Abstract
Background
Abuse liability is thought to possibly be lower in long- than in short-acting opioids because lower peak serum levels may be less likely to induce psychoactive effects.
Methods
We compared patient responses to extended-release morphine, hydrocodone plus acetaminophen, and placebo in a randomized, double-blind crossover study using markers of abuse liability. Patients indicated their craving for drugs on 5 visual analog scales (VASs), completed the Addiction Research Center Inventory, and underwent cue reactivity testing. To perform the latter, subjects watched a video intended to produce a positive or a negative affect, after which a vial of medication was or was not presented (the cue) and then indicated their craving for drugs on 5 different VASs (the reactivity).
Results
Differences in Addiction Research Inventory scores were statistically significant but clinically unimportant. Neuropsychological test results were mixed and unrelated to the medications studied. Cue reactivity did not differ among conditions but was uniformly high.
Conclusions
Using several markers of abuse liability, long-acting opioids do not have lower abuse potential than do short-acting opioids or placebo. Although cue reactivity did not differ among the conditions, uniformly high results in these patients suggest that it may have some value as a component of abuse liability testing.
Keywords: Prescription Drug Abuse, Long-Acting Opioids, Short-Acting Opioids, Abuse Liability, Cue-reactivity
Introduction
Prescription opioid abuse has increased greatly during the past decade (SAMHSA, 2004; Katz et al., 2008). This public health crisis challenges clinicians to deter prescription opioid abuse while providing appropriate access to prescription opioids for treating pain (Zacny et al., 2003). Although illicit activity cannot be condoned, the undertreatment of pain cannot be overlooked (Passik and Kirsh, 2008). The solution to these conflicting issues has long sought to employ opioid formulations that offer analgesia while minimizing euphoric effects. Purported advantages of long-acting opioids include more convenient dosing, uniform serum levels resulting in uninterrupted analgesia, and improved sleep without interruption. Even the potential for aberrant behaviors has been thought to be lower with long- versus short-acting opioids.(Heit, 2001; McCarberg and Barkin, 2001). However, the conventional wisdom that long-acting opioids have an inherently lower abuse liability than short-acting opioids has been challenged (Chou et al., 2003). Although the higher peak serum levels of short-acting opioids have been thought to be more likely to induce psychoactive effects, this claim has not been tested empirically.
To determine whether short- and long-acting opioids have different abuse liability risks, we conducted a randomized, double-blind, cross-over trial in chronic pain patients who had self-escalated their dose of opioids. These patients were selected for their propensity to have a high risk for abuse. We compared their self-reported effects after exposure to extended-release morphine (ER morphine), hydrocodone plus acetaminophen, and placebo. Neuropsychological effects of study medications were performed and abuse liability was assessed with standardized methods including “cue-reactivity,” a potential marker of abuse potential (Carter and Tiffany, 1999; Drummond, 2000).
Cue-reactivity often involves exposing addicted individuals to stimuli designed to elicit cravings (cues) and assessing concomitant psychological and physiological changes, such as self-reported urge to take the drug, (reactivity). Drugs with higher cue reactivity are believed to present a greater risk of abuse than are drugs with lower reactivity. Individuals commonly studied with cue-reactivity in the past include cigarette smokers, alcoholics, heroin addicts or cocaine addicts (Carter and Tiffany, 1999). Subjects have been exposed to a wide range of cues (e.g. the sight of drug paraphernalia (Field and Duka, 2004), observation of drug-use situations (Juliano and Brandon, 1998; Taylor et al., 2000) and cue presentation modes (e.g. photographic, imagery-based or in vivo presentations (Streeter et al., 2002; Tiffany and Drobes, 1990). The clinical utility of the cue-reactivity paradigm lies in its predictive capacity. For instance, an association between drug-related cues and relapse has been identified in the alcoholic population (Cooney et al., 1997).
Materials and Methods
Approval Process
The protocol was approved by the Institutional Review Boards of the UC Davis Medical Center (UCDMC) and the Northern California Veterans Administration Health Care System (VANCHCS) and was endorsed by the Research Advisory Panel of California, which approves investigations of Schedule II and III controlled substances. The National Institute of Drug Abuse granted a Certificate of Confidentiality. The trial was registered with ClinicalTrials.gov in April, 2006 (Identifier: NCT00314340).
Participants
Participants were recruited from the UCDMC and VANCHCS Pain Clinics following referral from primary care physicians after they manifested aberrant behavior (i.e, self-escalation of opioids). Patients were screened using a brief telephone interview and met with the primary author before being enrolled. All patients provided written informed consent before beginning the study, and each received $25 per hour for participating ($450 total).
Participants had to have had chronic pain for more than 3 months and to have self-escalated their dose of a short-acting opioid (i.e., a combination product containing hydrocodone, codeine, or oxycodone) prescribed to treat their pain. This increase in the number of pills consumed led to their being referred to a pain specialist because of problems with multiple telephone calls, showing up without an appointment, or other manifestations of running out of medications early. The dose escalation had to be more than 40 mg of morphine equivalents per day. For safety reasons, patients with uncontrolled hypertension, cardiovascular disease, chronic pulmonary disease (e.g., asthma, COPD), or chemical dependencies were not eligible for study participation. In addition, subjects with significant abnormalities detected during hematologic, blood chemistry, and/or urinary screening were also excluded.
Study Design
The study was a randomized, double-blind, crossover trial in which each patient was tested under each of three conditions: a long-acting opioid (ER morphine), a short-acting opioid (hydrocodone plus acetaminophen), and placebo. Patients received each treatment once, in random order, with the order of assignment determined using a web-based random number-generating program, “Research Randomizer” (http://www.randomizer.org/). The allocation schedule was kept in the research pharmacy and concealed from all other study personnel and patients to maintain blinding to treatment condition.
Participants arrived at the UC Davis Medical Center Clinical Translational Research Center (CCRC) at 9 am and remained in a hospital room for 6 h. Before taking the first medication dose of the day, participants completed the cue-reactivity protocol, which involved watching a 5-minute video (described below), the presentation (or not) of a physical cue, and completing 5 visual analog scales (VAS) to assess reactivity to the cue.
The first dose of the study medication was taken following collection of baseline measurements, and subsequent measurements were taken hourly thereafter (Figure 1). Respiration, heart rate, arterial oxygen saturation (pulse oximetry), and blood pressure were also monitored at these intervals to insure the safety of subjects.
Figure 1. Testing Schedule in a Randomized Cross-Over Trial Comparing Markers of Abuse Liability of Short- and Long-Acting Opioids in Chronic Pain Patients.
