Abstract
The arthropod cuticle is a multilayered extracellular matrix produced by the epidermis during embryogenesis and moulting. Molecularly and histologically, cuticle differentiation has been extensively investigated in the embryo of the insect Drosophila melanogaster. To learn about the evolution of cuticle differentiation, we have studied the histology of cuticle differentiation during embryogenesis of the amphipod crustacean Parhyale hawaiensis, which had a common ancestor with Drosophila about 510 million years ago. The establishment of the layers of the Parhyale juvenile cuticle is largely governed by mechanisms observed in Drosophila, e.g. as in Drosophila, the synthesis and arrangement of chitin in the inner procuticle are separate processes. A major difference between the cuticle of Parhyale and Drosophila concerns the restructuring of the Parhyale dorsal epicuticle after deposition. In contrast to the uniform cuticle of the Drosophila larva, the Parhyale cuticle is subdivided into two regions, the ventral and the dorsal cuticles. Remarkably, the boundary between the ventral and dorsal cuticles is sharp suggesting active extracellular regionalisation. The present analysis of Parhyale cuticle differentiation should allow the characterisation of the cuticle-producing and -organising factors of Parhyale (by comparison with the branchiopod crustacean Daphnia pulex) in order to contribute to the elucidation of fundamental questions relevant to extracellular matrix organisation and differentiation.
Keywords: Cuticle, Extracellular matrix, Chitin, Parhyale hawaiensis (Crustacea), Drosophila melanogaster (Insecta)
Introduction
The cuticle of arthropods is an extracellular matrix (ECM) with multiple functions. It protects the animal against environmental harm and dehydration and serves as an exoskeleton both allowing locomotion and supporting body shape. The functions of the cuticle are conferred by its stratified architecture (Locke 2001). The outermost lipid- and protein-containing envelope is involved in the control of water balance. The middle epicuticle, which is composed of a protein-catecholamine network, and the inner proteinchitin matrix called the procuticle together constitute the stiff, but elastic, exoskeleton. Despite the diversity of arthropods, the cuticle architecture that has been extensively described in the literature seems to be largely conserved.
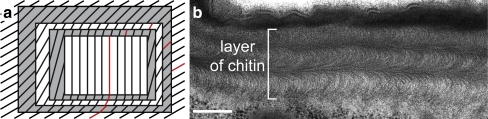
The cuticle is produced by the epidermis during embryogenesis and is renewed during moulting. Progress in understanding cuticle differentiation is currently being made in the model organism Drosophila melanogaster, an insect that has proved to be highly suitable for genetic and molecular methods. The well-defined timetable of development and the relatively small size of the eggs of Drosophila allow a thorough analysis of its ultrastructure after immobilisation by high-pressure freezing, a satisfactory histological preservative (McDonald and Morphew 1993; McDonald 1999; McDonald and Müller-Reichert 2002; Moussian et al. 2006a). Among others, we have shown that, during embryogenesis, the layers of the larval cuticle are not formed strictly sequentially, but partially simultaneously. For instance, procuticle production starts before epicuticle deposition. Interestingly, the arthropod-typical chitin arrangement with horizontally parallel (chitin laminae) and vertically twisted chitin microfibrils (Fig. 1), as described by Bouligand (1965), is established when chitin synthesis terminates just before hatching.
Fig. 1.
Arrangement of chitin in the cuticle of arthropods. In 1965, Bouligand presented a model to explain the arrangement of chitin microfibrils in the arthropod cuticle (a). Laminae (alternating in white and grey) of parallel chitin microfibrils (black and red lines) are stacked, with respect to the orientation of the microfibrils, in a helicoidal manner. In each sheet, one microfibril is highlighted in red to demonstrate that, in ultra-thin oblique sections, the illusion of a parabolic texture is provoked, as shown in a transmission electron micrograph of the Drosophila larval cuticle (b). Bar 500 nm
To promote comprehension of the processes involved in cuticle differentiation, several factors that are required for correct cuticle architecture have been isolated and genetically and molecularly characterised from Drosophila. The majority of factors, including the chitin synthase-1 Krotzkopf verkehrt (CS-1/Kkv), Knickkopf (Knk), Retroactive (Rtv), Piopio (Pio) and Papillote (Pot), are membrane-associated and function in the synthesis, arrangement and attachment of chitin to the cell during procuticle differentiation (Bökel et al. 2005; Moussian et al. 2005a, b, 2006b). In addition, some extracellular factors belonging to the TweedleD class of proteins are predicted to act within the epicuticle where they are needed for body-size regulation (Guan et al. 2006). Orthologues of these factors are encoded by all arthropod genomes sequenced to date, suggesting that basic processes of cuticle formation and function are evolutionary conserved. Indeed, we are beginning to understand the molecular and cellular mechanisms controlling cuticle differentiation in the Drosophila embryo.
In addition to following a genetic approach to extend our knowledge of cuticle differentiation, it is equally fruitful to study and compare its basic underlying mechanisms in distantly related organisms within the same taxon. Such a comparison is intended to uncover not only those characteristics that account for naturally occurring differences, but also those invariable factors ensuring features typical for all branches of the taxon. Within the arthropods, insects probably derive from crustaceans, together constituting the pancrustacea (Mallatt et al. 2004). Therefore, as a next logical step to learning about cuticle evolution, representatives of the insects and crustaceans seem to be predestined for comparative analyses of cuticle differentiation in arthropods, which in principle has been concisely described in several insects, but not in crustaceans.
