Abstract
Two innovative approaches in minimally invasive surgery that have been introduced recently are the da Vinci robotic platform and single port laparoscopic surgery (SPLS). Robotic surgery has many advantages such as 3-dimensional view, the wrist like motion of the robotic arm and ergonomically comfortable position for the surgeon. Numerous literatures have demonstrated the feasibility of robotic surgery in gynecologic oncology. However, further research should be performed to demonstrate the superiority of robotic surgery compared to conventional laparoscopy. Additionally, cost reduction of robotic surgery is needed to adopt robotic surgery into gynecologic oncology worldwide. SPLS has several possible benefits including reduced operative complications, reduced postoperative pain, and better cosmetic results compared to conventional laparoscopy. Although several authors have indicated that SPLS is a feasible approach for gynecologic surgery, there have been few reports demonstrating the potential advantages over conventional laparoscopy. Moreover, technical difficulties of SPLS still exist. Therefore, the advantages of a single port approach compared to conventional laparoscope should be evaluated with comparative study, and further technologic development for SPLS is also needed. These two progressive technologies take the lead in the development of MIS and further studies should be performed to evaluate the benefits of robot surgery and SPLS.
Keywords: Minimal surgical procedure, Endometrial neoplasm, Cervical neoplasm, Laparoscopic surgery
INTRODUCTION
Operative laparoscopy developed earlier on in the field of gynecology and the appearance of minimally invasive surgery (MIS) led to advances in general surgery as well. Operative laparoscopy was initiated in the 1970s, and tubal ligations for contraception were conducted with laparoscopy in women by the mid 1970s.1 Since laser and electric energy technology was integrated into laparoscopic surgery in the early 1980s, operative laparoscopy extended to complicated gynecologic procedures including hysterectomy, adnexal surgery and uterine myomectomy. Now, laparoscopic surgery has become an essential part of surgical treatment for gynecologic diseases, including gynecologic cancers. Compared with laparotomy, laparoscopic approach offers several advantages, such as faster return to normal activity, better cosmetic results, shorter length of hospital stay, lower cost, and reduced pain.
The technology and techniques related to laparoscopic surgery are still evolving to the direction of easier and less invasive laparoscopic surgery. Despite several advantages of laparoscopic surgery, the weakness of conventional laparoscopy including an unstable camera platform, the limited mobility of straight laparoscopic instruments, two-dimensional imaging, a poor ergonomic position for the surgeon, and a steep learning curve still remains. As easier laparoscopic approach, the robotic platforms that address many of the current limitations of conventional laparoscopy were developed and integrated into laparoscopic surgery. Recently, another innovative technique, single port laparoscopic surgery (SPLS), was also introduced in the field of MIS. Both robotic platform and SPLS are emerging concepts in MIS. The purpose of this study is to provide an overview of these cutting edge technologies in gynecologic oncology.
HISTORY OF ROBOTIC SURGERY
The term "robot" was first introduced to the public in 1921 when the Czech writer Karel Capek described the notion in his play Rossum's Universal Robots.2 The term "robot" originated from "robota", which means literally "work" or "forced labor" in the Czech language. For decades, robots have achieved substantial development from simple machines performing repetitive tasks to a highly sophisticated machine, capable of performing specific tasks requiring precision.
In the surgical field, automated endoscopic system for optimal positioning (AESOP) was launched as the first laparoscopic camera holder by Computer Motion Inc. (Computer Motion, Inc., Santa Barbara, CA, USA).3 Although AESOP has been used in over 10,000 laparoscopic surgeries, it was only designed to offer greater vision control to the surgeon and to eliminate the need for an assistant who manipulated the endoscope.
In 1992, ROBODOC (Integrated Surgical Supplies, Inc., Sacramento, CA, USA), the first commercially available robotic system, was introduced in orthopedic surgery, and numerous cases of total hip replacement surgery were successfully performed with this robot system.4 The ROBODOC utilized a robotic arm designed to make precise cuts in the femur bone for the insertion of surgical implants, based on the three-dimensional computerized tomography image. However, it is difficult to call these surgeries assisted by ROBODOC and AESOP as true robotic surgery because of the limited role in performing surgical procedures with these robotic systems.
