Abstract
The transport of drugs or drug delivery systems across the cell membrane is a complex biological process, often difficult to understand because of its dynamic nature. In this regard, model lipid membranes, which mimic many aspects of cell-membrane lipids, have been very useful in helping investigators to discern the roles of lipids in cellular interactions. One can use drug-lipid interactions to predict pharmacokinetic properties of drugs, such as their transport, biodistribution, accumulation, and hence efficacy. These interactions can also be used to study the mechanisms of transport, based on the structure and hydrophilicity/hydrophobicity of drug molecules. In recent years, model lipid membranes have also been explored to understand their mechanisms of interactions with peptides, polymers, and nanocarriers. These interaction studies can be used to design and develop efficient drug delivery systems. Changes in the lipid composition of cells and tissue in certain disease conditions may alter biophysical interactions, which could be explored to develop target-specific drugs and drug delivery systems. In this review, we discuss different model membranes, drug-lipid interactions and their significance, studies of model membrane interactions with nanocarriers, and how biophysical interaction studies with lipid model membranes could play an important role in drug discovery and drug delivery.
Keywords: Nanocarriers, Drug Transport, Lipids, Toxicity, Polymers
Introduction
Biological membranes contain lipid bilayers as their basic structural unit. Lipid bilayers are sheet-like assemblies of thousands of amphiphilic lipid molecules held together by hydrophobic interactions between their acyl chains. Such bilayers form the boundaries between intracellular cytoplasm and the cell’s outside environment, as well as between the interior of many of the cell’s organelles and their cytoplasm. Although this lipid bilayer structure was first recognized as the basis for cell membrane architecture in 1925,1 detailed study of the lipid structure of cell membranes began only three decades ago. In 1972, Singer and Nicholson2 first proposed a fluid mosaic model to explain the membrane structure. This model hypothesized that lipids and proteins diffuse freely within the plane of the cell membrane. Since then, the discovery of large membrane domains (e.g., basal, lateral and apical membrane regions of glandular, endothelial and epithelial cells) and lateral microdomain structures (e.g., lipid rafts, caveolae, and coated pits), revealed the complex nature of the cell membrane structure.3, 4
Membrane lipids can be divided into three groups based on their chemical structure: glycerol-based lipids (phospholipids), ceramide-based sphingolipids, and cholesterol. Phospholipids are further divided into different groups based on their hydrophilic head groups: phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine are the predominant lipids in the cellular membrane, whereas phosphatidylinositol and cardiolipin are present in smaller quantities. Sphingolipids have a sphingoid base as a backbone; these lipids impart greater hydrophobicity to the core of the lipid bilayer. Cholesterol has a hydrophilic hydroxyl group that interacts with the phospholipids’ hydrophilic head groups, and the bulky steroid groups interact with the hydrophobic acyl chains of lipids. These interactions influence the fluidity and packing of the lipid membrane.5, 6
It is very well known that membrane constituents are not always homogeneously arranged in the bilayer membrane of biological cells but rather organized in complex lateral microdomains (Figure 1-i). This polymorphic nature of lipid arrangement, in addition to a significant variety of lipids with distinctly different physical properties (i.e., cross-sectional area, fluidity, electric charge, molecular weight), is the reason that lipid membranes are extremely intricate structures.6, 7 Furthermore, the covalent association of proteins and carbohydrates adds to the complexity of this membrane’s structure.
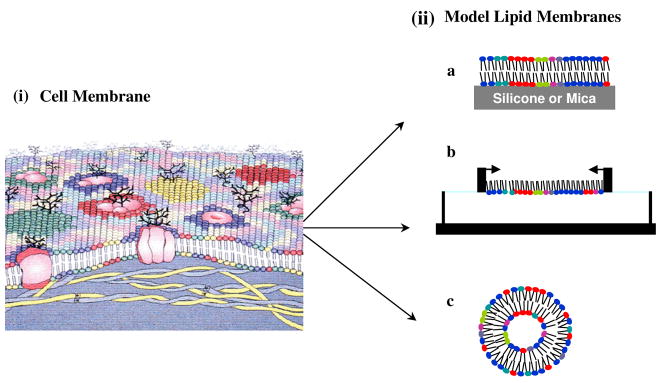
Fig. 1. Schematic illustration of cell membrane and different model membrane systems.
(i) This depiction of a cell membrane shows membrane lipid asymmetry as well as microdomains enriched in particular lipids and those induced by membrane proteins. (ii) Model membrane systems mimic the arrangement of lipid in a cell membrane. a, supported lipid bilayer; b, lipid monolayer; c, liposome. Figure 1. (i) Reproduced from ref (6) Copyright 2008 The Authors. Journal compilation copyright 2008 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
This intricacy of the cell membrane structure along with the highly dynamic nature of lipid-lipid and lipid-protein interactions in the cell membrane make the biophysical interactions with drugs and drug delivery systems very difficult to investigate. Therefore, simplified artificial membrane systems, which mimic the natural bilayer lipid membrane, have been developed. In this article, we review how studies of biophysical interactions with lipid model membrane systems elucidate the role of lipids in the cellular uptake of different drugs and drug delivery systems. Such interactions have also been explored in recent years to predict toxicity associated with drugs and nanoparticulate systems. We also discuss briefly the differences in lipid composition between diseased and normal cell membranes, and how that difference can influence the biophysical interactions of drug and drug delivery systems with the cell membrane.
Examples of Model Cell Membrane
Model cell membranes are systems in which the lipid organization mimics the arrangement of lipids in natural cell membranes. Supported lipid bilayers (SLBs)8 (Figure 1-iia), lipid monolayers9 (Figure 1-iib), and liposomes10 (Figure 1-iic) are widely used artificial model lipid membranes.
Supported lipid bilayers
SLBs are lipid bilayers formed on atomically smooth solid supports such as silicon or mica. Generally, SLBs are formed by fusion of lipid vesicles onto these solid supports or they can be formed by the Langmuir-Blodgett (LB) technique or by a combination of the LB and Langmuir-Schaeffer (LS) techniques. In the LB transfer technique,11, 12 a substrate is immersed in the subphase prior to formation of a Langmuir monolayer on the subphase; the Langmuir monolayer is then transferred by vertically lifting the substrate through the monolayer. In the LS technique,13 a hydrophobic substrate is positioned horizontal to the subphase; the monolayer is then transferred onto the hydrophobic substrate by bringing the substrate in contact with the Langmuir monolayer for a certain period of time (30–60 s). In the LB transfer technique, a bilayer is formed onto the substrate by a downward-then-upward dipping of a substrate through the Langmuir monolayer. In the combination transfer technique, the first layer is transferred by the LB technique and the second by the LS technique.14, 15 These model membrane systems typically allow only the investigation of interactions with lipid head groups. Changes in structure, morphology, and surface chemistry of SLBs following interaction with drugs or drug delivery systems can be investigated using various techniques, such as X-ray scattering, scanning electron microscopy, atomic force microscopy (AFM), transmission electron microscopy, Fourier transform infrared resonance (FTIR), and X-ray photoelectron spectroscopy.16, 17
Lipid monolayers
Lipid monolayers can be formed on the surface of water/buffer using a Langmuir film balance. In the Langmuir model system, various parameters such as lipid composition, subphase, and temperature can be chosen to imitate biological conditions.18, 19 In addition, lipid monolayers are very well-defined, stable, homogeneous bidimensional system with planar geometry. Typically, the interaction of drugs and drug delivery systems with lipids can be investigated in two ways. First, a lipid monolayer on the water/buffer surface is compressed to a surface pressure (SP) of 30 mN/m by applying lateral pressure. At this SP, lipid packing density is similar to that of a cell membrane, and the lipid monolayer mimics the outer surface of a cell membrane. By keeping the film area constant, changes in SP are recorded upon addition of drugs or drug delivery systems to the subphase. Alternatively, a lipid/drug or lipid/drug delivery system mixture is spread over a subphase (usually a buffer with pH 7.4 is used to mimic biological systems) to form a monolayer in a Langmuir trough. The monolayer is then compressed, and the SP-area isotherms of the pure lipid and the lipid with drug or drug delivery system are compared. Apart from changes in SP, changes in lipid morphology at the air-water interface can be studied by Brewster angle microscopy.20, 21; this method allows in situ study of the two-dimensional Langmuir monolayers at the air-water interface. This technique does not use probe compounds or any other modifications to sample for investigation; thus it ensures that the monolayer under investigation is in its original state. A lipid monolayer following interaction with drugs or drug delivery systems can be transferred to a molecularly smooth solid surface such as silicone or mica by the LB or LS technique. This transferred film can be surface characterized by several spectroscopic and microscopic techniques to investigate the effects of the interaction of drugs or drug delivery systems on lipids.
