Abstract
Alcoholic steatosis is a fundamental metabolic disorder in the progression of alcoholic liver disease. Zinc deficiency is one of the most consistently observed biochemical/nutritional manifestations of alcoholic liver disease. The purpose of this study is to determine whether or not dietary zinc supplementation to mice previously exposed to alcohol exposure could reverse alcoholic steatosis. Male 129S mice were pair-fed an alcohol or isocaloric maltose dextrin liquid diet for 16 weeks with or without dietary zinc supplementation for the last 4 weeks. Zinc supplementation significantly attenuated alcohol-mediated increases in hepatic triglyceride, cholesterol and free fatty acids in association with accelerated hepatic fatty acid oxidation and very low density lipoproteins (VLDL) secretion. Hepatic genes related to fatty acid oxidation and VLDL secretion were upregulated by zinc supplementation, which was accompanied by restoring activity of hepatocyte nuclear factor-4α (HNF-4α) and peroxisome proliferators activated receptor-α (PPAR-α). Zinc supplementation enhanced alcohol metabolism and attenuated oxidative stress and liver injury. Zinc supplementation also normalized alcohol-mediated increases in plasma triglycerides and partially reversed decrease in gonadal adipose depot (GAD) mass. Studies in HepG2 cells showed that zinc deprivation significantly suppressed the DNA binding activities of HNF-4α and PPAR-α, and reduced HNF-4α and PPAR-α target proteins. Consequently, zinc deprivation caused cellular accumulation of lipid droplets, triglycerides and free fatty acids in the HepG2 cells. Conclusions: Zinc supplementation reverses alcoholic steatosis, and reactivation of HNF-4α and PPAR-α by increasing zinc availability and inhibiting oxidative stress are potential mechanisms underlying these beneficial effects of zinc on hepatic lipid homeostasis.
Keywords: alcoholic fatty liver, fatty acid oxidation, VLDL secretion, trace element, zinc finger transcription factor
INTRODUCTION
Alcoholic steatosis (fatty liver) is one of the earliest pathological changes in alcoholic liver disease, and accumulation of lipid in the hepatocyte makes the liver susceptible to inflammatory mediators or other toxic agents, potentially leading to hepatitis and eventually fibrosis.1 Alcoholic steatosis is a reversible stage of liver damage, and reduction of steatosis will likely halt or slow the progression of alcoholic liver disease. While previous studies have shown that alterations in hepatic fatty acid synthesis, fatty acid oxidation, and triglyceride-rich VLDL secretion are involved the pathogenesis of alcoholic steatosis,2–4 the molecular mechanisms underlying the alcohol effects on hepatic lipid homeostasis have not been fully defined.
Zinc deficiency is one of the most consistently observed nutritional/biochemical manifestations in alcoholic liver disease.5 Clinical studies demonstrated that zinc concentrations in both serum and liver were significantly reduced in patients with alcoholic steatosis, hepatitis and cirrhosis.6–8 Animal studies showed that dietary zinc supplementation attenuates alcohol-induced liver injury,9–13 suggesting the importance of zinc in progression of alcoholic liver disease. While the link between zinc and alcoholic steatosis has not been determined, increasing evidence suggest that zinc plays a critical role in regulation of hepatic lipid metabolism. A lower hepatic zinc level was associated with steatosis in leptin receptor deficiency rats.14 Feeding rats with a zinc-deficient diet (a single nutrient deficiency) causes hepatic lipid accumulation in association with dysregulation of a large number of genes involved in lipid metabolism.15–17
Given the fact that dietary zinc deficiency modifies the lipid metabolic gene profile to facilitate hepatic lipid accumulation and given the evidence that alcohol causes hepatic zinc deficiency, we postulated that zinc supplementation would provide beneficial effects on alcoholic steatosis. The present study was undertaken to determine whether or not dietary zinc supplementation can reverse alcohol-induced steatosis in mice previously exposed to alcohol. PPAR-α and HNF-4α are known as the key regulators in hepatic fatty acid β-oxidation and VLDL secretion,18–20 and both PPAR-α and HNF-4α are zinc finger transcription factors. To define potential mechanisms of zinc action, the link between zinc and HNF-4α and PPAR-α was also evaluated.
MATERIALS AND METHODS
Animals and alcohol feeding experiments
Male 129S mice were obtained from Harlan (Indianapolis, IN). All the mice were treated according to the experimental procedures approved by the Institutional Animal Care and Use Committee. The mice were pair fed the Lieber-DeCarli alcohol liquid diet (Bio-Serv, Frenchtown, NJ), containing either ethanol (EtOH) or isocaloric maltose dextrin as control (Ctrl) for 12 weeks to produce liver injury as described previously.11 The mice were then divided into 4 groups, (1) Ctrl, (2) EtOH, (3) Zn and (4) EtOH+Zn, and continually fed for 4 weeks. For zinc supplementation, zinc sulfate was added to the liquid diet at 75 mg elemental zinc/L as described previously.9, 11 At the end of feeding experiment, the mice were anesthetized with Avertin (300 mg/kg) after 4 hrs fasting, and plasma, liver and GAD samples were harvested for assays.
HepG2 cell culture
HepG2 cells obtained from American Type Culture Collection (Rockville, MD) were grown in Dulbecco's modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin (100U/ml) / streptomycin sulfate (100 µg/ml) (Invitrogen, Carlsbad, CA). To evaluate the role of zinc in HNF-4α and PPAR-α function and in hepatic lipid metabolism, zinc deprivation was induced by adding N,N,N',N'-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN) at a final concentration of 2 µM, and cells were cultured for 4 days. Zinc sulfate was added at 25 and 50 µM elemental zinc 2 hrs after TPEN treatment to confirm the specificity of the zinc chelating action of TPEN.
Blood metabolites assay
Plasma zinc concentrations were determined by inductively coupled argon plasma emission spectroscopy. Alcohol concentrations were determined using a Ethanol Assay Kit (BioVison, Mountain View, CA). Alanine transferase (ALT) activities were measured with an Infinity ALT kit (Thermo Scientific, Waltham, MA). Plasma triglyceride and cholesterol concentrations were measured using Infinity Triglyceride Reagent and Infinity Cholesterol Reagent (Thermo Scientific), respectively. Plasma free fatty acids concentrations were determined with a Free Fatty Acid Quantification Kit (BioVision). Blood β-hydroxybutyrate (Ketone) was determined using a CardioCheck Brand Analyzer with PTS PANELS Ketone Test Trips (Polymer Technology System, Indianapolis, IN). Blood glucose was measured using a OneTouch Ultra2 Blood Glucose Meter (Life Scan, Milpitas, CA).
Determination of lipid accumulation and liver injury
Oil red O staining of neutral lipid was performed on cryostat liver sections and chamber slides of HepG2 cells. Quantitative assay of lipids was conducted by measuring the concentrations of triglycerides, cholesterol and free fatty acids in the liver tissue and hepatocytes as described above. To assess liver injury, liver tissue sections were prepared and stained with hematoxylin and eosin.
