Abstract
Impaired water excretion was noted to coincide with psychotic exacerbations in the first decades of the past century. In the ensuing decades, life-threatening water intoxication and elevated plasma levels of the antidiuretic hormone, arginine vasopressin (AVP) were reported in a subset of persons with schizophrenia. Subsequent studies demonstrated that the osmotic set point for AVP secretion was transiently reset in these patients by an unknown process and that this was further exacerbated by acute psychosis. More recent studies indicate that the AVP dysfunction is a manifestation of a hippocampal-mediated impairment in the regulation of both AVP and HPA axis responses to psychological, but not other types of, stimuli. Of potential significance, is that schizophrenic patients without water imbalance exhibit the opposite pattern of responses. Preliminary data indicate those with water imbalance also demonstrate a closely linked deficit in central oxytocin activity which may account for their diminished social function. These latter behavioral deficits are perhaps the most disabling and treatment resistant features of schizophrenia, which recent studies suggest, may respond to oxytocin agonists. Together these findings support the view that schizophrenia is a heterogeneous disorder, and provide novel biomarkers and approaches for exploring the pathophysiology and treatment of severe mental illness.
Keywords: vasopressin, oxytocin, HPA axis, stress, hippocampus, polydipsia, hyponatremia, schizophrenia, animal models
1. Introduction
1.1 History of primary polydipsia and water intoxication in severe mental illness
Impaired water excretion and water intoxication presenting concurrently with psychotic exacerbations were first noted in the initial decades of the 20th century (Targowla, 1923, Barahal, 1938). Investigators observed that over the course of a single day a patient could retain ten or more liters of fluid producing a profound dilutional hyponatremia. Furthermore, primary polydipsia was found to be the most common physiologic abnormality in chronic psychotic patients (Hoskins and Sleeper, 1933). No recognizable factors could account for the water imbalance, and over subsequent years the findings were both reproduced (De Leon et al., 1994; Hobson and English, 1963) and linked to fatal outcomes (Vieweg et al, 1988). Epidemiologic studies demonstrated that 10 to 20% of persons with schizophrenia were polydipsic (intake in excess of ∼ 3 liters/day) but only a fifth to a third of these (2-5% of total) experienced symptomatic hyponatremia (i.e. water intoxication: delirium, seizures, coma, lethargy, ataxia) (De Leon et al., 1994). Below we refer to the former group of patients as ‘polydipsic normonatremic schizophrenics’, the latter group as ‘polydipsic hyponatremic schizophrenics (PHS)’, and the two groups together as ‘schizophrenic patients with water imbalance’. Thus schizophrenics without water imbalance (nonpolydipsic normonatremic schizophrenics) constitute the majority (80-90%) of those with the mental illness. This review focuses primarily on studies addressing the CNS factors which account for the hyponatremia in the PHS and their relationship to the mental illness.
1.2 Evidence of antidiuretic hormone, arginine vasopressin (AVP) involvement
With the development of highly sensitive and specific radioimmunoassays, the role of excess levels of the antidiuretic hormone (arginine vasopressin: AVP) in diverse disorders of impaired water excretion became apparent during the latter half of the 20th century (Robertson, 1976). While basal AVP levels and water excretion often appeared to be normal in PHS (Gillum and Linas, 1984), more sensitive assessments of water balance, and other reports, revealed indirect evidence of AVP dysregulation (Hariprasad et al., 1980, Ragavan et al., 1984). Among these were reports of elevated plasma AVP concurrent with acute psychosis (Raskind et al., 1975). While primary polydipsia was commonly found across psychiatric disorders, water intoxication and AVP elevations appeared to be largely restricted to those with psychotic illnesses (De Leon et al., 1994). A recent study demonstrating that an AVP V-2 antagonist almost immediately normalizes plasma sodium and osmolality in PHS (Josiassen et al., 2008) underscores the critical role of the hormone in the hyponatremia.
2.0 Mechanism of impaired water excretion
2.1 Why AVP abnormalities are potentially powerful means of probing CNS dysfunction
The brain maintains the concentration of solute in tissues within a very narrow (∼ 1%) range primarily by controlling the secretion of AVP from the hypothalamus. Plasma AVP levels normally exhibit a tight linear relationship (r = ∼0.9) to plasma sodium/osmolality such that levels become undetectable when plasma sodium drops below about 130 mEq/l (∼275 mOsmoles/Kg). When AVP secretion is completely suppressed the human kidney can excrete more than 1000 ccs/hour of urine with an osmolality of ∼ 40 mOsmoles/Kg. Minimal increases (i.e. fentomolar) in plasma AVP rapidly reduce urine excretion to about 20 ccs/ hour of ∼ 1400 mOsmole/Kg urine. Urinary concentration peaks when plasma AVP levels reach only 5 pg/ml.
This system provides a rapid, robust and extremely precise means for maintaining the internal milieu regardless of levels of fluid intake. Note, however, that very slight increases in AVP can markedly diminish maximal water clearance (e.g. water clearance drops by 50% if maximal diluting capacity changes from 40 mOsmole/Kg to 80 mOsmole/Kg which is still very dilute urine). The precision and range of this system, its preeminent role in maintaining basic metabolic functioning, and the careful delineation of the many physiologic/pathological modulators and their associated neural pathways has made variations in AVP secretion incomparable windows into a wide range of CNS (dys)functions. The fact that the impairments in water excretion in PHS are life-threatening, predate the introduction of antipsychotic medications and coincide with exacerbations of psychosis suggest that their delineation and localization might provide insights into severe mental illness.
2.2 Reset osmostat in PHS
Hariprasad (et al. 1980) reported that evidence of urine concentration in PHS was apparent at plasma sodium/osmolality levels normally associated with maximal urinary dilution. The findings suggested a lowering of the osmotic set point for AVP secretion (‘reset osmostat’). In order to address the role and mechanism of AVP in these findings, Goldman (et al. 1988) systematically altered plasma osmolality with an oral water load and hypertonic saline infusion. This enables one to characterize the sensitivity, gain and set point of osmotic regulation (‘osmoregulation’) of AVP secretion and action. These and most of the other studies described below were performed in a clinical research center enabling careful assessment of other factors known or proposed to modulate AVP.
Subjects were PHS and nonpolydipsic (normonatremic) schizophrenic patients who were clinically stable and on similar doses of antipsychotic medication. Both patient groups demonstrated the normal tight linear relationship between plasma AVP and plasma osmolality, but those with hyponatremia exhibited a lower osmotic set point for vasopressin secretion (i.e. reset osmostat) as well as increased renal sensitivity to the hormone (Goldman et al., 1988, Table 1). Recognized modulators (e.g. nicotine activity, nausea, blood volume, blood pressure, renal function as well as plasma potassium, calcium, cortisol, and thyroxine) could not account for the findings. Shortly thereafter, a second group published data drawn from these same populations of patients confirming these results (Kishimoto et al., 1989). These reports thus demonstrated that the reset osmostat described by Hariprasad was attributable to an unexplained alteration in AVP activity.