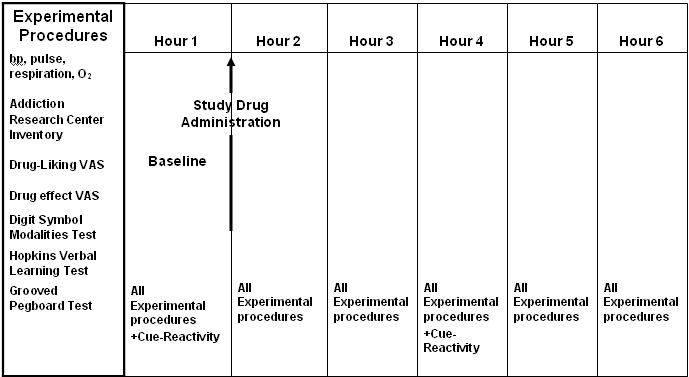
Study Medications
On the day of the study session, patients received ER morphine tablets, 45 mg (Mallinckrodt Pharmaceuticals, St. Louis, MO), hydrocodone 30 mg plus N-acetyl-para-aminophenol 975 mg (APAP; Qualitest Pharmaceuticals Inc, Huntsville, AL), or placebo. The dose of ER morphine sulfate (45 mg) was selected because of its approximate equianalgesic effect (Max et al., 1999) to the dose of hydrocodone-acetaminophen (30/925 mg). Both opioid medications and the placebo were administered in identical capsules.
Before the study, all participants underwent a urine toxicology screen (QuickScreen Pro 5 Drug Test Card) to detect evidence of illicit substance use (cannabis, cocaine metabolites and phencyclidine) and a breath alcohol test (Alcohawk ABI Digital Breathalyzer, KHN Solutions LLC, San Francisco, California). No one was disqualified for having indications of taking an illicit substance or for a positive breath alcohol test.
Between each testing session was a 3-to-10-day lead-in period during which blood levels of the drug to be tested were stabilized and the previous drug could be metabolized. Thus, patients being tested while taking the long-acting opioid began taking the ER morphine (15 mg q6h) at the beginning of the lead-in period and continued taking it through the day of testing. Patients being tested while taking either the short-acting opioid or the placebo began taking hydrocodone plus acetaminophen (10 mg/325 mg q6h) at the beginning of the lead-in period and continued through the day of testing. Participants were asked to take their other non-opioid medications as prescribed and to refrain from taking any opioid medication other than that provided during the study.
A registered nurse watched participants swallow the capsules at each testing session. Between testing procedures, participants were free to engage in sedentary recreational activities. After completing the sessions, participants were accompanied home by a responsible adult and were not to drive, operate machinery, or sign important documents for 24 hours.
Markers of Potential Abuse Liability Testing
The subjective effects of the study drug were evaluated with the short form of the Addiction Research Center Inventory (ARCI) (Haertzen and Hickey, 1987; Martin et al., 1971). This inventory consists of 49 true/false questions which survey major domains of drug effects. The subscales studied include Morphine-Benzedrine Group (euphoria), Phenobarbital-Chorpromazine-Alcohol (sedation), Lysergic Acid Diethylmide (dysphoria, agitation), Benzedrine (an empiric amphetamine scale), and Amphetamine (activation).
Participants indicated the pleasurable effects or desirability of the medications on 4 locally developed drug-liking ratings: craving, liking, strong desire, and “want more pain medication.” Ratings were made on a 100-mm VAS anchored with 0 at the low end and 10 at the high end.
Participants also responded to 11 locally developed drug-effect ratings to assess psychoactive effects. Ratings were again made on a 100-mm VAS anchored with 0 at the low end and 10 at the high end for: “on cloud 9,” “high,” “good drug effect,” “bad drug effect,” “impaired,” “stoned,” “sedated,” “confused,” “nauseated from,” “anxious,” and “down”.
Abuse liability was indicated by differences in these potential markers (i.e., ARCI and VAS scores) while on each treatment; the rating levels over the course of the day were examined to explore the timing of these effects.
Neurocognitive Testing
Neurocognitive assessments focused on three domains: attention and concentration, learning and memory, and fine motor speed. Participants completed the Wechsler Adult Intelligence Scale Digit Symbol Test (Wechsler, 1997), a test of concentration, psychomotor speed, and graphomotor abilities. In this pen-and-paper test, participants substitute a series of symbols with numbers as quickly and accurately as possible for 120 seconds. The results were expressed as the number of correct substitutions.
The Hopkins Verbal Learning Test Revised (Benedict et al., 1998) provided information on the ability to learn and immediately recall verbal information, as well as the ability to retain, reproduce, and recognize this information after a delay. Alternate forms (A through F) were used to minimize practice effects (Beglinger et al., 2005; Benedict and Zgaljardic, 1998). A list of 12 words (four words from each of three semantic categories) were presented, and the participant was asked to recall as many words as possible, in any order. After a 20-minute delay, the participant was asked to recall the words once again (i.e., delayed recall).
Utilizing the Grooved Pegboard Test (Klove, 1963) of fine motor coordination and speed, participants were required to place 25 small metal pegs into holes on a 3″ × 3″ metal board as quickly as possible. All pegs are alike and have a ridge on one side, which corresponds to a randomly oriented notch in each hole on the metal board. The dominant hand was tested first and the non-dominant hand second, and the total time for each test was recorded. The test was limited to 5 minutes, whether the task was completed or not.
Neurocognitive impairment was indicated by low scores on all three assessments.
Cue-Reactivity Testing
At each session, participants were exposed to different cues, watched a multimedia presentation on a computer screen and were instructed to vividly envision the imagery designed to induce a positive or negative affect. The videotaped imagery was presented either with or without a vial of opioid medication as a “perceived drug use opportunity”, a technique that has been shown to increase cue-reactivity in fMRI studies (Wilson et al., 2004) and maximized the chance that cue-reactivity would be demonstrated using the self-report measures examined in the present study.
The scripts (Appendix 1) were intended to stimulate 1) negative-affect imagery with explicit urges to abuse pain medications including the presentation of pain medication), 2) negative-affect imagery with explicit urges to abuse pain medications without the presentation of pain medication, and 3) positive-affect imagery with the presentation of pain medication. The latter was designed to serve as a control for the negative affect imagery.
Each session was begun with a 30-s rest period, followed by 30 s of instructions. Participants were seated in a comfortable chair in a darkened room to watch the video. After the video, subjects had 30 s to answer the 5 urge-to-use pain medications questions. To minimize social desirability bias, the research assistant told each participant “Your thoughts about drug liking are very sensitive and personal. We are interested in as honest an answer as you can supply, and we will make sure that your answers are kept confidential.” In the two instances in which the physical presentation of a vial of pain medication was performed, the research assistant added, “Please look at the vial in front of you before you answer the questions. The medicines in the vial will be provided to you within the hour.” This part of the experiment lasted about 10 min.
Responses to the 5 urge-to-use pain medications questions were recorded on 100-mm visual analog scales (VAS), anchored on the left with “not at all” and on the right with “extremely:”
“Do you have a craving for pain medication right now?”
“Do you have a liking for pain medication right now?”
“Do you have a strong desire for pain medication right now?”
“Do you want to take extra amounts of pain medication right now?”
“Do you have a need to control your pain with some pain medication right now?”
