Abstract
Echocardiography has emerged as the preferred modality by which diastolic function (DF) is assessed for clinical or research purposes. Echocardiographic indexes and parameters of diastolic function (DF) such as E/A, DT, E/E′, etc. deteriorate with advancing age. Whether the efficiency of filling depends on age is unknown.
To better characterize the filling process and DF in causal rather than correlative terms, we have previously modeled diastole kinematically. We introduced and validated a dimensionless measure of DF termed the kinematic filling efficiency index (KFEI).
In the current study, we determined the effect of aging on DF in terms of KFEI in 72 control subjects without cardiovascular related diseases or pathologies. We also evaluated the age dependence of other conventional parameters of DF. In concordance with other noninvasive DF measures known to decrease with age, KFEI decreases and correlates very strongly with age (R2=0.80). Multivariate analysis showed that age, is the single most important contributor to KFEI (p=0.003).
We conclude that KFEI provides novel insight into DF impairment mechanisms due to aging. These results support the clinical value of KFEI and advance our ability to characterize DF in mechanistic and quantitative terms based on the efficiency of filling.
Keywords: diastolic function, Doppler echocardiography, efficiency, aging
Introduction
The epidemic of heart failure with normal ejection fraction (Redfield 2002; Redfield 2004; Zile et al. 2002) reveals that about half of these subjects have normal left ventricular ejection fraction (LVEF) (Bhatia et al. 2006; Owan et al. 2006). Accordingly, these subjects are referred to as having heart failure with normal ejection fraction (HFNEF) or as having diastolic heart failure (DHF). As a consequence, the importance of quantitating diastolic function (DF) and of diagnosing DHF is increasingly recognized. Concomitantly, both the quantitative (Kovács et al. 2000), and the descriptive (Lester et al. 2008) characterization of DF has gained importance in clinical cardiology and cardiovascular physiology.
Diastolic Function Characterization
Currently, echocardiography is the preferred and most widely used non-invasive DF characterization method (Appleton et al. 2000; Lester et al. 2008). Conventional echocardiographic indexes of DF include the ratio of the transmitral E- and A- wave peaks and their velocity time integrals (Epeak/Apeak, VTIE/VTIA), E- wave deceleration time (DT), isovolumic relaxation time (IVRT), atrial filling fraction (AFF). DTI derived features related to (lateral or septal) mitral annular motion, such as its amplitude (E′) and velocity time integral (VTIE′) (Appleton et al. 2000) are also used to characterize DF. Recently, the presence or absence of longitudinal ‘ringing’ of the chamber as evidenced by annular oscillations after the initial E′-wave has been noted to provide information about relaxation related DF (Riordan et al. 2007). The gold standard for characterizing DF is cardiac catheterization which allows determination of LV end-diastolic pressure (LVEDP), isovolumic relaxation time constant (τ), and the end-diastolic pressure-volume relationship (EDPVR) (Kass 2000).
Diastolic Function and Normal Aging
While it is established that myocardial function changes with advancing age (Kitzman 2000; Lakatta 1993), mechanistic aspects of the change of DF with age are not well characterized. Although measured in various ways, DF becomes impaired during the course of normal aging (Benjamin et al. 1992; Downes et al. 1989; Gates et al. 2003; Hees et al. 2004; Kitzman 2000; Klein et al. 1994; Sveälv et al. 2006; Yang et al. 1999), the age associated change of isovolumic relaxation time constant (τ) remains controversial (Downes et al. 1989; Spirito et al. 1988; Yamakado et al. 1997).
The long axis motion of the mitral annulus determined via tissue Doppler imaging decreases (Sveälv et al. 2006) with age. End-diastolic pressure and ventricular chamber stiffness is higher in older humans and in animals (Pacher et al. 2004). The aging associated changes in DF also depend on gender (Hayward et al. 2000; Sveälv et al. 2006), but its onset may be delayed due to caloric restriction (Meyer et al. 2006).
Kinematic Modeling of Diastolic Filling
All human LVs initiate early, rapid-filling by functioning as a mechanical suction-pump (Katz 1930; Kovács et al. 1987; Zhang et al. 2008). As for any physical device, efficiency is a characteristic that conveys a measure of output relative to input. Though much work has been devoted to characterizing how E- and A- waves (and their features) change in both disease and normal aging, the effect of age on diastolic filling efficiency has not been considered.
Characterizing early rapid filling in efficiency terms is facilitated by the utilization of a parameterized diastolic filling (PDF) formalism by which the kinematics (i.e. how things move) of filling (Kovács et al. 1987; Kovács et al. 2001; Kovács et al. 2000) is modeled. Using this paradigm, a dimensionless kinematic filling efficiency index (KFEI) can be calculated (Zhang et al. 2007).
Hypothesis
In the current work, we investigated the effect of aging on the efficiency of early rapid filling. We hypothesized that KFEI decreases with age, in concordance with other invasive and noninvasive DF indexes. We tested our hypothesis by determining KFEI as well as other noninvasive and invasive DF parameters in 72 subjects.
Methods
This study was a cross sectional study where subjects of different ages were studied only once, rather than a longitudinal study where the same subjects were studied repeatedly as they aged.
Subject Selection
Seventy-two subjects were selected from the existing Cardiovascular Biophysics Laboratory database (Chung et al. 2008; Lisauskas et al. 2001).
Subjects were referred by their cardiologist for catheterization to rule out the presence of suspected coronary artery disease. The subjects selected are free from coronary artery disease or other cardiac pathologies. Additional selection criteria for inclusion were: normal LVEF (LVEF ≥ 55%), normal sinus rhythm, clearly discernible E-waves, and normal valvular function. None of the 72 subjects had previously diagnosed myocardial infarction, peripheral vascular disease, bundle branch block, ischemia, congestive heart failure or any other discernible cardiovascular co-morbidities. All subjects were normotensive at the time of data acquisition, although some of them have been treated for hypertension.
The 72 subjects were divided into 8 age groups, and the parameter values were averaged within the groups and compared. Unless otherwise stated, the parameter values refer to the average values. The numbers of subjects in each age group ranged from 5 to 16. This is a reflection of the cross-sectional and the retrospective nature of the study. The overall sample therefore reflects the age distribution encountered in clinical practice.
All subjects provided informed consent prior to the procedure in accordance with a protocol approved by the Washington University Human Research Protection Office (HRPO).
Data Acquisition
The method of simultaneous high fidelity, in-vivo pressure-volume and echocardiographic transmitral flow data recording has been previously detailed (Chung et al. 2006; Lisauskas et al. 2001; Lisauskas et al. 2001).
Immediately before the catheterization, a complete two-dimensional/Doppler examination was performed in the catheterization laboratory with a standard clinical imaging system (Acuson, Mountain View, CA and Philips, Eindhoven, the Netherlands) including DTI of the lateral mitral annulus and transmitral Doppler inflow according to American Society of Echocardiography criteria (Gottdiener et al. 2004).
Acquired data reflected steady-state physiology, and load was not varied, other than normal respiratory variation, in accordance with the HRPO approved protocol.
Kinematic Modeling of Diastolic Filling and the definition of KFEI
We have previously modeled and validated the kinematics of filling in terms of the motion of a damped simple harmonic oscillator (SHO), where the model predicted velocity of the SHO corresponds to the velocity contour of the clinically recorded E-wave (Kovács et al. 1987). From this paradigm, the kinematic filling efficiency index (KFEI) can be defined and calculated for each E-wave (Zhang et al. 2007). The kinematic model and KFEI are detailed in the Appendix.
