Abstract
The parafascicular nucleus of the intralaminar thalamus is implicated in the processing of pain affect in both animals and humans. Administration of morphine into nPf results in preferential suppression of the affective reaction to noxious tailshock in rats. The involvement of the ventrolateral periaqueductal gray in mediating the antinociceptive action of morphine injected into nPf was evaluated. Vocalizations that occur after tailshock offset (vocalization afterdischarges) are a validated rodent model of pain affect, and were preferentially suppressed by injection of morphine into nPf. Vocalizations that occur during tailshock were suppressed to a lesser degree, whereas, spinal motor reflexes (tail flick and hindlimb movements) were unaffected by injection of morphine into nPf. Inactivation of the vPAG via the microinjection of muscimol (GABAA agonist) produced dose-dependent antagonism of morphine-induced increases in vocalization thresholds. The results demonstrate that a functional link between the nPf and vPAG in generating the antinociceptive action of morphine injected into nPf.
Perspective: Microinjection of morphine into nucleus parafascicular preferentially suppressed rats’ affective reaction to noxious stimulation. This affective analgesia was reversed by inactivation of the ventrolateral periaqueductal gray. Understanding the neurobiology underlying the suppression of pain affect will provide insights into new treatments for pain and its associated affective disorders.
Keywords: nucleus parafascicularis, ventrolateral periaqueductal gray, morphine, pain affect, vocalizations
Introduction
The affective dimension of pain motivates those in pain to seek medical care, and contributes to development of emotional disturbances such as anxiety, fear, and depression that contribute to the suffering of patients in pain(35). Successful pain management therefore requires therapeutic strategies directed toward alleviating its affective attributes. The development of these strategies necessitates an understanding of the neurobiology that processes and modulates pain affect.
The intralaminar thalamic parafascicular nucleus (nPf) is implicated in the processing of pain affect. It receives nociceptive afferents(22, 33) and noxious peripheral stimulation evokes neural activity in nPf(17, 60). Ablation of nPf relieves the emotional suffering associated with chronic pain in humans(40, 61) and reduces affective responses of animals to noxious stimulation(18, 30, 53). Reduced pain reports of Alzheimer’s patients are correlated with neuronal degeneration in nPf(52, 55). Alternately, high frequency stimulation of nPf results in reports of intense pain and unpleasantness in humans(58, 59), and aversive pain-like reactions in animals(29, 51).
Mu-opioid receptors are localized within the nPf in rats and humans(21, 37), and the iontophoretic application of morphine into nPf of rats inhibited neural activity elicited by noxious stimulation of the tail(49). The significance of these receptors is indicated by reports that systemic administration of morphine suppressed noxious-evoked neural activity elicited in nPf of cats(32). Additionally, the intense pain accompanying nPf stimulation in humans was suppressed by the systemic administration of mu-opioid agonists(59).
We reported that microinjection of morphine into nPf preferentially suppressed rats’ affective reaction to noxious tailshock(27). The present study evaluated the contribution of the ventrolateral periaqueductal gray (vPAG) to this antinociceptive action. The vPAG is a core midbrain site that underlies antinociception(1, 6). Antinociception elicited from vPAG is mediated by descending projections that engage inhibitory spinopetal projections of the rostral ventromedial medulla (RVM) that suppress nociceptive transmission within the spinal dorsal horn. Ascending projections from the vPAG to the limbic forebrain and thalamus also contribute to the antinociception elicited by vPAG-stimulation or morphine injections into the vPAG. Conversely, antinociception elicited from the limbic forebrain (anterior cingulate cortex, amygdala, habenula) is mediated through projections to the vPAG(34, 46).
A functional interaction between vPAG and nPf is indicated by findings that suppression of pain affect in rats produced by morphine administered into vPAG was blocked by injecting methysergide (serotonin antagonist) into nPf(6). Morphine injected into vPAG also suppressed noxious-evoked neural activity in nPf that was reversed by iontophoretic application of methysergide into nPf(17). A reciprocal interaction between nPf and vPAG is indicated by reports that stimulation of nPf activates the vPAG(54), injection of morphine into nPf activates a subset of non-nociceptive responsive neurons in the nPf(16), and vPAG receives direct projections from nPf(38).
For the present study, the antinociceptive action of morphine injected into nPf was assessed following inactivation of the vPAG by microinjection of the GABAA agonist muscimol(41). Based on our earlier report, we predicted that intra-nPf administered morphine would preferentially suppress rats’ affective reaction to noxious tailshock. Previous research in this laboratory validated vocalization after discharges (VADs) as a rodent model of pain affect (see Discussion). VADs occur immediately following application of noxious tailshock, are organized within the forebrain, and have distinct spectrographic characteristics compared to vocalizations that occur during shock (VDSs). We predicted that morphine injected into the nPf would preferentially elevate VAD threshold compared to thresholds of tailshock-elicited behaviors organized at spinal (SMR = hindlimb movements and tail flexion) and medullary (VDS) levels of the neuraxis(8, 15). If the antinociceptive action of morphine injected into nPf is mediated via the vPAG, then it will be attenuated by administration of muscimol into vPAG.
Materials and Methods
Animals
Male Long-Evans rats (Charles River, Raleigh, NC) ranging from 90 to 150 days old were used. Rats were housed as pairs in plastic cages in a climate controlled vivarium (lights on 6 A.M. to 6 P.M.), and given ad libitum access to food and water. Testing occurred during the light portion of the cycle. Rats were handled one to two times per day for at least 1 week before testing to minimize possible effects of stress from human contact. All procedures were approved by the Animal Investigation Committee of Wayne State University and followed international guidelines.
Surgery & Histology
Surgeries were performed under aseptic conditions. Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) after pretreatment with atropine sulfate (1 mg/kg, i.p.), and positioned in a Kopf small animal stereotaxic frame. All implants were made with single or double stainless steel 22-gauge cannulae (Plastics One, Roanoke, VA) following stereotaxic coordinates adapted from the rat brain atlas of Paxinos and Watson(47) and measured relative to the bregma suture and the top of the level skull. Guides were implanted unilaterally at a 10 degree angle (lateral to medial) 2 mm above the vPAG. During the same surgical session bilateral guide cannulae were implanted 2 mm directly above the nPf. The following coordinates (in millimeters) were used: nPf (bilateral, AP = − 4.3, L = ± 1.2, DV = −4.0) and vPAG (unilateral, AP = − 4.3, L = ± 1.2, DV = −4.0). Guides were affixed to the skull with 4 stainless steel bone screws and cranioplastic cement. Each guide cannula was fitted with a 28-gauge dummy cannula that extended the length of the guide to keep it clear of debris. Rats were given 7–10 days to recover prior to initiation of testing.