The specific wording of these questions has not been validated for measuring cue-reactivity, but was intentionally similar to questions used by other researchers to measure the self-reported urge to consume abusable substances in response to cues (Ooteman et al., 2006) or to assess the use the term “craving” which may have variable meanings including liking, wanting, urges, desires, need, intention or compulsion to use (Drummond et al., 2000) (Kozlowski and Wilkinson, 1987).
Statistical Methods
Outcome measures are summarized in Table 1 and the testing schedule in Figure 1. Sample size was based on similar studies comparing a hydrocodone combination product to placebo (Zacny, 2003).
Table 1. Outcome Measures in Study of Markers of Abuse Liability of Short- and Long-Acting Opioids.
| Measure | Scoring |
|---|---|
| Markers of Abuse Liability Testing | |
| Addiction Research Center Inventory | 49 true-false questions |
| 4 drug-liking ratings | 100-mm visual analog scales |
| 11-drug-effect ratings | 100-mm visual analog scales |
| Neuropsychological Testing | |
| Wechsler Adult Intelligence Scale (WAIS-III) Digit Symbol Test | Number of correct symbol substitutions made in 120 s |
| Hopkins Verbal Learning Test Revised | Correct responses in immediate and delayed recall tasks |
| Grooved Pegboard Test | Number of pegs placed in 120 s |
| Cue Reactivity | |
| 5 urge-to-use pain medications questions | 100-mm visual analog scales |
Treatment effects at baseline, 60, 120, 180, 240, and 300 min were assessed with repeated measures ANOVA. A liner mixed-effects model with inclusion of interaction terms (1) treatment and time and (2) random order visit number and time was performed initially. If not significant, a liner mixed-effects model without interaction terms was then used for analysis. Log transformation of the response variable was used when appropriate. Alpha was set at 0.05, and P values were two-tailed. All analyses were performed with SAS Version 9.1.
Results
Of 55 patients approached, 18 were evaluated, and 14 met entry criteria and were enrolled (Table 2). Two withdrew before completing the study, one for insufficient pain relief from the study medication and other for “feeling drugged.” One patient missed a visit, leaving 35 visits by 12 patients for analysis (Figure 2).
Table 2. Characteristics of 12 Chronic Pain Patients Tested in a Study of Markers of Abuse Liability of Short- and Long-Acting Opioids.
| Characteristic | Value |
|---|---|
| Sex, n | |
| Male | 8 |
| Female | 4 |
| Age, y | |
| Median | 46 |
| Range | 35 to 59 |
| Years of Education | |
| Median | 14 |
| Range | 12 to 21 |
| Race | |
| Caucasian | 8 |
| Afro-American | 2 |
| Hispanic | 2 |
| Employment Status, n | |
| Employed | 3 |
| Unemployed | 3 |
| Disabled | 5 |
| Retired | 1 |
| Cause of pain, n | |
| Low Back Pain | 10 |
| Cervicalgia | 1 |
| RSD | 1 |
| Mean (SD) Baseline | 6.4 (2.2) |
| Pain Intensity, VAS measurement Duration of pain, yrs | |
| Mean | 11.3 |
| Range | 0.5 to 37 |
Figure 2. Participant Dispositions in a Randomized Cross-Over Trial Comparing Markers of Abuse Liability of Short- and Long-Acting Opioids in Chronic Pain Patients.
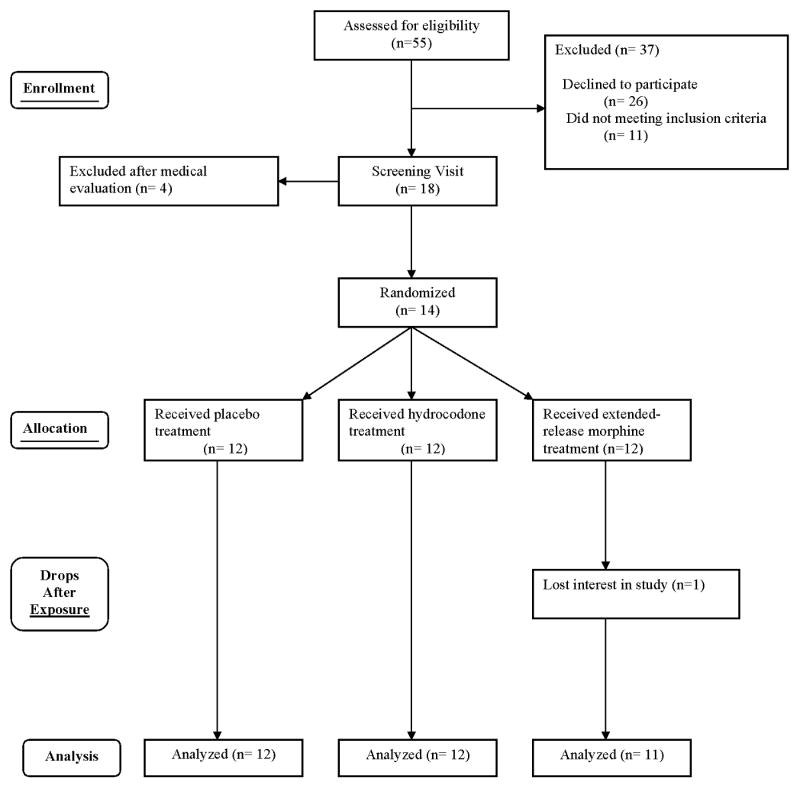
The intervals between study sessions ranged from 6 to 10 days with a mean (SE) of 7.1 (0.3) days.
Markers of Potential Abuse Liability Results
Measures of the 5 Addiction Research Center Inventory dimensions did not change significantly between baseline and 300 minutes (Table 3) under any treatment condition. Visit and/or carryover effects were statistically significant for all five subscales (Table 3). After controlling for these variables, significant treatment effects were observed for the Phenobarbital-Chorpromazine-Alcohol (sedation), Morphine-Benzedrine (euphoria), and Lysergic Acid Diethylmide (dysphoria, agitation) subscales. (Table 3).
Table 3. Statistical Significance of Results of Markers of Abuse Liability and Neuropsychological Testing of Short- and Long-Acting Opioids among 12 Chronic Pain Patients.
