Abstract
P73 plays an important role in modulating cell-cycle control, inducing apoptosis, and inhibiting cell growth. A novel non-coding p73 G4C14-to-A4T14 exon 2 polymorphism was associated with risk of squamous cell carcinoma of the head and neck (SCCHN). We hypothesized that p73 G4C14-to-A4T14 polymorphism modulates risk of second primary malignancies (SPM) in patients after index SCCHN. We followed a cohort of 1,384 patients diagnosed with incident SCCHN between May 1995 and January 2007 for SPM development. Log-rank test and Cox proportional hazard models were used to compare SPM-free survival and SPM risk between the different genotype groups. Our results showed that patients carrying the p73 variant AT allele were less likely to develop SPM compared to the patients with p73 GC/GC genotype (Log-rank test, P = 0.013). Compared to the p73 GC/GC genotype, there was a significantly reduced risk of SPM associated with the p73 GC/AT genotype (HR, 0.61, 95% CI, 0.40-0.93) and the combined p73 GC/AT+AT/AT genotypes (HR, 0.59, 95% CI, 0.39-0.89), but a non-significantly reduced risk for p73 AT/AT genotype (HR, 0.44, 95% CI, 0.14-1.41). The p73 AT allele was significantly associated with risk of SPM in an allele dose-response manner (P = 0.011 for trend). The risk of SPM associated with p73 variant genotypes (GC/AT+AT/AT) was more pronounced in several subgroups (e.g., older patients, men, minorities, ever smokers and ever drinkers). Our results support that this p73 polymorphism may be a marker for risk of SPM among patients with an incident SCCHN.
Keywords: p73 G4C14-to-A4T14, Squamous cell carcinoma of head and neck, Second primary malignancy, Genetic susceptibility, Polymorphism
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is the sixth most common malignancy worldwide, and is a cancer with moderately low survival and high recurrence rates, high second primary tumor rates, and high prevalence of comorbid illnesses. Each year, approximately 50,000 new patients are confirmed as having SCCHN, who are usually diagnosed at approximately 60 years of age 1. Smoking and alcohol and genetic variation have been linked to both SCCHN and SPM. Surgery, radiotherapy, and chemotherapy cure many SCCHN, but a significant cause of post-treatment morbidity and mortality is the development of SPM, estimated to occur in approximately 15% of SCCHN patients. Understanding susceptibility to tobacco-related malignancies holds great promise for primary cancer prevention, and identifying makers of risk for SPM among cancer survivors would greatly enhance secondary prevention, which is currently limited to rather simplistic clinical post-treatment screenings.
To achieve cure, aggressive multidisciplinary treatment has been adapted for SCCHN patients. However, second primary malignancy (SPM), which occurs in approximately 15% of SCCHN patients, is still a major factor contributing to poor overall survival of SCCHN patients 2, 3. While tobacco and alcohol exposure 4,5 and cancer treatment 6,7 have been found to be associated with the risk of SPM, only a small proportion of SCCHN patients will develop SPM, implying that there is inter-individual variation in genetic susceptibility to SPM among SCCHN patients 3. For instance, Foulkes et al has suggested that inherited genetic factors modify carcinogen activity or host resistance to carcinogens in the development of multiple head and neck tumors 8.
Functionally, p73, a family member of p53, activates transcription of p21- and p53-responsive genes, which participate in cell cycle control, DNA repair, and apoptosis and inhibits cell growth in a p53-like manner by inducing apoptosis or G1 cell cycle arrest 9,10. Studies in various tumors suggest that p73 mutations are rare 11-13. Therefore, this gene does not function as a traditional tumor-suppressor gene. Furthermore, increased p73 expression was found in human malignancies associated with p53 mutations 12,14-16, suggesting that p73 may act as a tumor suppressor with overlapping functions of p53 or compensating for the loss of function of p53 caused by mutations 12,14-16. Therefore, p73 may play an independent and/or compensatory functional role for p53 in SCCHN development and progression.
Genetic alteration of p53 has been shown to be associated with the development of SPM17,18. For instance, overexpression of p53 in the first primary cancer may be a valuable marker for identifying individuals at high risk of developing SPM 19, and the overexpression in tumor-distant epithelia in SCCHN patients is associated with the risk of development of a SPM. Therefore, this could be used as a marker to identify which groups of patients are at higher risk of developing SPM 20. Given the functional similarities between p73 and p53, genetic alteration of p73 could also contribute to the risk of SPM among SCCHN patients.
There are two completely linked non-coding exon 2 polymorphisms of p73 at positions 4 (G→A) and 14 (C→T) which are thought to affect p73 function by altering gene expression, perhaps by altering the efficiency of translational initiation 21. Whether this modifies the risk of SPM is unknown. Previous reports suggested that p73 G4C14-to-A4T14 polymorphism was associated with the risk of several types of cancers and survival of cancer patients 22-27. However, the role of p73 polymorphism in the etiology of SPM after index SCCHN has not been investigated. In order to test the hypothesis that the p73 G4C14-to-A4T14 polymorphism is associated with the risk of SPMs after index SCCHN, we evaluated this association in a cohort of 1384 incident SCCHN patients to compare SPM-free survival and the risk of SPM in different genotype groups.
