Abstract
Objective
Disability threatens the independence of older adults and has large economic and societal costs. This article examines the population impact of arthritis on disability incidence among older Americans.
Methods
The present study used longitudinal data (1998–2000) from the Health and Retirement Study, a national probability sample of elderly Americans. Disability was defined by the inability to perform basic activities of daily living (ADL). A total of 7,758 participants ages ≥65 years with no ADL disability at baseline were included in the analyses. Multiple logistic regression was used to measure the impact of baseline arthritis (self reported) on incidence of subsequent ADL disability after controlling for baseline differences in demographics, health factors, health behaviors, and medical access.
Results
Older adults who had baseline arthritis had a substantially higher incidence of ADL disability compared with those without arthritis (9.3% versus 4.5%). The strong relationship of arthritis and ADL disability was partially explained by demographic, health, behavioral, and medical access factors. However, even after adjusting for all other risk factors, arthritis remained as an independent and significant predictor for developing ADL disability (adjusted odds ratio 1.5, 95% confidence interval 1.2–1.8). Almost 1 in every 4 new cases of ADL disability was due to arthritis (adjusted population attributable fraction: 23.7%).
Conclusion
The high frequency of incident ADL disability attributable to arthritis points to the importance of intervention programs that address the entire spectrum of health and functional problems in persons with arthritis to prevent disability.
Keywords: Arthritis, ADL, Longitudinal data, Disability
INTRODUCTION
Disability compromises quality of life for older adults, and is often associated with hospitalization, institutionalization, and mortality (1-4). Informal or formal care services due to activity limitations impose considerable burdens on society and health care resources. In 1999, more than 44 million American adults had difficulty in ≥1 activities of daily living (ADL) (5). During 1996, direct medical costs for persons with disability were $260 billion (5). Prevention and intervention programs to reduce disability, therefore, are important to contain economic and societal costs.
Arthritis is the leading cause of disability in the United States (5,6). Approximately 1 (37.6%) in 3 adults with arthritis reported limitation in their usual activities (6). As the US population ages, the number of Americans ages ≥65 years with arthritis is projected to increase from ∼21.4 million in 2005 to 41.4 million by the year 2030 (7). Given the increasing numbers of older Americans with arthritis, understanding the role of arthritis in developing ADL disability becomes an urgent public health issue.
This article addresses 3 important questions. First, to what extent does arthritis contribute to the risk of ADL disability at the population level? Second, to what extent is the impact of arthritis explained by demographic, health, behavioral, or economic factors? Finally, in addition to arthritis, what other risk factors lead to ADL disability? Some of these questions have been addressed in the past (8-10), but there have been substantial changes in the population from the time of these original reports. In the present study, all of these questions were examined using a more recent national representative probability sample of community-dwelling Americans ages ≥65 years from longitudinal data of the 1998–2000 Health and Retirement Study (HRS). Because existing limitations in ADL tasks could confound the effect of arthritis on the development of ADL disability, we restricted our analyses to persons who initially reported no ADL disability at baseline.
MATERIALS AND METHODS
Data for this study were obtained from the 1998–2000 HRS. Funded by the National Institute of Aging and conducted by the University of Michigan, the HRS surveys a national probability sample of noninstitutionalized older Americans biannually, with over-samples of African Americans and Hispanics. Descriptions of HRS design are well documented in other sources (11). This sample included 9,707 nonproxy respondents ages ≥65 years at the 1998 interview. We then excluded by design 964 respondents with baseline ADL limitation, 521 decedents, 381 nonrespondents in 2000, and 83 persons with insufficient information on baseline explanatory variables. Our analyses were restricted to a final sample of 7,758 persons.
Outcome variable
ADL disability, based on the International Classification of Function, Disability and Health (12), was ascertained from self report of receiving help from other persons; cannot do; or do not do because of physical, mental, emotional, or memory problems in performing 6 ADL tasks (dressing, walking across a room, bathing, transferring from a bed, eating, or using the toilet). In addition, using mechanical assistance for walking or transferring from a bed was also defined as having ADL disability. Respondents were expected to have disability in ADL tasks for ≥3 months. This assessment of disability captures chronic dependency in basic self-care tasks that could be detrimental to a person's ability to live independently. Incidence of ADL disability was identified by the report of ≥1 ADL limitations at the subsequent 2000 interview.
Arthritis
Baseline (1998) arthritis was determined from a positive response to the following question: “Have you ever had or has a doctor ever told you that you have arthritis or rheumatism?”
