopters (SD†) | Refractive error range in diopters | ||
| Astigmatism | 31 (45%) | 2.42 (0.76) | 1.50 to 4.50 | 61 (72%) | 2.61 (0.96) | 1.50 to 5.25 | 0.0009 |
| Myopia | 31 (45%) | −1.60 (0.97) | −0.50 to −4.63 | 15 (18%) | −1.42 (0.86) | −0.63 to −3.50 | 0.0003 |
| Hyperopia | 18 (26%) | 4.44 (1.55) | 3.13 to 8.13 | 24 (28%) | 4.21 (0.99) | 3.00 to 6.38 | 0.86 |
Astigmatism: 2.0 diopters (D) or more, or 1.5 D or more if >36 months of age. Myopia: spherical equivalent (SE) 0.5 D or more. Hyperopia: SE 3.0 D or more.
16% of African-American children met both SE and astigmatic criteria.18% of Hispanic children met both SE and astigmatic criteria.
SD: standard devation.
Fisher’s exact test comparing proportions between ethnicities.
Because QC retesting was performed prior to retesting with correction in a subset of children with VI at presentation, we can estimate the proportion of resolving VI associated with refractive error that might have resolved due to repeated testing, rather than being attributable to refractive error. Among African-American children with resolving worse-eye VI associated with refractive error, 33 had QC testing, and 16 of these resolved with QC testing alone; by extrapolation, 48% of all refractive error-associated resolving VI might have resolved due to retesting, regardless of refractive correction. Adjusting the observed prevalence of 4.3% for refractive error-associated VI accordingly, a conservative estimate for the prevalence of resolving worse-eye VI actually attributable to refractive error rather than retesting would be 2.2%. For Hispanic children, 23 of 42 children (55%) had worse-eye VI that resolved with QC testing. Adjusting the observed prevalence of 5.3% for refractive error-associated VI accordingly, a conservative estimate for the overall prevalence of correctable worse-eye VI attributable to refractive error rather than retesting would be 2.4%.
Best-measured visual acuity
Best-measured VA was decreased in the worse eye of 3.1% of African-American children (52/1663) and 3.8% of Hispanic children (64/1701), and in the better eye of 1.4% of African-American children (24/1663) and 1.4% of Hispanic children (24/1701). For the worse eye, 58% of decreased best-measured VA in both African-American children (30/52) and Hispanic children (37/64) was attributable to an identifiable ophthalmic etiology and thus classified as VI. For the better eye, 54% of decreased best-measured VA in African-American children (13/24) and 50% in Hispanic children (12/24) was attributable to an identifiable ophthalmic etiology and thus classified as VI.
VI according to best-measured VA was seen in the worse eye of 1.8% of African-American children (30/1663) and 2.2% of Hispanic children (37/1701) (Table 4). It was seen in the better eye of 0.8% of African-American children (13/1663) and 0.7% of Hispanic children (12/1701) (Table 4). Multivariate logistic regression adjusting for gender, ethnicity, and age group found no association between best-measured VI in the worse eye and either gender (p=0.29), ethnicity (p=0.42), or age group (p=0.27). For the better eye, there was no association with either gender (p=0.46) or ethnicity (p=0.83), but there was a significant association with age group (p=0.04). Children in the 48–59 month-old age group were more likely to have VI in the better eye than children in the oldest (reference) age group (OR 2.70; 95% CI 1.05 – 6.92). Pooling results from all children, 51 of all 67 cases (76%) of worse eye VI were mild, as were 23 of 25 cases (92%) of better eye VI.
Table 4.
Prevalence of best-measured visual impairment (decreased visual acuity with an ophthalmic etiology) stratified by age group, ethnicity and severity of visual impairment.
| Visual Acuity level |
African-American | Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (months) | Age (months) | |||||||||
| 30–35 (n=128) |
36–47 (n=456) |
48–59 (n=540) |
60–72 (n=539) |
Total (n=1663) |
30–35 (n=145) |
36–47 (n=480) |
48–59 (n=534) |
60–72 (n=542) |
Total (n=1701) |
|
| Worse eye, stratified by severity: n (% of age group) (95% CI*) | ||||||||||
| >20/80 | 0 | 4 (0.9) | 12 (2.2) | 5 (0.9) | 21 (1.3) | 0 | 6 (1.3) | 14 (2.6) | 10 (1.9) | 30 (1.8) |
| 20/80 to 20/160 | 0 | 3 (0.7) | 3 (0.6) | 2 (0.4) | 8 (0.5) | 0 | 4 (0.8) | 0 | 1 (0.2) | 5 (0.3) |
| <=20/200 | 0 | 0 | 1 (0.2) | 0 | 1 (0.2) | 0 | 0 | 1 (0.2) | 1 (0.2) | 2 (0.1) |
| Total | 0 | 7 (1.5) (0.6–3.1) | 16 (3.0) (1.7–4.8) | 7 (1.3) (0.5–2.7) | 30 (1.8) (1.2–2.6) | 0 | 10 (2.1) (1.0–3.8) | 15 (2.8) (1.6–4.6) | 12 (2.2) (1.2–3.8) | 37 (2.2) (1.5–3.0) |
| Better eye, stratified by severity: n (% of age group) (95% CI) | ||||||||||
| >20/80 | 0 | 1 (0.2) | 9 (1.7) | 3 (0.6) | 13 (0.8) | 0 | 1 (0.2) | 7 (1.3) | 2 (0.4) | 10 (0.6) |
| 20/80 to 20/160 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.2) | 0 | 1 (0.2) | 2 (0.1) |
| <=20/200 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 0 | 1 (0.2) (0–1.2) | 9 (1.7) (0.8–3.1) | 3 (0.6) (0.1–1.6) | 13 (0.8) (0.4–1.3) | 0 | 2 (0.4) (0.1–1.5) | 7 (1.3) (0.5–2.7) | 3 (0.6) (0.1–1.6) | 12 (0.7) (0.4–1.2) |
CI: 95% confidence interval; calculated for total visual impairment in each age category.