Following testing, rats were sacrificed by carbon dioxide asphyxiation. Injection sites were marked by safrin-O dye and brains were extracted and placed in a 20% (w/v) sucrose formalin solution for 48–72 h. Brains were sectioned at 50 μm on a freezing microtome, and injection sites were localized with the aid of the Paxinos and Watson(47) brain atlas by an experimenter unaware of the behavioral outcomes.
Apparatus
Testing was controlled by custom computer programs via a multifunction interface board (DT-2801, Data Translation, Marlboro, MA) installed in a PC. Rats were placed into custom made Velcro body suits and restrained on a Plexiglas pedestal using Velcro strapping that passed through loops located on the underside of the suits (see photograph in (5)). This design maintained rats in a crouching posture throughout testing, enabled them to breathe and vocalize normally, and permitted unobstructed access to the head for intracerebral injections. Testing was conducted within a sound attenuating, lighted, and ventilated chamber equipped with a small window that enabled visual monitoring of rats during testing.
Tailshock (20 ms pulses at 25 Hz for 1,000 ms) was delivered by a computer controlled constant current shocker (STIMTEK, Arlington, MA) through electrodes (0-gauge stainless steel insect pins) placed intracutaneously (.5mm below the skin surface) on opposite sides of the tail, 7.0 cm (cathode) and 8.5 cm (anode) from the base. The utility of this form of tailshock as a noxious stimulus has been extensively discussed(4, 14). The intensity, duration, and timing of tailshocks were controlled by the computer. Current intensity was monitored by the computer via an analog-to-digital converter of the multifunction board that digitized (500 Hz sampling rate) an output voltage of the shocker that was proportional to the current delivered.
SMRs were measured with a semi-isotonic displacement transducer (Lafayette Instruments Model 76614, Lafayette, IN) attached to the rat’s tail with cotton thread. The output voltage of the transducer was amplified (x50) and then digitized (500 Hz sampling rate) by a second analog-to-digital converter of the multifunction board/computer. SMR was defined as movement of the transducer arm by at least 1.0 mm following shock onset. Once SMR criterion was exceeded the output voltage of the transducer was monitored for 2000 ms. The computer recorded the latency (ms), peak amplitude (mm), and magnitude (cm × ms) of tail movement on each trial. Displacement up to 100 mm could be detected.
Vocalizations were recorded by a pressure-zone microphone (Realistic model 33–1090, Tandy, Ft. Worth, TX) located on the wall of the testing chamber 15 cm from the rat’s head. The microphone was connected to an audio amplifier (Technics model SA-160, Tandy, Ft. Worth, TX) and a 10-band frequency equalizer adjusted to selectively amplify frequencies above 1500 Hz. The filtering of low frequencies prevented extraneous noise (i.e. rats’ respiration and movement artifacts) from contaminating vocalization records. The output of the amplifier was integrated by a Coulbourn Instruments (Allentown, PA) contour following integrator (2 ms time base) and digitized (500 Hz sampling rate) by a third analog-to-digital converter of the multifunction board/computer.
The audio system was calibrated by determining the relation between the peak digitized output of the analog-to-digital converter and the amplitude (SPL, B Scale) of a 3.0 kHz pure tone – the approximate fundamental frequency of pain-induced vocalizations of the rat(5, 12). The derived function was used to convert analog-to-digital inputs to decibels (dB). Sound intensities up to 113.0 dB could be measured. The most intense vocalization measured during any sampling period was 103.3 dB. The computer recorded the peak intensity (in decibels), latency (ms), and duration (ms) of vocalizations during the shock epoch (VDS) and for the 2,000 ms interval following shock termination (VAD).
Pain testing
Experimenters were blind to the group assignment of rats. For two consecutive days prior to testing, rats were adapted to the testing apparatus for a period of 20 min/day to minimize the effects of restraint stress. Testing began 10 – 12 min following completion of intracerebral injections. Test sessions consisted of 20 trials. On 16 trials tailshocks between 0.01 and 2.50 mA were delivered, and on 4 trials no current was delivered to permit assessment of false alarm rates. Trials were presented in a randomized order to control for the impact of any particular tailshock intensity on subsequent responding, and to prevent rats from anticipating the intensity of successive tailshocks. Trials were presented with a minimum intertrial interval of 30 s and each test session concluded within 20 min. These procedures caused no observable damage to the tail. Following each test session, the testing apparatus was cleaned with 5% ammonia hydroxide to eliminate stress odors(20).
Drugs & Drug Injections
Morphine sulfate and muscimol (free base) were purchased from Sigma-Aldrich (St. Louis, MO), and prepared in sterile normal saline. The dose of morphine (10 μg/side) administered into nPf was based on our previous report of the dose-dependent antinociceptive action of morphine injected into nPf(27). Intracerebral injections were administered at a constant rate over 2 min via 28-gauge injectors connected to a microinfusion pump (Harvard Model PHD 2000). Injectors extended 2 mm beyond the guide cannulae into the nPf or vPAG. Injectors were left in place for 2 min after the completion of injections to aid the diffusion of drugs into tissue. Injections into nPf and vPAG were made in volumes of 0.5 μl and 0.2 μl, respectively. Injections into vPAG preceded injections into nPf.
Procedures
Rats received two sessions of pain testing. Two groups (n = 5/group) received the following injections into vPAG and nPf prior to each test session: vPAG (muscimol = 50 ng or 100 ng) + nPf (morphine = 10 μg/side) and vPAG (saline) + nPf (morphine = 10 μg/side). An additional 9 rats received morphine injections into nPf but injections of saline and muscimol (50 ng, n = 4 and 100 ng, n = 5) were placed into the tegmentum adjacent to the vPAG. These animals were analyzed separately as anatomical controls. A separate control group (n = 5) was administered vPAG (saline) + nPf (saline) and vPAG (muscimol = 100 ng) + nPf (saline) prior to the two test sessions. Test sessions were separated by 5–7 days. Our experience is that this interval minimizes order effects on response thresholds. The order of treatments was counter balanced for all groups. For groups that received injections of morphine into nPf, rats were injected with either saline (n = 2) or muscimol (n = 3) into vPAG prior to the first test session. Prior to the second test session rats received the opposite treatment into vPAG (saline, n = 3; muscimol, n = 2). The same procedure was applied for the control group and the groups that severed as anatomical controls.