Data Analysis
For each subject, approximately 1–2 minutes of continuous transmitral flow data were recorded by pulsed-wave Doppler. An average of 5 (continuous) beats per subject were selected and analyzed to minimize the effect of respiratory variation.
All E- and A- waves were first analyzed using the conventional, triangle shape approximations (Appleton et al. 2000) providing the peak E-wave velocity (Epeak), E-wave acceleration and deceleration times (AT and DT), E-wave duration and velocity-time integral (Edur and VTIE), peak A-wave velocity (Apeak), and A-wave velocity-time integral (VTIA). The ratios of Epeak to Apeak (E/A) and VTIE to VTIA (VTIE/VTIA) were also calculated for all analyzed beats. Furthermore, the peak velocities of tissue Doppler E′- and A′- waves (E′peak, A′peak) were measured at the lateral segment and E/E′ for all the subjects was computed.
The presence or absence of the mitral annular oscillation was determined in all subjects. Since the presence or absence of annular oscillation in a particular patient is consistent, for each subject this value is either 1 (present) or 0 (absent). The percentages of the subjects having mitral annular oscillation among all subjects in each age group were computed.
All E-waves were also subjected to model-based image processing (MBIP) using the Parametrized Diastolic Filling (PDF) formalism to yield three PDF parameters (relaxation/viscoelasticity parameter c, stiffness parameter k, initial load parameter xo). Each E-wave velocity contour is uniquely defined by these parameters (Kovács et al. 1987; Kovács et al. 2000). The Model Based Image Processing (MBIP) method by which PDF parameters are obtained from digitized recordings of transmitral flow has been previously described (Kovács et al. 1987; Kovács et al. 2000) and is briefly reviewed in Appendix 1. Individual k, c, and xo (PDF) parameters were determined for each analyzed E-wave. The kinematic filling efficiency index (KFEI, see Appendix for details), a dimensionless parameter defined by the E-wave filling volume normalized to an idealized lossless filling volume, was computed for all E-waves according to Equation A1.3 (Zhang et al. 2007). In order to investigate the gender dependence of KFEI, the data was analyzed according to gender. The dependence of KFEI on age, τ, and LVEDP was also determined.
In addition, hemodynamic parameters were determined from the simultaneous high-fidelity Millar LVP data for each beat. A custom LABVIEW (National Instruments, Austin, TX) program was used to measure the end-diastolic pressure (LVEDP), peak-positive dP/dt (+dP/dtmax) and peak-negative dP/dt (−dP/dtmax). The time-constant of isovolumic relaxation (t) was calculated for all analyzed beats using previously reported methodology (Weiss et al. 1976).
LV mass was computed using short axis M-mode data according to established criteria (Devereux et al. 1986).
Statistical Analysis
The normality of parameters of interest was checked using one sample Kolmogorov-Smirnov test using SPSS (SPSS, Chicago, IL). In this analysis, the parameter values from all subjects were used together for all the parameters other than the numbers of subjects and the oscillation ratio. For these two parameters, the tests were performed on the binned data for all age groups.
The relations between age and DF parameters were evaluated using linear regression. The significance of the slope, the R2 value for the linear regression, and the 95% confidence intervals of the slopes and intercepts were also obtained. All linear regresion analyses were performed using SPSS (SPSS, Chicago, IL)..
The dependence of KFEI on age and gender was evaluated in all individual patients (n=72) using a multivariate linear model performed by SAS (GLM procedure) (SAS Institute, Cary, NC). The model used was KFEI = a1× Age + a2 Gender. The significance of the parameters a1 and a2 was evaluated. The order by which age and gender enters the model was changed and the corresponding significance was also evaluated.
Results
Subject Groups
The clinical descriptors for the group are shown in Table 1. For each DF parameter, the measurements from each subject were averaged for each age group to generate the average parameter value for the corresponding age group. The parameter values in this manuscript all refer to the averaged values.
Table 1.
Clinical descriptors, noninvasive DF parameters, invasive DF parameters, and kinematic DF parameters for all age groups.
| Age group | 30–40 | 41–45 | 46–50 | 51–55 | 56–60 | 61–65 | 66–70 | >70 | Average of all patients |
|---|---|---|---|---|---|---|---|---|---|
| Parameters | |||||||||
| Clinical Discriptors for all age groups | |||||||||
| N | 6 | 9 | 9 | 16 | 10 | 9 | 8 | 5 | 9±3 |
| Average age | 35 | 43 | 49 | 53 | 57 | 62 | 68 | 75 | 55±11 |
| Gender (m/f) | 3/3 | 8/1 | 5/4 | 9/7 | 7/3 | 6/3 | 4/4 | 3/2 | 45/27 |
| HR (bpm) | 75±17 | 68±8 | 72±10 | 67±8 | 64±10 | 69±14 | 66±12 | 68±8 | 68±11 |
| LVEF (%)* | 72±7 | 69±10 | 75±7 | 76±8 | 70±11 | 70±12 | 78±8 | 75±6 | 73±9 |
| LVEDV (ml) | 183±41 | 171±40 | 129±28 | 156±52 | 184±40 | 164±33 | 182±37 | 130±17 | 163±42 |
| LV mass (g) | 140±12 | 152±23 | 171±41 | 161±47 | 164±35 | 131±55 | 144±19 | 151±24 | 153±37 |
| Noninvasive DF parameters in all age groups | |||||||||
