Abstract
Aims
The influence of CYP2C9 and VKORC1 on warfarin dose, time to target INR, time to stabilization, and risk of over-anticoagulation (INR>4) was assessed after adjustment for clinical factors, intra-individual variation in environmental factors and unobserved heterogeneity.
Materials and Methods
Common CYP2C9 and VKORC1 polymorphisms were assessed in 302 European American and 273 African Americans on warfarin. Race-stratified multivariable analyses evaluated the influence of CYP2C9 and VKORC1 on warfarin response.
Results and Conclusion
CYP2C9 and VKORC1 accounted for up to 30% variability in warfarin dose among European Americans and 10% among African Americans. Neither CYP2C9 nor VKORC1 influenced time to target INR or stabilization among patients of either race or risk of over-anticoagulation among African Americans. The risk of over-anticoagulation was higher among European Americans with variant VKORC11173C/T (p<0.01) and marginally significant among those with variant CYP2C9 (p=0.08) genotype.
Although CYP2C9 and VKORC1 genotyping can facilitate individualized initiation of warfarin dose in African and European Americans, the ability to predict risk of over-anticoagulation is inconsistent across race. Identification of other factors that can predict such risk consistently in a racially diverse group will facilitate individualized maintenance of warfarin therapy.
Keywords: Warfarin, Pharmacogenetics, CYP2C9, VKORC1, over-anticoagulation, cohort study, African Americans, European Americans
Introduction
Although the efficacy of warfarin in the treatment and prevention of thromboembolic disorders (TEDs) is proven,[1, 2] it is underutilized with difficulties in management and risk of complications being the main deterrents.[3–6] Recognition of genetic regulation of warfarin response has stimulated efforts aimed at quantifying this influence, but past efforts have focused on a limited number of polymorphisms in populations of mainly European descent. [7–17]Race appears to influence warfarin dose requirements with African Americans requiring larger and Asians requiring lower doses compared to Europeans.[18–23] This variation in dose requirement by race may at least partially be explained by genetic differences. Cytochrome P4502C9 (CYP2C9)*2 and *3 variants are reported to have significant influence on warfarin dose among patients of European descent. [7–17] However among patients of African descent the CYP2C9-dose association has not been consistent.[24–26]African American populations contain known or putative poor-metabolizer alleles (CYP2C9*5, CYP2C9*6 and CYP2C9*11) which are rarely found in European Americans.[27] However the influence of these variants has not been extensively documented. In contrast to the influence of CYP2C9, Vitamin K epoxide reductase (VKORC1)1173C/T variants have been shown to influence warfarin dose requirements among patients of both racial groups.[28]
CYP2C9*2 and *3 VKORC11173C/T variants have been associated with an increased risk of over-anticoagulation among European Americans[7, 13, 25, 28–30] but not African Americans.[25, 28] It is recognized that while genes may influence inter-individual variation in warfarin response, for the intra-individual variation environmental factors may be more important.[2, 17] For example in any given patient, the International Normalized Ratio (INR) deviates from target (2.5, range 2–3) over time in response to many independent perturbations. Such intra-individual variation in INR due to unobserved factors introduces heterogeneity. Difficulties in capturing and accounting for unobserved heterogeneity may underestimate the environmental contribution (e.g. medication changes) and overestimate the genetic contribution in explaining variance in warfarin response and limit the ability to assess gene-environment interactions.[17]
Herein we evaluate the influence of CYP2C9 (*2 (rs1799853), *3 (rs1057910), *5 (rs28371686), *6 (rs9332131), and *11(rs28371685)) and four VKORC1 single nucleotide polymorphisms [SNPs: 1173C/T (rs9934438), 3730G/A (rs7294), 2255C/T (rs2359612), 1542G/C (rs8050894)] on warfarin maintenance dose. We also assess the influence of CYP2C9 and VKORC1 polymorphisms on anticoagulation status and risk of over-anticoagulation after accounting for unobserved heterogeneity and clinical factors.
Patients & methods/Materials & methods
The Pharmacogenetic Optimization of Anticoagulation Therapy (POAT) is an ongoing prospective cohort study aimed at defining the influence of CYP2C9 polymorphisms on warfarin response over a 2-year follow-up period.[26, 31, 32] The study is being conducted at the University of Alabama at Birmingham (UAB) enrolling patients from the anticoagulation clinic at The Kirklin Clinics (TKC-AC) and the Jefferson Clinic P.C., Jefferson County Health System (CGH-JC) under the approval of the respective Institutional Review Boards. Both clinics follow a standardized approach to manage anticoagulation therapy.[33]
Inclusion and Exclusion
Patients ≥20 years of age were identified at the initiation of warfarin therapy. Patients were considered eligible if the intended duration of anticoagulation therapy was at least 2 years, the target INR range was 2–3 and are managed at one of the two anticoagulation clinics.
Data Collection
A structured interview form was used at the time of enrollment to obtain a detailed medical lifestyle and concomitant medication history. Information on self-reported race, indication for therapy, demographics, height and weight, medications and co-morbid conditions was documented. Lifestyle and socioeconomic data included smoking, alcohol use, education, annual household income, medical insurance, physical activity, and dietary vitamin K intake. Medical history was then verified by medical records review.
All patients were followed at monthly intervals for up to two years from initiation of therapy. At each visit factors influencing warfarin response such as warfarin dose, INR, concurrent medications, dietary vitamin K (number of servings of foods rich in vitamin K consumed per week)[34] alcohol intake (number of alcoholic drinks per week), compliance and level of physical activity were documented.[35–37] Information on concurrent medications was updated at every clinic visit by self-report and verified by medical record review. Concomitant use of drugs such as non-steroidal anti-inflammatory drugs or antiplatelet agents, or drugs that alter warfarin pharmacokinetics, including CYP2C9 inhibitors (e.g., amiodarone), CYP2C9 inducers (e.g., rifampin), or CYP2C9 substrates (e.g., losartan) was recorded.[38, 39]
DNA extraction and genotyping methodology for CYP2C9 and VKORC1 polymorphisms were detailed in recent manuscripts.[26, 31] Blood (8ml) was collected in a Qaigen PAX gene tube and DNA was extracted using the PAXgene blood DNA extraction kits. Briefly CYP2C9 genotyping was conducted using pyrosequencing methods and PCR-RFLP methodology. VKORC1 genotyping (rs9934438, rs7294, rs2359612 and rs8050894) was conducted using the Sequenom iPLEX technology[40] at the Broad Institute.
Outcome definitions and Statistical Methods
Analysis of variance was used to assess group differences for continuous variables and χ2 test of independence for categorical variables. The assumption of Hardy Weinberg Equilibrium (HWE) was tested using the χ2 test of independence and exact statistics were obtained using a Markov Chain Monte Carlo algorithm.[41]
Dose was defined in two ways: the average maintenance dose required to maintain anticoagulation for the duration of therapy and stable dose defined as the first dose that leads to a stable INR over three consecutive visits following initiation of the drug. These INR measurements encompassing a period of at least 2 weeks, with a maximum difference between the mean daily dosages of 10%.[7] The distribution of dose was marginally skewed to the right therefore log transformation was done to attain normality. Race-stratified linear-regression analysis was conducted to assess the influence of CYP2C9, VKORC1 genotype on log-transformed dose after adjustment for age, gender, BMI, clinic, income, education, health insurance, smoking status, level of physical activity, alcohol intake, vitamin K intake, comorbid conditions (e.g. CHF, renal failure and cancer) and drug interactions (e.g. amiodarone, statins, NSAIDs, antiplatelet agents). We evaluated dose genotype associations using models that assessed the additive and dominant effects of VKORC1 and CYP2C9. Genotypes were included as covariates with 3-levels in the additive models and as covariates with 2 levels (wild-type versus variant) in the dominant models. The effect size associated with each predictor was calculated as the percentage of the variation in warfarin dose explained by the predictor, divided by the total variance in the regression model.
