Abstract
Dehydroepiandrosterone (DHEA) is used as a dietary supplement and can be metabolized to androgens and/or estrogens in the prostate. We investigated the hypothesis that DHEA metabolism may be increased in a reactive prostate stroma environment in the presence of pro-inflammatory cytokines such as TGFβ1 and further, whether red clover extract, which contains a variety of compounds including isoflavones, can reverse this effect. LAPC-4 prostate cancer cells were grown in coculture with prostate stromal cells (6S), and treated with DHEA +/- TGFβ1 or IL-6. PSA expression and testosterone (T) secretion in LAPC4/6S cocultures were compared with those in monocultured epithelial and stromal cells using real time PCR and/or ELISA. Combined administration of TGFβ1+DHEA to cocultures increased PSA protein secretion 2-4 times, and PSA gene expression up to 50-fold. DHEA + TGFβ1 also increased coculture production of testosterone over DHEA treatment alone. Red clover isoflavone treatment led to a dose-dependent decrease in PSA protein and gene expression and T metabolism induced by TGFβ1+DHEA in prostate LAPC-4/6S cocultures. In this coculture model of endocrine-immune-paracrine interactions in the prostate, TGFβ1 greatly increased stromal-mediated DHEA effects on T production and epithelial cell PSA production, whereas red clover isoflavones reversed these effects.
Keywords: DHEA, TGFβ1, stromal, prostate, PSA, testosterone, coculture, red clover isoflavones
Introduction
DHEA and its sulfated conjugate, DHEA-S, are present in adult men and women at plasma concentrations 100 to 500 times higher than those of testosterone, and 1000 to 10,000 times higher than those of estradiol (1). These levels decrease with age prompting the use of DHEA as a self-prescribed dietary supplement for its alleged anabolic and anti-aging effects, with unsubstantiated claims of beneficial effects as well as uncertain long-term safety (2). The controversy whether DHEA may be cancer promoting or cancer preventing in humans continues to be debated (3). In in vivo studies in rodents, DHEA has been found to be an effective inhibitor to carcinogen-induced prostate cancers (4). In humans, DHEA can be metabolized to androgens and/or estrogens in the prostate, (5), and thereby may affect prostate pathophysiology. Compared to serum levels of steroid hormones, the levels of intratissular androgens and estrogens metabolized from DHEA (5) are increasingly recognized as important targets of investigation We hypothesize that DHEA metabolism may be altered from that in the normal prostate during the various stages of cancer progression.
Stromal cell activation is a critical step in the progression of cancers. Prostate stroma may become activated in response to the progression of the co-localized carcinoma (6) or by various stimuli from tissue injury including growth factors and other cytokines (7). Once activated, stromal cells often secrete larger amounts of growth factors and extracellular matrix components and remodeling enzymes, similar to a wound repair response, thereby creating a growth promoting microenvironment that can alter epithelial function (8). Pro-inflammatory cytokines can modulate various cell functions in cancerous tissue and contribute to inducing stromal activation. Transforming growth factor beta-1 (TGFβ1), a pro-inflammatory cytokine that participates in many cellular processes, such as growth, proliferation, differentiation and apoptosis (9), is present in reactive stroma (10) and exerts multiple effects on carcinogenesis. In prostate cancer patients, TGFβ1 overproduction is associated with increased tumor grade, high vascularity and the presence of metastases (9). Interleukin-6 (IL-6), another pro-inflammatory cytokine secreted by T cells and macrophages, stimulates immune response to trauma, especially tissue damage leading to inflammation. IL-6 increases androgen responsiveness in prostate cancer cells in vitro (11). Other cytokines, IL-4 and IL-13 can increase expression of steroid metabolizing enzymes, potentially altering metabolism of hormones, including DHEA (12). We propose that reactive prostate stroma modulates DHEA hormone metabolism.
Increased dietary isoflavone consumption is associated with a decreased risk of prostate cancer. (13) Red clover, Trifolium pretense, is one source of isoflavones. The flowering tops of the red clover plant contain biochanin A, formononetin, daidzein and genestein. Red clover is available as a dietary supplement and standardized extracts are widely marketed to men as a treatment for symptoms of prostate enlargement. Red Clover isoflavones inhibit growth of prostate cancer cells (14), induce apoptosis in low to moderate grade prostate cancer (15) and inhibit 5α-reductase (16) and 17β-hydroxysteroid dehydrogenase (HSD) (17), two enzymes involved in steroid metabolism.
The current study utilizes a coculture model of human prostatic stromal plus epithelial cells to simulate endocrine-immune-paracrine interactions in the prostate. Addition of the pro-inflammatory cytokines, TGFβ1 and IL-6, facilitates investigations into mechanisms linking the immune, paracrine and endocrine influences on cancer growth and progression, including metabolism of DHEA to testosterone and induction of the epithelial specific secretory product prostate specific antigen (PSA) expression in prostate stromal plus epithelial cocultures. We hypothesized that combined cytokine + DHEA administration would increase PSA production and T metabolism in the cocultures, and that the addition of red clover isoflavones would inhibit these cytokine + DHEA-mediated effects.
Materials and Methods
Cell culture
LAPC-4 cells were generously provided by Dr. Charles Sawyers, UCLA. Primary human prostate cancer-derived stromal cells were isolated from radical prostatectomy specimens (“6S”; kindly provided by Dr. John Isaacs, Johns Hopkins School of Medicine) and have been previously described (18). Primary prostate stroma cells (PRSC) derived from normal prostate tissues were obtained from Cambrex-Clonetics (East Rutherford, NJ). All cell types were grown in DMEM:F12 (1:1) medium, (Invitrogen, Carlsbad, CA) with penicillin (100 units/mL), streptomycin (100 μg/mL), L-glutamine (292 μg/mL) (Invitrogen), and 5% Fetal Bovine Serum (FBS, HyClone Laboratories, Inc., Logan, UT) at 37°C in 5% CO2 and propagated at 1:5 dilutions. Cells were kept as frozen stocks and used within 7 passages after thawing.
Stromal Cell TGFβ1 Growth Studies
6S stromal cells were seeded in triplicate onto 12 well plates at a density of 15,000 cells per well in ‘Treatment Media’ consisting of Medium 199 (phenol red-free):F12 phenol red-reduced media (Invitrogen) (1:1) supplemented with penicillin (100 units/mL), streptomycin (100 μg/mL) and 1% CDS (charcoal-dextran treated FBS (CDS – Hyclone Labs). Cultures were incubated overnight for 24 hours and treated with TGFβ1 in concentrations ranging from 0.04pM to 400pM. Cells were trypsinized and counted at day 0, and daily thereafter for 5 days. using a Coulter cell counter (Z1 Dual, Beckman Coulter, California). This study was repeated three times, and the 6S stromal cells used were from passages 7-9.
