Abstract
We have reported recently that intranasal (i.n.) vaccination with chlamydial protease-like activity factor (CPAF) and interleukin-12 (IL-12) enhances protective immunity against genital chlamydial challenge. In this study, we show that i.n. or intraperitoneal (i.p.) vaccination with CPAF plus CpG deoxynucleotides (CpG), an alternative T helper 1 (Th1) adjuvant, induced robust CPAF-specific IFN-γ responses and elevated levels of serum antibody and vaginal IgA production. CPAF+CpG vaccinated animals displayed accelerated genital chlamydial clearance, and minimal hydrosalpinx and inflammatory cellular infiltration compared to mock-immunized (PBS) challenged animals. Together, CpG dexoynucleotides are an efficacious alternative Th1 adjuvant with CPAF to induce protective anti-chlamydial immunity.
Keywords: Chlamydial Protease-Like Activity Factor, CPAF, vaccine, Chlamydia, genital, CpG, adjuvant
1. Introduction
Chlamydia trachomatis is the leading cause of bacterial sexually transmitted disease worldwide [1, 2]. There currently is no licensed vaccine available for human use with only a few potential candidates being evaluated in murine models of genital chlamydial infection [1, 2]. Chlamydial protease-like activity factor (CPAF) is a bacterial protein secreted into the host cytosol that has been shown to degrade transcription factors required for major histocompatibility gene expression [3]. Additionally, CPAF has been demonstrated to induce the proteolytic degradation of cytoskeletal components such as keratin-8 [4] and pro-apoptotic proteins [5], and therefore is a putative chlamydial virulence factor. Recently, we have shown that intranasal (i.n.) vaccination with chlamydial protease-like activity factor (CPAF) plus interleukin-12 (IL-12), a mucosal T helper 1 (Th1) adjuvant, reduces the duration of chlamydial shedding and abrogates the development of oviduct pathology following intravaginal (i.vag.) chlamydial challenge [6]. Protection afforded by CPAF was highly dependent on CD4+ T-cells and endogenous IFN-γ production and required the use of interleukin-12 (IL-12) [6, 7]. These results suggest that CPAF may be a viable candidate for an anti-chlamydial vaccine and underscore the need to identify other adjuvants that may be appropriate for human use.
Oligodeoxynucleotides (ODN) containing CpG motifs mimic the ability of microbial DNA to activate the innate immune system [8]. CpG oligodeoxynucleotide (CpG) adjuvant has been shown to stimulate antigen presenting cells and to predominantly induce secretion of Th1-type cytokines such as IL-12 and IFN-γ [9-13]. Studies in mice and non-human primates have shown that DNA sequences containing CpG motifs can selectively promote antigen-specific cellular and/or humoral immune responses in vivo [9-12, 14]. In addition, early results from clinical studies indicate that CpG is well tolerated and improves the immune response to microbial vaccines [8, 14]. In the case of Chlamydia, CpG administered intramuscularly (i.m.) or subcutaneously (s.c.) with chlamydial major outer membrane protein (MOMP) has been shown to induce MOMP-specific Th1 type immune responses and to enhance protective immunity against i.n. C. muridarum challenge [15]. Additionally, transcutaenous immunization with MOMP plus cholera toxin and CpG induced a strong anti-MOMP immune response, including mucosal IgA, and induced protective immunity against genital C. muridarum challenge [16, 17]. Parenteral routes of vaccination induce robust systemic immunity, whereas mucosal immunization, orally or i.n., elicits systemic immune responses and robust mucosal IgA production [18-20]. Although the mechanisms are not currently well understood, intranasal immunization has been shown to induce optimal anti-chlamydial immunity in the genital tract when compared to other routes of vaccination, including vaginal immunization [17, 21].
In this study, we compared the protective efficacy of i.n. and intraperitoneal (i.p.) vaccination with CPAF plus CpG against genital chlamydial challenge. Both routes of CPAF+CpG vaccination induced robust CPAF-specific cellular and humoral immune responses, and comparably enhanced resolution of genital C. muridarum infection. Additionally, CPAF+CpG vaccination protected animals against the development of hydrosalpinx and oviduct dilatation in the genital tract. Given that early results from ongoing clinical studies indicate that CpG is well tolerated and improves the immune response to microbial vaccines, results from our study demonstrate the promise of using CpG as an alternative mucosal adjuvant against genital chlamydial infection.
2. Materials and Methods
2.1. Bacteria
Chlamydia muridarum was grown on confluent HeLa cell monolayers. Cells were lysed using a sonicator (Fisher, Pittsburgh, PA) and elementary bodies (EBs) purified on Renograffin gradients as described previously [22]. Aliquots of bacteria were stored at −70 °C in sucrose–phosphate–glutamine buffer.