Data analysis
Following each test session, data were reorganized in ascending order according to tailshock intensity. SMR, VDS, and VAD thresholds for each rat were calculated as the minimum current intensity from a string of at least two consecutive intensities that generated the response. Individual group comparisons were made with Student’s t-test for independent or correlated groups. The capacity of muscimol injected into vPAG to reduce increases in response thresholds generated by morphine injections into nPf was analyzed across doses of muscimol for each response by one-way ANOVA with post-hoc pairwise comparisons made using Dunnett’s test.
Results
Behavioral profile
As demonstrated by Carroll and Lim(15), SMR, VDS, and VAD reflect nociceptive processing at progressively higher levels of the neuraxis. Their analysis of rats that received transections of the neuraxis revealed that SMRs are organized at the spinal level (also see (8)), VDSs within the medulla below the pontomedullary border, and VADs within the forebrain (also see (28)). Consistent with our previous reports, responses organized rostrally within the neuraxis were rarely generated without those integrated more caudally. VAD generation, without concomitant elicitation of VDS and SMR occurred on 0.08% of all trials. VDS was elicited without SMR on 0.001% of the trials in which VDS was the most rostrally elicited response.
The effects of treatments on performance of each response at threshold were also analyzed. The capacity of monitored performance variables to detect decrements in performance that confound threshold measurement was established by previous studies8,14. Performance variables at threshold attained following vPAG(saline) + nPf(saline) treatment were compared to performance variables at threshold attained following all other treatments. Latency, amplitude, and magnitude of SMRs, and latency, amplitude, and duration of VDSs and VADs were not affected by any treatment (all ts < 1.2).
False alarm rates for each response were low (SMR = 0.08%, VDS = 0.02%, VAD = 0.01%). The low incidence of false alarms indicates that responses were not induced by drug treatments, were not occurring spontaneously, and were not conditioned responses to the context, but instead were generated by tailshock.
nPf to vPAG Interaction
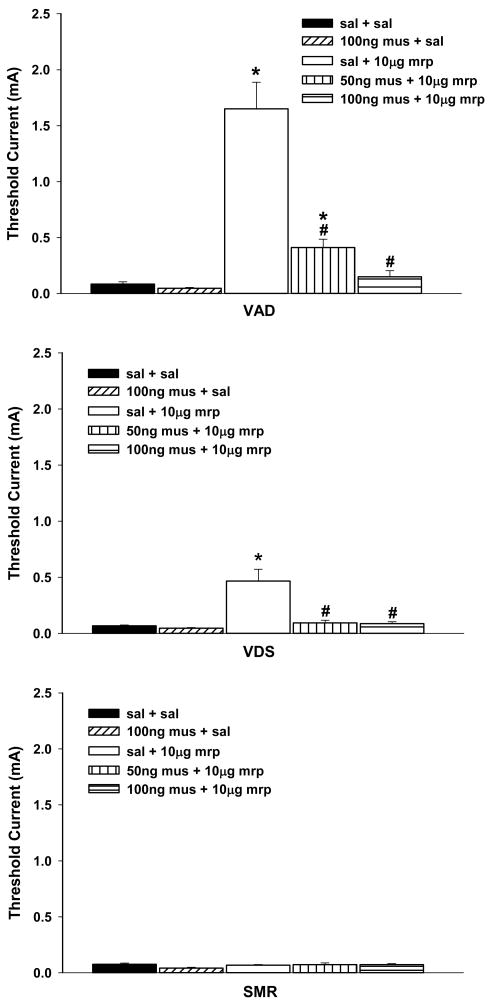
Consistent with our previous report, bilateral administration of morphine into nPf elevated VAD and VDS thresholds, but did not alter SMR threshold (Figure 1). The effects of morphine on response thresholds did not differ between the two groups that received vPAG(saline) + nPf(morphine) injections (t < 1), and these data from these groups were combined for statistical analyses. Comparison of response threshold following vPAG(saline) + nPf(saline) and vPAG(saline) + nPf(morphine) revealed significant increases in VAD and VDS thresholds, ts ≥ 2.69, ps < .05, but not SMR threshold (t < 1). Also consistent with our previous observation, morphine preferentially elevated VAD threshold. Although baseline thresholds of VAD, VDS and SMR did not differ, F(2,14) = 1.66, p > .23, VAD threshold was significantly higher than VDS threshold following vPAG(saline) + nPf(morphine) treatment (t = 4.55, p < .001).
Figure 1.
The effects of muscimol (50 ng or 100 ng) administered into ventrolateral periaqueductal gray (vPAG) on increases in response thresholds generated by the bilateral injection of morphine (5 μg/side) into nucleus parafascicularis (nPf). Caption next to each graph symbol in the legend indicates drug treatment in vPAG and nPf (saline = sal, mrp = morphine, mus = muscimol). Asterisks indicate significant increase in response thresholds when compared to following administration of saline into vPAG and saline into nPf (sal + sal). Pound signs indicate significant reduction in response thresholds when compared to following administration of saline into vPAG and morphine into nPf (sal + mrp). VAD = vocalization after discharge, VDS = vocalization during shock, SMR = spinal motor reflex (tail flick and hindlimb movements).
The effects of morphine injected into nPf on response thresholds were not altered by the order of drug treatments. Comparisons thresholds from subgroups that received vPAG (saline) + nPf (morphine) prior to the first versus second test session revealed no differences, ts < 1.
Pretreatment of the vPAG with muscimol resulted in the dose-dependent antagonism of increases in VAD and VDS thresholds produced by administration of morphine into nPf, Fs(2,19) ≥ 6.03, ps < 0.01 (Figure 1). Both doses of muscimol significantly reduced morphine-induced increases in VDS threshold, Dunnett’s test, ps <.05, and returned threshold to baseline levels, ts(8) ≤ 1.30, all ps > .22. Morphine-induced increases in VAD threshold were reduced after administration of 50 ng muscimol, Dunnett’s test, p < .05, but remained elevated relative to baseline, t(8) = 4.60, p < .01. Administration of 100 ng muscimol also reduced morphine-induced increases in VAD threshold, Dunnett’s test, p < .05, and returned threshold to baseline levels, t(8) = 1.48, p > .17.