TGFβ1+ DHEA induced Effects on PSA and T Secretion in Cocultures
LAPC-4 cancer epithelial cells were seeded in Treatment Media in triplicate onto Millipore PICM 12mm inserts (Billerica, MA) coated with a 1:10 dilution of Matrigel™:H2O, at a density of 5×105 cells/insert. Stromal cells (6S or PrSC) cells were seeded in Treatment Media in triplicate at 1×105 / well in 24 well plates. TGFβ1 was added to stromal cultures on the same day at 40pM to elicit the reactive stromal phenotype as previously reported (19). LAPC-4/6S coculture methods have been previously described (20) Epithelial and stromal cells were cultured separately in media containing 2% CDS for 3 days. Epithelial and stromal cultures were then combined in cocultures (CC) while monocultures (MC) remained separated. Hormones were added in Treatment Media containing 1% CDS and treated with ethanol control (< 0.02%), 100 nM DHEA +/- 40 pM TGFβ1, 10 nM R1881 and allowed to coculture for 3 days. Media containing hormones was replaced and allowed to condition for 48 hours. Conditioned media were collected from monocultures and cocultures (with media from epithelial and stromal compartments mixed together) and frozen at −80°C or assayed for PSA and T by ELISA. Total PSA ELISA kits (DSLabs, Webster, TX) were used to determine PSA concentrations as previously reported (21). Total T was also measured by an ELISA kit (ALPCO, Salem, NH). Each original triplicate experimental sample was assayed in duplicate. PSA and T values were normalized to cell numbers as determined by the modified MTT assay (Promega, Madison WI) as previously reported (21).
TGFβ1+DHEA induced Effects on PSA Gene Expression
Co-cultures were prepared as above with LAPC-4 seeded in triplicate in 30mm Millipore inserts pre-coated with Matrigel film at a density of 2×106 cells/6 well and 6S cells plated at 5×105/60 mm dish, using Treatment Media as described above. After 3 days, cells were either combined in coculture or left in monoculture and treated with hormone treatments as above for 2 days, and then harvested to extract RNA. Methods for RNA extraction, reverse transcription, real time PCR, as well as primers for RPLPO and PSA were previously described (21).
Preparation of Red Clover Isoflavones
Isoflavones present in red clover extract, biochanin A, formononetin, daidzein and genistein (Sigma) were dissolved in DMSO and combined in the same proportions as the published formulation in the clinically used Promensil™ (Novogen) (22). A 22.6mM 1mL stock solution was prepared from the four isoflavones based on the formulation: 61% biochanin A (15.3mg/250uL DMSO = 54mM), 20% formononetin (5mg/250uL DMSO = 18.6 mM), 9% daidzein (2.2mg/250uL DMSO = 8.68 mM) and 10% genistein (2.5mg/250uL DMSO = 9.25 mM).
Effects of Red Clover on TGFβ1+ DHEA mediated PSA and T Secretion
The same experimental procedure was used as above of the effects of TGFβ1+ DHEA on PSA and T, but in addition to treatments with 100 nM DHEA +/- 40 pM TGFβ1, and 10 nM R1881, cells were also treated with red clover (RC) isoflavones at 10nM, 30nM, 100nM or 300nM, 100nM E2, and 1μM ICI-182,780 (estrogen receptor antagonist).
Western Blot analysis of stromal cell expression of AR and smooth muscle proteins
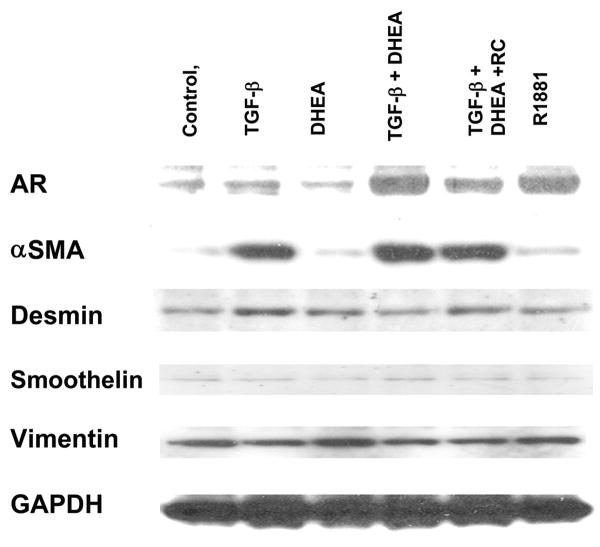
6S stromal cells were plated at a density of 5×105/well on 6-well plates, and 40 pM TGFβ1 was added on the same day. Cells were then grown in treatment media containing 2% CDS for 2 days, and treated with hormones (as above) and allowed to culture for 4 more days. Protein was extracted from cells and analyzed by Western Blot for androgen receptor (AR), α-smooth muscle actin, desmin, smoothelin, vimentin and GAPDH as reported previously (18).
Statistical analysis
All data are expressed as the mean values ± SEM derived from 3 replicates within each of three separate experiments. To delineate effects of hormones or inhibitors, one way analysis of variance (ANOVA) was performed using the Tukey-Kramer HSD adjustment for multiple comparisons. An adjusted P-value of 0.05 was considered significant. Probability designations are as follows: between hormone treatment *p = 0.05, **p=0.01, ***p=0.001; within hormone treatments (monoculture vs. coculture) + p=0.05, ++p = 0.01, +++ p< 0.001. In the red clover graphs, ◆ designates p = 0.05 of DHEA + TGFβ1 plus red clover-treated compared with DHEA + TGFβ1 alone.
Results
TGFβ1 effects on the growth of 6S cells
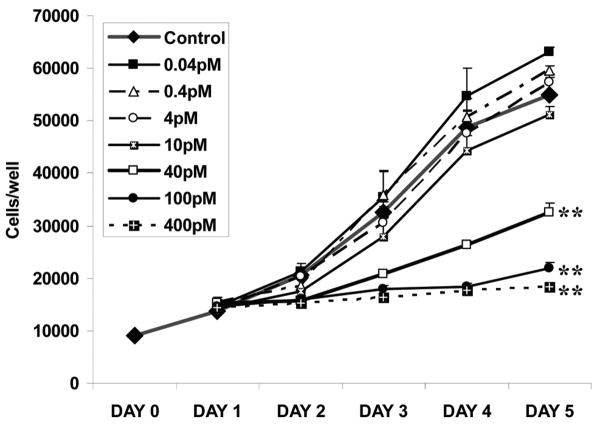
TGFβ1 effects on prostate stromal (6S) cell growth were tested using 7 concentrations for 5 days. The growth curve showed increased 6S proliferation with lower doses and growth arrest with higher doses (Figure 1). With increasing concentrations, the morphology of the stromal cells became more myofibroblastic with smooth-muscle-like prominent actin fibers present (data not shown). Statistical analysis verified that from Day 2 to Day 5, 40pM and higher doses significantly inhibited 6S cell growth (p=0.01), whereas growth stimulation by 10pM or lower doses was not significant (at both p=0.05 and p=0.01). The TGFβ1 concentration used in these experiments, 40pM, was 23.7% inhibitory by Day 2, the duration of the RNA experiment, and 40.7% inhibitory by Day 5, the usual duration of the ELISA experiment (P < 0.01).
Figure 1. Effects of TGFβ1 on Proliferation of 6S Prostate Stromal Cells.
6S stromal cells were plated in triplicate on 12 well plates at 15,000 cells per well. Cultures were incubated for 24 hours and treated with various concentrations of TGFβ1: Cells were trypsinized and counted at day 0 and daily for 5 days. Data represent the mean and SEM of three separate experiments, **p=0.01.