Hepatic fatty acid β–oxidation assay
Hepatic fatty acid β-oxidation activity was measured as described previously.21 Briefly, 100 mg liver was homogenized in 9 vol cold 10 mM-Hepes, pH 7.2, containing 300 mM-mannitol and 0.1 mM-EGTA. The homogenates were centrifuged at 600g for 10 min, and the resulting supernatants were used. Fatty acid β-oxidation was assayed as palmitoyl-CoA-dependent NAD+ reduction by using an assay mixture containing 50 mM-potassium phosphate, 0.5 mM NAD+, 0.2 mM CoA, 1 mM-dithiothreitol, 0.005 % (w/w) Triton X-100, pH 8.2. The reaction was started by addition of 50 µM palmitoyl-CoA, and the reduction of NAD+ was recorded at 340 nm.
Estimation of hepatic lipid export
Hepatic VLDL triglyceride production was determined with the Triton WR1339 method as described previously.22 In brief, mice after an overnight fast were anesthetized with Avertin (300 mg/kg body weight), and injected via tail vein with Triton WR1339 solution (Tyloxapol, Sigma Chemicals, St. Louis, MO) at 500 mg/kg body weight. Prior to injection and at 90 min after Triton WR1339 injection, 40 µl of blood was drawn by retro-orbital bleeding, and plasma was separated and measured for triglyceride concentration. The hepatic VLDL triglyceride secretion rate was expressed as mg/g liver/h.
Real time RT-PCR assay
The total RNA was isolated and reverse transcribed with the Moloney murine leukemia virus reverse transcriptase and oligo-dT primers. The forward and reverse primers were designed using Primer Express Software and listed in Table 1. The SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA) was used for real-time RT-PCR analysis. The relative differences of gene expression among groups were evaluated using cycle time values. The data were normalized to 18s and expressed as relatively changes, setting the values of control mice as one.
Table 1.
Primer sequences for real-time RT-PCR
| Gene | GeneBank Accession no. |
Sequences (Forward/Reverse) |
|---|---|---|
| Acadl | NM_007381 | GGCTTGCTTGGCATCAACA |
| GAGTACGCTTGCTCTTCCCAAGT | ||
| ApoB | BC100607 | GCGTCTGGGCTCAAGATGAA |
| ACACGTACTTTCGGAGGTGCTT | ||
| Cpta1 | NM_013495 | CCTGGGCATGATTGCAAAG |
| ACGCCACTCACGATGTTCTTC | ||
| HNF4a | NM_008261 | CGGAGCCCCTGCAAAGT |
| CCAGTCTCACAGCCCATTCC | ||
| Mttp | NM_008642 | TCAAGAGAGGCTTGGCTAGCTT |
| GCCTGGTAGGTCACTTTACAATCC | ||
| Ppara | X89577 | CCATACAGGAGAGCAGGGATTT |
| TTACCTACGCTCAGCCCTCTTC | ||
| Adh1 | M22611 | GGCCGCCTTGACACCAT |
| GCACTCCTACGACGACGCTTA | ||
| Adh4 | NM_011996 | GCAGTCCCCTTTGCATTGAA |
| CGTGGCGATTACCTGAATCC | ||
| Adh5 | NM_007410 | GGCAACGTGAAGGTCATGAGA |
| GCTACTCCCACTACCACACTGACA | ||
| Aldh2 | NM_009656 | AGCCAATTACCTGTCCCAAGCT |
| AGACTGGGCCCCAAACACA | ||
| 18s | X56974 | CGAACGTCTGCCCTATCAACTT |
| CCGGAATCGAACCCTGATT |
HNF-4α and PPAR-α DNA binding assay
Nuclear extracts from mouse liver and HepG2 cells were prepared using a kit from Active Motif (Carlsbad, CA). The HNF-4 α and PPAR-α function was assessed by measuring the DNA binding ability with a Trans-AM™ HNF Family Transcription Factor ELISA Kit from Active Motif (Carlsbad, CA) and a PPAR-α Transcription Factor ELISA Kit from Cayman Chemical (Ann Arbor, MI), respectively. Each kit consists of a 96-well plate into which a specific oligonucleotide sequence containing the consensus site of HNF-4α or PPAR-α is immobilized. The HNF-4α or PPAR-α bound to the consensus site is recognized by a rabbit polyclonal antibody against HNF-4α or PPAR-α, followed by incubation with a horseradish peroxidase-conjugated secondary antibody for the colorimetric quantification.
Measurement of hepatic oxidative stress
The activities of superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase were measured using assay kits form Cayman Chemical (Ann Arbor, MI). The lipid peroxidation product, malondialdehyde, was measured as thiobarbituric acid reactive substances (TBARS) with a Cayman TBARS kit.
Immunoblotting analysis
Aliquots containing 30 µg protein were loaded on to a 8–12% SDS-polyacrylamide gel. After electrophoresis, proteins were transferred to polyvinylidene fluoride membrane. The membrane was probed with rabbit or goat polyclonal antibodies against HNF-4α, PPAR-α, ACADM, MTTP, ApoB, Cu/Zn-SOD, GPx (Santa Cruz Biotechnologies, Santa Cruz, CA), ACADL (Proteintech, Chicago, IL) or ACADVL (Novus Biologicals, Littleton, CO). The membrane was then processed with HRP conjugated donkey anti-rabbit IgG or donkey anti-goat IgG (GE Healthcare, Piscataway, NJ). The protein bands were visualized by an ECL detection system (GE Healthcare) and quantified by densitometry analysis.
Statistics
All data are expressed as mean ± SE (n=4–6). The data were analyzed by analysis of variance (ANOVA) and Newman-Keuls’ Multiple-Comparison Test. Differences between groups were considered significant at p<0.05.
RESULTS
Effects of zinc supplementation on body weight, liver weight, GAD weight and plasma metabolites
As shown in Table 2, mice after 16 weeks of alcohol exposure showed a significantly lower body weight and body weight gain compared to the pair-fed controls. Zinc supplementation for the last 4 weeks did not significantly affect the body weight and body weight gain in either alcohol-fed or paired-fed mice. Alcohol exposure significantly increased the liver/body weigh ratio, which was normalized by zinc supplementation. In contrast to an increase in liver/body weight ratio, a significant decrease in GAD/body weight ratio was found in alcohol-fed mice due to a dramatic decrease in GAD weight. Zinc supplementation partially reversed the alcohol-reduced GAD/body weight ratio.
Table 2.