In 1998, Computer Motion which already had manufactured the AESOP developed the ZEUS surgical robot with a 2-dimensional imaging system similar to that of standard laparoscopy. Using the ZEUS robotic system, the first tele-robotic surgery was conducted by a surgeon in New York on a patient in France and was reported by Marescaux et al.5 On the other hand, the da Vinci surgical system was introduced by Intuitive Surgical, Inc. (Intuitive Surgical, Mountain View, CA, USA), and a more advanced da Vinci surgical system with four robotic arms obtained US Food and Drug Administration (FDA) approval in 2001. It is now being used in various surgical procedures throughout the world. The first tele-surgery using the da Vinci robotic system was conducted between the University of Cincinnati and Intuitive Surgical in California in 2006.6 The ongoing competition between the ZEUS and the da Vinci surgical systems ended when Computer Motion Inc. was merged into Intuitive Surgical Inc. in 2003. In Korea, the Korean FDA approved the da Vinci system in July 13th, 2005 and the first robot assisted laparoscopic hysterectomy was conducted by Kim et al.7 on January 31st in 2006. The da Vinci system is currently being used at the departments of gynecology, general surgery, urology and thoracic surgery in Korea. For 3 years, more than 2,000 cases of robotic surgeries in various fields have been performed at our institution.
ROBOT ASSISTED LAPAROSCOPIC SURGERY USING THE DA VINCI SURGICAL SYSTEM
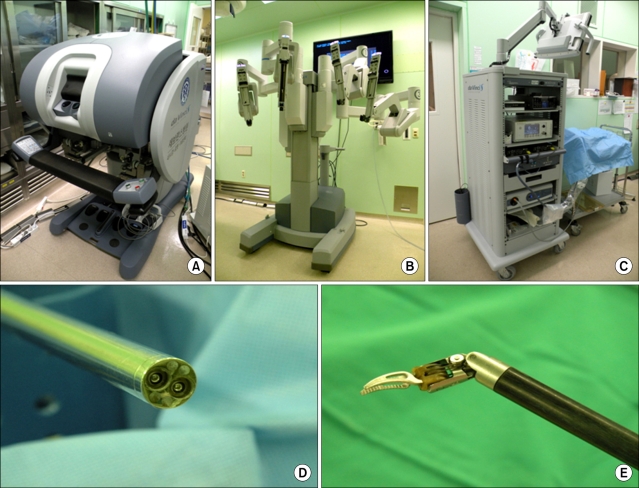
The da Vinci robotic system, which is the only FDA approved and commercially available robot in gynecology, consists of three main components: the robotic cart, the vision cart, and the operating console (Fig. 1). Four robotic arms are mounted on the robotic cart, which can be placed freely next to the patient. The robotic cart docked to the laparoscopic trocars on the patient's abdomen is connected to the operating console through a cable. The da Vinci surgical system is equipped with a 3-dimensional vision system, in which double endoscopes generate two images resulting in the perception of a 3D image. In addition, robotic arms with surgical instruments have three or four joints, which reproduce the range of motion and dexterity of the surgeon's hand. The surgeon sits at the surgical console and performs the surgery by manipulating the controller in it. The movement is translated from the surgeon's fingers to the tip of the surgical instruments. During this process, the physiologic tremor is eliminated by the robotic system. These instruments including the 3-D vision system and endowrist allow the surgeon to conduct more precise surgical procedures during surgery.
Fig. 1.
The da Vinci surgical system. (A) Surgical console, (B) Robotic cart, (C) Vision cart, (D) Three dimensional vision system with endoscope, (E) Endowrist.
ROBOT ASSISTED LAPAROSCOPIC SURGERY IN GYNECOLOGIC CANCER
Cervical cancer is the leading cause of cancer-related death in women worldwide and accounts for 5.7% of all new cancer cases in Korean women in 2005, with approximately 3,737 invasive cervical cancer cases being diagnosed.8 Endometrial cancer is also one of the most common malignancies of the female genital tract in developed countries.9 Surgery for these gynecologic cancers is considered to be one of the major management modalities for treating cancer, determining disease stage of patients, and obtaining the information for adjuvant treatment. However, laparotomic approaches in all patients with cervical or endometrial cancer have increased operative and post-operative morbidity. To reduce the surgical morbidity, robot assisted laparoscopic surgery was introduced as an alternative surgical method for laparotomic surgery in gynecologic cancers. Therefore, examining the surgico-feasibility of robotic approach in these cancers is an essential step for further discussion about the feasibility in all aspects.