Table 1.
Summary of major findings from investigations into the nature of altered neuroendocrine regulation in polydipsic hyponatremic schizophrenics
| Subject Groups* |
Methods/Procedure | Summary of Findings# | Reference |
|---|---|---|---|
| PHS^ | Water restriction post-ad lib intake |
■ Urine concentration begins at low plasma osmolalities |
Hariprasad et al., 1980 |
| PHS, NNS | Oral water load/ hypertonic saline inf. |
■ Lower osmotic set point for AVP ■ Enhanced renal response to AVP |
Goldman et al., 1988 |
| PHS, NNS HC |
Ad lib fluid intake | ■ Lower osmotic set point | Kishimoto et al., 1989 |
| PHS, PNS, NNC, HC |
Oral water load/ hypertonic saline inf. |
■ Enhanced renal response to AVP only in PHS ■ Neuroleptics enhance renal AVP response in all patient groups |
Goldman et al., 1996a |
| PHS, PNS | Intravenous psychotomimetic |
■ Enhanced AVP response to psychosis ■ Response predicted by basal psychosis |
Goldman et al., 1997 |
| PHS, PNS, NNC, HC |
Cold pressor test | ■ Enhanced AVP, ACTH, CORT response to cold stress ■ Blunted AVP, ACTH, CORT response to cold stress in NNS |
Goldman et al., 2007a |
| PHS, PNS, NNC, HC |
Structural MRI | ■ Smaller anterior hippocampus | Goldman et al., 2007b |
| PHS, PNS, NNC, HC |
Cortisol infusion post-metyrapone |
■ Blunted ACTH drop ■ Enhanced ACTH drop in NNS |
Goldman et al., 2007c |
| PHS, PNS, NNC, HC |
Cold pressor test | ■ Diminished oxytocin ■ Levels predicted by anterior hippo. volume, ACTH response to cortisol, and facial affect discrimination |
Goldman et al., 2008 |
PHS = polydipsic hyponatremic schizophrenic, NNS = nonpolydipsic normonatremic schizophrenic; PNS = polydipsic normonatremic schizophrenic, HN = healthy normals.
Polydipsic hyponatremic schizophrenics (PHS) compared to other groups unless otherwise stated.
Group compared to established norms.
2.3 Nature of altered AVP activity
A series of studies followed which addressed other putative factors that might reset the osmostat. In one study, osmoregulation was assessed in PHS and polydipsic normonatremic schizophrenic patients in order to assess claims that polydipsia (Hariprasad et al., 1980), per se, alters the set point for AVP release (Goldman et al., 1986a). The earlier reports arose from subjects who were studied immediately following transfer to a research unit, while these subjects were acclimated to the research unit for several weeks in order to diminish the possible role of acute stress/novelty on neuroendocrine release (Coccaro et al., 1984), In addition, patients all received the same antipsychotic medication (fluphenazine) to more closely examine putative medication effects on water excretion (Aljouni et al., 1974; Rider et al., 1995; Kishimoto et al., 1989; but see Suzuki et al., 1992). In a second study, oropharyngeal regulation of water balance (the acute drop in AVP observed immediately following fluid ingestion which facilitates physiologic diuresis, Applegren et al., 1991) was assessed.
The results demonstrated that adaptation to the research unit appeared to partly normalize the osmotic set point, consistent with the interpretation that an idiosyncratic response to the stress of hospitalization in PHS contributed to the earlier findings (Goldman et al., 1996b). None of the other putative factors (polydipsia, oropharyngeal influences, neuroleptic medication) could account for the results (Goldman et al., 1996a), though neuroleptic medication appeared to enhance renal responsiveness to AVP in all patient groups, and polydipsia seemed to blunt the gain of AVP secretion in both polydipsic patient groups (Table 1).
2.4 Impact of psychosis on AVP secretion
The lowered set point for AVP release uncovered in the initial studies was not only transient, but inadequate to produce water intoxication, indicating some other factor(s) must be involved (Berl 1988). As previously noted, psychotic exacerbations had been observed to coincide with enhanced AVP secretion and water intoxication (Ragavan et al., 1984; Raskind et al., 1975; Suzuki et al., 1992). Patients in the previous set of studies were all clinically stable. To confirm this previously noted association and address the mechanism (Fleischhacker et al., 1987), a brief psychotic exacerbation was induced with the psychotomimetic methylphenidate in PHS and polydipsic normonatremic patients.
The results confirmed the prediction that AVP levels rose more during exacerbations in the PHS. This occurred despite the fact that their plasma osmolality was much lower which in deed appeared appropriately suppress their AVP response (Goldman et al., 1997). No other recognized factors except for basal positive symptoms of psychosis could predict the finding (Table 1).
Thus the first two stages of the research indicated that life-threatening water intoxication in PHS was caused, in part, by enhanced AVP secretion that was linked to psychological stress and state of psychosis. Other aspects of AVP regulation appears to be intact in these and other schizophrenic patients. Polydipsia and a medication-induced enhanced renal response to AVP were contributory to the water imbalance but insufficient in themselves to produce hyponatremia.
3. Hippocampal modulation of neuroendocrine responses to stress
3.1 Neuroendocrine responses to psychological stress in PHS
Converging data indicate that the anterior hippocampal formation, a brain region consistently implicated in psychosis, normally constrains hypothalamic-pituitary-adrenal axis (HPA) and AVP responses to psychological (but not systemic) stressors (Herman and Mueller, 2006; Nettles et al., 2000; Pruessner et al. 2008). Preliminary evidence of a diminished cortisol response to dexamethasone (Goldman et al., 1993) and smaller anterior temporal lobe volumes (Elkashef et al., 1996, Luchins et al., 1997) in PHS were consistent with the interpretation that dysfunction of this function could account for the enhanced AVP secretion. To address this explanation the adrenocorticotropin (ACTH) and AVP responses to two different stimuli: hand immersion in ice water (‘cold pressor’) and upright posture were assessed. The study was conducted in the evening when hippocampal influences are most apparent. Previous studies indicated neuroendocrine responses to the cold pressor are likely attributable to emotional factors (Bullinger et al, 1984), while responses to upright posture are not (Goldman et al. 2007a).
As predicted, AVP and ACTH responses to the cold pressor were enhanced in PHS relative to healthy normals. In contrast, responses were blunted in schizophrenic patients without water imbalance, consistent with the extant literature (Jansen et al., 2000; Zahn and Pickar, 2005). Responses of polydipsic normonatremic patients were generally intermediate to the other two patient groups. Responses to the postural stimulus, on the other hand, did not differ across patient groups such that there were marked differences between patient groups in their relative responses to the two types of stress (Fig. 1). Thus these findings supported the view that there was a specific impairment in the neuroendocrine responses to psychological stress in PHS that was the opposite to the pattern seen in patients without water imbalance.