Effects of chronic alcohol exposure and zinc supplementation on body weight, liver weight, EWAT weight and blood metabolites
| Ctrl | EtOH | Zn | EtOH/Zn | |
|---|---|---|---|---|
| Body weight (g) | ||||
| Initial | 26.4 ± 0.5 | 26.3 ± 0.7 | 26.6 ± 0.8 | 26.4 ± 0.3 |
| End | 31.6 ± 0.5 a | 28.2 ± 1.5 b | 33.1 ± 1.4 a | 28.3 ± 0.8 b |
| Gain | 5.2 ± 0.4 a | 1.9 ± 1.0 b | 6.5 ± 0.7 a | 1.8 ± 0.7 b |
| Liver weight (g) | 1.24 ± 0.422 ab | 1.33 ± 0.07 a | 1.36 ± 0.10 a | 1.18 ± 0.73 b |
| Liver/BW ratio | 3.93 ± 0.15 a | 4.72 ± 0.33 b | 4.11 ± 0.27 a | 4.17 ± 0.21 b |
| GAD weight (g) | 1.31 ± 0.17 a | 0.23 ± 0.04 b | 1.34 ± 0.03 a | 0.59 ± 0.13 c |
| GAD/BW ratio | 4.15 ± 0.47 a | 0.84 ± 0.16 b | 4.06 ± 0.22 a | 2.02 ± 0.43 c |
| Blood metabolites | ||||
| Zinc (µg/dL) | 79.3 ± 2.9 a | 67.6 ± 3.6 b | 75.1 ± 3.6 a | 77.4 ± 6.5 a |
| Alcohol (mg/dL) | NA | 30.6 ± 8.2 a | NA | 15.5 ± 3.9 b |
| ALT activity (U/L) | 27.9 ± 5.3 a | 78.0 ± 6.3 b | 26.7 ± 7.6 a | 54.8 ± 7.7 c |
| Triglyceride (mg/dL) | 97 ± 7 a | 174 ± 15 b | 113 ± 16 a | 145 ± 15 c |
| Cholesterol (mg/dL) | 113 ± 13 a | 93 ± 13 b | 116 ± 14 a | 121 ± 12 a |
| Free fatty acids (mg/dL) | 26.6 ± 1.8 | 24.8 ± 4.4 | 24.6 ± 2.9 | 21.5 ± 1.4 |
| β-hydroxybutyrate (mg/dL) | 5.6 ± 1.2 a | 11.8 ± 3.4 b | 8.2 ± 2.7 ab | 17.8 ± 2.6 c |
| Glucose (mg/dL) | 145 ± 16 a | 106 ± 14 b | 153 ± 30 a | 110 ± 15 b |
Mice were pair-fed alcohol or isocaloric maltose dextran for 16 weeks with or without zinc supplementation for the last 4 weeks.
Results are means ± SD (n=4-6) Significant differences (p<0.05) among a, b and c are determined by ANOVA.
Ctrl: Control. EtOH: Alcohol. Zn: Zinc. BW: Body weight. GAD: Gonadal adipose depot.
Alcohol exposure caused a significant decrease in the plasma zinc level, which was normalized by zinc supplementation. The plasma alcohol levels of alcohol-fed mice after zinc supplementation were lower than alcohol without zinc. The plasma ALT activities, an indicator of liver injury, were elevated with alcohol exposure, and this was attenuated by zinc supplementation. Alcohol exposure elevated the plasma triglyceride and β-hydroxybutyrate, and decreased plasma cholesterol, but did not affect the plasma free fatty acids. Zinc supplementation significantly reduced the elevations in plasma triglycerides and normalized plasma cholesterol. On the other hand, zinc supplementation to alcohol-fed mice further increased the blood β-hydroxybutyrate in comparison with alcohol alone. Alcohol exposure also decreased the plasma glucose, and this reduction was not influenced by zinc supplementation.
Zinc supplementation partially attenuated alcohol-induced lipid accumulation and liver injury
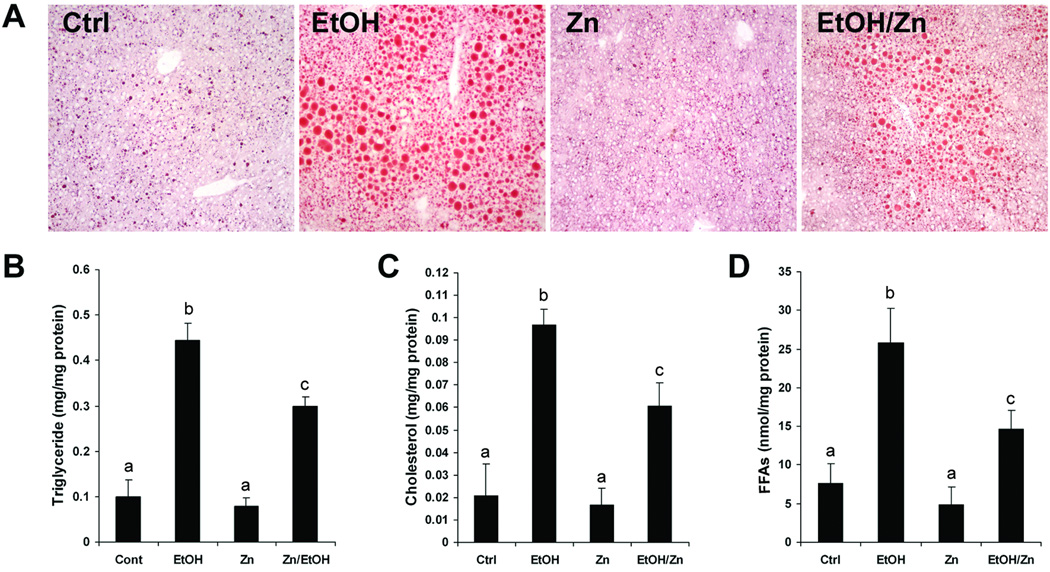
As shown in Figure 1A, Oil Red O stain demonstrated that alcohol exposure caused lipid droplets accumulation in the liver. Zinc supplementation to alcohol-fed mice remarkably reduced the number and the size of lipid droplets in the liver. Quantitative assay of hepatic lipid content showed alcohol exposure caused remarkable increases in hepatic triglyceride (Figure 1B), cholesterol (Figure 1C) and free fatty acids (Figure 1D), which was significantly reduced by zinc supplementation. In accordance with the plasma ALT activity (Table 2), zinc supplementation attenuated alcohol-induced liver pathological changes including neutrophil infiltration and necrotic cell death.
Figure 1.
Hepatic lipid accumulation in mice fed alcohol for 16 weeks with or without zinc supplementation for the last 4 weeks. A. Oil Red O staining of the neutral lipid in the liver. B. Hepatic Triglyceride. C. Hepatic cholesterol. D. Hepatic free fatty acids (FFAs). Results are means ± SD (n=4–6). Significant differences (p<0.05) among a, b and c are determined by ANOVA. Ctrl: control. EtOH: Ethanol. Zn: Zinc.