Muscimol alone did not alter baseline response thresholds. Comparison of thresholds of each response following vPAG(saline) + nPf(saline) and vPAG(muscimol = 100 ng) + nPf(saline) revealed no significant changes in thresholds, ts < 1. Repeated drug treatments did not alter baseline thresholds. Although the small sample size precluded statistical comparisons, no differences in response thresholds were observed in subgroups that were administered vPAG(saline) + nPf(saline) first or last in the testing sequence.
Anatomical Specificity
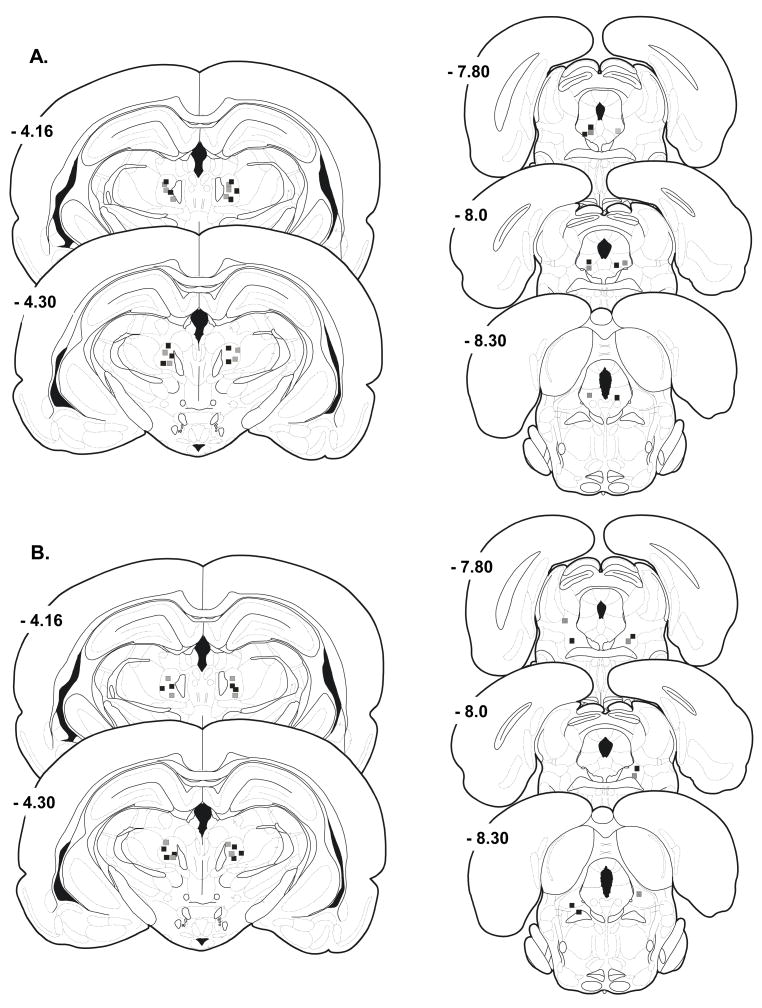
Histological evaluation revealed that the nPf was accurately targeted in all rats (Figure 2A). The effectiveness of morphine to elevate vocalization thresholds did not differ across sites within nPf. We previously demonstrated that the capacity of morphine to elevate vocalization thresholds when injected into nPf is limited to its action within nPf (27). Nine rats received morphine injections into nPf and muscimol (50 ng, n = 4 and 100 ng, n = 5) and saline injections into sites outside the vPAG (Figure 2B). Sites outside the vPAG (extra-vPAG sites) were located in the tegmentum lateral to the vPAG. Data from these rats were analyzed separately as anatomical controls for the effects of muscimol treatments.
Figure 2.
Schematic reproduction of injection sites directed toward nucleus parafascicularis (nPf) and ventrolateral periaqueductal gray (vPAG). Black squares in nPf represent sites injected with morphine following the injection of 100 ng muscimol into vPAG (black squares). Gray squares in nPf represent sites injected with morphine following injection of 50 ng muscimol into vPAG (gray squares). A) Injection sites in nPf and vPAG that accurately targeted vPAG. B) Injection sites in nPf and misplaced vPAG injections into the tegmentum. Coordinates are mm posterior to the bregma suture. Plates are taken from the rat brain atlas of Paxinos and Watson(47).
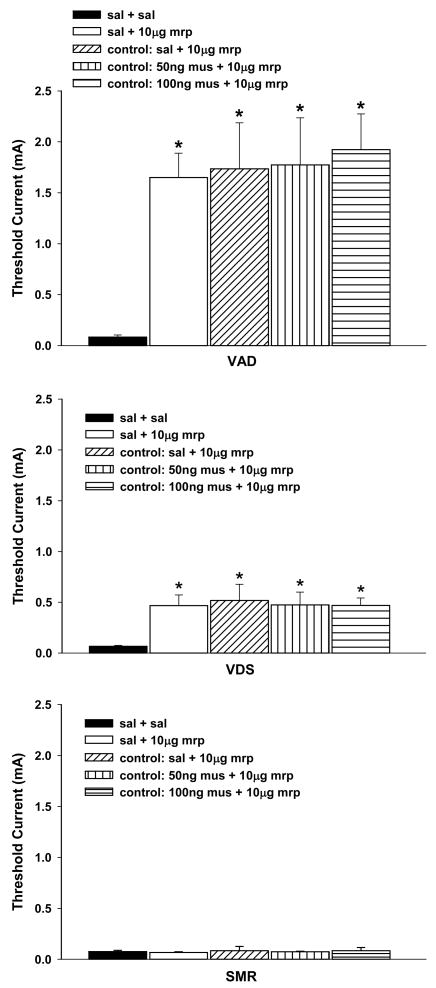
Administration of muscimol into extra-PAG sites failed to alter increases in vocalization thresholds generated by the injection morphine into nPf (Figure 3). The effects of extra-vPAG(saline) + nPf(morphine) injections on response thresholds did not differ between the two groups that also received muscimol injections (50 ng or 100ng) into extra-PAG sites (ts < 1), and these data were combined for statistical analyses. Comparison of thresholds following vPAG(saline) + nPf(saline) treatment with those following injections of 50 ng or 100 ng muscimol into extra-vPAG sites and morphine into nPf revealed significantly elevated VAD and VDS thresholds, Fs(2,13) ≥ 7.20, ps < .01, Dunnett’s test, ps < .05, but not SMR threshold, F(2,13) < 1. These increases in vocalization thresholds did not differ from those observed following injection of saline into extra-vPAG sites and morphine into nPf or vPAG(saline) + nPf(morphine) treatment, Fs < 1.
