Abstract
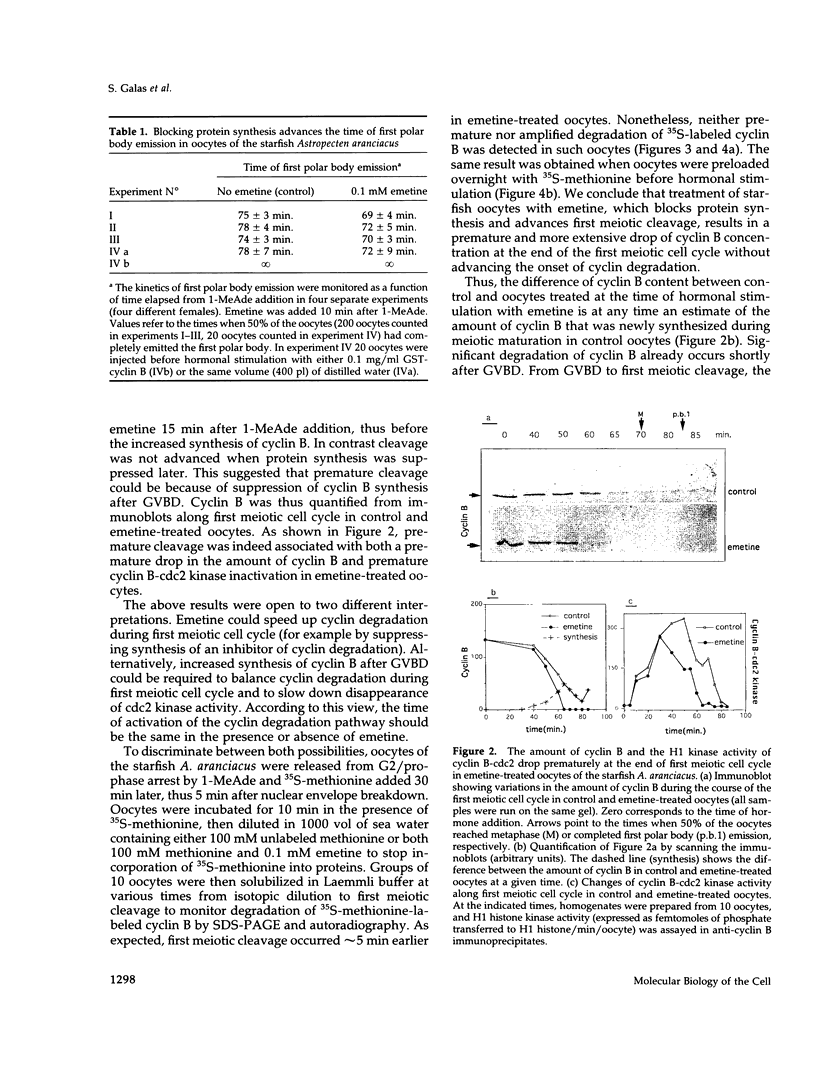
In most animals, the rate of cyclin B synthesis increases after nuclear envelope breakdown during the first meiotic cell cycle. We have found that cyclin B-cdc2 kinase activity drops earlier in emetine-treated than in control starfish oocytes, although the protein synthesis inhibitor does not activate the cyclin degradation pathway prematurely. Moreover, protein synthesis is required to prevent meiotic cleavage to occur prematurely, sometimes before chromosomes have segregated on the metaphase plate. In normal conditions, increased synthesis of cyclin B after germinal vesicle breakdown (GVBD) balances cyclin degradation and increases the time required for cyclin B-cdc2 kinase to drop below the level that inhibits cleavage. Taken together, these results point to cyclin B as a possible candidate that could explain the need for increased protein synthesis during meiosis I. Although direct experimental evidence was not provided in the present work, cyclin B synthesis after GVBD may be important for correct segregation of homologous chromosomes at the end of first meiotic metaphase, as shown by a variety of cytological disorders that accompany premature cleavage. Although the overall stimulation of protein synthesis because of cdc2 kinase activation is still observed in oocytes from which the germinal vesicle has been removed before hormonal stimulation, the main increase of cyclin B synthesis normally observed after germinal vesicle breakdown is suppressed. The nuclear factor required for specific translation of cyclin B after GVBD is not cyclin B mRNA, as shown by using a highly sensitive reverse transcription followed by polymerase chain reaction procedure that failed to detect any cyclin B mRNA in isolated germinal vesicles.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belyavsky A., Vinogradova T., Rajewsky K. PCR-based cDNA library construction: general cDNA libraries at the level of a few cells. Nucleic Acids Res. 1989 Apr 25;17(8):2919–2932. doi: 10.1093/nar/17.8.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankstein L. A., Kiefer B. I. The relation of DNA and protein synthesis to the meiotic-mitotic transition in the zygote of Urechis caupo. Dev Biol. 1977 Nov;61(1):1–10. doi: 10.1016/0012-1606(77)90336-0. [DOI] [PubMed] [Google Scholar]
- Doree M., Guerrier P. Site of action of 1-methyladenine in inducing oocyte maturation in starfish. Kinetic evidence for receptors localized on the cell membrane. Exp Cell Res. 1975 Mar 15;91(2):296–300. doi: 10.1016/0014-4827(75)90107-x. [DOI] [PubMed] [Google Scholar]
- Dorée M. Control of M-phase by maturation-promoting factor. Curr Opin Cell Biol. 1990 Apr;2(2):269–273. doi: 10.1016/0955-0674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- Dorée M., Peaucellier G., Picard A. Activity of the maturation-promoting factor and the extent of protein phosphorylation oscillate simultaneously during meiotic maturation of starfish oocytes. Dev Biol. 1983 Oct;99(2):489–501. doi: 10.1016/0012-1606(83)90298-1. [DOI] [PubMed] [Google Scholar]
- Dorée M. Protein synthesis is not involved in initiation or amplification of the maturation-promoting factor (MPF) in starfish oocytes. Exp Cell Res. 1982 May;139(1):127–133. doi: 10.1016/0014-4827(82)90326-3. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Freeman R. S., Pickham K. M., Kanki J. P., Lee B. A., Pena S. V., Donoghue D. J. Xenopus homolog of the mos protooncogene transforms mammalian fibroblasts and induces maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5805–5809. doi: 10.1073/pnas.86.15.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix M. A., Labbé J. C., Dorée M., Hunt T., Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990 Jul 26;346(6282):379–382. doi: 10.1038/346379a0. [DOI] [PubMed] [Google Scholar]