Materials and Methods
Study subjects
A cohort of 1,667 patients with incident SCCHN (newly diagnosed, histopathologically confirmed, and untreated squamous cell carcinomas of the oral cavity, oropharynx, hypopharynx or larynx) were consecutively recruited from May 1995 to January 2007 at The University of Texas M. D. Anderson Cancer Center as part of a ongoing prospective molecular epidemiologic study, which has been previously described elsewhere 28,29. All patients completed an IRB-approved informed consent and were recruited without discrimination regarding age, sex, ethnicity, or clinical stage. Patients with any prior cancer history excepting nonmelanoma skin cancer were not recruited. Also, patients with known distant metastases, primary sinonasal tumors, salivary gland tumors, cervical metastases of unknown origin, or tumors outside the upper aerodigestive tract were not recruited. Approximately 95% of contacted patients consented to enrollment in the parent study. Blood samples for genotyping data were not available for some patients recruited early in the study, and these patients were excluded from this genotype and SPM analysis, as were patients without follow-up and patients who underwent only palliative treatment. Consequently, 1,384 patients were available for this study of SPM risk.
Patients were monitored through their treatment and post-treatment course with regularly scheduled clinical and radiographic examinations. SPMs were distinguished from local recurrences based on modified criteria of Warren and Gates 30. Second lesions with different histopathologic type, or occurring more than 5 years following treatment for the primary tumor, or clearly separated by normal epithelium based on clinical and radiographic assessment were considered SPM. If there was discrepancy or differing opinion regarding the origin of the tumor, the second lesion was classified as a local recurrence rather than a SPM. Pulmonary lesions were considered SPM if they had a non-squamous histology; or if they were isolated squamous lesions greater than 5 years from initial SCCHN and felt to be SPM by the thoracic oncologist and thoracic surgeon. SPMs were then classified as tobacco-associated (SCCHN or cancers of the esophagus, lung, or bladder) and non tobacco-associated SPM.
Clinical data were obtained at initial presentation and through follow-up examinations and included overall stage at presentation of the index tumor, site of the index tumor, and treatment. Index cancer stage was then dichotomized into the early stage (including I and II clinical stage) and late stage (III and IV). We also grouped treatment into four categories: surgery only, surgery with radiotherapy and/or chemotherapy, radiotherapy, and radiotherapy plus chemotherapy. All patients completed at presentation an epidemiological questionnaire including data on alcohol and smoking status. Alcohol status was categorized as “ever drinkers” (those who had drunk at least one alcoholic beverage/per day for at least one year during their lifetime) and “never drinkers” (those who never had such a pattern of drinking). “Ever smokers” were those who had smoked at least 100 cigarettes in their lifetime, and “never smokers” were those who had smoked fewer than 100 cigarettes in their lifetime.
p73 genotyping
Genomic DNA was extracted from peripheral leukocyte pellets according to the manufacturer's instructions (QIAGEN Inc., Valencia, Calif) for the genotyping of the p73 polymorphism. The primers and PCR method for the polymorphism have been previously described 24. There was 100% concordance when 10% of the genotyping assays were repeated.
Statistical Analysis
For all analyses, statistical significance was set at P < 0.05, and all tests were two-sided. The Statistical Analysis System software (version 9.1.3; SAS Institute) was used to perform all statistical analyses. The primary endpoint of the study was SPM occurrence. The mean age and follow-up time of the patients who developed a second primary cancer and those who did not were compared using the Student's t test. The chi-squared test was used to evaluate differences in ethnicity, sex, smoking and alcohol status, tumor site, tumor stage, treatment, genotype distributions, and allele frequencies between the two groups. Time-to-event was calculated from the date of diagnosis of the index SCCHN to the date of SPM occurrence. Patients who were not known to have an event at the date of last contact, or who were lost to follow-up, or who died following the initial date of diagnosis of their first primary head and neck cancer, were censored. The associations between individual epidemiological risk factors, clinical characteristics including tumor site, staging, and treatment variables, and time to the occurrence of the SPMs, were initially assessed using univariate Cox proportional hazards regression models. The data were consistent with the assumptions of the Cox proportional hazards regression model from the examination of Kaplan-Meier survival curves and log-minus-log survival plots.
Epidemiological variables in the univariate analysis, assessed at the time of diagnosis, included age in years, ethnicity, sex, and smoking and alcohol status. Clinical characteristics included tumor site, tumor stage, and treatment. The first step in building a multivariable model for time to SPM occurrence did not incorporate any interaction terms. A multivariable proportional hazards model was built using the variables that had prognostic potential suggested by the univariate analysis (P < 0.25). Due to epidemiological and clinical considerations in building the model, age, sex, and ethnicity were always retained in the main-effects and final multivariable model. In building multivariable models, a stepwise search strategy was used. A threshold level of 0.25 for the likelihood ratio test was used as a cutoff to determine whether a variable could be entered into, or removed from, the regression model. Associations were quantified using hazard ratios and their 95% confidence intervals for developing a SPM. The final fully adjusted Cox regression models included age, sex, ethnicity, and smoking and alcohol status.