Figure 1.
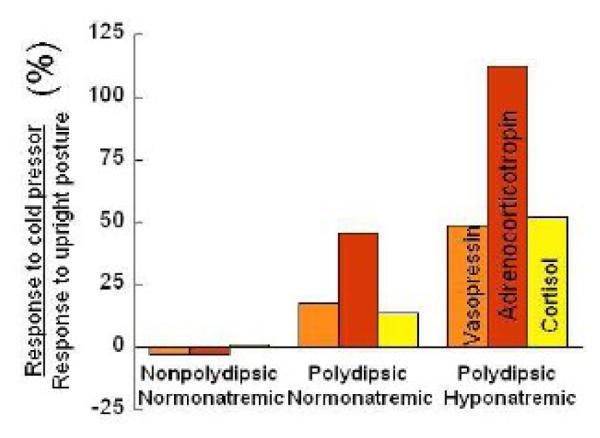
Ratio of peak plasma vasopressin, adrenocorticotropin and cortisol responses to a sixty second immersion in ice water (cold pressor) and a postural stimulus in nonpolydipsic normonatremic, polydipsic normonatremic, and polydipsic hyponatremic schizophrenic (PHS) patients. Responses of healthy normals (not shown) generally resemble those of the polydipsic normonatremics. The hippocampal formation is thought to normally constrain neuroendocrine responses to the cold pressor as it (unlike the postural stimulus) is determined by emotional factors. Thus the findings support the view that hippocampal-mediated responses to psychological stress are enhanced in PHS but blunted in patients without water imbalance. (Adapted from Goldman et al., 2007a)
3.2 Further evidence for hippocampal involvement in the hyponatremia
Two other studies also probed the involvement of the hippocampus. In the first, anterior and posterior hippocampal, as well as amygdala, volumes were compared across groups. As mentioned, the anterior hippocampus is a structure thought to normally constrain neuroendocrine responses to psychological stress. As predicted, this structure but not the amygdala or posterior hippocampus was smaller in PHS than the other groups (Goldman et al., 2007b, Table 1). In the second study, the ACTH response to cortisol (i.e. HPA negative feedback) was measured during the evening after endogenous cortisol levels were first lowered. The ACTH response under these conditions is particularly sensitive to hippocampal influences, and as predicted, the response was markedly blunted in the PHS (Goldman et al., 2007c). In contrast, the pattern in schizophrenic patients without water imbalance was enhanced relative to healthy controls, while polydipsic normonatremic subjects were again intermediate.
3.3 Synthesis with data from animal models of schizophrenia
Together this third phase of studies provides diverse evidence for the concept that PHS have a hippocampal-based impairment in the restraint of neuroendocrine responses to psychological stress. Additional support for this explanation comes from experiments in laboratory animals which disrupt the development of this brain region (ventral hippocampal formation in rodents, anterior hippocampus in primates), yielding adult animals with behavioral, electrophysiologic, anatomic and biochemical features of schizophrenia (Lipska, 2004; Lodge and Grace, 2007). Recent studies confirm these adult animals also exhibit enhanced AVP and HPA responses to stress (Chrapusta et al., 2003; Mitchell and Goldman, 2004) as well as blunted HPA negative feedback (Goursand et al., 2006), i.e. findings that parallel those in PHS. Thus, the findings are consistent with the view that anterior hippocampal pathology in schizophrenia is responsible for the enhanced AVP secretion required to produce life-threatening water intoxication.
4. Oxytocin and the underlying neuropsychiatric disorder
4.1 Is enhanced stress response directly linked to psychiatric illness?
As mentioned above, the anterior hippocampus is not only implicated in regulating neuroendocrine responses to psychological stress but also the pathophysiology of schizophrenia. Indeed, hippocampal-based enhanced stress vulnerability is thought to mediate key features of the mental illness (Conrad and Scheibel, 1987; Goldman and Mitchell, 2004; Gray, 1998; Walker et al., 2008), supporting the view that the former finding may be intimately related to the latter illness. The fourth phase of the research addresses this possibility. Oxytocin, a neurohormone closely linked to AVP and to the HPA axis has been the focus of this effort because of its role in modulating psychological stress responses as well as social behaviors that are markedly impaired in schizophrenia.
4.2 Brief review of central and peripheral oxytocin release
Oxytocin is secreted from the same hypothalamic nuclei that produce ACTH secretagogues and AVP. Furthermore, oxytocin release is likely modulated by the same hippocampal projection which influences AVP/ACTH secretagogue responses to psychological stress (Fig. 2) (Herman et al., 2002; Risold and Swanson, 1996). Oxytocin’s primary peripheral function is to facilitate labor and lactation which appear unrelated to the major functions of either the HPA axis or AVP. Oxytocin and AVP, however, are closely related nonapeptides differing in only two amino acids. While peripheral stimuli often increase both hormones (Olsson and Högberg, 2009), the two hormones have opposing actions in the central nervous system (CNS) (Legros, 2001). CNS AVP has been linked to aggression (Caldwell et al., 2008a; Legros, 2001) while CNS oxytocin diminishes psychological stress responses and enhances prosocial behaviors (Carter, 1999; Heinrichs et al, 2003; Keri et al., 2008; Ditzen et al., 2009;). Indeed some claim its efficacy on social behavior is because it reduces the stress associated with interpersonal proximity (Ross and Young, 2009). Thus the hippocampus could normally increase oxytocin as part of its stress-modulating function, and the hippocampal-mediated impairment in this response might contribute to both stress vulnerability and social deficits.
Figure 2.