Figure 3.
Anatomical specificity: Antagonism of increases in response thresholds generated by injection of morphine into nucleus parafascicularis (nPf) by pretreatment of the ventrolateral periaqueductal gray with muscimol. Caption next to each graph symbol in the legend indicates drug treatment in vPAG and nPf (saline = sal, mrp = morphine, mus = muscimol). The designation of ‘control’ in the legend identifies data from rats with misplaced vPAG injections into the tegmentum (see Figure 2). Asterisks indicate significant increase in response thresholds when compared to following administration of saline into vPAG and saline into nPf (sal + sal). VAD = vocalization after discharge, VDS = vocalization during shock, SMR = spinal motor reflex (tail flick and hindlimb movements).
Discussion
Consistent with our previous report, bilateral administration of morphine into nPf elevated VAD and VDS thresholds. Our previous research demonstrated that these elevations in vocalization thresholds reflect the action of morphine at mu-opioid receptors within nPf(27). Increases in vocalization thresholds were reversed in a dose-dependent manner by inactivation of the vPAG via pretreatment with muscimol. This attenuation of morphine antinociception reflected an action of muscimol within the vPAG as control injections made in the tegmentum adjacent to the vPAG failed to reduce morphine-induced increases in vocalization thresholds.
In agreement with our previous report, the administration of morphine into nPf preferentially elevated VAD threshold. Although baseline thresholds of VAD and VDS did not differ, VAD threshold was elevated to a greater extent compared to VDS threshold following intra-nPf injection of morphine. The preferential increase in VAD threshold after intra-nPf morphine administration reflects suppression of the affective reaction to noxious stimulation. As noted earlier, previous research in this laboratory validated VADs as a rodent model of pain affect. Systemically administered drug treatments that preferentially suppress the affective reaction of humans to pain(23, 48) also preferentially suppress production of VADs(9). Generation of VADs is suppressed by damage of or drug treatments into forebrain sites known to contribute to production of the affective response of humans to clinical and experimental pain(6, 12, 26–28, 39, 44, 57). Additionally, the capacity of noxious tailshock to support fear conditioning is directly related to its production of VADs(3, 5, 7).
The failure of morphine injected into nPf to elevate SMR threshold is also consistent with our previous report, and our reports that carbachol and 8-0H-DPAT administered into nPf selectively elevates VAD and VDS thresholds(25, 26). It is unlikely that the failure of nPf-administered morphine to elevate SMR threshold reflects resistance of this response to antinociceptive treatments or injection of an insufficient dose of morphine. Previous studies from this laboratory revealed that increases in VAD and VDS thresholds, comparable to those observed in the present study, produced by the administration of morphine into vPAG, RVM, or spinal subarachnoid space, were accompanied by significant increases in SMR threshold(4, 10, 11). The capacity of these central treatments to elevate SMR threshold also demonstrates that SMRs are not generated by direct stimulation of the tail musculature. These findings indicate that the capacity to elevate SMR threshold depends on the site within the CNS at which antinociceptive treatments are administered.
The present data are consistent with electrophysiological findings that stimulation of nPf activates neurons in the vPAG, and that these vPAG neurons project to the RVM(54). It was proposed that stimulation of the nPf activates (either directly or via disinhibition) vPAG neurons that engage neurons in RVM that provide descending projections that inhibit nociceptive transmission in the spinal dorsal horn. Application of morphine into nPf was shown to activate a subset of non-nociceptive neurons(16), and we speculate that projections of these nPf neurons activate antinociceptive projections of the vPAG. As noted, in the present study no increase in SMR threshold was observed following injection of morphine into nPf. This finding is consistent with our earlier report(27) and with the report that morphine injected into nPf elevated paw-lick latencies in the hot plate test, but not tail flick latencies in the tail-immersion test (56°C water; (19)). Therefore, injection of morphine into nPf activates antinociceptive projections from vPAG that exclusively inhibit nociceptive processing that contributes to production of pain behaviors organized at supraspinal levels of the neuraxis(6, 43).
We previously demonstrated that separate serotonergic projections from vPAG can be engaged that progressively suppress nociceptive processing at forebrain, medullary and spinal levels(6, 10). Administration of 1 μg morphine into vPAG produced a selective elevation of VAD threshold that was reversed by injection of methysergide into nPf or amygdaloid central nucleus (ACe), but not by intrathecal (i.t.) administration of serotonin, norepinephrine or μ-opioid antagonists. This finding is consistent with reports that electrical stimulation of the vPAG suppresses supraspinally organized pain behaviors in rats through inhibition of nociceptive processing at supraspinal levels(42), and that the vPAG provides serotonergic innervation of the nPf that suppresses nociceptive processing within the nPf(17, 50). Increases in VAD and VDS thresholds generated by administration of 2.5 μg morphine into vPAG were also reversed by injection of methysergide into nPf or ACe, but were not affected by i.t. administration of serotonin, norepinephrine or μ-opiate antagonists. Injection of methysergide into RVM also reversed the increase in VDS threshold and attenuated the increase in VAD threshold. Injection of 5 μ morphine into vPAG elevated VAD, VDS and SMR thresholds, but only the elevation of SMR threshold was reversed by i.t. administration of serotonin, norepinephrine or μ-opiate antagonists. Increases in VAD and VDS thresholds were reversed by injection of methysergide into nPf, ACe or RVM. Injection of 10μg morphine into vPAG also elevated VAD, VDS and SMR thresholds. Injection of i.t. antagonists again reversed increases in SMR threshold but only partially reduced increases in VAD and VDS thresholds. The failure of i.t. antagonists to reverse increases in VAD and VDS thresholds does not reflect different thresholds of inhibition of dorsal horn neurons involved in reflex generation (SMR) versus the rostral transmission of nociceptive information (VDS and VAD). Intrathecal administration of serotonin, norepinephrine or morphine produced equivalent dose-dependent increases in VAD, VDS and SMR thresholds(11). Residual increases in VDS and VAD threshold reflect continued inhibition of nociceptive processing at medullary and forebrain levels as they were eliminated following additional injection of methysergide into RVM, nPf or ACe.