| E/A | 1.5±0.3 | 1.2±0.2 | 1.1±0.2 | 1.2±0.2 | 1.1±0.2 | 1.0±0.2 | 1.0±0.2 | 1.0±0.3 | 1.1±0.2 |
| VTIE/VTIA | 2.6±0.7 | 1.8±0.3 | 1.8±0.4 | 1.8±0.4 | 1.7±0.3 | 1.6±0.2 | 1.6±0.2 | 1.4±0.2 | 1.8±0.4 |
| DT (ms) | 176±23 | 190±27 | 183±26 | 213±40 | 217±46 | 214± 25 | 231±30 | 205±40 | 205±36 |
| Epeak (cm/s) | 0.9±0.1 | 0.7±0.1 | 0.8±0.1 | 0.8±0.2 | 0.7±0.1 | 0.8±0.2 | 0.8±0.1 | 0.8±0.2 | 0.8±0.2 |
| Apeak (cm/s) | 0.6±0.1 | 0.6±0.1 | 0.7±0.1 | 0.7±0.1 | 0.7±0.1 | 0.8±0.1 | 0.8±0.2 | 0.8±0.1 | 0.7±0.1 |
| E/E′ | 4.5±0.4 | 4.1±1.3 | 4.5±1.6 | 4.7±1.3 | 5.2±2.8 | 4.0±1.1 | 3.8±1.8 | 5.7±2.2 | 4.5±1.7 |
| AFF** (%) | 29±6 | 37±3 | 37±6 | 37±4 | 37±3 | 38±4 | 38±3 | 42±4 | 37±5 |
| IVRT (ms) | 64±10 | 62±14 | 68±17 | 76±14 | 81±12 | 72±18 | 79±12 | 80±13 | 73±15 |
| Invasive DF parameters in all age groups | |||||||||
| LVEDP (mmHg) | 17±4 | 15±3 | 16±6 | 16±4 | 18±4 | 17±2 | 16±5 | 18±3 | 17±4 |
| τ (ms) | 42±8 | 44±9 | 45±8 | 46±6 | 54±13 | 44±7 | 43±5 | 45±8 | 46±9 |
| +dP/dtmax(mmHg/s)(103) | 1.0±0.0 | 1.2±0.3 | 1.2±0.3 | 1.3±0.2 | 1.2±0.1 | 1.3±0.3 | 1.5±0.4 | 1.4±0.2 | 1.3±0.3 |
| −dP/dtmax (mmHg/s)(103) | −1.4±0.2 | −1.6±0.4 | −1.5±0.2 | −1.6±0.3 | −1.4±0.2 | −1.6±0.3 | −1.8±0.2 | −1.7±0.3 | −1.6±0.3 |
| Kinematic DF parameters in all age groups | |||||||||
| Oscillation ratio | 0.67 | 0.7 | 0.44 | 0.44 | 0.5 | 0.11 | 0.13 | 0.2 | 0.5 |
| c (1/s) | 13.7±2.3 | 16.6±2.7 | 18.7±5.6 | 19.7±5.1 | 19.7±4.7 | 18.3±3.5 | 21.8±3.9 | 25.7±4.0 | 19.2±4.9 |
| k (1/s+) | 210±43 | 208±39 | 203±43 | 206±59 | 174±56 | 182±36 | 181±34 | 226±61 | 197±48 |
| 1/2kxo2 (cm2/s2) | 0.9±0.2 | 0.8±0.3 | 1.1±0.4 | 1.3±0.8 | 1.3±0.4 | 1.2±0.5 | 1.4±0.5 | 1.6±0.8 | 1.2±0.6 |
| EVTI/(1/2kxo2) (s2/cm) | 0.13±0.02 | 0.14±0.05 | 0.11±0.03 | 0.12±0.06 | 0.09±0.02 | 0.12±0.04 | 0.09±0.03 | 0.08±0.02 | 0.11±0.04 |
| kxo (cm/s2) | 19.1±2.2 | 17.7±3.6 | 19.8±3.1 | 21.5±7.8 | 20.2±3.3 | 20.1±6.0 | 22.3±4.0 | 27.7±9.3 | 20.8±5.8 |
| KFEI | 0.61±0.06 | 0.56±0.04 | 0.55±0.03 | 0.54±0.02 | 0.52±0.01 | 0.54±0.02 | 0.52±0.01 | 0.51±0.01 | 0.54±0.04 |
LVEF determined by ventriculography
AFF, atrial filling fraction. LVEDV = Left ventricular end-diastolic volume from ventriculography. Data are presented as mean ± (SD)
Conventional Echocardiographic Measurements vs. Age
In concordance with previous findings, E/A (p<0.01) and VTIE/VTIA (p<0.05) decrease with age. Deceleration time (DT) lengthens with advancing age (p=0.033). IVRT increases with age (R2= 0.69, p=0.011). See Table 2.
Table 2.
Linear relations between all measured parameters and age in all age groups.
| Parameter | Slope | Intercept | 95% confidence interval of slope | 95% confidence interval of intercept | Significance of slope | R2 |
|---|---|---|---|---|---|---|
| Clinical Descriptors | ||||||
| HR (bpm) | −0.178 | 78.5 | [−0.341, −0.153] | [69.3, 87.6] | 0.074 | 0.44 |
| LVEF* (%) | 0.108 | 67.0 | [−0.070, 0.286] | [56.9, 77.2] | 0.284 | 0.19 |
| LVEDV (ml) | −0.536 | 192.1 | [−1.841, 0.769] | [118.2, 266.0] | 0.452 | 0.10 |
| LV mass (g) | −0.123 | 158.5 | [−0.931, 0.685] | [112.8, 204.2] | 0.776 | 0.02 |
| Noninvasive DF parameters | ||||||
| E/A | 0.012 | 1.8 | [0.008, 0.016] | [1.5, 2.0] | 0.002 | 0.82 |
| VTIE/VTIA | −0.023 | 3.0 | [−0.034, −0.011] | [2.3, 3.8] | 0.012 | 0.68 |
| DT (ms) | 1.069 | 144.6 | [0.312, 1.823] | [101.7, 187.4] | 0.033 | 0.56 |
| Epeak (cm/s) | 0.015 | 78.0 | [−0.238, 0.267] | [63.6, 92.3] | 0.913 | 0.002 |
| Apeak (cm/s) | 0.692 | 32.9 | [0.492, 0.892] | [21.6, 44.3] | 0.001 | 0.88 |
| E/E ′ | 0.016 | 3.7 | [−0.022, 0.053] | [1.6, 5.8] | 0.437 | 0.10 |
| AFF** (%) | 0.246 | 23.4 | [0.134, 0.358] | [17.1, 29.7] | 0.005 | 0.76 |
| IVRT (ms) | 0.459 | 47.5 | [0.210, 0.708] | [33.5, 61.6] | 0.011 | 0.69 |
| Invasive DF parameters | ||||||
| LVEDP (mmHg) | 0.039 | 14.7 | [−0.016, 0.094] | [11.5, 17.8] | 0.215 | 0.24 |
| τ (ms) | 0.050 | 42.6 | [−0.184, 0.283] | [29.4, 55.8] | 0.691 | 0.03 |
| +dP/dtmax (mmHg/s) | 6.436 | 934.7 | [2.487, 10.385] | [711.3, 1158.2] | 0.019 | 0.63 |
| −dP/dtmax (mmHg/s) | −5.716 | −1259.3 | [−11.437, 0.005] | [−1583.1, −935.6] | 0.098 | 0.39 |
| Kinematic DF parameters | ||||||
| Oscillation ratio | −0.016 | 1.3 | [−0.021, 0.010] | [0.9, 1.6] | 0.004 | 0.78 |
| c (1/s) | 0.249 | 5.5 | [0.169, 0.330] | [0.9, 10.1] | 0.001 | 0.86 |
| k (1/s2) | −0.191 | 209.2 | [−1.261, 0.879] | [148.7, 269.7] | 0.739 | 0.20 |
| 1/2kxo2 (cm2/s2) | 0.019 | 0.2 | [0.013, 0.024] | [−0.2, 0.5] | 0.001 | 0.84 |
| EVTI/(1/2kxo2) (s2/cm) | −0.001 | 0.2 | [−0.002, −0.001] | [0.1, 0.2] | 0.007 | 0.73 |
| kxo (cm/s2) | 0.188 | 10.7 | [0.080, 0.296] | [4.5, 16.8] | 0.015 | 0.66 |
| KFEI | −0.002 | 0.67 | [−0.003, −0.001] | [0.62, 0.72] | 0.003 | 0.80 |
| KFEIFemale | −0.002 | 0.65 | [−0.003, −0.001] | [0.59, 0.71] | 0.011 | 0.69 |
| KFEIMale | −0.002 | 0.64 | [−0.002, −0.002] | [0.62, 0.66] | <0.001 | 0.94 |
LVEF determined by ventriculography,
AFF, atrial filling fraction. LVEDV = Left ventricular end-diastolic volume from ventriculography. Data are presented as mean ± (SD)
Hemodynamic Parameters vs. Age
LVEDP and isovolumic relaxation time constant (τ) are relatively age insensitive (p=0.22, 0.69, respectively). The magnitude of +dP/dtmax increases with age (p=0.02), and the magnitude of −dP/dtmax increase, but not significantly (p=0.098), with age. See Table 2.
