Abstract
A liver biopsy remains the gold standard to diagnose nonalcoholic steatohepatitis (NASH). We have recently demonstrated that plasma cytokeratin 18 (CK-18) fragment levels correlate with the magnitude of hepatocyte apoptosis and independently predict the presence of NASH. Our aim was to validate the utility of this novel biomarker for NASH diagnosis. The study was an ancillary study of the NASH Clinical Research Network (NASH CRN). Our cohort consisted of 139 patients with biopsy proven NAFLD from eight centers across the United States who are participants of the CRN and 150 age-matched healthy controls. CK-18 fragments were measured using a specific immunoELISA. Histology was assessed centrally by study pathologists.CK-18 fragments were markedly increased in patients with NASH as compared to not NASH and borderline diagnosis (Median (Q25, Q75): 335 (196, 511), 194 (151, 270), 200 (148, 284), respectively; P < 0.001). Moreover, the odds of having fibrosis on liver biopsy increased with increasing plasma CK-18 fragment levels (P < 0.001). On multivariable regression analysis, CK-18 fragments remained an independent predictor of NASH after adjusting for variables associated with CK-18 fragments or NASH on the univariable analysis (fibrosis, ALT, AST, age, biopsy length). The area under the ROC curve for NASH diagnosis was estimated to be 0.83 (0.75, 0.91).
Conclusion
Determination of CK-18 fragments in the blood predicts histological NASH and severity of disease in a large, diverse population of patients with biopsy-proven NAFLD, supporting the potential usefulness of this test in clinical practice.
Keywords: Nonalcoholic steatohepatitis, apoptosis, biomarker, cytokeratin 18
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease in both children and adults and threatens to become a serious public health problem (1, 2). Indeed, estimates show than about 80 million Americans may have a fatty liver. NAFLD encompasses a wide spectrum of conditions associated with over-accumulation of fat in the liver ranging from nonalcoholic fatty liver (NAFL) or simple steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis (3). Although NAFL typically follows a benign non-progressive clinical course, NASH is a potentially serious condition, since as many as 25% of patients may progress to cirrhosis and experience complications of portal hypertension, liver failure and hepatocellular carcinoma (4-6). At present, the available non-invasive tests to distinguish NASH from NAFL include clinical signs and symptoms, routine laboratory and radiological imaging tests and combinations of clinical and blood test results (7). Unfortunately, these tests are of limited utility, and liver biopsy remains the only reliable way of diagnosing NASH and grading the severity of liver damage. However, it is obvious that an invasive liver biopsy is poorly suited as a diagnostic test in such a prevalent condition. There is, therefore, an urgent need to develop and validate a simple, reproducible, non-invasive test that both accurately distinguishes NASH from NAFL and determines the stage and grade of the disease. Several investigators have tried to identify potential non-invasive markers for NASH diagnosis; however none of these markers have been externally validated(8-14). Validation and clinically availability of such a test would not only aid clinicians in the identification of patients with NASH, but also allow for non-invasive frequent monitoring of disease progression and response to therapy.
Emerging data suggest that hepatocyte apoptosis, a highly organized and genetically controlled form of cell death, may play an important role in liver injury and disease progression in NAFLD (15, 16). Increase in hepatocyte cell death by apoptosis is typically present in humans with NASH as well as animal models of NASH but absent in those with NAFL (17). A central consequence of the apoptotic process is the activation of the effector caspases (mainly caspase 3) which cleave a number of different substrates inside the cell including cytokeratin 18 (CK-18), the major intermediate filament protein in the liver, resulting in the characteristic morphologic changes of apoptosis (18). Recently in a “proof of concept” study, caspase generated CK-18 fragments were tested in the livers as well as in plasma of patients undergoing a liver biopsy for suspected NAFLD and healthy age-matched controls (19). CK-18 fragments were significantly elevated in the NAFLD patients as compared to controls and plasma levels correlated with the expression levels in the liver. Similar results were subsequently observed in an independent population of morbid obese patients undergoing bariatric surgery(20). The objective of the present study was to validate the clinical value of determination of the CK-18 fragment levels in blood for NASH diagnosis and assessment of disease severity in a large cohort of well characterized NAFLD patients from different regions across the United States.