Results
Patient Characteristics
Table I shows the demographics, exposure, and clinical variables for the 1,384 patients, of whom the 1,274 patients did not develop SPM, and the 110 (8.0%) patients developed SPM. In this patient cohort, the median follow-up time was 26 months (range 0 to 142.4 months). Of the 110 patients with SPM, 77 developed second tobacco-associated cancers, 29 developed other non tobacco-associated malignancies, and 4 had both a tobacco-associated and non-tobacco-associated SPM. Of 43 patients with second SCCHN, 24 (56%) were synchronous SCCHN primaries. Of these 24 patients with synchronous SCCHN, two had bilateral oral cavity cancers, three had bilateral oropharyngeal cancers, one had bilateral hypopharyngeal cancers, and the remainder had simultaneous cancers of more than one head and neck subsite.
Table I.
Distribution of Selected Characteristics of the Patient Cohort (n = 1384)
| Variable | Total | SPM-Free | SPM | P-values* | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Total patients | 1384 | 100 | 1274 | 92.1 | 110 | 7.9 | |
| Age | |||||||
| ≤ median (57 years) | 726 | 52.5 | 690 | 54.2 | 36 | 32.7 | < 0.001 |
| > median (57 years) | 658 | 47.5 | 584 | 45.8 | 74 | 67.3 | |
| Sex | 0.761 | ||||||
| Male | 1053 | 76.1 | 968 | 76.0 | 85 | 77.3 | |
| Female | 331 | 23.9 | 306 | 24.0 | 25 | 22.7 | |
| Ethnicity | 0.100 | ||||||
| Non-Hispanic White | 1160 | 83.8 | 1074 | 84.3 | 86 | 78.2 | |
| Other | 224 | 16.2 | 200 | 15.7 | 24 | 21.8 | |
| Smoking | 0.021 | ||||||
| Never | 382 | 27.6 | 362 | 28.4 | 20 | 18.2 | |
| Ever | 1002 | 72.4 | 912 | 71.6 | 90 | 81.8 | |
| Alcohol | 0.052 | ||||||
| Never | 373 | 26.9 | 352 | 27.6 | 21 | 19.1 | |
| Ever | 1011 | 73.1 | 922 | 72.4 | 89 | 80.9 | |
| Index Cancer Site | 0.114 | ||||||
| Oral cavity | 453 | 32.7 | 419 | 33.0 | 34 | 30.9 | |
| Oropharynx | 623 | 45.1 | 580 | 45.5 | 43 | 39.1 | |
| Larynx/Hypopharynx | 307 | 22.2 | 274 | 21.5 | 33 | 30.0 | |
| Index Cancer Stage | 0.819 | ||||||
| 1 or 2 | 352 | 25.4 | 323 | 25.4 | 29 | 26.4 | |
| 3 or 4 | 1031 | 74.6 | 950 | 74.6 | 81 | 73.6 | |
| Treatment | 0.983 | ||||||
| Surgery only | 247 | 17.9 | 228 | 17.9 | 19 | 17.3 | |
| Surgery + Adjuvant Tx1 | 343 | 24.8 | 314 | 24.6 | 29 | 26.4 | |
| XRT2 | 348 | 25.1 | 321 | 25.2 | 27 | 24.5 | |
| XRT + Chemotherapy | 446 | 32.2 | 411 | 32.3 | 35 | 31.8 | |
P values were calculated from chi-square test
Adjuvant Tx: adjuvant radiotherapy and/or chemotherapy
XRT: radiotherapy
The mean age at diagnosis for the index SCCHN patients was 57.3 years (range, 18-94 years, median, 57 years), and the mean age of patients at index SCCHN who developed SPM was significantly older than that of patients who did not develop a SPM (60.8 years vs. 57.0 years, respectively; P < 0.01). The participants in this study were predominantly male (76.1%) and non-Hispanic whites (83.8%), although sex and ethnicity were not associated with SPM development (P = 0.761 for sex and P = 0.100 for ethnicity; respectively). Compared with the SPM-free group, patients who developed SPM had similar characteristics with respect to index cancer site (P = 0.114), cancer stage (P = 0.819), and treatment (P = 0.870). However, compared with the SPM-free group, patients who developed SPM were more likely older (P < 0.001), smokers (P = 0.021), and drinkers (P = 0.052).
Association of p73 G4C14-to-A4T14 polymorphism with risk of SPM
Genotype data were available for 1,284 (92.8%) patients in the cohort. The p73 genotype distributions among the patients, the patients who developed SPM and the patients who did not, and the associations with risk of SPM are shown in Table II. The frequency of p73 wild type homozygous genotype (GC/GC) among SCCHN patients was 57.7%, and the minor or variant AT/AT homozygote only accounted for 4.6%. The distribution of p73 genotypes was significantly different between patients who developed SPM and those who did not (P = 0.047). Because the variant homozygotes for this p73 polymorphism were relatively uncommon, we combined the variant homozygotes with heterozygotes for SPM-free survival and SPM risk analyses. We found that the patients with p73 variant GC/AT or AT/AT genotype experienced a better SPM-free survival compared with patients with the p73 wild-type GC/GC genotype (log-rank, P = 0.013, Figure 1). When data were adjusted for age, sex, ethnicity, smoking, and alcohol (Table II), patients with the heterozygous (GC/AT) genotype had an approximately 39% significantly reduced risk for developing SPM compared to patients with the p73 wild-type genotype (GC/GC) (HR, 0.61; 95% CI, 0.40-0.93), and the combined variant genotypes (GC/AT+AT/AT) was associated with a significantly 41% reduced risk for SPM (HR, 0.59; 95% CI, 0.39-0.89). Moreover, the p73 AT/AT genotype was associated with an even lower risk of SPM (HR, 0.44; 95% CI, 0.14-1.41), although this association was not statistically significant likely due to the limited size of this subgroup. It appeared that the p73 AT allele was significantly associated with risk of SPM in a dose-response manner (P = 0.011 for trend).