Kinematic DF Parameters and KFEI vs. Age
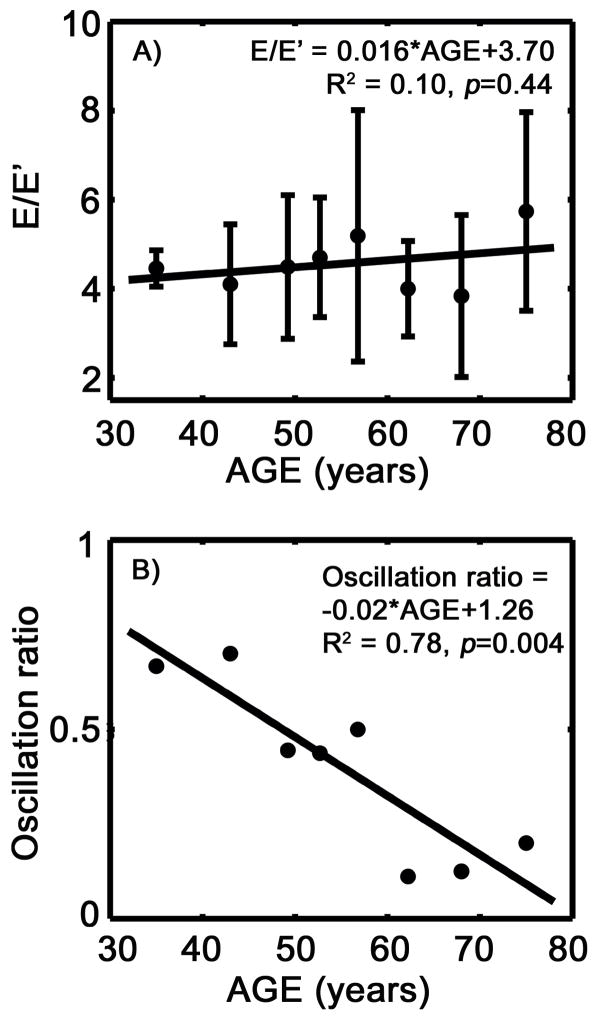
E/E′ remains fairly constant as age increases (p=0.44). However, the fraction of subjects with annular oscillation in each age group decreases with advancing age (p<0.01), as shown in Figure 1A and B and Table 2.
Figure 1.
Mitral annular motion via DTI as a DF index. A) E/E ′ vs. age. B) The percentage of subjects in each age group demonstrating mitral annular oscillation for all groups vs. age. See text for details.
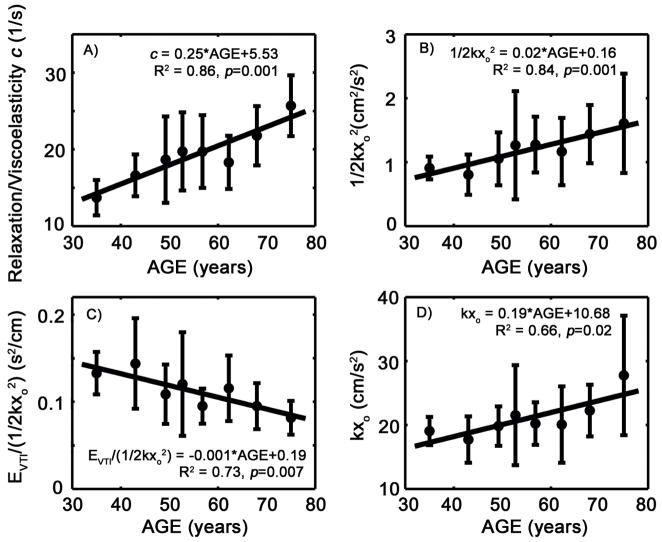
The damping/viscoelasticity parameter c in the PDF formalism increases with age (p<0.01), as shown in Figure 2A. The potential energy available to power the suction process, defined in kinematic modeling terms by 1/2kxo2, increases with age (p<0.01), as shown in Figure 2B. However, the volume that is delivered during early filling per unit amount of potential energy (EVTI/(1/2kxo2)) at the onset of diastole decreases (p<0.01), as shown in Figure 2C. Also, the peak atrio-ventricular pressure gradient that drives the blood flow during early filling (in terms of the PDF derived expression kxo) increases with age (p<0.05), as shown in Figure 2D.
Figure 2.
The relationship of selected PDF parameters and indexes computed from them vs. age. A) The PDF parameter c increases monotonically with age. B) Initial potential energy, defined by 1/2kxo2, increases with age. C) The volume delivered during early filling per unit amount of potential energy at the onset of diastole decreases with age. D) The peak atrio-ventricular pressure gradient during early filling (kxo) increases with age. See text for details
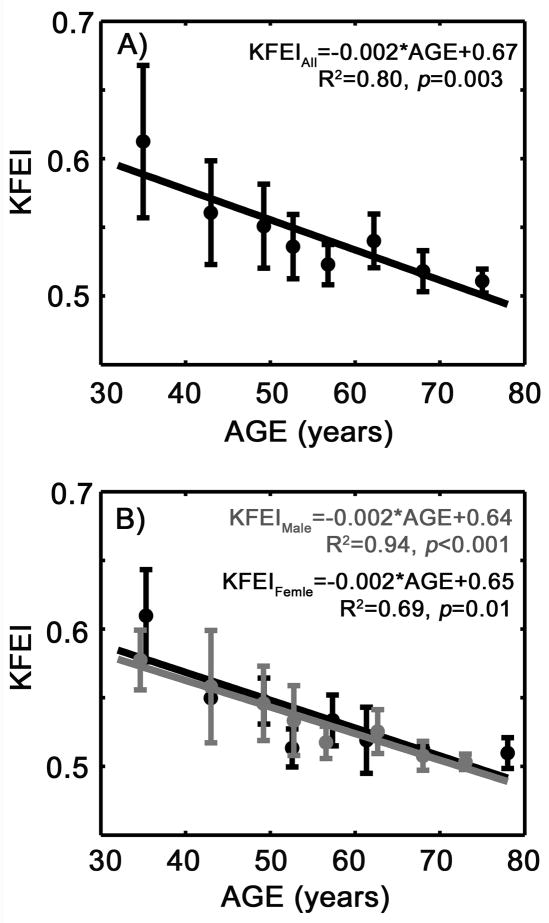
KFEI showed a very strong negative correlation with age (R2 = 0.80, p=0.003), as shown in Figure 3A. When broken down according to gender, the correlations between KFEI and age are similar (Figure 3B).
Figure 3.
A) The kinematic filling efficiency index (KFEI) decreases with age in all subjects. B) The correlations between KFEI and age do not show significant difference between genders. Gray solid circle, male. Gray straight line, linear regression for the male subjects. Black solid circle, female. Black straight line, linear regression for female subjects. See text for details.
LV Mass vs. Age
LV mass determined according to established criteria does not change with age (p=0.78, R2=0.02). The LV mass value (153±37g) was within the normal range published in the literature (Lang et al. 2006; Weyman 1994).
The Normality of the Parameter Values
The result indicates that other than VTIE/VTIA and +dP/dtmax, all the distributions of the parameter values are indistinguishable from a normal distribution, as shown in Table 3. The analysis of patient number (N) and oscillation ratio were done on the binned 8 groups.
Table 3.
