Abstract
Background and Aims
Repair responses define the ultimate outcomes of liver disease. This study evaluated the hypothesis that fibrogenic repair in nonalcoholic fatty liver disease (NAFLD) is mediated by Hedgehog (Hh) pathway activation and consequent induction of epithelial-to-mesenchymal transitions (EMT) in ductular-type progenitors.
Methods
Immature ductular cells were exposed to sonic hedgehog (Shh) in the presence or absence of the Hh inhibitor, cyclopamine, to determine if Hh-pathway activation directly modulates EMT in liver progenitors. Potential biological correlates of progenitor-cell EMT were assessed using mice fed methionine-choline deficient + ethionine (MCDE) diets with or without cyclopamine. The effects of increased Hh-signaling on EMT and fibrogenic repair during diet-induced NAFLD were also compared in wild-type (WT) and Patched haplo-insufficient (Ptc +/-) mice. Finally, evidence of Hh pathway activation and EMT was examined in liver sections from patients with NAFLD.
Results
In cultured progenitors, sonic hedgehog repressed expression of epithelial genes and EMT inhibitors, but induced genes that are expressed by myofibroblasts. Cyclopamine reversed these effects. In mouse NAFLD models, Hh pathway activation, EMT, expansion of myofibroblastic populations, and liver fibrosis occurred. Cyclopamine inhibited Hh pathway activation and induction of EMT. Ptc +/- mice, which have an over-active Hh pathway, exhibited sustained over-induction of Hh target genes and more EMT, myofibroblast accumulation, and fibrosis than WT mice. Numbers of Shh-producing cells and Hh-responsive ductular cells that expressed EMT markers increased in parallel with liver fibrosis in patients with NAFLD.
Conclusions
Hh-mediated EMT in ductular cells contributes to the pathogenesis of cirrhosis in NAFLD.
Keywords: epithelial-mesenchymal transition, fibrosis, hedgehog, non-alcoholic steatohepatitis
Introduction
The adult liver has tremendous regenerative capacity. After an acute noxious insult, dead hepatocytes are generally replaced within days and hepatic architecture and function are fully restored within weeks 1. Recovery from chronic liver injury tends to be more variable, however. Sometimes regeneration keeps pace with hepatocyte death, preserving liver structure and function despite ongoing injury. Other times, the rate of hepatocyte death outstrips repair capacity, and dead cells accumulate. The latter situation is often accompanied by hepatic accumulation of various types of cells that are rare in healthy adult livers, including liver progenitors and myofibroblasts, as well as deposition of excessive collagen matrix 1, 2. This process begins near canals of Herring, which house ductular-type progenitor cells along the edges of portal tracts. In individuals who are destined to become cirrhotic, fibrous septae that contain large numbers of immature ductular cells and fibroblastic cells extend out into lobules, ultimately forming bridges that separate islands of parenchyma 2, 3.
The cellular composition and migratory features of the fibroductular response to chronic liver injury suggested to us that this process may involve epithelial-to-mesenchymal transition (EMT) of immature ductular cells, thereby providing a mechanism to move these progenitors out of their normal niche and into the hepatic parenchyma to replace dead hepatocytes. We further reasoned that this repair process might be regulated by the Hedgehog (Hh) pathway because there is recent evidence that paracrine Hh signaling between myofibroblasts and ductular-type progenitors promotes EMT in immature ductular cells 4. To evaluate these hypotheses, we studied the direct effects of Sonic hedgehog (Shh) ligand on cultures of ductular-type progenitor cells, examined the consequences of Hh pathway activation in murine models of nonalcoholic fatty liver disease (NAFLD), and correlated levels of Hh pathway activity with severity of liver injury and fibrosis in patients with NAFLD. NAFLD was selected for scrutiny because it is one of the most common types of chronic liver disease in humans 5, and there is already strong evidence that increased hepatocyte apoptosis 6, 7 and the resultant fibroductular reaction3 play important roles in the evolution of cirrhosis in this disorder, but the fundamental mechanisms that regulate the latter process are not well understood.
Materials and Methods
Animals
C57BL/6 Patched-deficient (Ptc +/-) mice were obtained from P.A. Beachy (Johns Hopkins University, Baltimore, MD) and wild type mice (WT) were obtained from Jackson Laboratories (Bar Harbor, ME). Ptc +/- mice have only one copy of patched, a Hh pathway repressor. Therefore, these mice are unable to silence Hh signaling and exhibit excessive Hh pathway activity. WT and Ptc +/- mice were fed the methionine-choline deficient (MCD) diet (n = 4/group) to induce nonalcoholic steatohepatitis (NASH) and liver fibrosis, or control chow (n=4/group) for 4 weeks. All mice were then sacrificed to obtain serum and liver samples.
To assess progenitor-dependent liver regeneration during diet-induced NASH, mice (n = 4) were fed methionine choline-deficient diet supplemented with 0.15% ethionine (MCDE) 8. Mice were sacrificed after being fed MCDE diets for 1 week or 3 weeks (n=4/group) and results were compared to chow fed controls (n = 4). Another group of mice (n=4) were fed MCDE for 3 weeks, and then switched back to normal diet for a further 3 weeks to allow for liver recovery, before sacrifice.
To determine if inhibition of the Hh-pathway alters outcome, additional mice (n=12) were fed control chow (n=4 mice) or MCDE diet (n = 8 mice), with or without cyclopamine i.p., for 1 week (n = 4/group). Cyclopamine (Toronto Research Chemicals Inc., Toronto, Canada), an inhibitor of Hh-signaling, was administered at a dose of 0.6mg/mouse/day (1ml volume), according to an established in vivo protocol 9, 10. The first injection of cyclopamine was given 24 hours prior to commencing MCDE-diet.