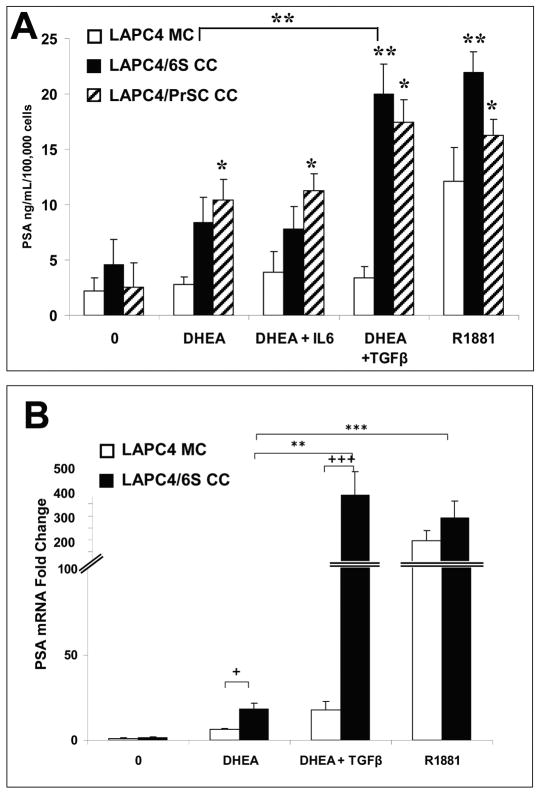
DHEA-induced PSA protein expression in LAPC4/6S or LAPC4/PrSC cocultures was increased by TGFβ1 but not by IL-6
In recent studies, we reported that 6S stromal cells in coculture with LAPC4 cells induce LAPC4 PSA expression in presence of DHEA, whereas LAPC-4 cells are unresponsive to DHEA in monocultures (20). This effect was replicated here, in that DHEA (100 nM) increased LAPC-4/6S coculture PSA protein secretion up to 8.4 ng/mL/100,000 cells, while in LAPC4 monocultures, PSA levels were similar to control at 3.9 ng.mL/100,000 cells (p=0.03; Figure 2A). The addition of TGFβ1 to DHEA-treated cocultured cells significantly enhanced PSA secretion (20.0 ng/mL/100,000 cells; p=0.01), to values similar to those induced by the positive control, the non-metabolized androgen R1881 (10nM) which induced PSA secretion in LAPC4 grown in monoculture (12.2 ng/mL/100,000 cells; p=0.05) and coculture conditions (22.0 ng/mL/100,000 cells; p=0.01). The combination of DHEA+IL6 did not alter PSA secretion in cocultures beyond that observed after DHEA alone. Parallel studies were performed with PrSC normal prostate stromal cells which produced similar pattern, where LAPC4-PSA production was increased by hormone treatments to 10.5, 11.3, 17.5 and 16.3 ng/mL/100,000 cells in DHEA, DHEA+ IL-6, DHEA + TGFβ and R1881 respectively (p> 0.05). As in the 6S co-cultures, the combination of DHEA+IL6 did not alter PSA secretion in PrSC cocultures beyond that observed after DHEA alone.
Figure 2. TGFβ1 Augmented DHEA-induced PSA Protein Production and gene expression in LAPC-4/6S or LAPC-4/PrSC cocultures.
A. LAPC-4 cells were seeded in treatment media in triplicate onto 30 mm inserts coated with a film of Matrigel at a density of 5×105 cells/well. Stromal cells (6S or PrSC) cells were seeded in triplicate at 1×105 / well in 24 well plates. TGFβ1 was added to stromal cultures on the same day at 40pM to elicit a reactive stromal phenotype. Cocultures were combined after 2 days while monocultures remained separated and hormones were added and allowed to coculture for 3 days. Media containing hormones was replaced and allowed to condition for 48 hours. Conditioned media was assayed for PSA by ELISA. Each original triplicate experimental sample was assayed in duplicate. PSA values were normalized to cell numbers as determined by the modified MTT assay. B. Co-cultures were prepared with LAPC-4 seeded in treatment media in triplicate in 30mm inserts pre-coated with Matrigel film at a density of 2×106 cells/6 well and 6S cells plated at 5× 105 /60 mm dish, using treatment media as described (see Methods). After 3 days, cells were either combined in coculture or left in monoculture and treated with hormone treatments for 2 days, and then harvested to extract RNA. PSA gene expression was assayed by real time PCR and normalized to RPLPO expression. Data represent the mean and SEM from three separate experiments. *p=0.05 **p=0.01 ***p=0.001, within monoculture or coculture; + p=0.05; +++ p< 0.001, between monoculture and coculture.
TGFβ1 effects on DHEA-induced PSA gene expression in LAPC4/6S cocultures
DHEA increased LAPC-4 PSA gene expression in LAPC4/6S cocultures (18.1 fold) as compared with the effect in LAPC-4 cells in monoculture (6.2 fold) (p<0.01; Figure 2B). Addition of TGFβ1 further enhanced DHEA-induced PSA gene expression in LAPC-4/6S cocultures (385 fold; p=0.01). In comparison, R1881 increased PSA expression in both monocultures (196 fold; p<0.001) and cocultures (292 fold; p<0.0015).
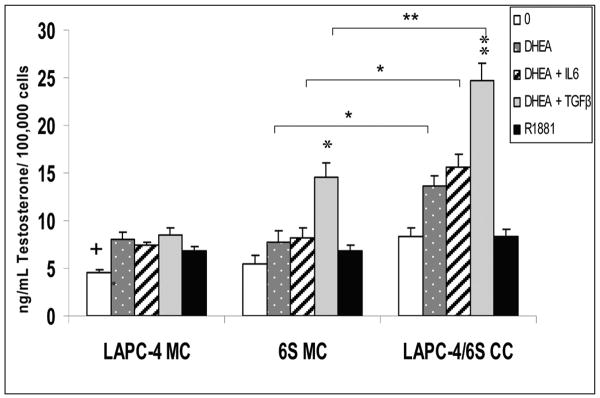
Testosterone secretion in DHEA-treated LAPC4/6S cocultures was increased by TGFβ1 but not by IL-6
In conditioned medium from hormone-treated cultures, a significant increase in T concentrations was detected in cocultures (LAPC4/6S CC) treated with 100 nM DHEA (13.6 pg/mL/100,000 cells; p=0.05) as compared with similarly treated LAPC4 or 6S cells in monoculture (7.8 for 6S MC and 8.0 for LAPC-4 MC) (Figure 3). With the addition of TGFβ1, DHEA treatment increased 6S MC stromal cell T production to 14.6 pg/mL/100,000 cells (p<0.01). In LAPC4/6S cocultures, TGFβ1+DHEA treatment produced even greater concentrations of T (24.7 pg/mL/100,000 cells; p<0.01) than after DHEA treatment alone. TGFβ1+DHEA induced higher T concentrations than did IL-6+DHEA in both 6S monocultures and cocultures (8.2 and 15.6 pg/mL/100,000 cells, respectively (p<0.01). T was increased in IL-6 + DHEA in 6S cocultures over 6S monocultures, (p=0.05) but the effect was not statistically different than in cocultures treated with DHEA alone. Control-treated monocultures of LAPC-4 cells secreted lower amounts of T than under all other treatment conditions (p=0.05). However, all values for LAPC-4 MC were less than or not different from the T concentrations found in R1881-treated cells and is likely attributable to background in the assay.