PATIENTS AND METHODS
Patient characteristics
The study was approved as an ancillary study of the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) and approved by the Cleveland Clinic Institutional Review Board. Our initial cohort consisted of 178 patients which included both adult and pediatric NAFLD. However, due to important difference between pediatric and adult NAFLD we decided to report the pediatric results on a separate manuscript. Thus, the population for the current study consisted of 139 well characterized biopsy proven adult NAFLD patients seen at eight different centers across the United States who are participants of the NASH CRN study. None of the patients included in this study were participants of the two previous studies on CK-18 levels from the Cleveland Clinic(19, 20). The diagnosis of NAFLD was based on the following criteria: 1) Liver biopsy features as assessed by NASH CRN pathologists; 2) appropriate exclusion of liver disease of other etiology including alcohol- or drug-induced, autoimmune, viral, cholestatic, metabolic or genetic disorders; and 3) plasma sample available within three months of baseline liver biopsy. As part of the initial screening evaluation for patient enrollment into any of the three protocols of the NASH CRN, patients undergo an extensive and thorough interrogation of alcohol consumption, including lifelong history and current consumption including the AUDIT questionnaire. Demographic, clinical and laboratory data were obtained from the NASH CRN database. In addition, 150 age-matched healthy controls from blood bank donors without clinical signs or symptoms of illness, normal transaminases and no history of chronic liver disease were analyzed.
Liver histology
The histological diagnosis of NAFLD was established by study pathologists according to their expertise. Patients were subdivided into three histological groups according to the consensus of the NASH CRN Pathology Committee: not NASH, borderline diagnosis, and NASH. The NAFLD activity score was determined for each patient(21). The stage of fibrosis was assessed using a 4-point scale (1= mild /moderate zone 3 perisinusoidal fibrosis, or portal fibrosis only; 2 = zone 3 and portal/periportal fibrosis; 3 = bridging fibrosis; 4 = cirrhosis).
Measurement of caspase-generated CK-18 fragments in the blood
For all patients in our cohort, a blood sample, taken within 3 months of the liver biopsy, was obtained from the NIH blood bank repository. All samples were originally processed to plasma and stored frozen at -80°C. The plasma was subsequently used for quantitative measurement of the apoptosis-associated neo-epitope in the C-terminal domain of CK-18 by the M30-Apoptosense ELISA kit (PEVIVA, Alexis, Grünwald, Germany). All assays were performed in duplicate and the absorbance was determined using a microplate reader (Molecular Devices M2, Sunnyvale, California, US).
Statistical Analysis
Descriptive statistics were computed for all variables. These include medians, as well as 25th and 75th percentiles for continuous factors. For categorical variables, frequencies and percentages were estimated. Kruskal-Wallis and Dunn's tests were used to assess whether there were any significant differences in terms of continuous clinical or serological characteristics between any of the three subject groups. Chi-square or Fisher's exact tests were used for categorical factors. Spearman's correlation coefficient was used to estimate the association of plasma CK-18 levels and several factors of interest. CK-18 levels were categorized into quartiles and a Cochran-Armitage trend test was used to assess whether the percentage of subjects with NASH increased with increasing level of CK-18; the same was done for fibrosis. Logistic regression analysis was used to assess the association between plasma levels of CK-18 fragments and the likelihood of having NASH as opposed to not having NASH (not NASH or Borderline). Receiver operating characteristic curve analysis was used to assess the utility of CK-18 in the diagnosis of NASH. A multivariable logistic regression analysis was performed in order to adjust for variables that were found to be associated with CK-18 fragments in the univariable analysis or those known to be associated with NASH severity (fibrosis, ALT, AST, age, biopsy length). Inclusion of variables was assessed using a stepwise selection method, which started with a model containing only one constant term and evaluated adding or deleting factors from the model until no additional terms could enter the model on the basis of a P value > 0.50 and no factors could be eliminated from the model on the basis of a P value < 0.20. The same was done to assess the utility of CK-18 levels in the prediction of having fibrosis. A P value of 0.05 was considered statistically significant. SAS version 9.1 software (SAS Institute, Cary, NC) and R 2.0.1 software (The R Foundation for Statistical Computing) were used to perform all analyses.