Human study
Formalin-fixed, paraffin-embedded liver sections from subjects with biopsy-proven non-alcoholic fatty liver (NAFL) (n=5), non-alcoholic steatohepatitis (NASH) (n=5), NASH-related cirrhosis (n=6) were obtained from the Duke University, Department of Pathology. Liver sections were also obtained from subjects with non-NAFLD diseases (alcoholic liver disease, ALD and primary biliary cirrhosis, PBC), to assess if a similar pattern of protein expression would be seen across a spectrum of liver disease. Control liver tissues were obtained from the Duke University School of Medicine Tissue Bank Shared Resource and studied in accordance with NIH and Institutional guidelines for human subject research.
Histopathologic Analysis
Serial sections were stained with H&E. NAFLD severity was assessed using criteria described by Brunt et al 11. (Supplemental Materials and Methods).
Immunohistochemistry
Formalin-fixed, paraffin-embedded livers were prepared for immunohistochemistry as described 12, 13. A detailed protocol and antibodies used are listed in Supplemental Materials and Methods.
Molecular techniques
Real time RT-PCR and Western-immunoblot were performed using established protocols 14; details in Supplemental Materials and Methods and Supplementary Table 1.
Cell culture experiments
The immortalized, but non-transformed, murine immature cholangiocyte cell line (603B) was maintained in 6-well, cell-culture cluster (Costar 3516, Corning Incorporated) in standard culture media as previously described 4, 15, 16. In order to evaluate the effect of exogenous Sonic Hedgehog on cholangiocytes, 603B cells were serum starved overnight, and then treated with recombinant Sonic hedgehog (0, 100, 1000 ng/ml) (StemCell Tech Inc, Canada) for an additional 24 hours. In separate experiments, 603B cells were cultured in Shh-containing medium (100 ng/ml) and treated with either cyclopamine (Toronto Research Chemicals Inc., Toronto, Canada), an inhibitor of Hh-signaling, at a concentration of 3uM 17, 18 or tomatidine 3uM (Calbiochem, San Diego, CA), a catalytically inactive analog of cyclopamine for 24 h. All experiments were performed in duplicate. Total RNA and protein were harvested and analyzed by QRTPCR and immunoblotting, respectively.
To validate changes observed in the 603B cells, some of the experiments were repeated using normal rat cholangiocyte line (NRC) 19.
Statistical Analysis
Groups were compared with baseline (control or vehicle) or between individual treatment groups. Results expressed as mean ± S.E.M (unless stated otherwise); analyses were performed using Student’s t-test or ANOVA (for multiple group comparisons) using PROC GLM in SAS 9.1. Significance of pair-wise comparison was established using the least squares means. P values were adjusted by Tukey’s multiple comparison procedure; significance was accepted at the 5% level. * p < 0.05; ** p < 0.005
Results
Exogenous Sonic hedgehog (Shh) promotes EMT in liver progenitors
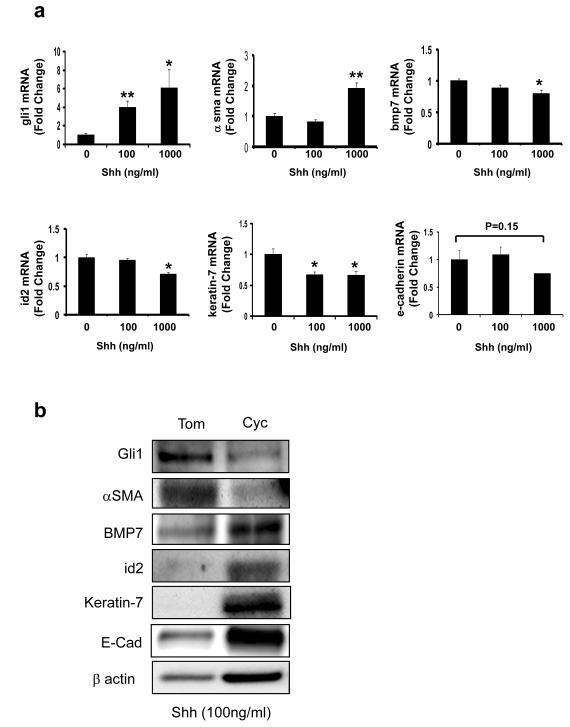
In order to examine the direct effects of Hh pathway activation on progenitor cell EMT, immature ductular cells (603B cells) were treated with N-terminal Shh ligand (0-1000 ng/ml) and RNA was analyzed by QRT-PCR (Fig 1a). Experiments were repeated by treating cells with Shh (100 ng/ml) with or without cyclopamine 3uM (a specific Hh pathway antagonist) or tomatidine 3uM (an inactive cyclopamine analog), and cellular RNA and protein were obtained for analysis (Fig 1b and Supplemental Fig 1). As expected, Shh increased expression of the Hh target gene, gli1. Expression of α-smooth-muscle actin (α-SMA), a marker of myofibroblasts 20, was also induced. Up-regulation of this mesenchymal marker was accompanied by down-regulation of bone morphogenic protein (bmp)7, an EMT inhibitor 21, 22, and its mediator, inhibitor of differentiation (id)2 23, and concomitant repression of epithelial genes, such as keratin (k)-7 and e-cadherin. The effects of Shh treatment on protein and mRNA expression of these factors were unaffected by tomatidine, but largely reversed by cyclopamine (Fig 1b and Supplemental Fig 1), demonstrating that changes resulted from specific activation of Hh signaling in the liver epithelial progenitors.
Figure 1. Treatment with Shh ligand induces Hh-target genes and EMT-related genes in liver-progenitors.