Figure 3. Effects of DHEA, TGFβ1, and IL-6 on Testosterone secretion in cocultured LAPC-4/6S cells.
LAPC-4 cells were seeded in treatment media in triplicate onto 30 mm inserts coated with a film of Matrigel at a density of 5×105 cells/insert. Stromal cells (6S) cells were seeded in triplicate at 1×105 / well in 24 well plates. TGFβ1 was added to stromal cultures on the same day at 40pM to stimulate a reactive stromal phenotype. Cocultures were combined after 2 days while monocultures remained separated and hormones were added and allowed to coculture for 3 days. Media containing hormones was replaced and allowed to condition for 48 hours. Conditioned media was assayed for Testosterone by ELISA. Each original triplicate experimental sample was assayed in duplicate. T values were normalized to cell numbers as determined by the modified MTT assay. Data represent the mean and SEM from three separate experiments. *p=0.05, **p=0.01, within monoculture or coculture; + p=0.05; +++ p< 0.001, between monoculture and coculture.
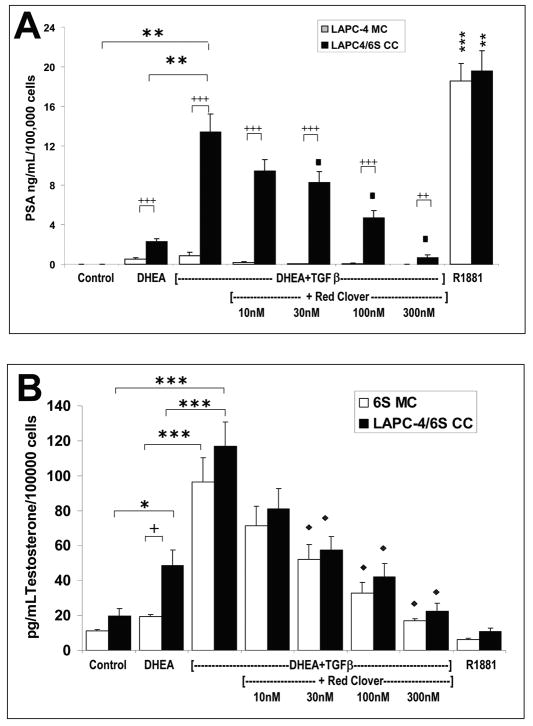
Red Clover Isoflavones decreased TGFβ1 + DHEA-induced PSA protein levels and gene expression in LAPC4/6S cocultures
A range of concentrations for the red clover (RC) isoflavones mixture was pretested for potential toxicity and found to be nontoxic up to 1μM for LAPC-4 cells and 10μM for 6S cells (data not shown). The dose of 100nM RC isoflavones was chosen because it was non-toxic and is similar to circulating concentrations of these isoflavones measured in patients treated with the red clover isoflavones (23). As before, DHEA treatment of LAPC-4 cells increased PSA protein secretion more in the presence of stromal cells (6S CC) versus monocultures (2.11 vs.1.01 ng/mL/100,000 cells; p=0.01; Figure 4A). TGFβ1 augmented DHEA-induced PSA protein secretion in cocultures (13.06 ng/mL/100,000 cells) (p<0.01). The addition of 100nM red clover isoflavones to the TGFβ1+DHEA combination inhibited TGFβ1+DHEA induction of PSA in cocultures, with resulting concentrations similar to those after DHEA treatment alone (4.11 ng/mL/100,000 cells). E2 (100nM) also inhibited TGFβ1+DHEA-induced PSA expression in LAPC4 cocultures (2.10 ng/mL/100,000 cells), whereas blocking estrogen receptors (ERα and ERβ) with the antagonist ICI 182,780 (100nM) did not reverse the red clover inhibition (3.47 ng/mL/100,000 cells). R1881 induced high levels of PSA in both monoculture and coculture (13.13 and 13.23 ng/mL/100,000 cells, respectively; p<0.01). Addition of TGFβ1 alone induced no discernable amounts of PSA in LAPC4 cells in monocultures or cocultures. RC alone induced no PSA and TGFβ1+/- RC did not alter R1881-induced PSA expression (data not shown).
Figure 4. Red Clover effects on TGFβ1 + DHEA-stimulated LAPC-4/6S co-cultures, A. LAPC-4 PSA production.
The same experimental procedure was used as in the PSA ELISA experiment depicted in Fig.2A, but in addition to hormone treatments of 100 nM DHEA +/- 40 pM TGFβ1, cells were also treated with DHEA/TGFβ1 + 100nM red clover isoflavones (RC); DHEA/TGFβ1 + RC plus 100nM ICI-182,780 (estrogen receptor antagonist), DHEA/TGFβ1 + 100nM E2, or TGF β-1 alone. or 10 nM R1881. Data represents average and SEM from three separate experiments. B. LAPC-4 PSA gene expression. LAPC-4 cells were plated in triplicate in monoculture and in coculture with 6S stromal cells, as described in figure 2B. Stromal cells were pretreated with 40 pM TGFβ1 for three days, then cultures were combined and treated with 100 nM DHEA +/- 40 pM TGFβ1, or DHEA/TGFβ1 + 100 nM red clover isoflavones (RC), DHEA/TGFβ1 + RC + 1μM ICI 182,780 (estrogen receptor antagonist) DHEA/TGFβ1 + 100 nM E2 or 10 nM R1881 for 48 hours. RNA was extracted and cDNA was reverse transcribed and probed by Real Time PCR for PSA expression, standardized to RPLPO expression. Data are expressed as the mean and SE from three experiments. C. Stromal Testosterone secretion in cocultured LAPC-4/6S cells. Testosterone concentrations were determined in conditioned media from stromal cell monocultures, compared to cocultures from the same experiments illustrated in Figure 3. Hormone treatments include 100 nM DHEA +/- 40 pM TGFβ1, DHEA/TGFβ1 + 100nM RC isoflavones; DHEA/TGFβ1 + RC plus 100nM ICI-182,780, DHEA/TGFβ1 + plus100nM E2, TGFβ1 alone, or 10 nM R1881. Data are expressed as the mean and SE from three experiments. *p = 0.05; ** p = 0.01; + p=0.05; ++ p=0.01; +++ p=0.001; ◆ p = 0.05 compared with DHEA + TGFβ1 alone
PSA gene expression in LAPC-4 cocultures was again found significantly increased 146 fold by TGFβ1+DHEA as compared to 40 fold increase by DHEA alone, (p=0.05); Figure 4B). Red Clover inhibited TGFβ1+DHEA induction of PSA mRNA in both monocultured (LAPC4 MC) and cocultured LAPC-4 cells (6S CC), by 24- fold and 41- fold, respectively. Addition of 100nM estradiol (E2) also inhibited PSA gene expression in both monoculture (90% inhibition to 7.93 fold) and coculture (83% inhibitory to 25.76 fold), whereas blocking ERβ or ER α with ICI 182,780 did not inhibit the red clover effect in either mono- or coculture (15.72 fold and 45.11 fold respectively). R1881 induced high levels of PSA gene expression in both mono- (p<0.01) and co-culture (p=0.01) (192- fold and 233-fold respectively).
