Abstract
Objectives
This work aims to develop the technique of histotripsy ultrasound therapy as a non-invasive treatment for benign prostatic hyperplasia (BPH). We have previously demonstrated the feasibility of prostate histotripsy fractionation in a canine model. The purpose of this paper is to examine the histotripsy dose - tissue response effect over time to provide insight for treatment optimization.
Methods
Various doses of histotripsy were applied trans-abdominally to the prostates of 20 canine subjects. Treated prostates were then harvested at interval time points from zero to 28 days and assessed for histologic treatment response.
Results
The lowest dose applied was found to produce only scattered cellular disruption and necrosis while higher doses produced more significant regions of tissue effect that resulted in sufficient fractionation of tissue so that the material could be voided with urination. Urethral tissue was more resistant to the lower histotripsy doses than was parenchymal tissue. Treatment of the urethra at the lowest doses appeared to heal with minimal long term sequelae.
Conclusions
Histotripsy was effective at fractionating parenchymal and urethral tissue in the prostate given a sufficient dose. Further development of this technique could lead to a non-invasive method for debulking the prostate to relieve symptoms associated with BPH.
Introduction
Benign prostatic hyperplasia (BPH) is a common urologic condition among older men that frequently manifests as lower urinary tract symptoms (LUTS). Moderate to severe LUTS were found to occur in 33%, 41%, and 46% of men in their 50s, 60s, and 70s, respectively [1]. The increased frequency of symptoms with age, coupled with an associated decrease in quality of life prompted 4.5 million physician visits for a primary diagnosis of BPH in 2000 [2]. In that same year, direct costs for non-pharmacologic treatment of BPH were estimated to be $1.1 billion [2].
Current therapies for BPH are lacking. Transurethral resection of the prostate (TURP) - long considered the gold standard - has a significant risk profile that may be less tolerable in older patients and those with co-morbidities [3, 4]. Minimally invasive treatments, although widely employed [5], are uniformly less effective than TURP due to their inability to provide sufficient tissue debulking [6, 7]. Although some patients with mild LUTS may derive durable benefit from minimally invasive thermal modalities, a significant portion will eventually fail and require definitive tissue debulking therapy when they are older, have increased co-morbidity, and thus are at greater risk of surgical complication [2, 8]. There is a need for development of new technologies that can replicate the anatomic effect and efficacy of TURP with an improved risk profile.
Histotripsy is a method of ultrasound therapy using intense bursts of ultrasound to destroy tissue by cavitation rather than thermal mechanisms [9]. Lake [10] demonstrated the feasibility of using histotripsy to fractionate canine prostate tissue transabdominally with transrectal ultrasound image guidance. This study is a follow-up to that work examining the tissue response to varying histotripsy doses within a chronic model. The overall end goal of this work is to develop histotripsy as a non-invasive (potentially office-based) therapy for the treatment of BPH with equivalent efficacy to TURP and a more favorable side effect profile.
Materials and Methods
20 mongrel canine subjects weighing 20–30 kg were used in this study. Subjects were screened with digital rectal examination to select those with suitably sized prostates, larger than approximately 20 cm3 (mean = 24.6 cm3). Subjects were anesthetized with acepromazine and sodium pentothal, intubated, and underwent digital rectal disimpaction and tap water enema to facilitate transrectal ultrasound examination. After transfer to the procedure room, they were placed supine on a v-shaped tray and anesthetic effect was maintained with 1–3 % inhaled isoflurane for the duration of histotripsy procedures. A guide wire was passed transurethrally into the bladder. The penis was retracted laterally to the right and the lower abdomen shaved to provide clear acoustic access through the abdominal wall to the prostate. With this configuration, the anterior surface of the prostate was found to be 1–3 cm below the skin surface.