Immature, murine ductular-type progenitor cells (603B) were maintained in standard culture conditions. Recombinant Sonic hedgehog (Shh) protein (0, 100, 1000 ng/ml) was added for 24 hours; cells were harvested, and changes in gene expression were assessed by QRT-PCR analysis. (a) gli1, α-sma, bmp7, inhibitor of differentiation (id)2, keratin-7 and e-cadherin. Results are expressed as fold change relative to vehicle-treated control cultures. Mean ± SEM of duplicate experiments are graphed. To determine if the effects of Shh were directly attributable to Hh-signaling, 603B cells were cultured in the presence of Shh (100 ng/ml) with 3uM cyclopamine (a specific hedgehog-pathway antagonist) or 3uM tomatidine (an inactive cyclopamine analogue) for 24 hours; protein was harvested and analyzed by Western-blot. (b) Quantitative data for these studies are shown in Supplemental Fig 1. *P < 0.05 or ** P< 0.005 vs 0 ng/ml Shh
To exclude the possibility that the observed EMT changes were 603B-specific, experiments were repeated using a well-characterized NRC-line that has a phenotype of mature bile ductular cells 19. The NRC-line responded similarly to 603B cells. Shh activated the Hh pathway and induced gene expression changes that are typical of EMT (data not shown). Compared to tomatidine, which had no effect on these responses, cyclopamine reduced the expression of gli1 and αsma while augmenting expression of the epithelial genes in Shh-treated cells (Supplemental Fig 2).
Progenitor-dependent repair of fatty liver damage is associated with evidence of EMT
To assess potential biological correlates of progenitor cell EMT, mice were fed MCD diets supplemented with ethionine (MCDE). MCD diets induce nonalcoholic steatohepatitis (NASH) in rodents 24 and the addition of ethionine exacerbates this liver injury while inhibiting replication of the mature hepatocytes that survive 25 - 27. Therefore, the MCDE diet model has been used to study regeneration of injured livers by progenitors 1, 26, 28. Liver progenitors, including the ductular-type cells that accumulate as NASH progresses, 8 possess the cellular machinery that is required for Hh signaling 14, 17, 29. However, it is not known if Hh signaling regulates progenitor-mediated repair of fatty liver damage, or if EMT occurs during this process.
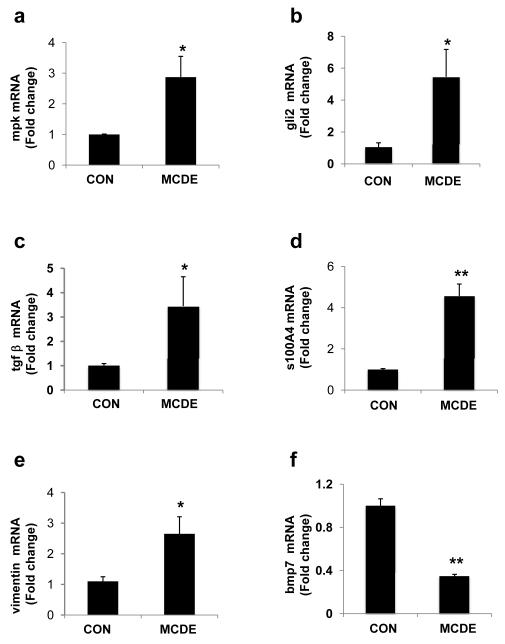
To begin to address these issues, Hh-pathway activation and changes in the expression of various EMT-related genes were evaluated in mice that were transiently fed control chow or MCDE diets. Mice were sacrificed after one week of dietary manipulation and liver RNA was obtained for analysis (Fig 2). Treatment with MCDE diet for one week increased hepatic expression of mpk, a well-established marker of liver epithelial progenitors 30. mRNA levels of the Hh-target gene, gli2, as well as the pro-fibrogenic cytokine, TGFβ, also increased significantly. Both Hh-pathway activation and accumulation of TGFβ have been shown to stimulate EMT 4, 12, 31 - 33. Consistent with those reports, we found that MCDE diet treatment increased hepatic mRNA content of s100A4, a marker of fibroblastic cells that are derived from epithelial cells 34, 35, as well as vimentin, while strongly down-regulating expression of the EMT inhibitor, bmp 7. These data demonstrate that the Hh-pathway is activated very early during progenitor cell-dependent liver regeneration, and suggest that this process is accompanied by EMT.
Figure 2. Rapid induction of EMT-related genes in mice treated with MCDE diets to induce progenitor cell-dependent liver regeneration.
C57BL/6 mice were fed control diet or MCD diet + 0.1% ethionine (in drinking water) for 1 week (MCDE diet; n=4 / group). At the end of the treatment period, total RNA was examined by QRT-PCR. (a) mpk, (b) gli2, (c) tgfβ, (d) s100A4, (e) vimentin, and (f) bmp7. Results are expressed as fold change relative to chow-fed controls. Mean ± SEM are graphed. * P < 0.05 or **P < 0.005 vs control
It would be logical if epithelial cells that undergo EMT as part of the process of repair revert to an epithelial phenotype to fill in gaps left by dead cells. To ascertain if reversal of EMT (i.e., mesenchymal to epithelial transition, MET) occurs on cessation of liver injury, additional mice were fed MCDE diets for 3 weeks, or fed the diets for 3 weeks and then switched to normal chow for a further 3 weeks. Expression of s100A4 and vimentin peaked after week 1, but declined by week 3 of the MCDE diet (Supplemental Fig. 3). In contrast, induction of epithelial genes, mpk and k7, lagged behind the early increase in mesenchymal genes, peaking in the third week of MCDE diet as mesenchymal gene expression declined. All of these markers eventually returned to basal levels when mice were removed from the MCDE diets. In aggregate, therefore, the data support the concept that EMT was followed by MET in this model of fatty liver damage and progenitor-mediated liver regeneration.