- Gallant P., Nigg E. A. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J Cell Biol. 1992 Apr;117(1):213–224. doi: 10.1083/jcb.117.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiara J. B., Richardson H. E., Sugimoto K., Henze M., Lew D. J., Wittenberg C., Reed S. I. A cyclin B homolog in S. cerevisiae: chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991 Apr 5;65(1):163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Guerrier P., Doree M. Hormonal control of reinitiation of meiosis in starfish. The requirement of 1-methyladenine during nuclear maturation. Dev Biol. 1975 Dec;47(2):341–348. doi: 10.1016/0012-1606(75)90288-2. [DOI] [PubMed] [Google Scholar]
- Hanocq-Quertier J., Baltus E. Phosphorylation of ribosomal proteins during maturation of Xenopus laevis oocytes. Eur J Biochem. 1981 Nov;120(2):351–355. doi: 10.1111/j.1432-1033.1981.tb05711.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol. 1988 Apr;126(2):242–252. doi: 10.1016/0012-1606(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Hirai T., Yamashita M., Yoshikuni M., Lou Y. H., Nagahama Y. Cyclin B in fish oocytes: its cDNA and amino acid sequences, appearance during maturation, and induction of p34cdc2 activation. Mol Reprod Dev. 1992 Oct;33(2):131–140. doi: 10.1002/mrd.1080330204. [DOI] [PubMed] [Google Scholar]
- Houk M. S., Epel D. Protein synthesis during hormonally induced meiotic maturation and fertilization in starfish oocytes. Dev Biol. 1974 Oct;40(2):298–310. doi: 10.1016/0012-1606(74)90132-8. [DOI] [PubMed] [Google Scholar]
- Hunt T., Luca F. C., Ruderman J. V. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992 Feb;116(3):707–724. doi: 10.1083/jcb.116.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery W. R. Polyadenylation of maternal and newly-synthesized RNA during starfish oocyte maturation. Dev Biol. 1977 May;57(1):98–108. doi: 10.1016/0012-1606(77)90357-8. [DOI] [PubMed] [Google Scholar]
- Kanatani H., Shirai H., Nakanishi K., Kurokawa T. Isolation and indentification on meiosis inducing substance in starfish Asterias amurensis. Nature. 1969 Jan 18;221(5177):273–274. doi: 10.1038/221273a0. [DOI] [PubMed] [Google Scholar]
- Kanatani H. Spawning of Starfish: Action of Gamete-Shedding Substance Obtained from Radial Nerves. Science. 1964 Nov 27;146(3648):1177–1179. doi: 10.1126/science.146.3648.1177. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Minshull J., Ford C., Golsteyn R., Poon R., Hunt T. On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J Cell Biol. 1991 Aug;114(4):755–765. doi: 10.1083/jcb.114.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Labbé J. C., Devault A., Fesquet D., Capony J. P., Cavadore J. C., Le Bouffant F., Dorée M. Dephosphorylation of cdc2 on threonine 161 is required for cdc2 kinase inactivation and normal anaphase. EMBO J. 1992 Jul;11(7):2381–2390. doi: 10.1002/j.1460-2075.1992.tb05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Labbé J. C., Devault A., Fesquet D., Strausfeld U., Nilsson J., Nygren P. A., Uhlen M., Cavadore J. C., Dorée M. Cyclin A-cdc2 kinase does not trigger but delays cyclin degradation in interphase extracts of amphibian eggs. J Cell Sci. 1992 May;102(Pt 1):55–62. doi: 10.1242/jcs.102.1.55. [DOI] [PubMed] [Google Scholar]