RESULTS
Characteristics of the patient population
The main clinical and laboratory characteristics of the patients are described in Table 1. Patient's age (median 48 years), gender (63% females), racial distribution (median of 79% of Caucasians) and BMI (median 34 kg/m2) did not statistically differ between the three histological NAFLD groups. There was no difference in the prevalence of hypertension, hyperlipidemia, or diabetes among groups. AST levels were significantly higher in subjects with NASH as compared to those with not NASH (P<0.01); there was no evidence to suggest a difference between subjects with not NASH and those with a borderline diagnosis (P of 0.33). ALT and AST/ALT ratio were not significantly different among the groups.
Table 1.
Clinical and Serological Characteristics of the patient population
| Factor | All Subjects (n = 139) | Not NASH (n = 44) | Borderline (n =26) | NASH (n = 69) | P value* |
|---|---|---|---|---|---|
| Age at enrollment (yr) | 48.0 (39.0, 55.0) | 49.0 (39.0, 57.0) | 46.5 (43.0, 55.0) | 47.0 (38.0, 54.0) | 0.86 |
| Months of plasma storage | 12.4 (9.0, 14.9) | 12.0 (8.4, 16.1) | 12.1 (8.8, 14.5) | 13.0 (9.5, 14.5) | 0.91 |
| BMI (Kg/m2) | 34.2 (30.3, 37.8) | 34.2 (30.1, 39.3) | 34.2 (29.8, 37.1) | 34.2 (30.7, 37.2) | 0.97 |
| AST (U/L) | 43.0 (31.0, 62.0) | 36.5 (27.5, 53.0) | 38.5 (31.0, 62.0) | 47.0 (36.0, 75.0) | 0.01 |
| ALT (U/L) | 66.0 (46.0, 109.0) | 56.5 (38.5, 89.0) | 68.5 (44.0, 111.0) | 68.0 (48.0, 120.0) | 0.14 |
| GGT (U/L) | 43.0 (28.5, 67.0) | 41.5 (28.0, 73.0) | 37.0 (22.0, 55.0) | 49.0 (33.0, 72.0) | 0.16 |
| AST/ALT ratio | 0.7 (0.5, 0.8) | 0.6 (0.5, 0.8) | 0.6 (0.5, 0.8) | 0.7 (0.6, 0.8) | 0.31 |
| Female gender | 88 (63.3) | 29 (65.9) | 14 (53.9) | 45 (65.2) | 0.54 |
| Race | 0.37 | ||||
| Caucasian | 110 (79.1) | 32 (72.7) | 23 (88.5) | 55 (79.7) | |
| Hispanic | 16 (11.5) | 8 (18.2) | 2 (7.7) | 6 (8.7) | |
| Other | 13 (9.4) | 4 (9.1) | 1 (3.9) | 8 (11.6) | |
| Diabetes | 26 (18.7) | 7 (15.9) | 4 (15.4) | 15 (21.7) | 0.66 |
| Hypertension | 59 (42.5) | 14 (31.8) | 13 (50.0) | 32 (46.4) | 0.21 |
| Hyperlipidemia | 84 (60.4) | 22 (50.0) | 16 (61.5) | 46 (66.7) | 0.21 |
| Biopsy length (mm) | 17.0 (12.0, 26.0) | 12.0 (9.0, 23.0) | 22.5 (14.0, 30.0) | 19.0 (13.0, 25.0) | 0.01 |
Statistics presented are Median (25th, 75th percentiles) or N (%).