To compensate for this discrepancy, we have studied the histology of cuticle differentiation in the embryo of the amphipod crustacean Parhyale hawaiensis by electron microscopy. We have chosen Parhyale as a model crustacean, as its entire development from zygote to the juvenile animal takes place within the eggcase, which is small enough to be immobilised by the high pressure freezing method prior to fixation. The cuticle in Parhyale is produced during the second half of embryogenesis (Browne et al. 2005). Soon after the formation of the layers has been initiated sequentially, as in Drosophila, the pro- and epicuticle differentiate simultaneously. Interestingly, unlike the overall uniform cuticle in Drosophila, the pro- and epicuticles at the ventral and the dorsal sides of Parhyale are dissimilar. The ventral epicuticle forms an even layer, whereas the ventral procuticle is eventually subdivided into two layers, the upper exo- and the lower endocuticle. By contrast, the dorsal epicuticle is interrupted and encloses electron-dense chambers that are coated by the envelope. Occasionally, similar but electron-lucid chambers are found within the dorsal procuticle. As in Drosophila, chitin microfibrils and laminae become visible long after chitin synthesis has been initiated suggesting that chitin synthesis and chitin arrangement may be separate processes. Based on the present framework of cuticle differentiation in Parhyale, we plan to investigate the function of its cuticle differentiation factors, which we are currently isolating by using the sequence information of the genome of the branchiopod crustacean Daphnia pulex (Colbourne et al. 2005, 2007).
Materials and methods
Animal maintenance and staging
Parhyale hawaiensis is a marine amphipod that is easy to maintain and breed in the laboratory. Its embryonic development is direct and takes approximately 10.5 days at 26°C.
Laboratory breeding cultures of Parhyale were maintained in shallow covered plastic trays on a day/night cycle. Water was circulated within trays by commercially available aquarium pumps. Phosphate-absorbing resin was used to control the accumulation of free phosphates and thus algal growth. Artificial seawater was prepared from commercial salt (Tropic Marin) to mimic natural seawater with a gravity of 1.018–1.022. About 50% of the water content per tray was changed every week. The animals received commercially available fish food (TetraRubin) every other day and dried yeast extract 3 times per week as a diet. For the methods described below, embryos were carefully taken from the ventral brood pouch of the mother manually. The developmental stages of the embryos were determined according to Browne et al. (2005).
Electron microscopy
Specimens for transmission electron microscopy (TEM) were prepared by high-pressure freezing followed by freeze-substitution and embedding in Epon following the protocol documented in Moussian et al. (2006a). In brief, embryos were immobilised within the eggcase in a high-pressure freezer (Bal-Tec HPM 010, Balzers, Liechtenstein) and freeze-substituted in 2% osmium tetroxide, 0.5% uranyl acetate and 0.5% glutaraldehyde in 97.5% acetone and 2.5% methanol at -90°C for 32 h, warmed up within 3 h to -60°C, kept at -60°C for 6 h, warmed up to -40°C within 2 h and kept for an additional 4 h at -40°C. After being washed with acetone, the samples were transferred into an acetone-Epon mixture at -30°C (1:1 for 4 h, 2:1 for 12 h), warmed up to room temperature, infiltrated in Epon (three changes within 30 h) and polymerised at 60°C for 48 h. Ultra-thin sections (50–70 nm) stained with 2% uranyl acetate in 70% methanol for 10 min and in 0.4% lead citrate in 0.1 N NaOH for 2 min were viewed in a Philips CM10 electron microscope at 60 kV.
For wheat germ agglutinin (WGA)-labelling, unstained Epon sections were incubated with biotinylated WGA (10 mg/ml; Vector Labs, Burlingame, USA) followed by rabbit anti-biotin antibodies (ENZO Life Sciences, Farmingdale, USA) and protein A conjugated to 10-nm gold (gift from Dr. York Stierhof, ZMBP Tübingen). Labelled sections were then stained with 1% aqueous uranyl acetate for 3 min and lead citrate.
For scanning electron microscopy (SEM), embryos were fixed for 5 h in 4% formaldehyde and 0.5% glutaraldehyde at 4°C. The chorion and vitelline membrane were dissected off the embryos manually with tungsten-wire needles placed in a syringe. They were osmium-treated (1% osmium tetroxide in 100 mM phosphate buffer, pH 7.2), dehydrated through an ethanol series, subjected to critical-point drying in CO2 and sputter-coated with 10-nm Au-Pd. Critical-point drying may have affected the appearance of the specimen’s surface. Nevertheless, the differences in the surface appearance of embryos at the different stages reflected changes in ECM composition during maturation. A Hitachi S100 field-emission scanning electron microscope was used to examine the samples.
Sequence analysis
To identify the protein sequences of CS-1/Krotzkopf verkehrt Knickkopf and Retroactive in Daphnia pulex (DpCs-1 to 3 DpKnk and DpRtv), the respective Drosophila sequences were “blasted” against the translated genome of Daphnia at wFleaBase http://wfleabase.org/blast/). The domains of the identified proteins DpKnk and DpRtv were predicted by the SOSUI, PSORT, GPI-SOM and big-PI predictor on-line programs listed at the Expert Protein Analysis System (Expasy) site (http://www.expasy.org/tools/).