Changes in testosterone secretion in the presence of Red Clover
Testosterone concentrations were measured in conditioned media of stromal 6S monocultures (MC) and LAPC-4/6S cocultures (CC). DHEA-treated cocultures exhibited greater increases in T (24.04 pg/mL/100,000 cells) than did DHEA-treated 6S monocultures (11.23pg/mL/100,000 cells; Figure 4C; (p<0.01). TGFβ1 augmented DHEA-metabolism to T in cocultures (58.24 pg/mL/100,000 cells; p<0.01). DHEA treated 6S cells in monoculture also exhibited increased T secretion (33 ng/mL p<0.05). Red Clover inhibited the TGFβ1+DHEA-induced T secretion in cocultures (14.51 pg/mL/100,000 cells, p= 0.05) to values similar to those after DHEA treatment alone. Blocking ERβ/or ER alpha with ICI 182,780 did not block the inhibitory effect on T secretion of Red Clover (22.46 pg/mL/100,000 cells). E2 did not significantly alter T secretion in TGFβ1+DHEA- treated cultures (56.22 pg/mL/100,000 cells). Addition of TGFβ1 alone or R1881 alone, or red clover isoflavone treatment alone elicited little or no change in T secretion (data not shown)., Cultures treated with R1881 plus TGFβ1 or R1881+TGFβ-1+red clover, showed no significant changes in T secretion (data not shown) as is expected as R1881 is not metabolizable to testosterone. Additionally, parallel ELISA assays for estradiol (E2) from the same samples showed no secretion of E2 by the cultures and E2 was present only in those samples treated with additional E2 (data not shown).
Red Clover Isoflavone inhibition of PSA and testosterone metabolism was dose responsive
Increasing doses of Red Clover isoflavones (from 0-300nM) resulted in progressive inhibition of the TGFβ-1-augmented increase in DHEA-induced PSA secretion (Figure 5A; 13.40 ng/mL/100,000 cells p<0.01) in LAPC4/6S cocultures, with significant reductions seen at concentrations of 30nM, 100nM and 300nM RC (at 38, 65 and 95% inhibition to 8.3, 4.7 and 0.71 ng/mL/100,000 cells, respectively (p=0.05). LAPC-4 cells cocultured with 6S cells exhibited significantly greater PSA production than did those in monoculture under all DHEA-treated conditions (p<0.01). Cultures treated with R1881 plus TGFβ-1 or R1881+TGFβ-1 + red clover, showed no change in PSA secretion (data not shown).
Figure 5. Dose-responsive effects of red clover isoflavones to inhibit DHEA+TGFβ-1-induced PSA expression in LAPC-4 cell monocultures and cocultures and T metabolism in 6S stromal cell monocultures and cocultures.
A. The same experimental procedure for PSA ELISA was used as in Figures 2 and 4, but in addition to hormone treatments of 100 nM DHEA +/- 40 pM TGFβ1, 10 nM R1881, cells were also treated with DHEA + TGFβ1 + RC isoflavones at 10nM, 30nM, 100nM or 300nM. B. Testosterone concentrations were determined in conditioned media from stromal cell monocultures, compared to cocultures from the same experiments represented in Figure 5A. Hormone treatments included 100 nM DHEA +/- 40 pM TGFβ1, DHEA/TGFβ1 + RC isoflavones at 10nM, 30nM, 100nM or 300nM; and10 nM R1881. Data are expressed as the mean and SE from three experiments. * p = 0.05; ** p = 0.01;*** p = 0.001; + p=0.05; ++ p=0.01; +++ p=0.001 ◆ p = 0.05 compared with DHEA + TGFβ1 alone.
Likewise increasing doses of Red Clover isoflavones (from 0-300nM) resulted in greater inhibition of the DHEA+TGFβ-1-augmented increase (p<0.01) in T secretion (116.78 pg/mL/100,000 cells) in 6S cells in cocultures, with significant (p=0.01) reductions observed at concentrations of 30nM, 100 nM and 300 nM with 51, 64 and 81 percent inhibition to 58, 42, and 23 pg/mL, respectively (Figure 5B, p = 0.01) Red Clover also inhibited T secretion in 6S monocultures where significant reductions of TGFβ-1+DHEA-induced T secretion (96.33 pg/mL/100,000 cells) occurred at concentrations of 30nM, 100 nM and 300 nM, with 46, 65, and 82 percent inhibition, to 52, 33 and 17 pg/mL, respectively (Figure 5B, p=0.05).
TGFβ-1 + DHEA effects on stromal cell expression of androgen receptor (AR) and smooth muscle cell markers in 6S cells
Western blot analysis revealed that the combination of TGFβ1+DHEA increased AR expression in 6S cells similar to that in cells treated with R1881 (Figure 6). Red clover decreased the AR expression induced by DHEA+TGFβ1. All treatments including TGFβ1 increased α-smooth muscle actin. Desmin, smoothelin and vimentin expression were unaffected by any of the treatments.
Figure 6. Expression of Androgen receptor (AR) and stromal cytoskeletal protein in 6S cells treated with TGFβ1 + DHEA and Red Clover.
6S prostate stromal cells were plated at a density of 5×105/well on 6-well plates. 40 pM TGFβ1 was added on same day as when cells were plated. Cells were treated with 100 nM DHEA +/- 40 pM TGFβ1, DHEA/TGFβ1 + RC isoflavones at 100nM and 10 nM R1881. and allowed to culture for 4 days. Protein was extracted from cells and analyzed by Western Blot for androgen receptor (AR), α-smooth muscle actin (αSMA), desmin, smoothelin, vimentin and GAPDH. Data shown are representative blot of three separate experiments.
Discussion
In this coculture model of endocrine (DHEA) - immune (TGFβ1) - paracrine (stromal-epithelial) interactions in the prostate, the addition of TGFβ1 to DHEA–treated prostate stromal plus epithelial cells reproduced a reactive stromal microenvironment and significantly increased the androgenicity of both cell types, as measured by increased PSA (epithelial) and T (stromal) production. These results expand our previous study showing that DHEA-treated LAPC4 cells do not secrete PSA in the absence of prostate stromal cells. Our developing hypothesis is that the presence of DHEA in the prostate may be benign in normal prostate tissues, as represented by epithelial cells cocultured with stromal cells and treated with DHEA only, but may promote more androgenic effects in a reactive stromal microenvironment as represented by addition of TGFβ to DHEA-treated cocultures. We investigated whether red clover isoflavones might reverse these TGFβ-1-mediated effects, and found that they may be beneficial in inhibition of androgenic effects in the prostate tissue microenvironment.
The results of this study suggest the involvement of at least three factors in TGFβ1+DHEA-treated stromal cells' effects on epithelial cells: 1- induction of a reactive stromal phenotype by TGFβ1, 2- increase in steroid (DHEA) metabolism, and 3-production of secondary paracrine mediators, to promote the changes in expression of PSA and T in prostate cocultures.