Table II.
SPM Risk Associated with p73 Polymorphism after the Index SCCHN
| P73 Genotype | Total (No. = 1284) |
SPM-free (No. = 1175) |
SPM (No. = 109) |
Pa | HR (95% CI)* | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| GC/GC (ref.b) | 741 | 57.7 | 666 | 56.7 | 75 | 68.8 | 1.00 | |
| GC/AT | 484 | 37.7 | 453 | 38.5 | 31 | 28.4 | 0.61(0.40-0.93) | |
| AT/AT | 59 | 4.6 | 56 | 4.8 | 3 | 2.8 | 0.047 | 0.44 (0.14-1.41) |
| Trend test | 0.011 | |||||||
| GC/AT + AT/AT | 543 | 42.3 | 509 | 43.3 | 34 | 31.2 | 0.014 | 0.59 (0.39-0.89) |
χ2 test for differences in the distribution of p73 genotypes between the patients who developed SPM and the patients who did not
Adjusted for age, sex, ethnicity, tobacco smoking and alcohol drinking in a Cox model.
Ref. = reference group.
Figure 1.
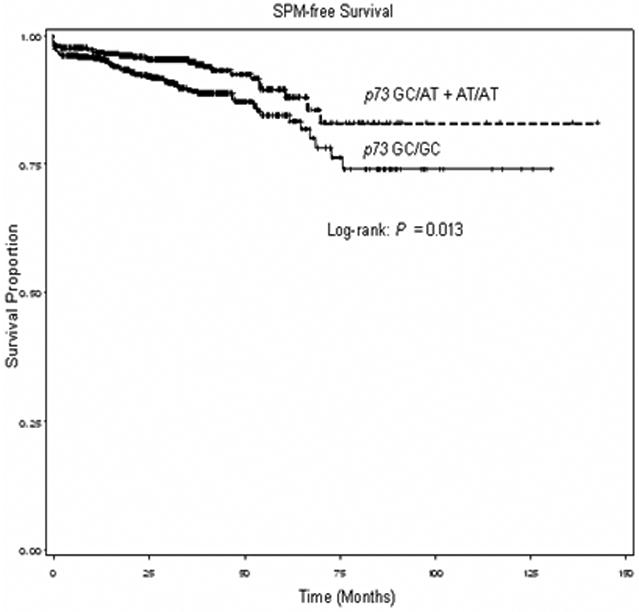
Kaplan-Meier SPM-free survival curve stratified by p73 homozygous wild-type genotype (GC/GC) and p73 combined variant genotypes (GC/AT+AT/AT)
Stratification Analysis
To evaluate the risk of SPM for subgroups, we further stratified the association between the combined p73 variant genotypes (GC/AT+AT/AT) and SPM risk by age, sex, ethnicity, smoking/drinking status, cancer treatment, index cancer stage, and SPM type as shown in Table III. We found a reduced SPM risk associated with the combined variant genotypes (GC/AT+AT/AT) for all subgroups, but this protective effect was significant only for older patients, men, minorities, ever smokers, ever drinkers, patients treated with DNA damaging therapeutic modalities, and patients with late stage of index SCCHN. Furthermore, we found that the patients with p73 combined variant genotypes had a 64% significantly reduced risk of non tobacco-associated SPM (HR = 0.36, 95% CI, 0.16-0.84).
Table III.