Results
Course of cuticle differentiation during Parhyale embryogenesis
Parhyale embryogenesis is completed after 240 h post-fertilisation (hpf); this period has been subdivided into 30 stages (S1–S30; Browne et al. 2005). Cuticle differentiation has been observed to start around stage 26 (S26) at 180 hpf. To trace the cellular mechanisms of cuticle differentiation in the Parhyale embryo, we have analysed the ultrastructure of the cuticle of staged embryos from 120 hpf (S21) to 240 hpf (S30) by SEM and TEM.
Stage 21
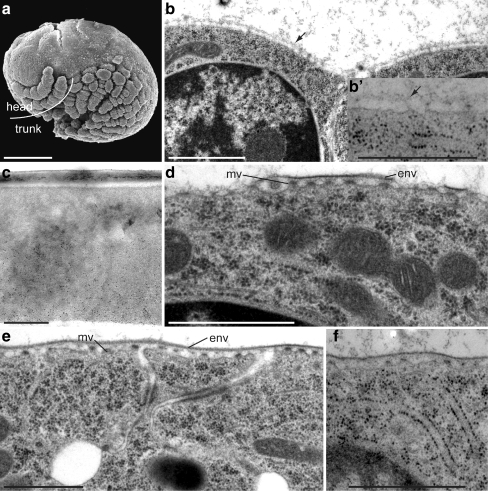
At S21 (120 hpf), the Parhyale embryo possesses all morphological features, such as the head and trunk appendages, of the adult animal (Fig. 2a). The epidermis is naked and resembles a cobblestone pavement. In cross sections, the epidermal cell has a smooth apical surface covered by a thin and squamous ECM (Fig. 2b). This ECM is also present at earlier stages (data not shown) and is devoid of chitin, as labelling with gold-conjugated WGA is negative (Fig. 2b inset). Interestingly, WGA detection is positive in the eggshell (Fig. 2c) and the signal persists throughout embryogenesis (data not shown).
Fig. 2.
Extracellular matrix (ECM) before cuticle differentiation and the differentiation of embryonic cuticle I. a The stage 21 (S21) embryo is devoid of visible ECM. Scanning electron microscopy (SEM). b A thin amorphous ECM (arrow) covers the epidermal cells but is not labelled by gold-conjugated WGA (inset b’). c By contrast, chitin is detected in the basal half of the eggshell (black dots). d At S22, the apical plasma membrane forms microvillus-like structures (mv) at the tips of which the envelope (env) is produced. e Later, at S23, the envelope is continuous. f No chitin can be detected at this stage with gold-conjugated WGA. Transmission electron microscopy (TEM), cross sections. Bars 100 μm (a), 1 μm (b, d–f), 500 nm (c)
Stages 22 and 23
At S22 (132 hpf), the apical surface of the epidermal cell starts to protrude microvillus-like structures (Fig. 2d). At the tips of these protrusions, fragments of a tripartite layer are formed that histologically resembles the insect envelope, which is also produced at microvillus-like structures. By S23 (144 hpf), the epidermal cells are unchanged compared with S22 but are now covered by a continuous envelope (Fig. 2e). Chitin has not yet been synthesised (Fig. 2f).
Stage 24 to early stage 26
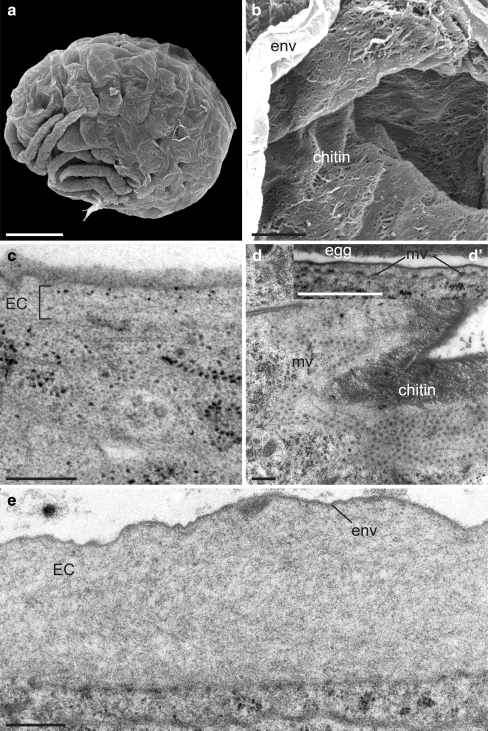
The surface of S24 (155 hpf) and S25 (168 hpf) embryos consists of a smooth but wrinkled membrane (Fig. 3a). When this membrane is torn apart, a fibrous matrix becomes visible underneath the membrane in S25 embryos (Fig. 3b). Labelling of ultra-thin sections with gold-conjugated WGA indicates that this ECM contains chitin (Fig. 3c). The fibrous consistency becomes apparent, especially in extreme oblique sections (Fig. 3d). At S24 and S25, the apical plasma membrane forms protrusions carrying electron-dense plaques (Fig. 3d). The chitinous ECM considerably thickens until S26 (180 hpf; Fig. 3e). The plasma membrane protrusions vanish at early S26. The envelope and the fibrous ECM together constitute the embryonic cuticle that has been described in many arthropods including crustaceans.