Alterations in stromal phenotype have been reported in many types of human cancer (24). Modified or activated prostate stromal cells have myofibroblastic characteristics, including increased levels of smooth muscle α actin (6). TGFβ1 and other pro-inflammatory cytokines mediate the reactive stromal response and promote a wound-repair-type reactive myofibroblast phenotype in prostate cancer (8, 25) (10) (26). It has been suggested that about 20% of human cancers are associated with chronic infection or inflammation (27). Such lesions have been characterized in the prostate as proliferative inflammatory atrophy (PIA) and illustrate the association between inflammation and unusually high proliferation (28). In addition to contributions from immune cells, TGFβ1 is also over-expressed in prostatic intraepithelial neoplasia (PIN) and prostate cancer cells, and may induce adjacent stroma to become reactive (8). By adding pro-inflammatory cytokines, like TGFβ1 or IL-6 to a coculture model of stromal plus epithelial cells from the prostate microenvironment, we aimed to mimic the increased levels of cytokines and characteristics of reactive stroma, such as are present in PIA, PIN and prostate cancer.
In the LAPC-4/6S cocultures, TGFβ+DHEA induced significantly more PSA protein and gene expression than did DHEA alone, whereas IL-6+DHEA did not produce similar additive effects on PSA. Both LAPC-4 and 6S cells contain TGFβ Receptors I, II and III (data not shown). Parallel LAPC4/PrSC cocultures produced similar results. TGFβ1+DHEA also resulted in higher metabolism to testosterone in both mono- and cocultured stromal cells, whereas IL-6+DHEA treatment failed to induce a similar, significant increase in T metabolism, in either cocultures or stromal monocultures. IL-6 also did not produce the reactive stroma cytoskeletal morphology observed after TGFβ1 treatment (data not shown). IL-6 induces HSD enzymes (12), and would be expected to increase DHEA metabolism in this model. Clinically both IL-6 and TGFβ1 are elevated in patients with prostate metastases and both have been found to be correlated with increased serum PSA concentrations (29). TGFβ1-treated stroma may produce distinct paracrine factors that contribute to the effect on epithelial cells. Because we found no significant additional responsivity with the IL-6 treatment, we focused our experiments on the effects of DHEA+TGFβ1 treatments.
TGFβ1 stimulated growth of prostate stromal cells at lower doses (0.001 to 0.01 ng/mL) and inhibited growth of prostate stromal cells and promotes differentiation into smooth muscle actin structures at higher doses (0.1 to 1.0ng/mL)(30). We performed growth experiments with varying doses of TGFβ1 to confirm that the 6S primary prostate stromal cells also respond as above, and to evaluate the stimulatory or inhibitory effect of 40pM of TGFβ1on 6S cell growth at the time points used for PSA gene and protein expression (Day 2 and 5, respectively). In the growth experiments, a concentration of 40pM TGFβ1 used in subsequent experiments, led to results between those of the control and the highest, growth static dose, 400pM. The 40pM dosage significantly inhibited 6S stromal cell growth with a trend similar to that reported (30). The increased stromal T secretion or stromal-induced PSA secretion and gene expression in epithelial cells by addition of TGFβ1 + DHEA (40pM) was associated with a decreased, and not an increased, number of 6S stromal cells. Also, addition of DHEA to 40pM TGFβ1did not affect the growth compared with use of 40pM TGFβ1 alone (data not shown), confirming our prior results of no change in stromal cell growth in the presence of DHEA or its metabolites (18).
Conditioned medium from TGFβ1 +DHEA-treated LAPC-4/6S cocultures contained increased PSA concentrations as well as enhanced metabolism of DHEA to T. DHEA can be metabolized to T and DHT via several enzymatic steps, including actions of 3β HSD, 17β HSD and 5α reductase. TGFβ1 decreases 3β HSD in adrenocortical cells (31) and 17β HSD in breast cancer cells (32). To our knowledge, there are no reports of TGFβ1 effects on prostatic metabolism of DHEA to testosterone. TGFβ1 can decrease activity of CYP7B, which metabolizes DHEA to 7-alpha OH-DHEA, a ligand for ERβ (33) as measured in inflammatory tissues. TGFβ1 modulation of the DHEA metabolic pathway may alter the balance of androgenic and estrogenic ligands affecting growth and function of the prostate.
PSA measurement in this coculture model is used a relative biomarker of androgenic activity. This is not to be confused with the diagnostic use of PSA in clinical settings. The expression is dependent on cell culture conditions that can be variable. TGFβ1+DHEA appears to have effects on the LAPC4 cell PSA gene expression even in monocultures as there was an increase in gene, but not protein, expression of PSA (Figures 2B and 4B). TGFβ1 may promote PSA gene expression via upregulating LAPC4 steroid metabolizing enzymes, increasing presence of androgenic metabolites of DHEA or Smad3 interactions with the AR-induced PSA expression (34). But, as Figure 3 indicates, there was no increase of T secretion in TGFβ1+DHEA-treated LAPC4 monocultures. Understanding the basis for the discrepancies between TGFβ1 induced PSA gene and protein expression in LAPC-4 monocultures will require further study.
As was the case with PSA production, TGFβ1+DHEA administration resulted in a greater increase in T metabolism over 6S cells in monoculture and coculture treated with DHEA alone. Additionally, increased T concentrations were found in cocultures versus stromal monocultures. Several possibilities may be that LAPC-4 cells provide paracrine reciprocal contributions to the stromal metabolism and/or stromal cells or TGFβ1 induce LAPC4 metabolic enzymes.
This coculture model has potential value for identifying natural or synthetic agents that may modulate endocrine-immune-paracrine interactions in the prostate. To this end, we treated cells with red clover isoflavones which were combined in the same proportions as in commercially available preparations. Red clover isoflavone administration significantly inhibited TGFβ-1+DHEA induction of expression of PSA and T in a dose-dependant manner at final concentrations similar to those achieved clinically (30-300nM) (35) (23).
Historically, red clover has been used in patients with cancer and various respiratory problems, and is currently given to manage menopausal symptoms as well as symptoms of prostate enlargement (http://nccam.nih.gov/health/redclover/). The safety and bioavailability of red clover extract have been evaluated to some extent. For example, 40mg red clover isoflavones taken twice daily for two weeks was well tolerated, and produced plasma concentrations of isoflavones similar to those seen in populations consuming high dietary amounts of isoflavones (35); moreover it was readily absorbed by the prostate (23). In vivo studies in mice fed a diet supplemented with 5% red clover isoflavones for 14 months reported significantly reduced expression of TGFβ1 in prostatic epithelium (36) suggesting that red clover isoflavones may modulate cytokine expression.
Red clover effects in the LAPC-4/6S cocultures could be mediated by the phytoestrogens contained in the mixture, including genistein and daidzein. Genistein comprises 10% of the red clover formulation (22) and is a potent anti-androgen, able to block >70% of DHT-induced PSA production in vitro (37). To compare red clover effects with those of a pure estrogen, E2 was added to TGFβ1+ DHEA treated LAPC-4/6S cocultures. E2 decreased PSA protein and gene expression, but exerted no significant effect on T metabolism. The addition of ICI-182,780, an estrogen receptor antagonist, did not reverse red clover's inhibitory effect on TGFβ1 + DHEA-stimulated PSA secretion or gene expression and T metabolism. These results suggest that the red clover effects we observed are not mediated by epithelial ER β or stromal ERα.
The red clover effects on inhibiting PSA in prostate cell cocultures reported herein are seemingly inconsistent with the results of a clinical study that found no change in serum PSA concentrations in men who consumed 160 mg of red clover daily prior to radical prostatectomy (15). The use of PSA as a marker for androgenicity in in vitro studies should be distinguished from the use of circulating PSA concentrations as a diagnostic tool for prostate cancer detection or management, and also reflects the difference between cellular expression of PSA vs leakage into the circulatory system.