Stratification Analysis of Association of p73 Polymorphism with SPM Risk
| GC/GC (Reference) | GC/AT + AT/AT | ||||
|---|---|---|---|---|---|
| SPM-free No. (%) |
SPM No. (%) |
SPM-free No. % |
SPM No. % |
HR (95%CI)* | |
| Age(years) | |||||
| ≤57 | 357 (53.6) | 23 (30.7) | 278 (54.6) | 12 (35.3) | 0.65 (0.32-1.31) |
| >57 | 309 (46.4) | 52 (69.3) | 231 (45.4) | 22 (64.7) | 0.60 (0.36-0.99) |
| Sex | |||||
| Male | 511 (76.7) | 59 (78.7) | 376 (73.9) | 25 (73.5) | 0.62 (0.39-0.98) |
| Female | 155 (23.3) | 16 (21.3) | 133 (26.1) | 9 (26.5) | 0.66 (0.29-1.49) |
| Ethnicity | |||||
| Non-Hispanic Whites | 575 (86.3) | 55 (73.3) | 435 (85.5) | 30 (88.2) | 0.75 (0.48-1.17) |
| Others | 91 (13.7) | 20 (26.7) | 74 (14.5) | 4 (11.8) | 0.26 (0.09-0.77) |
| Smoking Status | |||||
| Ever | 473 (71.0) | 61 (81.3) | 374 (73.5) | 29 (85.3) | 0.64 (0.41-0.99) |
| Never | 193 (29.0) | 14 (18.7) | 135 (26.5) | 5 (14.7) | 0.50 (0.18-1.42) |
| Drinking Status | |||||
| Ever | 481 (72.2) | 60 (80.0) | 375 (73.7) | 27 (79.4) | 0.61 (0.39-0.96) |
| Never | 185 (27.8) | 15 (20.0) | 134 (26.3) | 7 (20.6) | 0.60 (0.24-1.48) |
| Treatment | |||||
| Surgery only | 117 (17.6) | 15 (20.0) | 93 (18.3) | 4 (11.8) | 0.38 (0.12-1.15) |
| DNA damaging | 549 (82.4) | 60 (80.0) | 416 (81.7) | 30 (88.2) | 0.66 (0.43-1.00) |
| Stage | |||||
| Early (1 or 2) | 165 (24.8) | 20 (26.7) | 139 (27.3) | 9 (26.5) | 0.56 (0.25-1.24) |
| Late (3 or 4) | 501 (75.2) | 55 (73.3) | 370 (72.7) | 25 (73.5) | 0.62 (0.39-0.99) |
| SPM type | |||||
| TAa | 689 (49.1) | 52 (66.7) | 515 (49.0) | 28 (80.0) | 0.71 (0.45-1.12) |
| Non-TA | 715 (50.9) | 26 (33.3) | 536 (51.0) | 7 (20.0) | 0.36 (0.16-0.84) |
| Tumor site | |||||
| oropharnx | 319 (47.9) | 29 (38.7) | 219 (43.0) | 14 (41.2) | 0.72 (0.38-1.38) |
| Non-oropharynx | 347 (52.1) | 46 (61.3) | 290 (57.0) | 20 (58.8) | 0.53 (0.31-0.90) |
Adjusted for age, sex, ethnicity, tobacco smoking and alcohol drinking in a Cox model.
TA: Tobacco-Associated
Discussion
Previous studies have evaluated the association of this p73 polymorphism and risk of or survival from several types of cancer 22-27, but none of these focused on risk of SPM after index SCCHN. In the current study of 1,384 patients with SCCHN, we found that patients with p73 GC/AT heterozygotes or the combined p73 GC/AT+AT/AT genotypes had a significantly lower risk of developing SPM compared to patients with p73 GC/GC wild-type homozygous genotype, and the risk was significant only in older patients, men, minorities, ever smokers and ever drinkers, the patients who had late stage of index SCCHN, and the patients who received DNA damage-related treatment. Additionally, the protective effect seemed greater for non-tobacco-associated SPM.
Like p53, p73 plays a similar role in regulating critical cellular activities, such as cell cycle control, DNA repair, and apoptosis, thereby influencing tumor development, progression, and response to DNA damage. Although the exact functional relevance of the p73 G4C14-to-A4T14 genetic variants remains unknown, the GC to AT change may lead to formation of stem-loop structure and so may influence p73 translation efficiency and altered gene expression, which in turn would affect the regulation of cell-cycle control, apoptosis, response to DNA damage, and cell growth. The functional relevance of this p73 G4C14-to-A4T14 polymorphism from the epidemiologic findings may also imply linkage disequilibrium with other functional variants of p73 or with alleles at other nearby loci of other genes. However, this hypothesis remains to be tested.
Findings from previous studies regarding the association of this p73 G4C14-to-A4T14 polymorphism with cancer risk are inconsistent 22-27. We previously reported that this p73 polymorphism was associated with a significantly increased risk of lung and head and neck cancers 23,24, and similar associations were found for cervical cancer in a Japanese population 26 and colorectal cancer in a Swedish population 27. In contrast, two studies found an even protective effect on risk of lung cancer in a Chinese population 22 and esophageal cancer in an Irish population 25; additionally two other studies did not observe any association with risk of colorectal cancer and breast cancer in a Japanese population 31,32.
These conflicting results may be attributed to differences in the genotype frequencies between various populations, who may differ in demography, diet, primary risk exposures, social factors, and clinical characteristics. Furthermore, the effect of a low-penetrance susceptibility gene on disease risk might be influenced by other modifying genes and environmental factors through gene-gene or gene-environment interactions. Therefore, different genetic backgrounds and different risk factors might explain, to some extent, these inconsistent results in risk estimates associated with this p73 polymorphism in different cancers and populations. Other factors such as small sample size, inclusion of a single polymorphism, mixture of different ethnic groups in a single study, or inadequate adjustment for confounding factors could also cause the inconsistent results.