Best-measured visual acuity: etiology of visual impairment
The causes of VI according to best-measured VA are shown in Table 5. Structural eye anomalies or nystagmus (“ocular disease”, Table 5) causing bilateral VI were seen in only 2 African-American children (one case of foveal hypoplasia with aniridia and one case of isolated foveal hypoplasia) and 2 Hispanic children (one case of foveal hypoplasia with partial aniridia and one case of nystagmus). Two additional Hispanic children had unilateral VI from ocular disease (one case of unilateral optic nerve hypoplasia, and one corneal scar). The overall prevalence of VI from ocular disease was 0.2% (6/3835) for the worse eye and 0.1% (4/3835) for the better eye.
Table 5.
Cause-specific prevalence of best-measured visual impairment (decreased visual acuity with an ophthalmic etiology) stratified by age and ethnic group.
| African-American | Hispanic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age in months | 30–35 (n=128) |
36–47 (n=456) |
48–59 (n=540) |
60–72 (n=539) |
Total (n=1663) |
30–35 (n=145) |
36–47 (n=480) |
48–59 (n=534) |
60–72 (n=542) |
Total (n=1701) |
| Worse eye, stratified by etiology:* n (% of age group) (95% CI**) | ||||||||||
| Ocular disease | 0 | 0 | 1 (0.2) | 1 (0.2) | 2 (0.1) | 0 | 1 (0.2) | 2 (0.4) | 1 (0.2) | 4 (0.2) |
| Amblyopia | 0 | 4 (0.9) | 7 (1.3) | 5 (0.9) | 16 (1.0) | 0 | 6 (1.3) | 10 (1.9) | 8 (1.5) | 24 (1.4) |
| Prob. Amblyopia | 0 | 2 (0.4) | 4 (0.7) | 1 (0.2) | 7 (0.4) | 0 | 1 (0.2) | 1 (0.2) | 3 (0.6) | 5 (0.3) |
| Prob. Refr. Error | 0 | 1 (0.2) | 4 (0.7) | 0 | 5 (0.3) | 0 | 2 (0.4) | 2 (0.4) | 0 | 4 (0.2) |
| Total† | 0 | 7 (1.5) (0.6–3.1) | 16 (3.0) (1.7–4.8) | 7 (1.3) (0.5–2.7) | 30 (1.8) (1.2–2.6) | 0 | 10 (2.1) (1.0–3.8) | 15 (2.8) (1.6–4.6) | 12 (2.2) (1.2–3.8) | 37 (2.2) (1.5–3.0) |
| Better eye, stratified by etiology: n (% of age group) (95% CI) | ||||||||||
| Ocular disease | 0 | 0 | 1 (0.2) | 1 (0.2) | 2 (0.1) | 0 | 0 | 1 (0.2) | 1 (0.2) | 2 (0.1) |
| Amblyopia | 0 | 1 (0.2) | 5 (0.9) | 2 (0.4) | 8 (0.5) | 0 | 2 (0.4) | 5 (0.9) | 2 (0.4) | 9 (0.5) |
| Prob. Amblyopia | 0 | 0 | 3 (0.6) | 0 | 3 (0.2) | 0 | 0 | 0 | 0 | 0 |
| Prob. Refr. Error | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.2) | 0 | 1 (0.1) |
| Total† | 0 | 1 (0.2) (0–1.2) | 9 (1.7) (0.8–3.1) | 3 (0.6) (0.1–1.6) | 13 (0.8) (0.4–1.3) | 0 | 2 (0.4) (0.1–1.5) | 7 (1.3) (0.5–2.7) | 3 (0.6) (0.1–1.6) | 12 (0.7) (0.4–1.2) |
Amblyopia: unilateral or bilateral amblyopia. Prob. amblyopia: probable bilateral amblyopia; one line of acuity in one eye or 0.25 diopter (D) in one eye short of bilateral amblyopia criteria. Prob. Refr. Error: probable refractive error; significant refractive error at presentation, not testable on retest.
CI: 95% confidence interval; calculated for total visual impairment in each age category.
Numbers differ from Table 3 “not resolving” due to children untestable at presentation, and cases of better eye at presentation becoming worse eye for best measured acuity.
Untestable children 30–72 months old age who were excluded from analysis were reviewed for cases of probable bilateral VI. We identified 2 additional African-American children with probable bilateral VI (one case of optic atrophy, and one of severe myopic degeneration), and 3 additional Hispanic children (one case of optic atrophy, and twins with corneal and lens opacities and retinal scarring). Including these children would yield an overall prevalence of bilateral VI from ocular disease of 0.2% (9/3835) in children 30 to 72 months of age.
Amblyopia was a common cause of VI. All children with best-measured VI diagnosed with amblyopia had at least one retest of VA with refractive correction; 64% also completed return visit retesting. Amblyopia caused VI in the worse eye of 1% (16/1663) of African-American children, half of whom (0.5% of the cohort) had bilateral VI from bilateral amblyopia, usually from ametropia. An additional 0.4% of African-American children had probable bilateral amblyopia from bilateral ametropia. Amblyopia caused VI in the worse eye of 1.4% of Hispanic children (24/1701), 38% of whom (9 children) had bilateral VI from bilateral ametropic amblyopia. An additional 0.3% of Hispanic children had probable bilateral amblyopia from bilateral ametropia. Worse-eye amblyopic VI prevalence did not differ significantly by ethnicity (p=0.27).