Result of the one sample Kolmogorov-Smirnov test on all parameters of interest in all patients.
| Parameter | Significance (2- tailed) |
|---|---|
| Clinical Descriptors | |
| N | 0.672 |
| Age (years) | 0.657 |
| HR (bpm) | 0.626 |
| LVEF* (%) | 0.210 |
| LVEDV (ml) | 0.778 |
| LV mass (g) | 0.897 |
| Noninvasive DF parameters | |
| E/A | 0.769 |
| VTIE/VTIA | 0.018 |
| DT (ms) | 0.446 |
| Epeak (cm/s) | 0.396 |
| Apeak (cm/s) | 0.328 |
| E/E ′ | 0.101 |
| AFF** (%) | 0.367 |
| IVRT (ms) | 0.389 |
| Invasive DF parameters | |
| LVEDP (mmHg) | 0.980 |
| τ (ms) | 0.740 |
| +dP/dtmax (mmHg/s) | 0.009 |
| −dP/dtmax (mmHg/s) | 0.367 |
| Kinematic DF parameters | |
| Oscillation ratio | 0.928 |
| c (1/s) | 0.225 |
| k (1/s2) | 0.684 |
| 1/2kxo2 (cm2/s2) | 0.098 |
| EVTI/(1/2kxo2) (s2/cm) | 0.768 |
| kxo (cm/s2) | 0.361 |
| KFEI | 0.203 |
Multivariate Analysis of the Dependence of KFEI on Age and Gender
Multivariate analysis using age and gender as two independent variables showed (by type I sum of squares) that age is the main contributor to the change of KFEI regardless of the order that the parameters entered the model (p<0.001 for age and p=0.53 for gender if age enters first. p<0.001 for age and p=0.82 for gender if gender enters first).
Discussion
The major novel findings of the current study are: age significantly affects the (kinematic model based) PDF parameters and the kinematic filling efficiency index (KFEI) (R2 = 0.80, p=0.003); there was no gender effect on KFEI. These findings reveal the effect of aging on DF indexes, and shed mechanistic light on how diastolic function changes with age.
The Effect of Age on Kinematic DF Parameters
The kinematic filling efficiency index (KFEI) decreases with age (p<0.01). This implies the chamber aspirates less blood volume relative to the ideal scenario when the same (idealized) chamber recoils without energy loss. This decrease due to the increase in the relaxation/viscoelasticity parameter c as a function of age provides mechanistic insight as to how DF becomes impaired in filling efficiency terms.
No significant gender dependence of KFEI was observed. KFEI for both gender groups changed significantly among the low age groups (see Figure 3B) likely attributable to the fact that the sample sizes in these age groups were relatively small.
The relaxation/viscoelasticity PDF parameter c characterizes the energy loss in the system. This parameter differentiates between normal LVEF non-diabetic vs. diabetic animal and human subjects by being higher in diabetic ventricles (Dent et al. 2001; Riordan et al. 2005) indicating an increased viscous loss during filling (Riordan et al. 2005). Parameter c is also an important component of KFEI (Zhang et al. 2007) and E-wave deceleration time (DT) (Shmuylovich et al. 2007). The increase of the PDF parameter c with age provides, in part, the mechanistic and causal reason for the observed prolongation in DT and the associated decrease in KFEI.
In kinematic modeling terms, the potential energy (in ergs), defined by 1/2kxo2, represents the stored elastic energy available at end systole (Kovács et al. 2000) to power the early (suction initiated) filling. The decrease in early filling volume delivered per unit potential energy indicates that as the ventricle ages, it consumes more energy per unit of delivered filling volume. This is also consistent with the decrease of KFEI.
Importantly the PDF analog of the peak atrio-ventricular pressure gradient that drives the blood flow during early filling (kxo) increases with age indicating that the chamber requires greater recoil force to overcome the resistive/viscosity effects in order to deliver the same amount of blood.
We have previously proposed and validated the presence and absence of mitral annulus oscillation as an index for relaxation related diastolic dysfunction (Riordan et al. 2007). In the current study, the proportion of the subjects with mitral annular oscillation decreases with age. This implies increased prevalence of relaxation related diastolic dysfunction with aging (Riordan et al. 2007).
The Effect of Age on Noninvasive DF Indexes
We found that the conventional echocardiographic E- and A-wave parameters, E/A, VTIE/VTIA, both decrease with age. IVRT also lengthens with age. The relative contribution of atrial filling increases and ventricular relaxation becomes worse, as reflected by the age related prolongation of E-wave deceleration time (DT). All these result are consistent with the impairment of relaxation with aging (further discussed in the discussion).
The Effect of Age on Invasive DF Indexes
The age dependence of the isovolumic relaxation time constant τ is controversial (Downes et al. 1989; Spirito et al. 1988; Yamakado et al. 1997). In the current study, we found that LVEDP and τ are relatively insensitive to advancing age.
We found moderate correlations between +dP/dtmax, −dP/dtmax and age (R2=0.63, 0.39, p=0.019 and p=0.098, respectively), as shown in Table 2. These correlations suggest that the surrogate of contractility (+dP/dtmax) or of relaxation (−dP/dtmax) increase with age. The change in +dP/dtmax, −dP/dtmax, together with the decrease of early filling volume delivered by unit amount of initial potential energy at the onset of diastole (VTIE/(1/2kxo2)), suggests less efficient utilization of the energy available.
Relationship between Current Findings and Previous Work
We found that some echo based, kinematic parameters were age sensitive. It is established in the literature that filling related relaxation (E/A, EVTI/AVTI, IVRT) becomes impaired by aging (Appleton et al. 2000; Benjamin et al. 1992; Gardin et al. 1987; Klein et al. 1994; Miyatake et al. 1984; Prasad et al. 2005; Prasad et al. 2007; Spirito et al. 1988). We also observe this trend in our kinematic parameters, for example, the decrease of filling efficiency (KFEI), the increase in the PDF parameter c, the impairment of energy utilization (EVTI/(1/2kxo2)), the increase of the magnitude of +dP/dtmax indicating a stronger contraction is needed to deliver similar amount of stroke volume.
We found isovolumic relaxation time constant τ does not change with age. This finding was consistent with previous work by Yamakado et al. (Yamakado et al. 1997). It has also been shown that τ is causally determined jointly by stiffness (k-analog) and relaxation (c-analog) (Chung et al. 2008). Hence, due to confounding factors, τ may remain relatively age insensitive even though relaxation properties (c) during early filling worsen with aging. The kinematic approach has also been shown to be more sensitive than some of the classical echo derived DF parameters (Riordan et al. 2005; Zhang et al. 2007). So it is not surprising to see some kinematic parameters change with age while other conventional parameters remain fairly constant. The result that IVRT increases with age is in reminiscent to the previous work (Prasad et al. 2005; Prasad et al. 2007; Spirito et al. 1988).
In contrast to previous findings (Arbab-Zadeh et al. 2004; Downes et al. 1989; Templeton et al. 1979) which suggest LV passive stiffness increase with age, we found that chamber stiffness measured by the PDF stiffness parameter k, a validated invasive average stiffness analog (Lisauskas et al. 2001) remains relatively age insensitive in this sample of subjects. The differences between our findings and the literature can be attributed to two reasons. First, in the work done by Arbab-Zadeh et. Downes et al. and Templeton et al. stiffness measurements from a group of young subjects were compared with those from a group of elder subjects. In the current study, all patients were binned into 8 age groups and the dependence of stiffness on age was evaluated. Also, the sample sizes were larger than the current study (close or above 10 in each group vs. 5–6 in the elder group in the current study). Second, in the work mentioned above, end-diastolic pressure volume relationship (EDPVR) was used to characterize LV stiffness, which was not a feature of the current study. Rather, we used a noninvasive stiffness analog which correlates to an invasive average stiffness measurement, is different from EDPVR.