Fig. 3.
Differentiation of the embryonic cuticle II. a A wrinkled membrane covers the embryos at S24−S26 (here, S25). SEM. b Randomly oriented fibres that have a diameter of 100 nm are tightly packed underneath the membrane (env envelope) that has been disrupted manually (S25). SEM. c As detected with gold-conjugated WGA (black dots), this layer contains chitin (S24). TEM, cross section. d In tangential section, the apical plasma membrane during chitin synthesis carries regularly spaced microvillus-like structures (mv) that have electron-dense tips, the so-called plaques (S25). TEM, tangential section entering from the cuticle at the right side into the epidermal cell (left). Inset d’: Longitudinally sectioned microvillus-like structures (egg eggshell). TEM, longitudinal section. e In early S26, the apical plasma membrane of the epidermal cells is smooth and chitin production is terminated (EC embryonic cuticle). TEM, cross section. Bars 100 μm (a), 3 μm (b), 1 μm (c–e)
Late stage 26 to early stage 27
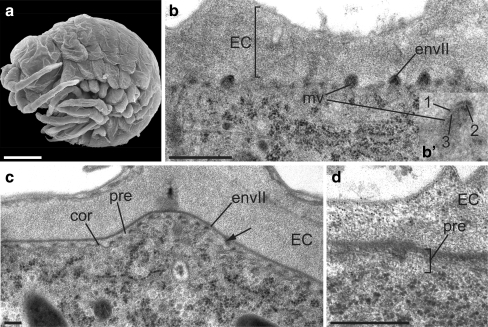
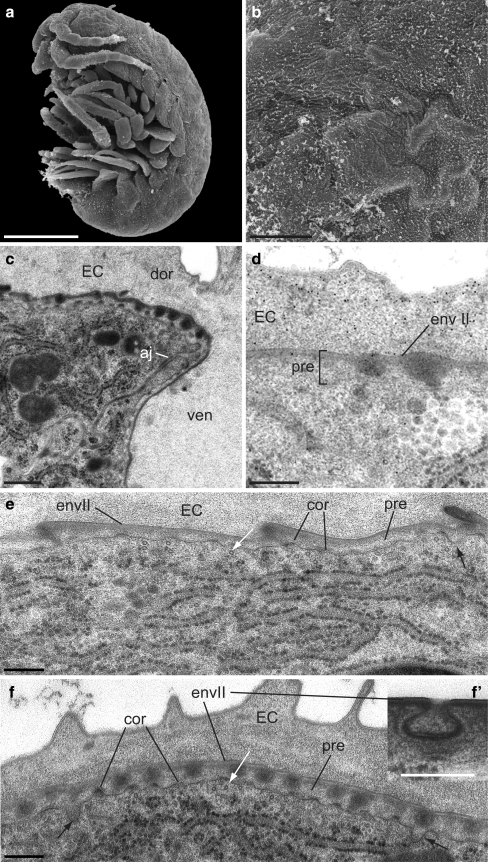
At late S26 and at early S27 (192 hpf), the surface of the embryo is still smooth but wrinkled (Fig. 4a). At late S26, a second tripartite envelope is produced at the tips of newly formed microvillus-like structures that do not contain microtubules (Fig. 4b). The formation of the second envelope is completed underneath the embryonic cuticle until early S27 (Fig. 4c). The apical plasma membrane starts to form shallow microtubule-containing corrugations carrying electron-dense plaques at their tips. A narrow space with moderate electron density separates the envelope and the apical plasma membrane. This layer is devoid of chitin; to distinguish it from the other layers, we call this layer the precuticle (Fig. 4d).
Fig. 4.
Differentiation of the juvenile cuticle I. a S26 and S27 embryos have a wrinkled surface (here, S27). SEM. b The apical plasma membrane of S26 embryos produces microvillus-like structures (mv) at the tips of which a second envelope (envII) is deposited (EC embryonic cuticle). Inset b’: Higher magnification of the envelope reveals that it is tripartite (1–3), one sub-layer with moderate electron density being sandwiched between two electron-dense sub-layers. c In S27 embryos, deposition of the envelope is completed, and microtubule-stabilised corrugations (cor) form at the apical plasma membrane. Material with moderate electron density is secreted (arrow) to establish the precuticle (pre) between the envelope (envII) and the apical plasma membrane. d Gold-conjugated WGA does not label the precuticle. TEM, cross sections. Bars 300 μm (a), 500 nm (b–d)
Late stage 27
At late S27, the surface of the embryo has roughened (Fig. 5a,b). Epicuticle differentiation is initiated, which is substantially different at the ventral side of the embryo compared with the dorsal side (Fig. 5c). As shown by WGA-gold labelling, chitin is not produced in the precuticle (Fig. 5d). At both the ventral and the dorsal sides, electron-dense epicuticular material is secreted in the valley between the plasma membrane corrugations that had emerged during the previous stages (Fig. 5e,f). At the ventral half, a thin electron-dense layer is formed underneath the envelope (Fig. 5e). The epicuticular material is regularly incorporated into small bulges. At the dorsal side, the epicuticular material traverses the precuticle as a ball-shaped structure contacting the envelope, which seems to invaginate at the sites of contact and delaminates to form inclusions within the epicuticle (Fig. 5f).