2.2. CPAF and CpG deoxynucleotides
CPAF from C. trachomatis L2 genome was cloned and expressed in a bacterial system as described previously [4]. CPAF has been shown to be highly conserved among the different biovars of Chlamydia, with 82% amino acid identity between L2 and C. muridarum CPAF [23]. Additionally, we have previously shown the protective efficacy of L2 CPAF against genital C. muridarum challenge [6]. Briefly, CPAF constructs cloned from C. trachomatis L2 genome with a 6-Histidine tag (His) were cloned into pBAD vectors and expressed in Escherichia coli with isopropyl-β-Dthiogalactopyranoside (IPTG) as an inducer. The fusion protein was purified using NiNTA agarose beads (Amersham Biosciences Corp.). The purified CPAF was identified by Western blot analysis using a monoclonal anti-CPAF antibody [24]. CPAF activity was determined by the ability to degrade the transcription factor RFX-5 in a concentration-dependent fashion, using a cell-free degradation assay, as described previously [3]. Purified CPAF was used as a source of protein for all experiments.
CpG oligodeoxynucleotides (5'-TCC ATG ACG TTC CTG ACG TT-3', designated CpG in this paper) or non-CpG control oligodeoxynucleotides (5'-TCC AGG ACT TTC CTC AGG TT-3', designated ODN in this paper) [25, 26] were synthesized and obtained from Sigma Genosys (St.Louis, MO).
2.3. Mice
Four week-old, female BALB/c mice were obtained from Charles River Laboratory (Bar Harbor, ME). Mice were housed and bred at the University of Texas at San Antonio and provided food and water ad libitum. Animal care and experimental procedures were performed in compliance with the Institutional Animal Care and Use Committee (IACUC) guidelines.
2.4. Immunization procedure
Mice were anesthetized i.n. with 3% isofluorane using a rodent anesthesia system (Harvard Apparatus, Holliston, MA). Mice were immunized i.n. or i.p. on day 0 with 15 μg rCPAF dissolved in 25 μl (for i.n.) or 100 μ (for i.p.) of sterile phosphate buffered saline (PBS). This was accompanied on days −1, 0, and +1 with 10 μg of CpG or 10 μg of ODN. Mice were boosted i.n. with 15 μg rCPAF + CpG or ODN on days 14 and 28. Some mice received only CpG or PBS (no CPAF vaccine). The dose of CPAF used was what provided optimal protection in our studies with CPAF+IL-12 in BALB/c mice [6].
2.5. Antigen-specific cytokine recall response
Spleens were removed 14 days after primary vaccination and single cell suspensions prepared to analyze the antigen-specific cytokine response as described previously [6, 27]. Collected cells [106/well] were incubated for 72 hr with 1μg CPAF per well or with an equal concentration of an unrelated antigen, hen egg lysozyme (HEL), or PBS alone in 96-well culture plates. Supernatants were assayed for levels of interleukin-12 (IL-12), gamma-interferon (IFN-γ) and interleukin-4 (IL-4) using BDOptEIATM kits (BD Pharmingen, San Diego, CA) according to manufacturer's instructions. Absorbance at 630 nm was measured using a μQuant ELISA microplate reader (Biotek Instruments, Winooski, VT).
2.6. Detection of antibody and isotype levels by ELISA
Ten days following final immunization, animals were bled for serum, or vaginal lavage fluids were obtained and analyzed by ELISA as described previously [6, 7, 27]. Microtiter plates (96-well) were coated overnight with 5 μg CPAF in sodium bicarbonate buffer (pH 9.5). Serial dilutions of serum or undiluted vaginal lavage fluids were added to wells followed by either goat anti-mouse total Ig, IgG1, IgG2a, IgG2b, IgM, or IgA conjugated to alkaline phosphatase (Southern Biotechnology Associates, Birmingham, AL). After washing, p-nitrophenyl phosphate substrate (Sigma, St. Louis, MO) was added for color development and absorbance (O.D.) monitored at 405 nm using a μQuant ELISA microplate reader (Biotek Instruments). Reciprocal serum dilutions corresponding to 50% maximal binding were used to obtain titers. Because of the low amounts of Ab in vaginal fluids and the large dilution involved in the lavage procedure, these samples were tested undiluted. No binding of immune serum was detected in plates coated with HEL.
2.7. Vaginal C. muridarum challenge and determination of bacterial shedding
One month following the final vaccination, animals were anesthetized using isofluorane (3%) and challenged i.vag. with 5×104 inclusion forming units (IFU) of C. muridarum in 5 μl of SPG buffer as described previously [6, 7, 27]. To synchronize the estrous cycle of animals prior to chlamydial challenge, two doses of depo-provera (Pharmacia Upjohn, Kalamazoo, MI) were injected subcutaneously on days -10 and -3 before challenge. To monitor bacterial shedding, vaginal swabs were obtained on the indicated days after vaginal challenge, followed by plating of the swab material on HeLa cell monolayers grown on culture coverslips. Chlamydial inclusions were detected using a anti-Chlamydia genus specific murine monoclonal primary antibody and goat anti-mouse IgG secondary antibody conjugated to Cy3 plus Hoescht nuclear stain. The average number of inclusions in 5 random microscopic fields was calculated for each animal for earlier time-points (until day 12 after challenge) and entire coverslips for later time-points (days 15-30 after challenge), and results expressed as average number of inclusions per animal group.