Liposomes
Liposomes are spherical lipid vesicles with an internal aqueous compartment.22 Three different kinds of unilamellar liposomes can be prepared: small (20–50 nm), large (50–100 nm), or giant (10–100 μm) unilamellar liposomes (SUVs, LUVs, or GUVs). GUVs are close to actual cell size. Typically, SUVs and LUVs are prepared by dissolving lipids in organic solvents (a 2:1 mixture of chloroform:methanol), then the solvents are evaporated under a vacuum to form thin films of lipids at the bottom of a round bottom flask. The dried lipid films are rehydrated in a desired buffer to form liposomes. The liposome suspension is subjected to vortexing until complete dissolution of the lipid film, followed by ultrasonication. This procedure usually results in multilammelar liposomes (MLVs), which are subjected to 3–5 cycles of freeze/thawing for the uniformity of liposome size. GUVs are prepared by electroformation.23–25 In this method, the lipid film is dried under an oscillating electric field. Typically, a standard wave generator is used to apply 1 V at 10 Hz between electrodes onto which a thin film of lipids has been dried in the presence of water to form GUVs. The liposome model membranes are highly suitable for the investigation of permeability of drugs and drug delivery systems and allow the use of various spectroscopic techniques, such as fluorescence and Raman spectroscopy, to study biophysical interactions.
Interactions of Model Lipid Membranes with Drugs
Many drug molecules have intracellular targets, and hence they must pass across one or more phospholipid bilayers to reach the intracellular targets and elicit a response to their pharmacological action; hence, drug-lipid interactions are inevitable. Studies in cell culture and in vivo have shown that drug-lipid interactions play a significant role in the pharmacokinetic properties of drugs, such as their transport, distribution, and accumulation, that ultimately influence the efficacy of the drugs used. Therefore, understanding the role of these interactions on the pharmacokinetic properties of drugs is critical in developing potent drugs. A variety of drugs – antibiotics,26–28 antihypertensive drugs,29, 30 antifungal drugs,21 antipsychotic drugs31, 32 and anticancer agents33–35 – have been investigated for their interaction with lipids using different biophysical techniques.
Antibiotics
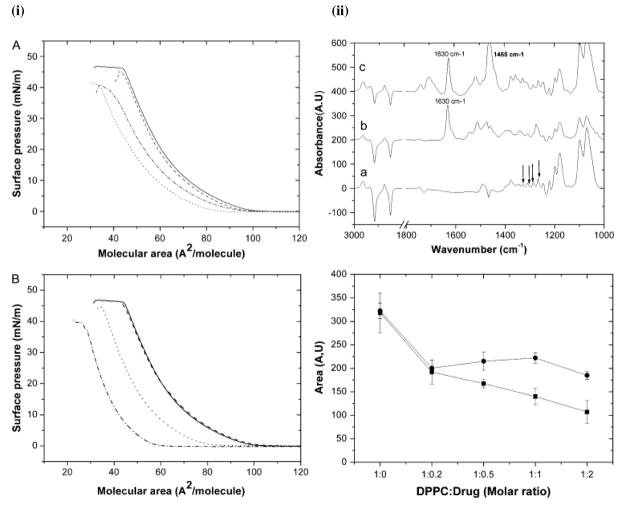
Biophysical investigations have provided better explanations for the observations made in cell culture studies and in vivo. For instance, Michot et al.36 observed significant differences in cellular accumulation and intracellular activity of four closely related fluoroquinolone derivatives in J774 macrophage cells in the following order: ciprofloxacin < levofloxacin < garenoxacin < moxifloxacin. Lower accumulation of ciprofloxacin compared to moxifloxacin was attributed to the efflux of ciprofloxacin in J774 macrophages37; however, the reason for the differential susceptibility to efflux by the ciprofloxacin transporter was not known. Biophysical investigations of the interactions of fluoroquinolone derivatives (ciprofloxacin and moxifloxacin) with lipid model membranes were used to explain the differences in cellular accumulation and intracellular activity of the two drugs.38–40 AFM of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)/dioleoylphosphatidylcholine (DOPC) SLBs showed that both ciprofloxacin and moxifloxicin can erode the DPPC domains within the DOPC fluid phase, whereas Langmuir studies showed a condensing effect (Figure 2-i). The comparison between the effects of the two drugs on lipids revealed that moxifloxacin has a higher condensing effect and greater ability to decrease the all-trans conformation of lipid acyl chains than ciprofloxacin (Figure 2-ii). The difference in lipid interaction was attributed to the difference in the molecular structure of the two drugs. The higher condensing effect of moxifloxacin was due to its higher lipophilicity as compared to ciprofloxacin. Based on biophysical interaction studies, the authors of the above study could explain that the greater cellular accumulation of moxifloxacin – is attributable to its increased interaction with the hydrophobic phase of lipid membranes – in turn resulted in slow release of moxifloxacin from the hydrophobic phase (membrane) to an aqueous phase (cytoplasm). This slow release of moxifloxacin from the membrane resulted in inactivation of efflux proteins, thus resulting in its greater cellular accumulation than ciprofloxacin.
Fig. 2. Effect of physiochemical properties of drug on biophysical interactions between drug and model membrane.
(i) SP-mean molecular area isotherms of DOPC/DPPC in the presence of ciprofloxacin (A) and moxifloxacin (B), on a subphase of 10 mM Tris at pH 7.4 and 25 °C. Mean molecular areas were corrected to take into account the percentage of fluoroquinolone remaining at the interface. DOPC/DPPC/drug molar ratios were 1:1:0 (continuous line), 1:1:0.4 (discontinuous line), 1:1:1 (dotted line), and 1:1:2 (dash-dotted line). (ii) Effect of fluoroquinolone on the orientation of the DPPC monolayer as revealed by attenuated total reflection-FTIR dichroic spectra. (Top panel) Polarized attenuated total reflection-FTIR spectra of DPPC (a), DPPC/ciprofloxacin (b), and DPPC/moxifloxacin (c). DPPC was used at 50 mg/ml; the molar ratio of lipid:drug was 1:1. Wagging γw(CH2) bands are indicated by arrows. (Bottom panel) Area evolution of dichroic peak of 1200 cm−1 of DPPC in the presence of fluoroquinolone. Integration area of dichroic γw(CH2) band (integrated between 1206 and 1193 cm−1) was plotted versus the DPPC/drug molar ratio as indicated, in the presence of ciprofloxacin (■) and moxifloxacin (●). Reproduced from ref (38). Copyright 2008 by the Biophysical Society.
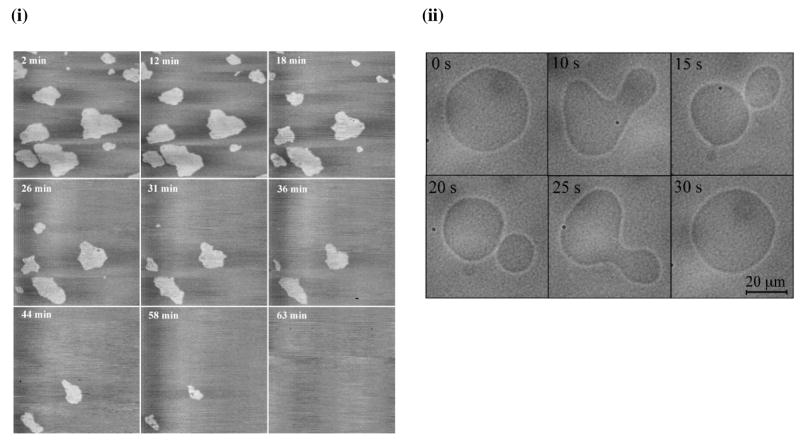
Azithromycin is another example of an antibiotic drug that has been widely investigated for its interactions with lipids. Studies using a variety of biophysical techniques such as AFM, fluorescence polarization, equilibrium dialysis,27, 28, 41 and differential scanning calorimetry42 demonstrated that the interaction of azithromycin with lipids is highly dependent on the chemical structure of lipid molecules. Azithromycin interacts with phospholipids but not with sphingomyelin (SM) and cholesterol. It was shown that azithromycin can merge the DPPC and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine gel domains in the DOPC fluid phase (Figure 3-i) but has no effect on SM or SM:cholesterol domains. Fa et al.23 investigated the effect of azithromycin on the elastic properties of DOPC GUVs. Microcinematographic and morphometric analyses revealed that the addition of azithromycin increases lipid membrane fluctuations, leading to eventual disruption of GUVs (Figure 3-ii). Thus, the data show that azithromycin can alter the elastic properties of DOPC lipid bilayers by decreasing the cohesion between DOPC molecules. Sogaard et al.43 showed that membrane protein function can be regulated by inducing changes in the elastic properties of the host lipid bilayer. For example, the structurally unrelated amphiphiles docosahexaenoic acid, polyunsaturated fatty acids, and Triton X-100 promoted GABAA receptor-muscimol binding by similar mechanism, i.e., by increasing the elasticity of lipid bilayers.