Therefore, administration of morphine into nPf directly suppresses nociceptive transmission within nPf and thereby suppresses nociceptive throughput from nPf to cortical areas (i.e., anterior cingulate cortex) involved in processing pain affect(56). Administration of morphine into nPf also activates descending projections to vPAG that activate antinociceptive projections of the vPAG that suppress nociceptive transmission at medullary levels and reciprocal antinociceptive projections of vPAG to nPf. The reciprocal connections between nPf and vPAG may serve to sustain activation of antinociceptive projections of the vPAG as stimulation of nPf induces increases in neural activity in vPAG and behavioral antinociception that exceed the duration of stimulation(45). Within this scheme, increases in VDS threshold reflects activation of descending projections of the vPAG that suppress nociceptive processing at medullary links in the spinoreticulothalamic pathway (i.e., nucleus reticularis gigantocellularis) that contribute to production of VDSs. Increases in VAD threshold also reflect the engagement of descending projections of the vPAG to the medulla that suppresses the throughput of nociceptive transmission to forebrain sites responsible for generating VADs. In addition, as the nPf contributes to the generation of VADs(28) administration of morphine into nPf directly suppresses nociceptive processing underlying the production of VADs. The dual inhibition of nociceptive processing at medullary and forebrain levels could account for the greater effect of nPf administered morphine on VAD versus VDS thresholds. It is unlikely that nPf-administered morphine engages spinopetal inhibitory projections that suppress dorsal horn neurons that contribute ascending nociceptive input to medullary and forebrain sites responsible for generating VDSs and VADs. Activation of these descending projections appears to require sufficient activation of vPAG and RVM to also engage descending inhibitory projections to dorsal horn neurons involved in SMR generation. As noted, SMR thresholds were not elevated in the present study.
The present findings support earlier reports that antinociception elicited from the limbic forebrain (anterior cingulate cortex, amygdala, habenula, nucleus accumbens) is mediated through projections to the vPAG(24, 34, 46, 62). Additional studies demonstrated that reciprocal interactions between vPAG and forebrain sites are critical for the antinociception elicited from the vPAG(13, 36). We reported that an interaction between nPf and ACe mediates increases in vocalization thresholds produced by injection of morphine into vPAG(13). Evidence for other antinociceptive circuits within the brain also exists. For example, the laterodorsal tegmental nucleus sends cholinergic projections to the ventral tegmental area and nPf(2), and administration of carbachol (cholinergic agonist) into these sites produced selective elevations of VAD and VDS thresholds similar to those observed in the present study(25, 31). Gaining a further understanding of brain circuits that contribute to suppression of nociceptive processing principally at supraspinal levels may provide insight into mechanisms that preferentially suppress affective responding to pain. As the affective reaction to pain underlies the emotional suffering associated with pain and contributes to development of secondary emotional disturbances (depression, anxiety) associated with the pain state an understanding of these brain antinociceptive circuits is of clinical importance and warrants additional study.
Acknowledgments
This research was supported by Grant R01 NS045720 from the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 2.Bolton RF, Cornwall J, Phillipson OT. Collateral axons of cholinergic pontine neurones projecting to midline, mediodorsal and parafascicular thalamic nuclei in the rat. J Chem Neuroanat. 1993;6:101–114. doi: 10.1016/0891-0618(93)90031-x. [DOI] [PubMed] [Google Scholar]
- 3.Borszcz GS. The capacity of motor reflex and vocalization thresholds to support avoidance conditioning in the rat. Behav Neurosci. 1993;107:678–693. doi: 10.1037//0735-7044.107.4.678. [DOI] [PubMed] [Google Scholar]
- 4.Borszcz GS. Increases in vocalization and motor reflex thresholds are influenced by the site of morphine microinjection: Comparisons following administration into the periaqueductal gray, ventral medulla, and spinal subarachnoid space. Behav Neurosci. 1995;109:502–522. doi: 10.1037//0735-7044.109.3.502. [DOI] [PubMed] [Google Scholar]
- 5.Borszcz GS. Pavlovian conditional vocalizations of the rat: A model system for analyzing the fear of pain. Behav Neurosci. 1995;109:648–662. doi: 10.1037//0735-7044.109.4.648. [DOI] [PubMed] [Google Scholar]
- 6.Borszcz GS. Differential contributions of medullary, thalamic, and amygdaloid serotonin to the antinociceptive action of morphine administered into the periaqueductal gray: A model of morphine analgesia. Behav Neurosci. 1999;113:612–631. doi: 10.1037//0735-7044.113.3.612. [DOI] [PubMed] [Google Scholar]
- 7.Borszcz GS. Contribution of the ventromedial hypothalamus to generation of the affective dimension of pain. Pain. 2006;123:155–168. doi: 10.1016/j.pain.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borszcz GS, Johnson CP, Anderson ME, Young BJ. Characterization of tailshock elicited withdrawal reflexes in intact and spinal rats. Physiol Behav. 1992;52:1055–1062. doi: 10.1016/0031-9384(92)90459-f. [DOI] [PubMed] [Google Scholar]
- 9.Borszcz GS, Johnson CP, Fahey KA. Comparison of motor reflex and vocalization thresholds following systemically administered morphine, fentanyl, and diazepam in the rat: assessment of sensory and performance variables. Pharmacol Biochem Behav. 1994;49:827–834. doi: 10.1016/0091-3057(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 10.Borszcz GS, Johnson CP, Thorp MV. The differential contribution of spinopetal projections to increases in vocalization and motor reflex thresholds generated by the microinjection of morphine into the periaqueductal gray. Behav Neurosci. 1996;110:368–388. doi: 10.1037//0735-7044.110.2.368. [DOI] [PubMed] [Google Scholar]