We also examined the time required to attain target INR (calculated as the difference in days from initiation of therapy to achieving the first INR in target range) and time to attain stable dose (calculated as the time from the initiation of therapy until attainment of stable dose) using the Cox Proportional Hazard (PH) models. To assess the risk of over-anticoagulation (INR>4) the hazard ratio (HR) and 95% CI were obtained using the counting process format in the PH model.[42, 43] This format allows individuals to contribute more than one event (INR>4). Valid confidence intervals were obtained by correction of dependence using robust variance estimation.[44]
Accounting for unobserved heterogeneity
In any given patient, INR deviates from target (2.5, range 2–3) over time in response to many independent perturbations. Such intra-individual variation in INR due to unobserved factors introduces heterogeneity, herein termed unobserved heterogeneity.
Although the fluctuation in INR control due to medication changes is accounted for in the counting process format analyses, inability to accurately quantify this interaction (e.g. plasma concentrations) limits our ability to account for all the heterogeneity introduced by such changes in drug regimen.
Changes in diet (e.g. vitamin K intake) are documented as change from the prior visit, not quantified using a full vitamin K inventory (or plasma concentrations) at each visit. This can potentially limit our ability in explaining the variance in INR due to more subtle changes in diet, thereby increasing heterogeneity.[45]
As the interval between INR measurements increases, the variation in INR increases.[46] Therefore by default patients who are evaluated more often (e.g. patients with higher comorbidity require more frequent consultations) may tend to have lower variation (heterogeneity) in the INR.[47]
We recognize that such unobserved heterogeneity may potentially influence the time to stabilization and over-anticoagulation. To capture its effect we computed a patient-specific variance growth rate (Vscore), a cumulative measure of time-weighted variance of the INR for each time interval (between visits) as proposed by Fihn et al,[48] with minor modification. This measure adjusts for the influence of the number of visits and the interval between visits on INR variation for each patient. The use of the Vscore from the preceding interval accounts for the patient-specific unobserved heterogeneity in the analyses of time to stabilization and time to over-anticoagulation.
| Equation 1 |
σ2 = variance growth rate (Vscore)
n = number of visits (n-1 will compute the Vscore up to the preceding visit)
τi = duration in weeks since preceding clinic visit (INR measurement)
INRi = International Normalized ratio at the ith visit (target INR was 2–3 for all participants)
Modified from Fihn et al [48]
All multivariable analyses included CYP2C9, VKORC1 genotype, age, race, gender, BMI, socio-demographic factors, clinic, indication and comorbid conditions. Changes in medications, Vscore, vitamin K and alcohol intake, and level of physical activity, warfarin dose and INRs were included as time-varying covariates. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC) at a non-directional alpha level of 0.05.
Results
Patients meeting eligibility criteria between August 2003 and April 2007 (n=621) were asked to participate in the study. Forty-three (6. 9%) patients declined participation. The cohort was comprised mainly of African Americans (47.2%) and European Americans (52.2%). As described in previous reports,[26, 31, 32] there were no significant differences in gender distribution, length of follow-up or BMI across race groups. European American patients were older, more physically active, and more likely to be light drinkers (1–7 drinks/week); enrolled from TKC-AC, have medical insurance, higher education and higher income (Table 1).
Table 1.
Socio-demographic and lifestyle characteristics of the POAT study participants at baseline
| Characteristic | African American (n=273) | European American (n=302) | p-value <0.0001 |
|---|---|---|---|
| Age, mean (SD) | 58.0 (±16.0) | 63.9 (±14.7) | |
| Follow-up (months, Mean ± SD) | 14.7 (±14.7) | 15.1 (±10.8) | 0.66 |
| Body Mass Index, mean (SD) | 30.1 (±7.3) | 29.3 (±7.2) | 0.18 |
| N (%) | N(%) | ||
| Gender | |||
| Female | 144 (52.7%) | 136 (45.0%) | 0.065 |
| Male | 129 (47.3%) | 166 (55.0%) | |
| Alcohol (drinks per week)1 | |||
| 0 | 213 (78.0%) | 186 (61.6%) | <0.0001 |
| 1–7 | 37 (13.6%) | 100 (33.1%) | |
| >8 | 21 (7.7%) | 16 (5.3%) | |
| Smoking Status1 | |||
| Current | 51 (18.7%) | 27 (8.9%) | 0.007 |
| Past | 90 (33.0%) | 119 (39.4%) | |
| Never | 123 (45.0%) | 147 (48.7%) | |
| Level of Physical Activity1 | |||
| Wheelchair bound | 18 (6.6%) | 10 (3.3%) | <0.0001 |
| Uses Walker/Cane | 46 (16.8%) | 30 (9.9%) | |
| Ambulates without assistance | 55 (20.1%) | 54 (17.9%) | |
| Physically active | 121 (44.3%) | 196 (64.9%) | |
| Consistent/Intensive exercise | 1 (0.4%) | 12 (4.0%) | |
| Education | |||
| < High School | 84 (30.8%) | 22 (7.3%) | 0.0007 |
| High School | 123 (45.0%) | 109 (36.1%) | |
| College | 63 (23.0%) | 132 (43.7%) | |
| Graduate School | 3 (1.1%) | 38 (12.6%) | |
| Annual Household Income1 | |||
| <15,000 | 98 (35.9%) | 29 (9.6%) | <0.0001 |
| 15,000–25,000 | 78 (23.6%) | 28 (9.3%) | |
| 25,000–50,000 | 90 (33.0%) | 107 (35.4%) | |
| 50,000–100,000 | 5 (1.8%) | 105 (34.8%) | |
| >100,000 | 1 (0.4%) | 31 (10.3%) | |
| Medical Insurance | |||
| Medicare | 85 (31.1%) | 126 (41.7%) | <0.0001 |
| Medicare Medicaid | 5 (1.5%) | 0 (0.0%) | |
| Medicaid | 18 (6.6%) | 10 (3.3%) | |
| Private | 100 (36.6%) | 142 (47.0%) | |
| None | 64 (23.4%) | 23 (7.6%) | |
| Site | |||
| UAB Anticoagulation clinic | 88 (32.2) | 19 (6.3) | <0.0001 |
| CGH-JC Anticoagulation clinic | 185 (67.8) | 283 (93.7) | |
3 Hispanic patients excluded, all significant p-values bolded
Mean (SD) displayed for continuous variables and frequency counts (column percent) for categorical variables
Information on missing for smoking (n=18), level of physical activity (n=2), alcohol (n=2), income (n=3), education (n=1) and Insurance (n=2). Private insurance includes various plans such as Blue Cross Clue Shield, Aetna, Travelers, etc.
Stroke and venous thromboembolism were more common indications for therapy in African American patients, while atrial fibrillation and valvular heart disease were more common in European Americans. The prevalence of individual comorbid conditions differed across racial groups. More European American patients had undergone coronary artery bypass grafting (CABG) or percutaneous coronary angioplasty (PTCA), had hyperlipidemia and malignancies while the prevalence of end stage renal disease and renal insufficiency was higher among African Americans. Other than the higher use of antiplatelet agents at baseline, the use of non-steroidal analgesics, CYP2C9 inhibitors or substrates was not significantly different across racial groups African American patients had a greater intra-individual variation in INR, captured by the variable VScore (p=0.026, Table 2).
Table 2.