Zinc supplementation accelerated hepatic fatty acid β-oxidation and lipid export
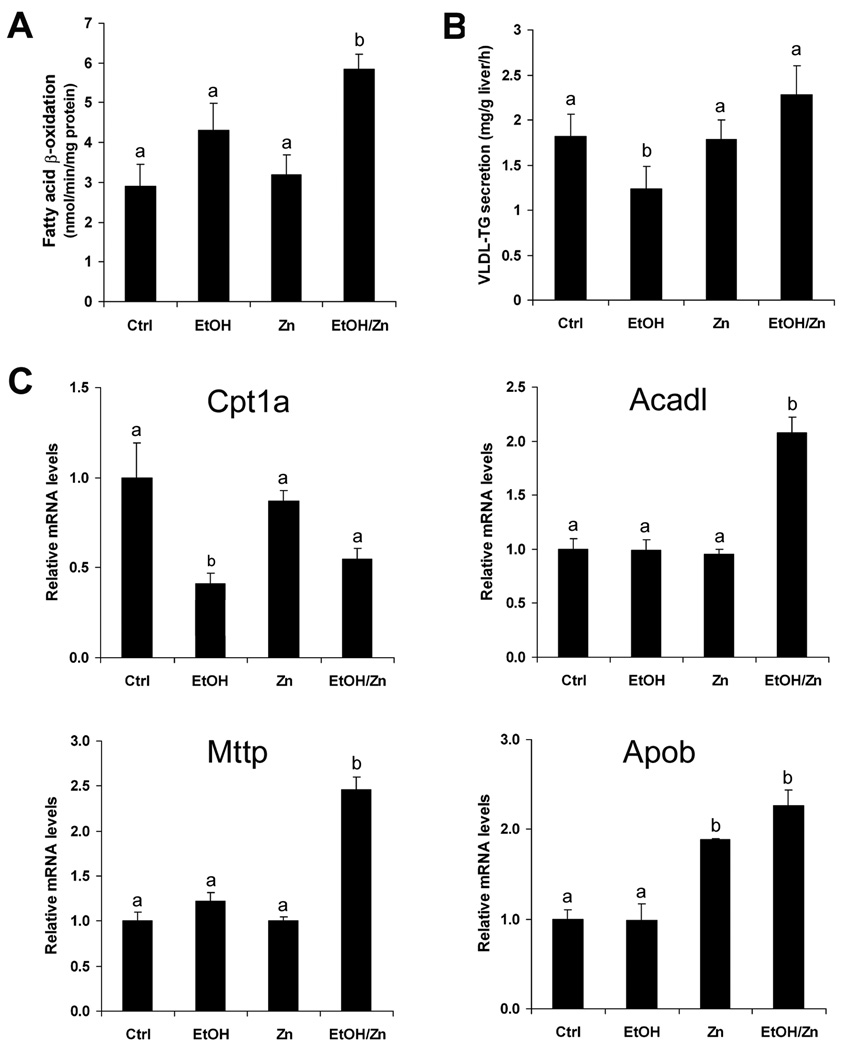
To understand the possible mechanism by which zinc supplementation attenuates alcohol-induced lipid accumulation in the liver, the effects of zinc supplementation on hepatic fatty acid β-oxidation and lipid export were determined. As shown in Figure 2A, hepatic fatty acid β-oxidation was not affected by alcohol exposure, and but accelerated by zinc supplementation. The VLDL export capacity was estimated by measuring VLDL triglyceride secretion to the blood after blocking lipolysis of lipoproteins by Triton WR1339. As shown in Figure 2B, alcohol exposure caused a decrease in VLDL triglyceride secretion, which was normalized by zinc supplementation.
Figure 2.
Hepatic fatty acid oxidation and VLDL secretion in mice fed alcohol for 16 weeks with or without zinc supplementation for the last 4 weeks. A. Hepatic fatty acid β-oxidation. B. Hepatic VLDL-TG (triglyceride) secretion rate measured by Triton WR1339 method. C. Real time RT-PCR assay of genes related to fatty acid β-oxidation and VLDL secretion. Results are means ± SD (n=4–6 in A and B; n=4 in C). Significant differences (p<0.05) among a and b are determined by ANOVA. Ctrl: control. EtOH: Ethanol. Zn: Zinc.
To determine how zinc supplementation modulates lipid metabolism, hepatic genes involved in fatty acid β-oxidation (Cpt1a and Acadl) and VLDL secretion (Mttp and Apob) were measured. As shown in Figure 2C, the Cpt1a mRNA was significantly decreased by alcohol exposure, and zinc supplementation did not alter the alcohol effect. The mRNA levels of Acadl, Mttp and ApoB were not affected by alcohol exposure, but were significantly increased by zinc supplementation.
Zinc supplementation reversed alcohol-inactivated hepatic HNF-4α and PPAR-α
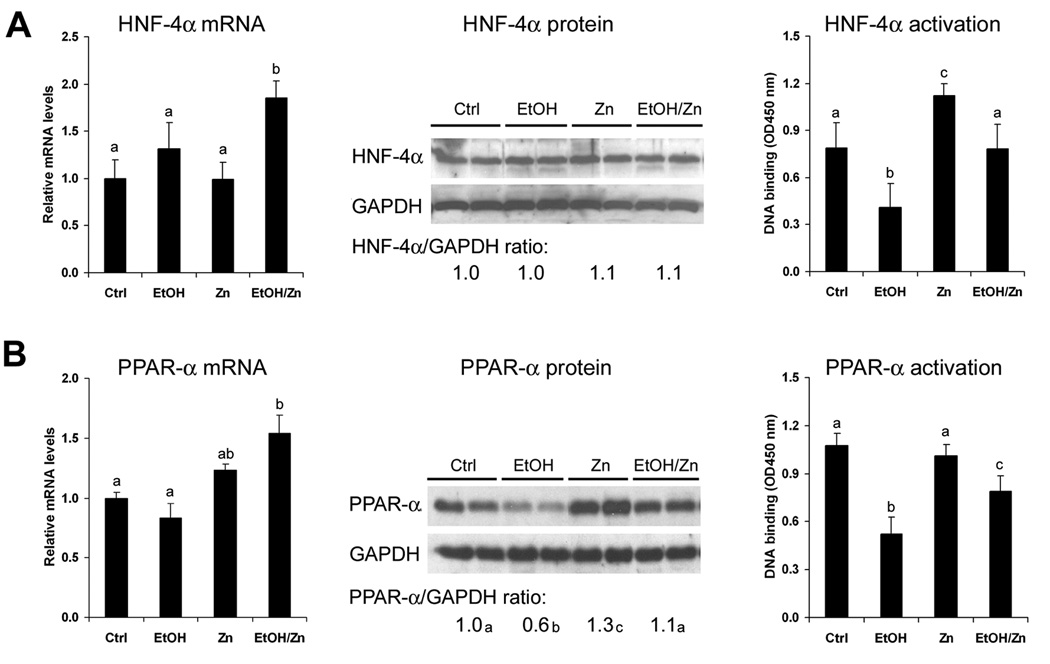
To determine if zinc action is related to HNF-4α and PPAR-α, the mRNA, protein and DNA binding activity of HNF-4α and PPAR-α were measured. As shown in Figure 3, the mRNA levels of HNF-4α and PPAR-α were not affected by alcohol exposure, but significantly increased by zinc supplementation. Alcohol exposure did not affect the HNF-4α protein but decreased the PPAR-α protein, and zinc supplementation normalized alcohol-reduced PPAR-α protein level. The DNA binding activities of HNF-4α and PPAR-α were significantly diminished by alcohol exposure, which was partially recovered by zinc supplementation.