VI in the worse eye caused by amblyopia or probable amblyopia was 6.5 times more common than that caused by ocular disease. For the better eye, VI caused by amblyopia or probable amblyopia was 5 times more common than that caused by ocular disease. The types of amblyopia and probable amblyopia causing VI are detailed in Table 6. Cases related to myopia were seen only in African-American children. VI from unilateral amblyopia was caused mostly by anisometropia rather than strabismus (6 of 9 cases in African-American children, and 13 of 15 cases in Hispanic children), and strabismic amblyopia was associated with bilateral hyperopia in 4 of 5 cases. Of all cases of amblyopia or probable amblyopia causing VI in the worse eye, 96% (50/52) were attributable to refractive error or refractive error-related strabismus. Only 4 of 23 African-American children (17%) and 4 of 29 Hispanic children (14%) with amblyopia or probable amblyopia in the worse eye already wore refractive correction. Considering just children with definite amblyopia due to refractive error or refractive esotropia, only 5/38 (13%) wore glasses.
Table 6.
Refractive error/ocular disease associated with type of amblyopia causing visual impairment, stratified by ethnicity.
| Unilateral amblyopia | Bilateral amblyopia (definite or probable) | |||
|---|---|---|---|---|
| African-American | Hispanic | African-American | Hispanic | |
| Myopia | 3* | 0 | 2* | 0 |
| Hyperopia | 1 | 8** | 6 | 8 |
| Astigmatism | 2 | 5 | 6 | 6 |
| History of congenital cataracts | 0 | 0 | 1 | 0 |
| Esotropia | 2† | 2† | - | - |
| Exotropia | 1 | 0 | - | - |
| Total (n) | 9 | 15 | 15 | 14 |
One myopic child met both unilateral and bilateral amblyopia criteria.
Three children also had smaller amounts of anisoastigmatism (1.5 D–1.75 D); one child with > 4.0 diopter (D) anisohyperopia also had esotropia. These children are classified under hyperopia.
All amblyopic esotropes were hyperopic (≥3.25 D to > 7.75 D bilaterally).
Nine children (4 Hispanic, 5 African-American) had decreased best-measured VA in one eye (in one case, both eyes) with significant uncorrected refractive error at presentation without ocular disease or amblyopia, but were unable to complete the same-day VA retest with correction and did not attend the scheduled return visit retest. They were never tested with refractive correction, but might have been classified as having resolving VI associated with refractive error had they been retested. Excluding these cases, 1.5% of African-American children (25/1663) had VI in the worse eye due to ocular disease or to definite or probable amblyopia (95% C.I. 1.0, 2.2). For Hispanic children, 1.9% of children (33/1701) had VI due to ocular disease or to definite or probable amblyopia (95% C.I. 1.3, 2.7). This difference was not statistically significant (p=0.36). In the better eye, the prevalence of VI due to ocular disease or to definite or probable amblyopia was 0.8% (13/1663) for African-American children (95% C.I. 0.4, 1.3) and 0.6% (11/1701) for Hispanic children (95% C.I. 0.3, 1.2) (p=0.69).
There were only three children (less than 0.1% of the sample) with worse eye VA poorer than 20/200 (two with amblyopia and one with optic nerve hypoplasia). No cases met the World Health Organization (WHO) definition37 of “severe VI,”1 i.e., VA worse than 20/200 bilaterally (although there may have been such cases among the 5 children with significant bilateral ocular disease who were untestable). Applying the WHO definition of any visual impairment - better-eye VA worse than 20/60 (herein, 20/63) - only 0.09% (3/3364 testable) to at most 0.2% of children (8/3835 age-eligible, including the 5 untestable children with suspected VI due to bilateral ocular disease) had this level of impairment.
Decreased visual acuity without an identifiable ophthalmic etiology
The proportion of all presenting decreased VA having no identifiable ophthalmic etiology (not classified as VI) decreased with increasing age group. For the worse eye, pooling ethnicities, 91%, 40%, 24% and 16% of decreased presenting VA was unexplained by examination findings for children aged 30–35, 36–47, 48–59 and 60–72 months, respectively. For the better eye, the proportions were 92%, 34%, 21% and 7%.
Decreased presenting VA without an identifiable etiology was stratified according to whether or not VA normalized with retesting (Table 7). Of worse-eye cases, 78% (76/98) resolved. Of the remainder, 36% (8/22) were untestable during retesting, and in 41% (9/22), the examiner commented on the child’s poor attention, poor cooperation, or poor understanding. Among better eye cases, 65% (22/34) resolved. Of the remainder, 42% (5/12) were untestable during retesting, and in 83% (10/12), the examiner commented on the child’s poor testability. This may underestimate the proportion attributable to poor testability, because the protocol did not mandate commenting on behavior. The overall prevalence of decreased presenting VA not attributable to ophthalmic causes decreased with increasing age group, from 10.1% of the youngest age group (20/199) to 1.1% of the oldest age group (12/1080) for the worse eye (p<0.0001), and from 5.5% of the youngest (11/199) to 0.2% of the oldest age group (2/1080) for the better eye (p<0.0001). Among children with unexplained decreased presenting VA in both eyes, the prevalence of premature birth by parental report (<32 weeks) was 3%, no greater than the prevalence in other children (8%).
Table 7.