2.8. Gross and histopathology
Genital tracts were removed from mice at day 80 after challenge [6, 7, 27], examined for presence of hydrosalpinx, then fixed in 10% neutral formalin, and embedded into paraffin blocks. Serial horizontal sections (5 μm) were prepared and stained using hematoxylin and eosin (H&E). Stained sections were visualized using a Zeiss Axioskop 2 Plus research microscope and images acquired using an Axiocam digital camera (Zeiss, Thornwood, NY).
2.9. Histological scoring
Sections stained with H&E were scored in blinded fashion as described previously [6]. Dilatation of oviducts was scored as follows: 0- no significant dilatation, 1- mild dilatation of single cross-section of oviduct, 2- 1-3 dilated cross-sections of oviduct, 3->3 dilated cross-sections of oviduct, 4- confluent pronounced dilatation of oviduct. Cellular parameters (polymorphonuclear cells (PMNs), mononuclear, and plasma cells) were individually scored as follows: 0- no significant presence of infiltration, 1- presence of infiltration at a single focus, 2- presence at 2-4 foci, 3- presence at more than 4 foci, or 4- confluent infiltration. Results are expressed as mean ± SD of scores from all animals in a group.
2.10. Statistical analyses
For comparison of two groups, the student's t test (for normally distributed values) or the Mann-Whitney Rank Sum test (for values not distributed normally) was used to compare values of continuous variables. For experiments with four groups of animals, analysis of variance (ANOVA) followed by multiple comparison of means (Kruskall-Wallis test) was used. To analyze differences in the time required for clearance, the Kaplan-Meier test was used. Differences between groups were considered statistically significant if P values were <0.05. All data shown are representative of 2–3 independent experiments and each experiment shown was analyzed independently.
3. Results
3.1 Cellular cytokine responses to CPAF vaccination
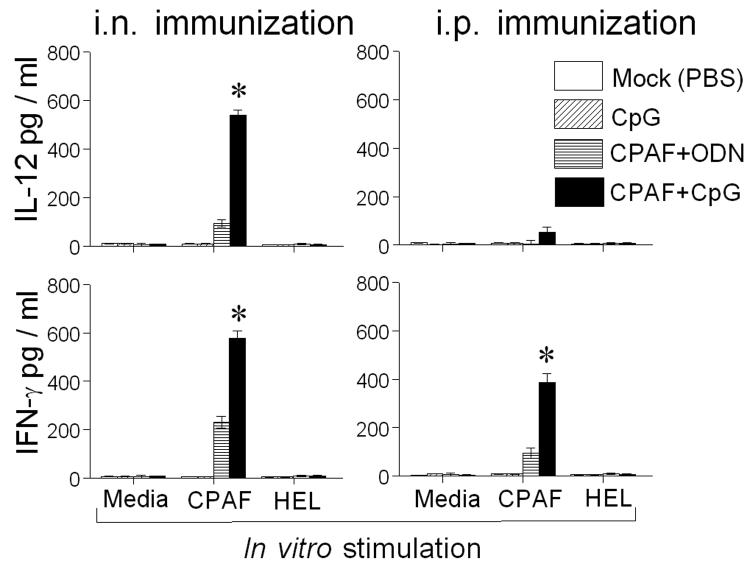
Groups of BALB/c mice were immunized i.n. or i.p. with CPAF+CpG, CPAF+ODN, or treated with PBS (mock). Fourteen days later, splenocytes were stimulated in vitro with CPAF and antigen specific cytokine production was measured by ELISA. As shown in Fig. 1, splenocytes from i.n. CPAF+CpG vaccinated mice stimulated with CPAF exhibited significantly greater IL-12 (538.84 ± 19.21 pg/ml) and IFN-γ production (577.60 ±30.6 pg/ml) as compared to those from CPAF+ODN immunized animals (93.2 ± 15.5 and 230.38 ± 24.8 pg/ml , respectively). In contrast, splenocytes from i.p. CPAF+CpG vaccinated mice exhibited significantly greater IFN-γ production (385.85 ± 35.36 pg/ml), but minimal levels of IL-12 induction (53.84 ± 19.21 pg/ml), as compared to those from i.p. CPAF+ODN (94.32 ± 20.2 and 3.2 ± 15.5 pg/ml, respectively) vaccinated animals. In either case, cells stimulated with an unrelated antigen, HEL, did not exhibit cytokine production. Furthermore, splenocytes from mock-immunized (PBS) or CpG treated animals did not exhibit CPAF-specific cytokine production. There was no detectable IL-4 production in splenocytes from any animal group (data not shown). These results demonstrate the induction of a robust CPAF-specific Th1 cellular response after i.n. or i.p. CPAF+CpG vaccination.
Fig. 1.