Baseline explanatory variables
Demographic characteristics included age, sex, race/ethnicity, marital status (married or not married), and living arrangement (living alone or not living alone). Age was coded in 3 categories: 65–74, 75–84, and ≥85 years. HRS race and ethnicity information was used to classify individuals into 4 mutually exclusive groups: (non-Hispanic) African American, Hispanic, (non-Hispanic) white, and other.
Health factors were assessed from other chronic medical conditions in addition to arthritis and higher-level functional limitations reported at the baseline interview. Chronic diseases were ascertained from a positive self report of physician diagnosis of cancer, diabetes, hypertension, heart problems (heart attack, coronary heart disease, angina, congestive heart failure, or other), pulmonary disease (chronic bronchitis or emphysema), or stroke. Obesity was defined as body mass index ≥30 ([weight (kg)]/[height (m)]2), calculated from self-reported height and weight. Bad vision was defined as poor or legally blind eyesight. To estimate a person's emotional health and psychological distress, we also included depressive symptoms as a risk factor. The presence of depressive symptoms was determined by an abbreviated Center for Epidemio-logic Studies Depression Scale (CES-D) assessment. To avoid confounding somatic depressive symptoms with arthritis-related symptoms, depressive symptoms were determined from the report of ≥1 nonsomatic CES-D mood items (felt depressed, not happy, felt lonely, did not enjoy life, felt sad), consistent with the work by Stump and colleagues (13). Higher-level task limitations, as surrogates of generic disease severity, were assessed from physical and instrumental activities of daily living (IADL) task limitations. Physical limitations were assessed from self-reported inability or avoidance of any of 4 tasks using lower or upper extremities: walking several blocks, climbing several stairs without rest, pulling or pushing large objects, and lifting or carrying weights >10 pounds. IADL limitations were ascertained from reports of receiving help; cannot do; or do not do because of physical, mental, emotional, or memory problems in any of 5 tasks: preparing hot meals, grocery shopping, using the telephone, taking medication, or managing money.
Health behavior factors included current smoking, alcohol consumption, and regular vigorous physical activity (RVPA). Current smoking was ascertained from a positive response to the question, “Do you smoke cigarettes now?” Alcohol consumption in the past 3 months was categorized as none, less than an average of 3 drinks per day, or ≥3 drinks per day. RVPA was based on a positive response to the question, “On average, over the last 12 months, have you participated in vigorous physical activity or exercise 3 times a week or more? By vigorous physical activity, we mean things like sports, heavy housework, or a job that involves physical labor.”
Medical access factors included education, wealth, and family income and health insurance. Education, a measure of human capital, was dichotomized as ≥12 versus <12 completed years of education. For analytic purposes, family income (all sources received by the respondent and spouse/partner during the preceding year) and wealth were dichotomized using the lowest 1998 HRS population-weighted quartiles of $16,800 and $44,800, respectively (14). If only partial income or wealth information was provided during the interview, dichotomized values were based on imputed estimates developed by the University of Michigan (15). Health insurance was classified as sole reliance on Medicare, private insurance coverage, other government insurance, and no coverage or missing.
Statistical analysis
The HRS is a national probability sample. All of our analyses used person-weights, stratum, and sampling error codes for the 1998 HRS data developed by the University of Michigan to provide valid inferences to the US population (16). We used SUDAAN version 9.0 software to account for the complex HRS sampling design (17). All statistical tests were conducted at a nominal 5% alpha significance level.
We calculated the proportion of ADL disability due to a risk factor from the fraction of population attributable risk with the following formula: Ie × [(ORadj − 1)/ORadj]/I × Pr, where Ie is the ADL disability incident rate among persons exposed to the risk factor, ORadj is the adjusted risk factor odds ratio measured from multivariate logistic model on incident ADL disability, I is the overall ADL disability incident rate, and Pr is the risk factor prevalence in the population (18). The fraction of population attributable risk represents the proportion of risk of developing ADL disability in the population that can be attributed to an exposure to a risk factor, making it a relevant public health measure.
Multiple logistic models were used to estimate the impact of arthritis on ADL disability onset, first controlling for demographic differences and then sequentially adjusting for additional differences in health factors, behaviors, and medical access factors. Direct standardization methods (19) were used to illustrate the potential mediating effect of risk factors on ADL disability incidence. This approach averages the expected ADL disability incidence probabilities across reference group members (those without arthritis) based on the estimated multiple logistic model given the actual characteristics of each person (demographics, health factors, health behaviors, and medical access factors). During a second stage, the risk for reference group members is then reestimated as though each referent reported arthritis by adding arthritis to the estimated model.