Bolding indicates statistical significance at the 0.05 level. Bolding with gray shading indicates a statistically significant interaction or treatment effect and is evaluated further in the next table by examining peak or trough scores.
| P Values | ||||
|---|---|---|---|---|
| Response Variable | Change Over Time within Each Treatment Group | Treatment Carry Over Effect on Response Variable | Visit Effect on Response Variable | Treatment Effect on Response Variable |
| Addiction Research Center Inventory Subscales | ||||
| Phenobarbital-Chorpromazine-Alcohol (sedation) |
0.4 | 0.4 | 0.02 | <0.01 |
| Morphine-Benzedrine (euphoria) |
0.7 | 0.1 | <0.001 | <0.001 |
| Lysergic Acid Diethylmide (dysphoria, agitation) |
0.2 | 0.01 | <0.001 | <0.001 |
| Benzedrine (euphoric stimulant) |
0.5 | 0.5 | 0.03 | 0.1 |
| Amphetamine (activation). |
1.0 | <0.01 | <0.001 | 0.6 |
| Drug-Liking VAS Ratings | ||||
| Craving | < 0.001 | 0.1 | 0.1 | 0.03 |
| Liking | < 0.001 | 0.3 | 0.7 | 0.3 |
| Strong Desire | < 0.001 | 0.5 | 0.7 | 0.03 |
| Want More Pain Medicine | < 0.01 | 0.3 | 0.5 | 0.1 |
| Drug-Effect VAS Ratings | ||||
| Sedated† | … | 0.8 | 0.4 | … |
| Confused‡ | … | <0.01 | 0.03 | … |
| Nauseated ¶ | … | 0.04 | 0.02 | … |
| Cloud Nine | 0.1 | 0.6 | 0.3 | 0.8 |
| High | < 0.001 | 0.1 | 0.2 | 0.1 |
| Good Drug Effect | < 0.001 | < 0.001 | 0.6 | <0.001 |
| Bad Drug Effect | 0.9 | 0.4 | 0.01 | 0.086 |
| Impaired | <0.01 | 0.6. | 1.0 | 0.02 |
| Stoned | < 0.001 | 0.01 | 0.4 | 0.02 |
| Anxious | 0.2 | 0.3 | 0.1 | 0.1 |
| Down | 0.02 | 0.4 | 0.3 | 0.1 |
| Neuropsychological Testing | ||||
| Digit Symbols Modality Test | <0.01 | <0.01 | < 0.001 | 0.087 |
| Hopkins Verbal Learning Trial | < 0.001 | 0.58 | 0.02 | 0.521 |
| Hopkins Verbal Learning - Delayed | < 0.001 | 0.6 | 0.8 | 0.4 |
| Grooved Pegboard Dominant | 0.1 | <0.05 | 0.3 | 0.6 |
| Grooved Pegboard Non- Dominant | 0.03 | 0.6 | 0.1 | 0.1 |
Treatment and time interaction: P = 0.02
Visit and time inter-action: P < 0.01
Treatment and time inter-action: P = 0.03
Mean Phenobarbital-Chorpromazine-Alcohol (sedation) levels were elevated at baseline, averaging 6 to 8 units on a scale of 0 to 15 for the various treatments. Trough levels were seen at 180 to 240 min after ingesting either opioid medication (Figure 3). The majority of the treatment effect on the Morphine-Benzedrine (euphoria) dimension resulted from values that peaked after the short-acting opioid was ingested. However, the 1.3-unit difference in peak effect size (which can range from 0 to 16 units; Table 4) between placebo and the short-acting opioid was inconsequential. Likewise, the 1.1-unit difference between placebo and the short-acting opioid on the Lysergic Acid Diethylmide (dysphoria, agitation) dimension (which ranges from 0 to 14 units), was also not clinically relevant (Table 4).
Figure 3. Sedation Levels over Time in a Randomized Cross-Over Trial Comparing Markers of Abuse Liability of Short- and Long-Acting Opioids in Chronic Pain Patients.
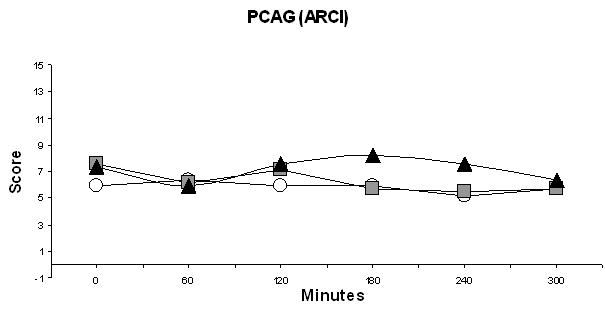
(Sedation levels were measured as the PCAG dimension of the Addiction Research Center Inventory.) ○ Morphine ■ Hydrocodone ▲ Placebo
Table 4. Mean peak or trough scores ratings (SE) of Response Variables sensitive to one or more of the active drug conditions.
The table utilizes statistically significant main effects or interactions from Table 3 (see key below)
| Placebo | Hydrocodone/Acetaminophen 30 mg/975 mg | Morphine 45 mg | |
|---|---|---|---|
| Mean (SD) Addiction Research Center Inventory Scores | |||
|
Phenobarbital-Chorpromazine-Alcohol* (sedation) |
7.6 (1.0) | 5.4 (0.9) | 5.2 (0.8) |
|
Morphine-Benzedrine ** (euphoria) |
3.3 (0.8) | 4.6 (0.9) | 3.5 (1.0) |
|
Lysergic Acid Diethylmide **↓ (dysphoria, agitation) |
4.7 (0.8) | 4.2 (0.6) | 3.6 (0.5) |
| Mean (SD) Drug Liking Ratings, mm | |||
| Craving*↓ | 23.0 (12.4) | 11.1 (4.2) | 7.5 (3.6) |
| Strong desire*↓ | 21.2 (8.2) | 12.5 (5.0) | 16.5 (7.8) |
| Mean (SD) Drug Effect Ratings, mm | |||
| Sedated*¶ | 18.0 (8.0) | 3.7 (1.5) | 1.9 (1.0) |
| Confused*¶ | 14.8 (6.8) | 5.6 (3.2) | 5.3 (2.2) |
| Nauseated*¶ | 10.2 (6.2) | 0.6 (0.3) | 1.5 (1.3) |
| Good Drug Effect** | 24.8 (10.0) | 10.2 (4.9) | 15.9 (8.0) |
| Impaired* | 16.2 (7.3) | 5.3 (3.5) | 9.6 (5.4) |
| Stoned* | 17.1 (9.2) | 12.4 (6.2) | 8.3 (6.6) |
P <0.05
P <0.001
Interaction Effect
Decline in trough score
All 4 drug-liking VAS ratings showed significant time effects (Table 3). All scores decreased after the medication was ingested, but only craving and strong desire had statistically significant treatment responses (Table 3 and Figures 4a and 4b). The mean peak trough levels for these two drug-liking measures were lower for the opioids than for placebo. This difference suggests that drug-liking does not differ substantially between ER morphine and hydrocodone-acetaminophen, a conclusions corroborated by the results of the ARCI Morphine-Benzedrine (euphoria) subscale.
Figure 4. Visual Analog Scores for a) Drug Craving and b) Strong Desire to Take Drugs in a Randomized Cross-Over Trial Comparing Markers of Abuse Liability of Short- and Long-Acting Opioids in Chronic Pain Patients.
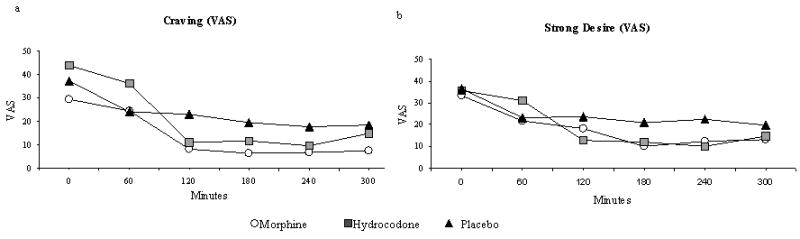
(These questions are part of the four Drug-Liking questions used to assess abuse liability.)