Figure 3.
Hepatic HNF-4α and PPAR-α status in mice fed alcohol for 16 weeks with or without zinc supplementation for the last 4 weeks. Protein and mRNA levels and activation of HNF-4α (A) and PPAR-α (B) measured by real time RT-PCR, immunoblotting and DNA binding ELISA, respectively. The immunoblotting bands were quantified by densitometry analysis and the ratio to GAPDH was calculated by setting the value of controls as one. Results are means ± SD (n=4-6). Significant differences (p<0.05) among a, b and c are determined by ANOVA. Ctrl: control. EtOH: Ethanol. Zn: Zinc.
Zinc supplementation enhanced enzymes involved in antioxidant defense and alcohol metabolism
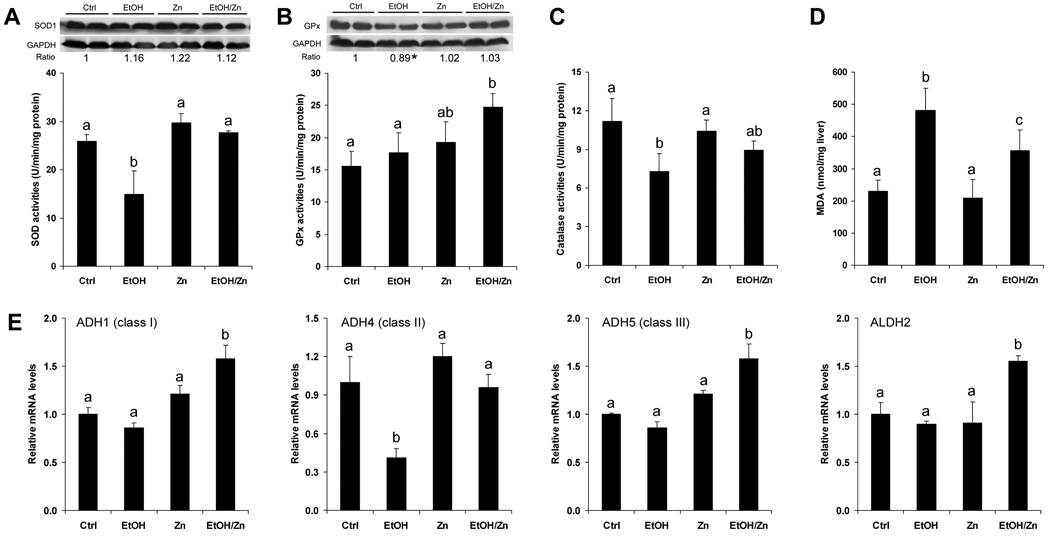
Oxidative stress has been shown to inactivate zinc finger transcription factors by mobilizing zinc. To determine if inhibition of oxidative stress is associated with the zinc action, the major cellular antioxidant enzymes, Cu/Zn-SOD, GPx and catalase, and the lipid peroxidation product, malondialdehyde, were measured. As shown in Figure 4A, no differences in Cu/Zn-SOD protein level were found among the treatments. However, the SOD activity was significantly reduced by alcohol exposure, which was normalized by zinc supplementation. Zinc supplementation did not affect the protein level of GPx, but increased the GPx activity (Fig. 4B). Alcohol exposure decreased the catalase activity, which was attenuated by zinc supplementation (Fig. 4C). As an indicator of oxidative stress, the lipid peroxidation product, malondialdehyde, was significantly increased by alcohol exposure, and this increase was significantly inhibited by zinc supplementation (Figure 4D). To determine if modulation of alcohol metabolism is involved in zinc action, the expression of hepatic alcohol dehydrogenase class I (Adh1), II (Adh4), and III (Adh5), and mitochondrial aldehyde dehydrogenase (Aldh2) were measured. As shown in Figure 4E, zinc supplementation upregulated expression of Adh1, adh5 and Aldh2, and normalized expression of Adh4.
Figure 4.
Hepatic antioxidant capacity and alcohol metabolic genes in mice fed alcohol for 16 weeks with or without zinc supplementation for the last 4 weeks. Protein and/or activities of Cu/Zn-SOD (A), GPx (B) and catalase (C). The bands in A and B were quantified by densitometry analysis and the ratio to GAPDH was calculated by setting the value of controls as one. * Significantly different from other groups. D. MDA concentrations. E. Real time RT-PCR assay of alcohol metabolic genes. Results are means ± SD (n=4–6). Significant differences (p<0.05) among a, b and c are determined by ANOVA. Ctrl: control. EtOH: Ethanol. Zn: Zinc.
In vitro zinc deprivation impaired HNF-4α and PPAR-α function and lipid homeostasis in HepG2 cells
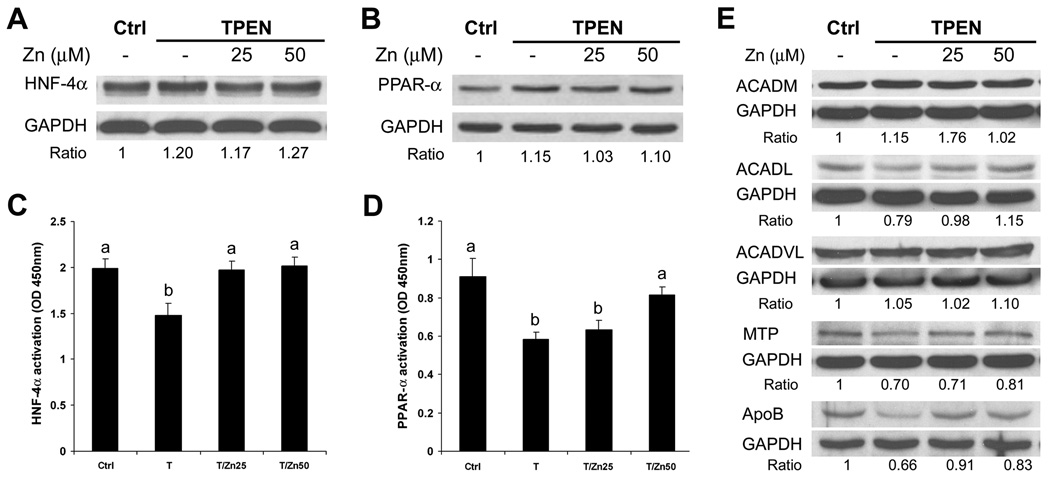
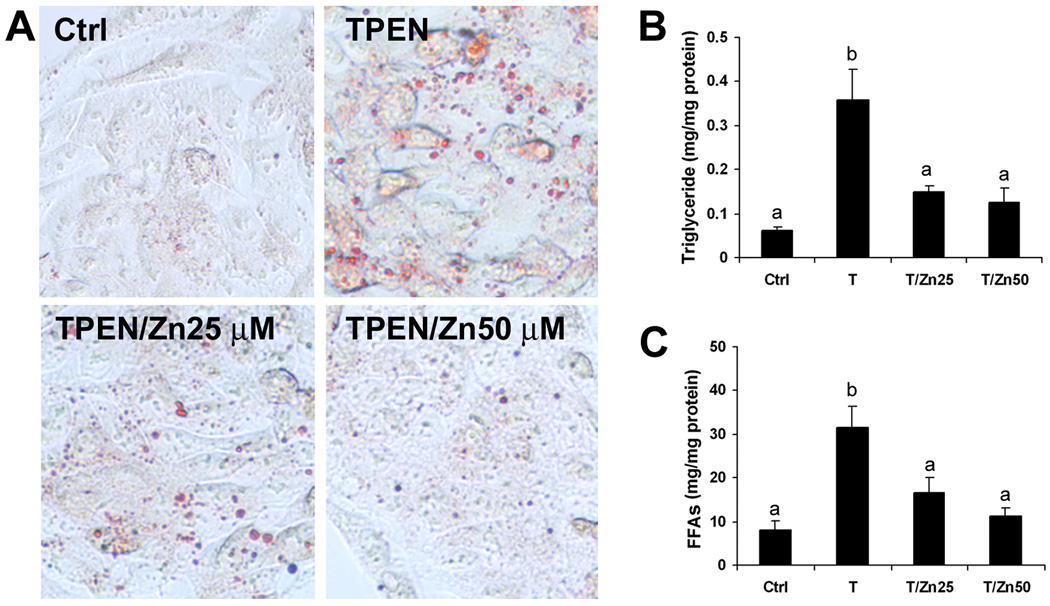
The effects of zinc deprivation by TPEN on HNF-4α and PPAR-α and their target proteins as well as cellular lipid accumulation were determined in HepG2 cell culture. Immunoblotting analysis showed that zinc deprivation with or without zinc supplementation did not significantly affect the protein levels of HNF-α (Figure 5A) anḍ PPAR-α (Figure 5B). However, the DNA binding activities of HNF-4α (Figure 5C) anḍ PPAR-α (Figure 5D) were significantly decreased by zinc deprivation. HNF-4α and PPAR-α regulatory proteins related to fatty acids β-oxidation (ACADM, ACADL, ACADVL) and VLDL secretion (MTTP, ApoB) were further determined by immunoblotting analysis, and zinc deprivation reduced the protein levels of ACADL, MTP and ApoB (Figure 5E). Oil red O stain showed that zinc deprivation caused lipid droplets accumulation in the HepG2 cells (Figure 6A). Zinc deprivation significantly increased the cellular concentrations of triglycerides (Figure 6B) and free fatty acids (Figure 6C). Zinc supplementation reversed of all the effects of TPEN, indicating that TPEN specifically induces zinc deprivation.