Two possible mechanisms may explain red clover's observed effects. Red clover inhibited the TGFβ-1+DHEA-induced increase of AR expression in 6S cells, supporting the idea that red clover might be modulating androgen activity in prostate cells. Also, red clover may affect one or more of the enzymes involved in DHEA metabolism, as it decreased production of the DHEA metabolite, T. Biochanin A, the predominant isoflavone in red clover has been shown to modulate 17β HSD type 5 in transfected bacteria (17), and genistein and biochanin A are potent inhibitors of 5α reductase and 17β HSD activity in genital skin fibroblasts (16). While it is premature to speculate that these enzymes are also modulated in prostate stromal and epithelial cells, the fact that they can be altered by isoflavones makes them attractive candidates for future study. Additional animal and clinical studies are absolutely necessary to determine usefulness of red clover extracts as safe or effective for human use.
We consistently observed morphological alterations in 6S cells following treatment with TGFβ1. TGFβ1-treated cells appeared to be more confluent, but were actually larger in size, and displayed an increase in cytoskeletal fibers (data not shown) which was confirmed by western blotting, which revealed increased expression of smooth muscle α actin, a hallmark of the activated phenotype (8) under all TGFβ1 treated conditions. Treatment with TGFβ1+DHEA resulted in increased expression of androgen receptor (AR) protein. A complex cross talk exists between androgen and TGFβ1 signaling where interactions can regulate activation of rat prostate stromal cells (38). This is consistent with our finding that AR expression was upregulated in 6S cells only with combined administration of TGFβ1+DHEA and not with TGFβ1 alone. Smooth muscle actin (αSMA) protein expression was also increased in all stromal cells treated with TGFβ1, independent of DHEA, as found in rat prostate stromal cells treated with TGFβ1+/-DHT (38).
The stromal 6S cells used in this study have previously been characterized to have more reactive phenotype (18) with an increased ability to secrete IGF-I in response to DHT, compared to other stromal cells lots from cancer or normal prostate. When compared to normal primary stromal cells, DHEA-treated stromal cell lots derived from cancer tissues showed increased ability to induce PSA expression in cocultures (20). The 6S cells in this study display increased “reactivity” with the addition of TGFβ-1. This increase in reactivity was also found in parallel coculture studies performed with PrSC normal prostate stromal cells which produced a similar increase in TGFβ-1 + DHEA-induced LAPC4/PrSC -PSA and T production over amounts induced by DHEA alone The comparison between 6S or PrSC without and with TGFβ-1 provides a better experimental representation of normal vs. reactive stroma, respectively, than the comparison of untreated primary normal vs. cancer assosciated stromal cells.
The reported increased PSA production in TGFβ1+DHEA-treated cocultures plus the significantly greater metabolism to T observed in stromal cells treated with TGFβ1+DHEA versus DHEA alone, suggest that reactive stroma as modeled by addition of TGFβ-1 responds differently to DHEA than does normal stroma, further supporting the hypothesis that DHEA's effects in the prostate depend on the prostate microenvironment. Further characterization of TGFβ1-treated stromal cells is needed to identify other factors involved in promoting DHEA metabolism, identify secreted paracrine factors that augment PSA production., and determine whether altered metabolism of DHEA may occur in various prostate cancer lesions in vivo and if so, its role in prostate pathology.
As is the case with any in vitro experiment, a coculture model is highly artificial and a highly variable technique. Variability was controlled through intra- and inter- experimental replicates, as is evidenced by the small SEM on the graphs as well as the consistency of effect pattern through out different experiments. The coculture model is useful in that it enables us to reproduce some of the variations in prostate cancer microenvironment in a controlled manner. However, there are a multitude of other in vivo and in vitro factors that undoubtedly play a role in carcinogenesis at the level of an organism, and thus, our ability to extrapolate potential clinical significance from the present data is limited. Nonetheless, stromal paracrine factors and steroid metabolizing enzymes that are identified to play a role in the effects described in this report will be further characterized in additional in vitro and in situ studies, and may offer insights to better inform the design of clinical and translational investigations.
In summary, we report that the addition of TGFβ1+DHEA to the stromal-epithelial cocultures increases PSA protein secretion and gene expression over DHEA treatment alone, while also enhancing metabolism of DHEA to T. Addition of TGFβ1 serves as a simulation of the reactive prostate stroma associated with the cancer tissue microenvironment. These results suggest that in cancer tissues compared to normal prostate, there may be a promotion of metabolism of DHEA to androgenic ligands, and production of stromal paracrine factors resulting in increased PSA and T production. Administration of red clover isoflavones decreased TGFβ1+DHEA-mediated PSA protein and gene expression and T metabolism in LAPC-4/6S cocultures in a dose dependent manner. This coculture model of endocrine-immune-paracrine interactions in the prostate provides a possible tool for identification of natural products or traditional medicines with multiple mechanisms that may prevent cancer progression by participating in stromal-epithelial cell interactions, such as by altering paracrine hormonal signals.
Acknowledgments
We wish to thank Dr. David Rowley (Baylor College of Medicine) for valuable discussions and Dr. Maria Merino (NIH-NCI) and Dr. Jeffrey Green (NIH- NCI) for their constructive comments upon reviewing this manuscript. This work was supported by the Intramural Research Program, National Center for Complementary and Alternative Medicine, National Institutes of Health, Bethesda, MD.