In cervical cancer, Kim et al.10 offered the evidence for the feasibility of performing robot assisted laparoscopic radical hysterectomy in their case series report. Since the study by Kim and colleagues was published, several authors elaborated on the surgical outcomes, which were obtained with robotic procedures compared to those of conventional laparoscopic or laparotomic surgeries.11-16 Magrina et al.17 reported that robotic and conventional laparoscopic surgeries are preferable to laparotomy for patients requiring radical hysterectomy in terms of blood loss and length of hospital stay. Boggess et al.16 conducted a case-control study of robot assisted laparoscopic radical hysterectomy compared with laparotomic approach, and this study showed that robot assisted laparoscopic radical hysterectomy is superior to open radical hysterectomy with regard to blood loss, operative time, hospital stay, and lymph node retrieval. Lowe et al.12 also reported the experience of multi-institution consortium which consists of five gynecologic oncologists in distinct geographical regions of the United States for radical hysterectomy using the da Vinci robotic platform. Through the analysis of 42 patients who underwent a type II or III robotic radical hysterectomy, the authors concluded that robot assisted laparoscopic type II/III radical hysterectomy is associated with a shortened hospital stay, few operative complications, acceptable lymph node yields, and acceptable operative times. Table 1 summarizes the current literature presenting surgical outcomes of robot assisted radical hysterectomy.
Table 1.
Literatures of robot assisted laparoscopic radical hysterectomy in cervical cancer
*Mean±standard deviation.
As many researchers demonstrated the feasibility of robotic radical hysterectomy in cervical cancer, several authors showed that the robot assisted staging surgery in endometrial cancer is comparable to conventional laparoscopic and laparotomic approach in terms of surgical outcomes.19-26 In addition, length of hospital stay, blood loss and peri-operative complication rates are significantly lower in patients who received robotic surgery than those who underwent laparotomic staging surgery. Comparing with laparoscopic staging, the advantages of robot assisted laparoscopic staging surgery are somewhat distinct in obese patients with regard to surgical outcomes.27,28 Gehrig et al.29 conducted a comparative study to examine which is the optimal minimally invasive surgical approach between conventional laparoscopy and robot for obese patients with endometrial cancer. The authors reported that robotic surgery was associated with shorter operative time, less blood loss, increased number of resected lymph nodes and shorter hospital stay compared to traditional laparoscopy. Seamon et al.27 also performed a comparative study in order to compare outcomes between robotic and laparoscopic staging for endometrial cancer. The authors showed that robotic staging surgery for endometrial carcinoma in heavier women resulted in shorter operation times, shorter hospital stay, lower transfusion rate, and less frequent conversion to laparotomy when compared to laparoscopic staging. Table 2 presents publications of robotic surgery for endometrial cancer staging.
Table 2.
Literatures of robot assisted laparoscopic staging surgery in endometrial cancer
*Total number of resected lymph nodes, †Mean±standard deviation.
Numerous investigators demonstrated that laparoscopic approach in performing radical hysterectomy or endometrial cancer staging operation was not only feasible, but also achievable.29-33 However, a recent survey investigating the use of MIS by the Society of Gynecologic Oncology (SGO) members showed that only 26% of SGO members considered laparoscopic radical hysterectomy as an appropriate approach to the management of cervical cancer.34 The Korean Gynecologic Oncology Group (KGOG) Survey also revealed that only 49% of KGOG members used laparoscopy for endometrial cancer staging surgery.35 The potential obstacles to the widespread acceptance of minimally invasive approach in gynecologic cancer are the technical difficulties of conventional laparoscopic surgery. Seamon et al.36 demonstrated that the learning curve for endometrial cancer staging with the robot platform required only 20 cases to reach the state of efficiency. The learning curve of achievement in endometrial cancer staging surgery using the da Vinci surgical system was shorter than that of conventional laparoscopic surgery. Although there was lack of data showing learning curve of robotic surgery in radical hysterectomy, all investigators who investigated the feasibility of robotic radical hysterectomy compared to laparotomy reported acceptable surgical outcomes in their initial experiences with the robot system.
Robotic surgery has many advantages, such as 3-dimensional view, the wrist like motion of the robotic arm and ergonomically comfortable position for the surgeon. These advantages offer significant technical ease in performing complicated surgical procedures, including suturing and tying of knots by the surgeon, providing a familiar environment similar to that of the laparotomic approach.