Fig. 3. Effect of chemical structure of lipid on biophysical interactions between drug and model membrane.
(i) AFM height images (7.5 × 7.5 μm; z-scale: 10 nm) of a mixed DPPC/DOPC (1:1, mol/mol) bilayer recorded in a Tris/NaCl/azithromycin solution at increasing incubation times. (ii) Qualitative evidence for the destabilization of GUVs by azithromycin. Typical fluctuations of a GUV exposed to 50 μM azithromycin over 30 s. Scale bar = 20 μm. Figure 3. (i) Reproduced from ref (41), copyright 2004 Elsevier. Figure 3. (ii) Reproduced from ref (23), copyright 2007 Elsevier.
Antifungal drugs
Corvis et al.21 used Langmuir model membranes to study the behavior of the antifungal agent griseofulvin at a biologically equivalent SP of 30 mN/m. It was speculated that the mechanism of action of griseofulvin may involve the ability of the cell membrane to recognize a particular molecule.
Antipsychotic drugs
Hidalgo et al.32 investigated the effects of the antipsychotic drugs trifluoperazine and chlorpromazine on lipid monolayers and found that the binding of even a very small amount of these drugs induces changes in SP and surface potential. From this observation, the authors concluded that the lipids must react to these drugs cooperatively, but binding of these drugs causes changes in a large number of lipids that extend far beyond the site at which the drug is bound. The cooperative effect of the lipid membrane observed in this study can possibly explain the mechanism of these drugs with relatively nonspecific effects upon the lipid membrane.
Liposome model membranes as predictors of drug efficacy
Liposome model membrane systems are widely used to estimate the efficacy of drugs. In general, drug effectiveness is measured by determining the partition coefficient of drugs, which is the measure of the amount of drug that will enter into and/or through a lipid membrane into the biological system. Partition coefficients are usually estimated for drugs by using an isotropic two-phase solvent system such as an octanol-water mixture.44 Several studies have shown that liposome model membranes are a better alternative than traditional methods used to estimate partition coefficients because those methods cannot account for the ionic interaction that may occur between drugs and lipids, particularly when drugs are charged. For instance, Rodrigues et al.45 showed that the drugs rifampicin and dibucaine, which are ionized at physiological pH, had different partition coefficient values with water-dimyristoyl-L-α-phosphatidylglycerol (DMPG) (anionic liposome) and water-DMPC (zwitterionic liposome) in comparison to neutral drugs, which showed similar partition coefficient values in both water-DMPG and water-DMPC systems. The difference in partition coefficient values was attributed to the electrostatic interactions between head groups of lipids and ionized drugs, i.e., cationic dibucaine interacted with anionic head groups of DMPG. These results show that liposomes are better systems in which to measure partition coefficients than octanol-water because liposomes mimic the hydrophobic part and the outer polar charged surface of the phospholipids of natural membranes.
Lipid model membranes can also be used to estimate the amount of drug transported into cells and also their mechanism of transport. Baciu et al.46 studied the interactions of cationic amphiphilic drugs (CADs) with lipid model membranes using different biophysical techniques. The results showed that CADs have a mechanism other than active transport and diffusion. In this mechanism, CADs were shown to cause the double-chain PCs to break into mono-chain PCs and fatty acid. At sufficient concentration, mono-chain PCs can form micelles, which then separate from the membrane and carry the drug to other intracellular membranes.
Model membranes have also been used to understand the mechanism of toxicity, particularly at drug concentrations that are known to be toxic in vivo. Amphotericin B (AmB) is a strong antifungal antibiotic known to be very toxic to mammalian cells.47, 48 Based on results from biophysical investigations, its nonselective toxicity was attributed to its close similarity in interaction with mammalian cell membrane cholesterol and fungal cell membrane ergosterol.48 It was proposed that AmB and membrane sterol complexes associate into transmembrane channels. These channels allow uncontrolled migration of ions and small molecules from the cell into the interior of the cell, resulting in cell death. However, N-methyl-N-D-fructopyranosylamphotericin B methyl ester (MF-AME), a derivative of AmB, retained fungicidal properties but showed reduced toxicity to mammals.47–49 Using the Langmuir monolayer approach, Hąc-Wydro et al.50 were able to explain the reasons for the improved selective toxicity of MF-AME. At low concentrations, the affinity of MF-AME to ergosterol is considerably stronger than its affinity to cholesterol, which correlated with improved selective toxicity to the fungus as compared to its parent compound AmB.
Maheswari et al.51, 52 measured the change in electrical properties of model membranes after the addition of methotrexate, an anticancer agent. The results correlated well qualitatively with the neurotoxicity of methotrexate, and it was suggested that the neurological side effects of methotrexate are caused by the drug’s interaction with the lipid bilayer. Kitagawa et al.53 found that the concentrations at which the anesthetics dodecyltrimethylammonium chloride, dibucaine, tetracaine, lidocaine, and procaine caused membrane disruption in DMPG lipid bilayers (as determined by a decrease in light-scattering intensity) corresponded closely with the concentrations reported to cause irreversible nerve injury. Thus, drug-lipid interactions could be used to predict drug toxicity or the concentration at which they could cause toxicity.
Interactions of Polymers and Drug Delivery Systems with Lipid Membrane
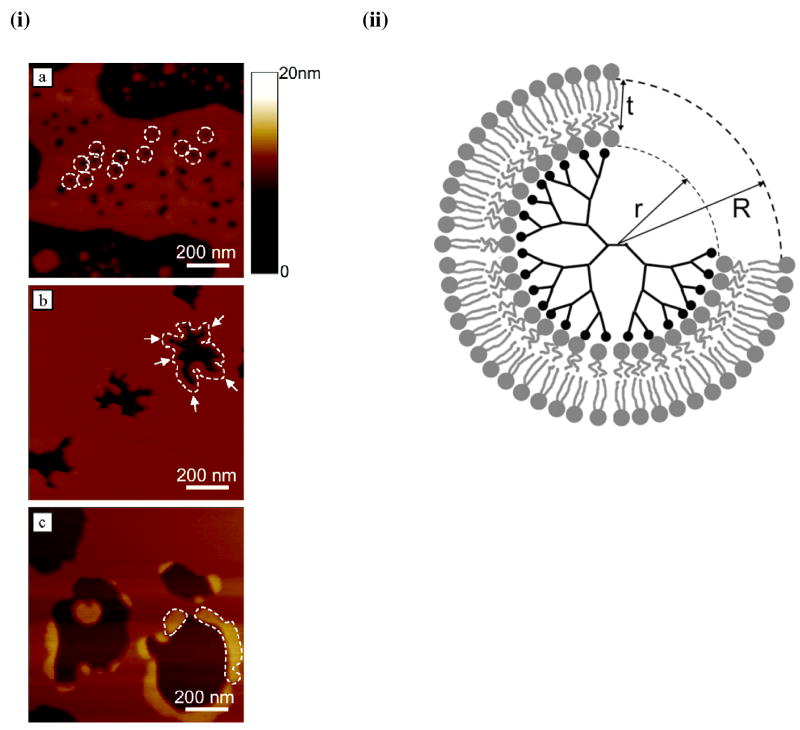
Interfacial properties of polymeric coatings used on drug delivery systems or the drug delivery systems themselves have been shown to influence the interactions with biological environments and consequently the efficiency of drug delivery systems in delivering biotherapeutic agents to cells and tissue. Poly(vinyl alcohol) (PVA),54 poly(ethylene oxide) (PEO),55 tocopheryl polyethylene glycol succinate (TPGS),56 dextran sulfate (DS),57 cell-penetrating peptides (CPPs),58, 59 PEO-b-PPO-b-PEO (Pluronics),60 and chitosan61 are some of the polymers used as coatings in the formulation of drug delivery systems. It has been shown that physical characteristics such as hydrophobicity, hydrophilicity, and surface charge of these polymers, as well as the size of drug delivery systems, can significantly influence their interactions with lipids. These polymers can either increase or decrease the efficiency of drug delivery systems in delivering biotherapeutic agents to cells/tissue. Therefore, a clearer understanding of the interactions of lipid-drug delivery systems with polymers is needed to develop efficient drug carrier systems and to better understand the mechanisms behind drug delivery system uptake or toxic effects. For instance, biophysical investigation of interactions between SLBs and amine-terminated generation 7 and 5 (G7 and G5) poly(amidoamine) (PAMAM) dendrimers revealed that PAMAM dendrimers form holes in lipid bilayers and that the degree of disruption depends on dendrimer’s size and charge.62–64 It was shown that G7 PAMAM dendrimers form holes of 15–40 nm diameter in DMPC SLBs, whereas G5 amine-terminated dendrimers do not initiate hole formation but can expand holes at existing defects (Figure 4-i). The results suggested that the dendrimers can remove lipid molecules from a membrane, leaving behind defects (Figure 4-ii). The above studies with dendrimers thus indicate the role of membrane lipids in their cellular uptake as well as toxicity.62, 63
Figure 4. Effect of physiochemical properties of a drug delivery system on biophysical interactions with model lipid membrane.