Abbreviations: N, number of subjects; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glytammyl transpeptidase; BMI; body mass index.
P values correspond to the comparison of the three subject groups. Kruskal-Wallis tests for continuous factors and Pearson's chisquare or Fisher's exact test for categorical factors were used.
Table 2 summarizes the histological characteristics of the patient population. There were 44 patients (31%) with not NASH on liver biopsy, 26 (19%) with borderline diagnosis and 69 (50%) with NASH.
Table 2.
Histological Characteristics
| Factor | All Subjects (%) (n = 139) |
|---|---|
| Steatosis | |
| <5% | 4 (2.9) |
| 5-33% | 46 (33.1) |
| 34-65% | 48 (34.5) |
| >=66% | 41 (29.5) |
| Lobular inflammation | |
| <2 under 20x | 73 (52.5) |
| 2-4 under 20x | 52 (37.4) |
| >4 under 20x | 14 (10.1) |
| Ballooning | |
| None | 56 (40.3) |
| Few | 48 (34.5) |
| Many | 35 (25.2) |
| Fibrosis | |
| 0 | 56 (40.3) |
| 1 | 39 (28.1) |
| 2 | 29 (20.9) |
| 3 | 15 (10.8) |
Abbreviations: N, number of subjects
CK-18 fragments are markedly increased in the blood of patients with NASH
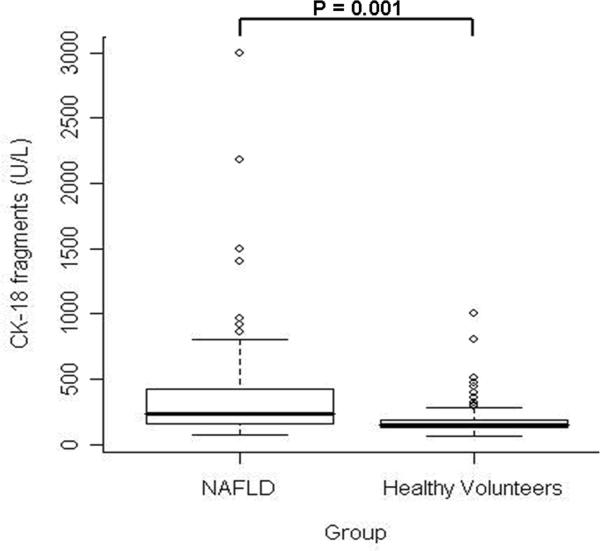
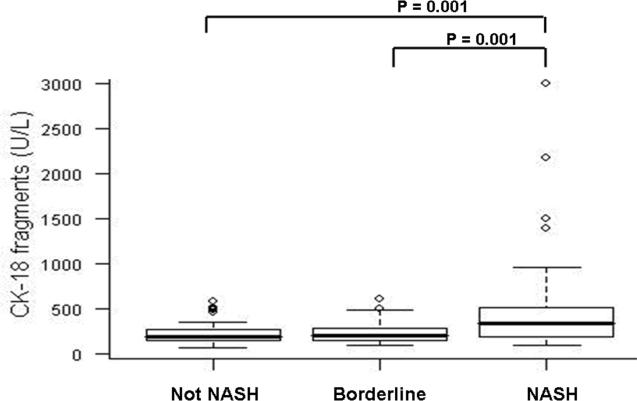
CK-18 fragment levels ranged from 68 to 3000 U/L (median (Q25, Q75): 244 U/L (161, 427)) and were significantly higher than in the 150 healthy volunteers (median (Q25, Q75): 145 U/L (126, 190); P<0.001 (Figure 1). More importantly, CK-18 fragment levels were significantly higher in patients with NASH as compared to those with not NASH or borderline diagnosis (median (Q25, Q75): 335 (196, 511), 194 (151, 270), 200 (148, 284), respectively; P < 0.001) (Figure 2). On the other hand, there was no evidence to suggest a difference in CK-18 fragment levels between subjects with not NASH and those with a borderline diagnosis (P=0.41).