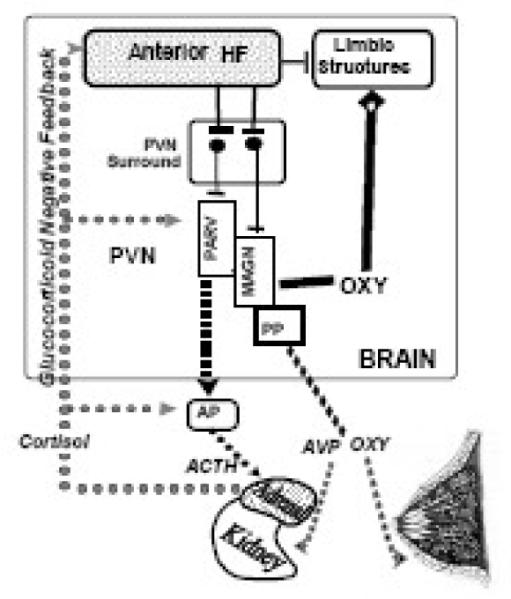
Mechanism of abnormal neuroendocrine regulation in PHS and its relationship to the underlying mental illness. The anterior hippocampal formation (Anterior HF) normally constrains hypothalamic-pituitary-adrenal axis (HPA), vasopressin and oxytocin responses to psychological stress via relays to the paraventricular surround (PVN surround) which in turn projects to the paraventricular nucleus (PVN) of the hypothalamus. The PVN contains parvacellular neurons (PARV) which secrete HPA secretagogues from the median eminence (not shown) and magnocellular neurons (MAGN) which secrete vasopressin (AVP) and oxytocin (OXY) from the posterior pituitary (PP). Peptide hormones from both types of neurons are secreted directly into blood vessels. The HPA secretagogues regulate adrenocorticotropin (ACTH) release from the anterior pituitary (AP) which then acts on the adrenal glands to release cortisol. Cortisol in turn feedsback at several levels of the HPA axis (glucocorticoid negative feedback), including the anterior hippocampal formation. AVP acts on the kidney to enhance water excretion and oxytocin enhances lactation. Oxytocin also acts centrally at limbic receptors to enhance social behaviors and to diminish responses to psychological stress. Central oxytocin is secreted from dendrites of magnocellular neurons directly into the brain, and this source may in fact account for much of brain oxytocin.
We propose the normal anterior hippocampal-mediated constraint of neuroendocrine responses to stress is impaired in PHS such that peripheral HPA axis and AVP responses are enhanced, while peripheral and central oxytocin responses are blunted. schizophrenic patients with water imbalance. Anterior hippocampal pathology disrupts the balance of regulatory influences emanating from the periventricular surround and this in turn alters central and peripheral AVP, HPA and oxytocin activity.
Central and peripheral oxytocin are released in a coordinated manner from the dendrites and axon terminals of the same magnocellular hypothalamic neurons (Neumann et al., 1993; Wotjak et al., 1998). Furthermore, the dendritic release may account for much of the oxytocin in the brain, as it is the most common neuropeptide found in the CNS (with many oxytocin receptors found particularly in limbic structures), yet few oxytocin containing projections have been identified (Ludwig and Leng, 2006; Neumann et al., 1994). Thus these hypothalamic neurons may be the source of both central and peripheral oxytocin, in which case peripheral measures of oxytocin could provide an indication of central activity. Indeed, evidence suggests that diverse factors known to increase peripheral oxytocin levels decrease stress responses which are presumably mediated by CNS oxytocin (Heinrichs et al., in press).
4.3 Possible relevance of CNS oxytocin to impaired social behaviors in PHS
Oxytocin knockout mice exhibit features of schizophrenia (Caldwell et al., 2008b) that are reversed with oxytocin (Feifel and Rez, 1999). Furthermore, deficits in social function in laboratory animals induced by diverse pharmacologic or environmental factors are normalized with oxytocin treatment (Lee et al., 2005; Lee et al., 2007), and are at least partly mediated by the ventral striatum (Ross and Young, in press), a brain region closely linked to psychosis. Social dysfunction, as noted, is a key feature of schizophrenia, reflected in the prominence of negative symptoms and the deficit syndrome (Addington et al., 2006; Goetz et al., 2007; Kirkpatrick et al., 2001; Kirkpatrick, 1997). Together these observations suggest that diminished CNS oxytocin activity could contribute to the social dysfunction in schizophrenia.
Keri (et al., 2008) addressed this possibility by comparing differences in peripheral oxytocin activity in schizophrenic patients and healthy controls following exchange of both personal and impersonal information. Enhancing trust between strangers is one of the means by which oxytocin appears to promote interpersonal proximity and hence prosocial behavior (Baumgartner et al., 2008; Kosfeld et al., 2005; Zak et al., 2005, 2007). Trusting behavior, per se, may also increase both CNS and plasma oxytocin levels (Keri et al., 2008). Keri et al. predicted that only the exchange of personal information would increase oxytocin levels. Indeed, such an increase was found in the healthy controls but not the schizophrenic subjects, while levels of oxytocin in schizophrenia were inversely correlated with indices of their social dysfunction (i.e. negative symptoms).
Social dysfunction is particularly prominent in PHS (Bralet, 2007) suggesting that oxytocin levels might be even lower in this subgroup. Indeed, preliminary data indicate this is true (Goldman et al., 2008). Furthermore, the diminished levels appear to be linked to the hippocampal-mediated neuroendocrine dysfunction identified in the third phase of the research summarized above (Table 1): plasma oxytocin was correlated with both anterior hippocampal volume loss and the ACTH response to cortisol (i.e. HPA negative feedback). Furthermore, oxytocin levels in this study were correlated with impairments in cognition linked to social behavior as well as social dysfunction, per se. Regarding the former, oxytocin improves the ability to attend to and identify the emotional state of others (Domes et al., 2007, Guastella et al., 2008; Savaskan et al., 2008) which is a cognitive function markedly impaired in schizophrenia (Addington et al., 2006; Bozikas et al., 2006, Kucharska-Pietura et al., 2005). Plasma oxytocin levels were highly predictive of this ability (Goldman et al., 2008) as well as indices of the deficit syndrome (Goldman, unpublished data) (a marker of profound social deficits). In summary, these early reports are consistent with the idea that social deficits in PHS may be directly related to their hippocampal-mediated neuroendocrine dysfunction.
4.4 Therapeutic implications
Recent studies suggest exogenous oxytocin reduces psychological stress responses and enhances social function in healthy controls (Heinrichs et al., in press). Intranasal oxytocin appears to cross the blood-brain barrier (Born et al., 2002; Pietrowsky et al., 1996; Riekkinen et al., 1987) and to bind receptors in sub-cortical limbic structures (Chen et al., 1998; Loup et al., 1991) supporting the view that these hormonal effects are centrally mediated. Trials has begun to address whether oxytocin can diminish the social dysfunction in anxiety disorders (Guastella et al., 2009) and autism (Heinrichs et al., 2009), but there is surprisingly little work in schizophrenia despite the prominence of social deficits in schizophrenia, their clinical significance and their general resistance to available therapies. Currently a trial is underway assessing whether intranasal oxytocin reverses the deficits in facial affect discrimination that were identified in the initial study summarized above. Preliminary findings are consistent with this view (Gomes et al., 2008). Testing has recently begun for oxytocin receptor agonists which cross the blood brain barrier. The current intention is to develop these agents for the treatment of anxiety and depression. Should the findings of intranasal oxytocin in PHS hold up, oxytocin agonists could provide a novel therapy for a core feature of schizophrenia, albeit possibly beneficial only for the subset of patients with water imbalance.