Drug effect ratings (Table 3) revealed a time-treatment interaction for sedation and nausea and a time-visit interaction for confusion. However, peak mean values of these adverse events were lower for active medications than for placebo (Table 4), implying that these effects were not clinically important for active medications.
Changes over the 6-hour study were evident, as were carryover effects for numerous other drug effects (Table 3). However, using repeated measures ANOVA, treatment effects were only statistically significant for good drug effect, impaired, and stoned. As was the case for the adverse events mentioned above, peak mean values for placebo were higher than for either study medication in all three of these variables (Table 4). These findings all argue against the hypothesis that the hydrocodone product induced a greater euphoric or reinforcing effect than that of ER morphine.
Neuropsychological Results
Neuropsychological testing revealed significant changes over time in several variables (Table 3). The slight improvement in the Digital Symbol Modality Test over time was not clinically important. Other neuropsychological variables paradoxically worsened somewhat during the study; this was surprising as neuropsychological test scores usually improve over time as a result of practice effects (Heaton and Marcotte, 2000). We controlled for practice effects by using a placebo group, alternate forms of the test (Benedict and Zgaljardic, 1998) where applicable (i.e., the Hopkins Verbal Learning Test), and exposure to the test before the study because the largest practice effects typically occur between the first and second test (Collie et al., 2003) (Beglinger et al., 2005). Using all of these maneuvers and controlling for carryover and visit effects using repeated measures ANOVA, we found no statistically significant main treatment effects on neuropsychological testing for the medications studied.
Cue-Reactivity Results
Participants completed the cue-reactivity protocol, which involved watching a 5-minute video (Appendix), the presentation (or not) of a physical cue, and completing 5 visual analog scales (VAS) to assess reactivity to the cue. There was evidence of cue-reactivity to all five urge-to-use pain medication variables at each of the 3 visits (Figure 5). However, after controlling for the order of the videos and treatment carryover effects, there was no statistically significant effect of either presenting a cue or different imageries on the reactivity variables (Table 5).
Figure 5. Results of the Urge-to-Use Questions from the Cue-Reactivity Test in a Randomized Cross-Over Trial Comparing Markers of Abuse Liability of Short- and Long-Acting Opioids in Chronic Pain Patients.
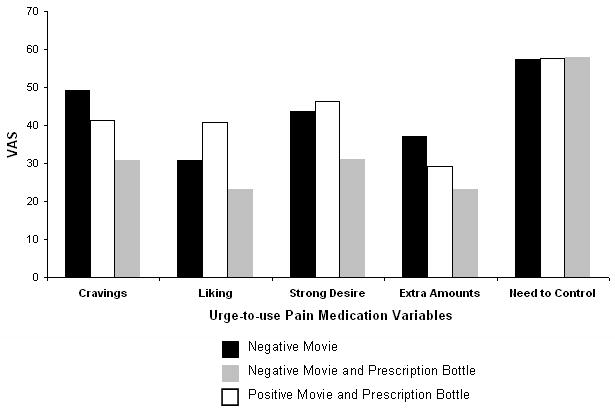
Table 5. Statistical Significance of Results of Cue Reactivity Testing of Short- and Long-Acting Opioids among 12 Chronic Pain Patients.
Bolding indicates a statistically significant effect
| P Value | |||
|---|---|---|---|
| Response to the question: “Do you ___ pain medication right now?” | Treatment Carry-Over Effect on Response Variable | Order of Movie | Type of Movie with or without Cue Effect on Response Variable |
| “have a craving for” | 0.3 | 0.7 | 0.08 |
| “have a liking for” | 0.9 | 0.2 | 0.09 |
| “have a strong desire for” | 0.2 | 0.3 | 0.08 |
| “want to take extra amounts of” | 0.9 | 0.5 | 0.1 |
| “have a need to control your pain with some” | 0.1 | 0.01 | 0.5 |
Discussion
There is a paucity of abuse liability testing of prescription opioids in patients with chronic pain, and methods are certainly needed to fill gaps in our knowledge in this area (Zacny et al., 2003). Here, we report data suggesting that long-acting opioids offer no advantage over short-acting ones in reducing abuse potential. Similarly, cognitive performance did not differ selectively across treatments. We also found no difference in cue-reactivity among our three different sets of cue and imagery presentations, although cravings were uniformly high across settings.
Markers of Abuse Liability Testing
Over the last decade, Zacny and collaborators have characterized the subjective and neuropsychological effects of a wide variety of opioids in normal healthy volunteers (Black et al., 1999; Walker et al., 2001; Zacny et al., 1997; Zacny et al., 1998). A comparison of (short-acting) hydrocodone and (immediate release) morphine revealed similar profiles of subjective effects, which included both pleasurable and unpleasant results (Zacny, 2003).
A more recent study employed the same type of double-blind, randomized, within-subject, placebo-controlled design as our study to examine the relative abuse potential and potency of oral oxycodone (10, 20, and 40mg), hydrocodone (15, 30, and 45 mg), hydromorphone (10, 17.5, and 25 mg), and placebo (Walsh et al., 2008). Unlike the studies of Zacny et al., participants were adult volunteers who used prescription opioids illicitly for psychoactive effects (but who were not physically dependent on opioids at the time of the study). They participated in 11 experimental sessions each 6.5 h long. The results suggest that the abuse liability profile and relative potency of these three commonly used short-acting opioids do not differ substantially from one another and that analgesic potencies may not accurately reflect relative differences in abuse liability of prescription opioids (Walsh et al., 2008).
Our results are similar to those of the above study involving volunteers who used prescription opioids illicitly (Walsh et al., 2008) in that we found little difference in the markers of abuse liability of the opioids studied. Our conclusions also contradict the belief that long acting opioids have different abuse liability profiles than short acting opioids. In our study, short- and long-acting opioids produced similar reinforcing effects that did not differ substantially from placebo (i.e., on the ARCI euphoria dimension, drug-liking VAS scales, and drug-effects VAS scales). Thus, we cannot assume that long acting opioid differentially reduce psychomimetic effects. However, these result cannot be generalized to all circumstances and therefore do not imply that opioid-naïve patients would not benefit from being placed on long-acting opioids in terms of avoidance of reinforcing effects. Generalization will require prospective studies of chronic pain patients who have not already been started chronic opioid therapy, as recently proposed by others (Turk et al., 2008). In addition, our results are hindered by not studying repeated dosing of short-acting opioids on a prn basis as often occurs in the clinical setting. Additional studies will be needed to understand how various dosing schedules affect abuse potential.