Our results indicated a protected effect of the p73 variant genotypes on risk of SPM or a better SPM-free survival in patients with SCCHN, an opposite effect to risk of primary SCCHN in the same population 24. Pfeifer et al. also found that carriers of the p73 variant genotypes have a greater risk of cancer (colorectal), while the patients who carried p73 variant genotypes had a better prognosis 27. The exact mechanism for these seemingly conflicting results remains unknown. It might be possible that this p73 polymorphism may cause less p73 expression for patients with variant genotypes in tumors because previous studies have shown that over-expression of p73 is a poor prognostic factor in colorectal cancer patients 33,34. It also might be that this p73 polymorphism's effect in normal epithelium of the head and neck differ from those in SCCHN tumor tissues which have numerous somatic changes. it might be that this p73 polymorphism may function differently in etiology (case-control study) and prognosis (case only study) because the normal epithelium of the head and neck and SCCHN tumor tissues have significant differences in genetic profiles such as somatic genetic changes (e.g., p53 mutation). This polymorphism of p73 may have different roles in etiology and prognosis through the interaction of this p73 variant with the normal genes in normal tissues, genetically altered genes in SCCHN tissues, smoking behavior, human papillomavirus (HPV), and other environmental risk factors, respectively. Therefore, definitive answers to the functionality of this p73 variant in the development of SPM and SCCHN will obviously require extensive genetic and biochemical studies. Since the p73 variant AT allele may play a critical role in the development or progression of early cancerous lesions, it may lead to different p73 functions, perhaps due to splice variant expression 25. The expressed patterns of any spliced isoforms of p73, such as the pro-apoptotic TA73 or the anti-apoptotic DNp73, may cause different expression of p73. Therefore, individuals with the AT allele may have an increased risk of developing SCCHN, but in the tumors, the AT allele may inhibit oncogenic functions, which may lead to a better SPM-free survival. This hypothesis needs to be tested in additional studies.
Several studies have demonstrated that occurrence of SPM is not related to clinical characteristics or treatment but, rather, to previous and continued exposure to risk factors 4,35 except one study showing that radiotherapy for oral cancer may be a risk factor for SPMs 36. In the current study, we did not find an association between the clinical characteristics (including cancer treatment, staging, and sites) and risk of SPM after adjustment for other covariates, but the results from the current study and others suggested that genetic factors, within the context of previous or continued exposure to smoking and alcohol, may affect the risk of SPM after index SCCHN 37-39. We observed that the p73 variant genotypes (GC/AT+AT/AT) were an independent risk factor for the risk of SPM in SCCHN patients who received previous treatment, even after controlling for smoking and alcohol status. We also observed this protective effect in all subgroups, though this effect was significant in only older patients, men, minorities, ever smoker and ever drinkers.
Our study has several inherent limitations, although it was a large and well-characterized cohort in SCCHN patients from a major multidisciplinary cancer center. This patient cohort included multiple ethnicities, however, 85% of patients were non-Hispanic whites. Secondly, while demographics, exposure, and clinical data for the cohort were collected prospectively, clinical outcomes including SPM were collected retrospectively without a strictly defined screening or follow-up regimen. Furthermore, follow-up time in this study was somewhat limited, and patients may not have had as much opportunity to develop SPM. This could result in a lower SPM rate than expected, and the smaller number of SPM limited statistical power, particularly in subgroup analyses. Our low SPM rate also could be due to our high prevalence of never smokers and our strict criteria in defining SPM. Finally, due to the retrospective nature of the original study design, we did not have information on HPV status, one of the major risk factors for SCCHN. We also did not have information on smoking behavior available (e.g., smoking intensity and duration). The lack of this information may bias the estimates of association in this study. Therefore, once this information becomes available, our future studies on the association between this and/or other genetic polymorphisms and risk of SPM will take HPV and smoking behavior into account as confounders. With the information available, the further analysis on the interaction effect between the p73 polymorphism and smoking behavior and/or HPV on the risk of SPM as well as SCCHN will become feasible. Despite these limitations, the current investigation supports a significant role of p73 G4C14-to-A4T14 polymorphism in the development of SPM after index SCCHN.
Acknowledgments
Funded by: Research Training Award, The American Laryngological, Rhinological, and Otological Society (to E.M.S.); U.T. M.D. Anderson Cancer Center Start-up Funds (to E.M.S.); N.I.H. Grant K-12 88084 (to E.M.S., faculty trainee; to R.C. Bast, P.I.); National Institute of Environmental Health Sciences Grant R01 ES-11740 (to Q.W.); N.I.H. Grant P-30 CA-16672 (to The University of Texas M.D. Anderson Cancer Center); and N.I.H. grant CA135679 (to G.L.) and CA133099 (to G.L.)