Prevalence of decreased presenting visual acuity without an identifiable ophthalmic cause.
| Age in months |
African-American | Hispanic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30–35 (n=101) |
36–47 (n=416) |
48–59 (n=537) |
60–72 (n=538) |
Total (n=1592) |
30–35 (n=98) |
36–47 (n=446) |
48–59 (n=529) |
60–72 (n=542) |
Total (n=1615) |
|
| Worse eye, stratified by resolution with retesting protocol:* n (% of age group) (95% CI**) | ||||||||||
| Non-resolving | 2 (2.0) | 3 (0.7) | 5(0.9) | 0 | 10 (0.6) | 3 (3.1) | 5 (1.1) | 3 (0.6) | 1 (0.2) | 12 (0.7) |
| Resolving on retesting | 8 (7.9) | 12 (2.9) | 9 (1.7) | 4 (0.7) | 33 (2.1) | 7 (7.1) | 18 (4.0) | 11 (2.1) | 7 (1.3) | 43 (2.7) |
| Total | 10 (9.9) (4.9–17.5) | 15 (3.6) (2.0–5.9) | 14 (2.6) (1.4–4.3) | 4 (0.7) (0.3–1.9) | 43 (2.7) (2.0–3.6) | 10 (10.2) (5.0–18.0) | 23 (5.2) (3.3–7.6) | 14 (2.6) (1.5–4.4) | 8 (1.5) (0.6–2.9) | 55 (3.4) (2.6–4.4) |
| Better eye, stratified by resolution with retesting protocol:* n (% of age group) (95% CI**) | ||||||||||
| Non-resolving | 1 (1.0) | 3 (0.7) | 1 (0.2) | 0 | 5 (0.3) | 3 (3.1) | 2 (0.4) | 1 (0.2) | 1 (0.2) | 7 (0.4) |
| Resolving on retesting | 4 (4.0) | 3 (0.7) | 5 (0.9) | 1 (0.2) | 13 (0.8) | 3 (3.1) | 3 (0.7) | 3 (0.6) | 0 | 9 (0.6) |
| Total | 5 (5.0) (1.6–11.2) | 6 (1.4) (0.5–3.1) | 6 (1.1) (0.4–2.4) | 1 (0.2) (0–1.0) | 18 (1.1) (0.7–1.8) | 6 (6.1) (2.3–12.9) | 5 (1.1) (0.4–2.6) | 4 (0.8) (0.2–1.9) | 1 (0.2) (0–1.0) | 16 (1.0) (0.6–1.6) |
Non-resolving: decreased acuity by best measured as well as presenting visual acuity, without an ophthalmic etiology. Resolving on retesting: resolved during retesting protocol (Figure 1), without significant refractive error at presentation (spherical equivalent (SE) hyperopia 3.0 diopter (D) or more, SE myopia 0.5 D or more, astigmatism 2.0 D or more at any age, or astigmatism 1.5 D or more for children >36 months of age).
CI: 95% confidence interval; calculated for total decreased visual acuity in each age category.
An additional 157 children, mostly (94%) <48 months old, could not complete VA testing in both eyes at presentation, but were testable during retesting, and analyzed for best-measured VA. Of these, 17.2% had decreased best-measured VA in the worse eye without an identifiable etiology, and 7.0% in the better eye. It is because of these poorly testable children that the proportion of decreased VA that is unexplained is higher for best-measured than for presenting VA (see above).
Discussion
The MEPEDS identified VI not immediately correctable with spectacles, due to ocular disease, unilateral or bilateral amblyopia, or probable bilateral ametropic amblyopia, in the worse eye of 1.5% of African-American children and 1.9% of Hispanic children. It was seen in the better eye of 0.8% of African-American and 0.6% of Hispanic children. We also found correctable presenting VI associated with unmet need for refractive correction in the worse eye of 4.3% of African-American children and 5.3% of Hispanic children, and in the better eye of 1.9% of African-American children and 1.7% of Hispanic children. There were no significant differences between ethnicities.
VI from ocular disease was rare, present in the worse eye of 0.2% of children, and better eye of 0.1%. Similarly, better-eye VI from ocular disease occurred in 0.1% of children (2/1347) in the BPEDS, using identical protocols.31 Worse-eye VI prevalence from ocular disease in children worldwide ranges from 0.1% in Australia,27 to 0.9% in India,18 where corneal (0.3%) and retinal diseases (0.4%) predominate. Corneal disease is rarer in the US thanks to nutrition, hygiene, and lower rates of infectious disease,38 while retinal causes are reduced through screening and treatment of retinopathy of prematurity.39
Most best-measured VI was from amblyopia or probable amblyopia, consistent with previous studies in children and into adulthood. 26 27 40 41 Definite or probable amblyopia caused 5 times more VI in the better eye and 6.5 times more VI in the worse eye than ocular disease. Anisometropia, bilateral ametropia, or refractive esotropia caused all but two cases of amblyopia. Thus, most VI persisting after refractive correction was nonetheless ultimately caused by refractive error. Fully 87% of the refractive errors causing amblyopia were uncorrected at the time of presentation.
VI in the worse eye from amblyopia was seen in 1% of African-American and 1.4% of Hispanic children. This is lower than the MEPEDS amblyopia prevalence reported previously,36 because this analysis excludes mild unilateral amblyopia (e.g. VA 20/32). Although total amblyopia prevalence was higher in Hispanic (2.6%) than African-American children (1.5%),36 due to excess mild amblyopia, there was no significant difference between ethnicities for amblyopia causing VI. The prevalence of better-eye VI from amblyopia, giving bilateral VI, was 0.5% in both Hispanic and African-American children.