(i) Interaction of poly(amidoamine) dendrimers with supported lipid bilayers: AFM study. (a) G7 amine terminated dendrimers cause formation of small holes (diameter 15–40 nm) in intact bilayers, (b) G5 amine terminated dendrimers remove lipid molecules from pre-existing defects in lipid bilayers, (c) G5 acetamide dendrimers do not cause hole formation or removal of lipid molecules, but rather adsorb on the edges of existing bilayer defects. (ii) Schematic cross section of the proposed dendrimer-lipid vesicle. Number of dendrimer end-groups (black circles) = M. Number of lipid headgroups in contact with dendrimer surface (inner ring of gray circles) = m. Figure 4. (i) Reproduced from ref (62), copyright 2004 American Chemical Society. Figure 4(ii). Reproduced from ref (64), copyright 2004 American Chemical Society.
Hydrophilic polymers with either a cationic or anionic charge (chitosan, DS, CPPs) have been shown to interact with lipids by electrostatic interactions. Pavinatto et al.65, 66 investigated the interaction between cationic chitosan and negatively charged dipalmitoyl phosphatidylglycerol and zwitterionic DPPC lipids, using a Langmuir balance and pendant drop technique. The pendant drop technique was used to measure the effects of chitosan on rheological properties of phospholipids. The results revealed that chitosan interacts with phospholipids mainly through electrostatic interactions and disrupt cell membranes. Another polymer, negatively charged DS was also shown to interact with phospholipid model membranes via electrostatic interactions.57
The interaction of cell CPPs with lipids has been extensively investigated using different biophysical techniques.58, 67–69 These biophysical investigations are focused on understanding the role of lipid-CPP interactions in translocation of CPP-conjugated drug delivery systems. It was shown that the peptide sequence, amino acid composition, and its conformational state strongly influence the lipid-CPP interactions, therefore the delivery of cargo.70–73 For instance, Deshayes et al.70 compared the interactions of two peptides, Pα and Pβ, with lipid model membranes using Langmuir monolayer studies. The peptides differ in their conformation: Pβ is nonstructured in water, but Pα is helical. This study provided some insight into the role of CPP structure in the formation of carrier/cargo complexes and their interactions with model membranes. The authors showed that the peptide Pβ undergoes a lipid-induced conformational transition into a sheetlike structure, whereas Pα remains helical. The penetration experiments showed that both peptides can spontaneously insert themselves into phospholipid membranes.
Analysis of compression isotherms indicated that both peptides interact with phospholipids in the liquid-expanded and liquid-condensed states. AFM observations of LB films revealed that the peptides strongly disrupt the lipid organization of the monolayers (Figure 5-i and ii). Conformational investigations indicated that the lipid-peptide interaction governs the conformational state of the peptides. Based on the ability of both peptides to promote ion permeabilization of natural as well as artificial membranes, authors have proposed a model illustrating the translocation process of the peptides through the cell membrane. Peptide Pβ is based on the formation of a barrel pore-like structure in the membrane; in contrast, Pα is based on the association of helices with the membrane. The mechanisms involved in cellular internalization mediated by CPPs thus can enable one to determine the criteria needed to design efficient peptide drug carrier systems.
Figure 5. Effect of peptide conformation on biophysical interactions of peptide with model lipid membrane.
AFM images of LB monolayers composed of (i) Pα, (ii) Pβ with DOPC (A), DOPG (B), DPPC (C), and dipalmitoyl phosphatidylglycerol (D) at a molar ratio of x[5] = 0.15. The scan size is 1 mm × 1 mm, and the z scale for A, B, and D is 3 nm, but for C is 5 nm. Reproduced with permission from ref (69), copyright 2004 American Chemical Society.
We have recently studied biophysical interactions of ritonavir-loaded poly(L-lactide) nanoparticles (RNPs) and those conjugated either to a trans-activating transcriptor-peptide (TAT-RNPs) or to a scrambled TAT peptide (sc-TAT-RNPs).74 A Langmuir model of the endothelial model membrane was formed on the buffer surface using a mixture lipids present typically present in endothelium using a Langmuir film balance. Among the different formulations, TAT-RNPs showed interactions with the model membrane, which was dependent on the amount of peptide bound to the nanoparticles (NPs) and the concentration of NPs, whereas sc-TAT-RNPs and RNPs did not show any interaction. Human vascular endothelial cells showed a higher uptake of the drug with TAT-RNPs than with sc-TAT-RNPs or RNPs. Thus, the biophysical interactions of NPs with model membrane correlated with the cellular uptake of the drug. TAT peptide alone did not interact with the lipid membrane, whereas TAT-RNPs showed evidence of interaction with the lipid membrane, suggesting that the interaction occurs when the peptide is conjugated to a carrier system. The overall data thus suggest that the biophysical interactions of NPs with cell-membrane lipids play a role in cellular internalization of NPs.
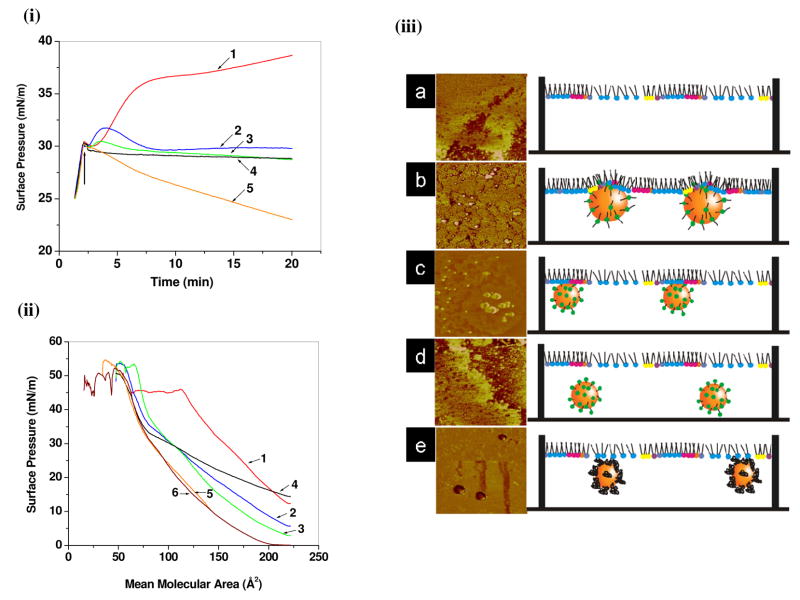
In another study, we investigated the biophysical interactions between polystyrene NPs of different physical and surface properties with a model membrane using the Langmuir technique and AFM.75, 76 In that study, we demonstrated that the physicochemical properties of polystyrene NPs, particularly their size and surface properties, influence their interactions with model lipid membrane.75 We found that small NPs (<60 nm in diameter) show greater interactions with the model membrane than large NPs. Furthermore, aminated and plain NPs have greater interactions than carboxylated NPs of the same size. In our recent study, we have shown that the surfactant molecular structure of the NP surface influences NP–lipid membrane interactions, which correlate with the uptake of NPs by human vascular endothelial cells.76 Among the different surfactants used, we found that the cationic di-chain didodecyldimethylammonium bromide (DMAB) and single-chain cetyltrimethylammonium bromide (CTAB) facilitated interaction with lipid model membrane, whereas cationic single-chain dodecyltrimethylammonium bromide (DTAB) and nonionic polymeric surfactant PVA did not facilitate interaction. Although the cellular uptake of the DMAB- and CTAB-modified NPs was similar, our biophysical investigations showed that these two surfactants interact by different means: DMAB-modified NPs showed penetration into the model membrane, whereas CTAB-modified NPs showed only electrostatic interactions (Figure 6). In the above study, all the cationic surfactant-modified NPs demonstrated a similar cationic zeta potential, but their interaction pattern with the model membrane was significantly different. Thus, it is not only the surface charge on the NPs that governs the interactions with the model membrane but also the structure of the molecule at the interface that interacts with lipids of the membrane. Mu and Seow77 have shown that the penetration of TPGS-coated poly(D,L-lactide-co-glycolide) NPs into the DPPC lipid membrane is greater than that of PVA-coated poly(D,L-lactide-co-glycolide) NPs.77 The greater penetration was attributed to greater hydrophobicity of the TPGS-coated NPs. Data from this interaction with model membrane correlated very well with results showing increased uptake of the TPGS-coated NPs compared to the PVA-coated NPs in human colon adenocarcinoma cells (Coca-2 and HT-29 cell lines).78 Thus, biophysical interactions with a model membrane could be effectively used in developing efficient functionalized nanocarrier systems for drug delivery applications.