5. Potential significance of findings and methodological approach
5.1 Summary of research to date
Disordered water balance in schizophrenia and its association with acute psychosis were first observed nearly a century ago. The studies reviewed here (Table 1) substantiate the view that these patients have a relatively subtle and transient impairment in water excretion attributable in part to resetting of the osmostat for AVP secretion. This resetting in turn appears to be caused by a hippocampal-mediated impairment in neuroendocrine regulation which produces enhanced AVP and HPA axis responses apparent only during psychological stress or acute psychosis. In addition, these patients exhibit related deficits in oxytocin activity that contribute to core, and potentially reversible, features of their neuropsychiatric illness. If confirmed these findings could lead to fundamental insights into the pathophysiology and treatment of severe mental illness.
5.2 Potential significance to stress-vulnerability hypothesis
Schizophrenic patients with water imbalance exhibit hippocampal dysfunction and elevated stress vulnerability which many have linked to the disorder (Conrad and Schiebel, 1987; Gray et al., 1998; Mednick and Schulsinger, 1968; Walker et al., 2008) but for which there has been minimal empirical support. Indeed, most evidence suggests the opposite, i.e. schizophrenia is associated with blunted stress responses ((Gundoz-Bruce et al., 2007; Jansen et al., 2000; Zahn and Pickar, 2005)). This apparent contradiction is addressed by the studies reviewed here which demonstrate that only a small subset of schizophrenic patients (i.e. those with water imbalance) exhibit ‘stress vulnerability’ whereas the majority do not. It remains to be shown, however, that this vulnerability extends to behavioral responses to stress.
This research supports the view of many that it is critical to identify endophenotypes in order to meaningfully address the pathophysiology of severe mental illness. The success of the strategy reported here is a product of the facts that patients with water imbalance can be reliably distinguished from other schizophrenic patients (de Leon et al., 1994; Goldman et al., 1992) and that AVP regulation is well-characterized and precise (Robertson, 1976).
Unfortunately, most studies of severe mental illness are not designed to account for heterogeneity. Moreover, the validity of putative endophenotypes, the knowledge needed to link them and recognized modulators to well-characterized neural functions/pathways, and the means of identifying subtle variations in neurofunction are often not established. Sadly, even when information about physiologic modulation of dependent measures is available, investigators may ignore it. For instance, of the 15 studies of psychiatric disorders published from 2000-2006 in which AVP is a major outcome variable, only six measured plasma osmolality or sodium. Only two of the fifteen reported easily obtainable measures of other physiologic modulators of plasma AVP (e.g. blood pressure, nicotine use). Only one of the articles acknowledged that the missing information was possibly relevant (Jahn et al., 2004) while another asserted that “plasma osmolality was not measured because it is not associated with plasma vasopressin in depression” (de Winter et al., 2003). Thus another implication of this work is that investigators should attend more closely to potential alterations in brain physiology in psychiatric disorders that may be linked to with well-characterized neural systems.
6. Limitations and caveats
6.1 Methodogical concerns
One of the challenges of matching subsets of patients with the same mental illness, as well as studying a relatively uncommon disorder, is generating a sufficient sample. Thus while some of the findings have been replicated and others seem logical extensions from earlier phases, most sample sizes are small with some cells containing only five subjects. In addition, since water imbalance is usually only apparent several years after the onset of schizophrenia (De Leon et al., 1994), it is nearly impossible to find patients at present who have not received antipsychotic medication. It is also very difficult to discontinue these medications because of cooperation required for the lengthy and somewhat intrusive procedures needed to fully characterize neuroendocrine regulation. Fortunately, impaired water excretion and its association with psychotic exacerbations occurred prior to the introduction of antipsychotic medication which argues against the possibility that the more recent findings are attributable to an idiosyncratic response to these medications.
6.2 Concepts require more empirical support
Some of the views presented here are based on concepts that have not been adequately tested, and a discounting of alternative explanations before they have been adequately addressed. For instance, the view that hippocampal-mediated responses to psychological stress are altered in PHS assumes that knowledge gained from other mammals and particularly rodents can be extrapolated to humans. Furthermore smaller anterior hippocampal volumes in PHS do not necessarily reflect a pathological process, and could alternatively be a consequence rather than the cause of enhanced HPA activity (Arango et al., 2001). In addition, other neurostructures such as the prefrontal cortex are implicated in both schizophrenia and neuroendocrine responses to stress (perhaps by their modulation of emotional resilience (Maier et al., 2006)) and their involvement has not been examined.
Another example is the conclusion that psychological, but not physical, stress responses vary across patient groups. The conclusion rests on a single psychological stressor (cold pressor) which contains both physical and psychological components. The problem is that schizophrenics tend to interpret pure psychological stresses (e.g. timed math problems, public speaking, mock job interviews) in an idiosyncratic manner, making them of very limited value. In any case, ideally one should administer diverse stressors before concluding that stress vulnerability, per se, is altered. Even if confirmed with other stressors, the enhanced HPA axis response in PHS could be a consequence of their enhanced AVP response, since magnocellular AVP appears capable of acting as an ACTH secretagogue (Engelman et al., 2004). Finally, as mentioned in the text, autonomic, emotional and cognitive responses to psychological stress have not been shown to be altered in PHS, which are likely more relevant to the concept of stress vulnerability in schizophrenia.
Another concept requiring more empirical support involves the possible significance and reversibility of oxytocin dysfunction in PHS. This view is again based on extrapolating animal data to humans. Additional propositions presented here include that the hippocampus modulates oxytocin release in a manner analogous to that of AVP and ACTH, that peripheral and central oxytocin regulation are closely linked, and that the effects of intranasally administered oxytocin are centrally mediated. Furthermore, as noted above, peripheral oxytocin and AVP respond similarly to most stimuli, and the finding that levels are instead opposed in PHS and perhaps other patient groups appears to be both paradoxical and unprecedented. Alternative explanations for the findings such as the possibility that diminished oxytocin is attributable to polydipsia (since it also blunts AVP secretion (Goldman et al., 1986a; Moses and Clayton, 1993)), or that diminished HPA responses to psychological stress are mediated by diminished parvocellular oxytocin release (Legros, 2001) have not been excluded.
7. Areas for further investigation
7.1 Polydipsia and associated features in patients with water imbalance
In addition to the need to confirm the scientific basis on which the findings have been interpreted, other aspects of the water imbalance have not been adequately addressed. The mechanism of the polydipsia, for instance, which is a strikingly abnormal behavior in these patients and plays at least an equal role in hyponatremia, remains unknown. Animal studies indicate that polydipsia and other repetitive behaviors (e.g. pica, pacing) (Devenport et al., 1981; Lee et al., 2007; Shutty et al., 1995) commonly seen in schizophrenic patients with water imbalance (Luchins et al., 1992; Arieti et al., 1974) are associated with both hippocampal lesions and blunted HPA feedback (Luchins, 1990; Mittleman et al., 1988). Moreover, it is possible that these behaviors could also be ameliorated with oxytocin, since oxytocin has been reported to reduce motor stereotypies in persons with autism and Aspergers Syndrome (Hollander et al., 2003).