- Lu X. P., Koch K. S., Lew D. J., Dulic V., Pines J., Reed S. I., Hunter T., Leffert H. L. Induction of cyclin mRNA and cyclin-associated histone H1 kinase during liver regeneration. J Biol Chem. 1992 Feb 15;267(5):2841–2844. [PubMed] [Google Scholar]
- Luca F. C., Ruderman J. V. Control of programmed cyclin destruction in a cell-free system. J Cell Biol. 1989 Nov;109(5):1895–1909. doi: 10.1083/jcb.109.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca F. C., Shibuya E. K., Dohrmann C. E., Ruderman J. V. Both cyclin A delta 60 and B delta 97 are stable and arrest cells in M-phase, but only cyclin B delta 97 turns on cyclin destruction. EMBO J. 1991 Dec;10(13):4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Clarke H. J. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Dworkin-Rastl E., Dworkin M. B., Richter J. D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989 Jun;3(6):803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- McGrew L. L., Richter J. D. Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J. 1990 Nov;9(11):3743–3751. doi: 10.1002/j.1460-2075.1990.tb07587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney J. D., Heintz N. Transcriptional regulation in the eukaryotic cell cycle. Trends Biochem Sci. 1991 Nov;16(11):430–435. doi: 10.1016/0968-0004(91)90170-z. [DOI] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Murray A., Colman A., Hunt T. Xenopus oocyte maturation does not require new cyclin synthesis. J Cell Biol. 1991 Aug;114(4):767–772. doi: 10.1083/jcb.114.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Subunit promiscuity among hemopoietic growth factor receptors. Cell. 1991 Oct 4;67(1):1–4. doi: 10.1016/0092-8674(91)90564-f. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., Thomas G., Maller J. L. Increased phosphorylation of ribosomal protein S6 during meiotic maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 May;79(9):2937–2941. doi: 10.1073/pnas.79.9.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris J., Richter J. D. Maturation-specific polyadenylation and translational control: diversity of cytoplasmic polyadenylation elements, influence of poly(A) tail size, and formation of stable polyadenylation complexes. Mol Cell Biol. 1990 Nov;10(11):5634–5645. doi: 10.1128/mcb.10.11.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucellier G. Initiation of meiotic maturation by specific proteases in oocytes of the polychaete annelid Sabellaria alveolata. Exp Cell Res. 1977 Apr;106(1):1–14. doi: 10.1016/0014-4827(77)90235-x. [DOI] [PubMed] [Google Scholar]
- Peaucellier G., Picard A., Robert J. J., Capony J. P., Labbe J. C., Doree M. Phosphorylation of ribosomal proteins during meiotic maturation and following activation in starfish oocytes: its relationship with changes of intracellular pH. Exp Cell Res. 1988 Jan;174(1):71–88. doi: 10.1016/0014-4827(88)90143-7. [DOI] [PubMed] [Google Scholar]
- Picard A., Doree M. The role of the germinal vesicle in producing maturation-promoting factor (MPF) as revealed by the removal and transplantation of nuclear material in starfish oocytes. Dev Biol. 1984 Aug;104(2):357–365. doi: 10.1016/0012-1606(84)90091-5. [DOI] [PubMed] [Google Scholar]
- Picard A., Harricane M. C., Labbe J. C., Doree M. Germinal vesicle components are not required for the cell-cycle oscillator of the early starfish embryo. Dev Biol. 1988 Jul;128(1):121–128. doi: 10.1016/0012-1606(88)90273-4. [DOI] [PubMed] [Google Scholar]
- Picard A., Labbe J. C., Doree M. The cell cycle can occur in starfish oocytes and embryos without the production of transferable MPF (maturation-promoting factor). Dev Biol. 1988 Jul;128(1):129–135. doi: 10.1016/0012-1606(88)90274-6. [DOI] [PubMed] [Google Scholar]