Although there are several advantages of robotic surgery, it still has disadvantages. The principal weak point is the high cost of robotic surgery, which prevents robotic surgery from spreading worldwide. The cost to install the da Vinci robotic platform in an institution ranges from $1,000,000 to $1,500,000 and a 10% annual maintenance fee is needed separately.37 The expense for robotic instruments, which can be used only ten times, adds significant charge to the total cost. According to the report by Bell et al.19 total average cost, including hospital charges, for endometrial cancer staging surgery with laparotomy, conventional laparoscopy and robot assisted laparoscopy were approximately $13,000, $7,600 and $8,200, respectively. Although there was no statistically significant difference in cost between laparoscopy and robotic surgery in the United States, the cost for surgical approach differs according to the medical insurance systems and cultures of each country. Persson et al.11 in Sweden raised a question about the cost efficiency of robotic radical hysterectomy compared with laparoscopy or laparotomy. In Korea, the government-driven medical insurance system is under strict control, such that the cost of medical treatment for patients who underwent robotic radical hysterectomy cannot obtain medical benefits from the government medical insurance system, and have to pay the high cost of surgery. A cost effectiveness analysis for hysterectomy using three surgical methods by Chung et al.38 revealed that laparoscopic approach is the most cost saving approach in Korea.
Other disadvantages included absence of tactile feedback of robotic arms, requirement of larger ports for robotic surgery compared to conventional laparoscopic staging surgery. Placement of a large sized port, more than 8-mm in diameter for robotic surgery, is larger than that of laparoscopic surgery, and causes aggravated postoperative pain and produce poor cosmetic results. Additionally, trocar site hernia through the 8-mm port may occur more frequently than the 5-mm port. Though trocar site herniation is a rare complication in gynecologic laparoscopic surgery, it develops more frequently when large ports (10mm in diameter or larger) are used, and rare in less than 10-mm ports, ranging from 0 to 0.09%.39 In fact, Seamon, et al. reported a case of small bowel evisceration through an 8-mm robotic port site after endometrial cancer staging surgery.40 Therefore, to achieve lower complication rates, port size must be reduced.
So far, most of the studies demonstrating the feasibility of robotic approach in performing radical hysterectomy or endometrial cancer staging surgery were retrospective in nature, or conducted in a single institution. In addition, several researchers demonstrated that the open approach was still superior to the robotic approach in terms of the number of resected lymph nodes.18 Literatures concerning long term survival in patients with these gynecologic cancers who underwent robot assisted laparoscopic surgery are limited. Therefore, the feasibility of robotic surgery compared to other approaches should be supported by prospectively designed multi-center studies, which are sufficient to evaluate the role of the robotic platform in conducting radical hysterectomy or endometrial cancer staging surgery. The research regarding long term survival in cervical or endometrial cancer patients, who underwent robot assisted laparoscopic surgery, should also be performed.
HISTORY OF SINGLE PORT LAPAROSCOPIC SURGERY IN GYNECOLOGY
Innovation in technology and techniques continues more minimal approach to be attained than traditional laparoscopic surgery; SPLS is one of those innovative techniques. As surgeons in gynecology were pioneers in laparoscopic surgery, the frontiers of SPLS were in gynecology. In fact, the single port approach had already been widely used in gynecology. Wheeless41 performed the first single incision tubal ligation in 1969. In the 1970s, several gynecologists conducted laparoscopic tubal ligations with Yoon's rings through a single umbilical incision.42 After that time, total hysterectomy with bilateral salpingo-oophorectomy using single puncture technique was performed by Pelosi43 in 1991. However, hysterectomy using SPLS did not gain widespread use due to technical difficulties. In 2005, Ghezzi et al.44 presented a novel technique for the treatment of tubal pregnancy; one trocar salpingectomy. The authors made only one incision below the umbilicus for a 10 mm operative laparoscope and inserted a 90 cm suture on a straight hand needle percutaneously to manipulate the distal tube. Ten cases of tubal pregnancies were successfully treated with this technique. However, technical challenges for more complicated gynecologic procedures still exist in SPLS, and originates from breakdown in triangulation, which is necessary for maintaining appropriate operative field during laparoscopic surgery. Technological innovations in the field of laparoscopic surgery have been remarkable, and complicated procedures, such as cholecystectomy and appendectomy using single port laparoscopy in the field of general surgery. At the same time, several gynecologists also demonstrated the feasibility of SPLS for hysterectomy and adnexal surgery.