Abbreviations
- SCCHN
squamous cell carcinoma of the head and neck
- SPM
second primary malignancies
- HR
hazard ratio
- 95% CI
95% confidence interval
- HPV
human papillomavirus
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Xu J, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Rennemo E, Zatterstrom U, Boysen M. Impact of second primary tumors on survival in head and neck cancer: An analysis of 2,063 cases. Laryngoscope. 2008;118:1350–6. doi: 10.1097/MLG.0b013e318172ef9a. [DOI] [PubMed] [Google Scholar]
- 3.Sturgis EM, Miller RH. Second primary malignancies in the head and neck cancer patient. Annals Otolog Rhinol Laryngol. 1995;104:946–54. doi: 10.1177/000348949510401206. [DOI] [PubMed] [Google Scholar]
- 4.Day GL, Blot WJ, Shore RE, McLaughlin JK, Austin DF, Greenberg RS, Liff JM, Preston-Martin S, Sarkar S, Schoenberg JB, Fraumeni JF. Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–7. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Do KA, Johnson MM, Lee JJ, Wu XF, Dong Q, Hong WK, Khuri FR, Spitz MR. Longitudinal study of smoking patterns in relation to the development of smoking-related secondary primary tumors in patients with upper aerodigestive tract malignancies. Cancer. 2004;101:2837–42. doi: 10.1002/cncr.20714. [DOI] [PubMed] [Google Scholar]
- 6.Ng AK, Travis LB. Second primary cancers: an overview. Hematol Oncol Clin North Am. 2008;22:271–89. doi: 10.1016/j.hoc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert ES, Stovall M, Gospodarowicz M, Van Leeuwen FE, Andersson M, Glimelius B, Joensuu T, Lynch CF, Curtis RE, Holowaty E, Storm H, Pukkala E, van't Veer MB, Fraumeni JF, Boice JD, Jr, Clarke EA, Travis LB. Lung cancer after treatment for Hodgkin's disease: focus on radiation effects. Radiat Res. 2003;159:161–73. doi: 10.1667/0033-7587(2003)159[0161:lcatfh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Foulkes WD, Brunet JS, Sieh W, Black MJ, Shenouda G, Narod SA. Familial risks of squamous cell carcinoma of the head and neck: retrospective case-control study. BMJ. 1996;313:716–21. doi: 10.1136/bmj.313.7059.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jost CA, Marin MC, Kaelin WG., Jr p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191–4. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- 10.Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–4. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 11.Mai M, Yokomizo A, Qian C, Yang P, Tindall DJ, Smith DI, Liu W. Activation of p73 silent allele in lung cancer. Cancer Res. 1998b;58:2347–9. [PubMed] [Google Scholar]
- 12.Yokomizo A, Mai M, Tindall DJ, Cheng L, Bostwick DG, Naito S, Smith DI, Liu W. Overexpression of the wild type p73 gene in human bladder cancer. Oncogene. 1999;18:1629–33. doi: 10.1038/sj.onc.1202474. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi H, Ichimiya S, Nimura Y, Watanabe M, Furusato M, Wakui S, Yatani R, Aizawa S, Nakagawara A. Mutation, allelotyping, and transcription analyses of the p73 gene in prostatic carcinoma. Cancer Res. 1998;58:2076–77. [PubMed] [Google Scholar]
- 14.Nimura Y, Mihara M, Ichimiya S, Sakiyama S, Seki N, Ohira M, Nomura N, Fujimori M, Adachi W, Amano J, He M, Ping YM, Nakagawara A. p73, a gene related to p53, is not mutated in esophageal carcinomas. Int J Cancer. 1998;78:437–40. doi: 10.1002/(sici)1097-0215(19981109)78:4<437::aid-ijc8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Zaika AI, Kovalev S, Marchenko ND, Moll UM. Overexpression of the wild type p73 gene in breast cancer tissues and cell lines. Cancer Res. 1999;59:3257–63. [PubMed] [Google Scholar]
- 16.Kang MJ, Park BJ, Byun DS, Park JI, Kim HJ, Park JH, Chi SG. Loss of imprinting and elevated expression of wild-type p73 in human gastric adenocarcinoma. Clin Cancer Res. 2000;6:1767–71. [PubMed] [Google Scholar]
- 17.Brachman DG, Graves D, Vokes E, Beckett M, Haraf D, Montag A, Dunphy E, Mick R, Yandell D, Weichselbaum RR. Occurrence of p53 gene deletions and human papilloma virus infection in human head and neck cancer. Cancer Res. 1992;52:4832–6. [PubMed] [Google Scholar]
- 18.Bénard J, Douc-Rasy S, Ahomadegbe JC. TP53 family members and human cancers. Hum Mutat. 2003;21:182–91. doi: 10.1002/humu.10172. [DOI] [PubMed] [Google Scholar]
- 19.Gallo O, Bianchi S. p53 expression: a potential biomarker for risk of multiple primary malignancies in the upper aerodigestive tract. Eur J Cancer B Oral Oncol. 1995;31B:53–7. doi: 10.1016/0964-1955(94)00031-x. [DOI] [PubMed] [Google Scholar]
- 20.Homann N, Nees M, Conradt C, Dietz A, Weidauer H, Maier H, Bosch FX. Overexpression of p53 in tumor-distant epithelia of head and neck cancer patients is associated with an increased incidence of second primary carcinoma. Clin Cancer Res. 2001;7:290–6. [PubMed] [Google Scholar]