Clinical characteristics of the POAT study participants at enrollment.
| Characteristic | African American (n=273) | European American (n=302) | p-value |
|---|---|---|---|
| Indication for warfarin therapy* | |||
| Deep Vein Thrombosis | 97 (35.5%) | 62 (20.5%) | <0.0001 |
| Pulmonary Thromboembolism | 49 (17.9%) | 27 (8.9%) | 0.0014 |
| Recurrent Venous Thromboembolism | 22 (8.1%) | 14 (4.6%) | 0.091 |
| Atrial Fibrillation | 87 (31.9%) | 174 (57.6%) | <0.0001 |
| Valvular Heart Disease | 32 (11.7%) | 54 (17.9%) | 0.04 |
| Low Left Ventricular Ejection Fraction | 42 (15.4%) | 29 (9.6%) | 0.03 |
| Cardiac Thrombus | 12 (4.4%) | 15 (5.0%) | 0.74 |
| Myocardial Infarction | 67 (24.5%) | 95 (31.5%) | 0.066 |
| Transient Ischemic Attack | 16 (5.9%) | 20 (6.6%) | 0.71 |
| Stroke | 57 (20.9%) | 38 (12.6%) | 0.007 |
| Peripheral Vascular Disease | 30 (11.0%) | 39 (12.9%) | 0.48 |
| Site of thromboembolism** | |||
| Arterial | 109 (39.9%) | 131 (43.4%) | 0.4 |
| Venous | 138 (50.5%) | 90 (29.8%) | <0.0001 |
| Both | 30 (11.0%) | 21 (6.9%) | 0.09 |
| None | 34 (12.4%) | 44 (14.6%) | 0.46 |
| Comorbidity | |||
| History of Myocardial Infarction | 9 (3.3%) | 30 (9.9%) | 0.0016 |
| History of CABG/ PTCA | 30 (11.0%) | 68 (22.5%) | 0.0002 |
| Cardiomyopathy | 32 (11.7%) | 43 (14.2%) | 0.37 |
| Coronary Artery Disease | 82 (30.0%) | 108 (35.8%) | 0.14 |
| Congestive Heart Failure | 69 (25.3%) | 59 (19.5%) | 0.10 |
| Hypertension | 134 (49.1%) | 132 (43.7%) | 0.20 |
| Hyperlipidemia | 63 (23.1%) | 118 (39.1%) | < 0.0001 |
| Diabetes Mellitus | 94 (34.4%) | 87 (28.8%) | 0.15 |
| Malignancy | 28 (10.3%) | 56 (18.5%) | 0.005 |
| Prior Hemorrhage | 24 (8.8%) | 16 (5.3%) | 0.1 |
| Renal insufficiency | 51 (18.7%) | 36 (11.9%) | 0.024 |
| End Stage Renal Disease | 36 (13.2%) | 10 (3.3%) | <0.0001 |
| Number of Comorbid Conditions | |||
| Low (0 or 1) | 88 (32.2%) | 74 (24.5%) | 0.03 |
| Medium (2 to 4) | 129 (47.2%) | 141 (46.7%) | |
| High (5 or more) | 56 (20.5%) | 87 (28.8%) | |
| Concurrent Medications | |||
| Antiplatelet agents | 133 (49.3%) | 189 (65.6%) | 0.001 |
| NSAIDS | 37 (13.7%) | 39 (12.9%) | 0.78 |
| CYP2C9 substrates | 91 (33.7%) | 96 (31.8%) | 0.62 |
| CYP2C9 inhibitors | 50 (18.3%) | 74 (24.4%) | 0.072 |
| VScoreΨ | 0.52 [0.27, 1.24] | 0.50 [0.25, 1.13] | 0.026 |
3 Hispanic patients excluded, significant p-values bolded
All patients had a prescribed target INR range of 2–3. Patients with orthopedic surgery excluded due to short (3–6 month) treatment duration, patients with mechanical heart valve and hypercoagulable state excluded due to higher intensity of anticoagulation required
Arterial thromboembolism includes patients with MI, Stroke & TIA. Venous thromboembolism includes patients with DVT & PE. Both include patients with venous and arterial events. None includes patients with no thromboembolic events (e.g. Atrial Fibrillation).
Patients can have more than one indication for therapy and comorbid conditions
CYp2C9 inhibitors included amiodarone, metronidazole, tamoxifen, propxyphene, co-trimoxazole, etc.. (Miners)
Variability Score captures the INR variation after accounting for the influence of the number of visits and the interval between visits on INR variation for each individual patient (presented as Median and Intraqaurtile range, with Wilcoxon p-value)
Genotype distributions for CYP2C9 and VKORC1 were in HWE among European Americans (all p-values >0.5) and African Americans (all p-values >0.25). Of the variant CYP2C9 alleles tested only CYP2C9*5, CYP2C9*6, and CYP2C9*11 were observed among African Americans while CYP2C9*2, and CYP2C9*3 were observed among European Americans and African Americans. European Americans had higher frequency of variant genotype for CYP2C9, 1173C/T, 2255C/T and 1542G/C, whereas African Americans had higher variant allele frequencies for 3730G/A (Table 3). Due to significant racial differences in covariates (Tables 1 and 2) and frequencies of CYP2C9 and VKORC1 variants (Table 3) all further analyses were stratified by race.
Table 3.
CYP2C9 and VKORC1 genotype distribution among African Americans (AA) and European Americans (EA).
| Characteristic | African American No. Positive (%) | European American No. Positive (%) | p |
|---|---|---|---|
| CYP2C9† | (n=268) | (n=292) | |
| *1/*1 | 237 (88.4) | 193 (66.9) | <0.0001 |
| *1/*2 | 7 (2.6) | 58 (19.9) | |
| *1/*3 | 9 (3.3) | 28 (9.6) | |
| *1/*5 | 3 (1.1) | 0 (0.0) | |
| *1/*6 | 2 (0.74) | 0 (0.0) | |
| *1/*11 | 7 (2.6) | 0 (0.0) | |
| *2/*2 | 0 (0.0) | 5 (1.7) | |
| *2/*3 | 0 (0.0) | 7 (2.4) | |
| *3/*3 | 0 (0.0) | 1 (0.3) | |
| *3/*6 | 1 (0.37) | 0 (0.0) | |
| *5/*6 | 1 (0.37) | 0 (0.0) | |
| *5/*11 | 1 (0.37) | 0 (0.0) | |
| VKORC1† | |||
| 1173C/T (rs9934438) | (n=259) | (n=278) | |
| CC | 207 (79.9) | 110 (39.6) | <0.0001 |
| CT | 49 (18.9) | 113 (47.8) | |
| TT | 3 (1.2) | 35 (12.6) | |
| 1542G/C (rs8050894) | (n=259) | (n=278) | |
| GG | 131 (50.6) | 109 (39.2) | <0.0001 |
| GA | 112 (43.2) | 134 (48.2) | |
| AA | 16 (6.2) | 35 (12.6) | |
| 2255C/T (rs2359612) | (n=259) | (n=278) | |
| CC | 167 (64.5) | 110 (39.6) | <0.0001 |
| CT | 79 (30.5) | 132 (47.5) | |
| TT | 13 (5.0) | 36 (12.9) | |
| 3730G/A (rs7294) | (n=261) | (n=275) | |
| GG | 72 (27.6) | 105 (38.2) | 0.021 |
| GA | 136 (52.1) | 130 (47.3) | |
| AA | 53 (20.3) | 40 (14.5) | |
Excludes 3 Hispanic patients. Significant p-values bolded
Genotype data not available for 15 patients (CYP2C9) 38 patients for rs9934438 (1173C/T), rs2359612 (2255C/T), rs8050894 and 39 patients for rs7294 (3730G/A). Missing genotype information did not differ by race (all p-values > 0.2).