We also found that E/E′, an LVEDP surrogate (Nagueh et al. 1997; Ommen et al. 2000), is also relatively age insensitive. This is consistent with studies that showed no change in pulmonary capillary wedge pressure (LVEDP surrogate) with age (Popović et al. 2006; Prasad et al. 2005). However, it contradicts some literature showing an increase in LVEDP with age (Downes et al. 1989). The difference is likely to be attributed to the methods as described above.
In summary, consistent with published work, we found that relaxation properties, evaluated by most of the conventional noninvasive parameters and the kinematic parameters (PDF parameter c), decrease with age. The stiffness properties, measured invasively (LVEDP, E/E′) and noninvasively (PDF parameter k), do not change with age in this sample of subjects. However, we don’t think that the age dependence of some parameters is artifact because relaxation and stiffness jointly (mathematically) determine filling efficiency (KFEI) and other kinematic parameters (kxo, EVTI/(1/2kxo2)). Their clinical or predictive values are evident because they provide insight into DF that may not be easily assessed by LVEDP or τ.
Mechanisms of Age-related Alterations of DF
The ventricle undergoes anatomical and structural changes with increasing age (Kitzman 2000). These changes alter the myocardial composition and structure and affect connective tissue (Eghbali et al. 1989; Villari et al. 1997), ventricular mass and thickness (Gardin et al. 1979; Gardin et al. 1995; Scholz et al. 1988; Shub et al. 1994), LV and LA chamber sizes (Gardin et al. 1979; Klein et al. 1994; Waller et al. 1983). These anatomical and structural changes together with reduced β-adrenergic tone (Gerstenblith et al. 1976) and calcium sequestration (Froehlich et al. 1978; Lye et al. 2000; Wei et al. 1984) result in increased ventricular stiffness (Miller et al. 1986; Miyatake et al. 1984) and the impairment of relaxation (Gardin et al. 1998). It is worth noting that relaxation/viscoelasticity parameter c is a lumped parameter so that any biologic component that slows relaxation is a possible contributing factor to c.
Limitations
Data acquisition was conducted while subjects were in physiologic steady-state during catheterization and alteration of load was not part of the HRPO approved protocol. Accordingly, the load dependence of KFEI cannot be assessed. Future studies will need to be performed to evaluate possible load dependence.
All the patients included in this study were normotensive at the time of data acquisition. It is true that some of them have been treated for hypertension. However, we found that LV mass didn’t change significantly with age (Table 2). The lack of age dependence of LV mass suggests that treatment of hypertension was effective. The LV mass values in this study were within the normal range published in the literature.
It is accepted that pulmonary venous flow and the difference in mitral A-wave and pulmonary venous A-wave duration are used for diastolic function characterization. Previous work indicates that these measures correlate with chamber stiffness, LVEDP, and relaxation properties (Appleton et al. 1988; Garcia et al. 1998; Gilbert et al. 1989; Nishimura et al. 1989; Rokey et al. 1985). Since we directly measured LVEDP and τ via a Millar catheter, the inclusion of other echocardiographic correlates as previously reported by others, provides minimal additional information.
It has been noted that the association between age and DF indexes are non-linear (Benjamin et al. 1992). An association, as characterized by linear, least-squares regression can be viewed as the dominant term in an association that is actually somewhat nonlinear. We selected linear regression because of its simplicity and the ease of comprehension.
In the current work, we only evaluated the gender dependence of KFEI. The gender dependences of other parameters have been addressed previously by other investigators (Hayward et al. 2000; Mitoff et al. 2007; Sveälv et al. 2006), hence were not included in this work.
Conclusion
Kinematic modeling can characterize DF and permits the derivation of new indexes. In this study, we determined the value of a filling-efficiency index (KFEI) and its dependence on age and gender in a large patient group (n=72). We also determined the change in noninvasive and invasive DF indexes associated with human aging with normal LV function. KFEI decreases monotonically with age with very high correlation (R2=0.80, p<0. 01). These results reinforce the clinical relevance of KFEI and the kinematic approach to the characterization of DF. KFEI provides mechanistic insight into age related diastolic dysfunction and can be used clinically to characterize the presence and severity of diastolic dysfunction in filling efficiency terms.
Acknowledgments
This work was supported in part by the National Institutes of Health, the Whitaker Foundation (Roslyn, VA), the Alan A. and Edith L. Wolff Charitable Trust (St Louis, MO), and the Barnes-Jewish Hospital Foundation. WZ is supported by the American Heart Association Predoctoral Fellowship (#0715729Z). We thank Peggy Brown for expert echocardiographic data acquisition, and the staff of the BJH Cardiac Catheterization Laboratory for their assistance. The comments from anonymous reviewers are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appleton CP, Firstenberg MS, Garcia MJ, Thomas JD. The Echo-Doppler Evaluation of Left Ventricular Diastolic Function - a Current Perspective. Cardiol Clin. 2000;18:513–546. doi: 10.1016/s0733-8651(05)70159-4. [DOI] [PubMed] [Google Scholar]
- Appleton CP, Hatle LK, Popp RL. Relation of Transmitral Flow Velocity Patterns to Left Ventricular Diastolic Function: New Insights from a Combined Hemodynamic and Doppler Echocardiographic Study. J Am Coll Cardiol. 1988;12:426–440. doi: 10.1016/0735-1097(88)90416-0. [DOI] [PubMed] [Google Scholar]
- Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of Aging and Physical Activity on Left Ventricular Compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Levy D, Anderson KM, Wolf PA, Plehn JF, Evans JC, Comai K, Fuller DL, St John Sutton M. Determinants of Doppler Indexes of Left Ventricular Diastolic Function in Normal Subjects (the Framingham Heart Study) Am J Cardiol. 1992;70:508–515. doi: 10.1016/0002-9149(92)91199-e. [DOI] [PubMed] [Google Scholar]
- Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- Chung CS, Ajo DM, Kovács SJ. Isovolumic Pressure-to-Early Rapid Filling Decay Rate Relation: Model-Based Derivation and Validation via Simultaneous Catheterization Echocardiography. J Appl Physiol. 2006;100:528–534. doi: 10.1152/japplphysiol.00617.2005. [DOI] [PubMed] [Google Scholar]
- Chung CS, Kovács SJ. Physical Determinants of Left Ventricular Isovolumic Pressure Decline: Model Prediction with in Vivo Validation. Am J Physiol. 2008;294:H1589–1596. doi: 10.1152/ajpheart.00990.2007. [DOI] [PubMed] [Google Scholar]