Activation of the Hedgehog (Hh) pathway, EMT, and liver fibrosis occur in diet-induced NASH and are exaggerated in Ptc+/- mice
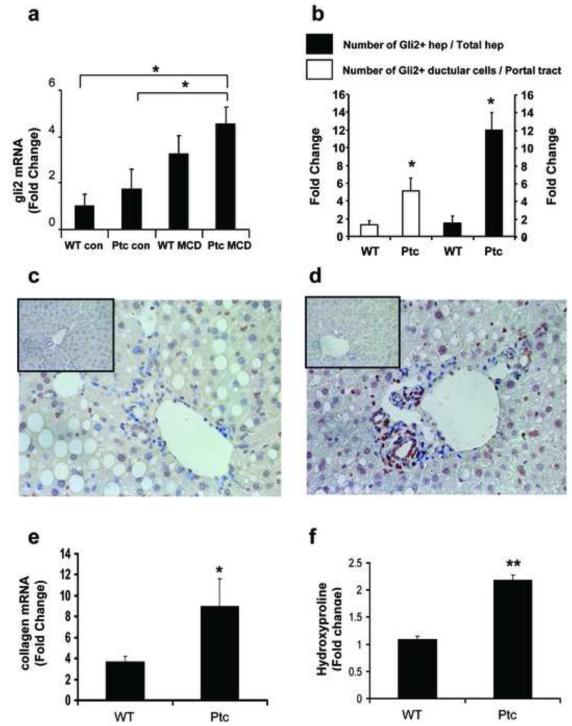
To more directly evaluate the role of Hh-mediated EMT in fibrogenic repair, Patched deficient (Ptc+/-) mice and their wild-type littermate controls (WT) were maintained on control (n=4/group) or MCD diet (n = 4/group) for 4 weeks. Chronic exposure to the MCD diet induced comparable liver injury in WT and Ptc+/- mice, as evidenced by similar histology (Supplemental Fig. 4a), as well as histologic grading and serum AST (Supplemental Table 2). Hepatic mRNA expression of sonic hedgehog (shh) was barely detected in either group of chowfed mice, but increased significantly and comparably (i.e., at least 4 fold) in both WT and Ptc +/- mice during consumption of the MCD diets (p < 0.05 vs. respective chow-fed control groups). Expression of gli2, a Hh-target gene, increased about 3 fold in WT mice, and 4 fold in Ptc+/- mice (Fig 3a). Similar to levels of gli2 mRNA, the numbers of ductular cells and hepatocytes that expressed Gli2 protein were also greater in the livers of Ptc+/- mice (Fig 3b-d and Supplemental Fig. 4b). Induction of gli1, another Hh-regulated transcription factor, also occurred, but was less robust (Supplemental Fig. 4c). Nevertheless, Ptc+/- mice tended to show greater induction of gli1 than WT mice, consistent with the fact that haplo-insufficiency of Ptc impairs normal silencing of Hh-signaling. Both WT and Ptc+/- mice exhibited fibrogenic responses to diet-induced NASH, as evidenced by 4 - 8 fold increases in collagen 1α1 gene expression (Fig 3e). Hepatic hydroxyproline content (Fig 3f), was also significantly greater than that of comparably treated WT controls (Fig 3f). These findings demonstrate that increased Hh-signaling enhanced fibrogenic liver repair in this model of chronic NASH.
Figure 3. Increased induction of Hh-target genes and liver fibrosis during diet-induced NASH in Ptc+/- mice with an overly-active Hh-pathway.
Ptc+/- mice and wild type control littermates (WT) were fed regular chow or methionine-choline deficient (MCD) diets for 4 weeks. At the end of the treatment period, mice (n = 4/group) were sacrificed. (a) QRT-PCR analysis of gli2 mRNA; (b) Accumulation of Gli-2-positive ductular cells (white bars) and Gli2-positive hepatocytes (solid bars) in WT and Ptc+/- groups. Results are expressed as fold change relative to the respective chow-fed controls and mean ± SEM are graphed; (c-d) immunohistochemistry for Gli2 in representative MCD diet fed-WT (c) and Ptc+/- (d) mice - small inserts in each photomicrograph display Gli2 staining in the respective chow-fed control; (e) collagen mRNA levels and (f) hepatic hydroxyproline content at the end of the treatment period. Results are expressed as fold change relative to the respective chow-fed control group. Mean ± SEM are graphed. * P <0.05 or ** P < 0.005 vs WT groups
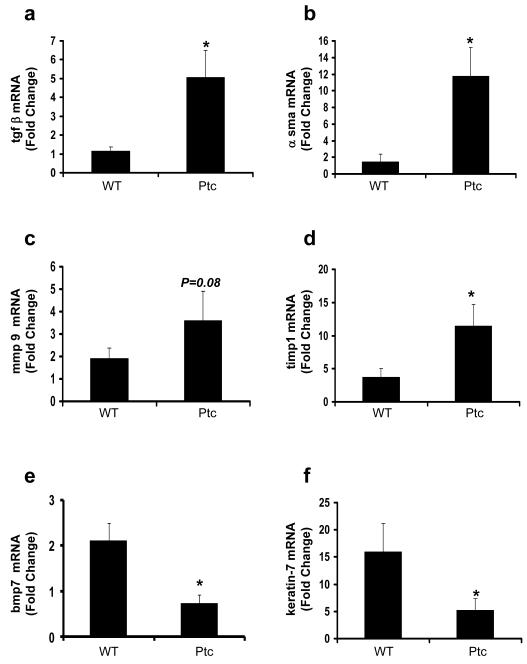
To assess the role of EMT in this process, differences in EMT-related genes were evaluated (Fig 4). Compared to WT controls, Ptc+/- mice exhibited greater induction of transforming growth factor β (tgfβ), α-sma, matrix metalloproteinase 9 (mmp9), and tissue inhibitor of metalloproteinase-1 (timp1), and greater repression of bmp7, and k-7 during chronic liver injury. Hence, in this model of NASH, the Ptc+/- mice exhibited more up-regulation of various mesenchymal genes and more down-regulation of epithelial genes than the WT controls. Given that BMP7 is a potent inhibitor of EMT 21, 22 and evidence that adenovirus-mediated over-expression of BMP7 in damaged livers represses hepatic fibrogenesis 36, the pattern of gene expression changes that occurred in response to MCD diet exposure suggest that chronic, diet-induced NASH provoked EMT and hepatic fibrogenesis. Moreover, because both responses were greater in Ptc+/- mice than WT controls, Hh-signaling was identified as an important regulator of fibrogenic repair in this animal model.