Influence of CYP2C9 and VKORC1 Genotype on warfarin dose
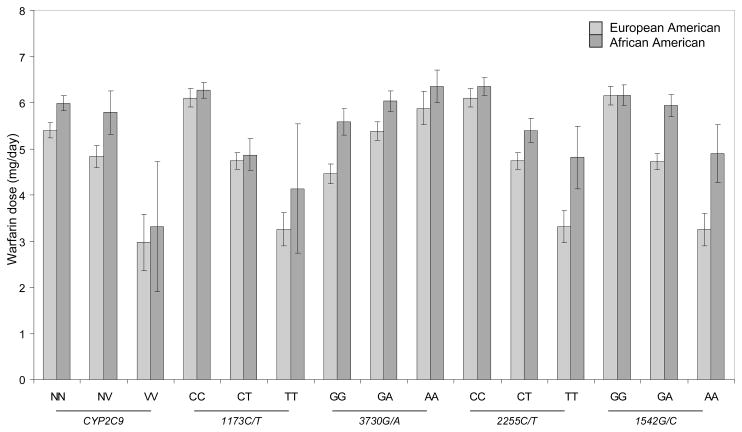
In univariate analysis, among European Americans, variant CYP2C9 and VKORC1 (1173C/T, 2255C/T and 1542G/C) genotypes were associated with lower (all p-values <0.01) and 3730G/A was associated with higher (p =0.008) warfarin dose. Among African Americans, variant VKORC1 (1173C/T, 2255C/T) genotypes were associated with lower dose (p <0.005) while variant CYP2C9 (p =0.12) and VKORC1 (1542G/C, p =0.15 and 3730G/A, p =0.2) genotypes did not have a significant influence. Univariate associations of CYP2C9 and VKORC1 variants with dose demonstrate the higher dose requirements among African Americans (Figure 1).
Figure 1.
Mean warfarin dose (mg/day) by CYP2C9 and VKORC1, stratified by race.
Among European Americans, prior reports indicate strong linkage disequilibrium between the four VKORC1 SNPs.[49, 50] Therefore multivariable analyses included the most informative VKORC1 SNP (1173C/T) along with CYP2C9 and clinical covariates. Since the LD structure for VKORC1 in African Americans is unknown, all four VKORC1 SNPs were included in the initial model. However the final model retained only VKORC11173C/T as a significant predictor.
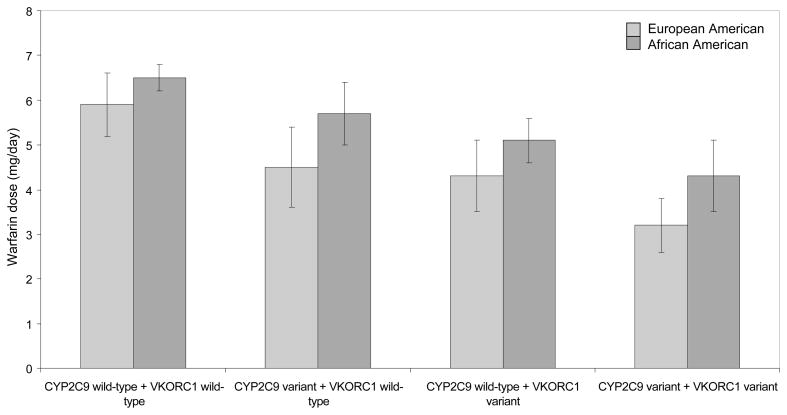
African Americans required higher warfarin maintenance doses compared to European Americans (Figure 2) after adjusting clinical and genetic covariates. The variability in dose explained by the VKORC11173C/T and CYP2C9 was lower in African Americans compared to European Americans (p<0.0001). After adjusting for potential confounders, variant CYP2C9 and VKORC11173C/T genotypes explained 30% variance in warfarin dose (20% in the dominant model) among European Americans compared to 10% variance in does (7% in the dominant model) among African Americans (Table 4). The variance in dose accounted for by CYP2C9 and VKORC11173C/T genotypes was similar for both dose definitions; average maintenance dose and first stable dose. The interaction between CYP2C9 and VKOR1173C/T genotype was not significant for either race group. Age, gender, BMI, vitamin K intake, concomitant therapy with CYP2C9 inhibitors also had significant influence on warfarin dose among patients of both race groups (p<0.05).
Figure 2.
Average warfarin dose (mg/day) by CYP2C9 and VKORC1, stratified by race after adjusting for clinical variables. The referent patient is a 61 year old man with BMI =30, current non-smoker, non-drinker, with no comorbidities (e.g. CHF, cancer, renal failure) and no inhibitors of CYP2C9 (e.g. amiodarone).
2C9 wt indicates CYP2C9 *1/*1 genotype; 2C9 v indicates one or more variant CYP2C9 allele (2, *3, *5, *6, *11). VKOR wt indicates VKORC11173’CC’ genotype; VKOR v indicates VKORC11173’CT’ or ‘TT’ genotype.
Table 4.
Multivariable analyses of the influence of CYP2C9 and VKORC1 polymorphisms on log warfarin dose
| Additive Model1 | Dominant Model2 | |||||
|---|---|---|---|---|---|---|
| r2model | r2vkor | r22C9 | r2model | r2vkor | r22C9 | |
| European Americans | ||||||
| Average dose | 53.8% | 16.7%* | 14.7%* | 41.5% | 9.6%* | 9.5%* |
| Stable Dose | 55.2% | 18.5%* | 11.8%* | 45.6% | 12.6%* | 8.3%* |
| African Americans | ||||||
| Average dose | 37.9% | 7.3%* | 3.0%* | 35.5% | 6.6%* | 1.0% |
| Stable Dose | 43.8% | 10.4%* | 0.6% | 40.8% | 7.4%* | 1.0% |
VKORC1 and CYP2C9 genotypes were included as covariates with 3-levels in the additive models.
VKORC1 and CYP2C9 genotypes were included as covariates with 2 levels (wild-type versus variant) in the dominant models.
r2model denotes percent variation in warfarin dose explained by the model containing clinical and genetic covariates
r2vkor is the semi-partial r2 denoting percent variation in warfarin dose explained by VKORC1 SNP
r22C9 is the semi-partial r2 denoting percent variation in warfarin dose explained by CYP2C9 (Variant includes *2, *3, *5, *6 and *11 alleles) genotype
Average dose is dose required to maintain anticoagulation for the duration of therapy after attainment of target INR
Stable dose defined as the first dose that leads to a stable INR over three consecutive visits following initiation of the drug, with these INR measurements encompassing a period of at least 2 weeks, with a maximum difference between the mean daily dosages of 10%.
Adjusted for age, gender, BMI, Follow-up clinic, income, smoking status, education, health insurance, drug interactions (e.g. amiodarone), alcohol intake, vitamin K intake, comorbid conditions (CHF and cancer) and CYP2C9 and VKORC11173C/T.
Genotype explains a statistically significant (at alpha level of 0.05) proportion of variance in warfarin dose
Influence of CYP2C9 and VKORC1173C/T on anticoagulation control
We assessed the effects of VKORC11173C/T on time to target INR, stabilization or risk of over-anticoagulation after accounting for clinical covariates and unobserved heterogeneity (Table 5).
Table 5.