Figure 1. CK-18 fragments are significantly increased in blood of patients with NAFLD compared to healthy volunteers.
Vertical axis represents plasma CK-18 levels in U/L and horizontal axis patient groups. The box represents the interquartile range (the 25th and 75th percentiles) from the median (the horizontal line), the bars the 95% confidence interval. Median and [interquartile ranges] are 244 U/L [161, 427], 145 U/L [126, 190], for NAFLD and healthy volunteers respectively (P<0.001).
Figure 2. CK-18 fragments are significantly increased in blood of patients with NASH compared to patients with simple steatosis and patients with borderline diagnosis.
Vertical axis is plasma CK-18 levels in U/L and horizontal axis patient groups. The box represents the interquartile range (the 25th and 75th percentiles) from the median (the horizontal line), the bars the 95% confidence interval. CK-18 levels were significantly higher in subjects with NASH as compared to those with not NASH or borderline diagnosis (median (Q25, Q75): 335 (196, 511), 194 (151, 270), 200 (148, 284), respectively; P < 0.001)
CK-18 fragment levels showed a significant positive correlation with NAS (r (95% CI): 0.51 (0.36, 0.65)), as well as the individual NAS components (Table 3), although the strength of the association with the later ones was only weak to moderate (Table 3). In addition, CK-18 fragment levels correlated with serum aminotransferases including ALT (r (95% CI): 0.55 (0.41, 0.69)), and AST (r (95% CI): 0.59 (0.45, 0.72)) and the stage of fibrosis (r (95% CI): 0.36 (0.21, 0.51)). However, CK-18 fragment levels did not differ significantly according to age of the patients or body mass index or the presence or absence of diabetes, hyperlipidemia, or hypertension (P >0.35).
Table 3.
Correlations with CK-18 Fragment Levels
| Factor | rho | 95% CI | P value |
|---|---|---|---|
| Age at enrollment | -0.08 | (-0.25,0.09) | 0.37 |
| BMI* | -0.07 | (-0.24,0.10) | 0.4 |
| NAS | 0.51 | (0.36,0.65) | <0.001 |
| ALT | 0.55 | (0.41,0.69) | <0.001 |
| AST | 0.59 | (0.45,0.72) | <0.001 |
| AST/ALT Ratio | -0.07 | (-0.24,0.10) | 0.42 |
| Steatosis | 0.28 | (0.12,0.44) | <0.001 |
| Lobular inflammation | 0.49 | (0.34,0.64) | <0.001 |
| Ballooning | 0.3 | (0.14,0.46) | <0.001 |
| Fibrosis | 0.36 | (0.20, 0.51) | <0.001 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI;body mass index.