Analyses were restricted to 7,758 respondents of the 1998–2000 HRS. We adjusted for potential bias due to nonresponse (6%) by handling respondents as an additional sampling stage to obtain adjusted sampling weights, using standard sampling methodology (20). The adjusted sample weight for each 1998–2000 respondent was the product of the respondent's 1998 HRS sample weight divided by the probability of participating in the 2000 interview given the 1998 characteristics. That probability was estimated from logistic regression that controlled for Spanish language, proxy response, phone interview, designated respondent for household questions, age, Hispanic race, education, withholding permission to additional records, changed residence, number of children, chronic diseases, avoiding sensitive questions, negative interview attitude, and geographic regions.
RESULTS
The 7,758 members of the HRS cohort represent a national population of community-dwelling Americans ages ≥65 years who were free of ADL disability in 1998. This population consisted of 7.2% African Americans, 4.3% Hispanics, 86.9% whites, and 1.6% members of other races. Approximately 57.0% of this cohort reported arthritis in 1998.
Overall, >7.2% of this cohort, free of ADL disability at baseline, reported ADL disability 2 years later. Participants with baseline arthritis had substantially higher 2-year incident ADL disability rates compared with those without arthritis (9.3% versus 4.5%) (Figure 1). Arthritis was a strong risk factor for the development of ADL disability regardless of race/ethnicity or sex. Rates of incident ADL disability among persons with arthritis were 1.8–3.4 times greater across race and ethnicity compared with their peers without arthritis (whites 8.7% versus 4.3%, African Americans 13.6% versus 5.9%, Hispanics 14.0 % versus 7.5%, and other races 7.5% versus 2.4%). Similarly, rates of incident ADL disability increased by 2.2 times for women (10.7% versus 5.1%) and 1.8 times for men (6.8% versus 3.9%) with arthritis compared with their nonarthritis peers.
Figure 1.
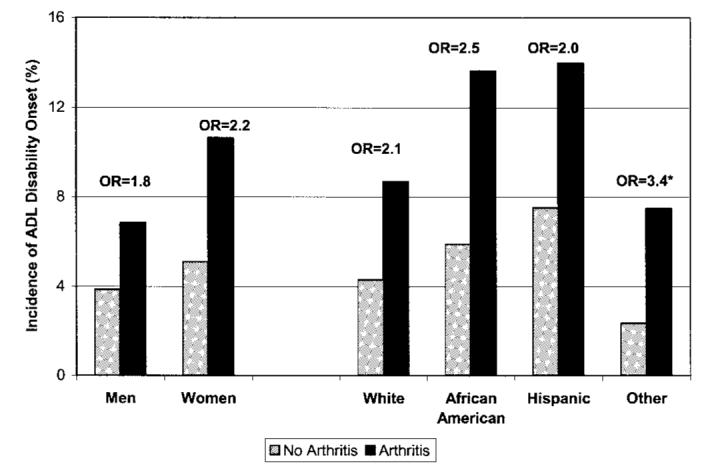
Incidence of 2-year activities of daily living (ADL) disability among persons ages ≥65 years from the 1998 Health and Retirement Study (n = 7,758), stratified by sex and race/ethnicity. * Not significant. OR = odds ratio.
ADL disability incident rates over the 2-year period for each individual baseline risk factor and associated univariate odds ratios (OR) are summarized in Table 1. All demographic factors, except being members of other races, were associated with elevated risk of subsequent incidence of ADL disability, with age ≥85 years being the dominating factor univariately (OR 4.3). It is evident that health factors had the strongest effects on subsequent incidence of ADL disability, increasing the risk by 1.2–7.6 times. Among health factors, baseline IADL limitation was the strongest univariate predictor for incident ADL disability (OR 7.6), followed by vision problems (OR 3.9), stroke (OR 3.5), and physical limitation (OR 2.7).
Table 1.