Adjusted risk ratios regarding the influence of CYP2C9 and VKORC1 on time to attaining anticoagulation endpoints
| Endpoint | CYP2C9 (variant vs. wild-type) | VKORC11173C/T (variant vs. wild-type) | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Time to first target INR | ||||
| European American | 1.23 (0.96, 1.58) | 1.09 (0.72, 1.65) | 1.11 (0.87, 1.42) | 1.09 (0.80, 1.49) |
| African American | 1.27 (0.87, 1.87) | 1.15 (0.73, 1.83) | 1.11 (0.80, 1.52) | 0.99 (0.69, 1.41) |
| Time to stable dosing* | ||||
| European American | 1.11 (0.85, 1.45) | 0.93 (0.59, 1.48) | 0.96 (0.74, 1.25) | 0.89 (0.63, 1.26) |
| African American | 0.98 (0.63, 1.53) | 0.85 (0.50, 1.43) | 0.98 (0.66, 1.46) | 1.41 (0.90, 2.22) |
| Time to INR >4 during first 30 days*$ | ||||
| European American | 1.23 (0.83, 1.81) | 2.84 (0.91, 8.85) | 2.67 (1.57, 4.57) | 3.75 (1.52, 9.30) |
| African American | 1.16 (0.56, 2.44) | 0.77 (0.19, 3.09) | 1.63 (0.93, 2.83) | 1.26 (0.57, 2.76) |
| Time to INR >4 prior to stabilization**$ | ||||
| European American | 1.33 (0.98, 1.81) | 2.15 (0.90, 5.13) | 2.28 (1.55, 3.35) | 2.69 (1.32, 5.47) |
| African American | 1.00 (0.60, 1.69) | 0.87 (0.34, 2.21) | 1.24 (0.83, 1.86) | 1.08 (0.69, 1.68) |
| Time to INR >4 during duration of follow-up*$ | ||||
| European American | 1.27 (1.03, 1.58) | 1.82 (0.97, 3.43) | 1.68 (1.33, 2.11) | 1.91 (1.27, 2.89) |
| African American | 1.11 (0.83, 1.49) | 0.66 (0.34, 1.29) | 0.93 (0.71, 1.23) | 0.72 (0.43, 1.20) |
CYP2C9 Variant genotype includes one or more (*2, *3, *5, *6 and *11) alleles.
Variant VKORC11173C/T includes ‘TT or CT’
All models adjusted for age, gender, BMI, CYP2C9, VKOR1173C/T, interaction between CYP2C9*VKOR1173C/T, number of comorbid conditions, vitamin K intake, drug interactions (e.g amiodarone), clinic site, education, income, indication, insurance and current smoking.
Models adjusted for Vscore, the variance growth rate, a measure of INR variability up to the preceding visit was also included in analyses of time to stable dosing and time to INR >4.
Models adjusted for correlation between repeat episodes of INR>4 within the patient
Median time to stabilization (95 days, Intraquartile range 42–175 days) was used to assess genotype related risk of over-anticoagulation. Analyses based on individual time to stabilization could not be conducted due to model non-convergence on stratification by race among African Americans.
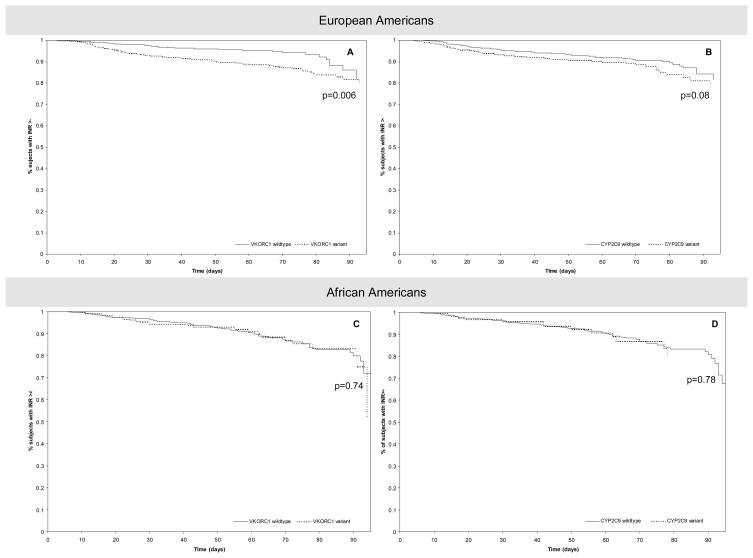
Neither CYP2C9 nor VKORC11173C/T influenced the time to attain target INR or time to stabilization among patients of either race. European Americans patients with variant VKORC11173C/T genotype showed a 2 to 4 fold higher risk of over-anticoagulation (Figure 3a). Those with variant CYP2C9 genotype were at a 2 to 3 fold higher risk; however, this finding was of marginal statistical significance (Table 5, Figure 3b). Neither VKORC11173C/T nor CYP2C9 was significantly associated with risk of over anticoagulation among African Americans (Table 5, Figure 3c and 3d).
Figure 3.
Estimated survival curves from Cox PH model for European Americans (A and B) and African Americans (C and D). Dotted line indicates variant genotype; Full line indicates wild-type genotype.
A. Adjusted for CYP2C9 genotype, age, sex, BMI, smoking, vitamin K intake, number of comorbid conditions, drug interactions, Vscore and socio-demographic factors
B. Adjusted for VKORC1 1173C/T genotype, age, sex, BMI, smoking, vitamin K intake, number of comorbid conditions, drug interactions, Vscore and socio-demographic factors.
C. Adjusted for CYP2C9 genotype, age, sex, BMI, smoking, vitamin K intake, number of comorbid conditions, drug interactions, Vscore and socio-demographic factors.
D. Adjusted for VKORC1 1173C/T genotype, age, sex, BMI, smoking, vitamin K intake, number of comorbid conditions, drug interactions, Vscore and socio-demographic factors.
The interaction of CYP2C9 and VKOR1173C/T genotypes was not significant for any endpoint. Time to stable dosing was not significantly influenced by Vscore prior to stabilization for patients of either race (p-values >0.1). Higher Vscore was associated with a significantly higher risk of over anticoagulation through out the duration of therapy (p<0.0001) among both racial groups. The risk was higher during the first 30 days of therapy (p=0.06 for European Americans, p=0.03 for African Americans) but not around the time when stabilization is first achieved (p=0.56 for European Americans, p=0.81 for African Americans).
Discussion
This prospective cohort study demonstrates the contribution of CYP2C9 and VKORC1 variants in determining warfarin dose in a racially diverse cohort. VKORC11173C/T genotype explains a higher proportion of variance in warfarin dose compared CYP2C9 for both race groups.
By evaluating both additive and dominant effects of VKORC1 and CYP2C9 we demonstrate the variance in warfarin dose explained is higher when VKORC1 and CYP2C9 genotype covariates were modeled on an additive scale compared to dominant scale. This finding was consistent among both European Americans and African Americans. Among African Americans, the variance in warfarin dose explained by CYP2C9 and VKORC1 (dominant model) was consistent with prior reports.[25, 26, 28] However, contrary to prior reports and concordant with those of Momary et al,[24] the additive model explained a higher percent variance and revealed statistically significant effects of CYP2C9 on warfarin dose in African Americans. Consistent with prior reports[24] and others age, gender, BMI, vitamin K intake, concomitant therapy with CYP2C9 inhibitors also had significant influence on warfarin dose among patients of both race groups.[8, 10–12, 15, 16, 24, 50–56]
By defining dose in two ways; dose at first stabilization and average maintenance dose we demonstrated consistent influence of VKORC1 and CYP2C9 genotype regardless of definition. VKORC1 and CYP2C9 along with clinical covariates explained up to 55% of the variance in dose in European Americans (up to 40% among African Americans) with VKORC1 and CYP2C9 accounting for up to 30% (10% among African Americans). Among European Americans these findings are consistent with the prior reports.[10, 11, 15, 19–21, 23, 49, 50, 57, 58] However the latter estimates, derived from mainly retrospective studies in homogeneous populations, may not hold in racially diverse populations as demonstrated by Schellman et al.[28] In concordance with their findings, the variability in dose explained by the VKORC11173C/T and CYP2C9 was lower in African Americans compared to European Americans.