Figure 6. Effect of molecular structure of cationic surfactants on biophysical interactions of model lipid membrane with surfactant-modified drug delivery systems.
(i) Changes in SP of endothelial cell model membrane (EMM) with time due to interaction with different surfactant-modified NPs. (ii) Changes in the SP-area (π-A) isotherm of the EMM lipid mixture in the presence of different surfactant-modified NPs. (iii) Schematic representation of interaction of (a) EMM-water (control), (b) DMAB-, (c) CTAB- (d) DTAB-, and (e) PVA-modified NPs with EMM. DMAB-modified NPs penetrate into the EMM, whereas CTAB-modified NPs interacts with phospholipids liquid condensed domains only through electrostatic interactions. DTAB- and PVA-modified NPs do not interact. Corresponding AFM phase images of LS films clearly shows that DMAB-modified NPs are embedded in between phospholipids, whereas CTAB-modified NPs are attached to the condensed phospholipid domains. Key: 1, DMAB-NPs; 2, CTAB-NPs; 3, DTAB-NPs; 4, PVA-NPs; 5 unmodified-NPs; 6, EMM alone. Reproduced from ref (75), copyright 2009 American Chemical Society
Block copolymers, Pluronics are known to interact with cell membranes. In cell culture studies, certain Pluronics have been shown to cause pronounced chemosensitization of tumor cells that exhibit drug resistance to anticancer drugs.79–81 This effect was attributed to inhibition of the P-glycoprotein responsible for drug efflux by interacting with the membrane lipids surrounding this protein.82 Demina et al.83 investigated the relationship between the structure of 19 different amphiphilic copolymers (polyglycerols, Pluronics, Brij surfactants) and their ability to accelerate the “flip-flop” of lipid molecules lipid molecule i.e., when lipids move from one interface of the bilayer to the other, and permeation of the antitumor drug doxorubicin in liposome model membranes. This biophysical study shed some light on developing novel amphiphilic copolymers capable of affecting the activity of membrane transporters in living cells. It was observed that copolymers containing polypropylene oxide cause higher acceleration of flip-flop and doxorubicin permeation than polysurfactants containing aliphatic chains. Both Pluronic bulk hydrophobicity and the chemical microstructure of the copolymer determine its membrane-disturbing ability.
Lipid Membrane from Normal vs. Diseased Tissue
Lipid alterations have been reported in cancer,84, 85 diabetes,86 and cardiovascular disorders such as hypertension, atherosclerosis, coronary heart disease, sudden cardiac death, aneurism, and thrombosis.87–89 It is believed that cancer cells acquire resistance to chemotherapy, in part due to changes in their membrane lipid composition.86 However, it is still not known whether the difference in lipid composition is the cause or the result of cancer. In gastric carcinoma, it has been suggested that elevated levels of PE might be of use as an additional marker of cancer.90, 91 Several studies have shown that membrane lipid composition and structure regulate the function and localization of membrane proteins.92 Nevertheless, current treatments for the above-mentioned diseases are not usually focused on membranes. Biophysical investigations have shown that lipid composition significantly influences the interaction of drugs or drug delivery systems, and thus their cellular uptake. Preetha et al.34, 35, 93 showed that paclitaxel penetrated much more readily in DPPC monolayers than in monolayers composed of lipid extract from cancerous cervical tissue. Subsequent experiments have provided evidence that this discrepancy is due to the behavior of cholesterol and SM present in the DPPC monolayers and lipid extract from cancerous cervical tissue.
Melik-Nubarov et al.94 reported that the same doses of Pluronic-P85 or -L61 exhibit contrasting effects in different cells. The study has shown increase in membrane microviscosity (“fluidized”) in the presence of Pluronic in cancerous cells but decrease in microviscosity (“solidified”) of the normal blood cell membranes. Using liposomes as a model membrane, Zhirnov et al.95 demonstrated that Pluronic-L61 interaction is dependent on the composition of lipids. In the presence of cholesterol, ganglioside or PE, membrane responsiveness to Pluronic was reduced, whereas it increased in the presence of phosphatidic acid.
Significance of Biophysical Interactions
The use of model membrane systems in biophysical investigations could help us to understand the critical role of membrane lipids in cellular uptake, predict toxicity of drugs, and optimize drug delivery systems. Model membranes offer advantages over the use of live cells in that they allow experimentation to be performed under conditions that cells may not be able to withstand and remain viable. Furthermore, the study allows analysis of data from which the mechanisms of transport can be more easily deduced. In addition, model membranes can predict and explain interaction of drugs or drug delivery system with biological membranes, which could be critical in developing new drug molecules or efficient drug delivery systems. Three different types of interactions are possible between drugs/drug delivery systems and lipids: interactions with the headgroups of lipids, with hydrophobic alkyl chains, and with both headgroups and hydrophobic acyl chains. In cell culture experiments, due to experimental limitations and the complexity of cell membranes, these interactions cannot be documented, whereas by using a model membrane system; these interactions can be explored to identify the probable basis for such interactions. Because of the lack of typical cell membrane characteristics in model membranes (such as the absence of anchoring membrane proteins, receptors, and the active process of endocytosis), the biophysical interactions of drugs/drug delivery systems with model membrane may not exactly replicate all the aspects of the cellular uptake process. However, all the studies discussed above have shown a good correlation between biophysical interactions and cellular uptake and/or therapeutic efficacy, suggesting the critical role of lipids in cellular interactions of drugs and drug carrier systems.
Lipid composition differences and their effect on the biophysical interactions between drugs and drug delivery systems show the importance of such studies. In addition, this illustrates the importance of choosing model membranes that closely resemble the tissue or cells under consideration to ensure that the behavior seen with lipid model membrane reflects the behavior cells in vitro and/or in vivo. Thus far, biophysical investigations of drug and drug delivery systems with lipid model membranes are retrospective, that is to say, the study is performed after the behavior in vitro or in vivo is known. However, if research in this area continues and good correlations are found between the behavior of drug and drug delivery systems in model membranes vs. biological membranes, then lipid model membranes have the potential to become a powerful, rapid, and relatively inexpensive predictive technique for drug discovery and for developing effective drug delivery systems.
Conclusions
Biophysical interaction studies with model membrane could provide a simple yet effective approach to understanding the role of membrane lipids in the transport of drugs and drug delivery systems across biological barriers. With better understanding of the mechanisms of interactions, biophysical interaction studies with model membranes could provide a rational approach for drug discovery and development as well as for developing efficient drug delivery systems.
Acknowledgments
The study reported here was funded by grants 1R01 EB 003975 from the National Institute of Biomedical Imaging and Bioengineering and R21 MH06 7525 from the National Institute of Mental Health of the National Institutes of Health (both to VL).