Prevalence rates and risks of ADL disability incidence by baseline characteristics among 7,758 participants ages ≥65 years in the 1998–2000 Health and Retirement Study*
| Baseline (1998) characteristics |
Risk factor prevalence, no. (population %) |
ADL disability onset, population % |
Univariate OR (95% CI) |
|---|---|---|---|
| Total | 7,758 (100.00) | 7.2 | − (−) |
| Demographic factors | |||
| Race/ethnicity | |||
| White | 6,370 (86.9) | 6.8 | 1.00 (−) |
| African American | 826 (7.2) | 10.7 | 1.65 (1.27–2.15) |
| Hispanic | 446 (4.3) | 10.9 | 1.69 (1.28–2.22) |
| Other | 116 (1.6) | 5.2 | 0.75 (0.28–1.97) |
| Female sex | 4,546 (59.4) | 8.5 | 1.64 (1.38–1.94) |
| Age ≥85 years | 607 (6.6) | 21.9 | 4.27 (3.48–5.22) |
| Not married | 3,095 (43.3) | 9.4 | 1.76 (1.47–2.11) |
| Live alone | 2,262 (32.1) | 9.6 | 1.63 (1.37–1.94) |
| Health factors | |||
| Chronic conditions | |||
| Arthritis | 4,450 (57.0) | 9.3 | 2.16 (1.80–2.60) |
| Cancer | 1,034 (13.4) | 8.4 | 1.22 (0.95–1.55) |
| Depressive symptoms | 2,584 (33.3) | 10.8 | 2.12 (1.81–2.48) |
| Diabetes | 1,102 (13.7) | 12.3 | 2.05 (1.56–2.68) |
| Heart disease | 1,788 (22.7) | 10.4 | 1.72 (1.37–2.17) |
| Hypertension | 3,927 (50.3) | 9.0 | 1.72 (1.48–2.01) |
| Pulmonary disease | 703 (9.3) | 13.4 | 2.19 (1.71–2.80) |
| Obesity | 1,343 (16.8) | 9.5 | 1.46 (1.20–1.77) |
| Stroke | 530 (6.9) | 19.0 | 3.45 (2.44–4.87) |
| Vision (poor/legally blind) | 382 (4.7) | 21.2 | 3.86 (2.76–5.38) |
| Higher level limitation(s) | |||
| Physical | 2,229 (27.6) | 12.7 | 2.71 (2.26–3.24) |
| IADL | 479 (5.8) | 31.6 | 7.59 (6.01–9.58) |
| Behavioral factors | |||
| Current smoking | 813 (10.8) | 6.8 | 0.93 (0.66–1.32) |
| Alcohol use ≥3 drinks/day | 171 (2.1) | 3.6 | 0.47 (0.28–0.78) |
| Lack of RVPA | 4,363 (55.8) | 10.3 | 3.41 (2.64–4.41) |
| Medical access factors | |||
| Education <12 years | 2,372 (29.1) | 10.0 | 1.73 (1.44–2.08) |
| Household income in the lowest quartile | 2,223 (28.6) | 11.9 | 2.40 (2.00–2.87) |
| Family wealth in the lowest quartile | 1,664 (20.3) | 11.9 | 2.11 (1.64–2.73) |
| Health insurance | |||
| Medicare only | 4,204 (54.7) | 8.1 | 1.76 (1.40–2.21) |
| Private | 2,890 (37.7) | 4.8 | 1.00 (−) |
| Other government | 582 (6.8) | 14.0 | 3.25 (2.53–4.17) |
| None or missing | 82 (0.8) | 2.8 | 0.57 (0.17–1.89) |
ADL = activities of daily living; OR = odds ratio; 95% CI = 95% confidence interval; IADL = instrumental activities of daily living; RVPA = regular vigorous physical activity.
Arthritis not only more than doubled the risk of incident ADL disability (OR 2.2), but it was the most prevalent chronic condition among this elderly population (57%). Among health behavior factors, lack of RVPA increased the risk of incident ADL disability by 3.4 times, and ADL disability was less likely to occur among persons who consume ≥3 drinks per day. Finally, less medical access, such as less education, low income or wealth, and lack of private health insurance coverage, was related to elevated risk of subsequent ADL disability.
It is well known that many risk factors associated with ADL disability are more common among individuals with arthritis. In this cohort, individuals with arthritis compared with their nonarthritis peers were more likely to be older and have other chronic conditions; physical limitations; IADL limitations; lack of RVPA; and fewer economic resources in terms of low education, low income, and low wealth, all of which were associated with higher incident rates of ADL disability (Table 2). Therefore, we investigated the effect of arthritis on the development of ADL disability controlling for the above influential factors.
Table 2.