Fig. 5.
Differentiation of the juvenile cuticle II. a, b The surface of late S27 embryos is not wrinkled, but rough with a few pleats. SEM. c The epicuticle differentiates with different modes at the ventral (ven) and dorsal (dor) halves of the animal (aj adherens junction). The border between the dorsal and the ventral cuticle coincides with the cell-cell boundaries (EC embryonic cuticle). d The precuticle (pre) lacks chitin. e, f Epicuticular electron-dense material is secreted between corrugations (cor) at the apical plasma membrane (black arrows in e and f) and deposited underneath the envelope (envII) at both the ventral and the dorsal side of the embryo, respectively. Microtubules (circles in cross section, white arrow) run through the plasma membrane corrugations. At the ventral side, cuticular denticle-like structures bulge out regularly (e). At the dorsal side, the envelope intrudes into the developing epicuticle to surround the electron-dense material (f). Inset f′: Higher magnification of the intruding envelope. TEM. Bars 200 μm (a), 40 μm (b), 500 nm (c–f, inset f′)
Stages 28 to 30
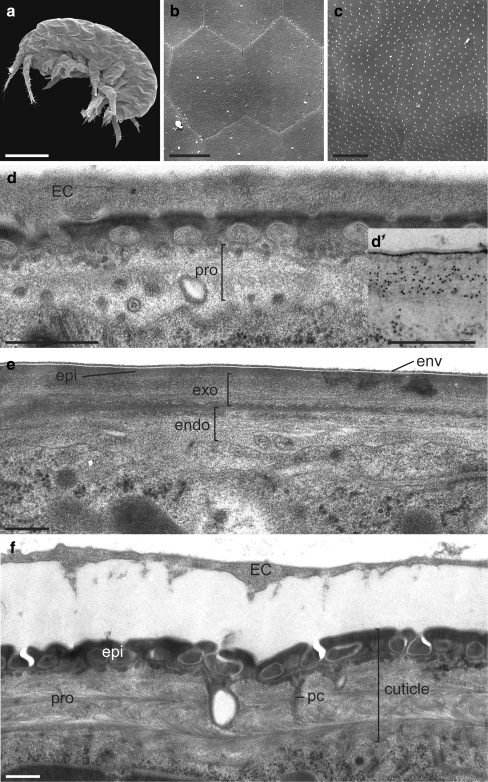
At late S28 (216 hpf), the surface of the mature embryo appears slightly wrinkled at low magnification (20×) by SEM and becomes tense and smooth by S30 (240 hpf; Fig. 6a). At higher magnification (3500×), a pattern of hexagons becomes apparent (Fig. 6b,c). In contrast to the dorsal surface of the head and thorax, the dorsal surface of the abdomen is littered with regularly spaced droplets. Procuticle differentiation, i.e. chitin synthesis, is initiated at early S28 at both sides of the embryo (inset in Fig. 6d). The precuticle has vanished. Chitin microfibrils are not yet arranged in a helicoidal manner along the apical-basal axis of the cuticle (Fig. 6d). At S30, the apical plasma membrane smoothens. Now, the procuticle shows the arrangement of chitin microfibrils typical for arthropods (Fig. 6e,f). Occasionally, inclusions encircled by an envelope-like membrane lie within the chitin laminae of the dorsal procuticle. Ventrally, the procuticle becomes bipartite, the lower portion (the endocuticle) being less electron-dense than the upper portion (the exocuticle). The dorsal epicuticle harbours several layers of inclusions. Pore canals with a wide lumen that originate from the surface of the epidermal cell run through the procuticle and fasciculate to contact and to run through the epicuticle. These pore canals are absent from the ventral cuticle. The ventral epicuticle is a homogeneous electron-dense layer that regularly forms bulges. In both regions, the substructure of the envelope does not change. The contact site of the dorsal and ventral cuticles is sharp and coincides with a cell-cell boundary. The embryonic cuticle starts to detach from the surface of the embryo and becomes thinner (Fig. 6f).
Fig. 6.
Differentiation of the juvenile cuticle III. a–c S28 to S30 embryos have a plain surface (a) displaying a hexagonal pattern (b, c). In contrast to the surface of the thorax and head (b), the surface of the abdomen carries randomly dispersed droplets (c). SEM. d At this stage, chitin is heavily synthesised marking the differentiation of the procuticle (pro). The embryonic cuticle (EC) is still attached. TEM, oblique section. Inset d’: Microfibrils of chitin detected with gold-conjugated WGA (black dots) do not have a specific texture. e, f At S30, chitin microfibrils are reorganised to establish Bouligand’s arrangement. e At the ventral side, the procuticle is bipartite with the outer exocuticle (exo) and the inner endocuticle (endo). TEM, cross section. f The epicuticle at the dorsal side at this stage further differentiates now housing several layers of inclusions. Predominantly at the dorsal site, shortly before hatching, pore canals (pc) are formed within the procuticle that ramify into the epicuticle (epi). The embryonic cuticle (EC) occasionally detaches from the juvenile cuticle. TEM, oblique section. Bars 200 μm (a), 4 μm (b), 5 μm (c), 500 nm (d–f)
Discussion
To gain wide-ranging information about basic mechanisms of cuticle differentiation by comparative morphology, a thorough analysis of the process in divergent, yet reasonably related, model animals is necessary. The evolutionary distance between insects and crustaceans promises a fruitful comparison of cuticle differentiation in these two major clades of arthropods. Cuticle differentiation has been well studied in the embryo of the insect Drosophila melanogaster (Hillman and Lesnik 1970; Moussian et al. 2006a). Here, we have presented our work on the course of cuticle differentiation in the embryo of the crustacean Parhyale hawaiensis in order to allow us to discuss some principles of cuticle differentiation.