Intranasal or i.p. CPAF+CpG vaccination induces robust cell-mediated immune responses. Animals (3 mice/group) were treated i.n. or i.p. with CPAF + CpG, CPAF + ODN, CpG, or PBS. On day 14, animals were euthanized and single cells prepared from spleens and analyzed for CPAF-specific recall cytokine responses by measuring IL-12, IFN-γ and IL-4 production by ELISA. Cells from each group were also stimulated with an unrelated antigen, hen egg lysozyme (HEL). * Significant differences in cytokine secretion between CPAF+CpG and CPAF+ODN immunization (P < 0.05, student's t test). Results are representative of two independent experiments.
3.2 Humoral response to CPAF vaccination
Groups of mice were vaccinated i.n. or i.p. with CPAF+CpG, CPAF+ODN, or with CpG or PBS (Mock) alone. Sera and vaginal fluids were assayed for the presence of anti-CPAF antibodies. Two weeks after the final i.n. or i.p. CPAF+CpG vaccination, mice exhibited high titers of serum anti-CPAF total IgG (5957.74 ± 533.73 and 7523.03 ± 677, respectively), IgG1 ( 6043.9 ± 269.2 and 8008.2 ± 501, respectively), IgG2a (4639.38 ± 409.14 and 3174.83 ± 652.22, respectively) and IgG2b (3811.12 ± 1120.98 and 4849.97 ± 737.35 respectively) (Fig. 2A), when compared to PBS (mock) or CpG alone immunized animals. Intranasal or i.p. immunization with CPAF+ODN induced comparable anti-CPAF total Ab and IgG1 to CPAF+CpG vaccination. However, titers following CPAF+CpG vaccination were significantly elevated for IgG2a (i.n.: 4639.38 ± 409.14 versus 327.87 ± 327.87, and i.p.: 3174.83 ± 652.22 versus 1119.63 ± 503.07, respectively) and IgG2b (i.n.: 3811.12 ± 1120.98 versus 661.41 ± 502.77, and i.p.: 4849.97 ± 737.35 versus 2523.02 ± 321.15, respectively). Vaginal fluid from i.n. or i.p. CPAF+CpG vaccinated mice exhibited significantly elevated levels of anti-CPAF IgA (0.45 ± 0.1 and 0.52 ± 0.05, respectively) when compared to mock vaccinated (PBS) animals (Fig. 2B). Animals immunized with CPAF+ODN exhibited intermediate levels (0.29 ± 0.06 and 0.27 ± 0.06, respectively) of anti-CPAF IgA as compared to animals vaccinated with CPAF+CpG or those treated with PBS (mock). Animals immunized with PBS (mock) or CpG alone displayed minimal levels of anti-CPAF IgA in the vaginal fluid. There were negligible levels of anti-CPAF IgA in the sera of each group of vaccinated animals (data not shown). Plates coated with HEL did not display detectable binding of sera or vaginal fluid from any animal group, indicating the specificity of the measured anti-CPAF antibody (data not shown). These results indicate that i.n. or i.p. vaccination with CPAF+CpG induces strong systemic and mucosal humoral responses against CPAF.
Fig. 2.
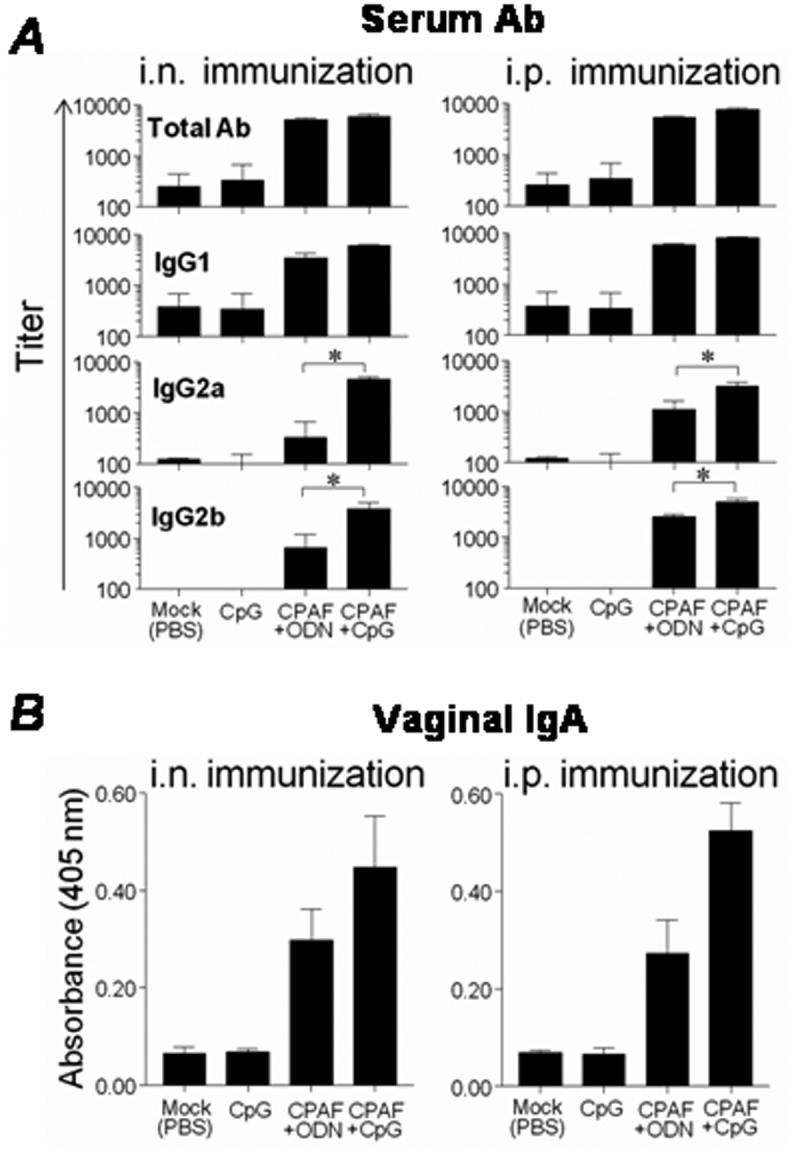