FEASIBILITY OF SPLS IN GYNECOLOGY
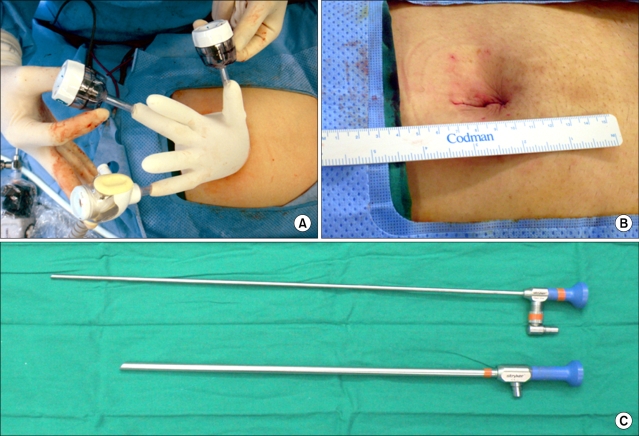
The principle concept of SPLS is to place all of the laparoscopic working ports through the same incision. However, this principle results in hand collisions out of the abdomen and clashing of instruments within the abdomen. Now, various devices designed to overcome the technical challenges for SPLS have been developed and introduced in gynecology.45 Those devices include laparoscopic ports designed to apply multiple instruments through a single incision, flexible/long endoscopes and articulating/variable length instruments. In addition, the da Vinci robotic platforms with articulating instruments can be integrated into SPLS for hysterectomy or salpingo-oophorectomy. Fig. 2 represents the devices which are used at our institution in performing SPLS for hysterectomy and adnexal surgery.
Fig. 2.
Single port laparoscopic surgery. (A) A single 3-channel port using a wound retractor, a surgical glove, and three conventional laparoscopic trocars, (B) Postoperative wound, (C) Long endoscope.
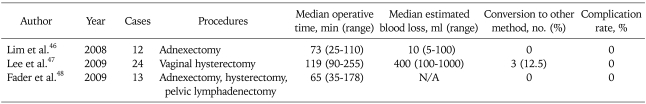
Recently, Korean gynecologic oncologists reported their initial experiences of SPLS in gynecology. Lim et al.46 demonstrated that SPLS is a promising approach for adnexal tumor. The median time of operation was 73 minutes (range, 25 to 110 minutes). Blood loss was 10 ml (range 5 to 100 ml). Lee et al.47 also successfully performed laparoscopic assisted vaginal hysterectomy (LAVH) in 21 patients with uterine fibroids using a single port access. The median operative time and the median blood loss were 119 minutes and 400 ml, respectively. These authors created a single port using a wound retractor, a surgical glove, and three conventional laparoscopic trocars. We have also submitted and are revising our initial experiences of total laparoscopic hysterectomy with SPLS. In a 6 month period, 29 patients with gynecologic disease underwent SPLS at our institution. The median operative time was 100 minutes and the median blood loss was 100 ml.
Fader and Escobar48 also conducted 13 cases of single port gynecologic surgery including 1 endometrial cancer staging, 1 ovarian cancer staging, 1 retroperitoneal pelvic lymph node dissection, 2 hysterectomy with bilateral salpingo-oophorectomy, 7 bilateral salpingo-oophorectomy and 1 ovarian cystectomy. The authors used the da Vinci robot system in 4 cases. Table 3 represents the literature demonstrating the feasibility of SPLS in gynecology.
Table 3.
Literatures of single port laparoscopic approach in gynecology
There are possible advantages of SPLS in gynecology. First, operative complications related trocar insertion such as epigastric vessel injury, visceral organ herniation, wound infection, and visceral organ damage might be reduced by eliminating the need of ancillary ports. Second, postoperative pain, which results from skin incision and penetrating muscle and fascia with the trocar, might be reduced. Third, a better cosmetic result may also be obtained. Fourth, in cases of adnexectomy, resected specimens can be easily extracted through the larger umbilical incision of the SPLS than that of conventional laparoscopy. However, there is no literature which examines the potential benefits of SPLS in gynecology compared to the conventional laparoscopic approach. Therefore, the advantages of a single port approach compared to conventional laparoscopy should be evaluated in a randomized prospective study.
Despite novel devices for SPLS, clashing of laparoscopic instruments and limited vision of in-line view are potential disadvantages of SPLS. These weaknesses which cause longer operative times and longer learning curves might be major obstacles for the popularity of SPLS. Therefore, increased efforts to develop surgical instruments which can overcome these technical problems should continue. Other disadvantages associated with SPLS are the need for special instruments, the increased risk of umbilical hernia due to larger umbilical incision, and the difficulty in training of residents/fellows for MIS.
SPLS is considered a feasible approach for hysterectomy and adnexectomy in gynecology. In the field of gynecologic oncology, SPLS may be applied to adnexal surgery in patients with adnexal tumors, prophylactic oophorectomy in patients with high risk of developing ovarian cancer, and hysterectomy in patients with preinvasive cervical neoplasia or microinvasive cervical cancer. If technical advances are achieved in laparoscopic instruments, including the robotic system, more complicated procedures in gynecologic oncology, such as radical hysterectomy and comprehensive endometrial cancer staging surgery might be conducted with SPLS in the near future.