- 11.Borszcz GS, Johnson CP, Williams DH. Increases in vocalization and motor reflex thresholds generated by the intrathecal administration of serotonin or norepinephrine. Behav Neurosci. 1996;110:809 – 822. doi: 10.1037//0735-7044.110.4.809. [DOI] [PubMed] [Google Scholar]
- 12.Borszcz GS, Leaton RN. The effect of amygdala lesions on conditional and unconditional vocalizations in rats. Neurobiol Learn Mem. 2003;79:212–225. doi: 10.1016/s1074-7427(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 13.Borszcz GS, Streltsov NG. Amygdaloid-thalamic interactions mediate the antinociceptive action of morphine microinjected into the periaqueductal gray. Behav Neurosci. 2000;114:574–584. doi: 10.1037//0735-7044.114.3.574. [DOI] [PubMed] [Google Scholar]
- 14.Bromm B, Meier W. The intracutaneous stimulus: A new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol. 1984;6:405–410. [PubMed] [Google Scholar]
- 15.Carroll MN, Lim KS. Observations on the neuropharmacology of morphine and morphinelike analgesia. Arch Int Pharmacodyn Ther. 1960;125:383–403. [PubMed] [Google Scholar]
- 16.Dafny N, Gildenberg P. Morphine effects on spontaneous, nociceptive, antinociceptive and sensory evoked responses of parafasciculus thalami units in morphine naive and morphine dependent rats. Brain Res. 1984;323:11–20. doi: 10.1016/0006-8993(84)90260-9. [DOI] [PubMed] [Google Scholar]
- 17.Dafny N, Reyes-Vazquez C, Qiao JT. Modification of nociceptively identified neurons in thalamic parafascicularis by chemical stimulation of dorsal raphe with glutamate, morphine, serotonin and focal dorsal raphe electrical stimulation. Brain Res Bull. 1990;24:717–723. doi: 10.1016/0361-9230(90)90128-m. [DOI] [PubMed] [Google Scholar]
- 18.Delacour J. Effects of medial thalamic lesions in the rat. A review and an interpretation. Neuropsychologia. 1971;9:157–174. doi: 10.1016/0028-3932(71)90040-6. [DOI] [PubMed] [Google Scholar]
- 19.Dupouy V, Zajac J-M. Neuropeptide FF receptors control morphine-induced analgesia in the parafascicular nucleus and the dorsal raphe nucleus. Eur J Pharmacol. 1997;330:129 – 137. doi: 10.1016/s0014-2999(97)01017-0. [DOI] [PubMed] [Google Scholar]
- 20.Fanselow MS. Odors released by stressed rats produce opioid analgesia in unstressed rats. Behav Neurosci. 1985;99:589–592. doi: 10.1037//0735-7044.99.3.589. [DOI] [PubMed] [Google Scholar]
- 21.Frost JJ, Wagner HN, Jr, Dannals RF, Ravert HT, Links JM, Wilson AA, Burns HD, Wong DF, McPherson RW, Rosenbaum AE, Kuhar MJ, Synder SH. Imaging opiate receptors in the human brain by positron tomography. J Comput Assist Tomogr. 1985;9:231–236. doi: 10.1097/00004728-198503000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Giesler GJ, Menetrey D, Bausbaum AJ. Differential origins of spinothalamic tract projections to medial and lateral thalamus in the rat. J Comp Neurol. 1979;184:107–126. doi: 10.1002/cne.901840107. [DOI] [PubMed] [Google Scholar]
- 23.Gracely RH, McGrath P, Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain. 1978;5:19–29. doi: 10.1016/0304-3959(78)90021-0. [DOI] [PubMed] [Google Scholar]
- 24.Hardy SG, Haigler HJ. Prefrontal influences upon the midbrain: a possible route for pain modulation. Brain Res. 1985;339:285–293. doi: 10.1016/0006-8993(85)90094-0. [DOI] [PubMed] [Google Scholar]
- 25.Harte SE, Hoot MR, Borszcz GS. Involvement of the intralaminar parafascicular nucleus in muscarinic-induced antinociception in rats. Brain Res. 2004;1019:152–161. doi: 10.1016/j.brainres.2004.05.096. [DOI] [PubMed] [Google Scholar]
- 26.Harte SE, Kender RG, Borszcz GS. Activation of 5-HT1A and 5-HT7 receptors in the parafascicular nucleus suppresses the affective reaction of rats to noxious stimulation. Pain. 2005;113:405–415. doi: 10.1016/j.pain.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Harte SE, Lagman AL, Borszcz GS. Antinociceptive effects of morphine injected into the nucleus parafascicularis thalami of the rat. Brain Res. 2000;874:78–86. doi: 10.1016/s0006-8993(00)02583-x. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmeister F. Effects of psychotropic drugs on pain. In: Soulariarc A, Cahn J, Charpentier J, editors. Pain. New York: Academic Press; 1968. [Google Scholar]
- 29.Kaelber WW, Mitchell CL. The centrum medianum - central tegmental fasciculus complex. A stimulation, lesion and degeneration study in the cat. Brain. 1967;90:83–100. doi: 10.1093/brain/90.1.83. [DOI] [PubMed] [Google Scholar]
- 30.Kaelber WW, Mitchell CL, Yarmat AJ, Afifi AK, Lorens SA. Centrum medianum-parafascicularis lesions and reactivity to noxious and non-noxious stimuli. Exp Neurol. 1975;46:282–290. doi: 10.1016/0014-4886(75)90135-1. [DOI] [PubMed] [Google Scholar]
- 31.Kender RG, Harte SE, Munn EM, Borszcz GS. Affective analgesia following muscarinic activation of the ventral tegmental area in rats. J Pain. 2008;9:597–605. doi: 10.1016/j.jpain.2008.01.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyama N, Nishikawa Y, Chen J. Differential inhibitory mechanisms in VPL versus intralaminar nociceptive neurons of the cat: II. Effects of systemic morphine and CCK. Jpn J Physiol. 1995;45:1029–1041. doi: 10.2170/jjphysiol.45.1029. [DOI] [PubMed] [Google Scholar]
- 33.Krout KE, Loewy AD. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2000;428:475–494. doi: 10.1002/1096-9861(20001218)428:3<475::aid-cne6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.LaBuda CJ, Fuchs PN. Attenuation of negative pain affect produced by unilateral spinal nerve injury in the rat following anterior cingulate cortex activation. Neuroscience. 2005;136:311–322. doi: 10.1016/j.neuroscience.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Loeser JD. Pain and suffering. Clin J Pain. 2000;16:S2–6. doi: 10.1097/00002508-200006001-00002. [DOI] [PubMed] [Google Scholar]