Two aspects of the baseline characteristics of patients in this study deserve mention. Unlike studies involving morphine and hydrocodone in normal volunteers (Zacny, 2003), the initial scores on the Phenobarbital-Chorpromazine-Alcohol (sedation) dimension, were consistently elevated before administration of study medicine, indicating that subjects were generally sedated. Chronic pain patients often have sleep disturbances (Marin et al., 2006; Roehrs and Roth, 2005; Zelman et al., 2006) and fatigue (Moldofsky, 2008); therefore, this finding is not particularly surprising. The other notable finding was the initial drug-liking VAS ratings of craving and strong desire which were also elevated (Figures 4a and b). Although the VAS ratings of active medications did not differ from placebo during the 6-hour sessions, drug-liking was nevertheless initially endorsed by patients who had self-escalated their opioid medications. Further recommendations to assess craving are discussed below, under cue-reactivity.
Neurocognitive Testing
A somewhat anticipated finding in our study was the lack of differences in neurocognitive test results between opioid and placebo test situations. It has been reported that the therapeutic use of opioids can be associated with altered cognition and impaired psychomotor function, particularly when first administered (Chapman et al., 2002; Kamboj et al., 2005). However, some studies reach the opposite conclusion when these medications are used long-term (Agarwal et al., 2007; Gaertner et al., 2006), even with reports of improved cognition (Lorenz et al., 1997; Panjabi et al., 2008). Concordantly, Brown and colleagues (Brown et al., 2006) developed a regression model in which psychological factors and pain severity predicted the decline in cognitive function better than specific opioid medications or daily opioid dose.
Cue-Reactivity Testing
Cue-reactivity assessment revealed moderate responses to all 5 of the craving probes (Figure 5) without detecting a significant influence of the positive or negative affect imagery (Table 5). This result is surprising, given the previous findings by our group (Wilsey et al., 2008) and others (Becker et al., 2008; Wasan et al., 2007) that psychological factors are associated with prescription opioid abuse. That the “perceived drug use opportunity” (i.e., the presentation of the vial of study medication) was not influential in increasing craving was also unexpected. However, this finding may have been mitigated by the fact that patients knew the study medication (or placebo) would be administered for the addiction liability testing.
Given the responses to the 5 craving probes seen in the present study (Figure 5), further exploration of cue-reactivity as a potential marker of abuse liability seems warranted. These findings are consistent with elevated baseline drug-liking VAS ratings of craving and strong desire (Figure 4a&b), again suggesting that additional evaluation of the reinforcing effects of opioids in this population is needed.
Cognitive task performance and physiological reactivity (heart rate, sweat gland activity, and skin temperature) should be evaluated by cue-reactivity testing in within-session manipulations of drug availability, in addition to assessing changes in self-reported urge as we did in the present study. Ostensibly, the level of cue-reactivity has intrinsic value in addiction studies. For instance, it has been found to be positively correlated with the degree of alcohol consumption (Streeter et al., 2002). Other investigations have found that the reactivity to drug-related cues correlates with maintenance of drug use and relapse (Juliano and Brandon, 1998). Perhaps similar experiments should be done with chronic pain patients with a propensity for prescription opioid abuse using a measure of the degree of aberrant behavior such as the SOAPP Score (Akbik et al., 2006; Butler et al., 2004; Butler et al., 2008). Alternatively, studies may seek to determine if cue-reactivity in chronic pain patients with a history of substance abuse differs from those without similar histories.
Despite the negative results in our study, the issue of emotional context of cue-reactivity needs to be reassessed because of findings in other addiction settings. One study used guided imagery designed to induce negative moods in alcoholics who were then exposed to either their favorite alcoholic beverage or to a glass of water (Cooney et al., 1997). Both alcoholic beverage presentation and negative affect imagery led to increased subjective reporting of desire to drink. These effects were additive but not multiplicative (i.e., the interaction of mood state with beverage type was not significant). The most notable finding in this study was that the reported urge to drink during the trial that combined negative mood imagery with alcoholic beverage exposure predicted time to relapse after discharge from an inpatient setting (Cooney et al., 1997). Similarly designed studies should be replicated in chronic pain patients who have been hospitalized or treated aggressively as outpatients for prescription opioid abuse.
The concept of “craving” has been central to addiction research for 5 decades, and cue-reactivity has become central to measuring craving. In the past 10 years, studies applying functional neuroimaging techniques to the study of cue-reactivity have identified specific brain regions in the pathogenesis of craving (Wilson et al., 2004). Unfortunately, the lack of internal consistency in these studies has perplexed investigators (Wilson et al., 2004). Perceived drug use may provide a way to reconcile contradictory findings among brain-imaging studies of cue-elicited craving (Wilson et al., 2004). If resolved, the prospect of imaging patients with prescription opioid abuse is one more reason why it is important to consider cue-reactivity in this population. Chronic pain and drug addiction presumably involve some shared neurobiologic anatomy. A better understanding of their CNS pathways via functional neuroimaging techniques may help the development of analgesics with less abuse potential, and improved treatments for addiction (NIDA, 2008).
The Addiction Research Center Inventory was developed by the Public Health Service in the 1960s to assess opioid abuse liability and has been the prototype for most abuse liability assessments in use today (Bigelow, 1991). Other techniques, such as self-reported measures that directly examine drug-taking behavior in laboratory experiments, may be useful in developing medications for treating substance abuse and for assessing the abuse liability of opioid configurations designed to reduce abuse and diversion (Comer et al., 2008). However, changes in self-reported measures and subjective effects induced by the same medication may not always agree (Comer et al., 2008). To this already complicated scenario of different methodologies, we suggest adding cue-reactivity to provide additional input into deciphering prescription opioid abuse in laboratory experiments.
Limitations of the Study
One limitation was the evaluation of only one dose of hydrocodone plus acetaminophen (as well as the single, equianalgesic dose of ER morphine). Although this dose of hydrocodone plus acetaminophen exceeded the dose that had been presented by others as a maximum strength at the time the present study was devised (Zacny, 2003; Zacny et al., 2005), a higher dose has subsequently been selected by one investigator (Walsh et al., 2008). Future studies might therefore benefit from employing a range of doses that include higher potencies of study medications. These studies might also employ a larger sample size and perform follow-up at more prolonged intervals than the brief six hour period allotted in the present clinical trial to understand how markers of abuse potential might vary over time.
Some authorities believe it necessary to prescreen subjects for their ability to detect and report subjective drug effects, and to distinguish the effects of an active drug from those of placebo (Busto et al., 1999). However, we found no evidence to suggest that this was a widely accepted practice and believe our results did not suffer from the absence of such screening.
Another limitation of our study was the relatively simple design of the cue-reactive presentation and measurement. Other investigators use multiple trials of either a lit cigarette or a glass of water and inform subjects of the probability (0%, 50%, or 100%) that they would be able to consume the cue on each trial. This trial-by-trial manipulation of drug availability had a pronounced impact on reactivity to cigarette cues (Carter and Tiffany, 2001). In addition, the “perceived drug use opportunity” in our study was not an isolated condition; subjects knew that they were going to receive study medication whether or not they viewed the prescription bottle.