The Parhyale embryo produces two cuticles
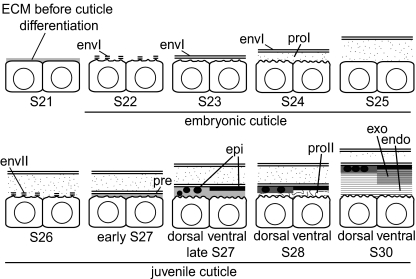
The Parhyale embryo like many other arthropods including crustaceans produces two cuticles prior to hatching (Fig. 7). The first embryonic cuticle consists of only two layers: the first envelope and the first procuticle containing unordered chitin. It is shed and degraded during the differentiation of the juvenile cuticle that is a typical arthropod cuticle composed of three functional layers: envelope, epicuticle and procuticle. As in Drosophila, the formation of the layers of the Parhyale juvenile cuticle is not strictly sequential. Before the completion of the epicuticle, procuticle differentiation is initiated, especially at the dorsal side of the embryo (see below). Calcification of the cuticle, especially at the dorsal side, starts after hatching (data not shown).
Fig. 7.
Representation of cuticle differentiation in the Parhyale embryo (endo endocuticle, envI embryonic envelope, envII juvenile envelope, pre precuticle, epi epicuticle, inc inclusion, exo exocuticle, proI embryonic procuticle, proII juvenile procuticle)
Chitin synthesis and arrangement
Chitin is a major constituent of the embryonic and juvenile cuticles and is also detected by WGA within Parhyale eggshell, which is produced by the somatic follicle cells of the mother. Chitin-binding proteins have consistently been isolated from the eggshell of other crustaceans including Fenneropenaeus chinensis and Marsupenaeus japonicus (Kim et al. 2004, 2005; Du et al. 2006). By contrast, in insects, the eggshell is devoid of chitin. Interestingly, a search for genes coding for chitin synthases in the recently sequenced genome of the branchiopod Daphnia pulex has revealed that Daphnia possesses three chitin synthase genes (Fig. 8a), in contrast to insects in which only two chitin synthases are present (Merzendorfer 2005). If the third chitin synthase in Daphnia and presumably in Parhyale is not a pseudogene, one may speculate that it is responsible for chitin synthesis in the follicle cells.
Fig. 8.
Chitin synthesis and orienting factors are conserved between insects (DmDrosophila) and crustaceans (DpDaphnia). Arthropod chitin is synthesised and extruded to the extracellular space by the large glycosyltransferase chitin synthase that resides in the apical plasma membrane of epidermal cells (chitin synthase-1) or in the epithelium of the midgut (chitin synthase-2). a In a similarity search with the amino acid sequence of Drosophila chitin synthase-1, we have found that the Daphnia genome encodes a third protein with a chitin synthase signature. b, c As illustrated by sequence comparison, crustaceans possess orthologues of the Drosophila chitin-orienting factors Rtv and Knk, respectively. b Retroactive proteins from Drosophila (DmRtv, 151 aa) and Daphnia pulex (DpRtv, 177 aa) share 63 from 138 aligned amino acids (46% identity). Both proteins have an N-terminal signal peptide (last residue marked by σ) and a C-terminal transmembrane domain (underlined). Those aromatic amino acids in DmRtv that are hypothesised to mediate association with chitin (Moussian et al. 2005b) are decorated by a star. Additional conserved aromatic amino acids are labelled by a question mark. No other sequence from Daphnia showed any similarity to DmRtv. cDrosophila Knk (DmKnk, 689 aa) and Daphnia Knk (DpKnk, 693 aa) are 52% identical (345/654 aa). Like DmKnk, DpKnk has an N-terminal signal peptide (last residue marked by σ) and is predicted to be inserted into the plasma membrane via a glycophosphatidyl-inositol (GPI)-anchor (predicted cleavage-site for GPI-modification marked by +)
The chitin arrangements in the eggshell and the two cuticles produced by the Parhyale embryo are different. In the eggshell and the embryonic cuticle, chitin does not adopt the stereotypic organisation that, according to Bouligand (1965), is characteristic for the second juvenile cuticle. In several instances, the topology of the apical plasma membrane has been proposed to play an important role in chitin orientation. Indeed, microtubule-stabilised corrugations reminiscent of the apical undulae in Drosophila are present during the differentiation of the juvenile procuticle and absent when the embryonic procuticle differentiates. In addition, the more complex arrangement of chitin in the juvenile cuticle might require factors that are not expressed or functioning in the follicle cells and during chitin assembly within the simpler embryonic cuticle. Candidates for these factors are Retroactive (Rtv) and Knickkopf (Knk), which have been hypothesised to be involved in chitin microfibril orientation in Drosophila (Moussian et al. 2005b, 2006b). Both factors are indeed present in the genome of Daphnia (Fig. 8b,c) stressing that crustaceans share these sequences with insects. The alternative view that chitin orientation may be a property of the chitin synthase itself is less likely, since chitin synthesis and chitin lamina rotation seem to be separate processes, also during procuticle differentiation in the juvenile cuticle. Thus, it will be exciting to unravel the expression pattern of Parhyale chitin synthases, knk and rtv during embryogenesis and to analyse the phenotype of Parhyale embryos lacking the function of the chitin synthases, Knk or Rtv as generated by RNA silencing or morpholinos.