- Picard A., Labbé J. C., Barakat H., Cavadore J. C., Dorée M. Okadaic acid mimics a nuclear component required for cyclin B-cdc2 kinase microinjection to drive starfish oocytes into M phase. J Cell Biol. 1991 Oct;115(2):337–344. doi: 10.1083/jcb.115.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard A., Peaucellier G., le Bouffant F., Le Peuch C., Dorée M. Role of protein synthesis and proteases in production and inactivation of maturation-promoting activity during meiotic maturation of starfish oocytes. Dev Biol. 1985 Jun;109(2):311–320. doi: 10.1016/0012-1606(85)90458-0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Raff J. W., Whitfield W. G., Glover D. M. Two distinct mechanisms localise cyclin B transcripts in syncytial Drosophila embryos. Development. 1990 Dec;110(4):1249–1261. doi: 10.1242/dev.110.4.1249. [DOI] [PubMed] [Google Scholar]
- Reed S. I. G1-specific cyclins: in search of an S-phase-promoting factor. Trends Genet. 1991 Mar;7(3):95–99. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Brandhorst B. P., Ruderman J. V. Translationally mediated changes in patterns of protein synthesis during maturation of starfish oocytes. Dev Biol. 1982 May;91(1):215–220. doi: 10.1016/0012-1606(82)90026-4. [DOI] [PubMed] [Google Scholar]
- Satterwhite L. L., Lohka M. J., Wilson K. L., Scherson T. Y., Cisek L. J., Corden J. L., Pollard T. D. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc2: a mechanism for the timing of cytokinesis. J Cell Biol. 1992 Aug;118(3):595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Letourneau G. E., Wassarman P. M. Meiotic maturation of mouse oocytes in vitro: protein synthesis in nucleate and anucleate oocyte fragments. J Cell Sci. 1978 Apr;30:251–264. doi: 10.1242/jcs.30.1.251. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E. The interaction of steroids with Rana pipiens Oocytes in the induction of maturation. Dev Biol. 1971 Jun;25(2):232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- Standart N., Minshull J., Pines J., Hunt T. Cyclin synthesis, modification and destruction during meiotic maturation of the starfish oocyte. Dev Biol. 1987 Nov;124(1):248–258. doi: 10.1016/0012-1606(87)90476-3. [DOI] [PubMed] [Google Scholar]
- Strausfeld U., Labbé J. C., Fesquet D., Cavadore J. C., Picard A., Sadhu K., Russell P., Dorée M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991 May 16;351(6323):242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Swenson K. I., Ruderman J. V. The role of cyclin B in meiosis I. J Cell Biol. 1989 Apr;108(4):1431–1444. doi: 10.1083/jcb.108.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield W. G., Gonzalez C., Maldonado-Codina G., Glover D. M. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990 Aug;9(8):2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. In the beginning is the end: regulation of poly(A) addition and removal during early development. Trends Biochem Sci. 1990 Aug;15(8):320–324. doi: 10.1016/0968-0004(90)90022-4. [DOI] [PubMed] [Google Scholar]
- Zindy F., Lamas E., Chenivesse X., Sobczak J., Wang J., Fesquet D., Henglein B., Bréchot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]
- van Loon A. E., Colas P., Goedemans H. J., Néant I., Dalbon P., Guerrier P. The role of cyclins in the maturation of Patella vulgata oocytes. EMBO J. 1991 Nov;10(11):3343–3349. doi: 10.1002/j.1460-2075.1991.tb04898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]