Transrectal ultrasound imaging and volume measurement (ellipsoid approximation) was performed using a model 3535 imaging system (BK Medical, Herlev, Denmark). The BK system was then replaced with a Logiq 9 ultrasound scanner (GE Healthcare, Chalfont St. Giles, United Kingdom) with a model I739 intraoperative imaging probe fixed in a custom housing and operated transrectally. The GE system was capable of significantly greater image quality than the BK system for targeting and therapy monitoring. Rotating the probe provided sagittal and oblique image planes of the prostate.
The therapeutic system consisted of a high power transducer on a three axis computer controlled positioning system. The transducer was a custom piezoceramic composite array of 18 elements in the shape of a focused annulus (Imasonic, Voray sur l’Ognon, France). The outer diameter was 14 cm, inner hole diameter was 5 cm, and overall radius of curvature (focal length) was 10 cm. All elements were driven in phase by custom electronic circuits with high power bursts of 3 cycles at 750 kHz and a repetition rate of 300 Hz. This system generated peak focal pressures exceeding negative 20 MPa in a 3 × 3 × 10 mm region in degassed water, however, cavitation precluded precise measurements of focal zone pressure or intensity.
The therapeutic system was acoustically coupled to the abdominal wall via a bath of degassed water. A large stainless steel bowl with a cutout and lined with a clear, thin plastic membrane was placed over the abdomen. The skin was wetted with a mixture of degassed water and acoustic coupling gel below the membrane and then the bowl was filled with water.
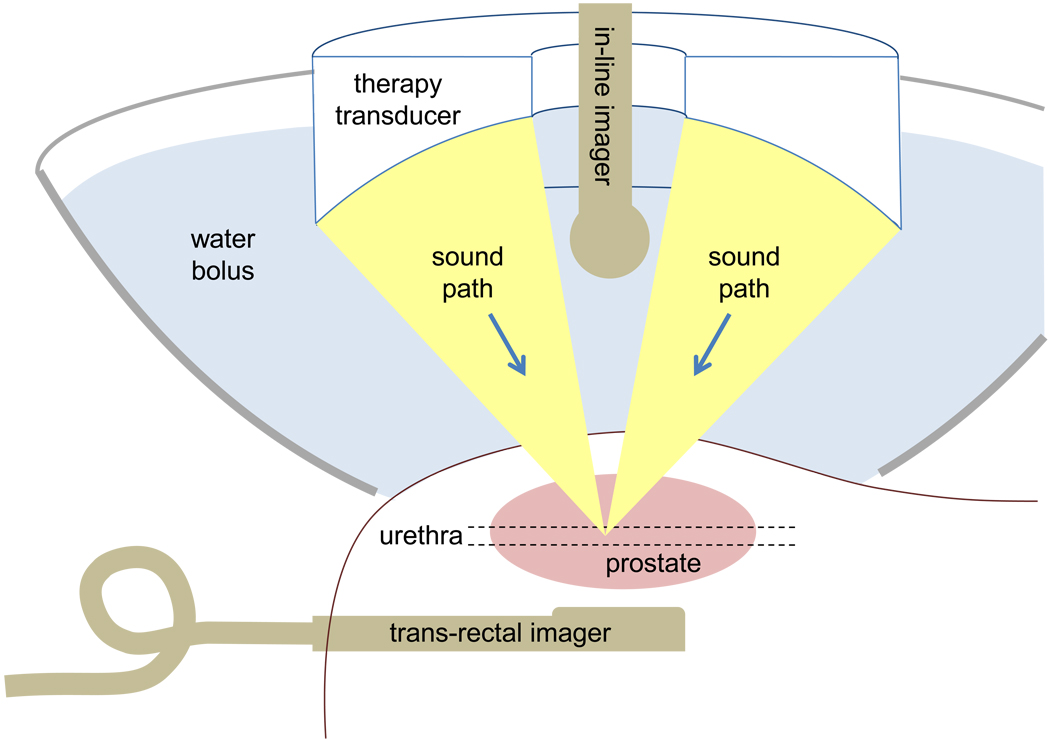
An imaging probe (E8C) was aligned confocally with the therapy transducer through the center hole. This was used for initial targeting. Exciting the therapy transducer briefly in the water bath away from the subject created a bubble cloud visible with the E8C imaging probe. This focal location was marked on the ultrasound screen as a reference point. The therapy system was then positioned such that the marked focal reference point corresponded to the center of the prostate. Intraprocedural imaging was obtained with the transrectal 1739 probe. A sketch of the setup is shown in figure 1.