In Australia, amblyopia caused worse-eye VI in 0.9% of children.27 In the RESC,15 worse-eye VI from amblyopia ranged in prevalence from 0.3% in South African children (82% African origin)22 to 1.4% in Chile.20 The Chilean result is similar to Hispanic children in MEPEDS, but our rate for African-American children is higher than in South Africa, possibly reflecting differences in refractive error prevalence (see below). BPEDS reported bilateral VI from amblyopia in 0.2% of children (3/1347);31 this is not significantly different from our estimate (p=0.22).
In addition to causing amblyopia, unmet need for refractive correction was associated with correctable VI in the worse eye in 4.3% of African-American and 5.3% of Hispanic children. Adjusting conservatively for the impact of practice on test performance (rarely addressed in studies of childhood VI), the prevalence of worse-eye VI from uncorrected refractive error was still 2.2% for African-American and 2.4% for Hispanic children. Myopia was more common in African-American children, and astigmatism in Hispanic children.
RESC prevalences of worse-eye refractive-error-related VI ranged from 1.6% in Nepal23 to 29.9% in China17 for 5–15 year-old children, with only Nepal23, rural India18 and South Africa22 reporting rates <6%. The RESC reports correctable VI that would be present if none of the children had glasses, but since only 1.6% of our participants had glasses, this does not explain our relatively lower prevalence. The RESC attributed VI to refractive error if it resolved with subjective refraction, regardless of the magnitude of the refractive error, and included older children with school-age myopia; the latter especially would contribute to higher prevalence estimates. Correctable VI prevalence in Chile (9.8%)20 was higher than in our Hispanic children. By contrast, the prevalence was lower (1.8%) in South Africa22 than in our African-American children, suggesting that South African children may be less prone to refractive error. Robaei et al, using a lower threshold for significant refractive error than MEPEDS, found it caused decreased worse-eye presenting VA in only 2.0% of 6- to 7-year-old Australian children (35/1738). Over 4% of their population presented wearing spectacles,42 but the total refractive correction need was still only 2.8% (49 children).27 This could reflect lower refractive error prevalence than MEPEDS, or, alternatively, better VA test performance in school-aged children.
Table 8 summarizes the prevalence of any decreased VA, with or without identifiable etiology, in African-American children in the MEPEDS and BPEDS.31 Decreased presenting VA was significantly more prevalent in MEPEDS, while worse-eye best-measured VA was more often decreased in BPEDS. Excluding unexplained cases, BPEDS found decreased better-eye presenting VA with an identifiable etiology in 1.1% of children (8/725) (95% C.I. 0.5–2.2),31 compared to 2.8% in MEPEDS (95% C.I. 2.0–3.7) (p=0.01). Better-eye amblyopia occurred in 0.3% (2/725) in BPEDS, and 0.4% (7/1592) in MEPEDS (p=0.73). Uncorrected refractive error was responsible in 0.8% (6/725) in BPEDS, compared to 1.9% (30/1592) in MEPEDS (p=0.07). This not quite significant difference is not attributable to glasses wear, which was similar in BPEDS (1.7%; 12/725) and MEPEDS children (1.6%); it may reflect differences in refractive error prevalence between the populations, and/or socioeconomic factors, which can impact VA test performance (Pan Y, Tarczy-Hornoch K, Cotter SA et al. Visual acuity norms in preschool children: the Multi-Ethnic Pediatric Eye Disease Study. Optom Vis Sci in press). The MEPEDS AND BPEDS both encountered more myopia-related VI and less from astigmatism in African-American children than in children of other ethnicities.
Table 8.
Comparison of the prevalence of decreased visual acuity in African American children in the Baltimore Pediatric Eye Disease Study to those in the Multi-Ethnic Pediatric Eye Disease Study, stratified by age.
| 30–35 months | 36–47 months | 48–59 months | 60–72 months | Total | |
|---|---|---|---|---|---|
| Decreased presenting visual acuity in worse eye: n (% of age group) (95% CI*) | |||||
| BPEDS AA | 0/47 (0) | 9/184 (4.9) | 10/250 (4.0) | 13/244 (5.3) | 32/725 (4.4) (3.0–6.2) |
| MEPEDS AA | 12/101 (11.9) | 38/416 (9.1) | 52/537 (9.7) | 35/538 (6.5) | 137/1592 (8.6) † (7.3–10.1) |
| Decreased presenting visual acuity in better eye: n (% of age group) (95% CI*) | |||||
| BPEDS AA | 0/47 (0) | 7/184 (3.8) | 3/250 (1.2) | 3/244 (1.2) | 13/725 (1.8) (1.0–3.1) |
| MEPEDS AA | 6/101 (5.9) | 19/416 (4.6) | 24/537 (4.5) | 13/538 (2.4) | 62/1592 (3.9) † (3.0–5.0) |
| Decreased best-measured visual acuity in worse eye: n (% of age group) (95% CI*) | |||||
| BPEDS AA | 4/62 (6.5) | 13/202 (6.4) | 10/251 (4.0) | 14/245 (5.7) | 41/760 (5.4) (3.9–7.3) |
| MEPEDS AA | 9/128 (7.0) | 15/456 (3.3) | 21/540 (3.9) | 7/539 (1.3) | 52/1663 (3.1) † (2.3–4.1) |
| Decreased best-measured visual acuity in better eye: n (% of age group) (95% CI*) | |||||
| BPEDS AA | 2/62 (3.2) | 4/202 (2.0) | 1/251 (0.4) | 2/245 (0.8) | 9/760 (1.2) (0.5–2.2) |
| MEPEDS AA | 5/128 (3.9) | 6/456 (1.3) | 10/540 (1.9) | 3/539 (0.6) | 24/1663 (1.4) (0.9–2.1) |
BPEDS: Baltimore Pediatric Eye Disease Study; MEPEDS: Multi-Ethnic Pediatric Eye Disease Study; AA: African-American.