Baseline characteristics among 7,758 participants ages ≥65 years in the 1998–2000 Health and Retirement Study, by baseline arthritis status*
| Baseline arthritis |
|||
|---|---|---|---|
| Baseline (1998) characteristics |
Yes (n = 4,450) |
No (n = 3,308) |
P† |
| Demographic factors | |||
| Race/ethnicity | |||
| White | 86.6 | 87.4 | 0.071 |
| African American | 7.9 | 6.2 | |
| Hispanic | 4.0 | 4.7 | |
| Other | 1.5 | 1.6 | |
| Female sex | 63.8 | 53.5 | < 0.001 |
| Age, years | |||
| 65–74 | 55.8 | 61.4 | < 0.001 |
| 75–84 | 36.6 | 33.3 | |
| ≥85 | 7.6 | 5.3 | |
| Not married | 45.5 | 40.5 | 0.001 |
| Live alone | 33.6 | 30.0 | 0.015 |
| Health factors | |||
| Chronic conditions | |||
| None | 14.7 | 22.6 | < 0.001 |
| Cancer | 14.2 | 12.2 | 0.003 |
| Depressive symptoms | 36.5 | 29.1 | < 0.001 |
| Diabetes | 15.4 | 11.4 | < 0.001 |
| Heart disease | 26.2 | 18.1 | < 0.001 |
| Hypertension | 53.9 | 45.6 | < 0.001 |
| Pulmonary disease | 11.3 | 6.5 | < 0.001 |
| Obesity | 20.2 | 12.2 | < 0.001 |
| Stroke | 7.1 | 6.5 | 0.258 |
| Vision (poor/legally blind) | 5.6 | 3.6 | 0.003 |
| Higher level limitation(s) | |||
| Physical | 31.8 | 22.0 | < 0.001 |
| IADL | 7.2 | 4.0 | < 0.001 |
| Behavioral factors | |||
| Current smoking | 9.7 | 12.1 | 0.005 |
| Alcohol use ≥3 drinks/day | 1.7 | 2.7 | 0.012 |
| Lack of RVPA | 59.5 | 50.9 | < 0.001 |
| Medical access factors | |||
| Education <12 years | 31.0 | 26.6 | < 0.001 |
| Household income in the lowest quartile | 31.8 | 24.4 | < 0.001 |
| Family wealth in the lowest quartile | 22.7 | 17.0 | < 0.001 |
| Health insurance | |||
| Medicare only | 55.0 | 54.3 | < 0.001 |
| Private | 36.3 | 39.6 | |
| Other government | 8.0 | 5.2 | |
| None or missing | 0.7 | 0.9 | |
Values are the population percentage unless otherwise indicated. See Table 1 for definitions.
Chi-square test.
The standardized incident rates of ADL disability for individuals with and without arthritis calculated from multiple logistic models are shown in Table 3. The adjusted OR and 95% confidence interval (95% CI) of arthritis from each model are also presented. The 2-year standardized incident rate of ADL disability for persons who had arthritis (9.3%) was twice that of those who did not have arthritis (4.5%). Controlling for 5 demographic factors reduced the standardized incident rate of the arthritis cohort from 9.3% to 8.3%. Controlling for 11 other health factors further reduced the standardized incident rate to 6.5%. Finally, controlling for 3 behavioral and 4 medical access factors slightly reduced the standardized incident rate to 6.3%. Table 3 also shows that after controlling for differences in demographic, other health factors, behavioral, and medical access factors, arthritis remained as a significant risk factor for the development of ADL disability (OR 1.5, 95% CI 1.2–1.8). The adjusted fraction of population attributable risk associated with arthritis after controlling for all investigated risk factors was 23.7%.
Table 3.
Two-year standardized incident rates of ADL disability by baseline arthritis status*
| Baseline arthritis |
|||
|---|---|---|---|
| Adjustment factors | No (reference group) |
Yes | Arthritis OR (95% CI) |
| Unadjusted | 4.5 | 9.3 | 2.16 (1.80–2.60) |
| + Demographics† | 4.5 | 8.3 | 1.96 (1.63–2.35) |
| + Other health needs‡ | 4.5 | 6.5 | 1.53 (1.26–1.86) |
| + Behavioral factors§ | 4.5 | 6.4 | 1.49 (1.22–1.82) |
| + Medical access factors¶ | 4.5 | 6.3 | 1.48 (1.21–1.80) |
See Table 1 for definitions.
Controlling for demographics (race/ethnicity, age, sex, marital status, living arrangement).
Controlling for demographics + other health factors (other chronic conditions: cancer, depressive symptoms, diabetes, heart disease, hypertension, obesity, pulmonary disease, stroke, vision problem; higher-level limitations: physical, instrumental activities of daily living).
Controlling for demographics + other health factors + behavioral factors (smoke, alcohol use, exercise).
Controlling for demographics + other health factors + behavioral factors + medical access factors (education, income, wealth, health insurance).
The details of the final multivariate model in Table 3 are presented in Table 4. Among demographic factors, the risk of developing ADL disability was significantly greater with each decade of age (adjusted OR 1.7 for age 75–84 years, adjusted OR 3.7 for age ≥85 years) and for women (adjusted OR 1.2). The strongest predictors of ADL disability incidence among other health, behavioral, and medical access factors were limitation in IADL tasks (OR 3.6), stroke (OR 2.2), and lack of RVPA (OR 2.1). Additional factors that significantly increased the likelihood of future ADL disability incidence included pulmonary disease, diabetes, physical limitation, obesity, depressive symptoms, and sole reliance on Medicare coverage. Taking the relative prevalence of these baseline factors into account, the only factor that had a greater population impact on the development of ADL disability than arthritis was lack of RVPA (adjusted population attributable risk fraction 42.1%), a factor that was strongly associated with arthritis.