Figure 4. Enhanced alterations in expression of EMT-related genes during diet-induced NASH in Ptc+/- mice.
QRT-PCR analysis was done to assess changes in expression of several EMT-related genes in total liver RNA from the mice described in the legend to Figure 3 (a) tgfβ, (b) α sma, (c) mmp9, (d) timp1, (e) bmp7, (f) keratin-7. Results are expressed as fold change relative to the respective chow-fed control group and graphed as mean ± SEM. *P < 0.05 vs WT group
Inhibition of Hh-signaling attenuates EMT-associated fibrogenesis in vivo
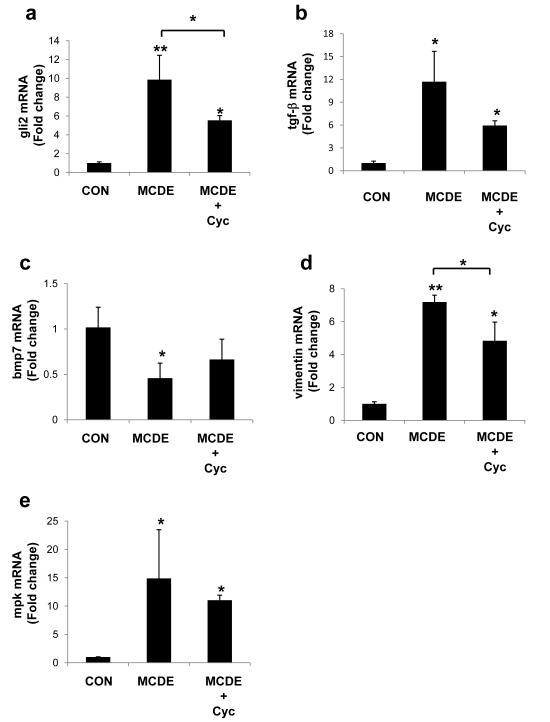
Because both mouse models of NAFLD suggested that Hh-pathway activation in hepatic progenitor populations modulated EMT-associated fibrogenesis, we investigated if inhibition of the Hh-pathway would reverse these effects. WT mice were fed either normal chow or MCDE diet (n = 4 / group) for 1 week, in the presence or absence of cyclopamine (0.6mg per mouse per day; i.p.), using a previously established protocol 9, 10. Cyclopamine inhibited MCDE-diet related induction of the Hh-target gene, gli2 (Fig 5a), as well as the pro-fibrogenic cytokine, tgf-β (Fig 5b). Both Hh and TGF-β modulate EMT 4, 12, 31 - 33. In contrast, expression of bmp7, the inhibitor of EMT, was somewhat increased by cyclopamine (Fig 5c). Cyclopamine-treated MCDE diet-fed mice also exhibited lower expression of the mesenchymal gene, vimentin (Fig 5d) and tended to have less induction of the epithelial progenitor marker, mpk (Fig 5e). The expense of cyclopamine, as well as its toxicity, prevented us from conducting a more protracted investigation of its effects in intact mice. Nevertheless, results from this short-term study support the concept that Hh-pathway activation plays a role in fibrogenic repair during fatty liver injury.
Figure 5. Inhibition of Hh-signaling attenuates EMT-associated fibrogenesis in vivo.