- 36.Ma QP, Shi YS, Han JS. Naloxone blocks opioid peptide release in nucleus accumbens and amygdala elicited by morphine injected into periaqueductal gray. Brain Res Bull. 1992;28:351–354. doi: 10.1016/0361-9230(92)90202-9. [DOI] [PubMed] [Google Scholar]
- 37.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 38.Marini G, Tredici G. Parafascicular nucleus-raphe projections and termination patterns in the rat. Brain Res. 1995;690:177 – 184. doi: 10.1016/0006-8993(95)00617-y. [DOI] [PubMed] [Google Scholar]
- 39.Mark VH, Ervin FR, Yakovlev PI. Correlation of pain relief, sensory loss, and anatomical lesion sites in pain patients treated with stereotactic thalamotomy. Trans Am Neurol Assoc. 1961;86:86–90. [PubMed] [Google Scholar]
- 40.Mark VH, Ervin FR, Yakovlev PI. The treatment of pain by stereotaxic methods. Confin Neurol. 1962;22:238–245. [Google Scholar]
- 41.Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- 42.Morgan MM, Sohn JH, Liebeskind JC. Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 1989;502:61–66. doi: 10.1016/0006-8993(89)90461-7. [DOI] [PubMed] [Google Scholar]
- 43.Morgan MM, Sohn JH, Liebeskind JC. Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 1990;502:61–66. doi: 10.1016/0006-8993(89)90461-7. [DOI] [PubMed] [Google Scholar]
- 44.Nandigama P, Borszcz GS. Affective analgesia following the administration of morphine into the amygdala of rats. Brain Res. 2003;959:343–354. doi: 10.1016/s0006-8993(02)03884-2. [DOI] [PubMed] [Google Scholar]
- 45.Oleson TD, Twombly DA, Liebeskind JC. Effects of pain-attenuating brain stimulation and morphine on electrical activity in the raphe nuclei of the awake rat. Pain. 1978;4:211–230. doi: 10.1016/0304-3959(77)90134-8. [DOI] [PubMed] [Google Scholar]
- 46.Pavlovic ZW, Cooper ML, Bodnar RJ. Opioid antagonists in the periaqueductal gray inhibit morphine and beta- endorphin analgesia elicited from the amygdala of rats. Brain Res. 1996;741:13–26. doi: 10.1016/s0006-8993(96)00880-3. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [Google Scholar]
- 48.Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 49.Prieto-Gomez B, Dafny N, Reyes-Vazquez C. Dorsal raphe stimulation, 5-HT and morphine microiontophoresis effects on noxious and nonnoxious identified neurons in the medial thalamus of the rat. Brain Res Bull. 1989;22:937–943. doi: 10.1016/0361-9230(89)90003-8. [DOI] [PubMed] [Google Scholar]
- 50.Reyes-Vazquez C, Qiao JT, Dafny N. Nociceptive responses in nucleus parafascicularis thalami are modulated by dorsal raphe stimulation and microiontophoretic application of morphine and serotonin. Brain Res Bull. 1989;23:405–411. doi: 10.1016/0361-9230(89)90181-0. [DOI] [PubMed] [Google Scholar]
- 51.Rosenfeld JP, Holzman BS. Effects of morphine on medial thalamic and medial bulboreticular aversive stimulation thresholds. Brain Res. 1978;150:436–440. doi: 10.1016/0006-8993(78)90297-4. [DOI] [PubMed] [Google Scholar]
- 52.Rub U, Del Tredici K, Del Turco D, Braak H. The intralaminar nuclei assigned to the medial pain system and other components of this system are early and progressively affected by the Alzheimer’s disease-related cytoskeletal pathology. J Chem Neuroanat. 2002;23:279–290. doi: 10.1016/s0891-0618(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 53.Saade NE, Al Amin H, Abdel Baki S, Chalouhi S, Jabbur SJ, Atweh SF. Reversible attenuation of neuropathic-like manifestations in rats by lesions or local blocks of the intralaminar or the medial thalamic nuclei. Exp Neurol. 2007;204:205–219. doi: 10.1016/j.expneurol.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Sakata S, Shima F, Kato M, Fukui M. Dissociated mesencephalic responses to medial and ventral thalamic nuclei stimulation in rats. Relationship to analgesic mechanisms. J Neurosurg. 1989;70:446–453. doi: 10.3171/jns.1989.70.3.0446. [DOI] [PubMed] [Google Scholar]
- 55.Scherder EJ, Sergeant JA, Swaab DF. Pain processing in dementia and its relation to neuropathology. Clin Neurophysiol. 2003;2:677–686. doi: 10.1016/s1474-4422(03)00556-8. [DOI] [PubMed] [Google Scholar]
- 56.Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1993;68:1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- 57.Sweet WH. Central mechanisms of chronic pain (neuralgias and certain other neurogenic pain) In: Bonica JJ, editor. Pain. New York: Raven Press; 1980. pp. 287–303. [PubMed] [Google Scholar]
- 58.Thoden U, Doerr M, Dieckmann G, Krainick JU. Medial thalamic permanent electrodes for pain control in man: an electrophysiological and clinical study. Electroencephalogr Clin Neurophysiol. 1979;47:582–591. doi: 10.1016/0013-4694(79)90259-1. [DOI] [PubMed] [Google Scholar]
- 59.Velasco M, Brito F, Jimenez F, Gallegos M, Velasco AL, Velasco F. Effect of fentanyl and naloxone on a thalamic induced painful response in intractable epileptic patients. Stereotact Funct Neurosurg. 1998;71:90–102. doi: 10.1159/000029653. [DOI] [PubMed] [Google Scholar]
- 60.Yen CT, Fu TC, Chen RC. Distribution of thalamic nociceptive neurons activated from the tail of the rat. Brain Res. 1989;498:118–122. doi: 10.1016/0006-8993(89)90405-8. [DOI] [PubMed] [Google Scholar]
- 61.Young RF, Jacques DS, Rand RW, Copcutt BC, Vermeulen SS, Posewitz AE. Technique of stereotactic medial thalamotomy with the Leksell Gamma Knife for treatment of chronic pain. Neurol Res. 1995;17:59–65. doi: 10.1080/01616412.1995.11740287. [DOI] [PubMed] [Google Scholar]
- 62.Yu L-C, Han J-S. Habenula as a relay in the descending pathway from nucleus accumbens to periaqueductal grey subserving antinociception. Intern J Neurosci. 1990;54:245–251. doi: 10.3109/00207459008986640. [DOI] [PubMed] [Google Scholar]