Intranasal or i.p. CPAF+CpG vaccination induces robust humoral immune responses. Animals (6 mice/group) were treated i.n. or i.p. with CPAF + CpG, CPAF + ODN, CpG, or PBS on days 0, 14, and 28. Sera or vaginal fluids were collected from animals two weeks after final immunization. (A) Systemic anti-CPAF Ab responses after immunization. Serum anti-CPAF antibody levels were analyzed by ELISA using CPAF-coated microtiter plates. Results are expressed as mean ± SD of reciprocal serum dilutions corresponding to 50% maximal binding. (B) Mucosal anti-CPAF IgA responses after immunization. Results are expressed as mean ± SD of the absorbance of undiluted vaginal fluids. * Significant differences between CPAF+CpG and CPAF+ODN immunized animals (P < 0.05, Kruskall-Wallis test). Results are representative of two independent experiments.
3.3 Protective efficacy against genital C. muridarum challenge after CPAF vaccination
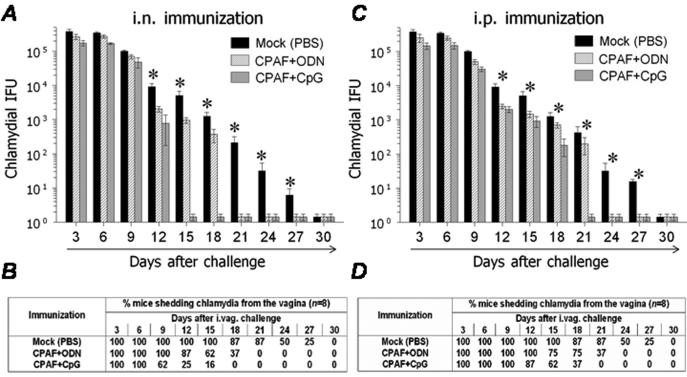
Groups of mice were vaccinated i.n. or i.p with CPAF+CpG, CPAF+ODN, or with CpG alone or PBS (mock) on days 0, 14, and 28. The mice were rested for a month and then challenged i.vag. with 5 × 104IFU C. muridarum. Vaginal bacterial recovery was measured at 3-day intervals post-challenge. Mice vaccinated i.n. with CPAF+CpG displayed significantly reduced chlamydial shedding compared to those immunized with PBS (mock) as early as day 12 after challenge (Fig. 3A). Complete resolution of infection was observed in 75% of i.n. CPAF+CpG vaccinated mice on day 12, 84% on day 15, and 100% on day 18 after challenge (Fig. 3A & 3B). In comparison, mice vaccinated with CPAF+ODN resolved the infection completely between days 15 and 21, whereas mock-immunized animals resolved the bacterial infection between 24 and 30 days post-challenge (Fig. 3A & 3B).
Fig. 3.
CPAF+CpG vaccination enhances the resolution of a genital challenge. Animals (6 mice/group) were treated i.n. or i.p. with three doses of CPAF + CpG, CPAF + ODN, or PBS. One month following final vaccination, mice were challenged i.vag. with 5 × 104 IFU of C. muridarum. At the indicated days following challenge, chlamydial shedding was measured. (A) & (C) Numbers of chlamydial inclusion forming units (IFUs) recovered from vaginal swabs at the indicated days after genital challenge. Results are expressed as Means ± SD. * Significant differences between PBS (mock) immunized animals and CPAF+CpG or CPAF+ODN vaccinated animals (P < 0.05, Kruskall-Wallis test). (B) & (D) Percentage of animals shedding Chlamydia after genital challenge. Significant differences were detected in the time required for resolution of infection between CPAF+CpG immunized mice and all other experimental groups (P = 0.0002, Kaplan-Meier test). Results are representative of three independent experiments.