Figure 1.
Sketch of the histotripsy treatment setup. A large bowl with a cutout in the bottom was filled with water and used to couple ultrasound from the therapy transducer through the skin and into the prostate. An in-line imaging transducer fixed with the therapy transducer provided initial targeting images for locating the prostate trans-abdominally. During treatment, a trans-rectal imaging transducer provided higher quality images for guidance.
Targeted volumes of 2 cm3 encompassed the urethra and parenchymal tissue in the left lobe of the prostate. The focal region of the therapy transducer was mechanically scanned through the target volume in a grid of 21 × 11 × 1 focal locations spaced by 1 mm. This was assumed to provide a reasonably uniform dose over the target volume. The total number of ultrasonic bursts applied to the targeted volume was either 56 000, 160 000, or 540 000. These doses used were established from published [10] and unpublished work by Lake and the present authors to encompass the wide range of tissue bioeffects produced by histotripsy. The highest dose (540 000) was known to yield nearly complete ablation. The lowest dose (56 000) was selected as approximately an order of magnitude less and the intermediate dose (160 000) as the approximate geometric mean. In 18 of the 20 subjects, one targeted volume was placed towards the apical end of the prostate and one towards the base. For the other two subjects, only one volume was placed approximately in the middle of the prostate.
Following histotripsy treatment, five subjects were euthanized immediately, seven were euthanized at 7 days, and eight at 28 days. Chronic subjects were catheterized with a 12-Fr urinary catheter placed immediately following treatment and left in place for 24–72 hours. Following euthanasia, the prostate, bladder, and adjacent rectum were surgically removed en bloc. The prostate, bladder and rectal tissues were inspected grossly for injury.
Harvested prostates were fixed in formalin for 1 week and then cut into 5 mm thick slabs. The slabs were dehydrated, paraffin embedded, and cut with a microtome in 5 micron sections at 1 mm increments. Sections were mounted and stained with hematoxylin and eosin. All slides were scanned with a flatbed scanner at 2400 dpi resolution before and after marking of therapy margins. The margin was defined by two boundaries. The inner boundary enclosed the region of complete cellular disruption and/or necrosis. The outer boundary separated the region of normal appearing tissue from regions containing any scattered cellular disruption, necrosis, or hemorrhage acutely and included inflammation and or granulation tissue at the more chronic time points.