Table 4.
ORs and associated 95% CIs from multiple logistic regression modeling the 2-year ADL disability incidence*
| Baseline (1998) characteristics |
Adjusted OR | 95% CI |
|---|---|---|
| Arthritis | 1.48† | 1.21–1.80† |
| Demographic factors | ||
| African American | 1.22 | 0.92–1.60 |
| Hispanic | 1.21 | 0.78–1.87 |
| Other race | 0.78 | 0.31–1.93 |
| Female sex | 1.21† | 1.00–1.47† |
| Age, years | ||
| 75–84 | 1.65† | 1.33–2.05† |
| ≥85 | 3.66† | 2.83–4.74† |
| Not married | 0.76 | 0.56–1.04 |
| Live alone | 1.19 | 0.89–1.61 |
| Health factors | ||
| Other chronic conditions | ||
| Cancer | 1.10 | 0.85–1.43 |
| Depressive symptoms | 1.39† | 1.17–1.65† |
| Diabetes | 1.53† | 1.15–2.04† |
| Heart disease | 1.14 | 0.89–1.47 |
| Hypertension | 1.18 | 0.98–1.44 |
| Pulmonary disease | 1.60† | 1.20–2.13† |
| Obesity | 1.41† | 1.17–1.69† |
| Stroke | 2.23† | 1.48–3.36† |
| Vision (poor/legally blind) | 1.40 | 0.95–2.06 |
| Higher level limitation(s) | ||
| Physical | 1.43† | 1.17–1.75† |
| IADL | 3.58† | 2.75–4.66† |
| Behavioral factors | ||
| Current smoking | 1.08 | 0.72–1.64 |
| Alcohol use ≥3 drinks/day | 0.81 | 0.45–1.47 |
| Lack of RVPA | 2.11† | 1.64–2.73† |
| Medical access factors | ||
| Education <12 years | 0.98 | 0.79–1.22 |
| Household income in the lowest quartile | 1.15 | 0.88–1.51 |
| Family wealth in the lowest quartile | 1.11 | 0.81–1.52 |
| Medicare only | 1.31† | 1.01–1.70† |
| Other government insurance | 1.36 | 0.96–1.94 |
| None/missing | 0.31 | 0.07–1.37 |
N = 7,758 participants in the 1998 Health and Retirement Study. See Table 1 for definitions.
Statistically significant.
DISCUSSION
Our study demonstrated a strong public health impact of arthritis on incidence of ADL disability. If arthritis were eliminated, almost 1 of every 4 new cases of ADL disability among older adults would be prevented. Arthritis substantially elevates the risk of developing ADL disability. Nearly 1 of every 10 persons who had arthritis developed ADL disability (9.3%) within 2 years. The odds of developing ADL disability were >2 times higher (OR 2.2) for persons who had arthritis than for those who did not. We found that even after controlling for demographic, health, behavioral, and medical access factors, arthritis remained as a significant predictor of ADL disability incidence (OR 1.5).
This study also demonstrated that a considerable proportion of the increased risk of ADL disability incidence associated with arthritis (∼20%) can be explained by the presence of other baseline chronic conditions and preexisting limitations in physical or IADL tasks. These health factors were more prevalent among adults who had arthritis than among their arthritis-free peers (21-23). Other literature on disability risk factors has found that these health factors are associated with elevated risks of functional limitation (9,24-26). It is further argued that physical and IADL limitations, often the secondary outcomes of arthritis and other chronic conditions, act as mediators in the pathway to incident ADL disability. Therefore, our findings suggest a complex link between arthritis and ADL disability development through other health factors.
The finding that arthritis is an independent predictor of incident ADL disability is consistent with other studies focusing on elderly Americans. Studies based on the Longitudinal Study on Aging have found that after accounting for demographic, health, and socioeconomic factors, arthritis persisted as a significant predictor of functional limitation incidence among older adults (ages ≥70 years) who were initially functionally independent (25,27). A more recent study, using the 1995–1998 Asset and Health Dynamics Among the Oldest Old survey, showed that arthritis was a strong predictor among older Americans, after baseline demographic, medical, and behavioral factors were taken into account (10). The current study demonstrated that this strong association of arthritis to disability, combined with the high prevalence of arthritis, has a substantial population impact: the adjusted fraction of population attributable risk was 23.7%. This result underlines the importance of raising awareness of the role of arthritis in developing disability and as a public health priority.