- Dent CL, Bowman AW, Scott MJ, Allen JS, Lisauskas JB, Janif M, Wickline SA, Kovács SJ. Echocardiographic Characterization of Fundamental Mechanisms of Abnormal Diastolic Filling in Diabetic Rats with a Parameterized Diastolic Filling Formalism. J Am Soc Echocardiogr. 2001;14:1166–1172. doi: 10.1067/mje.2001.115124. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic Assessment of Left Ventricular Hypertrophy: Comparison to Necropsy Findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- Downes TR, Nomeir A-M, Smith KM, Stewart KP, Little WC. Mechanism of Altered Pattern of Left Ventricular Filling with Aging in Subjects without Cardiac Disease. Am J Cardiol. 1989;64:523–527. doi: 10.1016/0002-9149(89)90433-5. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Robinson TF, Seifter S, Blumenfeld OO. Collagen Accumulation in Heart Ventricles as a Function of Growth and Aging. Cardiovasc Res. 1989;23:723–729. doi: 10.1093/cvr/23.8.723. [DOI] [PubMed] [Google Scholar]
- Froehlich JP, Lakatta EG, Beard E, Spurgeon HA, Weisfeldt ML, Gerstenblith G. Studies of Sarcoplasmic Reticulum Function and Contraction Duration in Young Adult and Aged Rat Myocardium. J Mol Cell Cardiol. 1978;10:427–438. doi: 10.1016/0022-2828(78)90364-4. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, Thomas JD, Klein AL. New Doppler Echocardiographic Applications for the Study of Diastolic Function. J Am Coll Cardiol. 1998;32:865–875. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Arnold AM, Bild DE, Smith V-E, Lima JAC, Klopfenstein HS, Kitzman DW. Left Ventricular Diastolic Filling in the Elderly: The Cardiovascular Health Study. Am J Cardiol. 1998;82:345–351. doi: 10.1016/s0002-9149(98)00339-7. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Drayer JI, Weber M, Rohan MK, Knoll M, Shu VW, Garcia R, Brewer D, Henry WL. Doppler Echocardiographic Assessment of Left Ventricular Systolic and Diastolic Function in Mild Hypertension. Hypertension. 1987;9:II90–II96. doi: 10.1161/01.hyp.9.2_pt_2.ii90. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Henry WL, Savage DD, Ware JH, Burn C, Borer JS. Echocardiographic Measurements in Normal Subjects: Evaluation of an Adult Population without Clinically Apparent Heart Disease. J Clin Ultrasound. 1979;7:439–447. doi: 10.1002/jcu.1870070606. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O’Leary D, Manolio TA. Sex, Age, and Disease Affect Echocardiographic Left Ventricular Mass and Systolic Function in the Free-Living Elderly: The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- Gates PE, Tanaka H, Graves J, Seals DR. Left Ventricular Structure and Diastolic Function with Human Ageing: Relation to Habitual Exercise and Arterial Stiffness. Eur Heart J. 2003;24:2213–2220. doi: 10.1016/j.ehj.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Gerstenblith G, Lakatta EG, Weisfeldt ML. Age Changes in Myocardial Function and Exercise Response. Prog Cardiovasc Dis. 1976;19:1–21. doi: 10.1016/0033-0620(76)90005-0. [DOI] [PubMed] [Google Scholar]
- Gilbert JC, Glantz SA. Determinants of Left Ventricular Filling and of the Diastolic Pressure- Volume Relation. Circ Res. 1989;64:827–852. doi: 10.1161/01.res.64.5.827. [DOI] [PubMed] [Google Scholar]
- Gottdiener JS, Bednarz J, Devereux R, Gardin JM, Klein AL, Manning WJ, Morehead A, Kitzman DW, Oh JK, Quinones MA, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography Recommendations for Use of Echocardiography in Clinical Trials a Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Echocardiography in Clinical Trials. J Am Soc Echocardiogr. 2004;17:1086. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hayward CS, Kelly RP, Collins P. The Roles of Gender, the Menopause and Hormone Replacement on Cardiovascular Function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- Hees PS, Fleg JL, Dong S-J, Shapiro EP. Mri and Echocardiographic Assessment of the Diastolic Dysfunction of Normal Aging: Altered Lv Pressure Decline or Load? Am J Physiol. 2004;286:H782–788. doi: 10.1152/ajpheart.01092.2002. [DOI] [PubMed] [Google Scholar]
- Kass DA. Assessment of Diastolic Dysfunction: Invasive Modalities. Cardiol Clin. 2000;18:571–586. doi: 10.1016/s0733-8651(05)70162-4. [DOI] [PubMed] [Google Scholar]
- Katz LN. The Role Played by the Ventricular Relaxation Process in Filling the Ventricle. Am J Physiol. 1930;95:542–553. [Google Scholar]
- Kitzman DW. Diastolic Dysfunction in the Elderly. Cardiol Clin. 2000;18:597–617. doi: 10.1016/s0733-8651(05)70164-8. [DOI] [PubMed] [Google Scholar]
- Klein AL, Burstow DJ, Tajik AJ, Zachariah PK, Bailey KR, Seward JB. Effects of Age on Left Ventricular Dimensions and Filling Dynamics in 117 Normal Persons. Mayo Clin Proc. 1994;69:212–224. doi: 10.1016/s0025-6196(12)61059-3. [DOI] [PubMed] [Google Scholar]
- Kovács SJ, Barzilai B, Pérez JE. Evaluation of Diastolic Function with Doppler Echocardiography: The PDF Formalism. Am J Physiol. 1987;252:H178–187. doi: 10.1152/ajpheart.1987.252.1.H178. [DOI] [PubMed] [Google Scholar]
- Kovács SJ, McQueen DM, Peskin CS. Modelling Cardiac Fluid Dynamics and Diastolic Function. Philos Trans R Soc Lond A. 2001;359:1299–1314. [Google Scholar]
- Kovács SJ, Meisner JS, Yellin EL. Modeling of Diastole. Cardiol Clin. 2000;18:459–487. doi: 10.1016/s0733-8651(05)70156-9. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular Regulatory Mechanisms in Advanced Age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for Chamber Quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the Mysteries of Diastolic Function Deciphering the Rosetta Stone 10 Years Later. J Am Coll Cardiol. 2008;51:679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- Lisauskas J, Singh J, Courtois M, Kovács SJ. The Relation of the Peak Doppler E-Wave to Peak Mitral Annulus Velocity Ratio to Diastolic Function. Ultrasound Med Biol. 2001;27:499–507. doi: 10.1016/s0301-5629(00)00357-4. [DOI] [PubMed] [Google Scholar]
- Lisauskas JB, Singh J, Bowman AW, Kovács SJ. Chamber Properties from Transmitral Flow: Prediction of Average and Passive Left Ventricular Diastolic Stiffness. J Appl Physiol. 2001;91:154–162. doi: 10.1152/jappl.2001.91.1.154. [DOI] [PubMed] [Google Scholar]
- Lye M, Donnellan C. General Cardiology: Heart Disease in the Elderly. Heart. 2000;84:560–566. doi: 10.1136/heart.84.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-Term Caloric Restriction Ameliorates the Decline in Diastolic Function in Humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Miller TR, Grossman SJ, Schectman KB, Biello DR, Ludbrook PA, Ehsani AA. Left Ventricular Diastolic Filling and Its Association with Age. Am J Cardiol. 1986;58:531–535. doi: 10.1016/0002-9149(86)90028-7. [DOI] [PubMed] [Google Scholar]
- Mitoff PR, Al-Hesayen A, Azevedo E, Newton GE, Mak S. Sex Differences in Basal Hemodynamics and Left Ventricular Function in Humans with and without Heart Failure. Am Heart J. 2007;154:575–580. doi: 10.1016/j.ahj.2007.04.047. [DOI] [PubMed] [Google Scholar]