CI: 95% confidence interval; calculated only for total visual impairment in each age category.
p<0.05 for comparison of proportions between studies (Fisher’s exact test).
In MEPEDS, 48–59 month-old children consistently showed more VI than the oldest age group. Older children may have been treated, or be better at VA testing.43 VI prevalence was low in the youngest children, as previously reported for amblyopia.36 Although strabismus prevalence increases with age,36 this would not account for rising VI prevalence between 20 and 59 months, since most VI in this population is not attributable to strabismus. Testability was lower in younger age groups, so children who had VI may simply have been untestable. Most VI was mild, and the proportion increased with age, probably reflecting improved test performance in older children.43
Profound VI was rare. No children had bilateral best-measured VA worse than 20/200 from an identifiable ophthalmic cause, and <0.1% had best-measured VI worse than 20/200 in either eye. Even if all five untestable children with severe bilateral ocular abnormalities had profound impairment, at most 0.1% of children had VA worse then 20/200 bilaterally. In Australia, no children had best-measured better-eye acuity worse than 20/200.27 In BPEDS, only one child had bilateral best-measured VA worse than 20/200 with an identifiable etiology.31 In the RESC and other studies,44 best-measured better-eye acuity below 20/200 ranged in prevalence from 0 to under 0.2%. Profound bilateral VI in multiply-disabled children could be underrepresented in MEPEDS if these children were less likely to participate; however, in-home interview data did not identify any such bias.
Around 30% of decreased presenting VA, whether in the better or worse eye, had no identifiable ophthalmic etiology. The proportion decreased with increasing age, from >90% in the youngest group, to 16% for the worse and 7% for the better eye in the oldest children. Similarly, 7% of decreased presenting VA was from “presumed lack of cooperation” in 6- to 7-year-old Australian children.27 Some unexplained decreased VA could have been physiological (e.g. cortical VI); however, the clear association with age, and resolution with retesting in 77% of cases, indicate that most cases reflect age-related limitations in test performance rather than visual deficits. At 3 years of age, when the American Academy of Pediatrics recommends initiating screening,45 40% of decreased presenting VA in at least one eye was unexplained; thus, variable test performance in preschool children limits the positive predictive value (PPV) of a single VA measurement. Since most unexplained presenting VA deficits resolved with retesting, retesting prior to referral would improve the PPV of VA tests. Currently, however, guidelines do not suggest repeated testing except when unable to complete testing on the first attempt.45
Variable test performance in young children also has implications for research studies. Since test performance improves with repetition, not all decreased presenting VA associated with refractive error is necessarily attributable to refractive error, even if it resolves during retesting with correction. Defining a threshold criterion for visually significant refractive error can reduce misattribution, but cannot prevent all misclassification. Retesting without correction in a subset of participants yields an estimate of the proportion of resolving cases attributable to retesting alone. Using data from QC retests, we can conservatively adjust our apparent prevalence of correctable VI (see above). Another approach would be to retest children without correction after they appear to improve with correction, to confirm that uncorrected VA remains decreased.
The validity of our findings is strengthened by a population-based study design, highly standardized protocol, large sample size, and analysis accounting for variable test performance in young children. The greatest weakness of the study is the low VA testability in 30–35 month-old children, limiting the conclusions that may be drawn in this age group. Also, the VI prevalence reported here may not reflect the burden in populations with better access to health care.
In conclusion, African-American and Hispanic preschool children in Los Angeles County have a substantial degree of unmet need for refractive correction resulting in VI that is easily corrected. In addition, refractive errors are the primary cause of most VI from amblyopia, and thus the major root cause of VI overall. The spectacle needs of amblyopic children in this population are largely unmet; improved early detection and treatment of significant refractive errors is needed. Although the VI we have identified is rarely severe enough to impact activities of daily living, this does not preclude a significant impact on high-level visual functions and early school performance; further study is needed to elucidate the long-term consequences of early childhood VI.
Acknowledgments
Supported by the National Eye Institute, National Institutes of Health, Bethesda, MD (grant nos. EY14472 and EY03040), and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Dr. Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
APPENDIX
Writing Committee: Lead author: Kristina Tarczy-Hornoch, MD, DPhil. Committee: Susan Cotter, OD, MS; Jesse Lin, MS; Arezoo Fozailoff, MD; Mark Borchert, MD; Stanley Azen, PhD; Rohit Varma, MD, MPH.
The Multi-Ethnic Pediatric Eye Disease Study Group
University of Southern California
Rohit Varma, MD, MPH (Principal Investigator); LaVina Abbott (2002–2005);George Ayala (2005–2006); Stanley P. Azen, PhD; Tal Barak, OD; Mark Borchert, MD; Jessica Chang, OD (2005–2008); Felicia K. Chen, OD (2005–2007); Rose Chon (2008); Susan Cotter, OD, MS (Co-Principal Investigator); Jennifer Deneen, MPH; Jackie Diaz; Anne DiLauro, MPH (2005–2007);Jill Donofrio, MPH (2003–2005); Claudia Dozal (2003–2004); Athena W. Foong; James Gardner; Regina Garriott, OD (2003–2005), Jackson Lau, OD (2006–2007); Jesse Lin, MS; George Martinez; Roberta McKean-Cowdin, PhD; Kisha Milo; Carlos Moya; Sylvia Paz, MS (2002–2005); Ana Penate; Amanda Reiner, MPH (2007); Claudia Salazar (2006–2008); Erin Song, OD (2003–2008); Kristina Tarczy-Hornoch, MD, DPhil; Mina Torres, MS; Natalia Uribe, OD (2003–2007); Ivania Verrico (2005–2007); Ying Wang, MS (2006–2007); Ge Wen, Peng Zhao, MS (2003–2007); Amy Zhu.