Consistent with Schellman et al[28] and Kealy et al,[25] our findings demonstrate the lack of influence of VKORC1 and CYP2C9 on time to stabilization in patients of either race. However these findings are discordant with those reported by Schalekamp et al.[7, 13, 29, 30] This discordance can potentially be explained by several factors including differences in the coumarin anticoagulant, dose initiation strategies, and important protocol differences such as exclusion of interacting drugs.
As reported by Schellman et al,[28] the VKORC11173’T’ allele was significantly associated with an increased risk of over-anticoagulation among European Americans but not African Americans. The CYP2C9 variant was marginally associated with an increased risk of over-anticoagulation among European Americans, which is consistent with previous studies[25]. Although we do not know the reason for the racial differences, we can speculate on the influence and interplay of various factors:
The contribution of unmeasured genetic/environmental factors to INR fluctuation may differ by race. This was supported by the significance of race-gene interactions in race adjusted analyses for time to stabilization (VKORC1 1173C/T x race, p=0.03) and risk of over anticoagulation (VKORC1 1173C/T x race, p=0.002; CYP2C9 x race, p=0.09). Recognizing race specific differences we chose to conduct stratified analyses.
We had to combine the ‘CT and TT’ genotypes for multivariable analyses because of the small number of patients with the ‘TT’ genotype thereby diluting the effect of VKORC1 polymorphism on risk of over-anticoagulation.
The association between the VKORC1 polymorphisms studied and the causative polymorphism(s) that determines warfarin response is weaker in African Americans compared with European Americans because of different haplotype structures.
Genetic and environmental factors other then those studied influence the risk of over-anticoagulation in African Americans. This idea is supported by the higher intra-individual variation in INR among African Americans compared to European Americans.
Among European Americans, the risk ratios in our study were lower than those reported by Kealy et al and Schellamn et al.[25, 28] This can perhaps be explained by the inclusion of a measure of unobserved heterogeneity in the analyses. Inability to account for such heterogeneity has been recognized as a limitation by several investigators.[9, 17, 28] Our results provide evidence that the higher risk of over-anticoagulation associated with variant VKORC11173C/T among European Americans is independent of such heterogeneity.
We recently reported a significantly increased risk of major hemorrhage conferred by variant CYP2C9 genotype but not by variant VKORC1 1173C/T genotype after adjusting for the influence of INR at the time of the event and other clinical covariates. The risk was statistically significant among European Americans but not in African Americans. The latter finding may be due to the lower frequency of the variant genotypes in African Americans.[31] Along with CYP2C9, an elevated INR significantly increased the risk of major hemorrhage. For every unit increase in INR the risk of major hemorrhage increased by 75% (HR 1.74, 95%CI: 1.5, 2.1). In both African American and European American patients these gene-response associations highlight two things; the risk of hemorrhage is higher among patients who possess CYP2C9 variants and the risk of hemorrhage is higher among patients who have elevated INR. These effects are independent of each other.
To our knowledge, our cohort represents the largest population of African Americans genotyped for CYP2C9 and VKORC1. Inclusion of the *5, *6 and *11 variants in the genotyping provides a robust estimate of the CYP2C9 allele frequencies in this previously underrepresented racial group. We examined only four SNPs in the VKORC1 gene (1173C/T, 3730G/A, 2255C/T, 1542G/C). We did not assess the -1639G/A polymorphism (rs9923231) as studies have demonstrated that the 1173C and -1639G allele are in linkage disequilibrium among both African Americans[28] and European Americans.[49] Three of these polymorphisms (1173C/T, 2255C/T, 1542G/C) are part of the haplotype that has been associated with a relatively low hepatic VKORC1 mRNA expression in the liver of European Americans.[49] Furthermore, Rieder et al showed that the 1173C/T polymorphism alone was as informative as VKORC1 haplotypes for predicting warfarin dose in a Caucasian population.[49] The haplotype structure differs significantly between persons of European versus African descent[20, 59] and may differ among African Americans across the US depending on the degree of racial admixture.[60–62] Therefore all four SNPs were included in the initial models for African Americans. Assessment of other VKORC1 polymorphisms will help determine haplotype structure and may identify other influential VKORC1 polymorphisms among African Americans.
We also recognize our sample-size was inadequate to detect significant CYP2C9-VKORC1 interaction in either race group. After adjusting for statistically significant and clinically relevant covariates a post-hoc assessment of power demonstrates the adequacy of the cohort size to detect significant dose differences (between variant and wild-type genotype) for VKORC1 among European Americans and African Americans and for CYP2C9 among European Americans (power >80%). However among African Americans significant dose difference was not detected for CYP2C9 (power ~40%) except when it was modeled on an additive scale for average dose. For most anticoagulation endpoints the risk ratios detected were consistent with the null for both European Americans and African Americans. Only risk ratios for INR >4 demonstrated an increased risk of over-anticoagulation among European Americans with variant VKORC1 genotype (power >80%) but not for variant CYP2C9 genotype (power ~70%). Documentation of vitamin K intake was based on patient report using vitamin K inventory and was not quantified by assay/measurements.[34] However, all measurements were used consistently; therefore, bias if any should be non-differential. We recognize that many factors including changes in vitamin K intake can contribute to INR fluctuation.[45, 63] The inclusion of the Vscore potentially accounts for the changes in unmeasured/unobserved environmental influences. We assessed the influence of only two genes (CYP2C9 and VKORC1) and recognize that other genes may influence warfarin response or modify the effect of these genes. ApoE has recently been shown to influence warfarin dose among African Americans. Other genes such as gamma-glutamyl carboxylase, calumenin, epoxide hydroxylase may influence warfarin dose in this race group. However, the extent to which variability in other genes in the warfarin pathways influences warfarin response is yet to be resolved.
Conclusion
In conclusion the CYP2C9 and VKORC1 variants are associated with lower warfarin dose requirements among both African Americans and European Americans. Although CYP2C9 and VKORC1 genotyping have the potential to facilitate the development of individually tailored warfarin dose in both African and European Americans, the ability to predict risk of over-anticoagulation is limited to European Americans. Perhaps future identification of other genetic/environmental factors that can predict such risk consistently in the racially diverse group of patients encountered in clinical practice will facilitate individually tailored maintenance of warfarin therapy.
Executive Summary
The limited representation of African Americans has hindered our understanding of genetic influences on warfarin response in this racial group.
In this prospective cohort study we assessed the effect of CYP2C9 *2, *3, *5, *6 and *11 and four VKORC1 polymorphisms in African Americans and European Americans after adjustment for numerous mediators of warfarin response.
We assessed the influence of CYP2C9 and VKORC1 polymorphisms on both stable dose and average maintenance dose. The evaluation of gene effects on both dominant and additive scale demonstrates the importance of both genes in determining warfarin dose in African Americans and European Americans.
In assessing the risk of over-anticoagulation we accounted for unmeasured heterogeneity.
VKORC1 variants are associated with a higher risk of over-anticoagulation (with CYP2C9 demonstrating a marginal significance) and in European Americans but not in African Americans.
Although CYP2C9 and VKORC1 variants influence warfarin dose in both African and European Americans, further research is needed to identify genetic and environmental determinants of over-anticoagulation in African Americans.
Acknowledgments
Supported in part by a grant from the National Institute of Neurological Disorders and Stroke (Grant Number: K23NS45598-01) and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences
Financial disclosure/ Acknowledgments
The authors thank Joyce Blaisdell for her work with CYP2C9 genotyping. The first author wishes to acknowledge Dr. Edward Faught for his support and mentorship. We are grateful to all the patients that participated in the study. We thank Janice Ware for her untiring efforts with patient recruitment and the staff of the Anticoagulation Clinic at The Kirklin Clinic, the Cooper Green Hospital and Jefferson Clinic P.C for their help with identification of potential participants. We also thank the physicians, especially Drs. Mark Wilson, and Melissa Baird; at the University of Alabama at Birmingham and the Health Service Foundation for their support of this research. Thanks to Steve Duncan and Darlene Green and the Office of Data Resources for their work with the POAT database and quality assurance.