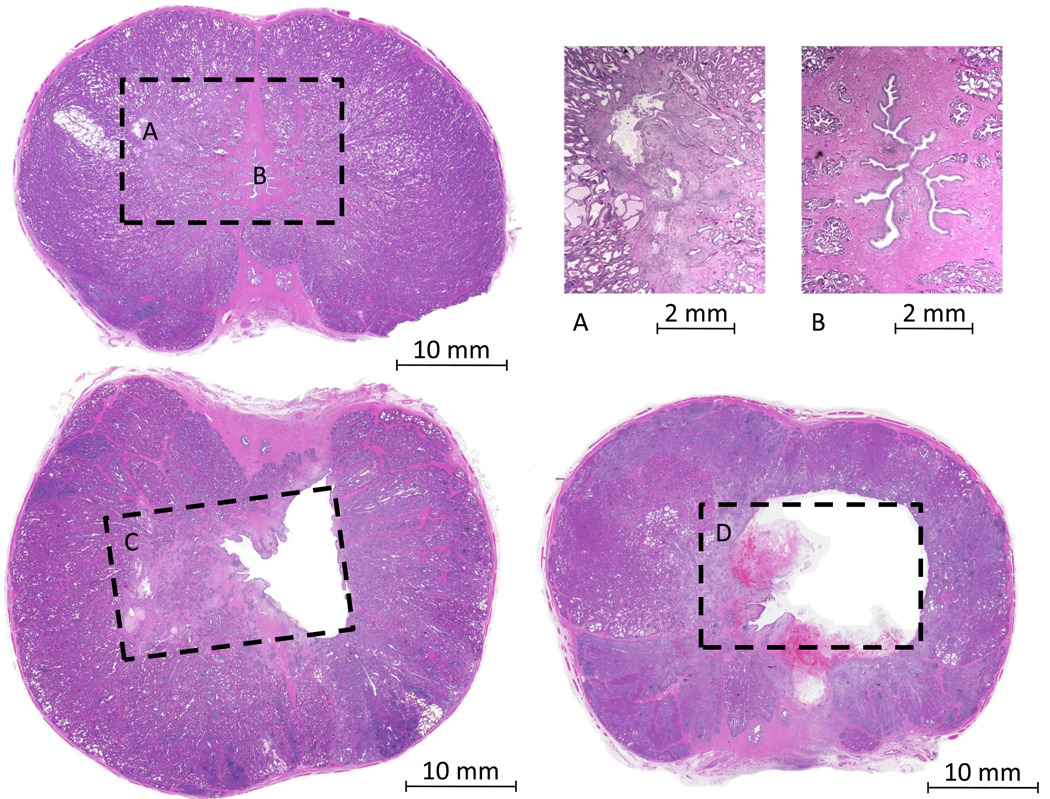
Urethra and prostate parenchymal tissues within each treatment volume were graded separately on a numeric scale from 0 to 3. Grade 0 represented no microscopic or macroscopic changes (no apparent treatment effect). Grade 1 represented the presence of only scattered microscopic cellular disruption, necrosis, or focal hemorrhage (Fig 2-A). Grade 2 represented at least one macroscopic region of cellular disruption, necrosis, or tissue absence, but encompassed only a portion of the targeted volume (Fig 2-C). Grade 3 represented complete fractionation (cellular disruption or necrosis) of nearly all the target volume with no remaining viable tissue and/or a void (Fig 2-D)
Figure 2.
Sample images to illustrate the treatment effect grading scale. For grade 1, effects were not apparent macroscopically (A - left), but at higher magnification (A - right), regions of cell disruption and necrosis were visible. For grade 0, no abnormal tissue was seen at low (B - left) or high (B - right) magnification. For grade 2, macroscopic regions of tissue fractionation or a void were seen, but the full target volume contained some viable tissue (C). For grade 3, the complete target volume was a void and/or necrotic. Note: dashed boxes represent approximate targeted volumes.
Results
The histotripsy cavitation process was successfully initiated within all prostate target volumes as determined by the observation of a bubble cloud on ultrasound imaging. Supplemental video 1 shows a portion of a typical treatment including the dynamic bubble cloud appearance indicative of histotripsy.
Macroscopic and microscopic examination revealed parenchymal tissue effects associated with every targeted volume ranging from grade 1 to grade 3. Urethral tissue effects ranged from grade 0 to grade 3.