Abbreviations
- AFM
Atomic force microscopy
- AmB
Amphotericin B
- CADs
Cationic amphiphilic drugs
- CPPs
Cell- penetrating peptides
- CTAB
Cetyltrimethylammonium bromide
- DMAB
Didodecyldimethylammonium bromide
- DMPC
Dimyristoyl-phosphatidylcholine
- DMPG
Dimyristoyl-L-α-phosphatidylglycerol
- DOPC
Dioleoylphosphatidylcholine
- DPPC
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine
- DTAB
Dodecyltrimethylammonium bromide
- FTIR
Fourier transform infrared resonance
- GUV
Giant unilamellar liposome
- LB
Langmuir-Blodgett (transfer technique)
- LS
Langmuir-Schaeffer (monolayer)
- LULV
Large unilamellar liposome
- MF-AME
N-methyl-N-D-fructopyranosylamphotericin B methyl ester
- NPs
Nanoparticles
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PEO
Poly(ethylene oxide)
- PVA
Poly(vinyl alcohol)
- RNPs
Ritonavir-loaded poly(L-lactide) nanoparticles
- Sc-TAT-RNP
Scrambled trans-activating transcriptor-peptide
- SLB
Supported lipid bilayers
- M
Sphingomyelin
- SP
Surface pressure
- SUV
Small unilamellar liposome
- TAT-RNP
Trans-activating transcriptor-peptide
- TPGS
Tocopheryl polyethylene glycol succinate
References
- 1.Gorter E, Grendel F. On bimolecular layers of lipoids on the chromocytes of the blood. J Exp Med. 1925;41:439–443. doi: 10.1084/jem.41.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 3.Escriba PV. Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol Med. 2006;12:34–43. doi: 10.1016/j.molmed.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Vereb G, Szollosi J, Matko J, Nagy P, Farkas T, Vigh L, Matyus L, Waldmann TA, Damjanovich S. Dynamic, yet structured: The cell membrane three decades after the Singer-Nicolson model. Proc Natl Acad Sci U S A. 2003;100:8053–8058. doi: 10.1073/pnas.1332550100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escriba PV, Gonzalez-Ros JM, Goni FM, Kinnunen PK, Vigh L, Sanchez-Magraner L, Fernandez AM, Busquets X, Horvath I, Barcelo-Coblijn G. Membranes: a meeting point for lipids, proteins and therapies. J Cell Mol Med. 2008;12:829–875. doi: 10.1111/j.1582-4934.2008.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escriba PV, Ozaita A, Ribas C, Miralles A, Fodor E, Farkas T, Garcia-Sevilla JA. Role of lipid polymorphism in G protein-membrane interactions: nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc Natl Acad Sci U S A. 1997;94:11375–11380. doi: 10.1073/pnas.94.21.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamm LK, McConnell HM. Supported phospholipid bilayers. Biophys J. 1985;47:105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maget-Dana R. The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim Biophys Acta. 1999;1462:109–140. doi: 10.1016/s0005-2736(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 10.Eytan GD. Use of liposomes for reconstitution of biological functions. Biochim Biophys Acta. 1982;694:185–202. doi: 10.1016/0304-4157(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 11.Lieser G, Mittler-Neher S, Spinke J, Knoll W. Electron microscopic investigations on free-standing mixed lipid Langmuir-Blodgett-Kuhn monolayers: phase separation and aging process. Biochim Biophys Acta. 1994;1192:14–20. doi: 10.1016/0005-2736(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 12.Clausell A, Busquets MA, Pujol M, Alsina A, Cajal Y. Polymyxin B-lipid interactions in Langmuir-Blodgett monolayers of Escherichia coli lipids: a thermodynamic and atomic force microscopy study. Biopolymers. 2004;75:480–490. doi: 10.1002/bip.20165. [DOI] [PubMed] [Google Scholar]
- 13.Ihalainen P, Peltonen J. Covalent immobilization of antibody fragments onto Langmuir-Schaefer binary monolayers chemisorbed on gold. Langmuir. 2002;18:4953–4962. [Google Scholar]
- 14.Hughes AV, Howse JR, Dabkowska A, Jones RA, Lawrence MJ, Roser SJ. Floating lipid bilayers deposited on chemically grafted phosphatidylcholine surfaces. Langmuir. 2008;24:1989–1999. doi: 10.1021/la702050b. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Conboy JC. Structure of a gel phase lipid bilayer prepared by the Langmuir-Blodgett/Langmuir-Schaefer method characterized by sum-frequency vibrational spectroscopy. Langmuir. 2005;21:9091–9097. doi: 10.1021/la051500e. [DOI] [PubMed] [Google Scholar]
- 16.Giocondi MC, Le Grimellec C. Temperature dependence of the surface topography in dimyristoylphosphatidylcholine/distearoylphosphatidylcholine multibilayers. Biophys J. 2004;86:2218–2230. doi: 10.1016/S0006-3495(04)74280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw JE, Slade A, Yip CM. Simultaneous in situ total internal reflectance fluorescence/atomic force microscopy studies of DPPC/dPOPC microdomains in supported planar lipid bilayers. J Am Chem Soc. 2003;125:11838–11839. doi: 10.1021/ja0370894. [DOI] [PubMed] [Google Scholar]
- 18.Brezesinski G, Mohwald H. Langmuir monolayers to study interactions at model membrane surfaces. Adv Colloid Interface Sci. 2003:100–102. 563–584. doi: 10.1016/s0001-8686(02)00071-4. [DOI] [PubMed] [Google Scholar]
- 19.Marsh D. Intrinsic curvature in normal and inverted lipid structures and in membranes. Biophys J. 1996;70:2248–2255. doi: 10.1016/S0006-3495(96)79790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amado E, Kerth A, Blume A, Kressler J. Infrared reflection absorption spectroscopy coupled with Brewster angle microscopy for studying interactions of amphiphilic triblock copolymers with phospholipid monolayers. Langmuir. 2008;24:10041–10053. doi: 10.1021/la801768m. [DOI] [PubMed] [Google Scholar]
- 21.Corvis Y, Barzyk W, Brezesinski G, Mrabet N, Badis M, Hecht S, Rogalska E. Interactions of a fungistatic antibiotic, griseofulvin, with phospholipid monolayers used as models of biological membranes. Langmuir. 2006;22:7701–7711. doi: 10.1021/la060998x. [DOI] [PubMed] [Google Scholar]
- 22.Sessa G, Weissmann G. Phospholipid spherules (liposomes) as a model for biological membranes. J Lipid Res. 1968;9:310–318. [PubMed] [Google Scholar]
- 23.Fa N, Lins L, Courtoy PJ, Dufrene Y, Van Der Smissen P, Brasseur R, Tyteca D, Mingeot-Leclercq MP. Decrease of elastic moduli of DOPC bilayers induced by a macrolide antibiotic, azithromycin. Biochim Biophys Acta: Biomembranes. 2007;1768:1830–1838. doi: 10.1016/j.bbamem.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Okumura Y, Zhang H, Sugiyama T, Iwata Y. Electroformation of giant vesicles on a non-electroconductive substrate. J Am Chem Soc. 2007;129:1490–1491. doi: 10.1021/ja068127x. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez N, Heuvingh J, Pincet F, Cribier S. Indirect evidence of submicroscopic pores in giant unilamellar [correction of unilamelar] vesicles. Biochim Biophys Acta. 2005;1724:281–287. doi: 10.1016/j.bbagen.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Grancelli A, Morros A, Cabanas ME, Domenech O, Merino S, Vazquez JL, Montero MT, Vinas M, Hernandez-Borrell J. Interaction of 6-fluoroquinolones with dipalmitoylphosphatidylcholine monolayers and liposomes. Langmuir. 2002;18:9177–9182. [Google Scholar]
- 27.Berquand A, Fa N, Dufrene YF, Mingeot-Leclercq MP. Interaction of the macrolide antibiotic azithromycin with lipid bilayers: Effect on membrane organization, fluidity, and permeability. Pharm Res. 2005;22:465–475. doi: 10.1007/s11095-004-1885-8. [DOI] [PubMed] [Google Scholar]
- 28.Fa N, Schanck A, Mingeot-Leclercq MP. Interaction of the macrolide antibiotic azithromycin with model of membranes. Biophys J. 2005;88:241a–241a. doi: 10.1007/s11095-004-1885-8. [DOI] [PubMed] [Google Scholar]
- 29.Barcelo F, Prades J, Funari SS, Frau J, Alemany R, Escriba PV. The hypotensive drug 2-hydroxyoleic acid modifies the structural properties of model membranes. Mol Membr Biol. 2004;21:261–268. doi: 10.1080/09687680410001716835. [DOI] [PubMed] [Google Scholar]
- 30.Cheng HY, Randall CS, Holl WW, Constantinides PP, Yue TL, Feuerstein GZ. Carvedilol-liposome interaction: Evidence for strong association with the hydrophobic region of the lipid bilayers. Biochim Biophys Acta: Biomembranes. 1996;1284:20–28. doi: 10.1016/0005-2736(96)00097-1. [DOI] [PubMed] [Google Scholar]