This work was supported in part by a grant from the National Institute of Neurological Disorders and Stroke (Grant number K23NS45598-01) and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. This study has contributed samples to the NINDS Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org/ninds), NINDS Repository sample numbers corresponding to the samples used are ND04466, ND04556, ND04604, ND04605, ND04626, ND04869, ND04907, ND04934, ND04951, ND05036, ND05108, ND05175, ND05176, ND05239, ND05605, ND05606, ND05701, ND05702, ND05735, ND06147, ND06207, ND06385, ND06424, ND06480, ND06706, ND06814, ND06871, ND06983, ND07057, ND07234, ND07304, ND07494, ND07602, ND07711, ND07712, ND08065, ND08596, ND08864, ND08932, ND09079, ND09172, ND09760, ND09761, ND09809.
The Broad Institute Center for Genotyping and Analysis is supported by grant U54 RR020278-01 from the National Center for Research Resources.
References
- 1.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57. [PubMed] [Google Scholar]
- 2.Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119:8S–21S. doi: 10.1378/chest.119.1_suppl.8s. [DOI] [PubMed] [Google Scholar]
- 3.Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–80. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 4.Stafford RS, Radley DC. The underutilization of cardiac medications of proven benefit, 1990 to 2002. J Am Coll Cardiol. 2003;41:56–61. doi: 10.1016/s0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]
- 5.Tapson VF, Hyers TM, Waldo AL, et al. Antithrombotic therapy practices in US hospitals in an era of practice guidelines. Arch Intern Med. 2005;165:1458–64. doi: 10.1001/archinte.165.13.1458. [DOI] [PubMed] [Google Scholar]
- 6.Wittkowsky AK. Effective anticoagulation therapy: defining the gap between clinical studies and clinical practice. Am J Manag Care. 2004;10:S297–306. discussion S12–7. [PubMed] [Google Scholar]
- 7.Schalekamp T, Brasse BP, Roijers JF, et al. VKORC1 and CYP2C9 genotypes and phenprocoumon anticoagulation status: interaction between both genotypes affects dose requirement. Clin Pharmacol Ther. 2007;81:185–93. doi: 10.1038/sj.clpt.6100036. [DOI] [PubMed] [Google Scholar]
- 8.Carlquist JF, Horne BD, Muhlestein JB, et al. Genotypes of the cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. J Thromb Thrombolysis. 2006;22:191–7. doi: 10.1007/s11239-006-9030-7. [DOI] [PubMed] [Google Scholar]
- 9.Wadelius M, Chen LY, Eriksson N, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sconce EA, Khan TI, Wynne HA, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–33. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 11.D’Andrea G, D’Ambrosio RL, Di Perna P, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–9. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 12.Herman D, Peternel P, Stegnar M, Breskvar K, Dolzan V. The influence of sequence variations in factor VII, gamma-glutamyl carboxylase and vitamin K epoxide reductase complex genes on warfarin dose requirement. Thromb Haemost. 2006;95:782–7. [PubMed] [Google Scholar]
- 13.Schalekamp T, Brasse BP, Roijers JF, et al. VKORC1 and CYP2C9 genotypes and acenocoumarol anticoagulation status: interaction between both genotypes affects overanticoagulation. Clin Pharmacol Ther. 2006;80:13–22. doi: 10.1016/j.clpt.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Bodin L, Verstuyft C, Tregouet DA, et al. Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood. 2005;106:135–40. doi: 10.1182/blood-2005-01-0341. [DOI] [PubMed] [Google Scholar]
- 15.Aquilante CL, Langaee TY, Lopez LM, et al. Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin Pharmacol Ther. 2006;79:291–302. doi: 10.1016/j.clpt.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell MD, Berg RL, Zhang KQ, et al. Evaluation of genetic factors for warfarin dose prediction. Clin Med Res. 2007;5:8–16. doi: 10.3121/cmr.2007.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Wadelius M, Pirmohamed M. Pharmacogenetics of warfarin: current status and future challenges. Pharmacogenomics J. 2006 doi: 10.1038/sj.tpj.6500417. Reviews all potential candidate genes in the warfarin pharmacodynamic and pharmacokinetic pathway. [DOI] [PubMed] [Google Scholar]
- 18.Dang MT, Hambleton J, Kayser SR. The influence of ethnicity on warfarin dosage requirement. Ann Pharmacother. 2005;39:1008–12. doi: 10.1345/aph.1E566. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Wilkinson GR, Nutescu EA, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics. 2006;16:101–10. doi: 10.1097/01.fpc.0000184955.08453.a8. [DOI] [PubMed] [Google Scholar]
- 20.Geisen C, Watzka M, Sittinger K, et al. VKORC1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thromb Haemost. 2005;94:773–9. doi: 10.1160/TH05-04-0290. [DOI] [PubMed] [Google Scholar]
- 21.Lee SC, Ng SS, Oldenburg J, et al. Interethnic variability of warfarin maintenance requirement is explained by VKORC1 genotype in an Asian population. Clin Pharmacol Ther. 2006;79:197–205. doi: 10.1016/j.clpt.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Veenstra DL, You JH, Rieder MJ, et al. Association of Vitamin K epoxide reductase complex 1 (VKORC1) variants with warfarin dose in a Hong Kong Chinese patient population. Pharmacogenet Genomics. 2005;15:687–91. doi: 10.1097/01.fpc.0000174789.77614.68. [DOI] [PubMed] [Google Scholar]
- 23.Yuan HY, Chen JJ, Lee MT, et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet. 2005;14:1745–51. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 24*.Momary KM, Shapiro NL, Viana MA, Nutescu EA, Helgason CM, Cavallari LH. Factors influencing warfarin dose requirements in African-Americans. Pharmacogenomics. 2007;8:1535–44. doi: 10.2217/14622416.8.11.1535. Demonstrates significant influence of CYP2C9 on warfarin dose among African Americans. [DOI] [PubMed] [Google Scholar]
- 25.Kealey C, Chen Z, Christie J, et al. Warfarin and cytochrome P450 2C9 genotype: possible ethnic variation in warfarin sensitivity. Pharmacogenomics. 2007;8:217–25. doi: 10.2217/14622416.8.3.217. [DOI] [PubMed] [Google Scholar]
- 26.Limdi NA, Goldstein JA, Blaisdell JA, Beasley TM, Rivers CA, Acton RT. Influence of CYP2C9 Genotype on warfarin dose among African American and European Americans. Personalized Medicine. 2007;4:157–69. doi: 10.2217/17410541.4.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaisdell J, Jorge-Nebert LF, Coulter S, et al. Discovery of new potentially defective alleles of human CYP2C9. Pharmacogenetics. 2004;14:527–37. doi: 10.1097/01.fpc.0000114759.08559.51. [DOI] [PubMed] [Google Scholar]
- 28.Schelleman H, Chen Z, Kealey C, et al. Warfarin Response and Vitamin K Epoxide Reductase Complex 1 in African Americans and Caucasians. Clin Pharmacol Ther. 2007 doi: 10.1038/sj.clpt.6100144. [DOI] [PubMed] [Google Scholar]