- Miyatake K, Okamoto M, Kinoshita N, Owa M, Nakasone I, Sakakibara H, Nimura Y. Augmentation of Atrial Contribution to Left Ventricular Inflow with Aging as Assessed by Intracardiac Doppler Flowmetry. Am J Cardiol. 1984;53:586–589. doi: 10.1016/0002-9149(84)90035-3. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler Tissue Imaging: A Noninvasive Technique for Evaluation of Left Ventricular Relaxation and Estimation of Filling Pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- Nishimura RA, Abel MD, Hatle LK, Tajik AJ. Assessment of Diastolic Function of the Heart: Background and Current Applications of Doppler Echocardiography. Part Ii. Clinical Studies. Mayo Clin Proc. 1989;64:181–204. doi: 10.1016/s0025-6196(12)65673-0. [DOI] [PubMed] [Google Scholar]
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical Utility of Doppler Echocardiography and Tissue Doppler Imaging in the Estimation of Left Ventricular Filling Pressures: A Comparative Simultaneous Doppler-Catheterization Study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Hasko G, Kollai M, Szabo C. Left Ventricular Pressure-Volume Relationship in a Rat Model of Advanced Aging-Associated Heart Failure. Am J Physiol. 2004;287:H2132–2137. doi: 10.1152/ajpheart.00405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popović ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, Greenberg NL, Levine BD, Thomas JD. Relationship among Diastolic Intraventricular Pressure Gradients, Relaxation, and Preload: Impact of Age and Fitness. Am J Physiol. 2006;290:H1454–1459. doi: 10.1152/ajpheart.00902.2005. [DOI] [PubMed] [Google Scholar]
- Prasad A, Okazaki K, Zadeh AA, Dijk E, Fu Q, Thomas JD, Levine BD. Abnormalities of Doppler Measures of Diastolic Function in the Healthy Elderly Are Not Related to Alterations of Left Atrial Pressure. Circulation. 2005;111:1499–1503. doi: 10.1161/01.CIR.0000159353.31271.F2. [DOI] [PubMed] [Google Scholar]
- Prasad A, Popović ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The Effects of Aging and Physical Activity on Doppler Measures of Diastolic Function. Am J Cardiol. 2007;99:1629–1636. doi: 10.1016/j.amjcard.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield MM. Heart Failure -- an Epidemic of Uncertain Proportions. N Engl J Med. 2002;347:1442–1444. doi: 10.1056/NEJMe020115. [DOI] [PubMed] [Google Scholar]
- Redfield MM. Understanding “Diastolic” Heart Failure. N Engl J Med. 2004;350:1930–1931. doi: 10.1056/NEJMp048064. [DOI] [PubMed] [Google Scholar]
- Riordan MM, Chung CS, Kovács SJ. Diabetes and Diastolic Function: Stiffness and Relaxation from Transmitral Flow. Ultrasound Med Biol. 2005;31:1589–1596. doi: 10.1016/j.ultrasmedbio.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Riordan MM, Kovács SJ. Absence of Diastolic Mitral Annular Oscillations Is a Marker for Relaxation-Related Diastolic Dysfunction. Am J Physiol. 2007;292:H2952–2958. doi: 10.1152/ajpheart.01356.2006. [DOI] [PubMed] [Google Scholar]
- Rokey R, Kuo L, Zoghbi W, Limacher M, Quinones M. Determination of Parameters of Left Ventricular Diastolic Filling with Pulsed Doppler Echocardiography: Comparison with Cineangiography. Circulation. 1985;71:543–550. doi: 10.1161/01.cir.71.3.543. [DOI] [PubMed] [Google Scholar]
- Scholz DG, Kitzman DW, Hagen PT, Ilstrup DM, Edwards WD. Age-Related Changes in Normal Human Hearts During the First 10 Decades of Life. Part I (Growth): A Quantitative Anatomic Study of 200 Specimens from Subjects from Birth to 19 Years Old. Mayo Clin Proc. 1988;63:126–136. doi: 10.1016/s0025-6196(12)64945-3. [DOI] [PubMed] [Google Scholar]
- Shmuylovich L, Kovács SJ. E-Wave Deceleration Time May Not Provide an Accurate Determination of LV Chamber Stiffness If LV Relaxation/Viscoelasticity Is Unknown. Am J Physiol. 2007;292:H2712–2720. doi: 10.1152/ajpheart.01068.2006. [DOI] [PubMed] [Google Scholar]
- Shub C, Klein AL, Zachariah PK, Bailey KR, Tajik AJ. Determination of Left Ventricular Mass by Echocardiography in a Normal Population: Effect of Age and Sex in Addition to Body Size. Mayo Clin Proc. 1994;69:205–211. doi: 10.1016/s0025-6196(12)61058-1. [DOI] [PubMed] [Google Scholar]
- Spirito P, Maron BJ. Influence of Aging on Doppler Echocardiographic Indices of Left Ventricular Diastolic Function. Br Heart J. 1988;59:672–679. doi: 10.1136/hrt.59.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveälv BG, Fritzon G, Andersson B. Gender and Age Related Differences in Left Ventricular Function and Geometry with Focus on the Long Axis. European Journal of Echocardiography. 2006;7:298–307. doi: 10.1016/j.euje.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Templeton G, Platt M, Willerson J, Weisfeldt M. Influence of Aging on Left Ventricular Hemodynamics and Stiffness in Beagles. Circ Res. 1979;44:189–194. doi: 10.1161/01.res.44.2.189. [DOI] [PubMed] [Google Scholar]
- Villari B, Vassalli G, Schneider J, Chiariello M, Hess OM. Age Dependency of Left Ventricular Diastolic Function in Pressure Overload Hypertrophy. J Am Coll Cardiol. 1997;29:181–186. doi: 10.1016/s0735-1097(96)00440-8. [DOI] [PubMed] [Google Scholar]
- Waller BF, Roberts WC. Cardiovascular Disease in the Very Elderly. Analysis of 40 Necropsy Patients Aged 90 Years or Over. Am J Cardiol. 1983;51:403–421. doi: 10.1016/s0002-9149(83)80072-1. [DOI] [PubMed] [Google Scholar]
- Wei JY, Spurgeon HA, Lakatta EG. Excitation-Contraction in Rat Myocardium: Alterations with Adult Aging. Am J Physiol. 1984;246:H784–791. doi: 10.1152/ajpheart.1984.246.6.H784. [DOI] [PubMed] [Google Scholar]
- Weiss J, Frederiksen JW, Weisfeldt ML. Hemodynamics Determinant of the Time Course of Fall in Canine Left Ventricular Pressure. J Clin Invest. 1976;58:751–760. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyman AE. Principles and Practice of Echocardiography. Lea & Febiger. 1994 [Google Scholar]
- Yamakado T, Takagi E, Okubo S, Imanaka-Yoshida K, Tarumi T, Nakamura M, Nakano T. Effects of Aging on Left Ventricular Relaxation in Humans: Analysis of Left Ventricular Isovolumic Pressure Decay. Circulation. 1997;95:917–923. doi: 10.1161/01.cir.95.4.917. [DOI] [PubMed] [Google Scholar]
- Yang B, Larson DF, Watson R. Age-Related Left Ventricular Function in the Mouse: Analysis Based on in Vivo Pressure-Volume Relationships. Am J Physiol. 1999;277:H1906–1913. doi: 10.1152/ajpheart.1999.277.5.H1906. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chung CS, Riordan MM, Wu Y, Shmuylovich L, Kovács SJ. The Kinematic Filling Efficiency Index of the Left Ventricle: Contrasting Normal Vs. Diabetic Physiology Ultrasound. Med Biol. 2007;33:842–850. doi: 10.1016/j.ultrasmedbio.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chung CS, Shmuylovich L, Kovács SJ. Viewpoint: Is Left Ventricular Volume During Diastasis the Real Equilibrium Volume and, What Is Its Relationship to Diastolic Suction? J Appl Physiol. 2008;105:1012–1014. doi: 10.1152/japplphysiol.00799.2007. [DOI] [PubMed] [Google Scholar]
- Zile MR, Brutsaert DL. New Concepts in Diastolic Dysfunction and Diastolic Heart Failure: Part I: Diagnosis, Prognosis, and Measurements of Diastolic Function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]