- 31.Agasosler AV, Tungodden LM, Cejka D, Bakstad E, Sydnes LK, Holmsen H. Chorpromazine-induced increase in dipalmitoylphosphatidylserine surface area in monolayers at room temperature. Biochem Pharmacol. 2001;61:817–825. doi: 10.1016/s0006-2952(01)00542-1. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo AA, Caetano W, Tabak M, Oliveira ON. Interaction of two phenothiazine derivatives with phospholipid monolayers. Biophys Chem. 2004;109:85–104. doi: 10.1016/j.bpc.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Feng SS, Gong K, Chew J. Molecular interactions between a lipid and an antineoplastic drug paclitaxel (taxol) within the lipid monolayer at the air/water interface. Langmuir. 2002;18:4061–4070. [Google Scholar]
- 34.Preetha A, Huilgol N, Banerjee R. Comparison of paclitaxel penetration in normal and cancerous cervical model monolayer membranes. Colloids Surf B Biointerfaces. 2006;53:179–186. doi: 10.1016/j.colsurfb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Preetha A, Huilgol N, Banerjee R. Effect of fluidizing agents on paclitaxel penetration in cervical cancerous monolayer membranes. J Membr Biol. 2007;219:83–91. doi: 10.1007/s00232-007-9064-6. [DOI] [PubMed] [Google Scholar]
- 36.Michot JM, Seral C, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob Agents Chemother. 2005;49:2429–2437. doi: 10.1128/AAC.49.6.2429-2437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michot JM, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Active efflux of ciprofloxacin from J774 macrophages through an MRP-like transporter. Antimicrob Agents Chemother. 2004;48:2673–2682. doi: 10.1128/AAC.48.7.2673-2682.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bensikaddour H, Fa N, Burton I, Deleu M, Lins L, Schanck A, Brasseur R, Dufrene YF, Goormaghtigh E, Mingeot-Leclercq MP. Characterization of the interactions between fluoroquinolone antibiotics and lipids: A multitechnique approach. Biophys J. 2008;94:3035–3046. doi: 10.1529/biophysj.107.114843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montero MT, Hernandez-Borrell J, Keough KMW. Fluoroquinolone-biomembrane interactions: Monolayer and calorimetric studies. Langmuir. 1998;14:2451–2454. [Google Scholar]
- 40.Bensikaddour H, Snoussi K, Lins L, Van Bambeke F, Tulkens PM, Brasseur R, Goormaghtigh E, Mingeot-Leclercq MP. Interactions of ciprofloxacin with DPPC and DPPG: Fluorescence anisotropy, ATR-FTIR and P-31 NMR spectroscopies and conformational analysis. Biochim Biophys Acta: Biomembranes. 2008;1778:2535–2543. doi: 10.1016/j.bbamem.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Berquand A, Mingeot-Leclercq MP, Dufrene YF. Real-time imaging of drug-membrane interactions by atomic force microscopy. Biochim Biophys Acta: Biomembranes. 2004;1664:198–205. doi: 10.1016/j.bbamem.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Fa N, Ronkart S, Schanck A, Deleu M, Gaigneaux A, Goonnaghtigh E, Mingeot-Leclercq MP. Effect of the antibiotic azithromycin on thermotropic behavior of DOPC or DPPC bilayers. Chem Phys Lipids. 2006;144:108–116. doi: 10.1016/j.chemphyslip.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Sogaard R, Werge TM, Bertelsen C, Lundbye C, Madsen KL, Nielsen CH, Lundbaek JA. GABA(A) receptor function is regulated by lipid bilayer elasticity. Biochemistry (Mosc) 2006;45:13118–13129. doi: 10.1021/bi060734+. [DOI] [PubMed] [Google Scholar]
- 44.Klopman G, Zhu H. Recent methodologies for the estimation of n-octanol/water partition coefficients and their use in the prediction of membrane transport properties of drugs. Mini Rev Med Chem. 2005;5:127–133. doi: 10.2174/1389557053402765. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues C, Gameiro P, Reis S, Lima JL, de Castro B. Derivative spectrophotometry as a tool for the determination of drug partition coefficients in water/dimyristoyl-L-alpha-phosphatidylglycerol (DMPG) liposomes. Biophys Chem. 2001;94:97–106. doi: 10.1016/s0301-4622(01)00227-7. [DOI] [PubMed] [Google Scholar]
- 46.Baciu M, Sebai SC, Ces O, Mulet X, Clarke JA, Shearman GC, Law RV, Templer RH, Plisson C, Parker CA, Gee A. Degradative transport of cationic amphiphilic drugs across phospholipid bilayers. Phil Trans R Soc A. 2006;364:2597–2614. doi: 10.1098/rsta.2006.1842. [DOI] [PubMed] [Google Scholar]
- 47.Gruszecki WI, Gagos M, Herec M, Kernen P. Organization of antibiotic amphotericin B in model lipid membranes. A mini review. Cell Mol Biol Lett. 2003;8:161–170. [PubMed] [Google Scholar]
- 48.Baginski M, Czub J, Sternal K. Interaction of amphotericin B and its selected derivatives with membranes: molecular modeling studies. Chem Rec. 2006;6:320–332. doi: 10.1002/tcr.20096. [DOI] [PubMed] [Google Scholar]
- 49.Matsumori N, Yamaji N, Matsuoka S, Oishi T, Murata M. Amphotericin B covalent dimers forming sterol-dependent ion-permeable membrane channels. J Am Chem Soc. 2002;124:4180–4181. doi: 10.1021/ja012026b. [DOI] [PubMed] [Google Scholar]
- 50.Hac-Wydro K, Dynarowicz-Latka P, Grzybowska J, Borowski E. Interactions of Amphotericin B derivative of low toxicity with biological membrane components - the Langmuir monolayer approach. Biophys Chem. 2005;116:77–88. doi: 10.1016/j.bpc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Maheswari KU. Lipid bilayer–methotrexate interactions: A basis for methotrexate neurotoxicity. Curr Sci. 2001;81:571–573. [Google Scholar]
- 52.Maheswari KU, Ramachandran T, Rajaji D. Interaction of cisplatin with planar model membranes - dose dependent change in electrical characteristics. Biochim Biophys Acta. 2000;1463:230–240. doi: 10.1016/s0005-2736(99)00189-3. [DOI] [PubMed] [Google Scholar]
- 53.Kitagawa N, Kaminoh Y, Takasaki M, Ueda I. Use of ion-exchange membranes to measure transfer free energies of charged local anesthetics: correlation to anesthetic potency. J Pharm Sci. 1990;79:344–348. doi: 10.1002/jps.2600790415. [DOI] [PubMed] [Google Scholar]
- 54.Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82:105–114. doi: 10.1016/s0168-3659(02)00127-x. [DOI] [PubMed] [Google Scholar]
- 55.Aqil A, Vasseur S, Duguet E, Passirani C, Benoit JP, Roch A, Muller R, Jerome R, Jerome C. PEO coated magnetic nanoparticles for biomedical application. Eur Poly J. 2008;44:3191–3199. [Google Scholar]
- 56.Mu L, Seow PH, Ang SN, Feng SS. Study on surfactant coating of polymeric nanoparticles for controlled delivery of anticancer drug. Colloid Polym Sci. 2004;283:58–65. [Google Scholar]
- 57.Huster D, Paasche G, Dietrich U, Zschornig O, Gutberlet T, Gawrisch K, Arnold K. Investigation of phospholipid area compression induced by calcium-mediated dextran sulfate interaction. Biophys J. 1999;77:879–887. doi: 10.1016/S0006-3495(99)76939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dennison SR, Baker RD, Nicholl ID, Phoenix DA. Interactions of cell penetrating peptide Tat with model membranes: a biophysical study. Biochem Biophys Res Commun. 2007;363:178–182. doi: 10.1016/j.bbrc.2007.08.162. [DOI] [PubMed] [Google Scholar]
- 59.Mano M, Henriques A, Paiva A, Prieto M, Gavilanes F, Simoes S, de Lima MC. Interaction of S413-PV cell penetrating peptide with model membranes: relevance to peptide translocation across biological membranes. J Pept Sci. 2007;13:301–313. doi: 10.1002/psc.842. [DOI] [PubMed] [Google Scholar]
- 60.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 61.Taetz S, Nafee N, Beisner J, Piotrowska K, Baldes C, Murdter TE, Huwer H, Schneider M, Schaefer UF, Klotz U, Lehr CM. The influence of chitosan content in cationic chitosan/PLGA nanoparticles on the delivery efficiency of antisense 2′-O-methyl-RNA directed against telomerase in lung cancer cells. Eur J Pharm Biopharm. 2008 doi: 10.1016/j.ejpb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Hong S, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi X, Balogh L, Orr BG, Baker JR, Jr, Banaszak Holl MM. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transport. Bioconjug Chem. 2004;15:774–782. doi: 10.1021/bc049962b. [DOI] [PubMed] [Google Scholar]