Figure 5.
Effect of zinc deprivation on HNF-4α and PPAR-α function in HepG2 cell culture. HepG2 cells were treated with TPEN at 2 µM with or without zinc supplementation at 25 or 50 µM for 4 days. Immunoblotting analysis of HNF-4α (A) and PPAR-α (B) protein. DNA binding activity of HNF-4α (C) and PPAR-4α (D). E. Immunoblotting of HNF-4α and PPAR-α target proteins. The bands were quantified by densitometry analysis and the ratio to GAPDH was calculated by setting the value of controls as one. Results in C and D are means ± SD (n=4). Significant differences (p<0.05) among a and b are determined by ANOVA. Ctrl: control. EtOH: Ethanol. Zn: Zinc.
Figure 6.
Effect of zinc deprivation on cellular lipid accumulation in HepG2 cell culture. HepG2 cells were treated with TPEN at 2 µM with or zinc supplementation at 25 or 50 µM for 4 days. A. Oil Red O staining of neutral lipid. B. Triglyceride concentrations. C. Free fatty acids (FFAs) concentrations. Results are means ± SD (n=4). Significant differences (p<0.05) between a and b are determined by ANOVA. Ctrl: control. T: TPEN.
DISCUSSION
The present study demonstrates that zinc supplementation reverses alcohol-induced steatosis in part by stimulating fatty acid oxidation and VLDL secretion. Reactivation of HNF-4α and PPAR-α represent one mechanism for the observed beneficial effects of zinc on fatty acid oxidation and VLDL secretion. Inhibition of oxidative stress by zinc is associated with reactivation of these zinc finger transcription factors. The antioxidant action of zinc most likely resulted from the enhancement of antioxidant enzymes. Furthermore, accelerated alcohol clearance by zinc may lead to reduction of hepatic reactive alcohol metabolites which can release zinc from zinc proteins. Cell culture studies further validated the link between zinc and HNF-4α and PPAR-α in lipid metabolism. Zinc deprivation significantly suppressed the DNA binding activities of HNF-4α and PPAR-α, and led to lipid accumulation in HepG2 cells. These results thus indicate that zinc deficiency may play a critical role in the pathogenesis of alcohol-induced steatosis by inactivating HNF-4α and PPAR-α. Furthermore, zinc supplementation normalized alcohol-elevated plasma triglycerides and partially reversed alcohol-reduced EWAT, suggesting that reversal of adipose tissue lipolysis may also be involved in the zinc beneficial effects on alcohol-induced steatosis.
Zinc finger transcription factors represent one of the largest classes of DNA-binding proteins, and elimination of zinc coordination by reactive oxygen species (ROS) or iron from the zinc finger can disassemble the secondary structure of these proteins, leading to defective DNA binding and decreased transcription of target genes.23–26 The present study showed that zinc supplementation reactivates the suppressed DNA binding activity of both HNF-4α and PPAR-α caused by alcohol exposure. Zinc is known to have antioxidant properties.27 Indeed, the present study showed that zinc supplementation preserves the major hepatic antioxidant enzymes, SOD, GPx and catalase, which may account for zinc inhibition of alcohol-induced ROS and lipid peroxidation products accumulation in the liver.11 In addition, zinc supplementation also normalizes or upregulated alcohol metabolic enzymes, thereby enhancing alcohol clearance. Because acetaldehyde, the major toxic metabolite of alcohol metabolism, has been shown to release zinc from zinc proteins,28 accelerated alcohol clearance may also contribute to zinc restoration of alcohol-inactivated PPAR-α and HNF-4α. While dietary zinc deficiency has been shown to interfere with lipid metabolism,15–17 the present study demonstrated that dysfunction of PPAR-α and HNF-4α may account for the deleterious effects of zinc deficiency on lipid metabolism.