7.2 Altered neuroendocrine responses in patients without water imbalance
The significance of the blunted HPA axis and AVP responses to psychological stress, the enhanced HPA feedback, and the generally higher plasma oxytocin in schizophrenics without water imbalance is consistent with the prior literature. In turn, these results provide powerful support that the changes in PHS cannot be attributed to chronicity of the illness, treatment of schizophrenia, or other nonspecific factors. The mechanism of these findings, however, is not apparent. It is tempting to attribute them to an appropriate compensation to the chronic stress of having a psychotic disorder, but there is no evidence to support this proposition.
7.3 Central vasopressin regulation in PHS
Finally, given the close association between central oxytocin and vasopressin, and the demonstration that peripheral AVP regulation is altered in these patients, it is reasonable to expect than any deficit in central oxytocin might be associated with excess central vasopressin activity. This in turn could contribute to the inability to trust and the difficulty modulating aggression (Thompson et al., 2004; Storm and Tecott, 2005) in schizophrenia. If this were the case, recent discovered CNS-penetrating AVP V1 antagonists could also provide new treatment options.
8. Summary
Information about neuroendocrine regulation in humans and other species has provided insights into the mechanisms of altered water metabolism in schizophrenia. The findings have lead to evidence of widespread neuroendocrine dysfunction in these patients that may be closely related to the underlying neuropsychiatric disorder, and contribute to disabling but reversible features of the illness. The reliance of this work on distinct clinical syndromes limited to a subset of patients, and their association with relatively well-characterized neurophysiologic systems suggests that similar strategies may lead to new insights into the significance of neural findings in mental disorders.
Acknowledgements
I am indebted to the patients and staff at the Psychiatric Clinical Research Center, University of Illinois at Chicago, Chicago, IL; the nursing, laboratory and support staffs of the Clinical Research Centers at University of Illinois at Chicago, the University of Chicago, and Northwestern University. Funding provided by Brain Research Foundation, an affiliate of the University of Chicago; State of Illinois funding of the Psychiatric Institute; NIH: RO1 MH56525 (MG), 1R21MH082295 (MG), General Clinical Research Center Grants: M01 RR00055 (UC), M01-RR-13987 (UIC).
Footnotes
Human subjects statement All experiments were undertaken with the understanding and written consent of each subject, with the approval of the Institutional Review Board, and in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (Declaration of Helsinki).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophren. Res. 2006;85:142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Ajlouni K, Kern MW, Tures JF, Theil GB, Hagen TC. Thiothixene-induced hyponatremia. Arch. Intern. Med. 1974;134:1103–1105. [PubMed] [Google Scholar]
- Appelgren BH, Thrasher TN, Keil LC, Ramsay DJ. Mechanism of drinking-induced inhibition of vasopressin secretion in dehydrated dogs. Am. J. Physiol. 1991;261:R1226–R1233. doi: 10.1152/ajpregu.1991.261.5.R1226. [DOI] [PubMed] [Google Scholar]
- Arango C, Kirkpatrick B, Koenig J. At issue: stress, hippocampal neuronal turnover, and neuropsychiatric disorders. Schizophr. Bull. 2001;27:477–480. doi: 10.1093/oxfordjournals.schbul.a006888. [DOI] [PubMed] [Google Scholar]
- Arieti S. Interpretation of Schizophrenia. 2nd Edition Basic Books; New York: 1974. pp. 415–422. [Google Scholar]
- Barahal HS. Water intoxication in a mental case. J. Psych. Quat. 1938;12:767–771. [Google Scholar]
- Baumgartner T, Heinrichs M, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Berl T. Psychosis and water balance. N. Engl. J. Med. 1988;318:441–442. doi: 10.1056/NEJM198802183180708. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Kosmidis MH, Anezoulaki D, Giannakou M, Andreou C, Karavatos A. Impaired perception of affective prosody in schizophrenia. J. Neuropsych. Clin. Neurosci. 2006;18:81–85. doi: 10.1176/jnp.18.1.81. [DOI] [PubMed] [Google Scholar]
- Bralet MC, Ton T, Falissard B. Schizophrenic patients with polydipsia and water intoxication more often have a form of schizophrenia first described by Kraepelin. Psychiatry Res. 2007;152:267–271. doi: 10.1016/j.psychres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Bullinger M, Naber D, Pickar D, Cohen RM, Kalin NH, Pert A, Bunney WE., Jr. Endocrine effects of the cold pressor test: relationships to subjective pain appraisal and coping. Psychiatry Res. 1984;12:227–233. doi: 10.1016/0165-1781(84)90028-3. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Stephens SL, Young WS. Oxytocin as a natural antipsychotic: a study using oxytocin knockout mice. Mol. Psychiatry. 2008b doi: 10.1038/sj.mp.4002150. 2008b (online: PMID: 18227836) [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS. Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008a;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1999;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Chen XQ, Fawcett JR, Rahman YE, Ala TA, Frey WH. Delivery of nerve growth factor to the brain via the olfactory pathway. J. Alzeheimer’s Dis. 1998;1:35–44. doi: 10.3233/jad-1998-1102. [DOI] [PubMed] [Google Scholar]
- Chrapusta SJ, Egan MF, Wyatt RJ, Weinberger DR, Lipska BK. Neonatal ventral hippocampal damage modifies serum corticosterone and dopamine release responses to acute footshock in adult Sprague-Dawley rats. Synapse. 2003;47:270–277. doi: 10.1002/syn.10179. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Prudic J, Rothpearl A, Nurnberg HG. Effect of hospital admission on DST results. Am. J. Psychiatry. 1984;141:982–985. doi: 10.1176/ajp.141.8.982. [DOI] [PubMed] [Google Scholar]
- Conrad AJ, Scheibel AB. Schizophrenia and the hippocampus: The embryological hypothesis extended. Schizophr. Bull. 1987;13:577–587. doi: 10.1093/schbul/13.4.577. [DOI] [PubMed] [Google Scholar]
- de Leon J, Verghese C, Tracy JI, Josiassen RC, Simpson GM. Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol. Psychiatry. 1994;35:408–419. doi: 10.1016/0006-3223(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Devenport LD, Devenport JA, Holloway FA. Reward-induced stereotypies: Modulation by the hippocampus. Science. 1981;212:1288–1289. doi: 10.1126/science.7195073. [DOI] [PubMed] [Google Scholar]