Conclusions
We found no difference in markers of abuse liability between short- and long-acting opioids prescribed to treat chronic pain. Cue-reactivity test results did not differ among the three presentations of emotional imagery even when combined with a “perceived drug use opportunity”. However, cue-reactivity scores were relatively high for all three treatments. The fact that these subjects endorsed craving of a pleasant subjective state is noteworthy. Given their experience with the medical profession, learned biases against reporting cravings were likely to have been overcome; it might even have been suppressed somewhat in this clinical research setting. Therefore, further evaluation of the cue-reactivity paradigm in the evaluation of prescription opioid abuse is warranted to confirm this measure's putative utility as a marker of abuse liability testing.
Appendix: Cue-Reactivity Scripts
1. Negative Affect + Urge, Paraphernalia (View Prescription Medicine Vial)
You have been sitting in a reception room, nervously waiting for a doctor's appointment. You're dreading asking your doctor for more pain pills, and things aren't being helped by the fact that you have run out of medicine over the past couple of days. As you sit in the chair feeling worried and tense, you suddenly realize that the two people across from you are taking pain medications out of vials. One of them is even taking the type of medicine that works well for you. You watch as this person pulls pill after pill from her medicine vial and puts them in her mouth. Her face tells you that her pain is melting away. Watching her, you begin to think of using pain medication like candy. As the time passes and your anxiety continues to build, the desire to take pain medication grows stronger and stronger. Your urge builds and you decide to ask this women for a pain pill. She agrees and hands you her vial of medications.
Off camera, the research assistant shows the patient his or her own vial of pain medication.
2. Positive Affect + Urge, Paraphernalia (View Prescription Medicine Vial)
As you are riding in a car on your way to visit some close friends, you are enjoying the nice weather and the interesting scenery. You've decided to try this trip without using pain medicine. Some of your favorite music is playing on the radio (music comes on softly), and you feel happy and carefree. You then stop at a red light and notice that two people on a park bench are taking pills from a medicine vial. They are both laughing and having a good time. You think that the medicine vial is similar to your own (slide of prescription medication vial). You can just imagine how good your medicine would make you feel, as it would take away your pain and make you feel relaxed. You think that now maybe you should go back home and get your medicine and take a pain pill. It's something to look forward to: something to make your day even more enjoyable. You turn your car around and drive back to your place. You're home now and ready to take your pain medicine.
Off camera, the research assistant shows the patient his or her own vial of pain medication.
3. Negative Affect Alone
You're outside on a cloudy fall day, raking leaves (picture of yard with leaves). There's only one small tree on this side of the lawn, so not much raking needs to be done. Some of the leaves are wet and matted into the grass. You have to press down hard on the rake to lift these leaves, so you are beginning to feel more pain. As you rake all of the brown leaves into a large pile, you can smell their musty odor. This reminds you of mildew. You start to feel a little warm, so you take your jacket off and drape it over one of the branches of the tree. You can feel the wind blowing through your clothing as you continue raking. Suddenly, you notice a car with a young driver speeding down your street (picture of sports car with sound of acceleration). You lean on your rake and watch as the car turns the corner. (Actors expression turns very serious). Suddenly, the car nearly hits a child playing hopscotch in the street. You regret that you cannot lecture that young driver about obeying the speed limit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barth L. Wilsey, Sacramento VA Medical Center, Associate Clinical Professor of Anesthesiology and Pain Medicine, UC Davis Medical Center.
Scott Fishman, Chief, Division of Pain Medicine, Department of Anesthesiology and Pain Medicine, UC Davis Medical Center.
Chin-Shang Li, UC Davis Clinical Translational Science Center, Division of Biostatistics, Department of Public Health Sciences, University of California, Davis
Jeanna Storment, Research Associate, Department of Anesthesiology and Pain Medicine, UC Davis Medical Center.
Anthony Albanese, Chief of the Hepatology and Chemical Dependency Programs, Sacramento VA Medical Center, Associate Clinical Professor of Medicine and Psychiatry, UC Davis Medical Center.
References
- Agarwal S, Polydefkis M, Block B, Haythornthwaite J, Raja SN. Transdermal fentanyl reduces pain and improves functional activity in neuropathic pain states. Pain Med. 2007;8:554–562. doi: 10.1111/j.1526-4637.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Akbik H, Butler SF, Budman SH, Fernandez K, Katz NP, Jamison RN. Validation and clinical application of the Screener and Opioid Assessment for Patients with Pain (SOAPP) J Pain Symptom Manage. 2006;32:287–293. doi: 10.1016/j.jpainsymman.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Beglinger LJ, Gaydos B, Tangphao-Daniels O, Duff K, Kareken DA, Crawford J, Fastenau PS, Siemers ER. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol. 2005;20:517–529. doi: 10.1016/j.acn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Benedict R, Schretlen D, Groninger L, Brandt J. Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist (Neuropsychology, Development and Cognition) 1998;12:43–55. [Google Scholar]
- Benedict RH, Zgaljardic DJ. Practice effects during repeated administrations of memory tests with and without alternate forms. J Clin Exp Neuropsychol. 1998;20:339–352. doi: 10.1076/jcen.20.3.339.822. [DOI] [PubMed] [Google Scholar]
- Bigelow GE. Human drug abuse liability assessment: opioids and analgesics. Br J Addict. 1991;86:1615–1623. doi: 10.1111/j.1360-0443.1991.tb01756.x. [DOI] [PubMed] [Google Scholar]
- Black ML, Hill JL, Zacny JP. Behavioral and physiological effects of remifentanil and alfentanil in healthy volunteers. Anesthesiology. 1999;90:718–726. doi: 10.1097/00000542-199903000-00013. [DOI] [PubMed] [Google Scholar]
- Brown RT, Zuelsdorff M, Fleming M. Adverse effects and cognitive function among primary care patients taking opioids for chronic nonmalignant pain. J Opioid Manag. 2006;2:137–146. doi: 10.5055/jom.2006.0023. [DOI] [PubMed] [Google Scholar]
- Busto UE, Zawertailo LA, Kaplan HL, Sellers EM. Identifying appropriate subjects for abuse liability studies using prestudy pharmacological testing. Can J Clin Pharmacol. 1999;6:103–110. [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112:65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain. 2008;9:360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Chapman SL, Byas-Smith MG, Reed BA. Effects of intermediate- and long-term use of opioids on cognition in patients with chronic pain. Clin J Pain. 2002;18:S83–90. doi: 10.1097/00002508-200207001-00010. [DOI] [PubMed] [Google Scholar]