Abbreviations
- 6S
primary prostate stromal cell lot
- ANOVA
analysis of variance
- AR
androgen receptor
- CC
coculture
- CDS
charcoal dextran-treated serum
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulphate
- DHT
dihydrotestosterone
- DMEM
Dulbecco's Modified Eagles Media
- DMSO
dimethylsulfoxide
- E2
17β estradiol
- ELISA
enzyme-linked immunosorbent assay
- ER
estrogen receptor
- FBS
fetal bovine serum
- HSD
hydroxysteroid dehydrogenase
- IGF-1
Insulin-like growth factor-1
- IL-6
Interleukin-6
- LAPC-4
Prostate cancer epithelial cell
- MC
Monoculture
- PrSC
primary normal prostate stromal cell
- PSA
prostate specific antigen
- RC
red clover
- RPLPO
(Ribosomal phosphoprotein PO)
- RT-PCR
reverse transcriptase polymerase chain reaction
- SEM
standard error
- T
testosterone
- TGFβ1
Transforming Growth Factor Beta 1
References
- 1.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–1090. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 2.Alesci S, Manoli I, Blackman MR. Dehydrodepiandrosterone (DHEA) In: Coates P, Blackman MR, Cragg G, Levine M, Moss J, White J, editors. Encyclopedia of Dietary Supplements. 1st. New York, NY: Marcel Dekker, Inc.; 2005. pp. 167–176. [Google Scholar]
- 3.Arnold JT, Blackman MR. Does DHEA Exert Direct Effects on Androgen and Estrogen Receptors, and Does It Promote or Prevent Prostate Cancer? Endocrinology. 2005;146:4565–4567. doi: 10.1210/en.2005-0901. [DOI] [PubMed] [Google Scholar]
- 4.Rao KV, Johnson WD, Bosland MC, Lubet RA, Steele VE, Kelloff GJ, McCormick DL. Chemoprevention of rat prostate carcinogenesis by early and delayed administration of dehydroepiandrosterone. Cancer Res. 1999;59:3084–3089. [PubMed] [Google Scholar]
- 5.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- 6.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- 7.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 8.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 9.Wikstrom P, Damber J, Bergh A. Role of transforming growth factor-beta1 in prostate cancer. Microsc Res Tech. 2001;52:411–419. doi: 10.1002/1097-0029(20010215)52:4<411::AID-JEMT1026>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17:411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 11.Lin DL, Whitney MC, Yao Z, Keller ET. Interleukin-6 induces androgen responsiveness in prostate cancer cells through up-regulation of androgen receptor expression. Clin Cancer Res. 2001;7:1773–1781. [PubMed] [Google Scholar]
- 12.Simard J, Gingras S. Crucial role of cytokines in sex steroid formation in normal and tumoral tissues. Mol Cell Endocrinol. 2001;171:25–40. doi: 10.1016/s0303-7207(00)00387-7. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Legume and isoflavone intake and prostate cancer risk: The Multiethnic Cohort Study. Int J Cancer. 2008 doi: 10.1002/ijc.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson G, Barnes S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate. 1993;22:335–345. doi: 10.1002/pros.2990220408. [DOI] [PubMed] [Google Scholar]
- 15.Jarred RA, Keikha M, Dowling C, McPherson SJ, Clare AM, Husband AJ, Pedersen JS, Frydenberg M, Risbridger GP. Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol Biomarkers Prev. 2002;11:1689–1696. [PubMed] [Google Scholar]
- 16.Evans BA, Griffiths K, Morton MS. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J Endocrinol. 1995;147:295–302. doi: 10.1677/joe.0.1470295. [DOI] [PubMed] [Google Scholar]
- 17.Krazeisen A, Breitling R, Moller G, Adamski J. Phytoestrogens inhibit human 17beta-hydroxysteroid dehydrogenase type 5. Mol Cell Endocrinol. 2001;171:151–162. doi: 10.1016/s0303-7207(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 18.Le H, Arnold JT, McFann KK, Blackman MR. Dihydrotestosterone and Testosterone, but not DHEA or Estradiol, Differentially Modulate IGF-I, IGFBP - 2 and IGFBP-3 Gene and Protein Expression in Primary Cultures of Human Prostatic Stromal Cells. Am J Physiol Endocrinol Metab. 2005 doi: 10.1152/ajpendo.00451.2005. [DOI] [PubMed] [Google Scholar]
- 19.Singh H, Dang TD, Ayala GE, Rowley DR. Transforming growth factor-beta1 induced myofibroblasts regulate LNCaP cell death. J Urol. 2004;172:2421–2425. doi: 10.1097/01.ju.0000138082.68045.48. [DOI] [PubMed] [Google Scholar]
- 20.Arnold JT, Gray NE, Jacobowitz K, Viswanathan L, PW C, McFann KK, Le H, Blackman MR. Human Prostate Stromal Cells Stimulate Increased PSA Production in DHEA-treated Prostate Cancer Epithelial Cells. J Ster Biochem and Mol Biol. 2008 doi: 10.1016/j.jsbmb.2008.06.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold JT, Le H, McFann KK, Blackman MR. Comparative effects of DHEA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells. Am J Physiol Endocrinol Metab. 2005;288:E573–584. doi: 10.1152/ajpendo.00454.2004. [DOI] [PubMed] [Google Scholar]
- 22.Nestel PJ, Pomeroy S, Kay S, Komesaroff P, Behrsing J, Cameron JD, West L. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J Clin Endocrinol Metab. 1999;84:895–898. doi: 10.1210/jcem.84.3.5561. [DOI] [PubMed] [Google Scholar]
- 23.Rannikko A, Petas A, Rannikko S, Adlercreutz H. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. Prostate. 2006;66:82–87. doi: 10.1002/pros.20315. [DOI] [PubMed] [Google Scholar]
- 24.Schedin P, Elias A. Multistep tumorigenesis and the mircroenvironment. Breast Cancer Research. 2004;6:93–101. doi: 10.1186/bcr772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peehl DM, Sellers RG. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp Cell Res. 1997;232:208–215. doi: 10.1006/excr.1997.3525. [DOI] [PubMed] [Google Scholar]
- 26.Deutsch E, Maggiorella L, Eschwege P, Bourhis J, Soria JC, Abdulkarim B. Environmental, genetic, and molecular features of prostate cancer. The Lancet Oncology. 2004;5:303–313. doi: 10.1016/S1470-2045(04)01468-8. [DOI] [PubMed] [Google Scholar]
- 27.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 30.Huang X, Lee C. Regulation of stromal proliferation, growth arrest, differentiation and apoptosis in benign prostatic hyperplasia by TGF-beta. Front Biosci. 2003;8:s740–749. doi: 10.2741/1093. [DOI] [PubMed] [Google Scholar]
- 31.Rainey WE, Naville D, Mason JI. Regulation of 3 beta-hydroxysteroid dehydrogenase in adrenocortical cells: effects of angiotensin-II and transforming growth factor beta. Endocr Res. 1991;17:281–296. doi: 10.1080/07435809109027202. [DOI] [PubMed] [Google Scholar]
- 32.Ee YS, Lai LC, Reimann K, Lim PK. Effect of transforming growth factor-beta1 on oestrogen metabolism in MCF-7 and MDA-MB-231 breast cancer cell lines. Oncol Rep. 1999;6:843–846. doi: 10.3892/or.6.4.843. [DOI] [PubMed] [Google Scholar]
- 33.Dulos J, Boots AH. DHEA metabolism in arthritis: a role for the p450 enzyme Cyp7b at the immune-endocrine crossroad. Ann N Y Acad Sci. 2006;1069:401–413. doi: 10.1196/annals.1351.038. [DOI] [PubMed] [Google Scholar]
- 34.Kang HY, Lin HK, Hu YC, Yeh S, Huang KE, Chang C. From transforming growth factor-beta signaling to androgen action: identification of Smad3 as an androgen receptor coregulator in prostate cancer cells. Proc Natl Acad Sci U S A. 2001;98:3018–3023. doi: 10.1073/pnas.061305498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howes J, Waring M, Huang L, Howes LG. Long-term pharmacokinetics of an extract of isoflavones from red clover (Trifolium pratense) J Altern Complement Med. 2002;8:135–142. doi: 10.1089/107555302317371424. [DOI] [PubMed] [Google Scholar]
- 36.Slater M, Brown D, Husband A. In the prostatic epithelium, dietary isoflavones from red clover significantly increase estrogen receptor beta and E-cadherin expression but decrease transforming growth factor beta1. Prostate Cancer Prostatic Dis. 2002;5:16–21. doi: 10.1038/sj.pcan.4500546. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg Zand RS, Jenkins DJ, Diamandis EP. Genistein: a potent natural antiandrogen. Clin Chem. 2000;46:887–888. [PubMed] [Google Scholar]
- 38.Gerdes MJ, Larsen M, Dang TD, Ressler SJ, Tuxhorn JA, Rowley DR. Regulation of rat prostate stromal cell myodifferentiation by androgen and TGF-beta1. Prostate. 2004;58:299–307. doi: 10.1002/pros.10327. [DOI] [PubMed] [Google Scholar]