WT mice were fed either normal chow (n= 4) or MCDE diet (n = 8) for 1 week with or without cyclopamine (n= 4/group; 0.6mg per mouse per day; i.p.). At the end of treatment, total liver RNA was harvested for QRT-PCR (a) gli2, (b) tgf-β, (c) bmp7, (d) vimentin, (e) mpk. Results are expressed as fold change relative to the respective chow-fed control group and graphed as mean ± SEM. * P < 0.05 vs control or MCDE group; **P < 0.005 vs control
Evidence of Hh-pathway activation and EMT in NAFLD patients
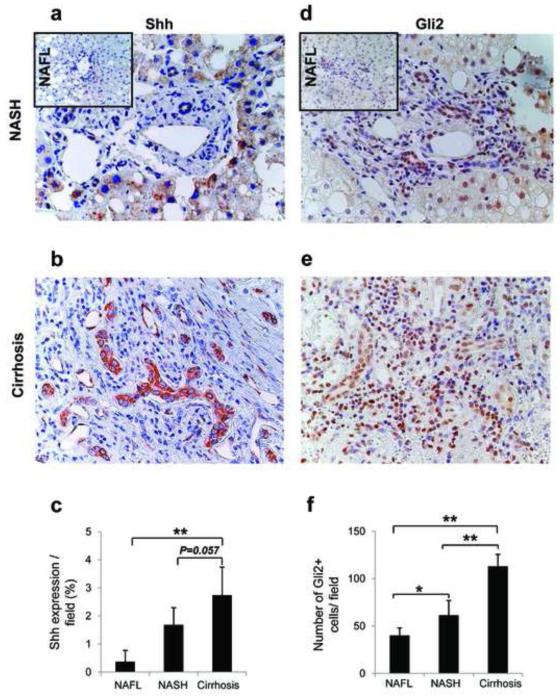
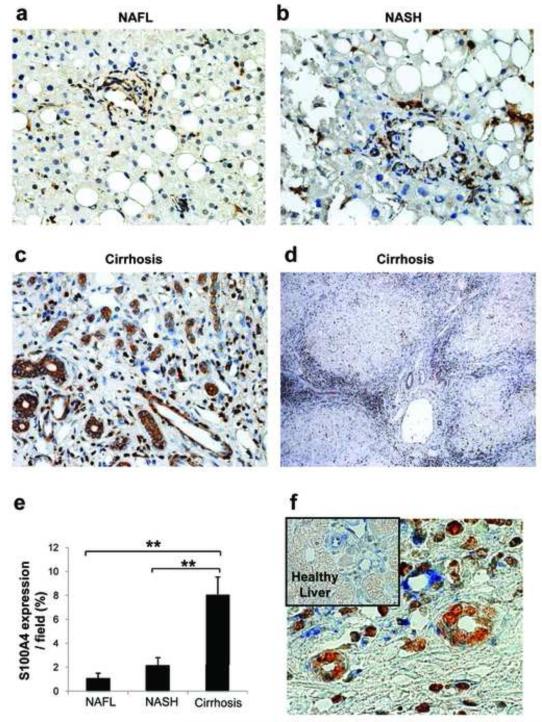
Because data in cultured liver progenitors and mouse models of NAFLD suggested that Hh-pathway activation in hepatic progenitor populations modulated epithelial to mesenchymal transitions that were involved in fibrogenic repair, we next examined human liver samples to determine if similar responses occurred during liver remodeling in NAFLD patients. Coded liver sections from 16 patients with well-characterized NAFL (n = 5), NASH (n = 5), and NASH-related cirrhosis (n=6) were stained to demonstrate Shh (Fig 6a-c), Gli2 (Fig 6d-f), and S100A4 (Fig 7a-e), and then analyzed by blinded observers and/or computer-assisted morphometry. Expression of Shh, Gli2 and S100A4 were lowest in patients with NAFL and highest in patients with cirrhosis, with intermediate levels of all three proteins occurring in patients with NASH. Thus, accumulation of Hh ligand and expression of Hh-target genes and EMT markers increased steadily as liver disease progressed, such that the livers of cirrhotic NAFLD patients exhibited about 3-fold more Shh- and Gli2-expressing cells (Fig 6 c, f), and at least 6-fold more S100A4(+) cells (Fig 7e) than the livers of patients with simple steatosis. A lower magnification photomicrograph of a representative cirrhotic liver demonstrates that S100A4-expressing cells tended to localize along fibroductular septae that extended around nodules of hepatic parenchyma (Fig 7d).
Figure 6. Hedgehog-pathway activation in NAFLD patients.
Coded liver sections from 16 patients with well-characterized NAFL (n = 5), NASH (n = 5), and NASH-related cirrhosis (n=6) were stained for the Hh-ligand, Shh and the Hh-target gene, Gli2. Shh staining was analyzed by computer-assisted morphometry. Photomicrographs are from representative patients with (a) NASH (small insert displays Shh staining in NAFL) and (b) NASH-related cirrhosis. (c) Quantitative analysis of Shh in all patients. Amount of Shh is expressed as % of stained cells per high-powered field (Magnification X400). The number of Gli2-stained cells was determined in 10 high-power fields / section on liver sections by blinded observers. Gli2 staining in (d) NASH (small insert displays Gli2 staining in NAFL), and (e) NASH-related cirrhosis. (f) Quantitative analysis of Gli2 in all patients. Data are graphed as mean ± numbers of Gli2 cells/high-powered field (Magnification X400). *P< 0.05 or **P < 0.005 vs NAFL
Figure 7. Evidence for EMT in patients with NAFL, NASH and NASH-related cirrhosis.
Coded liver sections from the patients described in Figure 6 legend were stained for S100A4, a marker of fibroblasts derived from epithelial cells. S100A4-staining was analyzed by computer-assisted morphometry. Photomicrographs are from representative patients with (a) NAFL, (b) NASH, and (c) NASH-related cirrhosis (Magnification X400). (d) Low-power view of S100A4 staining in a representative patient with NAFLD-related cirrhosis (Magnification x100). (e) Quantitative analysis of S100A4 in all patients. Amount of S100A4 is expressed as % of stained cells per high-powered field. *P < 0.05 or ** P < 0.005 vs NAFL. (f) Representative Gli2 (brown) and Vimentin (blue) - double-immuno-staining in NASH-related cirrhosis. Small Insert displays Gli2 and vimentin-double staining in human healthy liver (Magnification x630).
Previously, we showed that ductular cells were Hh-responsive and co-localized with EMT markers S100A44. Here, we demonstrate Gli2-positive ductular cells expressing the mesenchymal marker, Vimentin, in human NAFLD (Fig 7f and Supplemental Fig. 5d), ALD (Supplemental Fig. 5b & 5e) and PBC (Supplemental Fig. 5c & 5f). In aggregate, these data provide further evidence that Hh-induced EMT occurs in various human liver diseases, suggesting that it is a conserved response to chronic liver damage.