- Chou R, Clark E, Helfand M. Comparative efficacy and safety of long-acting oral opioids for chronic non-cancer pain: a systematic review. J Pain Symptom Manage. 2003;26:1026–1048. doi: 10.1016/j.jpainsymman.2003.03.003. [DOI] [PubMed] [Google Scholar]
- Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc. 2003;9:419–428. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95 2:S129–144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Litten RZ, Lowman C, Hunt WA. Craving research: future directions. Addiction. 2000;95 2:S247–255. doi: 10.1080/09652140050111816. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78:647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Gaertner J, Radbruch L, Giesecke T, Gerbershagen H, Petzke F, Ostgathe C, Elsner F, Sabatowski R. Assessing cognition and psychomotor function under long-term treatment with controlled release oxycodone in non-cancer pain patients. Acta Anaesthesiol Scand. 2006;50:664–672. doi: 10.1111/j.1399-6576.2006.01027.x. [DOI] [PubMed] [Google Scholar]
- Haertzen C, Hickey J. Addiction Research Center Inventory (ARCI): Measurement of euphoria and other drug effects. In: Bozarth M, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 489–524. [Google Scholar]
- Heaton R, Marcotte T. Clinical Neuropsychological Tests and Assessment Techniques. In: Boller F, Grafman J, Rizzolatti Ge, editors. Handbook of Neuropsychology. Elsevier Science BV; Amsterdam: 2000. [Google Scholar]
- Heit HA. The truth about pain management: the difference between a pain patient and an addicted patient. Eur J Pain. 2001;5 A:27–29. doi: 10.1053/eujp.2001.0276. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Exp Clin Psychopharmacol. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Tookman A, Jones L, Curran HV. The effects of immediate-release morphine on cognitive functioning in patients receiving chronic opioid therapy in palliative care. Pain. 2005;117:388–395. doi: 10.1016/j.pain.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Katz N, Fernandez K, Chang A, Benoit C, Butler SF. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008;24:528–535. doi: 10.1097/AJP.0b013e318167a087. [DOI] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. In: Forster F, editor. The Medical Clinics of North America. Saunders; New York, NY: 1963. [PubMed] [Google Scholar]
- Kozlowski LT, Wilkinson DA. Use and misuse of the concept of craving by alcohol, tobacco, and drug researchers. Br J Addict. 1987;82:31–45. doi: 10.1111/j.1360-0443.1987.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Beck H, Bromm B. Cognitive performance, mood and experimental pain before and during morphine-induced analgesia in patients with chronic non-malignant pain. Pain. 1997;73:369–375. doi: 10.1016/S0304-3959(97)00123-1. [DOI] [PubMed] [Google Scholar]
- Marin R, Cyhan T, Miklos W. Sleep disturbance in patients with chronic low back pain. Am J Phys Med Rehabil. 2006;85:430–435. doi: 10.1097/01.phm.0000214259.06380.79. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Max M, Payne R, Edwards W, Sunshine A. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 4th. American Pain Society; Chicago: 1999. [Google Scholar]
- McCarberg BH, Barkin RL. Long-acting opioids for chronic pain: pharmacotherapeutic opportunities to enhance compliance, quality of life, and analgesia. Am J Ther. 2001;8:181–186. doi: 10.1097/00045391-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Moldofsky H. The significance of the sleeping-waking brain for the understanding of widespread musculoskeletal pain and fatigue in fibromyalgia syndrome and allied syndromes. Joint Bone Spine. 2008;75:397–402. doi: 10.1016/j.jbspin.2008.01.021. [DOI] [PubMed] [Google Scholar]
- NIDA. Central Nervous System Intersections of Drug Addiction, Chronic Pain and Analgesia. [12/2/2008];2008 http://grants.nih.gov/grants/guide/rfa-files/RFA-DA-09-017.html.
- Ooteman W, Koeter MW, Vserheul R, Schippers GM, van den Brink W. Measuring craving: an attempt to connect subjective craving with cue reactivity. Alcohol Clin Exp Res. 2006;30:57–69. doi: 10.1111/j.1530-0277.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- Panjabi SS, Panjabi RS, Shepherd MD, Lawson KA, Johnsrud M, Barner J. Extended-Release, Once-Daily Morphine (Avinza) for the Treatment of Chronic Nonmalignant Pain: Effect on Pain, Depressive Symptoms, and Cognition. Pain Med. 2008 doi: 10.1111/j.1526-4637.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- Passik SD, Kirsh KL. The interface between pain and drug abuse and the evolution of strategies to optimize pain management while minimizing drug abuse. Exp Clin Psychopharmacol. 2008;16:400–404. doi: 10.1037/a0013634. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25:106–116. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Nonmedical Use of Prescription Pain Relievers. [11/20/2008];2004 May 21; http://oas.samhsa.gov/2k4/pain/pain.htm.
- Streeter CC, Gulliver SB, Baker E, Blank SR, Meyer AA, Ciraulo DA, Renshaw PF. Videotaped cue for urge to drink alcohol. Alcohol Clin Exp Res. 2002;26:627–634. [PubMed] [Google Scholar]
- Taylor RC, Harris NA, Singleton EG, Moolchan ET, Heishman SJ. Tobacco craving: intensity-related effects of imagery scripts in drug abusers. Exp Clin Psychopharmacol. 2000;8:75–87. doi: 10.1037//1064-1297.8.1.75. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. Imagery and smoking urges: the manipulation of affective content. Addict Behav. 1990;15:531–539. doi: 10.1016/0306-4603(90)90053-z. [DOI] [PubMed] [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP, Galva KE, Lichtor JL. Subjective, psychomotor, and physiological effects of cumulative doses of mixed-action opioids in healthy volunteers. Psychopharmacology (Berl) 2001;155:362–371. doi: 10.1007/s002130100723. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychologic adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin J Pain. 2007;23:307–315. doi: 10.1097/AJP.0b013e3180330dc5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale Administration and Scoring Manual. Harcourt Brace & Co; San Antonio, TX: 1997. [Google Scholar]
- Wilsey BL, Fishman SM, Tsodikov A, Ogden C, Symreng I, Ernst A. Psychological Comorbidities Predicting Prescription Opioid Abuse among Patients in Chronic Pain Presenting to the Emergency Department. Pain Med. 2008 doi: 10.1111/j.1526-4637.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Zacny JP. Characterizing the subjective, psychomotor, and physiological effects of a hydrocodone combination product (Hycodan) in non-drug-abusing volunteers. Psychopharmacology (Berl) 2003;165:146–156. doi: 10.1007/s00213-002-1245-5. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Marks S. Comparing the subjective, psychomotor and physiological effects of intravenous nalbuphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;280:1159–1169. [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S, Bolbolan SA. Profiling the subjective, psychomotor, and physiological effects of a hydrocodone/acetaminophen product in recreational drug users. Drug Alcohol Depend. 2005;78:243–252. doi: 10.1016/j.drugalcdep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Hill JL, Black ML, Sadeghi P. Comparing the subjective, psychomotor and physiological effects of intravenous pentazocine and morphine in normal volunteers. J Pharmacol Exp Ther. 1998;286:1197–1207. [PubMed] [Google Scholar]
- Zelman DC, Brandenburg NA, Gore M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin J Pain. 2006;22:681–685. doi: 10.1097/01.ajp.0000210910.49923.09. [DOI] [PubMed] [Google Scholar]