PPAR-α and HNF-4α belong to nuclear hormone superfamily, and play an important role in regulation of hepatic lipid homeostasis, in particular fatty acid β-oxidation and VLDL secretion, respectively. PPAR-α is one of the most well recognized transcription factors in mechanistic studies on the pathogenesis of alcoholic steatosis.3,19,20 The effects of chronic alcohol exposure on hepatic PPAR-α mRNA are controversial; some studies reported a decrease,29 while others showed no change.30,31 Chronic alcohol exposure in mice has been reported to suppress the DNA binding activity of PPAR-α, without affecting the protein level.31 Treatment with the PPAR-α agonist, WY14,643, upregulated PPAR-α mRNA and increased the PPAR-α protein.31 Importantly, WY14,643 treatment to alcohol-fed mice increased the DNA binding activity in association with upregulation of many PPAR-α target genes; in particular, the fatty acid β-oxidation-related genes. Treatment with the PPAR-α agonist, clofibrate, in chronic alcohol-fed rats also remarkably reduced alcoholic steatosis.32 These studies clearly demonstrated that dysfunction of PPAR-α is an etiologic factor in the development of alcoholic steatosis. The results in the present study showed that reactivation of PPAR-α is associated with zinc effects on alcoholic steatosis. The cell culture study further showed that zinc coordination is required for the DNA binding activity of PPAR-α; this may explain how alcohol reduces PPAR-α DNA binding activity without affecting its protein level, an unsolved question raised from previous studies.31
HNF-4α is a master regulator of hepatic gene expression,32 and it has been shown to bind to the reporters of more than 1,200 genes involved in most aspects of hepatocyte function.33 Liver-specific conditional knockout of HNF-4α in adult mice led to severe steatosis in association with disruption of expression of genes involved in VLDL secretion.18 Chronic alcohol exposure has been shown to inhibit VLDL synthesis and secretion rate.34–36 The present study demonstrated a link between zinc and HNF-4α and VLDL secretion. Because alcohol exposure decreased the DNA binding activity of HNF-4α without affecting its protein level, the alcohol effect on HNF-4α is likely at the post-transcriptional level. Zinc supplementation upregulated HNF-4α mRNA and restored DNA binding activity of HNF-4α but did not affect the HNF-4α protein. These results suggest that alcohol exposure inactivates HNF-4α, at least partially through mobilizing zinc from HNF-4α, and zinc may regulate HNF-4α at both transcriptional and post-transcriptional levels. Importantly, we also found that reactivation of HNF-4α by zinc supplementation is accompanied by upregulation of genes involved in both VLDL secretion (Mttp, Apob) and fatty acid β-oxidation (Acadl).
Previous studies have shown that alcohol exposure not only causes lipid accumulation in the liver, but also increases blood triglyceride and free fatty acids.31,37 Alcohol exposure also decreases the EWAT mass by increasing triglyceride degradation in adipose tissue, leading to increased release of free fatty acids into the circulation.38 Therefore, dysregulation of lipid homeostasis in both liver and adipose tissue may be involved in the development of alcoholic steatosis. Indeed, the present study demonstrated that reversal of alcoholic steatosis is associated with zinc actions in both the liver and adipose tissue. First, zinc supplementation increased hepatic fatty acid β-oxidation. Second, zinc supplementation increased hepatic VLDL secretion but normalized alcohol-elevated plasma triglyceride. Third, zinc supplementation partially reversed alcohol-reduced GAD weight. These results indicate that reduction by zinc of the alcohol-induced hepatic lipid accumulation may involve at least two mechanisms: (1) accelerating hepatic lipid utilization and export; and (2) promoting lipid deposition and/or inhibiting lipolysis in the adipose tissue.
In conclusion, the present study demonstrated that dietary zinc supplementation reverses alcoholic steatosis in mice previously exposed to alcohol by enhancing fatty acid β-oxidation and VLDL secretion. Alcohol exposure reduces the DNA binding activity of HNF-4α and PPAR-α at least partially through mobilizing zinc from these zinc finger transcription factors. Reactivation of HNF-4α and PPAR-α is likely the most important molecular mechanism underlying the zinc action on lipid homeostasis. Furthermore, reversal of alcohol-reduced white adipose tissue mass may also directly related to zinc reduction of hepatic lipid accumulation. These results suggest that zinc acts though multiple pathways to provide therapeutic efficacy in alcoholic steatosis.
ACKNOWLEDGEMENTS
We thank Xinguo Sun for excellent technical assistance and Marion McClain for critical review of this manuscript. YJK and CJM are Distinguished University Scholars of the University of Louisville. X. Kang is an awardee of the Postdoctoral Research Fellowship from the American Liver Foundation. W. Zhong (D.V.M.) is supported by the State Scholarship Fund of China Scholarship Council.
Financial Support
This research was supported in part by the National Institutes of Health, Office of Dietary Supplements grants, and the Veterans Administration.
List of Abbreviations
- VLDL
very low density lipoproteins
- HNF-4α
hepatocyte nuclear factor-4α
- PPAR-α
peroxisome proliferators activated receptor-α
- GAD
gonadal adipose depot
- TPEN
N,N,N',N'-tetrakis (2-pyridylmethyl) ethylenediamine
- Cpt1a
carnitine palmitoyl-CoA transferase-1α
- Acadl
long-chain acyl-coenzyme A dehydrogenase
- MTTP
microsomal triglyceride transfer protein
- ApoB
apoliporotein B
- ACADM
medium-chain acyl-coenzyme A dehydrogenase
- ACADVL
very long-chain acyl-coenzyme A dehydrogenase
Contributor Information
Xinqin Kang, Email: xqkang01@louisville.edu.
Wei Zhong, Email: zhongweicau@hotmail.com.
Jie Liu, Email: Jie@liuonline.com.
Zhenyuan Song, Email: zyong2008@uic.edu.
Craig J. McClain, Email: craig.mcclain@louisville.edu.
Y. James Kang, Email: yjkang01@louisville.edu.
Zhanxiang Zhou, Email: z0zhou01@louisville.edu.