Dorso-ventral differences of cuticle architecture
The juvenile dorsal and ventral cuticles of Parhyale are dramatically different. The dorsal epicuticle and procuticle are characterised by inclusions that are missing in the respective layers at ventral positions. Moreover, pore canals, which have been proposed to be mineralised or to be routes for mineralisation, are only present in the dorsal cuticle. Overall, the ventral cuticle resembles the cuticle described in other crustaceans including copepods (Anomalocera patersoni, Cletocamptus retrogressus and Porocellidium viride), branchiopods (Daphnia magna, Triops cancriformis and Leptestheria dahalacensis) and decapods (Homarus americanus; Gharagozlou-van Ginneken and Bouligand 1973, 1975; Halcrow 1976; Arsenault et al. 1984; Bresciani 1986; Freeman 1989), whereas the dorsal cuticle seems to be a specific trait of amphipods and isopods (see below). By contrast, in Drosophila, the architectures of the cuticle at dorsal and ventral positions are indistinguishable. Two conclusions can be drawn from these observations. First, differentiation of the cuticle in Parhyale responds to patterning information established early during embryogenesis, whereas in Drosophila, cuticle differentiation seems to occur independently from pattern formation. Second, the exact boundary between the different types of cuticle strongly indicates that, at this position, cuticle differentiation is a cell-autonomous process. Indeed, the boundary separating the dorsal and ventral cuticles corresponds to the sites of cell-cell contacts. Conceivably, the establishment of the boundary is driven by dorsal- and ventral-specific sets of regulating and differentiation factors that respect positional information. For the further elucidation of these crucial aspects of cuticle differentiation, we will attempt to identify the factors that are responsible for the organisation of the dorso-ventral cuticle boundary.
Modelling of the dorsal epicuticle
The dorsal epicuticle of Parhyale harbours inclusions surrounded by the envelope. A similar epicuticular architecture has not been described for other crustaceans or arthropods including insects, except for another amphipod, Hyale nilssoni, and, with some modifications, for Idotea baltica, which belongs to the isopods, the closest relatives of the amphipods (Halcrow 1985; Powell and Halcrow 1985). Intriguingly, the formation of these inclusions through the invagination of the envelope at S27 in Parhyale occurs in the ECM with no physical contact to the apical plasma membrane. Neither in Hyale nilssoni nor in Idotea baltica has the formation of these inclusions been described in detail. In Drosophila and in insects in general, the maturation of the epicuticle involves extracellular (i.e. cell-independent) cross-linking of cuticle proteins with catecholamines in a process called sclerotisation but no dramatic structural configuration beyond smooth layering. Hence, the modelling of the Parhyale dorsal epicuticle is a striking example for self-organisation during cuticle differentiation in arthropods. It will be a great challenge to analyse the molecular mechanisms of this fascinating process.
Concluding remarks
The comparison of cuticle differentiation in Parhyale (presented here) and in Drosophila (published recently) allows two major conclusions. First, cuticle differentiation naturally integrates information from the epidermal cell, especially that defined by its apical plasma membrane and intrinsic properties of the cuticular components themselves, which, for instance, direct laminae rotation within the procuticle. Second, the molecular mechanisms controlling envelope and procuticle differentiation are probably more conserved across arthropods than those governing epicuticle construction. Indeed, an obvious difference in cuticle structure between Parhyale and Drosophila is observed within the epicuticle, whereas their envelope and the procuticle are similar. A review of the literature concerning cuticle structure largely supports the conclusion of a variable epicuticle compared with the stereotypic envelope and procuticle. In other words, the epicuticle has been more sensitive to selective forces during evolution than have the envelope and the procuticle.
Footnotes
This work was supported by the German Research Foundation (DFG, grant number MO 1714/1-1).