Discussion
Hepatocyte injury and death are much more extensive in NASH than in simple steatosis, and this is thought to explain why individuals with NASH are significantly more likely to develop cirrhosis 6, 7. However, the fact that many patients with NASH do not become cirrhotic 37 suggests that inter-individual differences in the response to liver injury also play an important role in defining the ultimate outcomes of NASH. Results of the present studies demonstrate that in both mice and humans, the Hh-pathway becomes activated during fatty liver injury, and that the level of pathway activity correlates with fibrosis stage. These findings suggest that inter-individual differences in regulation of Hh-signaling may influence the intensity of fibrogenic responses to fatty liver injury. Evidence that genetic impairment of Hh-pathway silencing in mice results in excessive hepatic fibrogenesis during diet-induced NASH supports this concept. Moreover, a mechanism by which Hh-signaling could cause liver fibrosis has been identified, namely, epithelial-to-mesenchymal transition (EMT).
EMT describes the process by which epithelial cells disassemble cell-to-cell attachments that tether them to adjacent cells, and acquire a more mesenchymal phenotype that permits them to migrate into the stroma where they have improved access to various growth factors and may proliferate more freely 31. EMT occurs commonly in growing tissues during fetal development, and it is also a feature of invasive and metastatic malignant cells in adults. Hh-signaling regulates EMT during development 32, 38, as well as during tumor metastasis 33, 38. Thus, tissue growth and remodeling during embryogenesis and adult tumorigenesis involves Hh-mediated EMT.
There is accumulating evidence that EMT occurs in response to fibrogenic hepatic injuries, such as bile duct obstruction 4 and carbon tetrachloride-induced cirrhosis in rodents 39, as well as biliary cirrhosis 28 and alcohol-induced cirrhosis in humans 40. The present study demonstrates that EMT also occurs in NAFLD. Hepatocytes that undergo EMT become less vulnerable to apoptosis 39, suggesting that EMT is an adaptive response that ultimately promotes reconstruction/regeneration of a healthy liver. That concept is supported by results from the current study which show that Hh-pathway activation directly induces EMT in ductular-type progenitor cells in vitro and that a similar process also occurs in vivo during a classical rodent model of progenitor cell-dependent liver regeneration. Moreover, analysis of liver RNA at different time points during the repair process demonstrates that gene expression changes typical of EMT occur early during liver injury and then decline as markers of epithelial progenitors are up-regulated. The latter then regress back to baseline when liver regeneration has been completed. Such findings suggest that at least some of the fibroblastic cells in injured livers might be transitional epithelial cells that are being mobilized to replace dead hepatocytes. Indeed, another group has already reported that populations of immature ductular cells and liver fibroblastic cells expand in parallel with bridging fibrosis as NAFLD progresses to cirrhosis in humans 3. The current study demonstrates that these fibrous septae are replete with Hh-responsive cells, many of which express the protein S100A4, a pro-EMT factor that is expressed by fibroblastic cells that are derived from epithelial cells. Hence, the new data complement and extend other evidence, and in aggregate, demonstrate that excessive repair-related EMT contributes to fibrogenesis during the evolution of NASH-related cirrhosis.
TGFβ is the most established mediator of EMT21, 31, which it stimulates via Smad-dependent activation of snail and Smad-independent induction of mitogen-activated protein kinase / extracellular signal-regulated kinase (MEK) or PI3K pathways 41. Fetal hepatocytes undergo EMT when treated with TGFβ, increasing expression of vimentin and snail while repressing e-cadherin 42. TGFβ induced hepatocyte EMT has been confirmed in adult mice 43. In diet-induced NASH, we observed enhanced expression of TGFβ in Ptc +/- mice (with activated Hh-signaling). Moreover, inhibiting Hh signaling with cyclopamine reduced TGFβ levels. These findings suggest that Hh-related EMT may depend, at least partially, on induction of TGFβ. Preliminary studies support this possibility because TGFβ inhibitors significantly attenuate EMT-related gene expression in Hh-treated 3T3-NIH fibroblasts (data not shown).
NASH is a major cause of cryptogenic cirrhosis 44. In patients with idiopathic pulmonary fibrosis (which is generally considered to be the lung-equivalent of cryptogenic cirrhosis), most fibroblasts are derived from epithelial cells 45. Therefore, dysregulated EMT may play a role in the general pathogenesis of adult organ fibrosis, making it important to delineate the mechanisms involved. It is known that stressed and dying cells in injured adult tissues release factors that promote their replacement by triggering compensatory proliferation in surviving cell populations. Studies in Drosophila demonstrate that transient activation of the Hh-pathway in normally dormant multipotent progenitors is critical for this process because repair of acutely injured tissues fails to occur when Hh-signaling is abrogated 46. On the other hand, sustained (as opposed to transient) Hh-pathway activation occurs during several types of chronic liver injury in mammals 8, 12, 13. Our findings demonstrate that this perpetuates EMT and proliferation/accumulation of Hh-responsive cell types, leading to hepatic architectural distortion, cumulative fibrosis, and eventually cirrhosis. Consistent with this concept, in rodents with reversible biliary obstruction, Hh-signaling, EMT, and fibrosis all gradually subside after obstruction is relieved 47. The present study shows that the degree of EMT and severity of liver fibrosis are also dependent upon the level of Hh-pathway activity in fatty liver damage. Both observations raise the intriguing possibility that inter-individual differences in the tendency towards fibrogenic repair may reflect differences in production of injury-related factors that initiate/maintain Hh-signaling, as well as intrinsic variability in the sensitivity of the Hh-pathway itself to such regulation. Further research is needed to evaluate this concept, and to delineate the downstream mediators of Hh-TGFβ interactions, but could result in the identification of biomarkers for cirrhosis, as well as novel therapeutic targets to prevent cirrhosis from developing in sub-groups of patients who are at high risk for this complication.