- 21.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, Ferrara P, McKeon F, Caput D. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–19. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 22.Hu Z, Miao X, Ma H, Tan W, Wang X, Lu D, Wei Q, Lin D, Shen H. Dinucleotide polymorphism of p73 gene is associated with a reduced risk of lung cancer in a Chinese population. Int J Cancer. 2005;114:455–60. doi: 10.1002/ijc.20746. [DOI] [PubMed] [Google Scholar]
- 23.Li G, Wang LE, Chamberlain RM, Amos CI, Spitz MR, Wei Q. p73 G4C14-to-A4T14 polymorphism and risk of lung cancer. Cancer Res. 2004;64:6863–6. doi: 10.1158/0008-5472.CAN-04-1804. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Sturgis EM, Wang LE, Chamberlain RM, Amos CI, Spitz MR, El-Naggar AK, Hong WK, Wei Q. Association of a p73 exon 2 G4C14-to-A4T14 polymorphism with risk of squamous cell carcinoma of the head and neck. Carcinogenesis. 2004;25:1911–6. doi: 10.1093/carcin/bgh197. [DOI] [PubMed] [Google Scholar]
- 25.Ryan BM, McManus R, Daly JS, Carton E, Keeling PW, Reynolds JV, Kelleher DA. Common p73 polymorphism is associated with a reduced incidence of oesophageal carcinoma. Br J Cancer. 2001;85:1499–503. doi: 10.1054/bjoc.2001.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwa Y, Hamajima N, Atsuta Y, Yamamoto K, Tamakoshi A, Saito T, Hirose K, Nakanishi T, Nawa A, Kuzuya K, Tajima K. Genetic polymorphisms of p73 G4C14-to-A4T14 at exon 2 and p53 Arg72Pro and the risk of cervical cancer in Japanese. Cancer Lett. 2004;205:55–60. doi: 10.1016/j.canlet.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer D, Arbman G, Sun XF. Polymorphism of the p73 gene in relation to colorectal cancer risk and survival. Carcinogenesis. 2005;26:103–7. doi: 10.1093/carcin/bgh305. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Hu Z, Wang LE, Zhang W, El-Naggar AK, Sturgis EM, Wei Q. Polymorphic TP53BP1 and TP53 gene interactions associated with risk of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:4300–5. doi: 10.1158/1078-0432.CCR-07-0469. [DOI] [PubMed] [Google Scholar]
- 29.Zhen SL, Calderon J, Zhang Z, Sturgis EM, Spitz MR, Wei Q. Polymorphisms of vitamin D receptor gene protect against risk of head and neck cancer. Pharmacogenet Genomics. 2005;15:159–65. doi: 10.1097/01213011-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J Cancer. 1932;51:1358. [Google Scholar]
- 31.Hamajima N, Matsuo K, Suzuki T, Nakamura T, Matsuura A, Hatooka S, Shinoda M, Kodera Y, Yamamura Y, Hirai T, Kato T, Tajima K. No associations of p73 G4C14-to-A4T14 at exon 2 and p53 Arg72Pro polymorphisms with the risk of digestive tract cancers in Japanese. Cancer Lett. 2002;181:81–5. doi: 10.1016/s0304-3835(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 32.Huang XE, Hamajima N, Katsuda N, Matsuo K, Hirose K, Mizutani M, Iwata H, Miura S, Xiang J, Tokudome S, Tajima K. Association of p53 codon Arg72Pro and p73 G4C14-to-A4T14 at exon 2 genetic polymorphisms with the risk of Japanese breast cancer. Breast Cancer. 2000;10:307–11. doi: 10.1007/BF02967650. [DOI] [PubMed] [Google Scholar]
- 33.Sun XF. p73 overexpression is a prognostic factor in patients with colorectal adenocarcinoma. Clin Cancer Res. 2002;8:165–70. [PubMed] [Google Scholar]
- 34.Guan M, Peng HX, Yu B, Lu Y. p73 Overexpression and angiogenesis in human colorectal carcinoma. Jpan J Clin Oncol. 2003;33:215–20. doi: 10.1093/jjco/hyg045. [DOI] [PubMed] [Google Scholar]
- 35.Do KA, Johnson MM, Doherty DA, Lee JJ, Wu XF, Dong Q, Hong WK, Khuri FR, Fu KK, Spitz MR. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States) Cancer Causes Control. 2003;14:131–8. doi: 10.1023/a:1023060315781. [DOI] [PubMed] [Google Scholar]
- 36.Hashibe M, Ritz B, Le AD, Li G, Sankaranarayanan R, Zhang ZF. Radiotherapy for oral cancer as a risk factor for second primary cancers. Cancer Lett. 2005;220:185–95. doi: 10.1016/j.canlet.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328:184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 38.Ko Y, Abel J, Harth V, Bröde P, Antony C, Donat S, Fischer HP, Ortiz-Pallardo ME, Thier R, Sachinidis A, Vetter H, Bolt HM, Herberhold C, Brüning T. Association of CYP1B1 codon 432 mutant allele in head and neck squamous cell cancer is reflected by somatic mutations of p53 in tumor tissue. Cancer Res. 2001;61:4398–404. [PubMed] [Google Scholar]
- 39.Argiris A, Brockstein BE, Haraf DJ, Stenson KM, Mittal BB, Kies MS, Rosen FR, Jovanovic B, Vokes EE. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res. 2004;10:1956–62. doi: 10.1158/1078-0432.ccr-03-1077. [DOI] [PubMed] [Google Scholar]