P value corresponds to Ho: rho=0
138 subjects
CK-18 fragments as an independent predictor of NASH
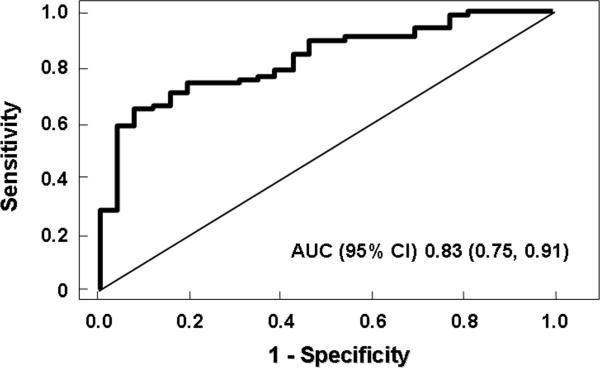
The risk of having NASH on liver biopsy increased with increasing CK-18 fragment levels (P=0.0001). For every 50 U/L increase in the plasma level of CK-18, the likelihood of having NASH increased 30% (OR (95% CI): 1.3 (1.1, 1.4)). Furthermore, CK-18 fragment levels were significantly higher in patients with fibrosis as compared to those without fibrosis (median (Q25, Q75): 305 (192, 493) vs. 193 (151, 261); P < 0.001). Similarly levels were significantly higher in those with moderate to severe fibrosis (stage 2-3) compared to those patients with no or mild fibrosis (stage 0-1) (median (Q25, Q75): 304 (211, 575) vs. 211 (154, 363); P < 0.002) (Table 4). To ascertain whether plasma CK-18 fragment levels independently predicted the presence of NASH, we used a multivariable logistic regression analysis. Variables associated with CK-18 fragment levels and those that are known to be associated with NASH severity (fibrosis stage, age, BMI, AST/ALT ratio, diabetes, and hyperlipidemia) were studied as possible confounders of the association between NASH and plasma levels of CK-18 fragments. The adjusted OR 1.2 (1.05, 1.5) was similar to the unadjusted OR, confirming that CK-18 fragment level is an independent predictor of NASH diagnosis (P < 0.01). Using the area under the receiver operating characteristic (ROC) curve approach we next calculated potential cutoff values to separate patients with NASH from those with not NASH or borderline diagnosis (Figure 3). The area under the ROC curve (AUC) was estimated to be 0.83 (0.61, 0.78). Prediction based on CK-18 fragments was significantly better than that based on ALT (AUC 0.58 (0.49, 0.68); p<0.01), AST (AUC 0.64 (0.55, 0.74); p=0.01), and GGT (AUC 0.57 (0.47, 0.67); p=0.01). There was no evidence to suggest that a model combining CK-18 fragments with routine laboratory tests (ALT or GGT) resulted in any significant improvement in AUCs (p≥0.20). Several cut off values were calculated to minimize the rate of false positive or false negative results (Table 4).
Table 4.
CK-18 Fragment Levels for NASH Prediction
| CK-18 Level (U/L) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| 216 | 77 (66, 85) | 65 (44, 83) |
| 230 | 76 (65, 84) | 69 (48, 86) |
| 246 | 75 (64, 83) | 81 (61, 93) |
| 279 | 71 (60, 80) | 85 (65, 96) |
| 281 | 67 (57, 77) | 89 (70, 98) |
| 287 | 65 (54, 75) | 92 (75, 99) |
All values are expressed as percentage and 95% confidence interval
Figure 3. CK-18 fragment levels for diagnoses of NASH.
CK-18 fragment levels accurately diagnose NASH in patients with NAFLD. The area under the ROC curve is shown for the performance of the CK-18 fragment levels for discriminating NASH from borderline and not NASH diagnosis.
DISCUSSION
NAFLD prevalence has grown to epidemic proportions affecting close to 30% of adults and 10% of children in the US (1, 22). Long-term longitudinal studies suggest NAFL has a benign non-progressive clinical course, while NASH is a serious condition with increased risk of both overall and liver related morbidity and mortality (4-6). Moreover, the prognosis of patients with NASH appears to be dictated by the presence and extent of fibrosis present on liver biopsy (4-6). Thus, at present time, an invasive liver biopsy is the only reliable way to diagnose the presence of NASH and assess the severity of liver damage present(23). There is, therefore, an urgent need to develop and validate a simple, reproducible, non-invasive test that accurately distinguishes NASH from NAFL and determines the stage of the disease. Such a test would not only aid clinicians in the identification of patients with NASH, but also allow for non-invasive frequent monitoring of disease status, response to therapy, and prediction of disease progression risk.