Mice vaccinated i.p. with CPAF+CpG also displayed significantly reduced chlamydial shedding as compared to mock-immunized animals as early as day 12 after challenge (Fig, 3C). Complete resolution in CPAF+CpG i.p. vaccinated mice was observed in 38% of animals on day 15, 63% on day 18, and 100% by day 21 after challenge (Fig. 3C & 3D). In comparison, mice vaccinated i.p. with CPAF+ODN displayed resolution between days 18-24, and mock-immunized animals between days 24-30 after challenge. These results demonstrate the comparable efficacy of i.n. and i.p. CPAF+CpG vaccination in accelerating clearance of Chlamydia from the genital tract. Additionally, vaccination with CPAF+ODN also enhanced the resolution of infection compared to mock-immunized animals, but with delayed kinetics in comparison to animals vaccinated with CPAF+CpG.
3.4 Histopathological analysis of genital tissues
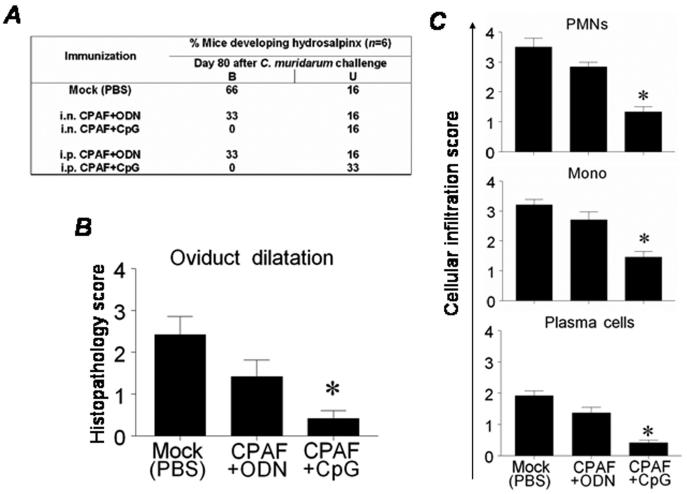
The effect of CPAF+CpG vaccination on the development of genital tract pathology (hydrosalpinx) was examined on day 80 following vaginal chlamydial challenge. This time point was chosen based on results from our previous studies [6, 7, 27]. As shown in Fig. 4A, mice vaccinated i.n. or i.p. with CPAF+CpG exhibited minimal development of hydrosalpinx (0% bilateral, 16% unlilateral or 0% bilateral, 33% unilateral, respectively) as compared to mock-immunized animals (66% bilateral, 16% unilateral). Mice vaccinated i.n. or i.p. with CPAF+ODN displayed intermediate degree of protection against hydrosalpinx (33% bilateral, 16% unilateral, for each) when compared to CPAF+CpG vaccinated and mock-immunized animals.
Fig. 4.
CPAF+CpG vaccination reduces the development of oviduct pathology. Animals (6 mice/group) were treated i.n. or i.p. with three doses of CPAF + CpG, CPAF + ODN, or PBS. One month following final vaccination, mice were challenged i.vag. with 5 × 104 IFU of C. muridarum. At day 80 following challenge, animals were euthanized and tissues collected for further analysis. (A) Percentage of animals developing hydrosalpinx in different immunization groups after genital chlamydial challenge: B-bilateral and U-unilateral. (B) Quantitative histopathological scoring of oviduct dilatation. (C) Quantitative estimation of cellular infiltration into the genital tracts following chlamydial challenge. Means ± SD of histopathology and cellular infiltration scores are shown. * Significant differences between CPAF+CpG and mock-immunized (PBS) animals (P < 0.05, Mann-Whitney Rank-Sum test). Results are representative of two independent experiments.
The development of histopathology and cellular infiltration at day 80 after challenge in vaccinated animals was further scored in a blinded fashion as described previously [6]. As shown in Fig. 4B, CPAF+CpG vaccinated animals displayed minimal oviduct dilatation (0.41 ± 0.19) as compared to mock-immunization with PBS (2.41 ± 0.43). In addition, CPAF+CpG vaccinated animals displayed significantly reduced infiltration of PMNs (1.33 ± 0.16), mononuclear cells (1.45 ± 0.18), and plasma cells (0.41 ± 0.08), as compared to mock-immunized animals (3.5 ± 0.28, 3.2 ± 0.17, 1.92 ± 0.15, respectively) (Fig. 4C). In addition, animals vaccinated with CPAF+ODN exhibited intermediate degrees of oviduct dilatation (1.41 ± 0.39), and inflammatory cellular infiltration (PMNs: 2.83 ± 0.15, mononuclear cells: 3 ± 0.01, plasma cells: 1.38 ± 0.17, respectively). These results indicate that apart from enhancing resolution of infection, vaccination with CPAF+CpG induces protection against oviduct pathology and reduces inflammatory cellular infiltration.
4. Discussion
We have recently shown that intranasal vaccination with CPAF+IL-12 enhances protective immunity against genital chlamydial infection [6] with an important contribution of CD4+ T-cells [7] and endogenous IFN-γ production [6]. In this study, we have shown that as an alternative Th1 adjuvant, CpG is equally efficacious when administered i.n. or i.p. with CPAF in inducing protective immunity.