- 63.Leroueil PR, Hong S, Mecke A, Baker JR, Jr, Orr BG, Banaszak Holl MM. Nanoparticle interaction with biological membranes: does nanotechnology present a Janus face? Acc Chem Res. 2007;40:335–342. doi: 10.1021/ar600012y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mecke A, Majoros IJ, Patri AK, Baker JR, Jr, Holl MM, Orr BG. Lipid bilayer disruption by polycationic polymers: the roles of size and chemical functional group. Langmuir. 2005;21:10348–10354. doi: 10.1021/la050629l. [DOI] [PubMed] [Google Scholar]
- 65.Pavinatto FJ, Pavinatto A, Caseli L, Santos DS, Jr, Nobre TM, Zaniquelli ME, Oliveira ON., Jr Interaction of chitosan with cell membrane models at the air-water interface. Biomacromolecules. 2007;8:1633–1640. doi: 10.1021/bm0701550. [DOI] [PubMed] [Google Scholar]
- 66.Pavinatto FJ, Caseli L, Pavinatto A, dos Santos DS, Jr, Nobre TM, Zaniquelli ME, Silva HS, Miranda PB, de Oliveira ON., Jr Probing chitosan and phospholipid interactions using Langmuir and Langmuir-Blodgett films as cell membrane models. Langmuir. 2007;23:7666–7671. doi: 10.1021/la700856a. [DOI] [PubMed] [Google Scholar]
- 67.Deshayes S, Heitz A, Morris MC, Charnet P, Divita G, Heitz F. Insight into the mechanism of internalization of the cell-penetrating carrier peptide Pep-1 through conformational analysis. Biochemistry (Mosc) 2004;43:1449–1457. doi: 10.1021/bi035682s. [DOI] [PubMed] [Google Scholar]
- 68.Deshayes S, Morris MC, Divita G, Heitz F. Interactions of amphipathic carrier peptides with membrane components in relation with their ability to deliver therapeutics. J Pept Sci. 2006;12:758–765. doi: 10.1002/psc.810. [DOI] [PubMed] [Google Scholar]
- 69.Deshayes S, Plenat T, Aldrian-Herrada G, Divita G, Le Grimellec C, Heitz F. Primary amphipathic cell-penetrating peptides: structural requirements and interactions with model membranes. Biochemistry (Mosc) 2004;43:7698–7706. doi: 10.1021/bi049298m. [DOI] [PubMed] [Google Scholar]
- 70.Deshayes S, Morris MC, Divita G, Heitz F. Interactions of primary amphipathic cell penetrating peptides with model membranes: consequences on the mechanisms of intracellular delivery of therapeutics. Curr Pharm Des. 2005;11:3629–3638. doi: 10.2174/138161205774580741. [DOI] [PubMed] [Google Scholar]
- 71.Deshayes S, Morris MC, Divita G, Heitz F. Interactions of amphipathic CPPs with model membranes. Biochim Biophys Acta. 2006;1758:328–335. doi: 10.1016/j.bbamem.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Magzoub M, Kilk K, Eriksson LE, Langel U, Graslund A. Interaction and structure induction of cell-penetrating peptides in the presence of phospholipid vesicles. Biochim Biophys Acta. 2001;1512:77–89. doi: 10.1016/s0005-2736(01)00304-2. [DOI] [PubMed] [Google Scholar]
- 73.Yandek LE, Pokorny A, Floren A, Knoelke K, Langel U, Almeida PF. Mechanism of the cell-penetrating peptide transportan 10 permeation of lipid bilayers. Biophys J. 2007;92:2434–2444. doi: 10.1529/biophysj.106.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peetla C, Rao KS, Labhasetwar V. Relevance of biophysical interactions of nanoparticles with a model membrane in predicting cellular uptake: study with TAT peptide-conjugated nanoparticles. Mol Pharmaceutics. 2009 Mar 27; doi: 10.1021/mp900011h. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peetla C, Labhasetwar V. Biophysical characterization of nanoparticle-endothelial model cell membrane interactions. Mol Pharmaceutics. 2008;5:418–429. doi: 10.1021/mp700140a. [DOI] [PubMed] [Google Scholar]
- 76.Peetla C, Labhasetwar V. Effect of molecular structure of cationic surfactants on biophysical interactions of surfactant-modified nanoparticles with a model membrane and cellular uptake. Langmuir. 2009;25:2369–2377. doi: 10.1021/la803361y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mu L, Seow PH. Application of TPGS in polymeric nanoparticulate drug delivery system. Colloids Surf B Biointerfaces. 2006;47:90–97. doi: 10.1016/j.colsurfb.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Z, Feng SS. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27:4025–4033. doi: 10.1016/j.biomaterials.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers for overcoming drug resistance in cancer. Adv Drug Deliv Rev. 2002;54:759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 80.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 82.Regev R, Assaraf YG, Eytan GD. Membrane fluidization by ether, other anesthetics, and certain agents abolishes P-glycoprotein ATPase activity and modulates efflux from multidrug-resistant cells. Eur J Biochem. 1999;259:18–24. doi: 10.1046/j.1432-1327.1999.00037.x. [DOI] [PubMed] [Google Scholar]
- 83.Demina T, Grozdova I, Krylova O, Zhirnov A, Istratov V, Frey H, Kautz H, Melik-Nubarov N. Relationship between the structure of amphiphilic copolymers and their ability to disturb lipid bilayers. Biochemistry (Mosc) 2005;44:4042–4054. doi: 10.1021/bi048373q. [DOI] [PubMed] [Google Scholar]
- 84.Patel PS, Shah MH, Jha FP, Raval GN, Rawal RM, Patel MM, Patel JB, Patel DD. Alterations in plasma lipid profile patterns in head and neck cancer and oral precancerous conditions. Indian J Cancer. 2004;41:25–31. [PubMed] [Google Scholar]
- 85.Franky DS, Shilin NS, Pankaj MS, Patel HR, Prabhudas SP. Significance of alterations in plasma lipid profile levels in breast cancer. Integr Cancer Ther. 2008;7:33–41. doi: 10.1177/1534735407313883. [DOI] [PubMed] [Google Scholar]
- 86.Vigh L, Escriba PV, Sonnleitner A, Sonnleitner M, Piotto S, Maresca B, Horvath I, Harwood JL. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res. 2005;44:303–344. doi: 10.1016/j.plipres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 87.Escriba PV, Sanchez-Dominguez JM, Alemany R, Perona JS, Ruiz-Gutierrez V. Alteration of lipids, G proteins, and PKC in cell membranes of elderly hypertensives. Hypertension. 2003;41:176–182. doi: 10.1161/01.hyp.0000047647.72162.a8. [DOI] [PubMed] [Google Scholar]
- 88.Ma QF, Yang FY. Lipids may not be involved in the recognition of apocytochrome C during its transportation. Biochem Mol Biol Int. 1997;41:487–496. doi: 10.1080/15216549700201511. [DOI] [PubMed] [Google Scholar]
- 89.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett. 1997;408:191–194. doi: 10.1016/s0014-5793(97)00420-1. [DOI] [PubMed] [Google Scholar]
- 90.Oremek GM, Sapoutzis N, Lorenz M. Phospholipids, tumour marker and beta-CrossLaps in diagnosis of gastric carcinoma. Anticancer Res. 2003;23:859–863. [PubMed] [Google Scholar]
- 91.Oremek GM, Sapoutzis N. Pro-gastrin-releasing peptide (Pro-GRP), a tumor marker for small cell lung cancer. Anticancer Res. 2003;23:895–898. [PubMed] [Google Scholar]
- 92.Faloia E, Garrapa GG, Martarelli D, Camilloni MA, Lucarelli G, Staffolani R, Mantero F, Curatola G, Mazzanti L. Physicochemical and functional modifications induced by obesity on human erythrocyte membranes. Eur J Clin Invest. 1999;29:432–437. doi: 10.1046/j.1365-2362.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- 93.Preetha A, Banerjee R, Huilgol N. Effect of temperature on surface properties of cervical tissue homogenate and organic phase monolayers. Colloids Surf B Biointerfaces. 2007;60:12–18. doi: 10.1016/j.colsurfb.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 94.Melik-Nubarov NS, Pomaz OO, Dorodnych T, Badun GA, Ksenofontov AL, Schemchukova OB, Arzhakov SA. Interaction of tumor and normal blood cells with ethylene oxide and propylene oxide block copolymers. FEBS Lett. 1999;446:194–198. doi: 10.1016/s0014-5793(99)00208-2. [DOI] [PubMed] [Google Scholar]
- 95.Zhirnov AE, Demina TV, Krylova OO, Grozdova ID, Melik-Nubarov NS. Lipid composition determines interaction of liposome membranes with Pluronic L61. Biochim Biophys Acta. 2005;1720:73–83. doi: 10.1016/j.bbamem.2005.11.010. [DOI] [PubMed] [Google Scholar]