Emerging data suggest that hepatocyte apoptosis may play an important role in the pathogenesis of NAFLD (15). Hepatocyte apoptosis is a prominent pathologic feature of human NASH (17) and the magnitude of apoptosis present correlates with degree of liver damage and stage of fibrosis. Experimental studies suggest that uncontrolled hepatocyte apoptosis may be a central mechanisms triggering liver fibrogenesis and fibrosis(24). For instance, attenuation of hepatocyte apoptosis also reduces fibrogenesis in animal models of cholestasis (25, 26), while hepatocyte-specific genetic disruption of the anti-apoptotic member of the Bcl-2 family, Bcl-xL results in hepatocyte apoptosis and liver fibrotic responses (27). This latter model is highly illustrative because it directly demonstrates that hepatocyte apoptosis is profibrogenic. Engulfment of apoptotic bodies by hepatic stellate cells stimulates the fibrogenic activity of these cells and may be one mechanism by which hepatocyte apoptosis promotes fibrosis(28). Recent data also demonstrated that DNA from apoptotic hepatocytes acts as an important mediator of hepatic stellate cell activation(29). Thus, non-invasive quantification of hepatocellular apoptosis represents a rational approach to assess the extend of liver damage and fibrosis present in the liver at a given time and also fibrogenesis and the risk for disease progression overtime In hepatocytes, regardless of the triggering stimuli, the apoptotic process tends to converge at the level of the mitochondria resulting in permeabilization of the mitochondrial outer membrane and release of multiple proteins from the mitochondrial intermembrane space into the cytosol (30, 31). The result of this process is the activation of the effector caspases (mainly caspase 3) which cleave different substrates inside the cell including cytokeratin 18 (CK-18), the major intermediate filament protein in the liver, resulting in apoptosis(23, 32). Recently, in a small pilot study using a specific immunoELISA assay we showed that these fragments were strikingly increased in the serum of patients with NASH and correlated with the presence of fibrosis (19). Moreover, we showed that this marker accurate predicted NASH in an independent population of morbid obese subjects(20), while subsequently other groups have reported similar results (33, 34). Using this novel approach in the present study we were able to demonstrate that determination of CK-18 fragments in blood accurately identifies the presence of NASH on liver biopsy. The CK-18 test is able to detect the presence of NASH with a specificity of more than 90%, or to exclude the presence of NASH with a sensitivity close to 80% by adopting different test thresholds.
The current study has several strengths. We included a large group of well characterized NAFLD patients followed at eight different centers across the U.S that form the NASH NIH Clinical Research Network. The cohort is composed of a large variety of different ages as well as different ethnic backgrounds. One limitation of our study is the fact that we used liver biopsy as the “gold standard” for assessing the utility of the CK-18 test. This technique has important limitations including those associated with sampling errors as well as intra- and inter-observer variability which at least in part may be linked to liver biopsy size (35). However, a series of studies looking at sampling error in NAFLD have demonstrated that this is more of an issue for the individual histological findings of necroinflammatory activity and hepatocyte ballooning but much less so for the diagnosis of NASH and staging of fibrosis (36, 37).
Similarly to what has been previously reported (7), in our study routinely available laboratory tests did not show sufficient sensitivity and specificity to diagnose NASH. Also, adding these tests to the CK-18 fragment determination to create a prediction model did not appear to improve further the diagnostic value of CK-18 fragment levels alone. Other approaches such as combining CK-18 fragment levels with other chemical markers(33, 38), prediction models such as NashTest(11), or imaging studies such as tissue elastography (39) warrants further investigation.
In summary, our findings suggest that non-invasive monitoring of hepatocyte apoptosis in blood of patients with NAFLD is a novel and reliable tool to diagnose NASH in patients with suspected NAFLD supporting its potential usefulness in clinical practice as a noninvasive NASH biomarker.
Table 5.
CK-18 Fragment Levels (U/L) & Fibrosis
| N | Median (Q25, Q75) | P value | |
|---|---|---|---|
| Fibrosis Grade | |||
| 0 | 56 | 192.7 (150.5, 261.2) | |
| 1 | 39 | 330.2 (170.4, 487.8) | |
| 2 | 29 | 279.6 (176.3, 575.1) | |
| 3 | 15 | 357.2 (240.3, 633.2) | |
| No(0) | 56 | 192.7 (150.5, 261.2) | <0.001 |
| Yes (1-3) | 83 | 305.3 (192.3, 492.7) | |
| Mild (0-1) | 95 | 210.9 (154.0, 363.0) | 0.002 |
| Moderate to Severe (≥ 2) | 53 | 318.5 (210.5, 575.1) | |
| Mild to Moderate (0-2) | 124 | 216.4 (157.7, 389.3) | 0.006 |
| Severe (≥ 3) | 15 | 357.2 (240.3, 633.2) |
Statistics presented are Median (25th, 75th percentiles).