REFERENCES
- 1.Purohit V, Russo D, Coates PM. Role of fatty liver, dietary fatty acid supplements, and obesity in the progression of alcoholic liver disease: introduction and summary of the symposium. Alcohol. 2004;34:3–8. doi: 10.1016/j.alcohol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Lakshman MR. Some novel insights into the pathogenesis of alcoholic steatosis. Alcohol. 2004;34:45–48. doi: 10.1016/j.alcohol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early alcohol-induced liver injury. Annu Rev Nutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 4.Crabb DW, Liangpunsakul S. Alcohol and lipid metabolism. J Gastroenterol Hepatol. 2006;21:S56–S60. doi: 10.1111/j.1440-1746.2006.04582.x. [DOI] [PubMed] [Google Scholar]
- 5.McClain CJ, Antonow DR, Cohen DA, Shedlofsky S. Zinc metabolism in alcoholic liver disease. Alcohol Clin Exp Res. 1986;10:582–589. doi: 10.1111/j.1530-0277.1986.tb05149.x. [DOI] [PubMed] [Google Scholar]
- 6.Kiilerich S, Dietrichson O, Loud FB, Naestoft J, Christoffersen P, Juhl E, Kjems G, Christiansen C. Zinc depletion in alcoholic liver diseases. Scand J Gastroenterol. 1980;15:363–367. doi: 10.3109/00365528009181484. [DOI] [PubMed] [Google Scholar]
- 7.Bode JC, Hanisch P, Henning H, Koenig W, Richter FW, Bode C. Hepatic zinc content in patients with various stages of alcoholic liver disease and in patients with chronic active and chronic persistent hepatitis. Hepatology. 1988;8:1605–1609. doi: 10.1002/hep.1840080622. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Moreno F, González-Reimers E, Santolaria-Fernández F, Galindo-Martín L, Hernandez-Torres O, Batista-López N, Molina-Perez M. Zinc, copper, manganese, and iron in chronic alcoholic liver disease. Alcohol. 1997;14:39–44. doi: 10.1016/s0741-8329(96)00103-6. [DOI] [PubMed] [Google Scholar]
- 9.Giménez A, Caballería J, Parés A, Alié S, Deulofeu R, Andreu H, Rodés J. Influence of dietary zinc on hepatic collagen and prolyl hydroxylase activity in alcoholic rats. Hepatology. 1992;16:815–819. doi: 10.1002/hep.1840160331. [DOI] [PubMed] [Google Scholar]
- 10.Cabré M, Folch J, Giménez A, Matas C, Parés A, Caballería J, Paternain JL, Rodés J, Joven J, Camps J. Influence of zinc intake on hepatic lipid peroxidation and metallothioneins in alcoholic rats: relationship to collagen synthesis. Int J Vitam Nutr Res. 1995;65:45–50. [PubMed] [Google Scholar]
- 11.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681–1690. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang X, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation enhances hepatic regeneration by preserving hepatocyte nuclear factor-ạ in mice subjected to a long-term alcohol administration. Am J Pathol. 2008;172:916–925. doi: 10.2353/ajpath.2008.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, Liu J, Song Z, McClain CJ, Kang YJ. Zinc supplementation inhibits hepatic apoptosis in mice subjected to a long-term alcohol exposure. Exp Biol Med. 2008;233:540–548. doi: 10.3181/0710-RM-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomita K, Azuma T, Kitamura N, Tamiya G, Ando S, Nagata H, Kato S, Inokuchi S, Nishimura T, Ishii H, Hibi T. Leptin deficiency enhances sensitivity of rats to alcoholic steatohepatitis through suppression of metallothionein. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1078–G1085. doi: 10.1152/ajpgi.00107.2004. [DOI] [PubMed] [Google Scholar]
- 15.tom Dieck H, Döring F, Fuchs D, Roth HP, Daniel H. Changes in rat hepatic gene expression in response to zinc deficiency as assessed by DNA arrays. J Nutr. 2003;133:1004–1010. doi: 10.1093/jn/133.4.1004. [DOI] [PubMed] [Google Scholar]
- 16.tom Dieck H, Döring F, Roth HP, Daniel H. Transcriptome and proteome analysis identifies the pathways that increase hepatic lipid accumulation in zinc-deficient rats. J Nutr. 2005;135:199–205. doi: 10.1093/jn/135.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI. Dietary zinc deficiency inducedchanges in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology. 2002;175:223–234. doi: 10.1016/s0300-483x(02)00049-5. [DOI] [PubMed] [Google Scholar]
- 18.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34:35–38. doi: 10.1016/j.alcohol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E10–E16. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 22.Uwe J.F. Tietge, Ahmed Bakillah, Cyrille Maugeais, Kazuhisa Tsukamoto, Mahmood Hussain, Daniel J Rader. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. Journal of Lipid Research. 1999;40:2134–2139. [PubMed] [Google Scholar]
- 23.Webster KA, Prentice H, Bishopric NH. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid Redox Signal. 2001;3:535–548. doi: 10.1089/15230860152542916. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox DE, Schenk AD, Feldman BM, Xu Y. Oxidation of zinc-binding cysteine residues in transcription factor proteins. Antioxid Redox Signal. 2001;3:549–564. doi: 10.1089/15230860152542925. [DOI] [PubMed] [Google Scholar]
- 25.Conte D, Narindrasorasak S, Sarkar B. In vivo and in vitro iron-replaced zinc finger generates free radicals and causes DNA damage. J Biol Chem. 1996;271:5125–5130. doi: 10.1074/jbc.271.9.5125. [DOI] [PubMed] [Google Scholar]
- 26.Sarkar B. Metal replacement in DNA-binding zinc finger proteins and its relevance to mutagenicity and carcinogenicity through free radical generation. Nutrition. 1995;11:646S–649S. [PubMed] [Google Scholar]
- 27.Powell SR. The antioxidant properties of zinc. J Nutr. 2000;130:1447S–1454S. doi: 10.1093/jn/130.5.1447S. [DOI] [PubMed] [Google Scholar]
- 28.Hao Q, Maret W. Aldehydes release zinc from proteins. A pathway from oxidative stress/lipid peroxidation to cellular functions of zinc. FEBS J. 2006 Sep;273(18):4300–4310. doi: 10.1111/j.1742-4658.2006.05428.x. [DOI] [PubMed] [Google Scholar]
- 29.Wan YJ, Morimoto M, Thurman RG, Bojes HK, French SW. Expression of the peroxisome proliferator-activated receptor gene is decreased in experimental alcoholic liver disease. Life Sci. 1995;56:307–317. doi: 10.1016/0024-3205(94)00953-8. [DOI] [PubMed] [Google Scholar]
- 30.Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPAR alpha)-regulated genes and is ameliorated by PPAR alpha activation. J Pharmacol Exp Ther. 2004;310:417–424. doi: 10.1124/jpet.103.064717. [DOI] [PubMed] [Google Scholar]
- 31.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor α (PPARα) agonist treatment reverses PPARα dysfunction and abnormalities in hepatic lipid metabolism in alcohol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 32.Watt AJ, Garrison WD, Duncan SA. HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 2003;37:1249–1253. doi: 10.1053/jhep.2003.50273. [DOI] [PubMed] [Google Scholar]
- 33.Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakshmanan MR, Felver ME, Veech RL. Alcohol and very low density lipoprotein synthesis and secretion by isolated hepatocytes. Alcohol Clin Exp Res. 1980;4:361–365. doi: 10.1111/j.1530-0277.1980.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesan S, Ward RJ, Peters TJ. Effect of chronic alcohol feeding on the hepatic secretion of very-low-density lipoproteins. Biochim Biophys Acta. 1988;960:61–66. doi: 10.1016/0005-2760(88)90009-4. [DOI] [PubMed] [Google Scholar]
- 36.Guzman M, Castro J. Zonal heterogeneity of the effects of chronic alcohol feeding on hepatic fatty acid metabolism. Hepatology. 1990;12:1098–1105. doi: 10.1002/hep.1840120504. [DOI] [PubMed] [Google Scholar]
- 37.Tomita K, Azuma T, Kitamura N, Nishida J, Tamiya G, Oka A, Inokuchi S, Nishimura T, Suematsu M, Ishii H. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology. 2004;126:873–885. doi: 10.1053/j.gastro.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic alcohol and triglyceride turnover in white adipose tissue in rats: inhibition of the antilipolytic action of insulin after chronic alcohol contributes to increased triglyceride degradation. J Biol Chem. 2007;282:28465–28473. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]