Lack of RVPA was the only risk factor that had a greater population-level impact on incident ADL disability than arthritis among this elderly population. If everyone could participate in RVPA, then 42% of ADL disability would be prevented. The effect of lack of RVPA is particularly important for persons with arthritis (28). A previous study by Wang et al, based on the 1987 National Medical Expenditure Survey, found that physical inactivity was associated with 12.4% ($1,250 in 2000 dollar) of the total medical costs among adults who had arthritis (29). It is worth noting that although physical activity intervention has long been advocated by the clinical literature as a safe and efficacious way to control arthritis consequences (30-32), our study still demonstrated that ∼60% of older adults who had arthritis did not engage in RVPA. From a public health point of view, additional effort should be made to educate persons with arthritis about the benefit of physical activity.
There are several limitations to our study that are common to secondary databases. First, we used self-reported rather than physician-confirmed assessment of chronic conditions, which may lack accuracy. However, this type of assessment is relevant from the public health perspective because many individuals with chronic illness do not seek a health provider. Therefore, to estimate the full burden of health conditions, we rely on self-reported measures. Second, because baseline factors were assessed cross-sectionally, it is not known which factors (e.g., depressive symptoms) may be consequences or causes of other concurrent factors (e.g., physical and IADL limitations). Third, this study did not control for disease severity or disease duration due to lack of such information from the HRS data. However, we used baseline functional limitations in physical or IADL tasks as surrogates of generic disease severity. Fourth, there could be other factors linking arthritis to incidence of ADL disability that we were unable to address in this study. For example, Moritz et al found that social isolation and lack of participation in social activities are related to developing ADL disability (33). Arthritis may play a role here if its symptoms, such as pain or fatigue, prevent patients from attending certain social activities. Finally, our findings are limited to a 2-year followup period, and only apply to adults ages ≥65 years.
Despite these limitations, our findings are based on a large nationally representative sample, and we examined a wide range of disability-relevant factors. This study demonstrated that because of its strong association with ADL disability and high prevalence, arthritis is associated with nearly one-fourth of new cases of ADL disability in older adults. The strong relationship of arthritis to ADL disability is only partially explained by other chronic conditions, higher-level activity limitations, and health behaviors, factors that are amenable to medical and public health intervention. These results point to the importance of intervention programs that address the entire spectrum of health and functional problems in persons with arthritis to prevent the development of ADL disability, which is crucial to promoting independence among the elderly.
Footnotes
Supported in part by funding from the NIH (National Institute for Arthritis and Musculoskeletal and Skin Diseases grant P60-AR48098 and National Center for Medical Rehabilitation Research grant R01-HD45412).
REFERENCES
- 1.Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Annu Rev Public Health. 1996;17:25–46. doi: 10.1146/annurev.pu.17.050196.000325. [DOI] [PubMed] [Google Scholar]
- 2.Mor V, Wilcox V, Rakowski W, Hiris J. Functional transitions among the elderly: patterns, predictors, and related hospital use. Am J Public Health. 1994;84:1274–80. doi: 10.2105/ajph.84.8.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493–8. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 4.Yelin E, Trupin L, Wong B, Rush S. The impact of functional status and change in functional status on mortality over 18 years among persons with rheumatoid arthritis. J Rheumatol. 2002;29:1851–7. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Prevalence of disabilities and associated health conditions among adults: United States, 1999 [published erratum appears in MMWR Morb Mortal Wkly Rep 2001;50:149] MMWR Morb Mortal Wkly Rep. 2001;50:120–5. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Racial/ethnic differences in the prevalence and impact of doctor-diagnosed arthritis: United States, 2002. MMWR Morb Mortal Wkly Rep. 2005;54:119–23. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Public health and aging: projected prevalence of self-reported arthritis or chronic joint symptoms among persons aged >65 years: United States, 2005–2030. MMWR Morb Mortal Wkly Rep. 2003;52:489–91. [PubMed] [Google Scholar]
- 8.Dunlop DD, Manheim LM, Yelin EH, Song J, Chang RW. The costs of arthritis. Arthritis Rheum. 2003;49:101–13. doi: 10.1002/art.10913. [DOI] [PubMed] [Google Scholar]
- 9.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–69. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds SL, Silverstein M. Observing the onset of disability in older adults. Soc Sci Med. 2003;57:1875–89. doi: 10.1016/s0277-9536(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 11.Heeringa SG, Conno RJ. Technical description of the Health and Retirement Study Sample Design. Population Studies Center, University of Michigan; Ann Arbor (MI): 1995. [Google Scholar]
- 12.World Health Organization . International Classification of Functioning, Disability and Health (ICF) World Health Organization; Geneva: 2001. [Google Scholar]
- 13.Stump TE, Clark DO, Johnson RJ, Wolinsky FD. The structure of health status among Hispanic, African American, and white older adults. (Spec No:49–60).J Gerontol B Psychol Sci Soc Sci. 1997;52 doi: 10.1093/geronb/52b.special_issue.49. [DOI] [PubMed] [Google Scholar]
- 14.Smith JP. Wealth inequality among older Americans. (Spec No:74–81).J Gerontol B Psychol Sci Soc Sci. 1997;52 doi: 10.1093/geronb/52b.special_issue.74. [DOI] [PubMed] [Google Scholar]
- 15.Cao H. (HRS/AHEAD Documentation Report No.: DR-007).IMPUTE: a SAS application system for missing value imputations: with special reference to HRS income/assets imputations. URL: http://hrsonline.isr.umich.edu/docs/userg/dr-007.pdf.