Intranasal or i.p. CPAF+CpG vaccination induced robust CPAF-specific Th1 type cellular responses. At day 14 after primary immunization, splenocytes from the i.n. CPAF+CpG vaccinated mice produced significant amounts of IFN-γ and IL-12 upon in vitro stimulation with CPAF, whereas i.p. CPAF+CpG vaccination induced elevated levels of IFN-γ, with minimal IL-12 production. Although the reason for reduced IL-12 expression after i.p. immunization is not known, it did not significantly affect the kinetics of chlamydial clearance in i.p. versus i.n. CPAF+CpG vaccinated animals. CD4+ Th1 cells have been shown to be important for the optimal resolution of genital chlamydial infection [28-30]. IL-12 is important for early clearance [28], whereas IFN-γ has been shown to be required for the later phases of chlamydial clearance and for the prevention of bacterial dissemination [28, 31-34].
Both routes of vaccination with CPAF+CpG induced high levels of anti-CPAF serum antibodies. Antibodies have been shown to play a predominant role in protective immunity against reinfection, but not against primary genital chlamydial infections [29, 35]. To this end, antibodies may function by neutralization [36, 37] or through Fc-receptor mediated mechanisms in clearance of chlamydial infection [38, 39]. Intranasal or i.p. vaccination also induced comparable levels of anti-CPAF vaginal IgA antibody. Mucosal IgA in particular has been thought to enhance resistance to chlamydial reinfection, but does not affect the resolution of primary infection [36]. We have found that CPAF vaccinated mice deficient in antibodies (μMT mice) or IgA (IgA−/− mice) resolve the genital chlamydial infection with kinetics comparable to similarly vaccinated wild type animals (Murthy and Arulanandam, unpublished observations). Interestingly, Chlamydia sero-positive humans have been shown to exhibit anti-CPAF antibodies [40], including those that neutralize the proteolytic effect of CPAF [41]. Therefore, the precise role of anti-CPAF antibodies in protective immunity against chlamydial infection has yet to be fully understood.
Animals vaccinated i.n. or i.p. with CPAF+CpG exhibited significantly accelerated resolution of the genital infection by as much as two weeks when compared to PBS (mock) immunized animals. In addition, CPAF+CpG vaccination also significantly reduced the development of oviduct pathology (hydrosalpinx and oviduct dilatation), and induced significant reduction in the numbers of inflammatory cells within the infected genital tracts. This is a highly desirable outcome since the development of oviduct pathology and complications are a major concern in individuals infected with Chlamydia [1, 2]. The resolution of infection in these animals was comparable to that of animals treated i.n. with CPAF+IL-12 [6], indicating that CpG was an effective alternative to IL-12 in this vaccination regimen. Mice treated with CpG alone displayed minimal CPAF-specific responses and comparable kinetics of chlamydial shedding and development of oviduct pathology to PBS (mock) immunized animals (data not shown), suggesting that there was no direct contribution of CpG to the observed protection apart from enhancing CPAF-specific immune responses. Our results are in agreement with Pal et. al [15]who have shown that CpG alone does not significantly alter the course of a genital chlamydial infection. In this study, we also found that non-CPG-containing ODN administered with CPAF also induced enhanced antigen-specific response and had a positive effect on bacterial clearance when compared to mock-immunized animals, but not to the extent of CpG ODN. The effect of CPAF+ODN was comparable to vaccination with CPAF alone as observed in our previous studies [6].
In summary, CpG dexoynucleotides are a potent Th1 adjuvant when delivered i.n. or i.p. with CPAF to enhance protective immunity against genital chlamydial challenge. Although the safety profile of CpG use in humans is still forthcoming [13, 14, 42], the utility of such adjuvants with microbial vaccines holds great promise [13]. These results together provide additional insight for the use of CPAF as a viable anti-chlamydial vaccine candidate.