- de Winter RF, van Hemert AM, DeRijk RH, Zwinderman KH, Frankhuijzen-Sierevogel AC, Wiegant VM, Goekoop JG. Anxious-retarded depression: relation with plasma vasopressin and cortisol. Neuropsychopharm. 2003;28:140–147. doi: 10.1038/sj.npp.1300002. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Leadbetter RA, Kirch DG. Structural brain imaging in patients with schizophrenia and polydipsia-hyponatremia syndrome. In: Schnur D, Kirch DG, editors. Water Balance in Schizophrenia. APA Press; Washington, USA: 1996. pp. 125–136. [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Feifel D, Rez T. Oxytocin modulates psychotomimetic-induced deficits in sensorimotor gating. Psychopharmacology (Berl.) 1999;141:93–98. doi: 10.1007/s002130050811. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Barnas C, Ledochowski M. Hyponatremia-induced organic mental disorder may mask paranoid schizophrenia. Biol. Psychiatry. 1987;22:650–652. doi: 10.1016/0006-3223(87)90192-2. [DOI] [PubMed] [Google Scholar]
- Gillum DM, Linas SL. Water intoxication in a psychotic patient with normal renal water excretion. Am. J. Med. 1984;77:773–774. doi: 10.1016/0002-9343(84)90384-x. [DOI] [PubMed] [Google Scholar]
- Goetz RR, Corcoran C, Yale S, Stanford AD, Kimhy D, Amador X, Malaspina D. Validity of a ‘proxy’ for the deficit syndrome derived from the Positive And Negative Syndrome Scale (PANSS) Schizophr. Res. 2007;93:169–177. doi: 10.1016/j.schres.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Luchins DJ, Robertson GL. Mechanisms of altered water metabolism in psychotic patients with polydipsia and hyponatremia, N. Engl. J. Med. 1988;318:397–403. doi: 10.1056/NEJM198802183180702. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Marks RC, Blake L, Petkovic M, Hedeker D, Luchins DJ. Estimating daily urine volume in psychiatric patients: Empiric conformation. Biol. Psychiatry. 1992;31:1228–1231. doi: 10.1016/0006-3223(92)90343-x. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Blake L, Marks RC, Hedeker D, Luchins DJ. Association of nonsuppression of cortisol on the DST with primary polydipsia in chronic schizophrenia. Am. J. Psychiatry. 1993;150:653–655. doi: 10.1176/ajp.150.4.653. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Robertson GL, Hedeker D. Oropharyngeal regulation of water balance in polydipsic schizophrenics. Clin. Endocrinol. 1996a;44:31–37. doi: 10.1046/j.1365-2265.1996.641463.x. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Robertson GL, Luchins DJ, Hedeker D. The influence of polydipsia on water excretion in hyponatremic, polydipsic schizophrenic patients. J. Clin. Endocrinol. Metab. 1996b;81:1465–1470. doi: 10.1210/jcem.81.4.8636352. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Robertson GL, Luchins DJ, Hedeker D, Pandey GN. Psychotic exacerbations and enhanced vasopressin secretion in schizophrenics with hyponatremia and polydipsia. Arch. Gen. Psychiatry. 1997;54:443–449. doi: 10.1001/archpsyc.1997.01830170069010. M.B. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Mitchell CP. What is the functional significance of the hippocampal pathology in schizophrenia? Schizophren. Bull. 2004;30:367–392. doi: 10.1093/oxfordjournals.schbul.a007086. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Hussain N, Gnerlich J. Neuroendocrine responses to a cold pressor stimulus in polydipsic hyponatremic and in matched schizophrenic patients. Neuropsychopharm. 2007a;32:1611–1621. doi: 10.1038/sj.npp.1301282. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Torres IJ, Keedy S, Marlow-O’Connor M, Beenken B, Pilla R. Reduced anterior hippocampal formation volume in hyponatremic schizophrenic patients. Hippocampus. 2007b;17:554–562. doi: 10.1002/hipo.20292. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Wood G, Goldman MB, Gavin M, Paul S, Zaheer S, Fayyaz G, Pilla RS. Diminished glucocorticoid negative feedback in polydipsic hyponatremic schizophrenic patients. J. Clin. Endocrinol. Metab. 2007c;92:698–704. doi: 10.1210/jc.2006-1131. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Marlow-O’Connor M, Torres I, Carter CS. Preliminary data on plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits in press. Schizophrenia Res. 2008;98:247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A, Lee R, Carter CS, Goldman MB. Effect of intranasal oxytocin on facial affect recognition in schizophrenia. Abstracet SFN; Washington, DC: 2008. [Google Scholar]
- Goursaud AP, Mendoza SP, Capitanio JP. Do neonatal bilateral ibotenic acid lesions of the hippocampal formation or of the amygdala impair HPA axis responsiveness and regulation in infant rhesus macaques (Macaca mulatta)? Brain Res. 2006;1071:97–104. doi: 10.1016/j.brainres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Gray JA. Integrating schizophrenia. Schizophr. Bull. 1998;24:249–266. doi: 10.1093/oxfordjournals.schbul.a033324. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell P, Dadds MR. Oxytocin Increases gaze to the eye region of human faces. Biol. Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Szeszko PR, Gueorguieva R, Ashtari M, Robinson DG, Kane JM, Bilder RM. Cortisol levels in relation to hippocampal sub-regions in subjects with first episode schizophrenia. Schizophr. Res. 2007;94:281–287. doi: 10.1016/j.schres.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Hariprasad MK, Eisinger RP, Nadler IM, Padmanabhan CS, Nidus BD. Hyponatremia in psychogenic polydipsia. Arch. Intern. Med. 1980;140:1639–1642. [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Dawans BV, Domes G. Oxytocin, Vasopressin, and Human Social Behavior. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2009.05.005. (in press) [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration glutamate-GABA connections. Pharm. Biochem. Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav. Brain. Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Hobson JA, English JT. Self-induced water intoxication. Ann. Int. Med. 1963;58:324–332. doi: 10.7326/0003-4819-58-2-324. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharm. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Hoskins RG, Sleeper FH. Organic functions in schizophrenia. Arch. Neurol. Psychiatry. 1933;30:123–140. [Google Scholar]
- Jahn H, Doring WK, Krampe H, Sieg S, Werner C, Poser W, Brunner E, Ehrenreich H. Preserved vasopressin response to osmostimulation despite decreased basal vasopressin levels in long-term abstinent alcoholics. Alcohol Clin. Exp. Res. 2004;28:1925–1930. doi: 10.1097/01.alc.0000148110.34917.c3. [DOI] [PubMed] [Google Scholar]
- Jansen LM, Gispen-de Wied CC, Kahn RS. Selective impairments in the stress response in schizophrenic patients. Psychopharmacology (Berl) 2000;149:319–325. doi: 10.1007/s002130000381. [DOI] [PubMed] [Google Scholar]