CONCLUSION
The numerous benefits of MIS are better cosmetic results, reduced operative morbidity, reduced postoperative pain, and shorter length of hospital stay compared with laparotomic surgery. MIS has taken the place of laparotomic approach and has become an imperative part of surgical approach in gynecologic oncology. However, technical difficulties have prevented the widespread adoption of MIS in gynecologic oncology. Over the last three decades, laparoscopic technologies have evolved remarkably, and robotic surgery using the da Vinci system has been introduced. Although it is not evident that robotic surgery is superior to conventional laparoscopic surgery in terms of surgical outcomes, current evidence demonstrates the positive feasibility of robot assisted laparoscopic surgery in gynecologic oncology. Robotic surgery is considered a stepping-stone to jump over the technical barriers of MIS, and contributes to widespread adoption of MIS. However, the economic feasibility of robotic surgery still remains as another obstacle to be solved. It is expected with further development of robotic technology and the emergence of a competitor to the da Vinci robotic platform, the issue of high cost will be resolved.
On the other hand, SPLS is a cutting edge technology requiring high degree of technique. Despite its demonstrated feasibility in gynecology and newly introduced devices, there are several matters that need to be solved, such as demonstrating superiority of the SPLS compared with conventional laparoscopic approach, and relieving technical difficulties. Therefore, further research should focus on the evaluation of the potential benefits of the SPLS and prompt technological progress. In the 21st century, these two innovative approaches; robot and SPLS takes the lead in the development of MIS.
Footnotes
This study was supported by the Brain Korea 21 Project for Medical Sciences, Yonsei University, and a grant from the Korean Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (0412-CR01-0704-0001).
References
- 1.Hulka JF. Current status of elective sterilization in the United States. Fertil Steril. 1977;28:515–520. doi: 10.1016/s0015-0282(16)42549-5. [DOI] [PubMed] [Google Scholar]
- 2.Wikipedia: The Free Encycolpedia [Internet] San Francisco: Wikimedia Foundation; [cited 2009 Aug 25]. Karel Čapek. Available from: http://en.wikipedia.org/wiki/Karel_%C4%8Capek. [Google Scholar]
- 3.Satava RM. Robotic surgery: from past to future--a personal journey. Surg Clin North Am. 2003;83:1491–1500. doi: 10.1016/S0039-6109(03)00168-3. [DOI] [PubMed] [Google Scholar]
- 4.Bann S, Khan M, Hernandez J, Munz Y, Moorthy K, Datta V, et al. Robotics in surgery. J Am Coll Surg. 2003;196:784–795. doi: 10.1016/S1072-7515(02)01750-7. [DOI] [PubMed] [Google Scholar]
- 5.Marescaux J, Leroy J, Gagner M, Rubino F, Mutter D, Vix M, et al. Transatlantic robot-assisted telesurgery. Nature. 2001;413:379–380. doi: 10.1038/35096636. [DOI] [PubMed] [Google Scholar]
- 6.Romano JA, Lam DM, Moses GR, Gilbert GR, Marchessault R. The future of military medicine has not arrived yet, but we can see it from here. Telemed J E Health. 2006;12:417–425. doi: 10.1089/tmj.2006.12.417. [DOI] [PubMed] [Google Scholar]
- 7.Kim YT, Kim SW, Yoon BS, Nahm EJ, Hur HW, Kim SH, et al. Robot-assisted total laparoscopic hysterectomy: initial experience in Korea. Korean J Obstet Gynecol. 2006;49:2620–2625. [Google Scholar]
- 8.Ministry for Heath Welfare and Family Affairs. Annual report of cancer incidence (2005) and survival (1993-2005) in Korea. Seoul: Ministry for Heath Welfare and Family Affairs; 2008. [Google Scholar]