Supplementary Material
Acknowledgements
The authors thank Dr Jiawen Huang for his assistance with animal care and Mr. Carl Stone for his administrative efforts.
The authors also thank Dr PA Beachy (Johns Hopkins University, Baltimore, MD) for providing the Patched-deficient (Ptc +/-) mice; Dr. G. J. Gores (Mayo Clinic, Rochester, MN) and Yoshiyuki Ueno (Tohoku University, Sendai, Japan) for providing the murine immature ductular cell line (603B); N. LaRusso (Mayo Clinic, Rochester, MN) for providing the normal rat cholangiocyte line (NRC).
Funding:
This work was supported by RO1 DK077794 and RO1 DK053792 to AMD.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- Hh
Hedgehog
- EMT
epithelial-to-mesenchymal transition
- Shh
Sonic Hedgehog
- K7
keratin 7
- α SMA
alpha smooth muscle actin
- BMP7
bone morphogenic protein 7
- Id
inhibitor of differentiation
- MCD (E) diet
methionine choline deficient (ethionine) diet
- Gli
glioblastoma
- Ptc
Patched
- TGFβ
transforming growth factor β
- MMP9
metalloproteinase 9
- TIMP1
tissue inhibitor of metalloproteinase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Authors declare that they have no conflict of interests or financial interests
References
- 1.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 2.Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. [PubMed] [Google Scholar]
- 3.Richardson MM, Jonsson JR, Powell EE, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 6.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 7.Wieckowska A, Zein NN, Yerian LM, et al. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 8.Fleig SV, Choi SS, Yang L, et al. Hepatic accumulation of Hedgehog-reactive progenitors increases with severity of fatty liver damage in mice. Lab Invest. 2007;87:1227–1239. doi: 10.1038/labinvest.3700689. [DOI] [PubMed] [Google Scholar]
- 9.Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 10.Lipinski RJ, Hutson PR, Hannan PW, et al. Dose- and Route-Dependent Teratogenicity, Toxicity and Pharmacokinetic Profiles of the Hedgehog Signaling Antagonist Cyclopamine in the Mouse. Toxicol Sci. 2008;104:189–197. doi: 10.1093/toxsci/kfn076. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 12.Omenetti A, Yang L, Li YX, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 13.Jung Y, McCall SJ, Li YX, et al. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091–1096. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Wang Y, Mao H, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yahagi K, Ishii M, Kobayashi K, et al. Primary culture of cholangiocytes from normal mouse liver. In Vitro Cell Dev Biol Anim. 1998;34:512–514. doi: 10.1007/s11626-998-0106-x. [DOI] [PubMed] [Google Scholar]
- 16.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 17.Sicklick JK, Li YX, Melhem A, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–870. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 18.Chen JK, Taipale J, Cooper MK, et al. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vroman B, LaRusso NF. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest. 1996;74:303–13. [PubMed] [Google Scholar]
- 20.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeisberg M, Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 22.Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094–8100. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 23.Kowanetz M, Valcourt U, Bergstrom R, et al. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol. 2004;24:4241–4254. doi: 10.1128/MCB.24.10.4241-4254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 25.Roskams T, Yang SQ, Koteish A, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhurst B, Croager EJ, Farley-Roche CA, et al. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology. 2001;34:519–522. doi: 10.1053/jhep.2001.26751. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Koteish A, Lin H, et al. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403–411. doi: 10.1002/hep.20082. [DOI] [PubMed] [Google Scholar]
- 28.Tee LB, Kirilak Y, Huang WH, et al. Differentiation of oval cells into duct-like cells in preneoplastic liver of rats placed on a choline-deficient diet supplemented with ethionine. Carcinogenesis. 1994;15:2747–2756. doi: 10.1093/carcin/15.12.2747. [DOI] [PubMed] [Google Scholar]
- 29.Sicklick JK, Li YX, Choi SS, et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85:1368–1380. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- 30.Tian YW, Smith PGJ, Yeoh GCT. The oval-shaped cell as a candidate for a liver stem cell in embryonic, neonatal and precancerous liver: identification based on morphology and immunohistochemical staining for albumin and pyruvate kinase isoenzyme expression. Hisochem Cell Biol. 1997;107:243–250. doi: 10.1007/s004180050109. [DOI] [PubMed] [Google Scholar]
- 31.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 33.Yoo YA, Kang MH, Kim JS, et al. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 34.Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada H, Danoff TM, Kalluri R, et al. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita K, Iimuro Y, Otogawa K, et al. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut. 2007;56:706–714. doi: 10.1136/gut.2006.092460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Bailey JM, Singh PK, Hollingsworth MA. Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem. 2007;102:829–839. doi: 10.1002/jcb.21509. [DOI] [PubMed] [Google Scholar]
- 39.Nitta T, Kim JS, Mohuczy D, et al. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology. 2008;48:909–919. doi: 10.1002/hep.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung Y, Brown KD, Witek RP, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;9:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 42.Valdés F, Alvarez AM, Locascio A, et al. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol Cancer Res. 2002;1:68–78. [PubMed] [Google Scholar]
- 43.Kaimori A, Potter J, Kaimori JY, et al. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007;282:22089–101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell SH, Oelsner DH, Iezzoni JC, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 45.Konigshoff M, Balsara N, Pfaff EM, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omenetti A, Popov Y, Jung Y, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275–1282. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.