Acknowledgment
Dr. Arthur McCullough was the liaison to the NASH CRN. The authors would like to thank the NASH CRN for providing us with the patients samples as well as extensive clinical, laboratory, and histological data and Teresa Markle and Michael Berk, General Clinical Research Center Technologists, for their excellent work and dedication.
This work was supported by the Nonalcoholic Steatohepatitis Clinical Research Network, GCRC grant MO1 RR-018390, and by NIH grant (DK076852) and the AGA Research Scholar Award (RSA) to AEF.
Footnotes
Financial Disclosures: None
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–641. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 3.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 6.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 7.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386–395. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 8.Campos GM, Bambha K, Vittinghoff E, Rabl C, Posselt AM, Ciovica R, Tiwari U, et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47:1916–1923. doi: 10.1002/hep.22241. [DOI] [PubMed] [Google Scholar]
- 9.Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954–960. doi: 10.1309/6VJ4DWGYDU0XYJ8Q. [DOI] [PubMed] [Google Scholar]
- 10.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–1971. e1962. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, Massard J, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solga SF, Alkhuraishe A, Cope K, Tabesh A, Clark JM, Torbenson M, Schwartz P, et al. Breath biomarkers and non-alcoholic fatty liver disease: preliminary observations. Biomarkers. 2006;11:174–183. doi: 10.1080/13547500500421070. [DOI] [PubMed] [Google Scholar]
- 13.Sookoian S, Castano G, Burgueno AL, Gianotti TF, Rosselli MS, Pirola CJ. A diagnostic model to differentiate simple steatosis from nonalcoholic steatohepatitis based on the likelihood ratio form of Bayes theorem. Clin Biochem. 2008 doi: 10.1016/j.clinbiochem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Sumida Y, Nakashima T, Yoh T, Furutani M, Hirohama A, Kakisaka Y, Nakajima Y, et al. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38:32–38. doi: 10.1016/s0168-8278(02)00331-8. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093–3099. doi: 10.2741/1765. [DOI] [PubMed] [Google Scholar]
- 16.Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 18.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 19.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 20.Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, Feldstein AE. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6:1249–1254. doi: 10.1016/j.cgh.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 23.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 24.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 25.Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 26.Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112:152–159. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takehara T, Tatsumi T, Suzuki T, Rucker EB, 3rd, Hennighausen L, Jinushi M, Miyagi T, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 30.Ricci JE, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Ricci JE, Waterhouse N, Green DR. Mitochondrial functions during cell death, a complex (I-V) dilemma. Cell Death Differ. 2003;10:488–492. doi: 10.1038/sj.cdd.4401225. [DOI] [PubMed] [Google Scholar]
- 32.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, Rafiq N, et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH) Obes Surg. 2008;18:1430–1437. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz Y, Dolar E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, Aker S, et al. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol. 2007;13:837–844. doi: 10.3748/wjg.v13.i6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 36.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 37.Janiec DJ, Jacobson ER, Freeth A, Spaulding L, Blaszyk H. Histologic variation of grade and stage of non-alcoholic fatty liver disease in liver biopsies. Obes Surg. 2005;15:497–501. doi: 10.1381/0960892053723268. [DOI] [PubMed] [Google Scholar]
- 38.Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, et al. Performance of ELF Serum Markers in Predicting Fibrosis Stage in Pediatric Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, Fruhwirth R, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442–448. doi: 10.1002/hep.22376. [DOI] [PubMed] [Google Scholar]