- 9.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 10.Kim YT, Kim SW, Hyung WJ, Lee SJ, Nam EJ, Lee WJ. Robotic radical hysterectomy with pelvic lymphadenectomy for cervical carcinoma: a pilot study. Gynecol Oncol. 2008;108:312–316. doi: 10.1016/j.ygyno.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Persson J, Reynisson P, Borgfeldt C, Kannisto P, Lindahl B, Bossmar T. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185–190. doi: 10.1016/j.ygyno.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Lowe MP, Chamberlain DH, Kamelle SA, Johnson PR, Tillmanns TD. A multi-institutional experience with robotic-assisted radical hysterectomy for early stage cervical cancer. Gynecol Oncol. 2009;113:191–194. doi: 10.1016/j.ygyno.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Estape R, Lambrou N, Diaz R, Estape E, Dunkin N, Rivera A. A case matched analysis of robotic radical hysterectomy with lymphadenectomy compared with laparoscopy and laparotomy. Gynecol Oncol. 2009;113:357–361. doi: 10.1016/j.ygyno.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008;109:86–91. doi: 10.1016/j.ygyno.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Fanning J, Fenton B, Purohit M. Robotic radical hysterectomy. Am J Obstet Gynecol. 2008;198:649.e1–649.e4. doi: 10.1016/j.ajog.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008;199:357.e1–357.e7. doi: 10.1016/j.ajog.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 17.Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008;109:86–91. doi: 10.1016/j.ygyno.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Maggioni A, Minig L, Zanagnolo V, Peiretti M, Sanguineti F, Bocciolone L, et al. Robotic approach for cervical cancer: comparison with laparotomy: a case control study. Gynecol Oncol. 2009;115:60–64. doi: 10.1016/j.ygyno.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 19.Bell MC, Torgerson J, Seshadri-Kreaden U, Suttle AW, Hunt S. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol. 2008;111:407–411. doi: 10.1016/j.ygyno.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199:360–369. doi: 10.1016/j.ajog.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 21.DeNardis SA, Holloway RW, Bigsby GE, Pikaart DP, Ahmad S, Finkler NJ. Robotically assisted laparoscopic hysterectomy versus total abdominal hysterectomy and lymphadenectomy for endometrial cancer. Gynecol Oncol. 2008;111:412–417. doi: 10.1016/j.ygyno.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Lowe MP, Johnson PR, Kamelle SA, Kumar S, Chamberlain DH, Tillmanns TD. A multiinstitutional experience with robotic-assisted hysterectomy with staging for endometrial cancer. Obstet Gynecol. 2009;114:236–243. doi: 10.1097/AOG.0b013e3181af2a74. [DOI] [PubMed] [Google Scholar]
- 23.Peiretti M, Zanagnolo V, Bocciolone L, Landoni F, Colombo N, Minig L, et al. Robotic surgery: changing the surgical approach for endometrial cancer in a referral cancer center. J Minim Invasive Gynecol. 2009;16:427–431. doi: 10.1016/j.jmig.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Seamon LG, Cohn DE, Henretta MS, Kim KH, Carlson MJ, Phillips GS, et al. Minimally invasive comprehensive surgical staging for endometrial cancer: robotics or laparoscopy? Gynecol Oncol. 2009;113:36–41. doi: 10.1016/j.ygyno.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Seamon LG, Cohn DE, Richardson DL, Valmadre S, Carlson MJ, Phillips GS, et al. Robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Obstet Gynecol. 2008;112:1207–1213. doi: 10.1097/AOG.0b013e31818e4416. [DOI] [PubMed] [Google Scholar]
- 26.Veljovich DS, Paley PJ, Drescher CW, Everett EN, Shah C, Peters WA. Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol. 2008;198:679.e1–679.e9. doi: 10.1016/j.ajog.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 27.Seamon LG, Bryant SA, Rheaume PS, Kimball KJ, Huh WK, Fowler JM, et al. Comprehensive surgical staging for endometrial cancer in obese patients: comparing robotics and laparotomy. Obstet Gynecol. 2009;114:16–21. doi: 10.1097/AOG.0b013e3181aa96c7. [DOI] [PubMed] [Google Scholar]
- 28.Gehrig PA, Cantrell LA, Shafer A, Abaid LN, Mendivil A, Boggess JF. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol. 2008;111:41–45. doi: 10.1016/j.ygyno.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Childers JM, Brzechffa PR, Hatch KD, Surwit EA. Laparoscopically assisted surgical staging (LASS) of endometrial cancer. Gynecol Oncol. 1993;51:33–38. doi: 10.1006/gyno.1993.1242. [DOI] [PubMed] [Google Scholar]