Battelle Survey Research Center
Charles Aders (2003–2006); Candace Kwong, MPH; Nancy Noedel; Michael Preciado; Karen Tucker, MA.
Footnotes
See Appendix 1(available at http://aaojournal.org) for writing committee and members/affiliations of the MEPEDS Group.
Conflicts of Interest: The authors have no proprietary or commercial interest in any materials discussed in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinkuller PG, Du L, Gilbert C, et al. Childhood blindness. J AAPOS. 1999;3:26–32. doi: 10.1016/s1091-8531(99)70091-1. [DOI] [PubMed] [Google Scholar]
- 2.Keeffe J, Taylor HR. Visual impairment in children. Br J Ophthalmol. 1995;79:624–5. doi: 10.1136/bjo.79.7.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahi J, Logan S, Timms C, et al. Risk, causes, and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population-based study. Lancet. 2002;360:597–602. doi: 10.1016/s0140-6736(02)09782-9. [DOI] [PubMed] [Google Scholar]
- 4.Jakobsson P, Kvarnstrom G, Abrahamsson M, et al. The frequency of amblyopia among visually impaired persons. Acta Ophthalmol Scand. 2002;80:44–6. doi: 10.1034/j.1600-0420.2002.800109.x. [DOI] [PubMed] [Google Scholar]
- 5.Dandona L, Dandona R. Revision of visual impairment definitions in the International Statistical Classification of Diseases. [Accessed March 14, 2009];BMC Med [serial online] 2006 4:7. doi: 10.1186/1741-7015-4-7. Available at: http://www.biomedcentral.com/1741-7015/4/7. [DOI] [PMC free article] [PubMed]
- 6.Kohler L, Stigmar G. Vision screening of four-year-old children. Acta Paediatr Scand. 1973;62:17–27. doi: 10.1111/j.1651-2227.1973.tb08060.x. [DOI] [PubMed] [Google Scholar]
- 7.Newman DK, Hitchcock A, McCarthy H, et al. Preschool vision screening: outcome of children referred to the hospital eye service. Br J Ophthalmol. 1996;80:1077–82. doi: 10.1136/bjo.80.12.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams C, Northstone K, Howard M, et al. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. Br J Ophthalmol. 2008;92:959–64. doi: 10.1136/bjo.2007.134700. [DOI] [PubMed] [Google Scholar]
- 9.Williams C, Harrad RA, Harvey I, Sparrow JM ALSPAC Study Team. Screening for amblyopia in preschool children: results of a population-based, randomised controlled trial. 0. Ophthalmic Epidemiol. 2001;8:279–95. doi: 10.1080/09286586.2001.11644257. [DOI] [PubMed] [Google Scholar]
- 10.Kvarnstrom G, Jakobsson P, Lennerstrand G. Screening for visual and ocular disorders in children, evaluation of the system in Sweden. Acta Paediatr. 1998;87:1173–9. doi: 10.1080/080352598750031176. [DOI] [PubMed] [Google Scholar]
- 11.Kvarnstrom G, Jakobsson P, Lennerstrand G. Visual screening of Swedish children: an ophthalmological evaluation. Acta Ophthalmol Scand. 2001;79:240–4. doi: 10.1034/j.1600-0420.2001.790306.x. [DOI] [PubMed] [Google Scholar]
- 12.Robinson B, Bobier WR, Martin E, Bryant L. Measurement of the validity of a preschool vision screening program. Am J Public Health. 1999;89:193–8. doi: 10.2105/ajph.89.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross E, Murray AL, Stead S. Prevalence of ambylopia in grade 1 schoolchildren in Saskatoon. Can J Public Health. 1977;68:491–3. [PubMed] [Google Scholar]
- 14.Oliver M, Nawratzki I. Screening of pre-school children for ocular anomalies. II. Amblyopia: prevalence and therapeutic results at different ages. Br J Ophthalmol. 1971;55:467–71. doi: 10.1136/bjo.55.7.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negrel AD, Maul E, Pokharel GP, et al. Refractive Error Study in Children: sampling and measurement methods for a multi-country survey. Am J Ophthalmol. 2000;129:421–6. doi: 10.1016/s0002-9394(99)00455-9. [DOI] [PubMed] [Google Scholar]
- 16.He M, Huang W, Zheng Y, et al. Refractive error and visual impairment in school children in rural southern China. Ophthalmology. 2007;114:374–82. doi: 10.1016/j.ophtha.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 17.He M, Zeng J, Liu Y, et al. Refractive error and visual impairment in urban children in southern china. Invest Ophthalmol Vis Sci. 2004;45:793–9. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- 18.Dandona R, Dandona L, Srinivas M, et al. Refractive error in children in a rural population in India. Invest Ophthalmol Vis Sci. 2002;43:615–22. [PubMed] [Google Scholar]
- 19.Goh PP, Abqariyah Y, Pokharel GP, Ellwein LB. Refractive error and visual impairment in school-age children in Gombak District, Malaysia. Ophthalmology. 2005;112:678–85. doi: 10.1016/j.ophtha.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Maul E, Barroso S, Munoz SR, et al. Refractive Error Study in Children: results from La Florida, Chile. Am J Ophthalmol. 2000;129:445–54. doi: 10.1016/s0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- 21.Murthy GV, Gupta SK, Ellwein LB, et al. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci. 2002;43:623–31. [PubMed] [Google Scholar]
- 22.Naidoo KS, Raghunandan A, Mashige KP, et al. Refractive error and visual impairment in African children in South Africa. Invest Ophthalmol Vis Sci. 2003;44:3764–70. doi: 10.1167/iovs.03-0283. [DOI] [PubMed] [Google Scholar]