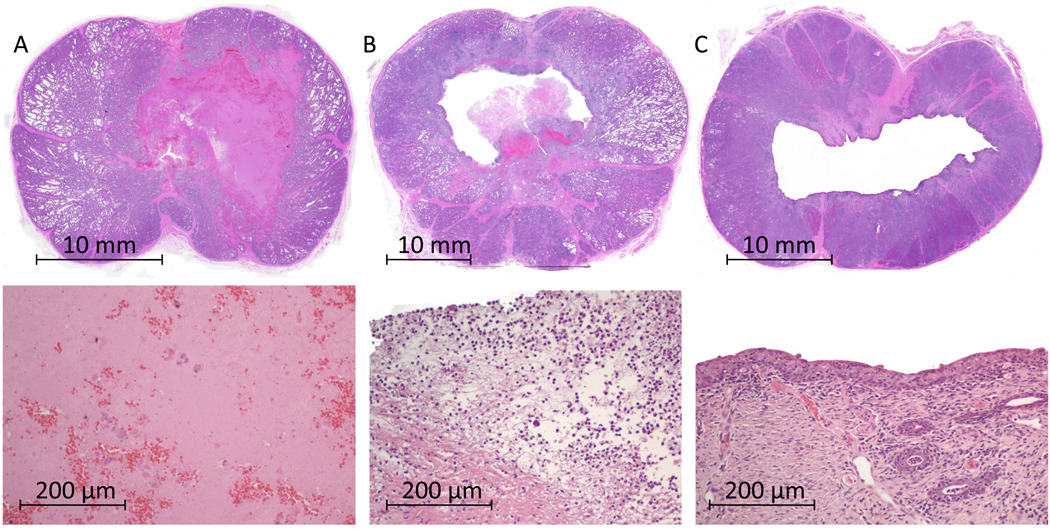
Figure 3 shows sample low and high magnification images of lesions resulting from the intermediate dose (160 000 bursts) zero to 28 days post-treatment. In the acute subject (3-A), a macroscopic region of complete cellular disruption in the parenchymal tissue was noted while the urethral architecture was largely preserved with microscopic regions of local disruption. High magnification of the targeted parenchymal tissue demonstrated a smooth, acellular residue with scattered erythrocytes. Seven days post treatment (3-B), much of the disrupted material in the targeted volume was absent leaving a cavity within the prostate. The urethral wall was absent or perforated in all cases. On high magnification, neutrophils were present with partial disintegration of fibrous tissue structures, consistent with a typical wound healing response. 28 days post treatment (3-C), a cavity was always present. The urethra was partially or completely absent and in communication with the cavity created within the prostate parenchyma. High magnification showed urothelialization of the entire cavity edge, continued presence of neutrophils, and minimal scar formation.
Figure 3.
Sample images of prostates at various times post histotripsy treatment (intermediate dose).Prostate (A) was harvested on the day of treatment and showed a large region of fractionated tissue. Higher magnification (bottom) revealed this to be completely liquefied tissue with a few identifiable scattered erythrocytes. Prostate (B) was harvested 7 days after treatment. A cavity was present with a thin border of necrosed tissue and neutrophils (bottom). Very minor inflammation was present in the rest of the prostate. Prostate (C) was harvested 28 days after treatment. A large cavity was present and the prostate had partially collapsed. The border of the treatment cavity was lined with urothelium and thin variably present margin of granulation tissue (bottom).