- Josiassen RC, Goldman M, Jessani M, Shaughnessy RA, Albazzaz A, Lee J, Ouyang J, Orlandi C, Czerwiec F. Double-blind, placebo-controlled, multicenter trial of a vasopressin V2-receptor antagonist in patients with schizophrenia and hyponatremia. Biol. Psychiatry. 2008;64:1097–1100. doi: 10.1016/j.biopsych.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Keleman O. Sharing secrets: Oxytocin and trust in schizophrenia. Soc. Neurosci. 2008;1:1–7. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B. Affiliation and neuropsychiatric disorders: the deficit syndrome of schizophrenia. Ann. N. Y. Acad. Sci. 1997;807:455–468. doi: 10.1111/j.1749-6632.1997.tb51939.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr. A separate disease within the syndrome of schizophrenia. Arch. Gen, Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Hirai M, Ohsawa H, Terada M, Matsuoka I, Ikawa G. Manner of arginine vasopressin secretion in schizophrenic patients--with reference to the mechanism of water intoxication. Jpn. J. Psychiatry Neurol. 1989;43:161–169. doi: 10.1111/j.1440-1819.1989.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kucharska-Pietura K, David AS, Masiak M, Phillips ML. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br. J. Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharm. 2005;30:1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–67. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros JJ. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- Lipska BK. Using animal models to test a neurodevelopmental hypothesis of schizophrenia. J. Psychiatry and Neurosci. 2004;29:282–286. [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Luchins DJ. A possible role of hippocampal dysfunction in schizophrenic symptomatology. Biol. Psychiatry. 1990;28:87–91. doi: 10.1016/0006-3223(90)90625-c. [DOI] [PubMed] [Google Scholar]
- Luchins DJ, Goldman MB, Dyson V, Lieb M. Excessive stereotypic behaviors in chronic schizophrenia. Schizophren. Res. 1992;8:119–123. doi: 10.1016/0920-9964(92)90027-3. [DOI] [PubMed] [Google Scholar]
- Luchins DJ, Nettles KW, Goldman MB. Anterior medial temporal lobe volumes in polydipsic schizophrenic patients with and without hypo-osmolemia: A pilot study. Biol. Psychiatry. 1997;42:767–770. doi: 10.1016/s0006-3223(96)00491-x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S, Schulsinger F. Some premorbid characteristics related to breakdown in children with schizophrenic mothers. In: Rosenthal D, Kety S, editors. The transmission of schizophrenia. Pergamon; Oxford, UK: 1968. pp. 267–293. [Google Scholar]
- Mitchell CP, Goldman MB. Neonatal lesions of the ventral hippocampal formation disrupt neuroendocrine responses to auditory stress in the adult rat. Psychoneuroendocrinology. 2004;29:1317–1325. doi: 10.1016/j.psyneuen.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Jones GH, Robbins TW. The relationship between schedule-induced polydipsia and pituitary-adrenal activity: pharmacological and behavioral manipulations. Behav. Brain Res. 1988;28:315–324. doi: 10.1016/0166-4328(88)90134-9. [DOI] [PubMed] [Google Scholar]
- Moses AM, Clayton B. Impairment of osmotically stimulated AVP release in patients with primary polydipsia. Am. J. Physiol. 1993;265:R1247–R1252. doi: 10.1152/ajpregu.1993.265.6.R1247. [DOI] [PubMed] [Google Scholar]
- Nettles KW, Pesold C, Goldman MB. Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic-pituitary-adrenal axis, and behavioral responses to novel acoustic stress. Brain Res. 2000;858:181–190. doi: 10.1016/s0006-8993(99)02281-7. [DOI] [PubMed] [Google Scholar]
- Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrin. 1993;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- Olsson K, Högberg M. Plasma vasopressin and oxytocin concentrations increase simultaneously during suckling in goats. J. Dairy Res. 2009;76:15–19. doi: 10.1017/S0022029908003658. [DOI] [PubMed] [Google Scholar]
- Pietrowsky R, Struben C, Molle M, Fehm HL, Born J. Brain potential changes after IN vs. intravenous administration of vasopressin: evidence for a direct nose-brain pathway for peptide effects in humans. Biol. Psychiatry. 1996;39:332–40. doi: 10.1016/0006-3223(95)00180-8. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Ragavan V, Verbalis J, Woods M. Psychogenic polydipsia and hyponatremia: evidence for a reset osmostat. Presented at the Seventh International Congress of Endocrinology; Quebec, Canada. 1984. [Google Scholar]
- Raskind MA, Orenstein H, Christopher TG. Acute psychosis, increased water ingestion, and inappropriate antidiuretic hormone secretion. Am. J. Psychiatry. 1975;132:907–910. doi: 10.1176/ajp.132.9.907. [DOI] [PubMed] [Google Scholar]
- Riekkinen P, Legros JJ, Sennef C, Jolkkonen J, Smitz S, Soininen H. Penetration of DGAVP (ORG 5667) across the blood-brain barrier in human subjects. Peptides. 1987;8:260–265. doi: 10.1016/0196-9781(87)90101-x. [DOI] [PubMed] [Google Scholar]
- Rider JM, Mauger TF, Jameson JP, Notman DD. Water handling in patients receiving haloperidol decanoate. Ann. Pharmacother. 1995;29:663–666. doi: 10.1177/106002809502907-801. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Robertson GL. The regulation of vasopressin function in health and disease. Recent Prog. Horm. Res. 1976;33:333–385. doi: 10.1016/b978-0-12-571133-3.50015-5. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. doi: 10.1016/j.yfrne.2009.05.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schächinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Shutty MS, Jr, McCulley K, Pigott B. Association between stereotypic behavior and polydipsia in chronic schizophrenic patients. J. Behav. Ther. Exp. Psychiatry. 1995;26:339–343. doi: 10.1016/0005-7916(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Storm EE, Tecott LH. Social circuits: peptidergic regulation of mammalian social behavior. Neuron. 2005;47:483–486. doi: 10.1016/j.neuron.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Takeuchi O, Mori I, Takegoshi K, Kurachi M. Syndrome of inappropriate secretion of antidiuretic hormone associated with schizophrenia. Biol. Psychiatry. 1992;31:1057–1061. doi: 10.1016/0006-3223(92)90098-k. [DOI] [PubMed] [Google Scholar]
- Targowla R. Des troubles fonctionnel du rein dans les maladies mentales. L’excretion del’eau (Kidney malfunction and mental illness: water excretion) Bulletins et mémoires de la Société médicale des hôpitaux de Paris. 1923;47:1711–1715. [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29:35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Vieweg WV, David JJ, Rowe WT, Wampler GJ, Burns WJ, Spradlin WW. Death from self-induced water intoxication among patients with schizophrenic disorders, J. Nerv. Ment. Dis. 1988;173:161–165. doi: 10.1097/00005053-198503000-00005. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Ann. Rev. Clin. Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Zahn TP, Pickar D. Autonomic activity in relation to symptom ratings and reaction time in unmedicated patients with schizophrenia. Schizophr. Res. 2005;79:257–270. doi: 10.1016/j.schres.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm. Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS ONE. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]