- 30.Cho YH, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic management of early uterine cancer: 10-year experience in Asan Medical Center. Gynecol Oncol. 2007;106:585–590. doi: 10.1016/j.ygyno.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Malzoni M, Tinelli R, Cosentino F, Perone C, Rasile M, Iuzzolino D, et al. Total laparoscopic hysterectomy versus abdominal hysterectomy with lymphadenectomy for early-stage endometrial cancer: a prospective randomized study. Gynecol Oncol. 2009;112:126–133. doi: 10.1016/j.ygyno.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Malzoni M, Tinelli R, Cosentino F, Fusco A, Malzoni C. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our experience. Ann Surg Oncol. 2009;16:1316–1323. doi: 10.1245/s10434-009-0342-7. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Xu H, Li Y, Wang D, Li J, Yuan J, et al. The outcome of laparoscopic radical hysterectomy and lymphadenectomy for cervical cancer: a prospective analysis of 295 patients. Ann Surg Oncol. 2008;15:2847–2855. doi: 10.1245/s10434-008-0063-3. [DOI] [PubMed] [Google Scholar]
- 34.Mabrouk M, Frumovitz M, Greer M, Sharma S, Schmeler KM, Soliman PT, et al. Trends in laparoscopic and robotic surgery among gynecologic oncologists: a survey update. Gynecol Oncol. 2009;112:501–505. doi: 10.1016/j.ygyno.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee TS, Kim JW, Kim SH, Seong SJ, Song E, Kim J, et al. Surgical practice patterns in endometrial cancer: results of the Korean Gynecologic Oncology Group survey. J Gynecol Oncol. 2009;20:107–112. doi: 10.3802/jgo.2009.20.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seamon LG, Fowler JM, Richardson DL, Carlson MJ, Valmadre S, Phillips GS, et al. A detailed analysis of the learning curve: robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Gynecol Oncol. 2009;114:162–167. doi: 10.1016/j.ygyno.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Mendivil A, Holloway RW, Boggess JF. Emergence of robotic assisted surgery in gynecologic oncology: American perspective. Gynecol Oncol. 2009;114:S24–S31. doi: 10.1016/j.ygyno.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Chung SM, Jung YW, Lee SH, Nam EJ, Kim SW, Kim JH, et al. Cost-effectiveness analysis of hysterectomy via laparotomy, laparoscopy and robotic assisted laparoscopy [abstract] J Gynecol Oncol. 2009;20(Suppl 1):150S. [Google Scholar]
- 39.Tonouchi H, Ohmori Y, Kobayashi M, Kusunoki M. Trocar site hernia. Arch Surg. 2004;139:1248–1256. doi: 10.1001/archsurg.139.11.1248. [DOI] [PubMed] [Google Scholar]
- 40.Seamon LG, Backes F, Resnick K, Cohn DE. Robotic trocar site small bowel evisceration after gynecologic cancer surgery. Obstet Gynecol. 2008;112:462–464. doi: 10.1097/AOG.0b013e3181719ba8. [DOI] [PubMed] [Google Scholar]
- 41.Wheeless CR. Outpatient tubal sterilization. Obstet Gynecol. 1970;36:208–211. [PubMed] [Google Scholar]
- 42.Quinones GR, Alvarado DA, Ley Ch E. Tubal ligation using Yoon's ring. Ginecol Obstet Mex. 1976;40:127–136. [PubMed] [Google Scholar]
- 43.Pelosi MA. Laparoscopic hysterectomy with bilateral salpingo-oophorectomy using a single umbilical puncture. N J Med. 1991;88:721–726. [PubMed] [Google Scholar]
- 44.Ghezzi F, Cromi A, Fasola M, Bolis P. One-trocar salpingectomy for the treatment of tubal pregnancy: a 'marionette-like' technique. BJOG. 2005;112:1417–1419. doi: 10.1111/j.1471-0528.2005.00665.x. [DOI] [PubMed] [Google Scholar]
- 45.Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc. 2009;23:1419–1427. doi: 10.1007/s00464-009-0463-x. [DOI] [PubMed] [Google Scholar]
- 46.Lim MC, Kim TJ, Kang S, Bae DS, Park SY, Seo SS. Embryonic natural orifice transumbilical endoscopic surgery (E-NOTES) for adnexal tumors. Surg Endosc. doi: 10.1007/s00464-009-0408-4. Epub 2009 Apr 3. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y, Kim T, Kim CJ, Kang H, Choi CH, Lee J, et al. Single-port access laparoscopic-assisted vaginal hysterectomy: a novel method with a wound retractor and a glove. J Minim Invasive Gynecol. 2009;16:450–453. doi: 10.1016/j.jmig.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 48.Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol. 2009;114:157–161. doi: 10.1016/j.ygyno.2009.05.020. [DOI] [PubMed] [Google Scholar]