- 16.Health and Retirement Study Sampling weights revised for tracker 2.0 and beyond. URL: http://hrsonline.isr.umich.edu/meta/tracker/desc/wghtdoc.pdf.
- 17.Research Triangle Institute . SUDAAN language manual, release 9.0. Research Triangle Institute; Research Triangle Park (NC): 2004. [Google Scholar]
- 18.Rothman K. Epidemiology: an introduction. Oxford University Press; New York: 2002. [Google Scholar]
- 19.Korn EL, Graubard BI. Analysis of health surveys. John Wiley and Sons; New York: 1999. [Google Scholar]
- 20.United States Bureau of the Census . The current populations survey: a report on methodology. US Government Printing Office; Washington, DC: 1963. [Google Scholar]
- 21.Verbrugge LM. Women, men, and osteoarthritis. Arthritis Care Res. 1995;8:212–20. doi: 10.1002/art.1790080404. [DOI] [PubMed] [Google Scholar]
- 22.Dunlop DD, Manheim LM, Song J, Chang RW. Health care utilization among older adults with arthritis. Arthritis Rheum. 2003;49:164–71. doi: 10.1002/art.11003. [DOI] [PubMed] [Google Scholar]
- 23.Yelin EH, Trupin LS, Sebesta DS. Transitions in employment, morbidity, and disability among persons ages 51–61 with musculoskeletal and non-musculoskeletal conditions in the US, 1992–1994. Arthritis Rheum. 1999;42:769–79. doi: 10.1002/1529-0131(199904)42:4<769::AID-ANR22>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Sands LP, Yaffe K, Lui LY, Stewart A, Eng C, Covinsky K. The effects of acute illness on ADL decline over 1 year in frail older adults with and without cognitive impairment. J Gerontol A Biol Sci Med Sci. 2002;57:M449–54. doi: 10.1093/gerona/57.7.m449. [DOI] [PubMed] [Google Scholar]
- 25.Dunlop DD, Manheim LM, Sohn MW, Liu X, Chang RW. Incidence of functional limitation in older adults: the impact of gender, race, and chronic conditions. Arch Phys Med Rehabil. 2002;83:964–71. doi: 10.1053/apmr.2002.32817. [DOI] [PubMed] [Google Scholar]
- 26.Verbrugge LM, Patrick DL. Seven chronic conditions: their impact on US adults' activity levels and use of medical services. Am J Public Health. 1995;85:173–82. doi: 10.2105/ajph.85.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boult C, Kane RL, Louis TA, Boult L, McCaffrey D. Chronic conditions that lead to functional limitation in the elderly. J Gerontol. 1994;49:M28–36. doi: 10.1093/geronj/49.1.m28. [DOI] [PubMed] [Google Scholar]
- 28.Dunlop DD, Semanik P, Song J, Manheim LM, Shih V, Chang RW. Risk factors for functional decline in older adults with arthritis. Arthritis Rheum. 2005;52:1274–82. doi: 10.1002/art.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Helmick CG, Macera C, Zhang P, Pratt M. Inactivity-associated medical costs among US adults with arthritis. Arthritis Rheum. 2001;45:439–45. doi: 10.1002/1529-0131(200110)45:5<439::aid-art363>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Hakkinen A, Sokka T, Kotaniemi A, Hannonen P. A randomized two-year study of the effects of dynamic strength training on muscle strength, disease activity, functional capacity, and bone mineral density in early rheumatoid arthritis. Arthritis Rheum. 2001;44:515–22. doi: 10.1002/1529-0131(200103)44:3<515::AID-ANR98>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359–70. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 32.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in over-weight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 33.Moritz DJ, Kasl SV, Berkman LF. Cognitive functioning and the incidence of limitations in activities of daily living in an elderly community sample. Am J Epidemiol. 1995;141:41–9. doi: 10.1093/oxfordjournals.aje.a117344. [DOI] [PubMed] [Google Scholar]