5. Acknowledgements
This work was supported by National Institutes of Health grants AR048973 and GM08194. The authors thank Dr. Jieh-Juen Yu for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect.Immun. 2002;70(6):2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat.Rev.Immunol. 2005;5(2):149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 3.Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J.Exp.Med. 2001;193(8):935–42. doi: 10.1084/jem.193.8.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong F, Su H, Huang Y, Zhong Y, Zhong G. Cleavage of host keratin 8 by a Chlamydia-secreted protease. Infect.Immun. 2004;72(7):3863–8. doi: 10.1128/IAI.72.7.3863-3868.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirbhai M, Dong F, Zhong Y, Pan KZ, Zhong G. The secreted protease factor CPAF is responsible for degrading pro-apoptotic BH3-only proteins in Chlamydia trachomatis-infected cells. J.Biol.Chem. 2006;281(42):31495–501. doi: 10.1074/jbc.M602796200. [DOI] [PubMed] [Google Scholar]
- 6.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology and is highly dependent upon endogenous IFN-{gamma} production. Infect.Immun. 2006 doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphey C, Murthy AK, Meier PA, Guentzel MN, Zhong G, Arulanandam BP. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4(+) T cells. Cell Immunol. 2006 doi: 10.1016/j.cellimm.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verthelyi D, Klinman DM. Immunoregulatory activity of CpG oligonucleotides in humans and nonhuman primates. Clin.Immunol. 2003;109(1):64–71. doi: 10.1016/s1521-6616(03)00202-x. [DOI] [PubMed] [Google Scholar]
- 9.Weiner GJ. The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides. J.Leukoc.Biol. 2000;68(4):455–63. [PubMed] [Google Scholar]
- 10.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J.Exp.Med. 1997;186(10):1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat.Med. 1997;3(8):849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 13.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu.Rev.Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 14.Verthelyi D. Adjuvant properties of CpG oligonucleotides in primates. Methods Mol.Med. 2006;127:139–58. doi: 10.1385/1-59745-168-1:139. [DOI] [PubMed] [Google Scholar]
- 15.Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect.Immun. 2002;70(9):4812–7. doi: 10.1128/IAI.70.9.4812-4817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry LJ, Hickey DK, Skelding KA, et al. Transcutaneous immunization with combined cholera toxin and CpG adjuvant protects against Chlamydia muridarum genital tract infection. Infect.Immun. 2004;72(2):1019–28. doi: 10.1128/IAI.72.2.1019-1028.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skelding KA, Hickey DK, Horvat JC, et al. Comparison of intranasal and transcutaneous immunization for induction of protective immunity against Chlamydia muridarum respiratory tract infection. Vaccine. 2006;24(3):355–66. doi: 10.1016/j.vaccine.2005.07.104. [DOI] [PubMed] [Google Scholar]
- 18.Meeusen EN, Scheerlinck JP, Wattegedera S, Entrican G. Advances in mucosal vaccination. Anim Health Res.Rev. 2004;5(2):209–17. doi: 10.1079/ahr200470. [DOI] [PubMed] [Google Scholar]
- 19.Lencer WI. Mucosal vaccination: “all politics are local”. Gastroenterology. 1999;116(2):497–9. doi: 10.1016/s0016-5085(99)70152-6. [DOI] [PubMed] [Google Scholar]
- 20.Walker RI. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12(5):387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 21.Igietseme JU, Uriri IM, Kumar SN, et al. Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect.Immun. 1998;66(9):4030–5. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murthy AK, Sharma J, Coalson JJ, Zhong G, Arulanandam BP. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell Immunol. 2004;230(1):56–64. doi: 10.1016/j.cellimm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Dong F, Zhong Y, Arulanandam B, Zhong G. Production of a proteolytically active protein, chlamydial protease/proteasome-like activity factor, by five different Chlamydia species. Infect.Immun. 2005;73(3):1868–72. doi: 10.1128/IAI.73.3.1868-1872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong F, Pirbhai M, Zhong Y, Zhong G. Cleavage-dependent activation of a Chlamydia-secreted protease. Mol.Microbiol. 2004;52(5):1487–94. doi: 10.1111/j.1365-2958.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 25.Wongratanacheewin S, Kespichayawattana W, Intachote P, et al. Immunostimulatory CpG oligodeoxynucleotide confers protection in a murine model of infection with Burkholderia pseudomallei. Infect.Immun. 2004;72(8):4494–502. doi: 10.1128/IAI.72.8.4494-4502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J.Immunol. 2001;166(5):3451–7. doi: 10.4049/jimmunol.166.5.3451. [DOI] [PubMed] [Google Scholar]
- 27.Murthy AK, Cong Y, Murphey C, et al. Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele. Infect.Immun. 2006;74(12):6722–9. doi: 10.1128/IAI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J.Immunol. 1997;158(7):3344–52. [PubMed] [Google Scholar]
- 29.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect.Immun. 2000;68(12):6979–87. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect.Immun. 1995;63(9):3302–8. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito JI, Lyons JM. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect.Immun. 1999;67(10):5518–21. doi: 10.1128/iai.67.10.5518-5521.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect.Immun. 1997;65(6):2145–52. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect.Immun. 1997;65(3):1032–44. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rank RG, Ramsey KH, Pack EA, Williams DM. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect.Immun. 1992;60(10):4427–9. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect.Immun. 2001;69(4):2643–9. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect.Immun. 1997;65(6):1993–9. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15(5):575–82. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 38.Moore T, Ekworomadu CO, Eko FO, et al. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J.Infect.Dis. 2003;188(4):617–24. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- 39.Moore T, Ananaba GA, Bolier J, et al. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105(2):213–21. doi: 10.1046/j.0019-2805.2001.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma J, Bosnic AM, Piper JM, Zhong G. Human antibody responses to a Chlamydia-secreted protease factor. Infect.Immun. 2004;72(12):7164–71. doi: 10.1128/IAI.72.12.7164-7171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma J, Dong F, Pirbhai M, Zhong G. Inhibition of proteolytic activity of a chlamydial proteasome/protease-like activity factor by antibodies from humans infected with Chlamydia trachomatis. Infect.Immun. 2005;73(7):4414–9. doi: 10.1128/IAI.73.7.4414-4419.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tokunaga T, Yamamoto H, Shimada S, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J.Natl.Cancer Inst. 1984;72(4):955–62. [PubMed] [Google Scholar]