- 23.Pokharel GP, Negrel AD, Munoz SR, Ellwein LB. Refractive Error Study in Children: results from Mechi Zone, Nepal. Am J Ophthalmol. 2000;129:436–44. doi: 10.1016/s0002-9394(99)00453-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Pan X, Sui R, et al. Refractive Error Study in Children: results from Shunyi District, China. Am J Ophthalmol. 2000;129:427–35. doi: 10.1016/s0002-9394(99)00452-3. [DOI] [PubMed] [Google Scholar]
- 25.Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K. The prevalence of refractive errors among schoolchildren in Dezful, Iran. Br J Ophthalmol. 2007;91:287–92. doi: 10.1136/bjo.2006.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robaei D, Huynh SC, Kifley A, Mitchell P. Correctable and non-correctable visual impairment in a population-based sample of 12-year-old Australian children. Am J Ophthalmol. 2006;142:112–8. doi: 10.1016/j.ajo.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 27.Robaei D, Rose K, Ojaimi E, et al. Visual acuity and the causes of visual loss in a population-based sample of 6-year-old Australian children. Ophthalmology. 2005;112:1275–82. doi: 10.1016/j.ophtha.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 28.Robaei D, Kifley A, Rose KA, Mitchell P. Refractive error and patterns of spectacle use in 12-year-old Australian children. Ophthalmology. 2006;113:1567–73. doi: 10.1016/j.ophtha.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 29.Preslan MW, Novak A. Baltimore Vision Screening Project. Ophthalmology. 1996;103:105–9. doi: 10.1016/s0161-6420(96)30753-7. [DOI] [PubMed] [Google Scholar]
- 30.Flom MC, Neumaier RW. Prevalence of amblyopia. Public Health Rep. 1966;81:329–41. [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman DS, Repka MX, Katz J, et al. Prevalence of decreased visual acuity among preschool-aged children in an American urban population: the Baltimore Pediatric Eye Disease Study, methods, and results. Ophthalmology. 2008;115:1786–95. doi: 10.1016/j.ophtha.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varma R, Deneen J, Cotter S, et al. Multi-Ethnic Pediatric Eye Disease Study Group. The Multi-Ethnic Pediatric Eye Disease Study: design and methods. Ophthalmic Epidemiol. 2006;13:253–62. doi: 10.1080/09286580600719055. [DOI] [PubMed] [Google Scholar]
- 33.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the Amblyopia Treatment Study visual acuity testing protocol. Am J Ophthalmol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 34.Holmes JM, Beck RW, Repka MX, et al. Pediatric Eye Disease Investigator Group. The Amblyopia Treatment Study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 35.Cotter SA, Tarczy-Hornoch K, Wang Y, et al. Multi-Ethnic Pediatric Eye Disease Study Group. Visual acuity testability in African-American and Hispanic children: the Multi-Ethnic Pediatric Eye Disease Study. Am J Ophthalmol. 2007;144:663–7. doi: 10.1016/j.ajo.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Multi-ethnic Pediatric Eye Disease Study Group. Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months: the Multi-ethnic Pediatric Eye Disease Study. Ophthalmology. 2008;115:1229–36. doi: 10.1016/j.ophtha.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The prevention of blindness: report of a WHO study group. World Health Organ Tech Rep Ser. 1973;518:1–18. [PubMed] [Google Scholar]
- 38.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020--the right to sight. Bull World Health Organ. 2001;79:227–32. [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Kessel L, Hougaard JL, Mortensen C, et al. Visual acuity and refractive errors in a suburban Danish population: Inter99 Eye Study. Acta Ophthalmol Scand. 2004;82:19–24. doi: 10.1111/j.1395-3907.2004.0179.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang JJ, Foran S, Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2000;28:268–73. doi: 10.1046/j.1442-9071.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 42.Robaei D, Rose K, Kifley A, Mitchell P. Patterns of spectacle use in young Australian school children: findings from a population-based study. J AAPOS. 2005;9:579–83. doi: 10.1016/j.jaapos.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Drover JR, Felius J, Cheng CS, et al. Normative pediatric visual acuity using single surrounded HOTV optotypes on the Electronic Visual Acuity Tester following the Amblyopia Treatment Study protocol. J AAPOS. 2008;12:145–9. doi: 10.1016/j.jaapos.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorairaj SK, Bandrakalli P, Shetty C, et al. Childhood blindness in a rural population of southern India: prevalence and etiology. Ophthalmic Epidemiol. 2008;15:176–82. doi: 10.1080/09286580801977668. [DOI] [PubMed] [Google Scholar]
- 45.Committee on Practice and Ambulatory Medicine Section on Ophthalmology, American Association of Certified Orthoptists, American Association for Pediatric Ophthalmology and Strabismus and American Academy of Ophthalmology. Eye examination in infants, children, and young adults by pediatricians. Pediatrics. 2003;111:902–7. [PubMed] [Google Scholar]