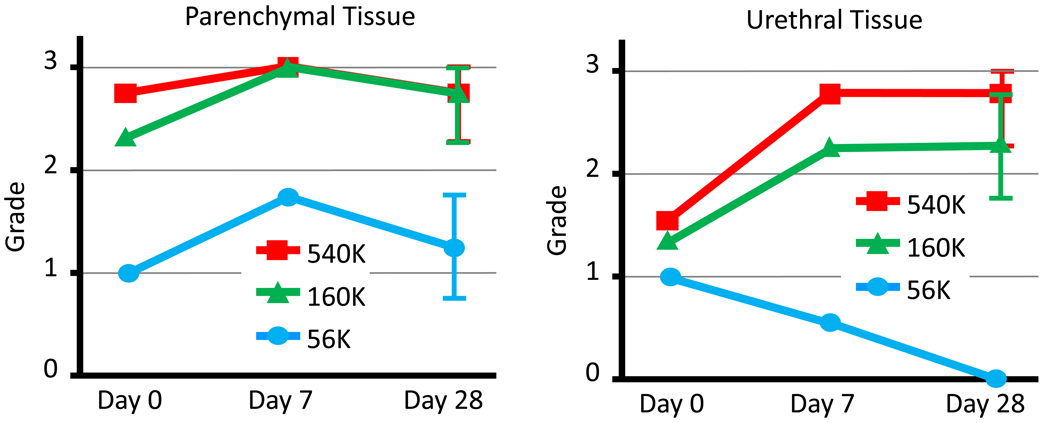
Figure 4 summarizes grading of the treated tissue at various treatment doses and evaluation times. Parenchymal tissue was nearly completely fractionated with moderate and high histotripsy doses and only partly fractionated with the low dose. No trend was observed with respect to the time of evaluation post procedure. Urethral tissue was partially fractionated with the moderate histotripsy dose and more completely by the high dose. The low dose produced little or no effect. These differences became more pronounced for subjects evaluated at 7 or 28 days post procedure. Parenchymal tissue effects graded at 28 days demonstrated a value (mean ± std) of 1.25 ± 0.5, 2.75 ± 0.5, and 2.75 ± 0.5 for low, medium, and high doses. Urethral tissue grades were 0 ± 0, 2.25 ± 0.5, and 2.75 ± 0.5.
Figure 4.
Summary of average lesion grades (tissue response) for various doses and various harvest times. Error bars represent one standard deviation. Grades of 2 – 3 were considered to represent clinically significant tissue effect. The 56k dose produced significantly lower grades of tissue response than 160k or 540k for both parenchymal and urethral tissue. Urethral tissue exhibited a pronounced difference in tissue response based on dose at harvest times of 7 or 28 days.
Histotripsy was well tolerated by all subjects without any treatment related complications. Hematuria was minimal and not clinically significant. Only minor elevation in pain scores and transient abnormalities in blood laboratory values were noted, consistent with previously reported data on the safety of prostate histotripsy [7].
Comment
In an attempt to reduce complications associated with TURP, minimally invasive and non-invasive techniques including microwave therapy [11] and transurethral needle (RF) ablation [7] were developed. These thermal ablation methods are designed to spare the urethra and thus do not mechanically debulk the prostate. The coagulated tissue resulting from thermal treatment is probably not well reabsorbed as has been seen in the literature on RF ablation of renal masses [12]. Deviation from the principle of immediate mechanical tissue debulking achieved with TURP is likely the reason for inferior long term outcomes, lengthy post-procedure catheterization requirements and delayed relief of symptoms [13, 14], when compared to TURP.
The mechanism of histotripsy tissue effect is through cavitation action of microbubbles rather than absorption of heat. A large focused ultrasound transducer is used to emit brief, intense acoustic bursts at a rate up to several kilohertz. At the focus of this sound field, microscopic voids form (microbubbles) which oscillate violently causing disruption of cellular membranes and tissue structures ultimately leading to the fractionation and liquefaction of the targeted tissue. This is distinct from HIFU where focused ultrasound energy is applied to induce thermal tissue coagulation of the prostate. Histotripsy appears to be a more efficient debulking process because the fractionated (liquefied) tissue is easily eliminated when in communication with a natural channel or relatively quickly reabsorbed by the body [15] where undrained. In the case of prostate debulking, where a portion of the prostatic urethra is destroyed, liquefied prostate tissue can be simply voided with urination.
The histotripsy process is readily imaged with standard diagnostic ultrasound imaging as the microbubbles are very strong scatterers of ultrasound. This produces a cloud of twinkling points appearing on the ultrasound image at the site of cavitation. To build up the desired ablation volume, the sound field focus is slowly scanned about under ultrasound guidance. In addition to these appearance changes during treatment, fractionated tissue often appears hypoechoic [16] and the fluid motion of the liquefied tissue may be visible. Very high quality imaging of the prostate is possible with transrectal ultrasound particularly when using probes design for brachytherapy guidance. This makes precise treatment control possible and provides feedback of effectiveness during histotripsy treatment of the prostate.