References
- Arsenault AL, Castell JD, Ottensmeyer FP (1984) The dynamics of exoskeletalepidermal structure during molt in juvenile lobster by electron microscopy and electron spectroscopic imaging. Tissue Cell 16:93–106 [DOI] [PubMed]
- Bökel C, Prokop A, Brown NH (2005) Papillote and Piopio: Drosophila ZP-domain proteins required for cell adhesion to the apical extracellular matrix and microtubule organization. J Cell Sci 118:633–642 [DOI] [PubMed]
- Bouligand Y (1965) On a twisted fibrillar arrangement common to several biologic structures. C R Acad Sci D 261:4864–4867 [PubMed]
- Bresciani J (1986) The fine structure of the integument of free-living and parasitic Copepods. A review. Acta Zoologica 67:125–145
- Browne WE, Price AL, Gerberding M, Patel NH (2005) Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis 42:124–149 [DOI] [PubMed]
- Colbourne JK, Singan VR, Gilbert DG (2005) wFleaBase: the Daphnia genome database. BMC Bioinformatics 6:45 [DOI] [PMC free article] [PubMed]
- Colbourne JK, Eads BD, Shaw J, Bohuski E, Bauer DJ, Andrews J (2007) Sampling Daphnia’s expressed genes: preservation, expansion and invention of crustacean genes with reference to insect genomes. BMC Genomics 8:217 [DOI] [PMC free article] [PubMed]
- Du XJ, Wang JX, Liu N, Zhao XF, Li FH, Xiang JH (2006) Identification and molecular characterization of a peritrophin-like protein from fleshy prawn (Fenneropenaeus chinensis). Mol Immunol 43:1633–1644 [DOI] [PubMed]
- Freeman JA (1989) The integument of Artemia during early development. In: MacRae TH, Bagshaw JC (eds) Biochemistry and cell biology of Artemia. CRC Press, Boca Raton, pp 233–256
- Gharagozlou-van Ginneken ID, Bouligand Y (1973) Ultrastructure of the skin of the crustacean copepod Cletocamptus retrogressus. Tissue Cell 5:413–439 [PubMed]
- Gharagozlou-van Ginneken ID, Bouligand Y (1975) Studies on the fine structure of the cuticle of Porcellidium, Crustacea Copepoda. Cell Tissue Res 159:399–412 [DOI] [PubMed]
- Guan X, Middlebrooks BW, Alexander S, Wasserman SA (2006) Mutation of TweedleD, a member of an unconventional cuticle protein family, alters body shape in Drosophila. Proc Natl Acad Sci USA 103:16794–16799 [DOI] [PMC free article] [PubMed]
- Halcrow K (1976) The fine structure of the carapace integument of Daphnia magna Straus (Crustacea Branchiopoda). Cell Tissue Res 169:267–276 [DOI] [PubMed]
- Halcrow K (1985) The fine structure of the pore canals of the talitrid amphipod Hyale nilssoni Rathke. J Crust Biol 5:606–615 [DOI]
- Hillman R, Lesnik LH (1970) Cuticle formation in the embryo of Drosophila melanogaster. J Morphol 131:383–396 [DOI]
- Kim YK, Kawazoe I, Tsutsui N, Jasmani S, Wilder MN, Aida K (2004) Isolation and cDNA cloning of ovarian cortical rod protein in kuruma prawn Marsupenaeus japonicus (Crustacea: Decapoda: Penaeidae). Zool Sci 21:1109–1119 [DOI] [PubMed]
- Kim YK, Tsutsui N, Kawazoe I, Okumura T, Kaneko T, Aida K (2005) Localization and developmental expression of mRNA for cortical rod protein in kuruma prawn Marsupenaeus japonicus. Zool Sci 22:675–680 [DOI] [PubMed]
- Locke M (2001) The Wigglesworth lecture: Insects for studying fundamental problems in biology. J Insect Physiol 47:495–507 [DOI] [PubMed]
- Mallatt JM, Garey JR, Shultz JW (2004) Ecdysozoan phylogeny and Bayesian inference: first use of nearly complete 28S and 18S rRNA gene sequences to classify the arthropods and their kin. Mol Phylogenet Evol 31:178–191 [DOI] [PubMed]
- McDonald K (1999) High-pressure freezing for preservation of high resolution fine structure and antigenicity for immunolabeling. Methods Mol Biol 117:77–97 [DOI] [PubMed]
- McDonald K, Morphew MK (1993) Improved preservation of ultrastructure in difficult-to-fix organisms by high pressure freezing and freeze substitution. I. Drosophila melanogaster and Strongylocentrotus purpuratus embryos. Microsc Res Tech 24:465–473 [DOI] [PubMed]
- McDonald K, Müller-Reichert T (2002) Cryomethods for thin section electron microscopy. Methods Enzymol 351:96–123 [DOI] [PubMed]
- Merzendorfer H (2005) Insect chitin synthases: a review. J Comp Physiol [B] 176:1–15 [DOI] [PubMed]
- Moussian B, Schwarz H, Bartoszewski S, Nüsslein-Volhard C (2005a) Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J Morphol 264:117–130 [DOI] [PubMed]
- Moussian B, Söding J, Schwarz H, Nüsslein-Volhard C (2005b) Retroactive, a membrane-anchored extracellular protein related to vertebrate snake neurotoxin-like proteins, is required for cuticle organization in the larva of Drosophila melanogaster. Dev Dyn 233:1056–1063 [DOI] [PubMed]
- Moussian B, Seifarth C, Müller U, Berger J, Schwarz H (2006a) Cuticle differentiation during Drosophila embryogensis. Arthropod Struct Dev 35:137–152 [DOI] [PubMed]
- Moussian B, Tang E, Tonning A, Helms S, Schwarz H, Nüsslein-Volhard C, Uv AE (2006b) Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 133:163–171 [DOI] [PubMed]
- Powell CVL, Halcrow K (1985) Formation of the epicuticle in a marine isopod, Idotea baltica (Pallas). J Crust Biol 5:439–448 [DOI]