- 29.Schalekamp T, Oosterhof M, van Meegen E, et al. Effects of cytochrome P450 2C9 polymorphisms on phenprocoumon anticoagulation status. Clin Pharmacol Ther. 2004;76:409–17. doi: 10.1016/j.clpt.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Schalekamp T, van Geest-Daalderop JH, de Vries-Goldschmeding H, Conemans J, Bernsen Mj M, de Boer A. Acenocoumarol stabilization is delayed in CYP2C93 carriers. Clin Pharmacol Ther. 2004;75:394–402. doi: 10.1016/j.clpt.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 31*.Limdi N, McGwin G, Goldstein J, et al. Influence of CYP2C9 and VKORC1 1173C/T Genotype on the Risk of Hemorrhagic Complications in African-American and European-American Patients on Warfarin. Mol Ther. 2008;83:312–21. doi: 10.1038/sj.clpt.6100290. Demonstrates influence of CYP2C9 on risk of hemorrhagic complications among African Americans and European Americans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limdi NA, Beasley TM, Allison DB, Rivers CA, Acton RT. Racial differences in the prevalence of Factor V Leiden mutation among patients on chronic warfarin therapy. Blood Cells Mol Dis. 2006;37:100–6. doi: 10.1016/j.bcmd.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansell JEOL, Wittkowsky AK. Managing Oral Anticoagulation Therapy: Clinical and Operational Guidelines. 2. St. Louis, MO: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 34.Booth SLSJ, Weihrauch JL, Ferland G. Vitamin K (phylloquinone) content of foods. J Food Consump Anla. 1993;6:109–20. [Google Scholar]
- 35.Lenz TL, Lenz NJ, Faulkner MA. Potential interactions between exercise and drug therapy. Sports Med. 2004;34:293–306. doi: 10.2165/00007256-200434050-00002. [DOI] [PubMed] [Google Scholar]
- 36.Shibata Y, Hashimoto H, Kurata C, Ohno R, Kazui T, Takinami M. Influence of physical activity on warfarin therapy. Thromb Haemost. 1998;80:203–4. [PubMed] [Google Scholar]
- 37.Wittkowsky AK, Devine EB. Frequency and causes of overanticoagulation and underanticoagulation in patients treated with warfarin. Pharmacotherapy. 2004;24:1311–6. doi: 10.1592/phco.24.14.1311.43144. [DOI] [PubMed] [Google Scholar]
- 38.Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–38. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Rettie AE, Jones JP. Clinical and toxicological relevance of CYP2C9: drug-drug interactions and pharmacogenetics. Annual Review of Pharmacology & Toxicology. 2005;45:477–94. doi: 10.1146/annurev.pharmtox.45.120403.095821. Reviews clinical significance of drug interactions involving CYP2C9. [DOI] [PubMed] [Google Scholar]
- 40.Sequenom, Inc. http://www.sequenom.com.
- 41.Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–72. [PubMed] [Google Scholar]
- 42.Ake CF, Carpenter AL. Proceedings of the 11th Annual Western Users of SAS. Cary, NC: SAS Institute Inc; 2003. Extending the Use of PROC PHREG in Survival Analysis. [Google Scholar]
- 43.Therneau TM, Grambsch PM. The Cox Model. In: Therneau TM, Grambsch PM, editors. Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health) 1. New York, New York: Springer-Verlag; 2001. pp. 39–79. [Google Scholar]
- 44.Therneau TM, Grambsch PM. Multiple Events per Subject. In: Therneau TM, Grambsch PM, editors. Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health) 1. New York, New York: Springer-Verlag; 2001. pp. 169–229. [Google Scholar]
- 45.Couris R, Tataronis G, McCloskey W, et al. Dietary vitamin K variability affects International Normalized Ratio (INR) coagulation indices. Int J Vitam Nutr Res. 2006;76:65–74. doi: 10.1024/0300-9831.76.2.65. [DOI] [PubMed] [Google Scholar]
- 46.Ansell J, Hollowell J, Pengo V, Martinez-Brotons F, Caro J, Drouet L. Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: the international study of anticoagulation management (ISAM) J Thromb Thrombolysis. 2007;23:83–91. doi: 10.1007/s11239-006-9022-7. [DOI] [PubMed] [Google Scholar]
- 47.Shalev V, Rogowski O, Shimron O, et al. The interval between prothrombin time tests and the quality of oral anticoagulants treatment in patients with chronic atrial fibrillation. Thromb Res. 2007;120:201–6. doi: 10.1016/j.thromres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Annals of Internal Medicine. 1993;118:511–20. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 49*.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–93. doi: 10.1056/NEJMoa044503. Describes VKORC1 haplotype structure among European Americans and their influence on warfarin dose. [DOI] [PubMed] [Google Scholar]
- 50.Wadelius M, Chen LY, Downes K, et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5:262–70. doi: 10.1038/sj.tpj.6500313. [DOI] [PubMed] [Google Scholar]
- 51.Anderson JL, Horne BD, Stevens SM, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116:2563–70. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 52*.Caldwell MD, Awad T, Johnson JA, et al. CYP4F2 genetic variant alters required warfarin dose. Blood. 2008 doi: 10.1182/blood-2007-11-122010. Describes the influence of CYP2F4 on warfarin dose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Mol Ther. 2008 doi: 10.1038/clpt.2008.10. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura R, Miyashita K, Kokubo Y, et al. Genotypes of vitamin K epoxide reductase, gamma-glutamyl carboxylase, and cytochrome P450 2C9 as determinants of daily warfarin dose in Japanese patients. Thromb Res. 2007;120:181–6. doi: 10.1016/j.thromres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Wu AH. Use of genetic and nongenetic factors in warfarin dosing algorithms. Pharmacogenomics. 2007;8:851–61. doi: 10.2217/14622416.8.7.851. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y, Shennan M, Reynolds KK, et al. Estimation of Warfarin Maintenance Dose Based on VKORC1 (−1639 G>A) and CYP2C9 Genotypes. Clin Chem. 2007 doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 57.Vecsler M, Loebstein R, Almog S, et al. Combined genetic profiles of components and regulators of the vitamin K-dependent gamma-carboxylation system affect individual sensitivity to warfarin. Thromb Haemost. 2006;95:205–11. doi: 10.1160/TH05-06-0446. [DOI] [PubMed] [Google Scholar]
- 58*.Veenstra DL, Blough DK, Higashi MK, et al. CYP2C9 haplotype structure in European American warfarin patients and association with clinical outcomes. Clin Pharmacol Ther. 2005;77:353–64. doi: 10.1016/j.clpt.2005.01.019. Describes CYP2C9 haplotype structure among European Americans and their influence on warfarin dose. [DOI] [PubMed] [Google Scholar]
- 59.Marsh S, King CR, Porche-Sorbet RM, Scott-Horton TJ, Eby CS. Population variation in VKORC1 haplotype structure. J Thromb Haemost. 2006;4:473–4. doi: 10.1111/j.1538-7836.2006.01759.x. [DOI] [PubMed] [Google Scholar]
- 60.Kuffner T, Whitworth W, Jairam M, McNicholl J. HLA class II and TNF genes in African Americans from the Southeastern United States: regional differences in allele frequencies. Hum Immunol. 2003;64:639–47. doi: 10.1016/s0198-8859(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 61.Reed TE. Caucasian genes in American Negroes. Science. 1969;165:762–8. doi: 10.1126/science.165.3895.762. [DOI] [PubMed] [Google Scholar]
- 62.Reitnauer PJ, Go RC, Acton RT, et al. Evidence for genetic admixture as a determinant in the occurrence of insulin-dependent diabetes mellitus in U.S. blacks. Diabetes. 1982;31:532–7. doi: 10.2337/diab.31.6.532. [DOI] [PubMed] [Google Scholar]
- 63.Schurgers LJ, Shearer MJ, Hamulyak K, Stocklin E, Vermeer C. Effect of vitamin K intake on the stability of oral anticoagulant treatment: dose-response relationships in healthy subjects. Blood. 2004;104:2682–9. doi: 10.1182/blood-2004-04-1525. [DOI] [PubMed] [Google Scholar]