Specific treatment methods for BPH using histotripsy have yet to be explored. One strategy would be to fractionate the entire transition zone and urethra as is done with TURP. However, this may make the procedure unacceptably long. Shortening the time required compared to a traditional TURP would likely contribute to the success of this technique. Other strategies might take advantage of the different dose thresholds found for the urethra and parenchymal tissues. Parenchymal prostate tissue was found to be more readily fractionated with lower histotripsy doses than urethral tissue. While there does not appear to be any adverse effects caused by super threshold doses, the extra treatment time required is undesirable. A low treatment dose appears to be sufficient to rapidly fractionate parenchymal tissue, though largely sparing urethral tissue (or causing only reversible damage). Reabsorption of liquefied tissue in a few days or weeks would result in size reduction relieving symptoms. Alternatively, the urethra could also be specifically targeted with a high dose in one region to cause a perforation allowing immediate drainage of the liquefied parenchymal tissue. Partial treatments of the whole transition zone may also be effective. A number of small treatment volumes placed regularly in the prostate could reduce the overall tissue density sufficiently to relieve symptoms. It is also important to consider that a quick and mostly painless non-invasive procedure might be repeated as necessary as is often done using lithotripsy for patients with recurring stones.
Urethral tissue was found to have a sharp difference in treatment effect between the low and higher histotripsy doses. However, this was not apparent in prostates harvested on the day of treatment. The small amount of damage seen immediately with the lowest dose may be reversible within 28 days while permanent damage from higher doses can be difficult to identify immediately. Additionally, it is possible that vascular damage may also contribute to urethral necrosis and eventual tissue removal.
One concern for translating this work to a clinical trial is the anatomical differences between canines and humans. Canines are one of very few mammals with more than a vestigial prostate and the only anatomically practical model for development of BPH therapies. The human prostate is located much deeper in the pelvis which would make trans-abdominal histotripsy very challenging. Instead, it would seem a trans-perineal approach would be favored. Preliminary research [17] examining perineal ultrasound therapy access to the prostate is encouraging.
Conclusions
Histotripsy is effective at fractionating parenchymal and urethral tissue in the prostate with a greater dose required for urethral tissue. Sufficient treatment doses allow immediate drainage or rapid reabsorption of fractionated tissue which may minimize or eliminate urinary catheterization requirements. Further development of this technique could lead to a non-invasive method for debulking the prostate to relieve symptoms associated with BPH.
Supplementary Material
This video clip shows a portion of a typical histotripsy treatment as seen with transrectal ultrasound imaging. The dynamic hyperechoic bubble cloud shows the active site of ablation at the transducer focus. As this focus is slowly scanned through the tissue, the previously treated region appears hypoechoic.
Acknowledgments
This research was funded in part by grants from the Wallace H. Coulter Foundation and NIH 1K08DK081656-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chute CG, Panser LA, Girman CJ, Oesterling JE, Guess HA, Jacobsen SJ, et al. The prevalence of prostatism: A population-based survey of urinary symptoms. J Urol. 1993;150:85–89. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 2.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: Benign prostatic hyperplasia. J Urol. 2005;173:1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 3.Mebust WK, Holtgrewe HL, Cockett AT, Peters PC. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol. 1989;141:243–247. doi: 10.1016/s0022-5347(17)40731-2. [DOI] [PubMed] [Google Scholar]
- 4.Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: A prospective multicenter evaluation of 10,654 patients. J Urol. 2008;180:246–249. doi: 10.1016/j.juro.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 5.Yu X, Elliot SP, Wilt TJ, McBean AM. Practice patterns in benign prostatic hyperplasia surgical therapy: The dramatic increase in minimally invasive technologies. J Urol. 2008;180:241–245. doi: 10.1016/j.juro.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Tunuguntla HS, Evans CP. Minimally invasive therapies for benign prostatic hyperplasia. World J Urol. 2002;20(4):197–206. doi: 10.1007/s00345-002-0283-2. [DOI] [PubMed] [Google Scholar]
- 7.Zlotta AR, Giannakopoulos X, Maehlum O, Ostrem T, Schulman CC. Long-term evaluation of transurethral needle ablation of the prostate (TUNA) for treatment of symptomatic benign prostatic hyperplasia: clinical outcome up to five years from three centers. Eur Urol. 2003;44:89–93. doi: 10.1016/s0302-2838(03)00218-5. [DOI] [PubMed] [Google Scholar]
- 8.Elhilali MM. Minimally invasive therapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177:820–821. doi: 10.1016/j.juro.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Kieran K, Hall TL, Parsons JE, Wolf JS, Jr, Fowlkes JB, Cain CA, Roberts WW. Refining histotripsy: defining the parameter space for the creation of nonthermal lesions with high intensity, pulsed focused ultrasound of the in vitro kidney. J Urol. 2007;178(2):672–676. doi: 10.1016/j.juro.2007.03.093. [DOI] [PubMed] [Google Scholar]
- 10.Lake AM, Hall TL, Kieran K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy: minimally invasive technology for prostatic tissue ablation in an in vivo canine model. Urology. 2008;72(3):682–686. doi: 10.1016/j.urology.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman RM, MacDonald R, Monga M, Wilt TJ. Transurethral microwave thermotherapy vs transurethral resection for treating benign prostatic hyperplasia: A systematic review. Br J Urol. 2004;94:1031–1036. doi: 10.1111/j.1464-410X.2004.05099.x. [DOI] [PubMed] [Google Scholar]
- 12.Hegarty NJ, Gill IS, Desai MM, Remer EM, O’Malley CM, Kaouk JH. Probeablative nephron-sparing surgery: cryoablation versus radiofrequency ablation. Urology. 2006;68 suppl 1A:7–13. doi: 10.1016/j.urology.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 13.Kellner DS, Armenakas NA, Brodherson M, Heyman J, Fracchia JA. of high-energy transurethral microwave thermotherapy in alleviating medically refractory urinary retention due to benign prostatic hyperplasia. Urology. 2004 Oct;64(4):703–706. doi: 10.1016/j.urology.2004.04.074. [DOI] [PubMed] [Google Scholar]
- 14.Minardi D, Garofalo F, Yehia M, Cristalli AF, Giammarco L, Galosi AB, Muzzonigro G. Pressure-flow studies in men with benign prostatic hypertrophy before and after treatment with transurethral needle ablation. Urol Int. 2001;66(2):89–93. doi: 10.1159/000056577. [DOI] [PubMed] [Google Scholar]
- 15.Hall TL, Kieran K, Ives K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy of rabbit renal tissue in vivo: temporal histologic trends. J Endourol. 2007;21(10):1159–1166. doi: 10.1089/end.2007.9915. [DOI] [PubMed] [Google Scholar]
- 16.Hall TL, Fowlkes JB, Cain CA. A real-time measure of cavitation induced tissue disruption by ultrasound imaging backscatter reduction. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54(3):569–575. doi: 10.1109/tuffc.2007.279. [DOI] [PubMed] [Google Scholar]
- 17.Häcker A, Köhrmann KU, Back W, Kraut O, Marlinghaus E, Alken P, Michel MS. Extracorporeal application of high-intensity focused ultrasound for prostatic tissue ablation. BJU Int. 2005;96(1):71–76. doi: 10.1111/j.1464-410X.2005.05570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video clip shows a portion of a typical histotripsy treatment as seen with transrectal ultrasound imaging. The dynamic hyperechoic bubble cloud shows the active site of ablation at the transducer focus. As this focus is slowly scanned through the tissue, the previously treated region appears hypoechoic.